Abstract
Fanconi anemia is a complex heterogeneous genetic disorder with a high incidence of bone marrow failure, clonal evolution to acute myeloid leukemia and mesenchymal-derived congenital anomalies. Increasing evidence in Fanconi anemia and other genetic disorders points towards an interdependence of skeletal and hematopoietic development, yet the impact of the marrow microenvironment in the pathogenesis of the bone marrow failure in Fanconi anemia remains unclear. Here we demonstrated that mice with double knockout of both Fancc and Fancg genes had decreased bone formation at least partially due to impaired osteoblast differentiation from mesenchymal stem/progenitor cells. Mesenchymal stem/progenitor cells from the double knockout mice showed impaired hematopoietic supportive activity. Mesenchymal stem/progenitor cells of patients with Fanconi anemia exhibited similar cellular deficits, including increased senescence, reduced proliferation, impaired osteoblast differentiation and defective hematopoietic stem/progenitor cell supportive activity. Collectively, these studies provide unique insights into the physiological significance of mesenchymal stem/progenitor cells in supporting the marrow microenvironment, which is potentially of broad relevance in hematopoietic stem cell transplantation.
Introduction
The hematopoietic system is built upon the ordered self-renewal and differentiation of hematopoietic stem cells (HSC) within the bone marrow (BM). This process involves intrinsic and extrinsic cues including both cellular and humoral regulatory signals generated by the HSC microenvironment, termed as “niche”. The cellular composition of this “niche” is heterogeneous, including endothelial cells,1 osteoblasts,2 adipocytes, and mesenchymal stem/progenitor cells (MSPC), a common progenitor for many of the cell lineages comprising the HSC niche.3–5 For fate decisions, regulatory signals from the BM microenvironment are transmitted to HSC through intercellular interactions within the proximity of the endosteal surface, the perivascular space, soluble factors, and the extracellular matrix.6 These cellular and humoral regulatory signals dictate the fates of HSC, including self-renewal, proliferation, differentiation, and apoptosis.7 In addition, there is increasing evidence suggesting a role of the hematopoietic microenvironment in hematopoietic disorders, such as myeloproliferative neoplasms8,9 and myelodysplastic syndrome.10
Fanconi anemia (FA) is a complex inherited disorder caused by germline mutations in at least one of 16 genes including FANCA, -B, -C, -D1, -D2, -E, -F, -G, -I, - J, -L, -M, -N, -O, –P, and –Q.11–19 Clinically, FA is a chromosomal fragility disorder characterized by progressive BM failure (BMF), variable developmental anomalies, and a strong propensity to develop cancer. The risk of developing BMF by 40 years of age is as high as 90%, and the cumulative incidence of hematologic and non-hematologic malignancies was reported to be as high as 33% and 28%, respectively.20 In the natural course of the disease, FA patients develop progressive pancytopenia, indicating that the defect occurs at the level of HSC.21–23 FA patients have a high incidence of inherited skeletal malformations and osteoporosis,20,24 suggesting a role of FA proteins in osteogenesis and bone maintenance. Despite these clinical observations of multiple mesenchymal defects in FA and our increasing awareness of the interdependence between the BM niche and hematopoiesis, relatively little attention has been directed to investigating the putative association between abnormal HSC function and the BM niche in FA.
MSPC are a major component of the hematopoietic niche and have been shown to serve a critical function as hematopoiesis-supporting stromal cells.25 Here, we report that defective MSPC are pivotal mediators in the pathogenesis of hematopoietic defects in Fancc−/−;Fancg−/− double knockout (DKO) mice. Our studies provide detailed cellular and molecular evidence implicating mesenchymal cells as contributory to the BMF in FA, indicating the potential utility of MSPC/HSC co-transplantation, which may improve treatment of BMF in FA.
Methods
Animals and reagents
The Fancc and Fancg double heterozygous mice used in this study have been described previously.26–28 These mice were back-crossed into a C57BL/6J strain and were then bred to produce Fancc−/−;Fancg−/− (DKO) and wild-type (WT) mice. Age- and gender-matched DKO and WT mice were used for all experiments. All protocols were approved by the Institutional Animal Care and Use Committee at Indiana University School of Medicine. Chemicals were obtained from Sigma (St. Louis, MO, USA) unless otherwise indicated.
Isolation and expansion of mesenchymal stem/progenitor cells
MSPC from mice were generated as previously described.29 Briefly, BM mononuclear cells (BMMNC) were separated by low-density gradient centrifugation from 6- to 8-week-old, age- and gender-matched WT and DKO mice, then cultured in complete mouse MesenCult medium (Stem Cell Technologies Inc, Vancouver, Canada) at 37°C in 5% CO2. MSPC between passage five to ten were used for the following experiments. The phenotypic analyses of MSPC were performed by evaluating the expression of surface markers including CD44, CD105, CD146, CD29 on a FACS Calibur flow cytometer as previously described.30 For human MSPC isolation, whole BM cells from FA patients and healthy donors were cultured in Dulbecco modified Eagle medium (DMEM)/F12 (Gibco, Carlsbad, USA), containing 10% fetal bovine serum (Hyclone, South Logan, USA), 1× Insulin transferrin selenium-A (Life Technologies, Carlsbad, USA), 10 ng/mL human epidermal growth factor (Peprotech, Rocky Hill, NJ, USA), and 10 ng/mL human platelet-derived growth factor-BB (Peprotech) at 37°C in 5% CO2 and 5% O2 in a fully humidified atmosphere. MSPC at passage three to five were used for the following experiments.
Micro-computed tomography
To evaluate trabecular microarchitecture in the distal femoral metaphysis, fixed femora were scanned using a high-resolution desktop micro-computed tomography imaging system (μCT-20; Scanco Medical AG, Basserdorf, Switzerland). The region of interest was defined as 15% of the total femur length measured from the tip of the femoral condyle and extending proximally for 200 slices with an increment of 9 μm, and was subsequently reconstructed, filtered (σ= 0.8 and support = 1.0), and thresholded (at 22% of the possible gray scale value) for analysis, as described elsewhere.31 Trabecular bone was contoured manually within the trabecular compartment, excluding the cortical shell. The parameter of micro-architecture for bone volume fraction (BV/TV, %) was measured.
Histomorphometric measurements
Upon sacrifice, the isolated bones were fixed in 10% neutral buffered formalin for 48 h, dehydrated in graded ethanol, and embedded undecalcified in methyl methacrylate. Sagittal sections (5 μm thick) were cut from the middle of the femur. Tartrate-resistant acid phosphatase (TRAP) staining was performed using a leukocyte acid phosphatase kit (Sigma Diagnostics, St. Louis, MO, USA) and McNeal staining was performed using the McNeal tetrachromat kit (Polysciences, Warrington, PA, USA), both according to the manufacturers’ protocols. One section per femur was viewed at 100× magnification on a Leitz DMRXE microscope (Leica Mikroskopie und System GmbH, Wetzlar, Germany). Images were captured using a QImaging camera and QCapture-Pro software (Fryer Company Inc., Cincinnati, OH, USA). The region of interest for the metaphysis was defined by a rectangular area, which begins 0.5 mm proximal to the midpoint of the growth plate, non-inclusive of cortical bone, and extends proximally for a total area of approximately 2.8 mm2.
