Abstract
A significant proportion of hematopoietic stem cell transplants are performed with ABO-mismatched donors. The impact of ABO mismatch on outcome following transplantation remains controversial and there are no published data regarding the impact of ABO mismatch in acute myeloid leukemia patients receiving haploidentical transplants. Using the European Blood and Marrow Transplant Acute Leukemia Working Group registry we identified 837 patients who underwent haploidentical transplantation. Comparative analysis was performed between patients who received ABO-matched versus ABO-mismatched haploidentical transplants for common clinical outcome variables. Our cohort consisted of 522 ABO-matched patients and 315 ABO-mismatched patients including 150 with minor, 127 with major, and 38 with bi-directional ABO mismatching. There were no significant differences between ABO matched and mismatched patients in terms of baseline disease and clinical characteristics. Major ABO mismatching was associated with inferior day 100 engraftment rate whereas multivariate analysis showed that bi-directional mismatching was associated with increased risk of grade II–IV acute graft-versus-host disease [hazard ratio (HR) 2.387; 95% confidence interval (CI): 1.22–4.66; P=0.01). Non-relapse mortality, relapse incidence, leukemia-free survival, overall survival, and chronic graft-versus-host disease rates were comparable between ABO-matched and -mismatched patients. Focused analysis on stem cell source showed that patients with minor mismatching transplanted with bone marrow grafts experienced increased grade II–IV acute graft-versus-host disease rates (HR 2.03; 95% CI: 1.00–4.10; P=0.04). Patients with major ABO mismatching and bone marrow grafts had decreased survival (HR=1.82; CI 95%: 1.048 – 3.18; P=0.033). In conclusion, ABO incompatibility has a marginal but significant clinical effect in acute myeloid leukemia patients undergoing haploidentical transplantation.
Introduction
As the full potential of haploidentical hematopoietic stem cell transplantation (HCT) is gaining appreciation in the field of transplantation, and its capacity to provide an alternative donor source for a substantial segment of the population of patients lacking a matched related donor (estimated recently to be as large as 70%1) is being realized, efforts aimed at optimizing donor-recipient compatibility are gaining traction. Indeed, emerging data from patients with acute myeloid leukemia (AML) undergoing haploidentical HCT is establishing this approach as a viable option for patients lacking an HLA-matched donor.2–4 While the extensive applicability of haploidentical HCT was limited initially by a significant component of graft-versus-host disease (GvHD) contributing to increased non-relapse mortality,5,6 recent innovative approaches employing novel immunosuppression techniques are significantly improving patients’ outcome in this setting.7–9 Although ABO incompatibility is found in up to one-half of HLA-matched transplants,10,11 and has the potential to put the recipient at risk of significant complications, its overall effect on clinical outcome measures has been debated extensively. Publications involving multiple datasets of patients with various disease states, donor sources, and conditioning regimens have shown conflicting results in this regard.12–17 In this analysis of data in the European Society for Blood and Marrow Transplantation (EBMT) registry we set out to determine whether ABO compatibility has a significant role in influencing the outcome of AML patients undergoing haploidentical HCT.
Methods
Study population
This was a retrospective, multicenter analysis. Data were provided and approved for this study by the Acute Leukemia Working Party (ALWP) of the EBMT group registry. The latter is a voluntary working group of more than 500 transplant centers that are required to report all consecutive stem cell transplants and follow-ups once a year. Audits are routinely performed to determine the accuracy of the data. The study protocol was approved by the institutional review board at each site and complied with country-specific regulatory requirements. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent authorizing the use of their personal information for research purposes. Using the EBMT registry, we identified adult patients (age >18 years) with AML and the following inclusion criteria: transplanted between 2005 and 2014, and HLA haploidentical donor with bone marrow or granulocyte colony-stimulating factor-mobilized peripheral blood stem cell grafts. All donors were HLA-mismatched at least at two loci (≤8/10) (-A, -B, -C, DRB1, -DQB1). Exclusion criteria were previous allogeneic or cord blood transplantation. Major ABO incompatibility was defined as serological evidence of recipient-derived antibodies directed against donor red cells, minor ABO incompatibility was defined as serological evidence of donor-derived antibodies directed against the recipient’s red cells, while bi-directional incompatibility comprised cases with serological evidence of both donor- and recipient-derived red cell directed antibodies. Engraftment was defined as sustained achievement of an absolute neutrophil count of over 0.5×109/L. Conditioning regimens were classified as myeloablative or reduced intensity based on previously published criteria.18 Grading of acute and chronic GvHD was performed using established criteria.19 Chronic GvHD was classified as limited or extensive according to usual criteria.20 The list of institutions reporting data included in this study is provided in the Online Supplementary Data.
