Abstract
The formin protein mDia2 plays a critical role in a number of cellular processes through its ability to promote nucleation and elongation of actin filaments. In erythroblasts, this includes control of cytokinesis and enucleation by regulating contractile actin ring formation. Here we report a novel mechanism of how mDia2 is regulated: through acetylation and deacetylation at lysine 970 in the formin homology 2 domain. Ectopic expression of an acetyl-mimic mDia2 mutant in mouse erythroblasts is sufficient to abolish contractile actin ring formation at the cleavage furrow and subsequent erythrocyte cytokinesis and enucleation. We also identified that class II histone deacetylase 6 deacetylates and subsequently activates mDia2. Knockdown or inhibition of histone deacetylase 6 impairs contractile actin ring formation, and expression of a non-acetyl-mimic mDia2 mutant restores the contractile actin ring and rescues the impairment of enucleation. In addition to revealing a new step in mDia2 regulation, this study may unveil a novel regulatory mechanism of formin-mediated actin assembly, since the K970 acetylation site is conserved among Dia proteins
Introduction
Formin proteins play important roles in many different cellular processes such as cell adhesion, cell migration, cytokinesis, phagocytosis, endosomal trafficking, synaptic growth and the maintenance of cell polarity by directing actin nucleation and polymerization.1,2 The Diaphanous-related formins are a subfamily of formins that are effectors of Rho-family GTPases.1,3 The mammalian Diaphanous-related formin (mDia2) is important for cell motility, cell polarity, vesical trafficking and cytokinesis.1,3–6 All formin proteins contain the formin homology 2 (FH2) domain, which directly mediates actin assembly.3,7,8 Two FH2 domains form a doughnut-shaped dimer that nucleates new, unbranched actin filaments and moves along the actin filaments to promote the addition of further actin monomers.2,9–11 The adjacent formin homology 1 domain accelerates actin assembly by recruitment of profilin-bound G-actin.2,11,12
The process of erythropoiesis starts with the multipotent hematopoietic stem cell. The erythroid progenitor cells derived from these multipotent cells undergo terminal erythroid differentiation through a series of maturation stages to produce enucleated reticulocytes which subsequently mature into red blood cells. At the late stage of differentiation, the nuclear chromosomes become highly condensed and F-actin bundles accumulate between the extruding nucleus and nascent reticulocyte. Resembling the process in cytokinesis, non-muscle myosin and F-actin form a contractile actin ring (CAR) at the cleavage furrow between the incipient reticulocyte and pyrenocyte, resulting in subsequent enucleation.13,14 Mice deficient in mDia2 survive until E11.5 and exhibit severe anemia with multi-nucleated erythroblasts. The mDia2-deficient erythroid cells fail to complete cytokinesis and have decreased accumulation of F-actin in the cleavage furrow during late differentiation from proerythroblasts.15 Conditional knockout of mDia2 at adult stage shows ineffective erythropoiesis with bi-and multi-nucleated erythroblasts,16 demonstrating the critical roles of mDia2 in erythropoiesis.
Lysine acetylation is a widely occurring post-translational protein modification involved in various functions in transcriptional regulation and cellular processes in eukaryotic cells.17 The enzymes responsible for acetyl group addition to or removal from the ε-amino groups of target proteins are known as histone acetyltransferases and histone deacetylases (HDAC), respectively. HDAC6, a class IIb member of HDAC, has been shown to play essential roles in regulating cell migration, protein trafficking and accumulation of misfolded proteins into the aggressome.17,18 HDAC6 is a primary tubulin deacetylase and regulates tubulin-mediated cell motility, migration and cytokinesis.19–21 HDAC6 also regulates cell motility and endocytosis through regulating actin remodeling.22,23 The role of HDAC6 in hematopoiesis, especially erythropoiesis, is completely unknown.
In this study, we found that knockdown of HDAC6 or inhibition of HDAC6 activity prevents cytokinesis and enucleation in cultured mouse fetal erythroblasts through disruption of CAR formation. We further discovered that HDAC6 regulates these processes through deacetylation of mDia2. The overexpression of non-acetyl-mimic mDia2 rescues the HDAC6 knockdown defect in enucleation. Our study therefore unveils a novel regulatory mechanism of formin protein by which mDia2 mediates actin organization and subsequently controls terminal erythroblast maturation.
Methods
Retroviral production and infection
The generation of retroviral particles and viral infection have been described previously.24 The infection rate was determined at around 60% by infection of green-fluorescent protein (GFP)-containing virus. For cells co-infected with MSCV–GFP-mDia2 shRNA and MICD4 mDia2, positive cells were selected by GFP and CD4 surface marker. For cells co-infected with pSuper HDAC6 shRNA and MICD4 mDia2, positive cells were selected by CD4 surface marker.
Purification and culture of mouse fetal liver cells
All mouse procedures were conducted in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals for research purposes and the collection of mouse fetal liver cells was approved by the University of Florida Institutional Animal Care and Use Committee (IACUC) (#201309309; #201609309). Mouse fetal liver cells were obtained from E13.5 C57BL/6 embryos. The purification and in vitro culture of mouse fetal liver erythroblast precursors (Ter119-negative cells) were performed as previously described.25 Purified cells were seeded in fibronectin-coated wells (BD Pharmingen) and differentiation induced in Iscove modified Dulbecco medium (IMDM) containing 1 U/mL erythropoietin (Amgen). The medium was changed to erythropoietin-free IMDM 24 h after induction.
Bone marrow transplantation
Bone marrow cell infection and transplantation have been described elsewhere16 and were approved by the Northwestern University IACUC (2015-IS00001416). Briefly, c-Kit-positive stem/progenitor cells from mDia2 knockout mice were purified by mouse CD117 (c-KIT)-positive selection kit (STEMCELL Tech.). The cells were cultured in serum-free StemSpan SFEM medium and spin-infected with concentrated virus supernatants supplied with 8 μg/mL of polybrene at 2500 rpm for 90 min. The infected cells were recovered for 24 h at 37°C and then 2×106 cells were injected retro-orbitally into lethally irradiated CD45.1-positive recipient mice (gamma-irradiation, 1000 rad). One month after transplantation, reconstitution was achieved and recipient mice were sacrificed for the flow cytometric bone marrow enucleation assay staining with Ter119 and Hoechst 33342.
