Abstract
The presence of circulating plasma cells in patients with multiple myeloma is considered a marker for highly proliferative disease. In the study herein, the impact of circulating plasma cells assessed by cytology on survival of patients with multiple myeloma was analyzed. Wright-Giemsa stained peripheral blood smears of 482 patients with newly diagnosed myeloma or plasma cell leukemia were reviewed and patients were classified into 4 categories according to the percentage of circulating plasma cells: 0%, 1–4%, 5–20%, and plasma cell leukemia with the following frequencies: 382 (79.2%), 83 (17.2%), 12 (2.5%) and 5 (1.0%), respectively. Median overall survival according to the circulating plasma cells group was 47, 50, 6 and 14 months, respectively. At multivariate analysis, the presence of 5 to 20% circulating plasma cells was associated with a worse overall survival (relative risk 4.9, 95% CI 2.6–9.3) independently of age, creatinine, the Durie-Salmon system stage and the International Staging System (ISS) stage. Patients with ≥5% circulating plasma cells had lower platelet counts (median 86×109/L vs. 214×109/L, P<0.0001) and higher bone marrow plasma cells (median 53% vs. 36%, P=0.004). The presence of ≥5% circulating plasma cells in patients with multiple myeloma has a similar adverse prognostic impact as plasma cell leukemia.
Introduction
Plasma cell leukemia (PCL) was originally defined by the presence of both >20% circulating plasma cells (PCs) and an absolute count >2 × 109/L PCs,1,2 although in many studies the presence of only 1 of the above criteria was required.3–5 PCL may be classified as primary when it presents de novo in patients without previous evidence of multiple myeloma (MM) or secondary when it is presented as a leukemic transformation of a previously recognized MM. Primary PCL is a rare entity with an incidence of 2 to 4% of MM6–8 and is associated with a worse prognosis than MM. Its median survival, in a large epidemiological study, was only 4 months.7 However, with the use of novel drugs upfront, median survival ranging from 18 to 36 months has been reported.9–15
The presence of circulating PCs, identified by cytology,16 multiparameter flow cytometry17,18 or slide-based immunofluorescence,19 is also associated with a worse prognosis in myeloma patients not fulfilling the criteria of PCL. The presence of circulating PCs is also a risk factor of progression to active disease in patients with monoclonal gammopathy of undetermined significance20 and smoldering MM.21 It has been suggested that MM patients with circulating PCs, even below 20%, could have the same bad prognosis as patients with PCL. Indeed, a lower cutoff of ≥5% or ≥0.5 ×109/L of nucleated peripheral blood cells to redefine PCL has been proposed.3 In the present study, the impact of the presence of circulating PCs assessed by cytology on the survival of patients with MM was analyzed.
Methods
Requirements to enter the study were a diagnosis of symptomatic MM or primary PCL according to the International Myeloma Working Group (IMWG) criteria22 between January 2008 and December 2013 in 5 University Hospitals from Catalonia, and to have peripheral blood smears at diagnosis available for review. The study was approved by the Ethic Committee of the Hospital de la Santa Creu i Sant Pau and was conducted according to the declaration of Helsinki. Clinical data including age, sex, myeloma isotype, percentage of bone marrow plasma cells, lactate dehydrogenase (LDH), Durie-Salmon and ISS stages, as well as initial treatment and follow up, were collected from medical records. Cytogenetic analysis was performed according to local policies and patients were treated according to regional protocols. Wright-Giemsa stained peripheral blood smears were reviewed by 5 experienced hematologists on peripheral blood cytology. A minimum of 100 nucleated cells per smear were systematically counted. Each sample was analyzed by a single morphologist, with each following the same common criteria.
The primary endpoint was overall survival (OS) measured from the date of diagnosis to the date of death or last follow up. Differences in demographics and baseline characteristics were compared using the two-sided Fisher’s exact test for categorical variables and the Mann-Whitney U test for continuous variables. Survival analysis was performed using the Kaplan-Meier method and differences were tested for statistical significance using the log-rank test. Multivariate analysis was conducted using the Cox proportional hazards model. All calculations were performed using the software SPSS® statistics version 22.