Bone remodeling measurement
Fluorochrome labeling of the bones was performed by intraperitoneal injections of calcein (20 mg/kg, 8 days before sacrifice) and alizarin (20 mg/kg, 4 days before sacrifice), as previously described.32 Trabecular bone turnover was assessed by measuring the extent of single-labeled surface (sLS), double-labeled surface (dLS) and the surface of the bone (BS) between the calcein and alizarin labels using Image Pro Plus version 4.1 software (Media Cybernetics, Silver Spring, MD, USA). Derived histomorphometric parameters included: (i) mineralizing surface (MS/BS), a measure of active bone-forming surface, calculated as (dLS+sLS/2)/BS; (ii) mineral apposition rate (MAR, μm/day), a measure of the rate of radial expansion of new bone, calculated as Thickness/4 day; and (iii) bone formation rate, an overall measure of bone formation that combines MS/BS and MAR, calculated as MS/BS × MAR.
Other methods
Methods for the clonogenic assay, bone mineral density quantification, annexin V/propidium iodide staining, senescence assay, thymidine incorporation assay, purification of HSPC, long-term culture of HSPC on MSPC monolayers, detection of reactive oxygen species (ROS), osteoblast and adipocyte differentiation of MSPC, reciprocal transplantation and co-transplantation of FA BMMNC and MSPC in NS2 mice are described in detail in the Online Supplementary Methods.
Statistics
Survival curves were compared using the log-rank test. Differences between two groups with equal variances were assessed by two-tailed Student t-tests. Multiple comparisons were conducted with one- or two-way analysis of variance (ANOVA) followed by an appropriate post-hoc correction. P values less than 0.05 were considered statistically significant. Data are presented as mean ± standard error of mean (SEM). Statistical analyses were performed with Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA).
Study approval
FA MSPC were generated from BM cells of five FA patients from the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College and Indiana University School of Medicine. Written informed consent was obtained from all patients. The study was approved by the Ethics Committee of the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences, and Indiana University School of Medicine according to guidelines of the 1975 Helsinki Declaration.
Results
Hematopoietic defects in double knockout mice are associated with a dysfunctional bone marrow microenvironment
We have previously reported that Fancc/g DKO mice develop progressive hematologic abnormalities, including BMF, acute myeloid leukemia, and myelodysplastic syndrome, which closely mimic hematopoietic disorders observed in FA patients.27 To assess the contribution of the microenvironment to the hematopoietic phenotype of DKO mice, we performed reciprocal transplantation experiments whereby hematopoietic cells from WT and DKO mice were transplanted into lethally irradiated WT or DKO recipients. The survival rate was lowest in the cohort of DKO mice reconstituted with DKO BM cells among the four groups (Figure 1A). DKO BM transplanted into WT recipients also caused reduced survival as compared to WT BM transplanted into WT recipients, due to the occurrence of BM dysplasia and BMF. Intriguingly, DKO recipient mice transplanted with WT BM cells also exhibited impaired survival and BM dysplastic phenotypes indicated by hematologic analysis, suggesting a putative role for an impaired marrow niche in DKO mice. In addition, an expanded granulocyte-macrophage progenitor compartment was observed in DKO recipient mice transplanted with WT BM cells compared to WT recipients (Online Supplementary Figure S1A). Furthermore, we observed a 25% or 60% reduction in the total number of colony-forming unit-cells (CFU-C) in DKO as compared to WT recipient mice transplanted with WT or DKO BM cells, respectively (Figure 1B). Consistently, a significantly hypoplastic BM was observed in WT or DKO recipients transplanted with either DKO or WT BM cells, respectively, with the most severe BM hypoplasia occurring in DKO recipients reconstituted with DKO BM cells (Figure 1C). The histology of the spleens of DKO recipients with either WT or DKO BM cells revealed disrupted architecture. Lymphoid aggregates in the white pulp (disrupted architecture) of DKO recipient spleens were also smaller than those of WT recipients (Figure 1D). May-Grünwald-Giemsa stained peripheral blood smears prepared from DKO recipients showed dysplastic features, including hyposegmented (bilobed) neutrophils with fine nuclear bridging consistent with pseudo-Pelger-Huët cells (Figure 1E, b–d, red arrows, Online Supplementary Figure S1B) and monocytes (Figure 1E, f–h, green arrows), whereas blasts were rare. In addition, dysplastic megakaryocytes (multinuclear megakaryocytes and hyposegmented megakaryocytes) were observed in the BM of DKO recipients transplanted with WT BM cells, but not in WT recipient mice (Online Supplementary Figure S1C). These data suggest that DKO recipient mice transplanted with WT or DKO BM cells develop a myelodysplastic syndrome-like disease33 and provide strong in vivo evidence that the niche plays a cooperative role in the pathogenesis of impaired marrow engraftment in the DKO FA mouse model.
Figure 1.
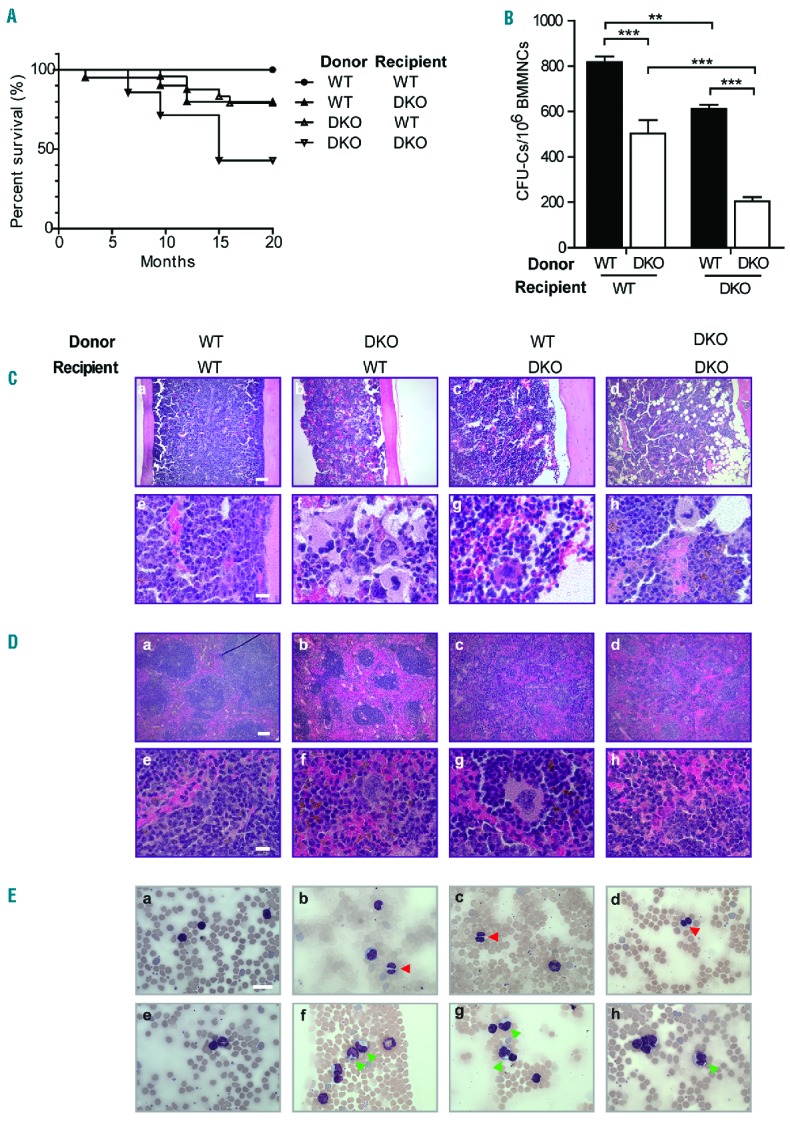
Poor survival and hematopoietic defects in double knockout mice. (A) Survival curves of WT and DKO recipients following transplantation with either WT (n = 11) or DKO (n = 11) BM cells were monitored over a duration of 20 months. (P<0.01, log-rank test). (B) Total number of colony-forming unit-cells (CFU-C) in five sets of DKO as compared to WT recipient mice transplanted with either WT or DKO BM cells; data are presented as mean ± SEM, **P<0.01, ***P<0.001, two-way ANOVA followed by the Bonferroni test. (C) Representative photomicrographs demonstrated BM histology of recipient mice 15 months post-transplantation at low (10×, a–d) and high (40×, e–h) magnification. Scale bar: 100 μm for a–d, and 10 μm for e–h. (D) Representative H&E-stained sections of spleens from transplanted recipients are shown at low (10×, a–d) and high (40×, e–h) magnification. Scale bar: 100 μm for a–d, and 10 μm for e–h. (E) Representative May-Grünwald-Giemsa stained peripheral blood smears of transplanted recipient mice are shown. The peripheral blood smear of DKO recipient mice with WT or DKO BM cells showed dysplastic features including monocytes (f–h, green arrows) and bilobed neutrophils (b–d, red arrows) which were consistent with pseudo Pelger-Huët cells. Scale bar: 10 μm.