Immunocytochemistry and confocal microscopy
Immunocytochemistry is described in the Online Supplementary Methods. Staining was examined with a Leica TCS-SP5 confocal imaging system (Leica Microsystems Inc.). To quantify polarized florescent signals, a line, with a thickness of 20 pixels, was drawn across the center of the cell towards the polarization direction using Fiji software. The polarization of the target signal was defined as a more than 2-fold change of florescence intensity between the maximal peak values from the side of the polarized cytoplasm and the nuclear side. At least five random pictures were taken from one slide, and more than 30 cells were measured for each experiment.
Flow cytometric analysis
For the in vitro analysis of differentiation, the mouse fetal erythroid progenitors were cultured in vitro for 24 h with erythropoietin and then the culture was continued for an additional 24 h without erythropoietin. Detached cells from culture were subjected to FACS analysis using antibodies against Ter119 PE and CD71 FITC (BD Pharmingen). For the in vitro analysis of enucleation, cells were stained with Hoechst 33342 (Sigma-Aldrich) and Ter119 PE. The FACS analysis was carried out using a BD LSR II (BD Biosciences).
Statistics
Statistical significance was determined by a Student t test. P values <0.05 are considered statistically significant.
Results
Inhibition of HDAC6 impairs cytokinesis and erythrocyte enucleation
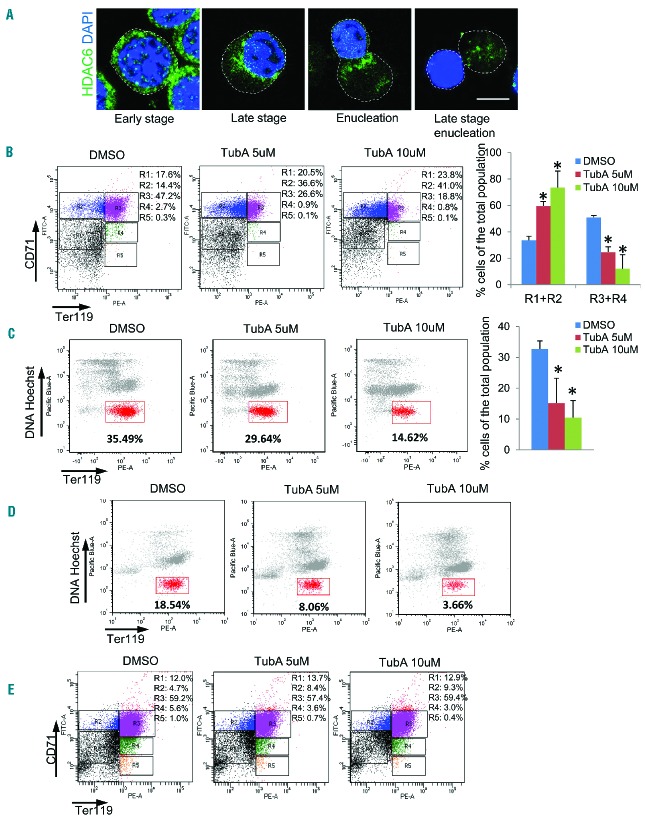
During erythropoiesis, erythroid progenitors undergo a series of differentiation stages with cell division, nuclear condensation and eventually enucleation, a type of cytokinesis which requires actin remodeling.26–28 It has been shown that the class IIb HDAC6 plays a role in cytokinesis and endocytosis.20,23 We, therefore, investigated whether HDAC6 is also involved in erythrocyte maturation. We first examined the expression and localization of HDAC6 during erythroid differentiation using Ter119-negative erythroid progenitor cells purified from E13.5 mouse fetal livers. The progenitor cells were cultured and induced to differentiate into erythroblasts in vitro by addition of erythropoietin.25 HDAC6 was evenly distributed in cytoplasm during the early stage of erythropoiesis. As the nucleus became polarized during the later stage of erythropoiesis, HDAC6 gradually accumulated at the boundary of the cytoplasm and nucleus (Figure 1A). During enucleation, HDAC6 localized at the sites of CAR formation between the incipient reticulocytes and pyrenocytes (Figure 1A). The total protein and gene expression level of HDAC6 did not change significantly during erythropoesis (Online Supplementary Figure S1A, S1B). The dynamic localization of HDAC6 to the cytokinetic furrow suggests that it may play an important role there. To determine whether HDAC6 is involved in erythroid cell maturation, the mouse erythroid progenitor cells were induced to differentiate by addition of erythropoietin and treated with tubastatin A (TubA), a HDAC6-specific inhibitor.29 The expression level of HDAC6 was not affected by TubA treatment (Online Supplementary Figure S1A–C); however the deacetylase activity of HDAC6 was inhibited (Online Supplementary Figure S1B). FACS analysis was utilized to study the erythrocyte differentiation. Cell populations were gated and quantified based on expression levels of CD71 and Ter119.25 The population of differentiated erythrocytes, which were positive for both CD71 and Ter119 (R3 + R4), was profoundly reduced by TubA (Figure 1B). The enucleation efficiency was also analyzed by FACS using co-staining with Hoechst for DNA and Ter119. The population of enucleated cells, which were positive for Ter119 and negative for Hoechst, was also reduced by TubA treatment (Figure 1C). These results demonstrate that inhibition of HDAC6 affects erythroid progenitor cell differentiation and enucleation. An alternate explanation for these results could be that the loss of enucleated cells is due to a reduction of differentiated cells. However, when the erythroid progenitor cells were induced to differentiate by erythropoietin for 36 h prior to treatment with TubA for 12 h, there was a significant decrease in the enucleated cell population (Figure 1D) without the R3 + R4 population being affected significantly (Figure 1E). It is known that the class III HDAC SIRT2 and HDAC6 have an overlapping function as both enzymes can deacetylate tubulin.30 We, therefore, tested whether SIRT2 also affects enucleation. However, treatment with class III HDAC inhibitors, sirtinol or nicotinamide, did not affect enucleation (Online Supplementary Figure S1D,E). These results reveal a functional significance of HDAC6 in differentiation and enucleation during the late stage of terminal erythropoiesis.
Figure 1.