Results
Clinical characteristics
The study cohort included 482 patients diagnosed with MM between January 2008 and December 2013. The median age at diagnosis was 69 years (range 28 to 92 years). Two hundred and sixty (53.9%) of the patients were males. The median follow up was 28 months for the whole cohort and 38 months for the patients alive. Two hundred and thirty-one (47.9%) patients died during follow up. First-line therapy was based on bortezomib combinations in 230 (47.7%) patients, alkylating agents with glucocorticoids in 114 (23.7%) patients, vincristine, doxorubicin (adriamycin), and dexamethasone (VAD) or VAD-like chemotherapy in 60 (12.4%), immunomodulatory-based combinations in 26 (5.4%), high-dose dexamethasone in 4 (0.8%), and only palliative care in 48 (9.9%) patients. One hundred and fifty-six (32.4%) patients received autologous stem cell transplantation as part of their first-line treatment. Twelve (2.5%) patients received allogeneic stem cell transplantation during the course of the disease.
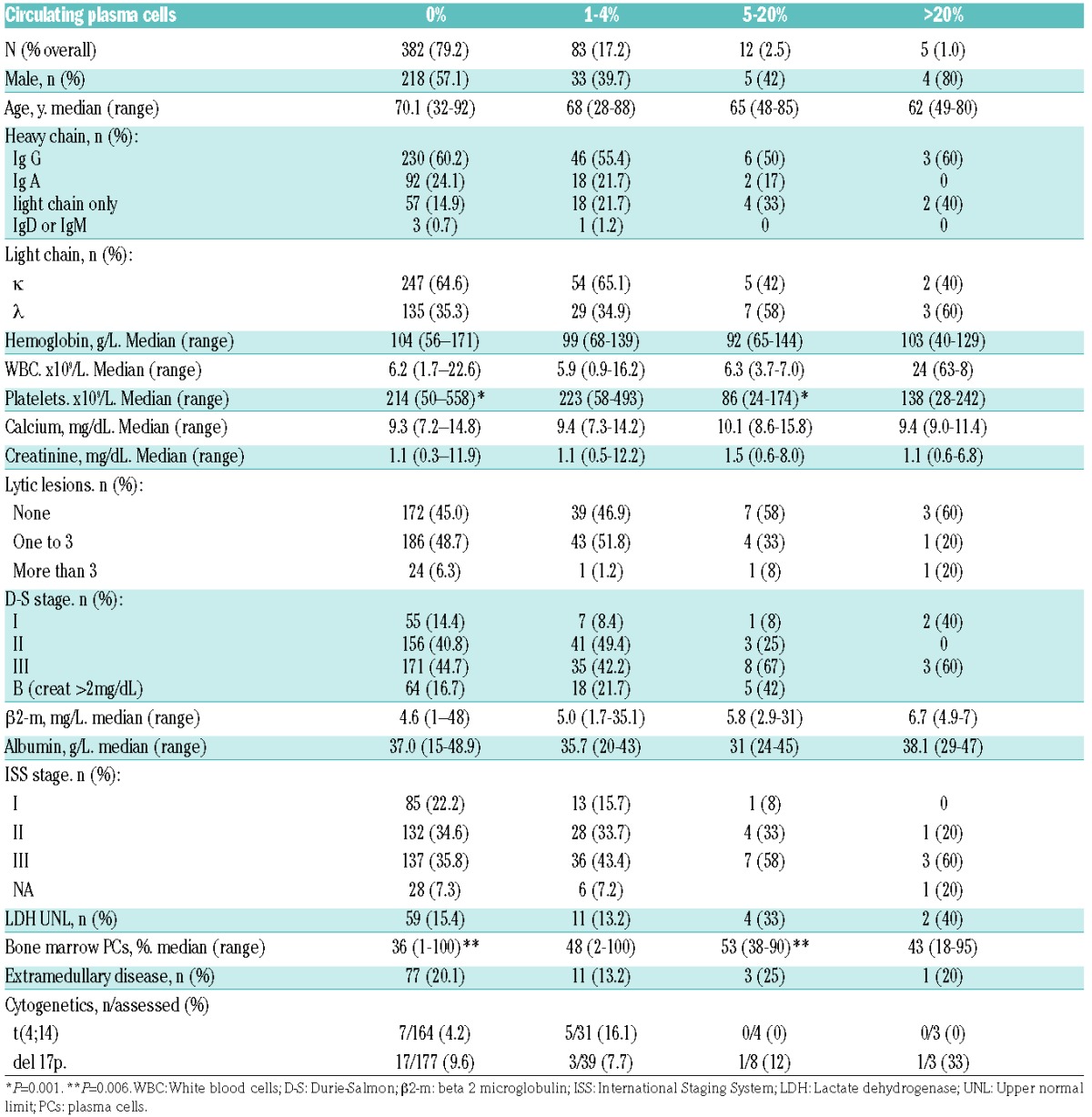
Clinical characteristics at diagnosis according to the circulating PCs group are summarized in Table 1. Differences in age, sex, myeloma isotope, LDH, Durie-Salmon and ISS stages between the 4 groups were not statistically significant. However, patients within the 5 to 20% circulating PCs group had lower platelet counts (median 86×109/L vs. 214×109/L, P<0.0001) and a higher proportion of bone marrow PCs (median 53% vs. 36%, P=0.004).
