The development of acute lymphoblastic leukemia (ALL) typically involves the acquisition of at least two genetic events. The initial mutation leads to the formation of a persistent, but clinically covert, preleukemic clone. Additional genetic changes then lead, in a stepwise manner, to complete leukemic transformation and promote the clinical onset of the disease.1 Although the leukemic cells have been extensively studied over the past decades, little is known about the presence, dynamics and evolution of the (pre)leukemic cells before the clinical onset of leukemia. Typically, the only biological material available in which we can search for the presence of leukemic cells is archived dried neonatal blood spots.2 Rarely, stored cord blood from patients later diagnosed with leukemia is available3 or in-utero-originated preleukemic cells can be preserved in a healthy monozygotic twin of the leukemia patient.4 However, these samples only represent the characteristics of preleukemic cells/clone and its presence/absence at birth, hence providing a very limited potential to characterize the premalignant period and its dynamics. The situation is more favorable in secondary leukemias in which the preleukemic period can be monitored via archived samples collected during the follow-up of the primary disease.5,6
In a small (~2%) proportion of ALL cases the diagnosis of leukemia is preceded by clinical symptoms of fever, infections and transient non-specific anemia.7 As the clinical conditions resemble aplastic anemia, bone marrow (BM) aspiration is usually indicated.
We analyzed eight children with B-cell precursor ALL who had a prodromal period of atypical anemia along with fatigue and infections ≥6 weeks before the clinical diagnosis of leukemia (Table 1). As a hematologic disorder was suspected, BM aspirates were investigated by morphology and flow cytometry. Initially, the diagnostic criteria of ALL were not fulfilled and thus no antileukemic treatment was started except for low doses of corticosteroids in three patients (Table 1). However, continuous follow-up was indicated and after a premalignant period lasting from 42 days up to 17 months the diagnostic criteria were met and the diagnosis of B-cell precursor ALL was finally confirmed in all eight patients. Using a combination of several strategies we tried to backtrack leukemia-specific markers and retrospectively follow the kinetics of the (pre)leukemic cells before the clinical manifestation of ALL in these patients.
Table 1.
Patients’ characteristics at the time of the first prediagnostic bone marrow aspiration.