Impaired skeletal development and bone mass deficits in double knockout mice
Skeletal anomalies including short stature and osteopenia/osteoporosis are widespread among the FA population.34 We, therefore, sought to ascertain the impact of Fancc/g genetic inactivation on skeletal development. The body length and body weight (Online Supplementary Figure S2A,B) of DKO mice were significantly reduced as compared to those of age- and sex-matched WT littermates. Whole body bone mineral density, determined by pDEXA, was also reduced in DKO mice as compared to WT controls (Figure 2A), with an even more substantial reduction in femoral bone mineral density of the DKO mice versus WT controls (Figure 2B). Consistent with the decreased bone mass in DKO mice determined by bone mineral density analysis, micro-computed tomography analysis of the animals at 6 months of age revealed decreased bone volume in the mid-shaft of DKO femora compared to WT controls (Figure 2C,D). Quantitative histomorphometric analysis also revealed significantly reduced bone volume in DKO femora versus WT ones (Online Supplementary Figure S2C).
Figure 2.
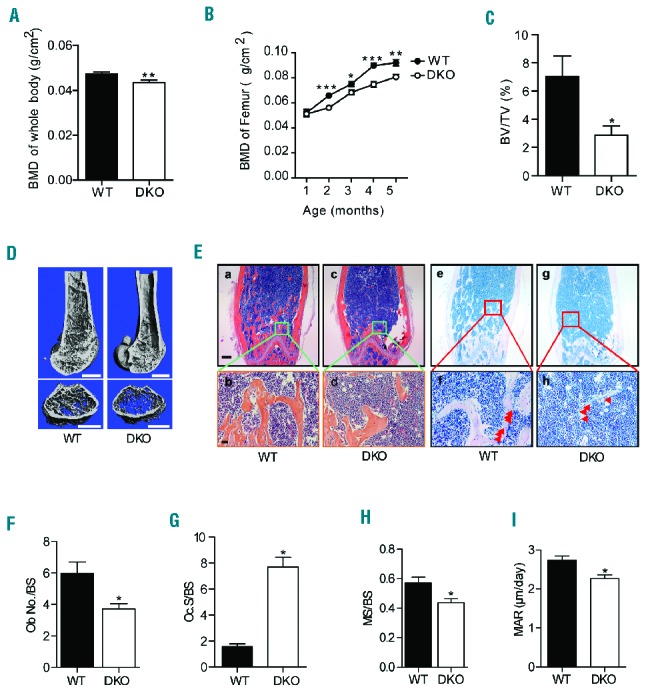
Double knockout mice had retarded growth and impaired bone mineralization. (A) DKO mice had decreased whole body bone mineral density (BMD) (n=20 mice per genotype) as compared to WT mice. Data are presented as mean ± SEM, **P<0.01, two-tailed Student t-test. (B) DKO mice had decreased BMD as compared to WT littermates at varying ages. Data are presented as mean ± SEM, *P<0.05, **P<0.01, ***P<0.001, two-tailed Student t-test. (C) Micro-computed tomography demonstrated that DKO mice had reduced femoral trabecular bone volume as compared to WT mice (n=8 mice per genotype). Data are presented as mean ± SEM of bone volume per tissue volume (BV/TV), *P<0.05, two-tailed Student t-test. (D) Representative micro-computed tomography reconstructions of WT and DKO mouse femora. Scale bar: 1 mm. (E) Representative H&E (a–d) and McNeal staining (e–h) analysis of the femora of WT and DKO mice. Red arrows indicate the osteoblasts on the bone surface. Scale bar: 200 μm for a, c, e, g and 50 μm for b, d, f, h. (F) DKO mice had reduced numbers of osteoblasts on the trabecular bone surface (Ob.No./BS) as compared to WT mice (n=20 mice per genotype). Data are presented as mean ± SEM, *P<0.05, two-tailed Student t-test. (G) DKO mice had increased osteoclasts along the trabecular bone surface (Oc.S/BS) as compared to WT mice (n=8 mice per genotype). Data are presented as mean ± SEM, *P<0.05, two-tailed Student t-test. (H, I) Bone remodeling studies demonstrated that DKO mice had reduced MS/BS and MAR as compared to WT mice (n=3 mice per genotype). Data are presented as mean ± SEM, *P<0.05, two-tailed Student t-test.
Alterations in skeletal homeostasis can occur secondary to imbalances between bone-forming osteoblast activity and osteoclast-mediated bone resorption. To further assess osteoblast and osteoclast development in vivo, quantitative histomorphometry was performed on histological sections from the distal femoral metaphysis stained with hematoxylin and eosin (H&E), McNeal, and the osteoclast enzyme TRAP (Figure 2E,F, Online Supplementary Figure S2D,E). Sections stained with H&E revealed a marked reduction in trabecular and cortical bone in DKO femora as compared to WT control femora (Figure 2E, a–d). Manually counting osteoblasts in the femoral trabecular bone on McNeal-stained sections revealed that the number of osteoblasts was singnificantly lower in DKO mice than in WT controls (Figure 2E, e–h, Figure 2F). In addition, a significantly increased osteoclast surface to bone surface ratio was observed in DKO mice than in WT controls, as assessed by scoring the TRAP-positive staining osteoclast surface in the femoral trabeculae normalized to the trabecular bone surface (Figure 2G, Online Supplementary Figure S2D). To study dynamic changes in bone remodeling, WT and DKO mice were injected with fluorochrome markers to label the bone surface (Online Supplementary Figure S2E).35 A 23% reduction in the mineralizing surface (MS/BS, Figure 2H, Online Supplementary Table S1), a 17% reduction in mineral apposition rate (MAR, Figure 2I, Online Supplementary Table S1), and a 36% reduction in bone formation rate (BFR)/BS were observed in DKO mice as compared to age- and sex-matched WT controls (Online Supplementary Figure S2F, Online Supplementary Table S1). Collectively, these data suggest that abnormal osteoblast-and osteoclast-mediated bone turnover in DKO mice leads to pathological bone remodeling.