Statistical analysis
Five outcomes were evaluated: (i) non-relapse mortality, defined as death without previous relapse; (ii) relapse incidence, defined on the basis of morphological evidence of leukemia in bone marrow or other extramedullary organs; (iii) leukemia-free survival, defined as the time from transplantation to first event (either relapse or death in complete remission); (iv) overall survival; and (v) GvHD-free/relapse-free survival, defined as events including grade III–IV acute GvHD, chronic GvHD requiring systemic therapy, relapse, or death in the first year following the HCT. Cumulative incidence curves were used for relapse incidence and non-relapse mortality in a setting of competing risks, since death and relapse are competing events. Probabilities of overall survival and leukemia-free survival were calculated using the Kaplan–Meier estimate. All tests were two-sided with the type I error rate fixed at 0.05. Statistical analyses were performed with SPSS 19 (SPSS Inc., Chicago, IL, USA), and R 3.0.1 (R Development Core Team, Vienna, Austria) software packages.
Results
Patients’ characteristics
In all, 837 patients were transplanted between 2005–2014 with a median follow-up period of 35 months (range, 1.2–125.4 months). The characteristics of the patients, their diseases and transplants are summarized in Table 1. There were no significant differences between ABO-matched and ABO-mismatched patients in terms of disease status at transplant, high-risk cytogenetics, donor and recipient cytomegalovirus status, conditioning intensity, graft source, and rates of T-cell depletion.
Table 1.
Baseline characteristics of the study population.

As shown in Online Supplementary Table S1, leukemia, GvHD, and infection were the major causes of death across all ABO incompatibility categories.
Major ABO incompatibility is associated with decreased engraftment in haploidentical stem cell transplantation
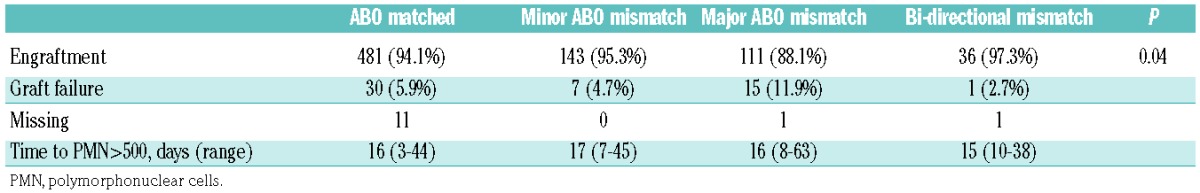
Since previous data indicated that ABO mismatching affected stem cell engraftment,11 we analyzed engraftment data per ABO category. As summarized in Table 2, day 100 engraftment rates were significantly lower in patients with major ABO incompatibility compared to those with other ABO mismatch categories. An analysis focused on graft source revealed that while the engraftment rate of peripheral blood grafts did not differ between subgroups, in bone marrow grafts major ABO incompatibility was again associated with inferior engraftment (data not shown).
Table 2.
Engraftment rate according to ABO incompatibility category.