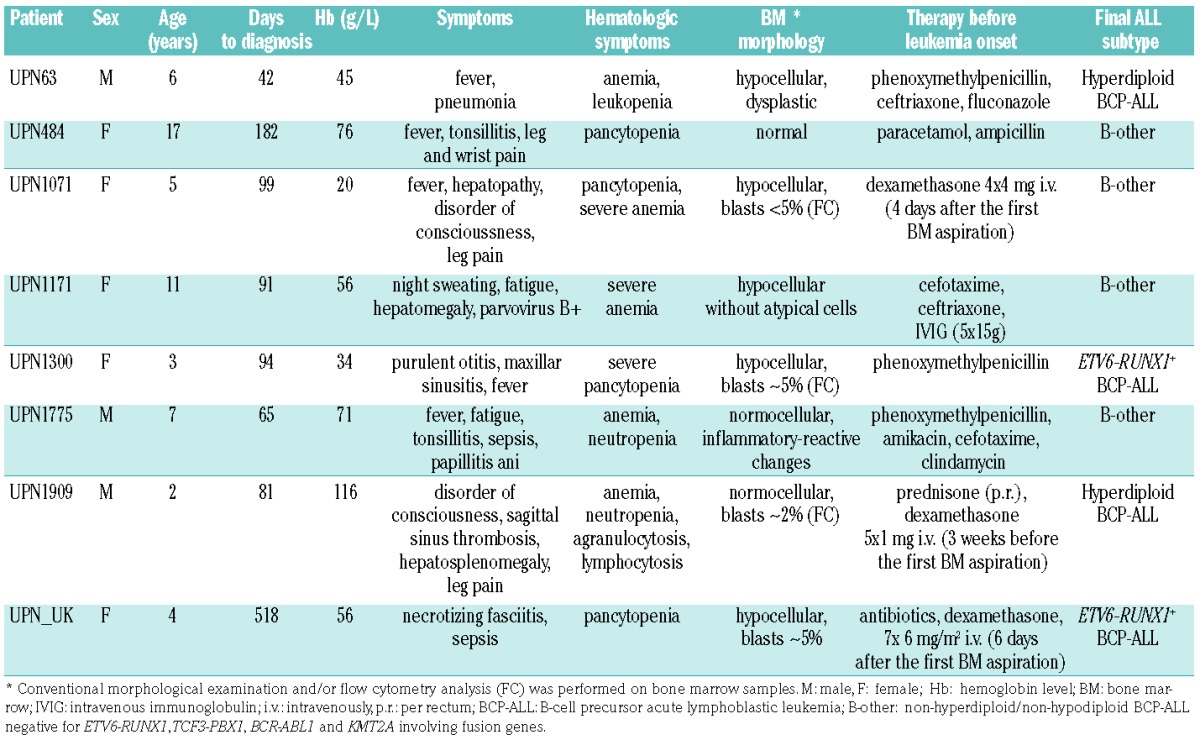
In one case with ETV6-RUNX1-positive leukemia (UPN1300), we were able to assess the clonal evolution/selection 3 months before the definitive diagnosis of leukemia. Single nucleotide polymorphism array performed on the diagnostic BM identified six distinct copy number alterations that were further validated by fluorescence in situ hybridization. At diagnosis, five of the aberrations [del(12)(p13) including the ETV6 gene, del(12)(q24.32), del(12)(q24.11), del(14)(q24) and del(1)(p33)] were harbored by 100% of the ETV6-RUNX1-positive leukemic cells (8–60 cells evaluated); the sixth, del(4)(q31), was confirmed in 86% (12/14 ETV6-RUNX1-positive cells). All ETV6-RUNX1-positive (pre)leukemic cells 92 days before the diagnosis harbored the deletion of non-translocated ETV6 allele and 14q24 deletion (167/167 and 5/5 cells, respectively). The presence of other deletions within the (pre)leukemic clone ranged between 28% and 96%, thus demonstrating a significant clonal selection shortly before the clinical onset of leukemia (Figure 1). Our observations build upon previous case reports showing similar data.7,8
Figure 1.
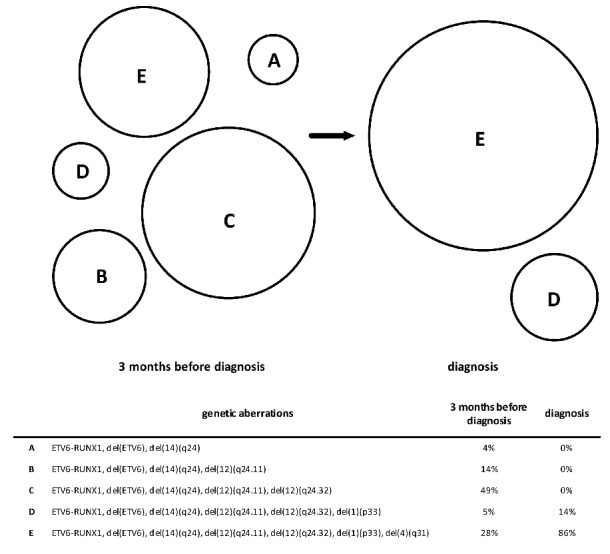
Clonal architecture of (pre)leukemic and leukemic cells in patient UPN1300. A proportional scheme of distinct subclones bearing different aberrations (A–E) and their relative representation within the population of (pre)leukemic cells in bone marrow taken 3 months before leukemia onset and at diagnosis. Only cells harboring the ETV6-RUNX1 fusion were considered as (pre)leukemic and further analyzed.
In all eight patients we were able to backtrack diagnostic immunoglobulin/T-cell receptor gene rearrangements using quantitative polymerase chain reaction (PCR) analysis. In five patients, two or more pre-diagnostic samples were available. Interestingly, instead of a linear progression/expansion of the cells harboring the leukemia-specific immunoglobuin/T-cell receptor gene rearrangement(s), the (pre)leukemic clone dynamics followed a characteristic pattern: initial high levels followed by a significant decrease (>1 log to >3 logs), and finally a rise just before diagnosis (Figure 2). A lack of samples prevented us formally demonstrating similar clonal dynamics in the remaining three cases. However, a similar “V-shape” course could be inferred in the cases with high initial levels of (pre)leukemic cells (17% and 36% in patients UPN484 and UPN1775, respectively), detected several months before the clinical diagnosis.
Figure 2.