Table 1.
Clinical characteristics according to the number of circulating plasma cells group.
The 5 patients with >20% circulating PCs were initially treated with bortezomib and dexamethasone (2 patients); bortezomib, cyclophosphamide and dexamethasone (1 patient); melphalan and prednisone (1 patient); and bortezomib, melphalan and prednisone (1 patient). Of the 12 patients with 5–20% circulating PCs, 1 died the day after diagnosis and only received supportive care, the remaining 11 patients were initially treated as follows: bortezomib and dexamethasone (6 patients); bortezomib, thalidomide and dexamethasone (2 patients); VAD (1 patient); bortezomib, melphalan and prednisone (1 patient); and cyclophosphamide and steroids (1 patient). One patient with >20% circulating PCs and 3 patients with 5–20% circulating PCs received an autologous stem cell transplantation as consolidation.
Risk factors for overall survival in the overall cohort
According to the percentage of circulating PCs, 4 groups were considered for the analysis of survival: no circulating plasma cells, 382 (79.2%) patients; 1 to 4% circulating plasma cells, 83 (17.2%) patients; 5 to 20% circulating plasma cells, 12 (2.5%) patients; and classical PCL group (>20% or >2×109/L plasma cells), 5 (1%) patients. A patient with 15% circulating PCs but an absolute circulating PC count of 2.7×109/L was included in the classical PCL group.
The median OS of patients with no circulating PCs, 1 to 4%, 5 to 20% and >20% were 47 (95%CI 38.6–55.4) months, 50 (95%CI 31.0–68.9) months, 6 (95%CI 0.9–11.1) months and 14 (95%CI 9.7–18.3) months, respectively (Figure 1) (P<0.001).
Figure 1.
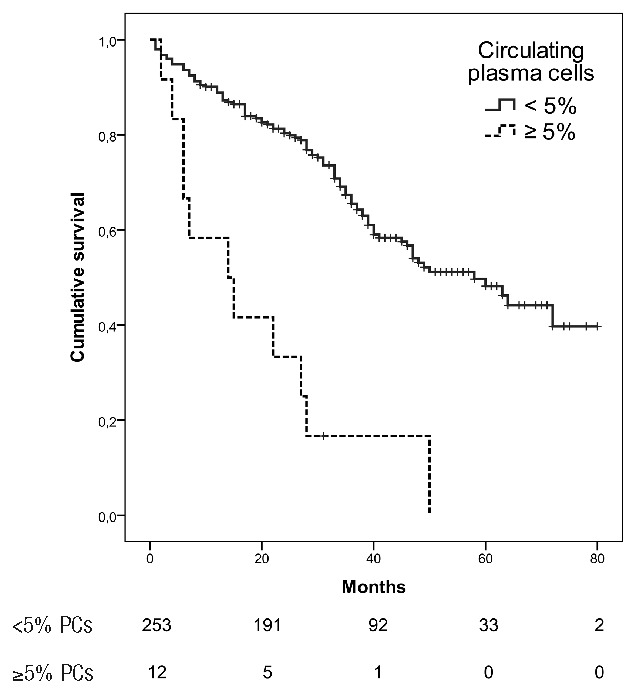
Overall survival according to the circulating plasma cells (PCs) group in patients with multiple myeloma (P<0.001).
