Antigen presenting cells (APCs) play a key role in the uptake of antigens, and processing and presentation of antigen-derived peptides in the context of MHC molecules to T cells. The co-stimulatory molecules CD80 and CD86 provide signals for T-cell activation and proliferation. Dendritic cells (DCs), macrophages/monocytes, and memory B cells are classically known for their role as APCs. In contrast to this dogma, recent reports from mice show that basophils can also function as APCs. These reports were revolutionary as basophils were previously known as accessory cells that produce IL-4 required for the Th2 responses mediated by classical APCs.1,2 In fact, it was shown that murine basophils express MHC class II, and co-stimulatory molecules CD80 and CD86 required for the T-cell stimulation.3–5 Additionally, these basophils were also reported to process and present antigens that are associated with classical Th2 responses such as allergens and proteases, and mediate Th2 differentiation in vitro and in vivo. Although in vivo data were later contradicted,6,7 the ex vivo and in vitro data clearly demonstrated functioning of murine basophils as APCs.
In contrast to mice, several data from humans have shown that basophils, either from healthy donors or from allergic and lupus patients, lack the features of APCs.8–12 These basophils were negative for the expression of antigen presenting molecule HLA-DR, and co-stimulatory molecules CD80 and CD86, and failed to polarize Th2 as well as Th17 responses when co-cultured with CD4+ T cells. However, all these reports were based on basophils from the circulation, while APC functions of murine basophils were demonstrated from the secondary lymphoid organs, like spleen and lymph nodes. One of the hypotheses is that basophils, upon migration to the secondary lymphoid organs, might gain characteristics of APCs. Therefore, the debate on whether human basophils display the features of APCs or not remains an unresolved issue unless and until their features are probed in secondary lymphoid tissues of humans.
In order to unequivocally address whether human basophils, like their murine counterparts, display features of APCs or not, we studied basophils from human secondary lymphoid tissues such as spleen, lung-draining lymph nodes and tonsils (See Online Supplementary Methods). In general, c-kit (CD117) and BDCA-4 (CD304) markers were used to distinguish basophils from mast cells and plasmacytoid DCs (pDCs), while CD203c distinguished basophils from other immune cells. Basophils were identified as CD203c+FcεRI+BDCA-4− c-kit− cells. As myeloid DC population was also reported to express FcεRI and that pDCs also express BDCA-2 (CD303), BDCA-1 (CD1c), BDCA-3 (CD141) and BDCA-2 markers were additionally used to distinguish splenic basophils from different DC subsets. The phenotype of basophils was analyzed in steady state and under various stimulatory conditions. Basophils were also sorted from the spleen, and the morphology of cytospin preparations of sorted basophils was analyzed by May-Grünwald Giemsa staining. The APC features of sorted splenic basophils were investigated in the functional assays by probing their ability to stimulate CD4+ T-cell activation, proliferation and cytokine production (See Online Supplementary Methods).
Basophils are rare leukocytes and represent <1% blood leukocytes in the blood.1 However, basophils were sparsely present in the human lung-draining lymph nodes (0.0013% (±0.0002 SD), n=3) and tonsils (0.004±0.0024%, n=4) (Figure 1 A–B). Therefore, we could not analyze APC features of basophils from these secondary lymphoid organs.
Figure 1.