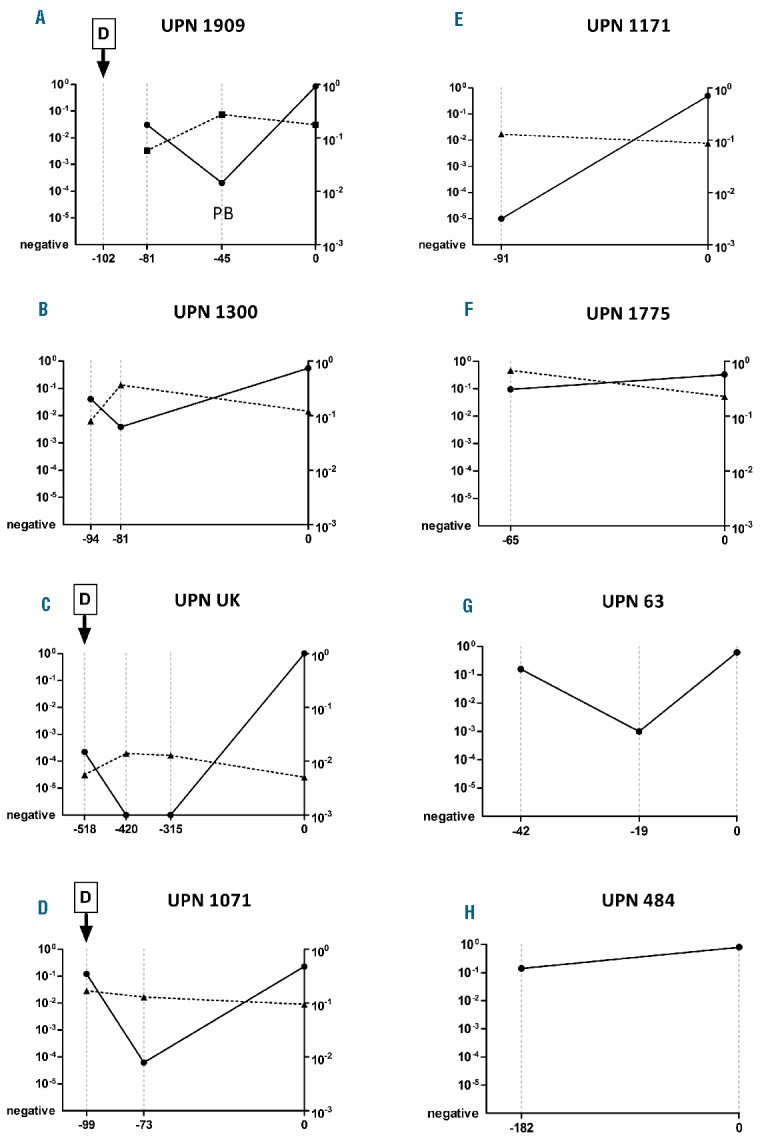
Dynamics of the (pre)leukemic clone and non-malignant T-lymphocytes harboring the specific Vγ9-Jγ1.2 rearrangement during the pre-diagnostic phase. Quantitative levels of (pre)leukemic clones are connected by a solid line (left Y axis in each patient). Representations of the T-cell clone (defined by the specific Vγ9-Jγ1.2 rearrangement) within non-malignant cells are connected by a dashed line (right Y axis in each patient). Time points of sampling are shown on the X axis, relative quantification normalized to a housekeeping gene (ALB) is shown on the Y axis. Data are presented for individual patients (A–H). In patients UPN63 and UPN484 no biological material was available to analyze the T-lymphocytes of interest. All samples are from bone marrow except for the second pre-diagnostic sample from patient UPN1909 taken from peripheral blood (PB). D = dexamethasone treatment [(4 × 4 mg (UPN1071), 5 × 1 mg (UPN1909), 7 × 6 mg/m2 (UPN_UK)].
In three patients the temporary reduction of (pre)leukemic burden can be related to the administration of intravenous corticosteroids (dexamethasone) shortly before or after the initial BM aspiration. Corticosteroids alone are capable of inducing temporary remission; however, without further treatment these remissions last only several weeks (median 9 weeks).9 Thus, although administered in a significantly lower amount than the dose used in the leukemia treatment prephase, the corticosteroid therapy probably contributed to the observed reduction of the (pre)leukemic clone in the patients treated after the first aspiration (UPN1071, UPN_UK). It is less likely to explain the >2 log decrease of (pre)leukemic clone in patient UPN1909, who received a low dose of dexamethasone 3 weeks before the first BM aspirate. Moreover, the short corticosteroid treatment can hardly explain a remission lasting for 17 months (and PCR-negativity for at least 6 months) in patient UPN_UK. Importantly, no antileukemic treatment was used in the remaining two patients (UPN1300, UPN63), in whom an initial decrease of the (pre)leukemic clone is also apparent.