Inhibition of HDAC6 impairs cytokinesis and erythrocyte enucleation. (A) Confocal microscopy analysis of HDAC6 cellular distribution during Ter119-negative mouse fetal progenitor cell differentiation. The Ter119-negative erythroid progenitor cells were harvested from E13.5 mouse fetal livers, and cultured in vitro with erythropoietin for 24 h, and then the culture continued for an additional 24 h without erythropoietin. The cells were fixed at 0, 6 h, 12 h, and 48 h during the culture and immunostained with anti-HDAC6 conjugated with Alexa Fluor 488 and DAPI. Scale bar is 5 μm. The dashed line indicates the cell boundary. (B) Flow cytometric analysis of cultured Ter119-negative mouse fetal progenitors. DMSO, TubA (5 μM) or TubA (10 μM) was added at the time of erythropoietin induction and the cells were treated for 48 h. Cells were stained with Ter119 and CD71, and analyzed by FACS. The percentages of cells in an undifferentiated state (R1+R2) and differentiated state (R3+R4) were analyzed. (C) Flow cytometric analysis of induced Ter119-negative mouse fetal progenitors. DMSO, TubA (5 μM) or TubA (10 μM) was added at the time of erythropoietin induction and the cells were treated for 48 h. Cells were stained with Ter119 and Hoechst 33342, and analyzed by FACS. The percentage of the gated cells (Ter119-positive and Hoechst-negative cells), which represent the pool of enucleated reticulocytes, was analyzed. (D and E) DMSO, TubA (5 μM) or TubA (10 μM) was added 36 h after erythropoietin induction and the cells were treated for an additional 12 h. Cells were stained with (D) Ter119 and Hoechst 33342, or (E) Ter119 and CD71, and analyzed by FACS. The error bars represent mean +SD (n=3), *P<0.05 compared to DMSO treatment.
Inhibition of HDAC6 blocks recruitment of mDia2 at the contractile actin ring
Remodeling of actin filaments is critical to erythroblast enucleation.27,28 The formation of a CAR at the boundary between the incipient reticulocyte and pyrenocyte (condensed nucleus) is a phenomenon distinct to enucleation. At the late erythroblast stage, HDAC6 gradually moves toward the boundary of the nucleus and cytoplasm (Figure 1A), which led us to further examine HDAC6 function on the formation of the CAR. During the early stages of erythropoiesis, actin filaments are distributed at the plasma membrane and are partially co-localized with HDAC6 (Figure 2A). During nuclear condensation, actin filaments gradually accumulate at the cleavage furrow to form the CAR (Figure 2A). In cells treated with TubA, F-actin remains at the cell periphery and CAR formation is blocked, even though there is no significant defect in nuclear polarization (Figure 2B,C, Online Supplementary Figure S2A,B). To further confirm the role of HDAC6 in enucleation, HDAC6 was knocked down with retrovirus encoding shRNA. Knockdown of HDAC6 also significantly blocked F-actin polarization to the CAR (Figure 2D,E). Interestingly, a few erythroblasts with inactivated HDAC6 or HDAC6 knockdown eventually enucleated (Figure 2F,G). However, even in these cells, CAR formation was disrupted. Taken together, these results suggest that HDAC6 is required for the organization of actin filaments into the CAR.
Figure 2.
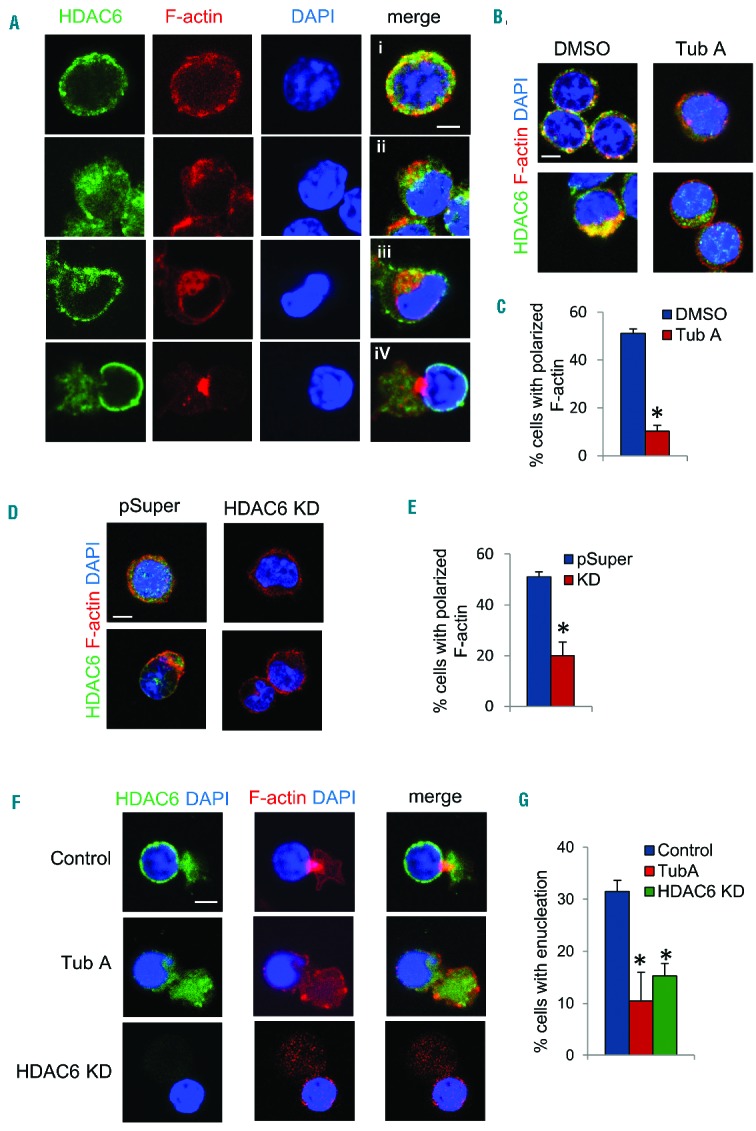
Inhibition of HDAC6 blocks contractile actin ring formation. (A) Immunostaining of HDAC6 and F-actin in cultured Ter119-negative mouse fetal progenitors. i–iv indicate progressive stages during enucleation. (B) Immunostaining of HDAC6 and F-actin in differentiating Ter119-negative mouse fetal progenitors treated with DMSO or TubA. (C) Quantification of cells with polarized F-actin [as shown in stages ii to iv in (A)] in cells treated with DMSO or TubA. (D) Immunostaining of HDAC6 and F-actin in Ter119-negative mouse fetal progenitors infected with retrovirus harboring pSuper vector or HDAC6 shRNA. (E) Quantification of polarized F-actin in cells with HDAC6 knockdown. (F) Immunostaining of HDAC6 and F-actin in cultured Ter119-negative mouse fetal progenitors treated with TubA or HDAC6 shRNA. (G) Quantification of enucleated cells in induced mouse fetal liver progenitor cells with HDAC6 shRNA or treated with TubA. Scale bar is 5 μM. The error bars represent mean +SD (n=3), *P<0.05 compared to DMSO.