Fancc/g genetic ablation alters mesenchymal stem/progenitor cell fates, favoring adipogenic versus osteoblastic differentiation
As osteoblasts, the principal cells mediating bone formation were deficient in DKO mice (Figure 2F), we hypothesized that Fancc/g deficiency alters the proliferative and/or differentiative capacity of MSPC, which give rise to mature osteoblasts and their precursors. We, therefore, performed colony-forming unit-fibroblast (CFU-F) assays on BM cells of the mice to determine the frequency of MSPC in WT versus DKO mice in vivo. DKO BM exhibited a significant reduction in the number of CFU-F compared to the marrow of WT littermates (Figure 3A,B). Consistently, flow cytometric analysis showed that the frequency of CD45−CD146+Nestin+CD105+ MSPC was significantly decreased in the BM of DKO mice compared to WT controls (Online Supplementary Figure S3A). Phenotypically defined MSPC from BM of DKO and WT mice were used to conduct the following experiments (Online Supplementary Figure S3B), and a significant reduction of CD146 expression was observed in DKO MSPC compared to WT MSPC. A thymidine incorporation assay demonstrated that DKO MSPC had significantly less proliferative potential compared to WT MSPC (Online Supplementary Figure S3C).
Figure 3.
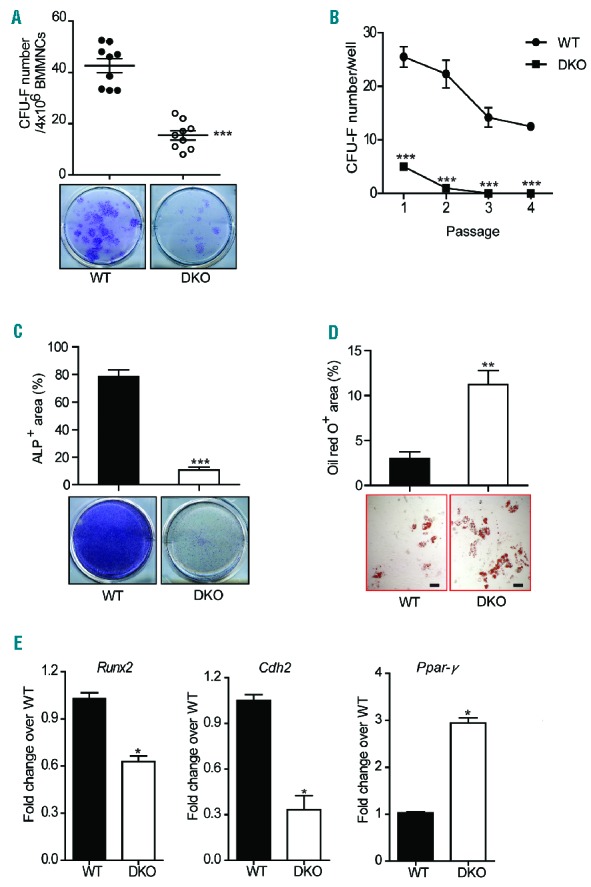
In vitro analysis of mesenchymal stem progenitor cell frequency and osteoblast differentiation. (A) The frequency of CFU-F per 4×106 BMMNC from WT and DKO mice is shown (n=9 mice per genotype). Data are presented as mean ± SEM, ***P<0.001, two-tailed Student t-test. (B) Significant reduction of CFU-F in DKO mice at different cell passages (n=9 mice per genotype). Data are presented as mean ± SEM, ***P<0.001, two-tailed Student t-test. (C) The frequency of CFU-osteoblasts in WT and DKO mice is shown (n=5 mice per genotype). Alkaline phosphatase staining of WT and DKO BMMNC cultured in osteogenic medium. Data are presented as mean ± SEM, ***P<0.001, two-tailed Student t-test. (D) The ratio of Oil Red O-positive adipocytes to total colonies demonstrated enhanced adipocyte differentiation in DKO mice compared to WT ones (n=5 mice per genotype). Data are presented as mean ± SEM, **P<0.01, two-tailed Student t-test. Scale bar: 100 μm (E) Significantly reduced Runx2, Cdh2 and increased Ppar-γ gene expression in DKO MSPC as compared to WT controls (n=5 mice per genotype). Data are presented as mean ± SEM, *P<0.05, two-tailed Student t-test.
As one of the fundamental properties of MSPC is their capacity to differentiate into multiple lineages under specific culture conditions,36 we next determined whether Fancc/g deletion altered MSPC lineage commitment by performing osteoblast and adipogenic differentiation assays. Alkaline phosphatase activity is an indicator of successful differentiation of MSPC into osteoblasts.37 Compared to WT MSPC, DKO MSPC exhibited markedly reduced alkaline phosphatase staining following incubation in osteogenic differentiation medium, indicating impaired osteoblast differentiation (Figure 3C). In contrast, when MSPC were cultured in adipogenic medium for 14 days, a significantly increased Oil Red O-positive area was observed in DKO cultures compared to WT controls (Figure 3D), suggesting that DKO MSPC had an increased capacity of adipocyte differentiation. These results indicate that Fancc/g deficiency leads to impaired MSPC proliferation (as determined by CFU-F) and lineage skewing, favoring adipocyte commitment over osteoblast differentiation.
To delineate the molecular basis of impaired cell fate determination in DKO MSPC, we used quantitative polymerase chain reaction analysis to examine the expression of critical genes governing lineage commitment of MSPC, including osteoblasts, and adipocytes in WT versus DKO cells. Our results indicated that the expression of genes controlling osteoblast differentiation, such as Runx2 and N-cadherin (Cdh2), were significantly reduced in DKO MSPC compared to WT controls (Figure 3E). In contrast, a marked increase in the expression of the adipogenic transcription factor, Ppar-γ, was observed in DKO MSPC compared to WT MSPC (Figure 3E). These data indicate that Fancc/g deletion alters gene expression programs governing lineage commitment of MSPC, leading to deregulated adipocyte and osteoblast lineage commitment.
Osteoclasts are specialized cells derived from the monocyte/macrophage hematopoietic lineage which adhere to the bone surface, secreting acid and lytic enzymes that degrade the bone matrix. To determine whether genetic ablation of Fancc/g alters osteoclast development, we established osteoclast cultures from BMMNC in the presence of the osteoclast differentiating cytokines M-CSF and RANK-L. DKO BMMNC exhibited a significantly increased propensity to osteoclast differentiation compared to WT BMMNC, as quantified by TRAP staining (Online Supplementary Figure S3D). Collectively, these data indicate that functional imbalances between osteoblast and osteoclast differentiation in the context of Fancc/g deficiency might cooperate to alter bone remodeling in vivo, contributing to short stature and osteoporosis in DKO mice as shown in Figure 2.
Double knockout mesenchymal stem/progenitor cells exhibit defective hematopoietic supportive activity in vitro
MSPC and their progeny, such as osteoblasts and adipocytes, are widely recognized to play a critical role in supporting hematopoietic cells within the BM niche.38,39 Given that DKO MSPC exhibit impaired expansion and differentiation, we sought to explore further the role of Fancc/g in maintaining MSPC hematopoietic supportive activity. We began by performing cobblestone area-forming cell (CAFC) assays to evaluate the hematopoietic supportive activity of DKO MSPC. When hematopoietic progenitors (LK cells) were co-cultured for 4 weeks on MSPC feeder layers, significantly reduced CAFC numbers were observed when using DKO MSPC compared to WT MSPC, suggesting impaired hematopoietic supportive activity by DKO MSPC (Figure 4A,B). To further assess whether Fancc/g deficiency alters the capacity of MSPC to maintain hematopoietic cell differentiation, the percentage of Gr1+/Mac1+ cells following 4 weeks of co-culture was determined by flow cytometry. As shown in Figure 4C, significantly increased percentages of Gr1+/Mac1+ cells were observed in DKO MSPC-supported WT and DKO LK hematopoietic cell cultures compared to WT MSPC, indicating an enhancement of myeloid differentiation. Consistently, a significantly increased percentage of Gr1+/Mac1+ cells was observed in the peripheral blood of DKO recipients transplanted with either WT or DKO BM cells, compared with the WT recipients (Online Supplementary Figure S4A). In addition, DKO MSPC-supported cultures contained increased percentages of apoptotic CD45+ cells (Figure 4D). Collectively, these findings indicate that Fancc/g-deleted MSPC increase myeloid cell differentiation in vitro compared to WT MSPC.