The (pre)leukaemic clonal dynamics described here are consistent with our observations in secondary leukemias,5,6 and the fluctuation in the percentage of ETV6-RUNX1-positive cells detected by fluorescence in situ hybridization described in another ALL patient with a preleukemic phase.7 This suggests that some immunological mechanisms might be capable of temporary surveillance over the pre-malignant clones, at least in some leukemia subtypes.
Deep sequencing of the T-cell receptor-gamma repertoire in diagnostic and pre-diagnostic samples in five cases (UPN1171, UPN1300, UPN1775, UPN1909, UPN_UK) revealed that a significant proportion of the cells were from a specific non-malignant clone with a V-J fusion sequence 5´-TGTGCCTTGTGGGAGGTGCAGAGTTGGGCAAAA-3´ identified as the Vγ9-Jγ1.2 rearrangement. This is the most abundant clonotype among circulating Vγ9Vδ2 T-lymphocytes which are often described to play an important role in tumor immune surveillance.10 We designed a quantitative PCR system for the quantification of T-lymphocytes harboring this specific Vγ9-Jγ1.2 rearrangement. In six patients (UPN1071, UPN1171, UPN1300, UPN1775, UPN1909, UPN_UK), the levels of the Vγ9-Jγ1.2 clone were scrutinized in both diagnostic and pre-diagnostic samples and in all but one case the trend in kinetics confirmed the deep sequencing data. The Vγ9-Jγ1.2 T-lymphocytes inversely mirrored the dynamics of the (pre)leukemic cells, even after adjustment for the proportion of non-leukemic cells in the sample (Figure 2). In the remaining case (UPN1071) the Vγ9-Jγ1.2 levels were virtually constant.
We analyzed 167 diagnostic and follow-up leukemic samples and ten normal BM specimens by the same clone-specific quantitative PCR. The representation of the Vγ9-Jγ1.2 clone within the non-leukemic proportion of BM cells at diagnosis of both ALL and acute myeloid leukemia (AML) (n=98 and n=29, respectively) was significantly higher compared to that in healthy BM samples (P=0.0005 and P=0.012, respectively; median value for ALL=0.17, AML=0.11 and healthy BM=0.035; Online Supplementary Figure S1) and very similar to the highest levels found in pre-diagnostic samples of our patients presenting with an aleukemic prodrome (median 0.22; P>0.8). In 14 patients with B-cell precursor ALL analyzed during treatment (diagnosis, days 8, 15 and 33 and week 12 of treatment) the size of this clone within the non-malignant hematopoietic cells did not differ significantly in the samples taken between the diagnosis and day 33. At week 12, the level of this specific clone decreased significantly, to the levels seen in normal BM (P=0.007) (Online Supplementary Figure S1).
There is compelling evidence of the cytotoxic properties of Vγ9Vδ2 cells and their involvement in anti-tumor surveillance.11 Physiologically, Vγ9Vδ2 T-lymphocytes represent the most abundant subset of circulating γδT cells. Stimulation by several cancer-derived cell lines (including the Daudi cell line derived from a B-cell lymphoma) elicits a proliferative response and clonal expansion10 and human immunodeficiency virus-related depletion of circulating Vγ9Vδ2 T cells contributes to the increased risk of developing leukemia/lymphoma in individuals infected by this virus.12 The involvement of Vγ9Vδ2 cells in immune surveillance has been shown in several malignancies so far, including hematologic malignancies.11 Importantly, Vγ9Vδ2 cells efficiently kill leukemic blasts in AML.13 According to our data, the Vγ9Vδ2 T-lymphocytes separated from healthy peripheral blood and expanded by phosphoantigen isopentenyl pyrophosphate showed only very limited, yet consistent capacity to lyse ALL cell lines (Online Supplementary Figure S2). Even without a major direct killing effect as described in AML, the Vγ9Vδ2 cells may execute their immunosurveillance capacity in (pre-)ALL via regulating other immune cells, especially natural killer cells with more potent cytotoxic ability.11 Moreover, we cannot exclude that while Vγ9Vδ2 cells might be effective in the suppression of preleukemic cells, after the cells become fully leukemic, the Vγ9Vδ2 clone is no longer able to control their expansion effectively. Furthermore, the protracted activation of Vγ9Vδ2 cells in developing tumor microenvironment can result in the accumulation of dysfunctional Vγ9Vδ2 lymphocytes that are no longer capable of controlling the smoldering disease.14 Alternatively, a cytokine release accompanying the acute infection/sepsis period in some of the patients may induce a significant cytotoxic reaction (possibly also involving Vγ9Vδ2 expansion) including leukemia cytotoxicity and cause or contribute to a temporary reduction of the (pre)leukemic clone.15
In conclusion, we have demonstrated that the dynamics of the (pre)leukemic clone are non-linear and result in significant clonal selection shortly before the diagnosis of ALL. Moreover, our data point towards immunological mechanisms that might be involved in temporary surveillance over the (pre)leukemic burden.