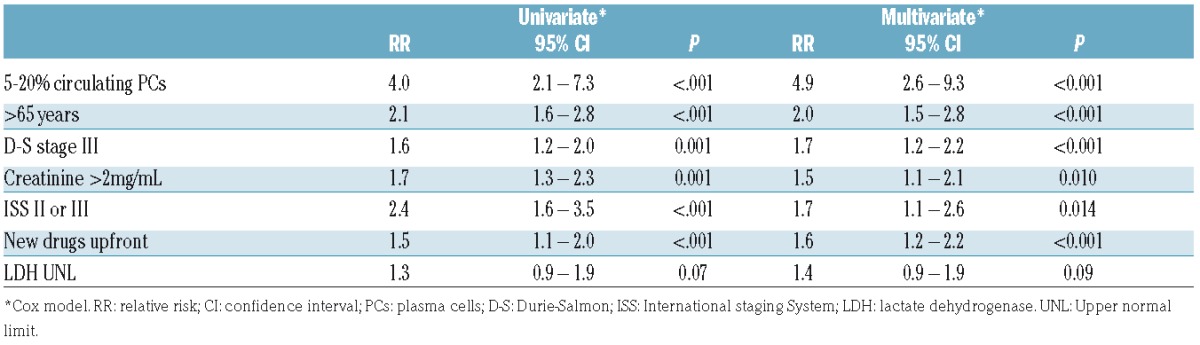
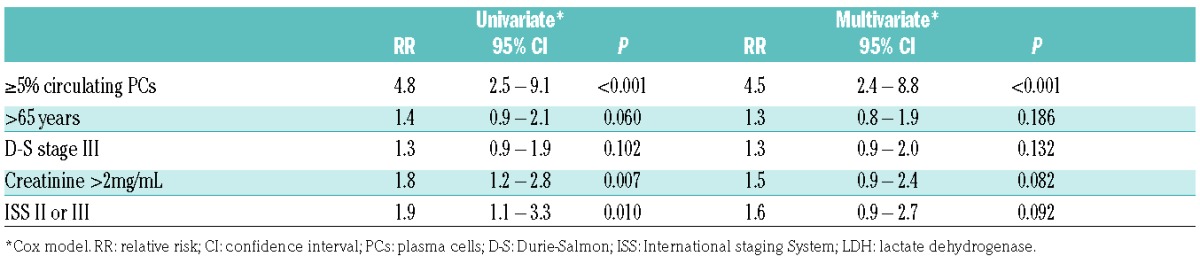
In the univariate analysis, the other factors associated with a worse survival together with circulating PCs were age older than 65 years at diagnosis, creatinine >2 mg/dL, treatment with new drugs upfront (proteasome inhibitors or immunodulatory drugs), and Durie-Salmon and ISS advanced stages. Cytogenetic data was not included in the survival analysis due to the lack of such data in most patients. The 5 patients with classical PCL were excluded from the multivariate analysis. The finding of 5 to 20% circulating plasma cells, age older than 65 years, Durie-Salmon III, creatinine >2 mg/dL, and ISS 3 retained their significance in the multivariate analysis (Table 2).
Table 2.
Risk factors for overall survival, overall cohort.
Risk factors for overall survival in patients treated with novel agents upfront
Two hundred and sixty five of the 482 (54.9%) patients were treated upfront with proteasome inhibitors and/or inmunomodulatory drugs. Of these, 192 (72.5%) patients had 0% circulating PCs, 61 (23.0%) patients had 1 to 4% circulating PCs, 9 (3.4%) patients had 5 to 20% circulating PCs and 3 (1.1%) patients had a diagnosis of classical PCL. One hundred and ten (41.5%) patients treated with novel agents upfront died during follow up and their median OS was 50 (95% CI 38–61) months.
In patients treated with novel agents upfront, the median OS in cases with 0%, 1 to 4%, 5 to 20% and PCL were 58 (95% CI NR) months, 60 (95% CI 33–86) months, 22 (95% CI 0–65) months and 14 (95% CI 1–26) months, respectively. When only 2 groups were considered, <5% and ≥5% circulating PCs, median OS were 58 (95% CI 46 – 69) months and 14 (95% CI 0.4 – 27) months (Figure 2).
Figure 2.