Figure 4.
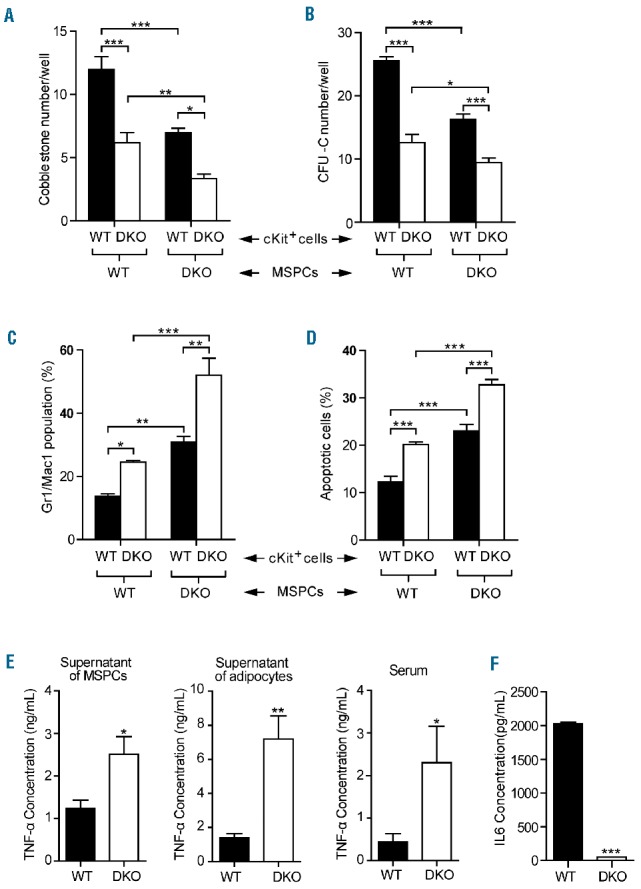
Co-culture of double knockout mesenchymal stem/progenitor cells with hematopoietic stem/progenitor cells showed the decreased hematopoietic supportive activity. (A, B) DKO MSPC had reduced hematopoietic supportive activity as compared with WT MSPC (n=3 mice per genotype). Data are presented as mean ± SEM, *P<0.05, **P<0.01, ***P<0.001, two-way ANOVA followed by the Bonferroni test. (C, D) DKO MSPC supported HSC cultures contained higher percentages of the Gr1+/Mac1+ population and apoptotic CD45+ cells (n=3 mice per genotype). Data are presented as mean ± SEM, *P<0.05, **P<0.01, ***P<0.001, two-way ANOVA followed by the Bonferroni test. (E) MSPC-conditioned medium, adipocyte-conditioned medium, and the serum of DKO mice contained significantly increased concentrations of tumor necrosis-α (TNF-α) as compared with WT mice (n=3 mice per genotype). Data are presented as mean ± SEM, *P<0.05, **P<0.01, two-tailed Student t-test. (F) A marked reduction of the interleukin-6 (IL6) level was observed in DKO MSPC conditioned medium (n=3 mice per genotype). Data are presented as mean ± SEM, ***P<0.001, two-tailed Student t-test.
Secretion of trophic and paracrine factors within the BM niche is regarded to be a central mechanism by which MSPC function to maintain hematopoiesis. We, therefore, hypothesized that deregulated secretion of paracrine factors might account for the impaired hematopoietic supportive activity of DKO MSPC. Tumor necrosis factor-alpha (TNF-α) is an inflammatory cytokine, which has been shown to preferentially induce apoptosis in FA hematopoietic cells.40,41 An enzyme-linked immunosorbent assay showed a significantly increased level of TNF-α in DKO MSPC supernatants (Figure 4E). In addition to MSPC, adipocytes are known to be another major source of TNF-α production.42,43 Consistent with these data, we observed a 4.5-fold increase in the concentration of TNF-α in conditioned media collected from DKO adipocyte cultures compared to WT controls (Figure 4E). As further validation, significantly increased concentrations of TNF-α were also observed in the serum of DKO mice as compared to that of WT mice (Figure 4E). By contrast, levels of the hematopoietic supportive cytokine interleukin-6 were found to be markedly reduced in DKO MSPC conditioned medium (Figure 4F). HSC can lose stem cell capacity and die after exposure to ROS; DKO MSPC exposed to H2O2 produced increased ROS compared to their WT counterpart (Online Supplementary Figure S4B). This enhanced ROS production has also been identified along with the senescence and adipocyte differentiation of MSPC,44,45 which is consistent with the characteristics of the DKO MSPC.
Taken together, these data suggest that loss of Fancc/g in MSPC alters the production of multiple cytokines and ROS, which may serve to perpetuate dysfunctional hematopoiesis in DKO mice.
Human Fanconi anemia patient-derived and double knockout mesenchymal stem/progenitor cells exhibit similar cellular phenotypes
To examine whether MSPC derived from FA patients exhibit similar phenotypes to those observed in DKO mice, MSPC were isolated from four patients with a clinical diagnosis of FA (Online Supplementary Table S2) and healthy volunteers by culturing BM cells in human MesenCult medium and phenotypically validating the cells by flow cytometry (Online Supplementary Figure S5A). Sensitivity to mitomycin C was determined as previously described30 and was greater in FA MSPC than in healthy control MSPC (Figure 5A). Cellular senescence is a key pathophysiological phenomenon characterized by cell cycle arrest and upregulation of senescence-associated β-galactosidase activity. A 3-fold increase in the percentage of senescent cells was observed in FA MSPC compared to control MSPC (Figure 5B, Online Supplementary Figure S5B). Like DKO MSPC, human FA MSPC exhibited markedly defective osteoblast differentiation (Figure 5C,D) and increased adipocyte differentiation (Online Supplementary Figure S5C). Furthermore, osteoblast numbers were significantly reduced in BM biopsy sections from FA patients compared to those from healthy controls (Online Supplementary Figure S5D,E).
Figure 5.