Supplementary Material
Acknowledgments
The authors would like to thank to all centers of the Czech Pediatric Hematology Working Group (CHP) for taking care of the patients included in the study. This study was supported by grants from the Czech Ministry of Health, 15-28525A, IGA MZ NT/12428-5 and IGA MZ NT/14350-3, by MSMT NPU I nr.LO1604, by EU-Prague project CZ.2.16/3.1.00/24022 and by projects for conceptual development of research organization 00064203 (University Hospital Motol, Prague, Czech Republic) and 64165 (General University Hospital in Prague, Czech Republic).
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381(9881):1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gale KB, Ford AM, Repp R, et al. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proc Natl Acad Sci USA. 1997;94(25):13950–13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maia AT, van der Velden VH, Harrison CJ, et al. Prenatal origin of hyperdiploid acute lymphoblastic leukemia in identical twins. Leukemia. 2003;17(11):2202–2206. [DOI] [PubMed] [Google Scholar]
- 4.Greaves MF, Maia AT, Wiemels JL, Ford AM. Leukemia in twins: lessons in natural history. Blood. 2003;102(7):2321–2333. [DOI] [PubMed] [Google Scholar]
- 5.Zuna J, Burjanivova T, Mejstrikova E, et al. Covert preleukemia driven by MLL gene fusion. Genes Chromosomes Cancer. 2009;48(1):98–107. [DOI] [PubMed] [Google Scholar]
- 6.Metzler M, Staege MS, Harder L, et al. Inv(11)(q21q23) fuses MLL to the Notch co-activator mastermind-like 2 in secondary T-cell acute lymphoblastic leukemia. Leukemia. 2008;22(9):1807–1811. [DOI] [PubMed] [Google Scholar]
- 7.Horsley SW, Colman S, McKinley M, et al. Genetic lesions in a preleukemic aplasia phase in a child with acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2008;47(4):333–340. [DOI] [PubMed] [Google Scholar]
- 8.Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356–361. [DOI] [PubMed] [Google Scholar]
- 9.Gehan EA, Freireich EJ. The 6-MP versus placebo clinical trial in acute leukemia. Clin Trials. 2011;8(3):288–297. [DOI] [PubMed] [Google Scholar]
- 10.Sherwood AM, Desmarais C, Livingston RJ, et al. Deep sequencing of the human TCRgamma and TCRbeta repertoires suggests that TCRbeta rearranges after alphabeta and gammadelta T cell commitment. Sci Transl Med. 2011;3(90):90ra61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braza MS, Klein B. Anti-tumour immunotherapy with Vgamma9Vdelta2 T lymphocytes: from the bench to the bedside. Br J Haematol. 2013;160(2):123–132. [DOI] [PubMed] [Google Scholar]
- 12.Cummings JS, Cairo C, Armstrong C, Davis CE, Pauza CD. Impacts of HIV infection on Vgamma2Vdelta2 T cell phenotype and function: a mechanism for reduced tumor immunity in AIDS. J Leukoc Biol. 2008;84(2):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gertner-Dardenne J, Castellano R, Mamessier E, et al. Human Vgamma9Vdelta2 T cells specifically recognize and kill acute myeloid leukemic blasts. J Immunol. 2012;188(9):4701–4708. [DOI] [PubMed] [Google Scholar]
- 14.Coscia M, Vitale C, Peola S, et al. Dysfunctional Vgamma9Vdelta2 T cells are negative prognosticators and markers of dysregulated mevalonate pathway activity in chronic lymphocytic leukemia cells. Blood. 2012;120(16):3271–3279. [DOI] [PubMed] [Google Scholar]
- 15.Fidanza M, Seif AE, DeMicco A, et al. Inhibition of precursor B-cell malignancy progression by toll-like receptor ligand-induced immune responses. Leukemia. 2016;30(10):2116–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