An earlier study indicated that formin protein mDia2 is required for the formation of the CAR and subsequent enucleation.6 We therefore examined whether inhibition of HDAC6 affects mDia2 function. In normal cultured mouse fetal erythroblasts, mDia2 gradually polarizes to one side of the plasma membrane and partially co-localizes with actin filaments where the CAR is formed during the enucleation process (Figure 3A). Treatment with TubA significantly impaired the polarization of mDia2 (Figure 3A,B). Since mDia2 is also important for cytokinesis,4,15 we then tested whether HDAC6 is also important for mDia2-mediated cytokinesis. Control dividing cells form the CAR at the cleavage furrow (Figure 3C, left panel). mDia2 is polarized to the front edge of the CAR (Figure 3C, left panel). TubA-treated cells failed to form the cleavage furrow or CAR and lacked mDia2 localization at the cleavage site. Some TubA-treated cells had two or more nuclei, indicating that nuclear division had occurred (Figure 3C, top right panel; Figure 3D; Online Supplementary Figure S3A). These cells grew at a slower rate (Online Supplementary Figure S3B,C), supporting the notion that cytokinesis is blocked. A few treated cells did manage to go through complete cytokinesis without the formation of an actin ring (Figure 3C, bottom right panel), suggesting an alternative mechanism for cytokinesis without the CAR. Other cell types, such as NIH3T3 fibroblasts, also require HDAC6 activity for cytokinesis (Online Supplementary Figure S3D). Our study therefore demonstrates that HDAC6 is important for mDia2-mediated cytokinesis and enucleation.
Figure 3.
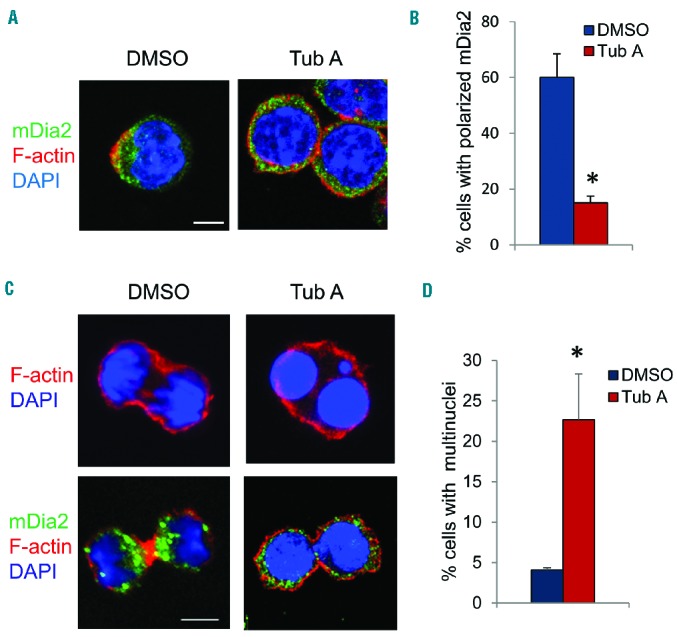
HDAC6 is required for recruitment of mDia2 at the contractile actin ring. (A) Immunostaining of mDia2 and F-actin in cultured erythropoietin-induced Ter119-negative mouse fetal progenitors treated with DMSO or TubA. (B) Quantification of cells with polarized mDia2. (C) Immunostaining of mDia2 and F-actin in cultured uninduced Ter119-negative mouse fetal progenitors treated with DMSO or TubA. (D) Quantification of multi-nuclear cells as described in (C). Scale bar is 5 μm. The error bars represent mean +SD (n=3), *P<0.05 compared to DMSO.
mDia2 is a novel target of HDAC6
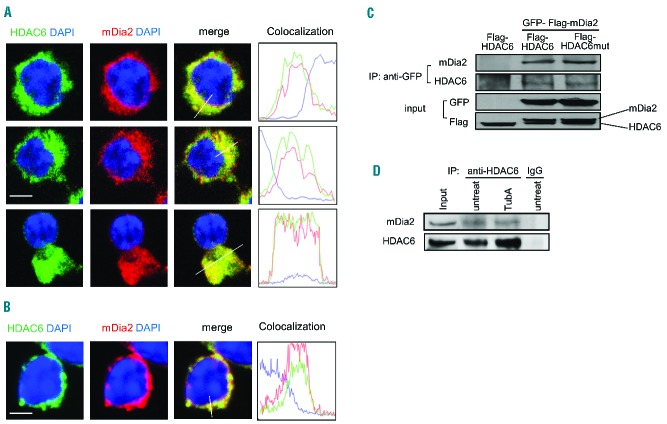
To further investigate how HDAC6 affects mDia2-mediated enucleation, we first examined whether HDAC6 interacts with mDia2 in erythroid cells. mDia2 co-localized with HDAC6 during both early and late stages of erythropoiesis in mouse fetal erythroid progenitors, suggesting that these two proteins may have an interaction (Figure 4A). The co-localization was not affected by TubA treatment (Figure 4B). The interaction between HDAC6 and mDia2 was confirmed by immunoprecipitation analysis. GFP-Flag-mDia2 was co-transfected into 293T cells with either wild-type Flag-HDAC6 or catalytically inactive Flag-HDAC6 mutant. HDAC6 was co-precipitated with GFP mDia2 (Figure 4C). Consistent with the imaging results described above, both wild-type HDAC6 and catalytically inactive HDAC6 were found in the mDia2 complex (Figure 4C), indicating that deacetylase activity is not required for the interaction. We further confirmed this interaction with endogenous proteins in cells of the murine erythroleukemia (MEL) line. The endogenous HDAC6 protein interacted with mDia2 in both TubA-treated and control cell lysates (Figure 4D). These results indicate that mDia2 forms a complex with HDAC6 and that the inhibition of deacetylase activity does not interfere with the integrity of the complex.
Figure 4.
mDia2 interacts with HDAC6 during erythropoiesis. Confocal microscopy analysis of HDAC6 and mDia2 co-localization in cultured Ter119-negative mouse fetal progenitors treated with (A) DMSO or (B) TubA. The florescent intensity was measured using Volocity imaging software. Scale bar is 5 μm. (C) 293T cells were transfected with GFP-Flag-tagged mDia2 and Flag-tagged HDAC6 or HDAC6 mutant as indicated. The cell lysate was immunoprecipitated with GFP antibody. Associated proteins were detected by western blotting with antibodies as indicated. (D) Endogenous HDAC6 in MEL cells treated with TubA and immunoprecipitated with HDAC6 antibody. Associated mDia2 was detected by western blotting using anti-mDia2 antibody.