Bi-directional ABO incompatibility increases the incidence of acute graft-versus-host disease in haploidentical transplants
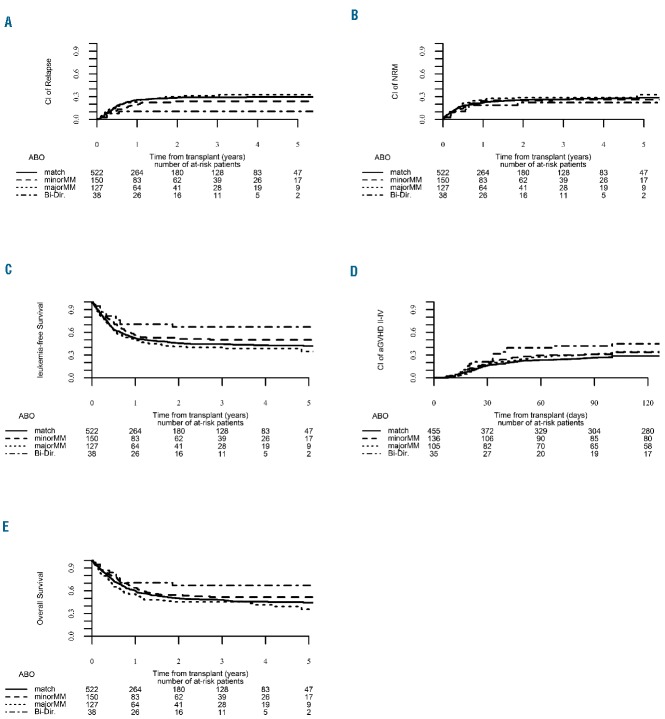
To evaluate whether ABO compatibility affects clinical outcome, a univariate analysis was initially carried out and, as shown in Online Supplementary Table S2, demonstrated that patients with bi-directional ABO mismatching had a significantly higher 3-year leukemia-free survival rate compared to patients with major ABO mismatching who had the lowest rate (67.2% and 40.1%, respectively). A similar finding was also observed with regard to 3-year GvHD-free/relapse-free survival rates which were increased in bidirectional mismatched patients and significantly lower in patients with a major ABO mismatch. A subsequent subgroup analysis of ABO-matched versus ABO-mismatched patients followed by focused analysis of specific mismatching patterns did not show any statistically significant differences between groups (Online Supplementary Table S2). To validate our findings we performed a multivariate analysis using the group of ABO-compatible patients as the reference group (Table 3). Interestingly, bi-directional ABO mismatching (n=38) was found to be associated with a significantly increased risk of grade II–IV acute GvHD [hazard ratio (HR)=2.38, 95% confidence interval (95% CI): 1.22 – 4.66; P=0.01) (Figure 1).
Table 3.
Multivariable analysis per ABO mismatch category of the entire cohort.
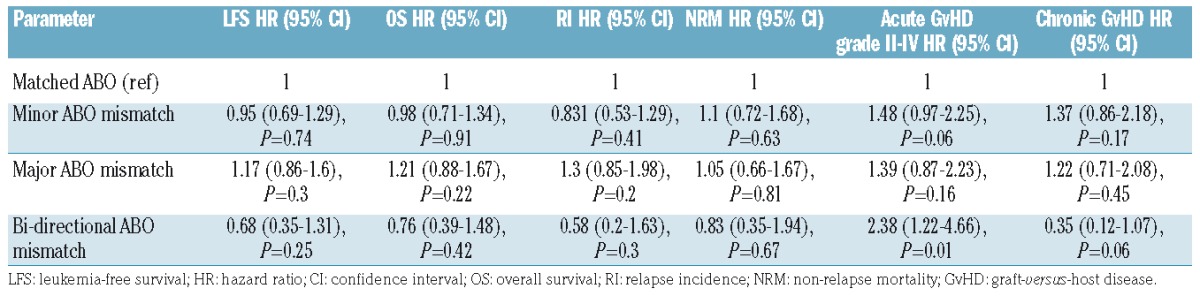
Figure 1.