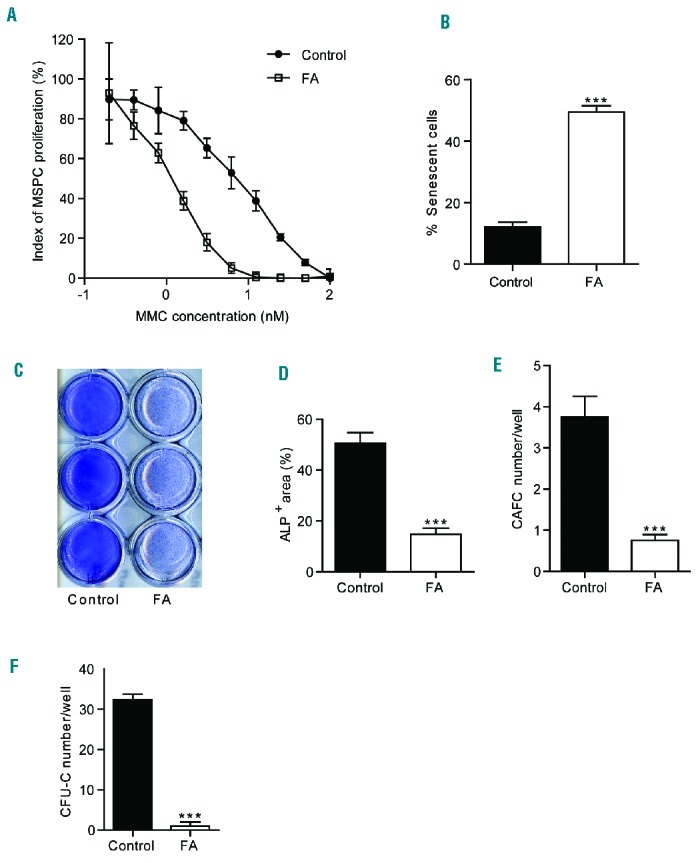
Impaired cellular functions of Fanconi anemia patient-derived mesenchymal stem/progenitor cells. (A) FA MSPC were more sensitive to mitomycin C (MMC). Data are presented as mean ± SEM and represent one of four independent experiments. (B) FA MSPC had an increased rate of senescence as compared to control MSPC. Data are shown as mean ± SEM from triplicate wells (5 fields/well) and represent one of four independent experiments. ***P<0.001, two-tailed Student t-test. (C) Representative images demonstrating alkaline phosphatase (ALP) staining of a healthy donor and FA MSPC cultured in the osteogenic medium surface. (D) FA MSPC had impaired osteoblast differentiation. Data are presented as mean ± SEM from triplicate wells (6 fields/well) and represent one of four independent experiments. ***P<0.001, two-tailed Student t-test. (E, F) FA MSPC had impaired hematopoietic supportive activity. Data are presented as mean ± SEM from triplicate wells and represent one of four independent experiments. *** P<0.001, two-tailed Student t-test. Each experiment was performed with a different MSPC culture isolated from the individual patient.
To evaluate the hematopoietic supportive activity of these MSPC, MSPC from FA patients were co-cultured with cord blood CD34+ cells. After 5 weeks of co-culture, CAFC were counted, and CFU-C assays were performed. Significantly lower numbers of CAFC (Figure 5E) and CFU-C (Figure 5F) were observed in the co-cultures of FA MSPC with CD34+ cells than in those of healthy control MSPC with CD34+ cells. These data suggest that MSPC derived from FA patients exhibit impaired HSPC supportive activity, which is consistent with the findings in MSPC from DKO mice.
To test whether MSPC derived from healthy donor BM, compared to FANCG-deficient MSPC, would enhance the engraftment of human FANCG-deficient BM cells in vivo, MSPC were injected intratibially into sub-lethally irradiated NOD.Cg-Prkdcscid IL2rgtm1Wjl/Sz (NS2) recipient mice. Twenty-four hours later, BMMNC from a human FANCG-deficient patient were delivered via tail vein injection. Four months following co-transplantation, human (h) CD45+ cell engraftment in the BM of recipient mice was analyzed by flow cytometry. Injection of healthy MSPC dramatically enhanced FANCG BMMNC engraftment (19% of hCD45+ cells, Online Supplementary Figure S5F, right panel), while the percentage of hCD45+ cells in the mice that received FANCG MSPC and FANCG BMMNC was only 0.9% (Online Supplementary Figure S5F, left panel).
Discussion
MSPC act as an essential component of the BM hematopoietic microenvironment and have been proven to be involved in the pathogenesis of several hematologic malignancies.46,47 A recent translational study by Dong et al. found that Ptpn11-activating mutations in the BM MSPC and osteoprogenitors cause a juvenile myelomonocytic leukemia-like cancer in mice through profound, detrimental effects on HSC.48 Several previous studies indicated that MSPC from FA patients display reduced long-term proliferation ability and spontaneous chromosome breakages.49–51 In addition, we have previously reported that MSPC from the murine Fancg−/− model exhibit impaired proliferative capacity.30 Amarachintha et al. also reported that MSC from Fanca−/− or Fancd2−/− mice impaired WT HSPC self-renewal and induced myeloid expansion.52 Although another study showed that BM MSPC did not have impaired function and contribute to the pathogenesis of the disease in acquired BMF such as aplastic anemia,53 recent studies by Zambetti et al. demonstrated that mesenchymal niche-derived inflammatory signaling induces oxidative and genotoxic stress in HSPC in Shwachman-Diamond syndrome, a rare inherited BMF syndrome.54 Whether defects of BM MSPC are involved in the pathophysiology of FA deserves further in vivo investigation.
FA is caused by a mutation in genes encoding proteins required for the FA pathway. Although FA patients are clinically characterized by congenital mesenchymal anomalies, and a uniformly progressive and fatal BMF which begins in infancy or childhood,20,55–58 the impact of the loss of FA genes on other stem cell compartments and the role of the BM niche in the pathogenesis of FA-dependent BMF have received limited attention. To date, more than ten FA genes have been deleted or mutated in the mouse, but none of these mouse models with single FA gene deficiency spontaneously develops severe hematologic abnormalities like FA patients.59,60 We have previously reported that Fancc/g DKO mice spontaneously develop more aggressive hematopoietic deficits including BMF, acute myeloid leukemia, and myelodysplastic syndrome.27 Here, using the DKO murine model, we provided evidence that Fanc/g-deficient MSPC within the hematopoietic microenvironment cooperate to engender dysfunctional hematopoiesis. These results provide new insights regarding the fundamental mechanisms by which the BM “niche” contributes to the pathogenesis of FA-dependent BMF. Furthermore, we demonstrated that Fancc/g-deficient MSPC lead to impaired osteoblast differentiation with concomitant lineage skewing toward adipocyte commitment. Genetic ablation of Fancc/g also altered the production of critical inflammatory cytokines including interleukin-6 and TNF-α, the latter of which is known to induce HSPC apoptosis and contribute to BMF. Collectively, this study reveals an intimate relationship between FA HSPC and the BM niche in the pathogenesis of hematopoietic deficits.
Our study provides strong evidence that Fancc/g deficiency results in multiple skeletal pathologies, including reduced body size and low bone mass phenotypes. These phenotypes in mice recapitulate the clinical features of short stature and osteoporosis common in FA patients.61 Bone remodeling is a dynamic process controlled by the coordinated activity of osteoblast-mediated bone formation and osteoclast-mediated bone resorption. We attribute the bone mass deficits in DKO mice in part to reduced osteoblast activity, as evidenced by the reduced osteoblast numbers and impaired bone remodeling in vivo.
Like HSC, MSPC have the potential to differentiate into multiple lineages, including osteoblasts, adipocytes, and chondrocytes. The balance between osteogenesis and adipocyte formation is required for normal niche activity to maintain hematopoiesis.62–65 Our in vitro and in vivo studies indicate that Fancc/g deficiency impairs the differentiation of MSPC into osteoblasts while favoring adipocyte differentiation. Meanwhile, DKO mice exhibit reduced MSPC numbers and impaired self-renewal, suggesting an association between dysfunctional DKO MSPC and abnormal skeletal development/homeostasis in the DKO mouse model. DKO MSPC displayed dysregulated expression of multiple key genes controlling osteoblast versus adipocyte lineage commitment including Runx2, Cdh2, and Ppar-γ. Cdh2 has been identified as a negative regulator of adipogenesis.66 Therefore, skewed lineage commitment of DKO MSPC away from osteoblast differentiation and toward adipocyte commitment may be associated with reduced Cdh2 expression. In addition, we observed a significant reduction of CD146 expression in DKO MSPC compared to WT MSPC. Since CD146 has been reported to be a marker for multilineage differentiation capacity,67 the fewer CD146+ cells in DKO MSPC might be associated with the defective MSPC functions. Consistent with murine data, MSPC obtained from BM biopsies of FA patients also revealed impaired proliferation and osteoblast differentiation capacity.