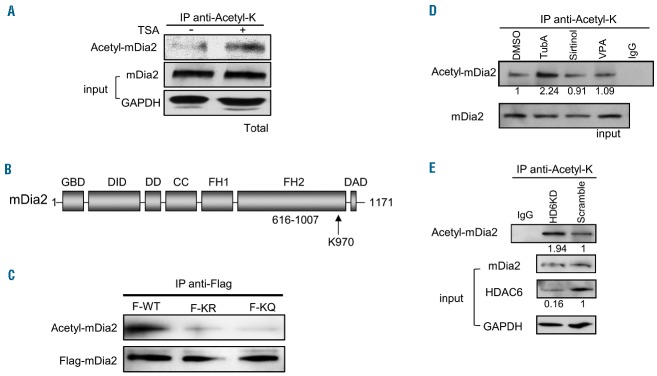
As HDAC6 is a deacetylase for a number of cellular proteins, we hypothesized that mDia2 is a novel target of HDAC6. First, we examined whether mDia2 can be acetylated in vivo. In mouse fetal erythroid progenitors, mDia2 was acetylated at a low level and the acetylation was dramatically increased after treatment with trichostatin A (TSA), a broad-spectrum HDAC inhibitor (Figure 5A). This shows that mDia2 may be dynamically acetylated and deacetylated during erythropoiesis and inhibition of HDAC activity enhances mDia2 acetylation in vivo. Next, we wanted to identify the acetylation site on mDia2. Since TubA treatment blocks the recruitment of mDia2 and F-actin at the CAR, it is conceivable that the acetylation site is localized at the FH2 domain, the domain that mediates actin binding and polymerization.2 Through a protein alignment study with mDia proteins among model organisms, we found that lysine 970 of the FH2 domain of mDia2 is highly conserved (Figure 5B, Online Supplementary Figure S4). To determine whether K970 is a potential acetylation site, we generated Flag-tagged K970R and K970Q mDia2 mutants to mimic unacetylated and acetylated forms, respectively, and expressed them in 293T cells. The wild-type (WT) mDia2 in 293T cells was acetylated. Mutations on lysine 970 abolished the acetylation (Figure 5C). Taken together, these results suggest that mDia2 is an acetylated protein and lysine 970 in the FH2 domain of mDia2 is a major acetylation site in vivo.
Figure 5.
mDia2 is acetylated in vivo and HDAC6 is responsible for deacetylation of mDia2. (A) Total acetylated protein was immunoprecipitated by anti-acetyl-K antibody from the cell extracts treated with or without TSA in Ter119-negative mouse fetal progenitors. mDia2 protein was detected by specific anti-mDia2 antibody using western blotting. (B) Schematic representation of mDia2 protein structure. K970 is the acetylation site in the FH2 domain of mDia2. (C) 293T cells were transfected with Flag-tagged mDia2 WT (F-WT), mDia2 K970R (F-KR), or mDia2 K970Q (F-KQ). The cell extracts were incubated with anti-Flag antibody and acetylated mDia2 and total Flag-tagged mDia2 were detected by anti-acetyl-lysine (anti-acetyl-K) and anti-Flag antibodies. (D) Total acetylated protein was immunoprecipitated by anti-acetyl-K antibody from the cell extracts treated with or without inhibitors in MEL cells as indicated. Acetylated mDia2 protein was detected by anti-mDia2 antibody. The numbers indicate the relative density of the bands. (E) Total acetylated protein was immunoprecipitated by anti-acetyl-K antibody from the cell extracts of stable HDAC6 knockdown (KD) or scramble control MEL cells. mDia2 protein was detected by anti-mDia2 antibody. The numbers indicate the relative density of the bands. Each experiment was repeated three times.
To examine whether HDAC6 is responsible for mDia2 deacetylation, we treated MEL cells with various HDAC inhibitors, TubA, valproic acid (a class I and IIa inhibitor), or sirtinol (a class III HDAC inhibitor). Total acetylated lysine proteins were immunoprecipitated with an acetyl-lysine antibody from cell lysates and the presence of mDia2 protein was detected. mDia2 acetylation was dramatically increased in TubA-treated cells, but not in the other inhibitor-treated cells (Figure 5D). Furthermore, mDia2 acetylation was significantly increased with HDAC6 knockdown (Figure 5E), indicating that HDAC6 is indeed a major deacetylase for mDia2 in vivo.
HDAC6 regulates enucleation through deacetylation of mDia2
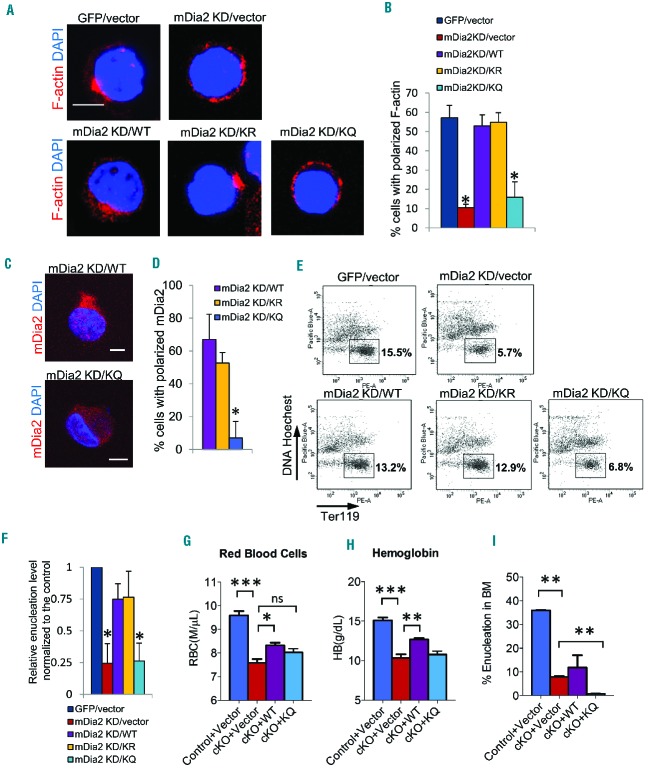
In order to determine whether deacetylation of mDia2 is required for CAR formation, we simultaneously knocked down mDia2 with shRNA (Online Supplementary Figure S5A,B) and re-expressed shRNA resistant mDia2 WT, non-acetyl-mimicking mDia2 K970R, or acetyl-mimicking mDia2 K970Q (Online Supplementary Figure S5C). The WT and K970R mutant, but not the K970Q mutant, rescued the defective F-actin polarization phenotype in mDia2 knockdown cells (Figure 6A,B). The K970Q mutant also failed to rescue the mDia2 polarization phenotype (Figure 6C,D). Importantly, WT or K970R rescued the enucleation in mDia2 knockdown cells (Figure 6E,F). As expected, the mDia2 K970Q mutant was not able to rescue the defects of enucleation in mDia2 knockdown cells (Figure 6E,F). To test whether this is also the case in vivo, bone marrow cells from mDia2 knockout mice were infected with retrovirus encoding WT mDia2 or K970Q mutant. Infected bone marrow cells were transplanted into lethally irradiated recipient mice and bone marrow was collected 1 month later. The mice with mDia2 knockout had low red blood counts and low hemoglobin levels. This phenotype was not rescued in mice transplanted with K970Q bone marrow, while it was partially rescued in those transfected with WT mDia2 (Figure 6G,H). Importantly, the number of enucleated red cells in bone marrow was significantly reduced in K970Q-rescued mice (Figure 6I). The more severe effect of K970Q in bone marrow than in the in vitro assay may be due to the fact that K970Q affects both cytokinesis and enucleation in bone marrow. We, thus, demonstrated that deacetylation of mDia2 is required for terminal erythropoiesis in vitro and in vivo.