Clinical outcome according to ABO compatibility status for the entire cohort. (A) Relapse incidence. (B) Non-relapse mortality. (C) Leukemia-free survival. (D) Acute graft-versus-host disease. (E) Overall survival.
To ensure that T-cell graft composition, namely T-cell-replete versus T-cell-depleted grafts, was not affecting our results, separate analyses were performed for patients transplanted with T-cell-replete and T-cell-depleted grafts. As detailed in Online Supplementary Table S3, in T-cell-replete grafts, univariate analysis revealed that the 3-year leukemia-free survival and GvHD-free/relapse-free survival rates were significantly increased in bi-directional ABO-mismatched patients compared to those in ABO-compatible patients and both ABO major and minor mismatched patients. Of note, chronic GvHD rates were increased in ABO-mismatched patients compared to ABO-matched patients. However, multivariate analysis failed to corroborate a statistically significantly association between ABO mismatch status and clinical outcome.
Since there were only four patients with bi-directional ABO mismatching in the T-cell-depleted cohort, these were excluded from the analysis. Neither univariate nor multivariate analysis showed that ABO mismatching status is a significant independent predictor of patients’ outcome following haploidentical HCT.
ABO mismatching status does not affect the clinical outcome of peripheral blood-derived grafts used for haploidentical transplant
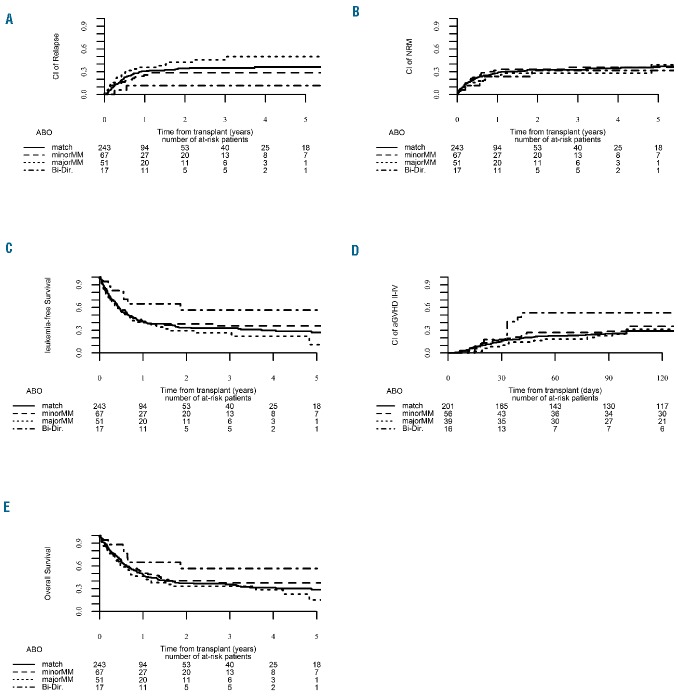
While previous publications indicated that clinical outcome is independent of stem cell source used for haploidentical HCT,21,22 we wondered whether ABO mismatching would have a differential effect on outcome in peripheral blood-mobilized grafts compared to bone marrow grafts. To this end, an analysis of the 378 patients transplanted with peripheral blood grafts (243 ABO-matched, 67 minor ABO-mismatched, and 68 major ABO-mismatched patients) was carried out. As shown in Online Supplementary Table S4 and Table 4, there was no statistically significant association between ABO incompatibility status and clinical outcome following transplantation of peripheral blood-derived grafts (Figure 2).
Table 4.
Multivariable analysis of patients’ outcome following transplantation with peripheral blood-derived grafts.