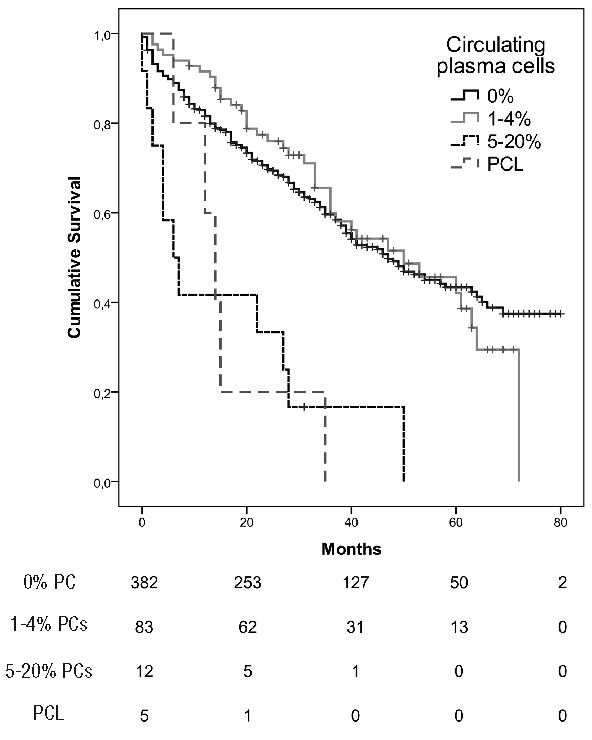
Overall survival according to the circulating plasma cells (PCs) in patients with multiple myeloma and plasma cell leukemia (PCL) treated with novel drugs upfront (P<0.001).
Together with the percentage of circulating PCs, the other factors associated with a worse OS in univariate analysis were creatinine >2mg/dL and ISS stage II or III. A trend was observed in patients >65 years old and Durie-Salmon stage III. Taking into account the aforementioned variables, having ≥5% circulating PCs alone was the only fact which maintained statistical significance in the multivariate analysis (Table 3). LDH was not included in the multivariate analysis due to the lack of statistical significance or even a trend in univariate analysis (RR 1.1, 95% CI 0.7–1.9, P=0.467).
Table 3.
Factors associated with overall survival in patients treated with novel drugs upfront.
Discussion
This study aimed to address the impact of circulating PCs on the survival of patients with MM. Seventeen per cent of patients had between 1 and 4% circulating plasma cells. That finding was not associated with other clinical characteristics and had no impact on survival. There was a completely different picture for the 2.5% of patients with 5 to 20% circulating PCs. Such patients had lower platelet counts, higher bone marrow infiltration and, importantly, a shorter survival independent of other known clinical prognostic factors. In fact, the median OS of 6 months observed in these patients is closer to that of patients with the “classical” definition of PCL. When the analysis was restricted to patients treated with novel agents upfront, the impact of circulating PCs was consistent with the whole cohort. The differences in OS observed between patients with 5–20% circulating PCs and >20% circulating PCs (6 vs. 14 months) may be explained by the low number of cases in both groups.
Although the presence of circulating PCs has been previously associated with survival, conventional cytology has been used for their assessment in only one study.16 In that case, patients with circulating plasma cells constituted 14.1% of the overall series and had a median survival of 25 months. The results of the present study are consistent with the ominous prognostic impact of peripheral blood plasmacytosis; however, the definition of a high-risk group found was different; ≥2% circulating PCs in the study by An et al. and ≥5% circulating PCs in the study herein.
Using multiparameter flow cytometry, the presence of circulating PCs has also been associated with survival.17,18,23 In the study from the Mayo Clinic,17 24% of patients had more than 400 circulating PCs. Such patients had a median OS of 32 months versus not reached in patients with 400 or less circulating PCs. In another study from the same institution, which in this case used slide-based immunofluorescence microscopy for plasma cell quantification,19 54% of patients with >4% PCs were identified. These patients had a median survival of 2.4 years compared to 4.4 years in patients with fewer circulating PCs. The aforementioned studies used much more sensitive techniques than those used in the present; this may explain the different percentages of patients with circulating PCs identified in those studies in comparison with the present one.