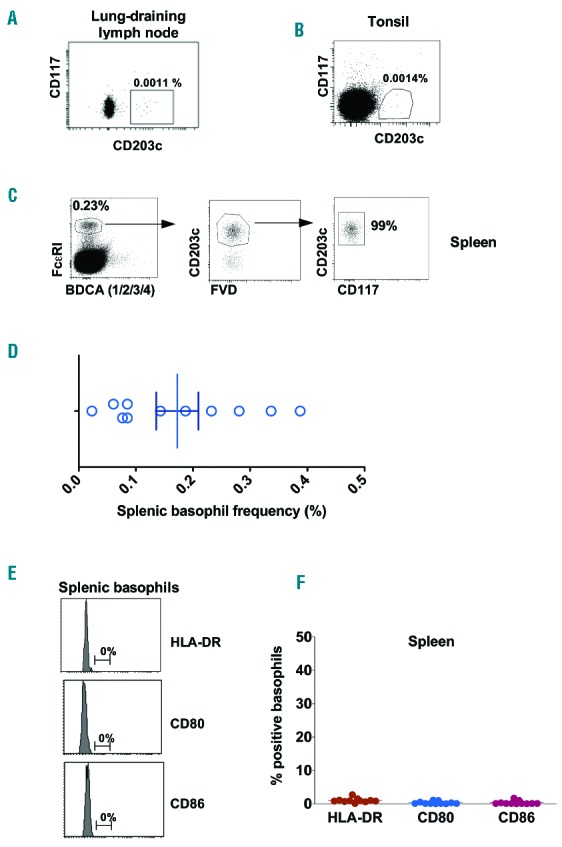
Human basophils from secondary lymphoid organs lack the phenotypic features of APCs. (A, B) The frequency of basophils in lung-draining lymph nodes and tonsils. Representative data of three or four subjects. (C) CD203c+FcεRI+BDCA-(1/2/3/4)−CD117− splenic basophils negative for fixable viable dye (FVD). (D) The frequency of splenic basophils from 11 subjects. (E, F) Representative histograms and mean±SEM (n=11 subjects) of HLA-DR, CD80 and CD86 expressions on steady-state splenic basophils.
Basophils in the spleen (0.172±0.122%, n=11) (Figure 1 C–D) were nearly 130 times higher than lung-draining lymph nodes. We found that splenic basophils under steady state condition did not express HLA-DR, CD80 and CD86 (Figure 1 E–F). On the other hand, splenic DCs expressed high levels of HLA-DR and varying degrees of CD80 and CD86 (Online Supplementary Figure S1). These results thus indicate that under steady state, human basophils residing at secondary lymphoid tissues do not display phenotypic characteristics of APCs.
Basophils receive signals from various sources such as cytokines, toll-like receptor (TLR) agonists and IgE-bound antigens. Therefore, we explored if basophils express phenotypic markers of APCs upon activation by these different categories of stimulation. IL-3, in addition to supporting differentiation and survival of basophils, also primes their activation.2 Previous reports have shown that murine basophils cultured in IL-3-conditioned medium express MHC class II and co-stimulatory molecules.3–5 On the other hand, GM-CSF and IFN-γ were previously reported to induce HLA-DR and co-stimulatory molecules on human innate cells. Therefore, IL-3 and cytokine cocktail containing IL-3, GM-CSF and IFN-γ were used for the stimulation of basophils (See Online Supplementary Methods).
Human basophils express various TLRs, including TLR4 and 2, and undergo activation upon TLR signaling.13 Therefore, we stimulated basophils with lipopolysaccharide from E. coli (LPS, TLR4 agonist), FSL-1 (Pam2CGDPKHPKSF, TLR2/6 agonist), or CpG ODN (TLR9 agonist). Furthermore, we also used papain as a model allergen in our experiments, and this cysteine protease allergen can directly activate basophils and promote murine basophil-mediated Th2 responses.4 Since, IgE-bound antigens/allergens provide the most-potent activation signal for basophils,13,14 this stimulation condition was mimicked by crosslinking of surface FcεRI-bound IgE by using anti-IgE antibodies (See Online Supplementary Methods).
Splenocytes were stimulated for 4 to 24 hours and analyzed for the expression of HLA-DR and B7 molecules on the basophils. Notably, expression of HLA-DR, CD80 and CD86 on basophils did not change significantly despite stimulation with IL-3, LPS, papain or FSL-1. The expression of these markers on stimulated basophils was on par with steady state cells (Figure 2 A–D). We confirm that basophils were indeed activated by these stimuli as analyzed by the expression levels of FcεRI and CD203c (Figure 2 E–F). The expression of FcεRI was significantly increased on splenic basophils irrespective of stimulatory conditions. Although expression of CD203c was also enhanced upon stimulation, due to variations among the donors, significance was not reached in all stimulatory conditions. Similar results were also obtained when splenocytes were stimulated with anti-IgE antibodies or cytokine cocktail (Figure 2 G–L). These results thus provide a pointer that human splenic basophils lack the phenotypic characteristics of APCs irrespective of activation signals they receive.