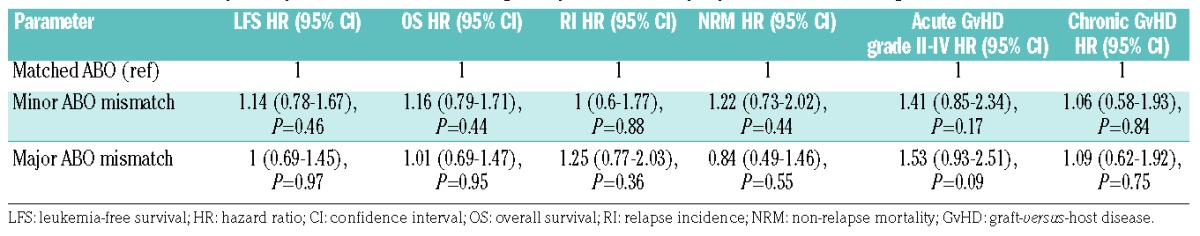
Figure 2.
Clinical outcome according to ABO compatibility status for patients transplanted with peripheral blood mobilized grafts. (A) Relapse incidence. (B) Non-relapse mortality. (C) Leukemia-free survival. (D) Acute graft-versus-host disease. (E) Overall survival.
Since our cohort grafted with peripheral blood included only 17 patients with bi-directional ABO mismatching we repeated the univariate and multivariate analyses with exclusion of these patients, again confirming that ABO mismatching does not influence clinical outcome in peripheral blood-mobilized grafts.
ABO incompatibility affects overall survival and graft-versus-host disease rates in haploidentical stem cell transplantation with bone marrow-derived grafts
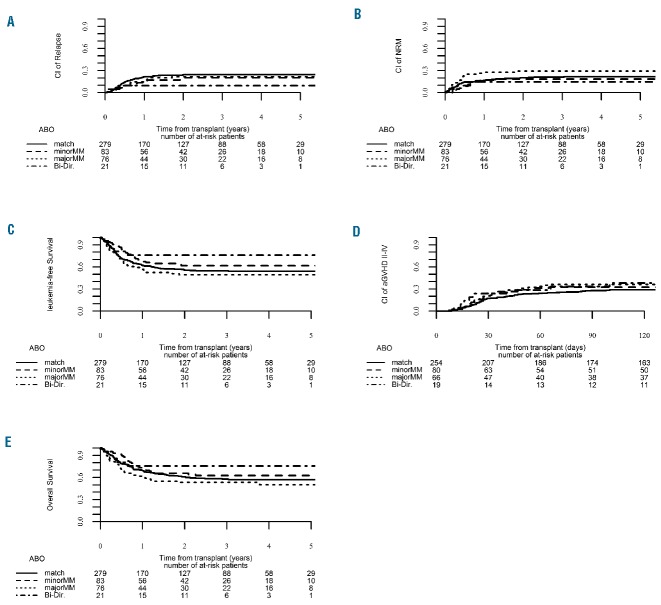
We then repeated the abovementioned analysis for the group of patients transplanted with bone marrow grafts (n=459). Univariate analysis (Online Supplementary Table S5) revealed that 3-year chronic GvHD rates were highest in patients with a minor ABO mismatch and lowest in ABO-matched patients (45.5% and 29.1%, respectively). Notably, in multivariate regression analysis with matched ABO patients as the reference group, minor ABO mismatching increased the risk of grade II-IV acute GvHD (HR=2.03; 95% CI: 1.007 – 4.1; P=0.047) (Table 5 and Figure 3).
Table 5.
Multivariable analysis of patients’ outcome following transplantation with bone marrow grafts.

Figure 3.
Clinical outcome according to ABO compatibility status for patients transplanted with bone marrow grafts. (A) Relapse incidence. (B) Non-relapse mortality. (C) Leukemia-free survival. (D) Acute graft-versus-host disease. (E) Overall survival.
Subsequently the analysis was repeated with exclusion of the small group of 21 patients with bi-directional mismatching. On univariate analysis with matched ABO patients as the reference group, the chronic GvHD rate was again increased in patients with a minor ABO mismatch compared to ABO-matched patients (45.5% and 29.1%, respectively). Notably, in multivariate regression analysis with matched ABO patients as the reference group, patients with major ABO mismatching had decreased overall survival (HR=1.82; 95% CI: 1.048 – 3.18; P=0.033) while there was a trend for increased 3-year grade II–IV acute GvHD rates in patients with minor ABO incompatibility (HR=2.01; 95% CI: 0.99 – 4.07; P=0.0504) (Online Supplementary Table S6).