Figure 6.
Acetylation of mDia2 impairs enucleation. (A) Ter119-negative mouse fetal progenitors were infected with retrovirus carrying GFP or mDia2 shRNA (KD), and shRNA resistant mDia2 WT, mDia2 K970R (KR), mDia2 K970Q (KQ), or control vector as indicated. Cells were fixed and subjected to immunostaining at 48 h after erythropoietin induction. Scale bar is 5 μm. (B) Quantification of cells with polarized F-actin in cells described in (A). The error bars represent mean +SD (n=3), *P<0.05 compared to the GFP/vector control. (C) Immunostaining of mDia2 in cultured Ter119-negative mouse fetal progenitors infected with mDia2 shRNA (KD) and shRNA resistant mDia2 WT, or mDia2 K970Q (KQ). Representative images are shown. Scale bar is 5 μm. (D) Quantification of cells with polarized mDia2 in cells described in (C). The error bars represent mean +SD (n=3), *P<0.05 compared to mDia2 KD/WT. (E) Flow cytometric analysis of cultured Ter119-negative mouse fetal progenitors described in (A). Cells were harvested 48 h after erythropoietin induction and GFP and CD4-positive cells, which harbor mDia2 shRNA and mDia2 KD, were collected and subjected to FACS analysis. The percentage of enucleated reticulocytes from a representative experiment is indicated. (F) Quantification of enucleation in cultured Ter119-negative mouse fetal progenitors described in (E) at 48 h. The enucleation rate was normalized to the GFP/vector control. The error bars represent mean +SD (n=3), *P<0.05 compared to the vector control. (G to I) c-Kit-positive stem/progenitor cells from control or mDia2 conditional knockout (cKO) mice were infected with empty retroviral vector or retrovirus encoding mDia2 WT and KQ mutant as indicated. The cells were then transplanted into lethally irradiated CD45.1-positive recipient mice. One month after bone marrow transplantation, mice were sacrificed for analysis of (G) circulating red blood cell count; (H) hemoglobin level and (I) flow cytometry analysis of enucleation in bone marrow cells by Ter119/Hoechst 33342 staining. Results are representative of three experiments. Data were shown as mean ± SEM. *P<0.05; **P<0.01 and ***P<0.001.
Since we identified that HDAC6 is the deacetylase for mDia2, and mDia2 deacetylation is essential for CAR formation, we hypothesized that HDAC6 mediates erythroblast enucleation through deacetylation of mDia2. If this is the case, then overexpression of an unacetylated mDia2 mimic mutant should rescue CAR and enucleation defects in HDAC6 knockdown cells. To test this, we overexpressed mDia2 K970R or control vector in HDAC6 knockdown Ter119-negative erythroid progenitors (Online Supplementary Figure S6). The re-expression of mDia2 K970R mutant rescued F-actin polarization at the cleavage furrow in the HDAC6 knockdown cells (Figure 7A,B). These results provide direct evidence that mDia2 acts downstream of the HDAC6-regulated pathway in erythroblast enucleation and deacetylation of mDia2 by HDAC6 is required for CAR formation and proper enucleation during erythropoiesis (Figure 7C).
Figure 7.
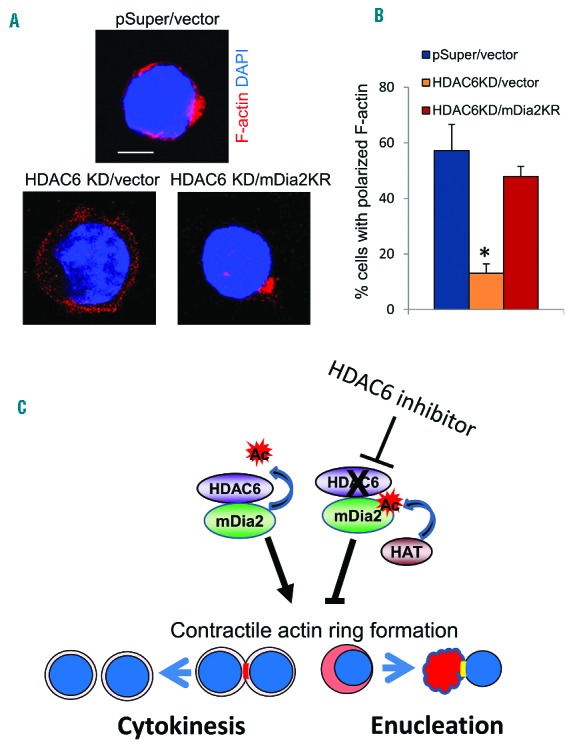
mDia2 rescues enucleation in HDAC6 knockdown mouse fetal progenitors. (A) Immunostaining of actin in cultured Ter119-negative mouse fetal progenitors infected with HDAC6 shRNA (KD) and mDia2 K970R or control viruses. Representative images are shown. Scale bar is 5 μm. (B) Quantification of cells with polarized F-actin in cells described in (A). (C) Schematic representation of the HDAC6-mDia2 pathway in cytokinesis and enucleation. Deacetylation of mDia2 by HDAC6 promotes formation of the CAR and subsequent cytokinesis and enucleation. Inhibition of HDAC6 could cause the accumulation of acetylated mDia2, which impairs CAR formation and subsequent cytokinesis and enucleation processes.