Figure 2.
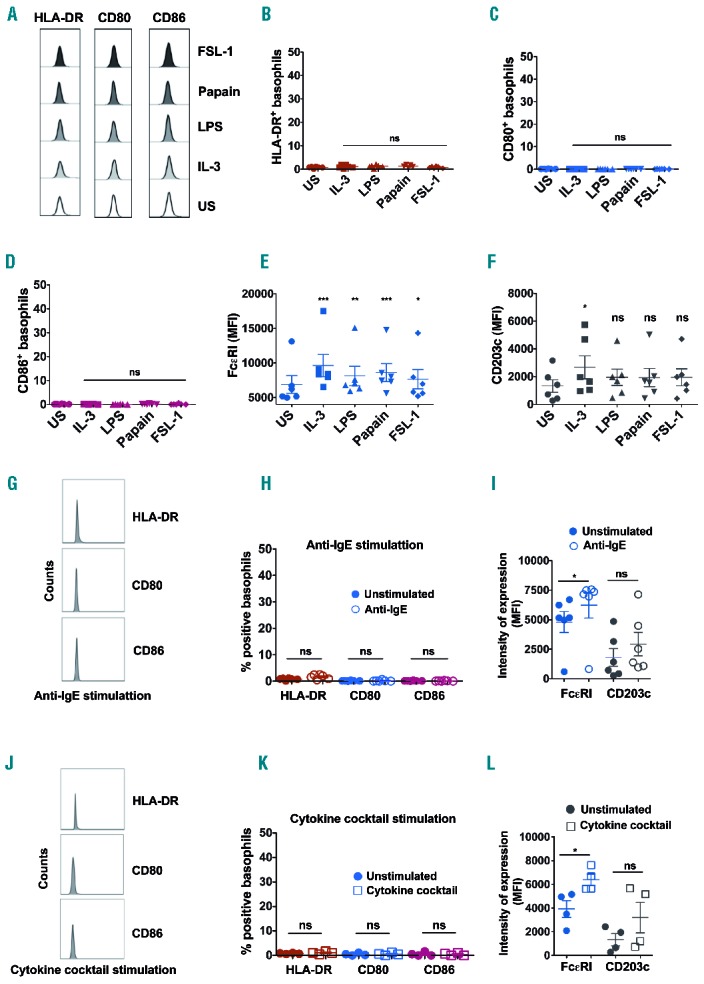
Expression of HLA-DR, B7 co-stimulatory molecules and activation-associated markers on human splenic basophils under various stimulatory conditions. (A–L) Spleenocytes were stimulated with IL-3, LPS, papain, FSL-1 (A–F), or anti-IgE (G–I), or cytokine cocktail (IL-3, GM-CSF and IFN-γ) (J–L) for 4 to 24 hours. Representative histograms showing the expression of HLA-DR, CD80 and CD86 on splenic basophils (A, G, J); mean± SEM values of % of splenic basophils positive for HLA-DR, CD80 and CD86 (B, C, D, H, K); and mean± SEM values of intensity of expression (MFI) of FcεRI and CD203c (E, F, I, L) on stimulated splenic basophils are shown. Data are from four to six subjects. US, unstimulated cells; *P<0.05; **P<0.01; ***P<0.001; ns, not significant; by One-way analysis of variance (B–F) or two-tailed Mann-Whitney test (H, I, K, L).