Discussion
Haploidentical HCT is an innovative approach aimed to fill a substantial therapeutic gap for the significant population of patients without a related donor or a matched unrelated donor. Since initial experience with this approach showed that there is considerable risk of transplant-related complications,5 optimizing donor-recipient compatibility is of prime importance. In this analysis, the first of its kind for haploidentical HCT, we demonstrate that patients with major ABO incompatibility have inferior polymorphonuclear cell engraftment compared to both ABO-matched and minor-mismatched patients. Additionally, our data suggest that bi-directional ABO mismatching is associated with a significantly increased risk of grade II–IV acute GvHD. Furthermore our data indicate that patients transplanted with bone marrow grafts have an increased incidence of acute GvHD if there is minor ABO incompatibility, and decreased overall survival when major ABO incompatibility is present.
Donor-recipient ABO incompatibility is nearly ubiquitous in transplantation as up to one-half of transplants involve some degree of mismatching.10,11 This places patients at an increased risk of acute and delayed hemolytic reactions, and delayed recovery of red blood cell function. While secondary clinical parameters such as gender, donor age, parity, and cytomegalovirus status are clearly minor factors in dictating patients’ outcome following transplantation in general,23 the precise role ABO incompatibility holds in this regard is unclear.
In the present analysis we found that bi-directional ABO mismatching, namely the presence of antibodies directed against red blood cells in both donor and recipient, was associated with a significantly increased risk of grade II–IV acute GvHD. We do cautiously note the small number of patients with bi-directional incompatibility in our analysis (n=38) may limit the generalizability of these results to some degree. Our results are consistent with recently published data by Hefazi and colleagues12 who showed, in a cohort of 127 patients with AML or myelodysplastic syndromes (47 of whom were ABO mismatched), that the composite of major and bi-directional mismatching was also associated with a higher incidence of grade II–IV acute GvHD. However, when we analyzed the entire study cohort we were unable to find statistically significant associations between ABO mismatching and inferior nor-relapse mortality and overall survival rates which the abovementioned group did find. These differences probably reflect differences in the populations of patients, graft sources, and possibly cohort sizes.
An additional noteworthy finding in our study is the observation that patients with major ABO mismatching transplanted with bone marrow grafts had a lower overall survival rate than that of their ABO-matched counterparts. These findings are comparable with the recently published Center for International Blood and Marrow Transplant Research (IBMTR) experience with a large data set from over 5000 patients with AML or myelodysplastic syndromes indicating that major ABO incompatibility is associated with decreased overall survival (using related and unrelated matched donors).16 Our data also concur with their results in terms of the impact of ABO status on peripheral blood-mobilized grafts since neither analysis found any detrimental effect of ABO mismatching on clinical outcome following transplantation in this subgroup of patients. Notably, in a separate single institution (Stanford) retrospective analysis presented by the same authors,16 it was suggested that minor ABO incompatibility was closely associated with bone marrow grafts and these in turn were correlated with inferior overall survival and event-free survival, as well as increased non-relpase mortality rates. We did not find minor ABO incompatibility of bone marrow grafts to be associated with these clinical outcome measures but rather we did find that minor ABO mismatching was correlated significantly with grade II–IV acute GvHD. These variances could be accounted for by considering the differences in the cohorts analyzed, ours being a uniform cohort of AML patients transplanted with haploidentical HCT while the Stanford analysis was not limited to AML and consisted of standard matched related and unrelated donors.