MSPC-HSPC co-culture assays further revealed that MSPC from DKO mice and FA patients have defective hematopoietic supportive activity, as evidenced by reduced CAFC and CFU-C formation. Dysregulated skeletal remodeling and impaired osteoblast differentiation in DKO mice may thus contribute to the defective BM microenvironment, which is unable to sustain adequate HSPC numbers. These results are consistent with findings by Morad et al. who showed that the hematopoietic supportive activity of MSPC is influenced by lineage determination.68
It has been previously demonstrated that FA HSPC are hypersensitive to TNF-α induced apoptosis and increased levels of TNF-α and other inflammatory cytokines have been observed in FA patients.26,69,70 Here we observed reduced levels of interleukin-6 and significantly higher levels of inflammatory cytokines, including TNF-α, in the DKO model. Nagajyothi et al. previously reported that adipocytes are the major source of circulating inflammatory cytokines.71,72 It is also known that adipocytes negatively regulate hematopoietic activity and that TNF-α plays an important role in the pathogenesis of BMF.38,73,74
In addition, we observed that co-transplantation of healthy donor MSPC enhanced the engraftment and expansion of human BMMNC from an FANCG patient in vivo in NS2 mice while FA MSPC failed to support FA BM cell reconstitution. Nevertheless, studies in a larger cohort of NSG mice with the co-transplantation of MSPC and FA BM cells are warranted in the future.
Collectively, these data suggest that the pathogenesis of hematopoietic deficits in FA is complex and likely depends on the interplay between abnormal hematopoietic cells and a dysfunctional BM niche. Our study indicates that FA MSPC have skewed differentiation capacity, altered cytokine secretion and defective hematopoietic supportive activity, providing scientific insight into the role of FA mutations in impairing the hematopoietic niche and their involvement in the pathogenesis of BMF. Clinically, a more detailed understanding of the disease might lead to rethink about the niche for the development of new therapies for FA. While MSPC are increasingly appreciated to be a critical part of the niche, due to the anatomical structure of the niche, future studies to dissect the role of other niche components, e.g., the endothelial niche or MSPC progenies, with specific deletion of FA genes using Cre/loxP technology are warranted.
Supplementary Material
Acknowledgments
The authors thank Heather Daniel for administrative support.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/6/1017
Funding
This work was supported in part by the Leukemia Lymphoma Society (LLS 6234-12 to FCY), NIH (R01CA155294-05 to DWC, F-C Y and HH), Ministry of Science and Technology of China (2016YFA0100600) and National Natural Science Foundation of China (81570113, 81270575, 81421002). SDR was supported in part by pre-doctoral training grants from the Indiana CTSI (NCCR 5TL1RR025759-03) and Children’s Tumor Foundation.
References
- 1.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. [DOI] [PubMed] [Google Scholar]
- 3.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. [DOI] [PubMed] [Google Scholar]
- 4.Devine SM, Hoffman R. Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol. 2000;7(6):358–363. [DOI] [PubMed] [Google Scholar]
- 5.Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20(3):161–171. [DOI] [PubMed] [Google Scholar]
- 6.Schepers K, Campbell TB, Passegue E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16(3):254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DA, Cancelas JA. Leukaemia: niche retreats for stem cells. Nature. 2006;444(7121):827–828. [DOI] [PubMed] [Google Scholar]
- 8.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129(6): 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YW, Koo BK, Jeong HW, et al. Defective Notch activation in microenvironment leads to myeloproliferative disease. Blood. 2008;112(12):4628–4638. [DOI] [PubMed] [Google Scholar]
- 10.Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garaycoechea JI, Patel KJ. Why does the bone marrow fail in Fanconi anemia¿ Blood. 2014;123(1):26–34. [DOI] [PubMed] [Google Scholar]
- 12.Reid S, Renwick A, Seal S, et al. Biallelic BRCA2 mutations are associated with multiple malignancies in childhood including familial Wilms tumour. J Med Genet. 2005;42(2):147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sims AE, Spiteri E, Sims RJ, 3rd, et al. FANCI is a second monoubiquitinated member of the Fanconi anemia pathway. Nat Struct Mol Biol. 2007;14(6):564–567. [DOI] [PubMed] [Google Scholar]
- 14.Smogorzewska A, Matsuoka S, Vinciguerra P, et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129(2):289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorsman JC, Levitus M, Rockx D, et al. Identification of the Fanconi anemia complementation group I gene, FANCI. Cell Oncol. 2007;29(3):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Shin-ya K, Brosh RM., Jr FANCJ helicase defective in Fanconia anemia and breast cancer unwinds G-quadruplex DNA to defend genomic stability. Mol Cell Biol. 2008;28(12):4116–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaz F, Hanenberg H, Schuster B, et al. Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat Genet. 2010;42(5): 406–409. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y, Lach FP, Desetty R, et al. Mutations of the SLX4 gene in Fanconi anemia. Nat Genet. 2011;43(2):142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogliolo M, Schuster B, Stoepker C, et al. Mutations in ERCC4, encoding the DNA-repair endonuclease XPF, cause Fanconi anemia. Am J Hum Genet. 2013;92(5):800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutler DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003;101(4):1249–1256. [DOI] [PubMed] [Google Scholar]
- 21.Ceccaldi R, Parmar K, Mouly E, et al. Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell. 2012;11(1):36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geiselhart A, Lier A, Walter D, Milsom MD. Disrupted signaling through the Fanconi anemia pathway leads to dysfunctional hematopoietic stem cell biology: underlying mechanisms and potential therapeutic strategies. Anemia. 2012;2012:265790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamimae-Lanning AN, Goloviznina NA, Kurre P. Fetal origins of hematopoietic failure in a murine model of Fanconi anemia. Blood. 2013;121(11):2008–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wajnrajch MP, Gertner JM, Huma Z, et al. Evaluation of growth and hormonal status in patients referred to the International Fanconi Anemia Registry. Pediatrics. 2001; 107(4):744–754. [DOI] [PubMed] [Google Scholar]
- 25.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. [DOI] [PubMed] [Google Scholar]
- 26.Si Y, Ciccone S, Yang FC, et al. Continuous in vivo infusion of interferon-gamma (IFN-gamma) enhances engraftment of syngeneic wild-type cells in Fanca−/− and Fancg−/− mice. Blood. 2006;108(13):4283–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulliam-Leath AC, Ciccone SL, Nalepa G, et al. Genetic disruption of both Fancc and Fancg in mice recapitulates the hematopoietic manifestations of Fanconi anemia. Blood. 2010;116(16):2915–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Kuang Y, Montes De Oca R, et al. Targeted disruption of the murine Fanconi anemia gene, Fancg/Xrcc9. Blood. 2001;98(12):3435–3440. [DOI] [PubMed] [Google Scholar]