One of the hallmarks of APCs is their ability to stimulate CD4+ T-cell proliferation and cytokine production. Therefore, to prove indisputably that human basophils lack the functions of APCs, we sorted splenic basophils for co-culture with CD4+ T cells (See Online Supplementary Methods). Basophils from splenocytes were first enriched using basophil isolation kit II (Miltenyi Biotec). Enriched cells were labelled, and live basophils were sorted on FACSAria III flow cytometer (BD Biosciences) as cells positive for FcεRI and CD203c, and negative for BDCA-4 and c-kit. The purity of sorted basophils was 98–99 %. May-Grünwald Giemsa staining of cytospin preparations confirmed morphology of basophils with basophilic granules in the cytoplasm. Nucleus was partly covered by the basophilic granules. Cells presented relatively narrow cytoplasm that was loosely permeated by intensely basophilic granules. The basic color of the cytoplasm was pale blue to pale pink (Figure 3 A). Isolated splenic basophils were functionally viable. Upon IL-3 stimulation, isolated cells presented with enhanced CD69 expression and released histamine in response to degranulation stimuli.
Figure 3.
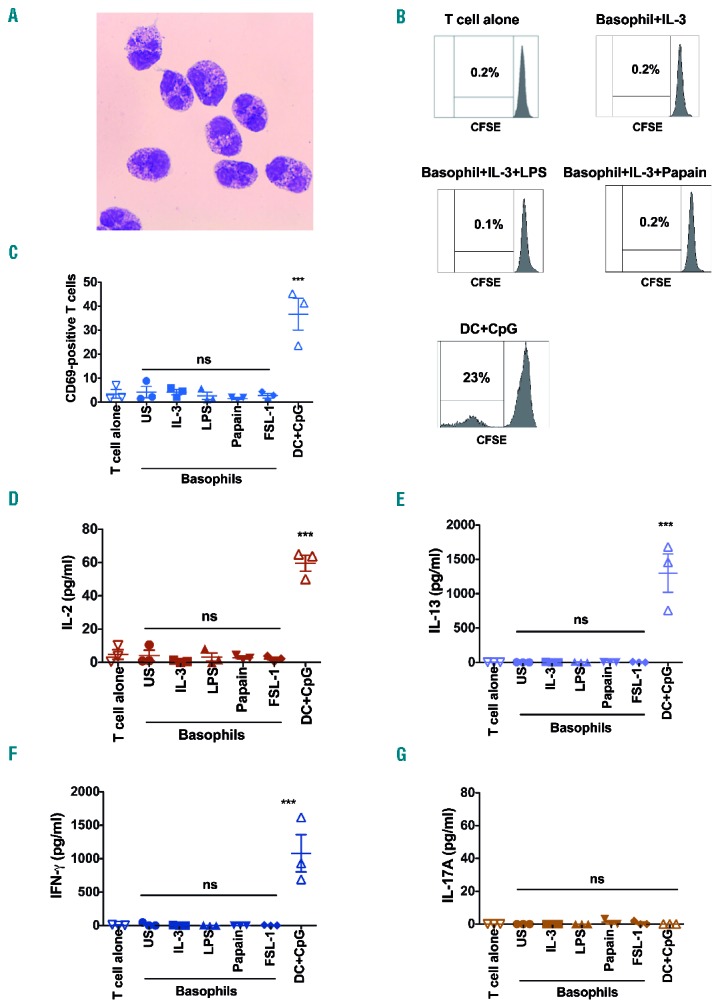
Human splenic basophils lack the functional features of APCs. (A) May-Grünwald Giemsa staining of cytospin preparations of sorted splenic basophils. (B) Ability of splenic basophils to stimulate CD4+ T-cell proliferation as analysed by CFSE staining. Representative of three experiments. (C) CD69 expression on CD4+ T cells co-cultured with sorted splenic basophils. (D–G) Induction of IL-2, and Th2 (IL-13), Th1 (IFN-γ) and Th17 (IL-17A) cytokines in sorted splenic basophil-CD4+ T-cell co-cultures (n=3). US: unstimulated cells; ***P<0.001; ns: not significant; by One-way analysis of variance.