Discussion
HDAC6 regulates various cellular processes, such as cell migration, vesicle trafficking, endocytosis and cytokinesis.18,19,23,31–33 Our study here demonstrates that HDAC6 can regulate actin nucleation through deacetylation of the formin protein mDia2, which reveals a Rho-GTPase-independent mechanism that regulates mDia2 activity. Other reports show that HDAC6 plays an important role in regulating actin-mediated endocytosis and osteoclast maturation.20,25,34 It is yet to be determined whether other HDAC6-dependent processes, such as endocytosis or cell motility, also involve deacetylation of mDia2 or other formin proteins.
It has been well demonstrated that HDAC6 interacts with and deacetylates cytoplasmic α-tubulin and subsequently affects microtubule organization.19,25,33,35 Although there is no direct evidence showing that tubulin acetylation affects cytokinesis, it has been shown that inhibition of Arl3 results in increased tubulin acetylation and failure of cytokinesis.36 In addition, mDia2 stabilizes microtubules by enhancing HDAC6 tubulin deacetylase activity.34,37 It is, therefore, possible that tubulin deacetylation by HDAC6 may also be involved in cytokinesis. The F-actin binding protein cortactin is an acetylated protein and is important for CAR formation.22,38 Acetylated cortactin dissociates from actin filaments and HDAC6 deacetylates cortactin.22 It is, therefore, conceivable that HDAC6-mediated cortactin deacetylation may also play an important role in cytokinesis. Thus, HDAC6 may have multiple roles in regulating cytokinesis and enucleation through modulating acetylation levels of various proteins that are involved in the regulation of actin and tubulin networks. However, tubulin and cortactin deacetylation may not play an essential role in cytokinesis or enucleation as non-acetyl-mimicking mDia2 can rescue HDAC6 knockdown defects.
Enucleation is considered as an asymmetric cytokinesis event.13 In mammalian cells, formation of the CAR is required for successful cytokinesis. However, in our study, we found that although in low percentage, erythrocytes without functional HDAC6 can divide or enucleate without apparent accumulation of mDia2 or F-actin bundles at the cleavage farrow (Figures 2F and 3C). This is interesting but consistent with a recent finding in vivo using a mDia2 whole body knockout mouse model. These mice die as embryo on day 13.5 with severe defects in primitive erythropoiesis. Similar to what we observed here, although enucleation is significantly influenced, some primitive erythroblasts managed to enucleate.15 We suspect that an alternative pathway may be utilized when the CAR is absent. Indeed, yeast and Dictyostelium cells are able to divide without actin or myosin II, two key components of the CAR.39–41 Furthermore, it has been shown that an actin-binding protein, cortexillin, may rescue cytokinesis in the absence of the CAR.42 It remains unclear what mechanism is involved in erythrocyte enucleation in the absence of the CAR in mammalian cells.
It is worth mentioning that HDAC6 knockout mice are viable with no developmental defect.23,43 Although the mice have hyperacetylated tubulin and elevated acetylated HSP90, they do not have significant cellular defects, as shown in transient knockdown cell lines,18,43 suggesting that germline deletion of HDAC6 may result in a compensation of HDAC6 function. Consistent with this notion, except no response to HDAC6 inhibitor, the Ter119-negative fetal progenitor cells isolated from knockout mice differentiate and enucleate normally (data not shown). These cells can form a normal CAR and mDia2 recruitment is also normal (data not shown). Since HDAC6 is a unique deacetylase for cellular functions, it remains to be determined which protein or deacetylase plays a role in compensating HDAC6 function.
Formin proteins play critical roles in cell morphology, motility, polarity, and division, which are essential for cell growth and differentiation.2 Formin protein activities are regulated through interactions with various binding proteins. Post-translational modifications are also important in modulating formin activity. There is one report describing that phosphorylation of mDia2 on the diaphanous autoregulatory domain (DAD) enhances its activity by reducing the interaction with the diaphanous inhibitory domain (DID).44 In this study, we uncovered a novel post-translational modification for formin proteins at their FH2 domain. We found that mDia2 is acetylated and that the acetylation abolishes mDia2-mediated CAR formation. We further discovered that the acetylation is reversible and HDAC6-mediated deacetylation of mDia2 restores CAR formation. Interestingly, the acetylation site of mDia2, K970, is located in the FH2 domain, the key domain that forms dimers and promotes actin filament nucleation and actin polymerization through an interaction with the barbed end of actin filaments. The FH2 domain is highly conserved throughout formin proteins (Online Supplementary Figure S4).2 Importantly, the acetylation site on FH2 is sequence- and structurally-conserved within model organisms, indicating that acetylation may have a conserved function (Online Supplementary Figure S4).11,45 The crystal structure shows that FH2 domains form a stable, but flexible dimeric “donut” structure.11,12 Interestingly, the crystal structure of the FH2 domain with actin suggests that the acetylation site may not be important for direct actin binding and FH2 dimerization.12 The function of FH2 domain acetylation on actin nucleation does, therefore, require further investigation. In addition, the acetylation site is also conserved in mDia1 and the acetylation may, therefore, have a more general effect on actin nucleation beyond cytokinesis and erythrocyte enucleation processes.
Supplementary Material
Acknowledgments
We thank Dr. Narumiya (Kyoto University, Kyoto, Japan) for the mDia2 plasmid. We are very grateful to Tao Yang, Yuanjing Liu for their preliminary study and technical assistance, and to Tracy Read for technical assistance.