- 29.Wu X, Estwick SA, Chen S, et al. Neurofibromin plays a critical role in modulating osteoblast differentiation of mesenchymal stem/progenitor cells. Hum Mol Genet. 2006;15(19):2837–2845. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Chen S, Yuan J, et al. Mesenchymal stem/progenitor cells promote the reconstitution of exogenous hematopoietic stem cells in Fancg−/− mice in vivo. Blood. 2009;113(10):2342–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munugalavadla V, Vemula S, Sims EC, et al. The p85alpha subunit of class IA phosphatidylinositol 3-kinase regulates the expression of multiple genes involved in osteoclast maturation and migration. Mol Cell Biol. 2008;28(23):7182–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien CA, Plotkin LI, Galli C, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008;3(8):e2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 34.Giri N, Batista DL, Alter BP, Stratakis CA. Endocrine abnormalities in patients with Fanconi anemia. J Clin Endocrinol Metab. 2007;92(7):2624–2631. [DOI] [PubMed] [Google Scholar]
- 35.Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146(2):685–693. [DOI] [PubMed] [Google Scholar]
- 36.Lee HS, Huang GT, Chiang H, et al. Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells. 2003;21(2):190–199. [DOI] [PubMed] [Google Scholar]
- 37.Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96(1):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naveiras O, Nardi V, Wenzel PL, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960): 841–846. [DOI] [PubMed] [Google Scholar]
- 40.Koh PS, Hughes GC, Faulkner GR, Keeble WW, Bagby GC. The Fanconi anemia group C gene product modulates apoptotic responses to tumor necrosis factor-alpha and Fas ligand but does not suppress expression of receptors of the tumor necrosis factor receptor superfamily. Exp Hematol. 1999;27(1):1–8. [DOI] [PubMed] [Google Scholar]
- 41.Dufour C, Corcione A, Svahn J, et al. TNF-alpha and IFN-gamma are overexpressed in the bone marrow of Fanconi anemia patients and TNF-alpha suppresses erythropoiesis in vitro. Blood. 2003;102(6):2053–2059. [DOI] [PubMed] [Google Scholar]
- 42.Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582(1): 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoareau L, Bencharif K, Rondeau P, et al. Signaling pathways involved in LPS induced TNFalpha production in human adipocytes. J Inflamm (Lond). 2010;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang DY, Pan Y, Zhang C, et al. Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol Cell Biochem. 2013;374(1–2):13–20. [DOI] [PubMed] [Google Scholar]
- 45.Kanda Y, Hinata T, Kang SW, Watanabe Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011;89(7–8):250–258. [DOI] [PubMed] [Google Scholar]
- 46.Schepers K, Pietras EM, Reynaud D, et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell. 2013;13(3):285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menendez P, Catalina P, Rodriguez R, et al. Bone marrow mesenchymal stem cells from infants with MLL-AF4+ acute leukemia harbor and express the MLL-AF4 fusion gene. J Exp Med. 2009;206(13):3131–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong L, Yu WM, Zheng H, et al. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature. 2016;539(7628):304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mantelli M, Avanzini MA, Rosti V, et al. Comprehensive characterization of mesenchymal stromal cells from patients with Fanconi anaemia. Br J Haematol. 2015;170(6):826–836. [DOI] [PubMed] [Google Scholar]
- 50.Lecourt S, Vanneaux V, Leblanc T, et al. Bone marrow microenvironment in fanconi anemia: a prospective functional study in a cohort of fanconi anemia patients. Stem Cells Dev. 2010;19(2):203–208. [DOI] [PubMed] [Google Scholar]
- 51.Barroca V, Mouthon MA, Lewandowski D, et al. Impaired functionality and homing of Fancg-deficient hematopoietic stem cells. Hum Mol Genet. 2012;21(1):121–135. [DOI] [PubMed] [Google Scholar]
- 52.Amarachintha S, Sertorio M, Wilson A, Li X, Pang Q. Fanconi anemia mesenchymal stromal cells-derived glycerophospholipids skew hematopoietic stem cell differentiation through toll-Like receptor signaling. Stem Cells. 2015;33(11):3382–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bueno C, Roldan M, Anguita E, et al. Bone marrow mesenchymal stem cells from patients with aplastic anemia maintain functional and immune properties and do not contribute to the pathogenesis of the disease. Haematologica. 2014;99(7):1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zambetti NA, Ping Z, Chen S, et al. Mesenchymal inflammation drives genotoxic stress in hematopoietic stem cells and predicts disease evolution in human pre-leukemia. Cell Stem Cell. 2016;19(5):613–627. [DOI] [PubMed] [Google Scholar]
- 55.Kook H. Fanconi anemia: current management. Hematology. 2005;10(Suppl 1):108–110. [DOI] [PubMed] [Google Scholar]
- 56.Bagby GC, Jr, Segal GM, Auerbach AD, et al. Constitutive and induced expression of hematopoietic growth factor genes by fibroblasts from children with Fanconi anemia. Exp Hematol. 1993;21(11):1419–1426. [PubMed] [Google Scholar]
- 57.Auerbach AD, Buchwald M, Joenje H. Fanconi anemia. In: Vogelstein B, Kinzler KW, eds. The Genetic Bases of Human Cancer. 2002; 2nd Edition (New York: McGraw-Hill, Inc.):289–306. [Google Scholar]
- 58.Mathew CG. Fanconi anaemia genes and susceptibility to cancer. Oncogene. 2006;25(43):5875–5884. [DOI] [PubMed] [Google Scholar]
- 59.Parmar K, D’Andrea A, Niedernhofer LJ. Mouse models of Fanconi anemia. Mutat Res. 2009;668(1–2):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bakker ST, de Winter JP, te Riele H. Learning from a paradox: recent insights into Fanconi anaemia through studying mouse models. Dis Model Mech. 2013;6(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glanz A, Fraser FC. Spectrum of anomalies in Fanconi anaemia. J Med Genet. 1982;19(6):412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Omatsu Y, Sugiyama T, Kohara H, et al. The essential functions of adipoosteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33(3): 387–399. [DOI] [PubMed] [Google Scholar]
- 63.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117(20):5281–5288. [DOI] [PubMed] [Google Scholar]
- 65.Visnjic D, Kalajzic Z, Rowe DW, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–3264. [DOI] [PubMed] [Google Scholar]
- 66.van Oostrom AJ, van Wijk JP, Sijmonsma TP, Rabelink TJ, Castro Cabezas M. Increased expression of activation markers on monocytes and neutrophils in type 2 diabetes. Neth J Med. 2004;62(9):320–325. [PubMed] [Google Scholar]
- 67.Xu J, Wang W, Kapila Y, Lotz J, Kapila S. Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev. 2009;18(3):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morad V, Pevsner-Fischer M, Barnees S, et al. The myelopoietic supportive capacity of mesenchymal stromal cells is uncoupled from multipotency and is influenced by lineage determination and interference with glycosylation. Stem Cells. 2008;26(9):2275–2286. [DOI] [PubMed] [Google Scholar]
- 69.Li J, Sejas DP, Zhang X, et al. TNF-alpha induces leukemic clonal evolution ex vivo in Fanconi anemia group C murine stem cells. J Clin Invest. 2007;117(11):3283–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pang Q, Keeble W, Christianson TA, Faulkner GR, Bagby GC. FANCC interacts with Hsp70 to protect hematopoietic cells from IFN-gamma/TNF-alpha-mediated cytotoxicity. EMBO J. 2001;20(16):4478–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagajyothi F, Desruisseaux MS, Thiruvur N, et al. Trypanosoma cruzi infection of cultured adipocytes results in an inflammatory phenotype. Obesity (Silver Spring). 2008;16(9):1992–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 73.Touw I, Lowenberg B. No stimulative effect of adipocytes on hematopoiesis in long-term human bone marrow cultures. Blood. 1983;61(4):770–774. [PubMed] [Google Scholar]
- 74.Yokota T, Oritani K, Takahashi I, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96(5):1723–1732. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