We co-cultured sorted splenic basophils with allogeneic CD4+ T cells at a ratio of 1:10 under various stimulatory conditions, such as IL-3, LPS, papain or FSL-1. CD4+ T cells were isolated from peripheral blood mononuclear cells of healthy donors using CD4 MicroBeads (Miltenyi Biotec). Consistent with the phenotypic characteristics, stimulated basophils failed to induce CD4+ T-cell proliferation (Figure 3 B), T-cell activation marker CD69 (Figure 3 C) and secretion of IL-2 (Figure 3 D). In addition, basophils did not induce Th2 (IL-13), Th17 (IL-17A) or, as expected, Th1 (IFN-γ) cytokines (Figure 3 E–G). CpG or cytokine cocktail-stimulated basophils also presented similar results. Splenic DCs used as control, induced CD4+ T-cell proliferation, expression of CD69, and secretion of IL-2, IFN-γ and IL-13 (Figure 3 B–F).
Several models of allergy or parasitic infection have demonstrated induction of Th2 response in vivo in the spleen7 suggesting that the spleen has an important role in Th2 responses. Furthermore, previous studies in helminth-infected or papain-immunized mice have used splenic basophils to demonstrate their APC features3–5 and capacity to induce Th2 responses.3,5 These lines of evidence thus validate the use of human splenic basophils for the analysis of APC characteristics. Altogether, our results indicate that in humans, the role of basophils in Th2 responses is restricted to provision of IL-4 in the microenvironment,15 but not as APCs.
Supplementary Material
Acknowledgments
We thank Ms. Anupama Karnam and Dr. Jonathan Pol for their help; and Dr. V Languillat-Fouquet and Pr. H Martelli, Service Chirurgie Pédiatrique, Hôpital Bicêtre, France for providing spleen sections of patients with spherocytosis.
Footnotes
Funding: supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Université Pierre et Marie Curie and Université Paris Descartes. ES-V is a recipient of fellowship from Indo-French Center for Promotion of Advanced Research (CEFIPRA) and CG. is a recipient of fellowship from La Fondation pour la Recherche Médicale (FDM20150633674), France.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T. Nonredundant roles of basophils in immunity. Annu Rev Immunol. 2011;29:45–69. [DOI] [PubMed] [Google Scholar]
- 2.Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13(5):362–375. [DOI] [PubMed] [Google Scholar]
- 3.Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009; 10(7):697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10(7):713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshimoto T, Yasuda K, Tanaka H, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10(7):706–712. [DOI] [PubMed] [Google Scholar]
- 6.Hammad H, Plantinga M, Deswarte K, et al. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207(10):2097–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phythian-Adams AT, Cook PC, Lundie RJ, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207(10):2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkstra D, Hennig C, Witte T, Hansen G. Basophils from humans with systemic lupus erythematosus do not express MHC-II. Nat Med. 2012;18(4):488–489. [DOI] [PubMed] [Google Scholar]
- 9.Eckl-Dorna J, Ellinger A, Blatt K, et al. Basophils are not the key antigen-presenting cells in allergic patients. Allergy. 2012; 67(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitzmuller C, Nagl B, Deifl S, et al. Human blood basophils do not act as antigen-presenting cells for the major birch pollen allergen Bet v 1. Allergy. 2012;67(5):593–600. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M, Hegde P, Aimanianda V, et al. Circulating human basophils lack the features of professional antigen presenting cells. Sci Rep. 2013;3:1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma M, Stephen-Victor E, Poncet P, Kaveri SV, Bayry J. Basophils are inept at promoting human Th17 responses. Hum Immunol. 2015;76(2–3):176–180. [DOI] [PubMed] [Google Scholar]
- 13.Suurmond J, Stoop JN, Rivellese F, et al. Activation of human basophils by combined toll-like receptor- and FcεRI-triggering can promote Th2 skewing of naive T helper cells. Eur J Immunol. 2014;44(2):386–396. [DOI] [PubMed] [Google Scholar]
- 14.Voehringer D. Basophil modulation by cytokine instruction. Eur J Immunol. 2012;42(10):2544–2550. [DOI] [PubMed] [Google Scholar]
- 15.Falcone FH, Haas H, Gibbs BF. The human basophil: a new appreciation of its role in immune responses. Blood. 2000;96(13):4028–4038. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