We were also interested in specifically examining the incidence of extensive chronic GvHD in our study as recent work from the UK in 594 patients undergoing reduced intensity conditioning with alemtuzumab suggested that the incidence of extensive chronic GvHD was increased in ABO-mismatched patients. We did not find a similar association in our analysis, possibly because of the difference in patient composition between the analyses with the UK study also including patients with non-malignant conditions.17
One of the strengths of our analysis is the uniformity of the analyzed cohort since we focused our analysis solely on AML patients, differing to a significant degree from most prior publications in the field in which heterogeneous disease entities were analyzed with regard to the impact of ABO incompatibility. This may help to explain the divergence between some recent publications and ours. For example, an analysis of 414 patients with both malignant and non-malignant diagnoses using bone marrow, peripheral blood-, and cord blood-derived grafts failed to show a significant effect of ABO mismatching on patients’ outcome;15 in the same vein a study from Sweden looking at 310 patients with various hematologic diagnoses who underwent reduced intensity conditioning transplantation also did not show a substantial correlation between ABO status and clinical outcome.14 Interestingly, graft source may modify the influence of ABO mismatching, as emerging data with cord blood transplants in both the adult and pediatric setting also did not support a prognostic role for ABO status.13,24 To substantiate our findings we also conducted a sub-analysis of the impact of ABO status on T-cell-depleted grafts versus T-cell-replete grafts to determine whether there was a possible bias related to T-cell composition of the graft; as shown above, the T-cell-repletion status of the transplanted grafts had no effect on clinical outcome.
The limitations of our study include that it is a multicenter, retrospective analysis with the inherent biases involved in analyzing retrospective datasets. In addition, it is conceivable that additional modifying factors which were not analyzed, such as the ABH secretor status,25 graft mononuclear cell content26 or the presence of donor-specific anti-HLA antibodies which significantly affect graft failure and rejection,27–29 mediate the effect of ABO incompatibility on the final clinical outcome of patients undergoing haploidentical HCT.
Supported by a recently published clinical algorithm for donor selection in haploidentical transplantations which incorporates consideration of ABO compatibility,30 we cautiously propose that our findings may have future implications for clinical practice in terms of optimizing donor selection for AML patients undergoing haploidentical HCT, a supposition which would have to be confirmed in a controlled clinical trial.
In conclusion, our findings suggest that in AML patients undergoing haploidentical HCT major ABO mismatching is associated with inferior engraftment and overall survival when bone marrow grafts are used. Additionally, patients with minor ABO mismatching may experience increased acute GvHD rates when transplanted with bone marrow-derived grafts. Thus, ABO incompatibility status may hold prognostic significance and should be considered and assessed routinely during evaluation for the optimal donor prior to haploidentical HCT.
Supplementary Material
Acknowledgments
We thank all the European Group for Blood and Marrow Transplantation (EBMT) centers and national registries for contributing patients to the study and data managers for their excellent work. Supplementary information is available at the EBMT Web site.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/6/1066
References
- 1.Appelbaum FR. Pursuing the goal of a donor for everyone in need. N Engl J Med. 2012;367(16):1555–1556. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125(25):3956–3962. [DOI] [PubMed] [Google Scholar]
- 3.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Stasi A, Milton DR, Poon LM, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014;20(12): 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzieri DA, Koh LP, Long GD, et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol. 2007;25(6):690–697. [DOI] [PubMed] [Google Scholar]
- 6.Ciurea SO, Saliba R, Rondon G, et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant. 2010;45(3):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10): 1310–1316. [DOI] [PubMed] [Google Scholar]