This work was supported by the National Institutes of Health (grants R01 HL095674 to YQ; DK102718 to PJ and DK110108 to SH). The work was also supported by the Department of Defense grant CA140119 to PJ.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/6/984
References
- 1.Bogdan S, Schultz J, Grosshans J. Formin’ cellular structures: physiological roles of Diaphanous (Dia) in actin dynamics. Commun Integr Biol. 2013;6(6):e27634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallar BJ, Alberts AS. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 2003;13(8):435–446. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe N, Madaule P, Reid T, et al. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16(11):3044–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe S, Ando Y, Yasuda S, et al. mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol Biol Cell. 2008;19(5):2328–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallar BJ, Deward AD, Resau JH, Alberts AS. RhoB and the mammalian Diaphanous-related formin mDia2 in endosome trafficking. Exp Cell Res. 2007;313(3):560–571. [DOI] [PubMed] [Google Scholar]
- 6.Ji P, Jayapal SR, Lodish HF. Enucleation of cultured mouse fetal erythroblasts requires Rac GTPases and mDia2. Nat Cell Biol. 2008;10(3):314–321. [DOI] [PubMed] [Google Scholar]
- 7.Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119(3):419–429. [DOI] [PubMed] [Google Scholar]
- 8.Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP-and ADP-actin by formins and profilin. Cell. 2006;124(2):423–435. [DOI] [PubMed] [Google Scholar]
- 9.Higgs HN. Formin proteins: a domain-based approach. Trends Biochem Sci. 2005;30(6): 342–353. [DOI] [PubMed] [Google Scholar]
- 10.Paul AS, Pollard TD. Review of the mechanism of processive actin filament elongation by formins. Cell Motil Cytoskeleton. 2009;66(8):606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada A, Nyitrai M, Vetter IR, et al. The core FH2 domain of diaphanous-related formins is an elongated actin binding protein that inhibits polymerization. Mol Cell. 2004;13(4):511–522. [DOI] [PubMed] [Google Scholar]
- 12.Thompson ME, Heimsath EG, Gauvin TJ, Higgs HN, Kull FJ. FMNL3 FH2-actin structure gives insight into formin-mediated actin nucleation and elongation. Nat Struct Mol Biol. 2013;20(1):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji P, Murata-Hori M, Lodish HF. Formation of mammalian erythrocytes: chromatin condensation and enucleation. Trends Cell Biol. 2011;21(7):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An X, Mohandas N. Erythroblastic islands, terminal erythroid differentiation and reticulocyte maturation. Int J Hematol. 2011;93(2): 139–143. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe S, De Zan T, Ishizaki T, et al. Loss of a Rho-regulated actin nucleator, mDia2, impairs cytokinesis during mouse fetal erythropoiesis. Cell Rep. 2013;5(4):926–932. [DOI] [PubMed] [Google Scholar]
- 16.Mei Y, Zhao B, Yang J, et al. Ineffective erythropoiesis caused by binucleated late-stage erythroblasts in mDia2 hematopoietic specific knockout mice. Haematologica. 2016;101(1):e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31(4):449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenzuela-Fernandez A, Cabrero JR, Serrador JM, Sanchez-Madrid F. HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 2008;18(6):291–297. [DOI] [PubMed] [Google Scholar]
- 19.Hubbert C, Guardiola A, Shao R, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417(6887):455–458. [DOI] [PubMed] [Google Scholar]
- 20.Wickstrom SA, Masoumi KC, Khochbin S, Fassler R, Massoumi R. CYLD negatively regulates cell-cycle progression by inactivating HDAC6 and increasing the levels of acetylated tubulin. EMBO J. 2010;29(1):131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Li N, Caron C, et al. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003;22(5):1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Yuan Z, Zhang Y, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27(2):197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao YS, Hubbert CC, Lu J, Lee YS, Lee JY, Yao TP. Histone deacetylase 6 regulates growth factor-induced actin remodeling and endocytosis. Mol Cell Biol. 2007;27(24): 8637–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Peng L, Seto E, Huang S, Qiu Y. Modulation of histone deacetylase 6 (HDAC6) nuclear import and tubulin deacetylase activity through acetylation. J Biol Chem. 2012;287(34):29168–29174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102(12):3938–3946. [DOI] [PubMed] [Google Scholar]
- 26.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112(10):3939–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chasis JA, Prenant M, Leung A, Mohandas N. Membrane assembly and remodeling during reticulocyte maturation. Blood. 1989;74(3): 1112–1120. [PubMed] [Google Scholar]
- 28.Koury ST, Koury MJ, Bondurant MC. Cytoskeletal distribution and function during the maturation and enucleation of mammalian erythroblasts. J Cell Biol. 1989;109(6 Pt 1):3005–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem Soc. 2010;132(31):10842–10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11(2):437–444. [DOI] [PubMed] [Google Scholar]
- 31.Gao YS, Hubbert CC, Yao TP. The microtubule-associated histone deacetylase 6 (HDAC6) regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation. J Biol Chem. 2010;285(15): 11219–11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serrador JM, Cabrero JR, Sancho D, Mittelbrunn M, Urzainqui A, Sanchez-Madrid F. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity. 2004;20(4):417–428. [DOI] [PubMed] [Google Scholar]
- 33.Matsuyama A, Shimazu T, Sumida Y, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. Embo J. 2002;21(24):6820–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Destaing O, Saltel F, Gilquin B, et al. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci. 2005;118(Pt 13):2901–2911. [DOI] [PubMed] [Google Scholar]
- 35.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA. 2003;100(8):4389–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou C, Cunningham L, Marcus AI, Li Y, Kahn RA. Arl2 and Arl3 regulate different microtubule-dependent processes. Mol Biol Cell. 2006;17(5):2476–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, Gundersen GG. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J Cell Biol. 2008;181(3):523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S, Tang DD. c-Abl tyrosine kinase regulates cytokinesis of human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2014;50(6):1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bi E, Maddox P, Lew DJ, et al. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol. 1998;142(5):1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naqvi NI, Eng K, Gould KL, Balasubramanian MK. Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. EMBO J. 1999;18(4):854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber I, Niewohner J, Faix J. Cytoskeletal protein mutations and cell motility in Dictyostelium. Biochem Soc Symp. 1999;65:245–265. [PubMed] [Google Scholar]
- 42.Stock A, Steinmetz MO, Janmey PA, et al. Domain analysis of cortexillin I: actin-bundling, PIP(2)-binding and the rescue of cytokinesis. EMBO J. 1999;18(19):5274–5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Kwon S, Yamaguchi T, et al. Mice lacking histone deacetylase 6 have hyper-acetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28(5):1688–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staus DP, Taylor JM, Mack CP. Enhancement of mDia2 activity by Rho-kinase-dependent phosphorylation of the diaphanous autoregulatory domain. Biochem J. 2011;439(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otomo T, Tomchick DR, Otomo C, Panchal SC, Machius M, Rosen MK. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature. 2005;433(7025):488–494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.