Despite these findings, the definition of PCL has been based on standard morphological examination of peripheral blood.3 In comparison with flow cytometry and immunofluorescence, conventional cytology identifies a smaller number of patients with an extremely poor prognosis. Additionally, conventional cytology has the advantage of being a simple and inexpensive technique that can be applied in any clinical laboratory worldwide. However, it has a limitation; conventional cytology is not able to identify the clonality of PCs, as can be achieved by flow cytometry and immunofluorescence. This may hamper the specificity of conventional cytology since polyclonal reactive PCs may be rarely detected in some patients with MM.24,25
The presence of t(4;14), del(17p), amp(1q21) and del(1p21) in malignant PCs are adverse prognostic factors in MM.26,27 Indeed, adverse cytogenetics together with ISS 3 and/or high LDH identifies a group of patients with an ultra-high risk of MM.28 Several of these genetic abnormalities, particularly del(17p)9 and chromosome 1 alterations29 are more frequent in PCL than in MM. In the series presented herein, del(17p) by fluorescence in situ hybridization (FISH) was observed in 1 of 7 patients with 5 to 20% circulating PCs and 1 of 2 patients with >20% circulating PCs. A limitation of the present study is that, due to the lack of cytogenetic data in most patients, the prognostic impact of unfavorable cytogenetic abnormalities and the revised ISS28 could not be analyzed.
As highlighted in the last consensus by IMWG,3 the diagnosis of PCL has been classically done on the basis of the presence of >20% circulating PCs and/or an absolute count >2 × 109/L PCs. However, lower peripheral blood PC counts, as showed in our study (that is, ≥5% peripheral blood plasma cells), should be considered as a diagnostic criteria of PCL (“PCL-like” myeloma or early PCL), due to the independent and strong prognostic impact. Prospective multicenter analysis with translational and correlative studies into the biology of these patients is encouraged as well as risk-oriented therapeutic strategies.30,31 Careful examination of peripheral blood by conventional microscopy should be done for all patients with MM in daily clinical practice.
In conclusion, the presence of ≥5% circulating PCs by conventional cytology easily identifies a group of patients with myeloma with a prognosis as poor as that of PCL, suggesting that the diagnosis of PCL should be revisited. If confirmed in other series, especially in prospective studies of uniformly treated patients, such patients may benefit from a distinct and more intensified therapeutic approach.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/6/1099
Funding
This study was supported in part by grants AGAUR 2014SGR-1281 and 2014SGR-552 (Generalitat de Catalunya), and RD12/0036/0071, RD12/0036/0046 and PI16/00423 (Instituto de Salud Carlos III) and Fondo Europeo de Desarrollo Regional (FEDER) as well as a grant from the Cellex Research Foundation, Barcelona, Spain.
References
- 1.Kyle RA, Maldonado JE, Bayrd ED. Plasma cell leukemia. Report on 17 cases. Arch Intern Med. 1974;133(15):813–818. [DOI] [PubMed] [Google Scholar]
- 2.Noel P, Kyle RA. Plasma cell leukemia: an evaluation of response to therapy. Am J Med. 1987;83(6):1062–1068. [DOI] [PubMed] [Google Scholar]
- 3.Fernández de Larrea C, Kyle RA, Durie BGM, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria, and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27(4):780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van de Donk NW, Lokhorst HM, Anderson KC, Richardson PG. How I treat plasma cell leukemia. Blood. 2012;120(12):2376–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jelinek T, Kryukov F, Rihova L, Hajek R. Plasma cell leukemia: from biology to treatment. Eur J Haematol. 2015;95(1):16–26. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Palumbo A, Delasalle KB, Alexanian R. Primary plasma cell leukemia. Br J Haematol. 1994;88(4):754–759. [DOI] [PubMed] [Google Scholar]
- 7.Ramsingh G, Mehan P, Luo J, Vij R, Morgensztern D. Primary plasma cell leukemia: a Surveillance, Epidemiology, and End Results database analysis between 1973 and 2004. Cancer. 2009; 115(24):5734–5739. [DOI] [PubMed] [Google Scholar]
- 8.García-Sanz R, Orfao A, González M, et al. Primary plasma cell leukemia: clinical, immunophenotipic, DNA ploidy, and cytogenetic characteristics. Blood. 1999; 93(3):1032–1037. [PubMed] [Google Scholar]