- 8.Luo Y, Xiao H, Lai X, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124(17):2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Bartolomeo P, Santarone S, De Angelis G, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121(5): 849–857. [DOI] [PubMed] [Google Scholar]
- 10.Booth GS, Gehrie EA, Bolan CD, Savani BN. Clinical guide to ABO-incompatible allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(8): 1152–1158. [DOI] [PubMed] [Google Scholar]
- 11.Kimura F, Sato K, Kobayashi S, et al. Impact of AB0-blood group incompatibility on the outcome of recipients of bone marrow transplants from unrelated donors in the Japan Marrow Donor Program. Haematologica. 2008;93(11):1686–1693. [DOI] [PubMed] [Google Scholar]
- 12.Hefazi M, Litzow M, Hogan W, et al. ABO blood group incompatibility as an adverse risk factor for outcomes in patients with myelodysplastic syndromes and acute myeloid leukemia undergoing HLA-matched peripheral blood hematopoietic cell transplantation after reduced-intensity conditioning. Transfusion. 2016;56(2):518–527. [DOI] [PubMed] [Google Scholar]
- 13.Kudek MR, Shanley R, Zantek ND, McKenna DH, Smith AR, Miller WP. Impact of graft-recipient ABO compatibility on outcomes after umbilical cord blood transplant for nonmalignant disease. Biol Blood Marrow Transplant. 2016;22(11):2019–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watz E, Remberger M, Ringden O, et al. Analysis of donor and recipient ABO incompatibility and antibody-associated complications after allogeneic stem cell transplantation with reduced-intensity conditioning. Biol Blood Marrow Transplant. 2014;20(2):264–271. [DOI] [PubMed] [Google Scholar]
- 15.Blin N, Traineau R, Houssin S, et al. Impact of donor-recipient major ABO mismatch on allogeneic transplantation outcome according to stem cell source. Biol Blood Marrow Transplant. 2010;16(9):1315–1323. [DOI] [PubMed] [Google Scholar]
- 16.Logan AC, Wang Z, Alimoghaddam K, et al. ABO mismatch is associated with increased nonrelapse mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(4): 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brierley CK, Littlewood TJ, Peniket AJ, et al. Impact of ABO blood group mismatch in alemtuzumab-based reduced-intensity conditioned haematopoietic SCT. Bone Marrow Transplant. 2015;50(7):931–938. [DOI] [PubMed] [Google Scholar]
- 18.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2009;15(3):367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. [DOI] [PubMed] [Google Scholar]
- 21.Castagna L, Crocchiolo R, Furst S, et al. Bone marrow compared with peripheral blood stem cells for haploidentical transplantation with a nonmyeloablative conditioning regimen and post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2014;20(5):724–729. [DOI] [PubMed] [Google Scholar]
- 22.Bradstock K, Bilmon I, Kwan J, et al. Influence of stem cell source on outcomes of allogeneic reduced-intensity conditioning therapy transplants using haploidentical related donors. Biol Blood Marrow Transplant. 2015;21(9):1641–1645. [DOI] [PubMed] [Google Scholar]
- 23.Confer DL, Abress LK, Navarro W, Madrigal A. Selection of adult unrelated hematopoietic stem cell donors: beyond HLA. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S8–S11. [DOI] [PubMed] [Google Scholar]
- 24.Konuma T, Kato S, Ooi J, et al. Effect of ABO blood group incompatibility on the outcome of single-unit cord blood transplantation after myeloablative conditioning. Biol Blood Marrow Transplant. 2014;20(4):577–581. [DOI] [PubMed] [Google Scholar]
- 25.Holbro A, Stern M, Infanti L, et al. Impact of recipient ABH secretor status on outcome in minor ABO-incompatible hematopoietic stem cell transplantation. Transfusion. 2015;55(1):64–69. [DOI] [PubMed] [Google Scholar]
- 26.Reshef R, Huffman AP, Gao A, et al. High graft CD8 cell dose predicts improved survival and enables better donor selection in allogeneic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2015;33(21):2392–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang YJ, Zhao XY, Xu LP, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gladstone DE, Zachary AA, Fuchs EJ, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19(4):647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshihara S, Maruya E, Taniguchi K, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47(4):508–515. [DOI] [PubMed] [Google Scholar]
- 30.Chang YJ, Luznik L, Fuchs EJ, et al. How do we choose the best donor for T-cell-replete, HLA-haploidentical transplantation? J Hematol Oncol. 2016;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.