- 9.Tiedemann RE, González-Paz N, Kyle RA, et al. Genetic aberrations and survival in plasma cell leukemia. Leukemia. 2008; 22(5):1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katodritou E, Terpos E, Kelaidi C, et al. Treatment with bortezomib-based regimens improves overall response and predicts for survival in patients with primary or secondary plasma cell leukemia: Analysis of the Greek myeloma study group. Am J Hematol. 2014;89(2):145–150. [DOI] [PubMed] [Google Scholar]
- 11.D’Arena G, Valentini CG, Pietrantuono G, et al. Frontline chemotherapy with bortezomib-containing combinations improves response rate and survival in primary plasma cell leukemia: a retrospective study from GIMEMA Multiple Myeloma Working Party. Ann Oncol. 2012; 23(6):1499–1502. [DOI] [PubMed] [Google Scholar]
- 12.Musto P, Simeon V, Martorelli MC, et al. Lenalidomide and low-dose dexamethasone for newly diagnosed primary plasma cell leukemia. Leukemia. 2014;28(1):222–225. [DOI] [PubMed] [Google Scholar]
- 13.Pulte D, Jansen L, Castro FA, et al. Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. Br J Haematol. 2015;171(2):189–196. [DOI] [PubMed] [Google Scholar]
- 14.Gonsalves WI, Rajkumar V, Go RS, et al. Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood. 2014;124(6):907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royer B, Minvielle S, Diouf M, et al. Bortezomib, doxorubicin, cyclophosphamide, dexamethasone induction followed by stem cell transplantation for primary plasma cell leukemia: a prospective phase II study of the intergroupe francophone du myélome. J Clin Oncol. 2016; 34(18):2125–2132. [DOI] [PubMed] [Google Scholar]
- 16.An G, Qin X, Acharya C, et al. Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann Hematol. 2015;94(2):257–264. [DOI] [PubMed] [Google Scholar]
- 17.Nowakowski GS, Witzig TE, Dingli D, et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood. 2005;106(7):2276–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonsalves WI, Rajkumar SV, Gupta V, et al. Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: implications for redefining high-risk myeloma. Leukemia. 2014;28(10):2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witzig TE, Gertz MA, Lust JA, Kyle RA, O’Fallon WM, Greipp PR. Peripheral blood monoclonal plasma cells as a predictor of survival in patients with multiple myeloma. Blood. 1996;88(5):1780–1787. [PubMed] [Google Scholar]
- 20.Kumar S, Rajkumar AV, Kyle RA, et al. Prognostic value of circulating plasma cells in monoclonal gammopathy of undetermined significance. J Clin Oncol. 2005; 23(24):5668–5674. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi G, Kyle RA, Larson DR, et al. High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia. 2013;27(3):680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- 23.Vagnoni D, Travaglini F, Pezzoni V, et al. Circulating plasma cells in newly diagnosed symptomatic multiple myeloma as a possible prognostic marker for patients with standard-risk cytogenetics. Br J Haematol. 2015;170(4):523–531. [DOI] [PubMed] [Google Scholar]
- 24.Shtalrid M, Shvidel L, Vorst E. Polyclonal reactive peripheral blood plasmacytosis mimicking plasma cell leukemia in a patient with Staphylococcal sepsis. Leuk Lymphoma. 2003;44(2):379–380. [DOI] [PubMed] [Google Scholar]
- 25.Touzeau C, Pellat-Deceunynck C, Gastinne T, et al. Reactive plasmacytoses can mimick plasma cell leukemia: therapeutical implications. Leuk Lymphoma. 2007; 48(1):207–208. [DOI] [PubMed] [Google Scholar]
- 26.Avet-Loiseau H, Campion L, et al. Long-term analysis of the IFM 99 trials for myeloma: cytogenetic abnormalities [t(4;14), del(17p), 1q gains] play a major role in defining long-term survival. J Clin Oncol. 2012;30(16):1949–1952. [DOI] [PubMed] [Google Scholar]
- 27.Chang H, Ning Y, Qi X, Yeung J, Xu W. Chromosome 1p21 deletion is a novel prognostic marker in patients with multiple myeloma. Br J Haematol. 2007;139(1):51–54. [DOI] [PubMed] [Google Scholar]
- 28.Moreau P, Cavo M, Sonneveld P, et al. Combination of international scoring system 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol. 2014; 32(20):2173–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang H, Qi X, Yeung J, Reece D, Xu W, Patterson B. Genetic aberrations including chromosome 1 abnormalities and clinical features of plasma cell leukemia. Leuk Res. 2009;33(2):259–262. [DOI] [PubMed] [Google Scholar]
- 30.Musto P. Progress in the treatment of primary plasma cell leukemia. J Clin Oncol. 2016;34(18):2082–2084. [DOI] [PubMed] [Google Scholar]
- 31.Neri A, Todoerti K, Lionetti M, et al. Primary plasma cell leukemia 2.0: advances in biology and clinical management. Expert Rev Hematol. 2016;9(11):1063–1073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


