Abstract
In the present study, production of rhamnolipid biosurfactant by Pseudomonas aeruginosa OG1 was statistically optimized by response surface methodology. Box–Behnken design was applied to determine the optimal concentrations of 52, 9.2, and 4.5 g/L for carbon source (waste frying oil), nitrogen source (chicken feather peptone), and KH2PO4, respectively, in production medium. Under the optimized cultivation conditions, rhamnolipid production reached up to 13.31 g/L (with an emulsification activity of 80%), which is approximately twofold higher than the yield obtained from preliminary cultivations. Hence, rhamnolipid production, noteworthy in the literature, was achieved with the use of statistical optimization on inexpensive waste materials for the first time in the present study.
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0774-x) contains supplementary material, which is available to authorized users.
Keywords: Rhamnolipid, Pseudomonas aeruginosa, Waste frying oil, Chicken feather peptone, Response surface methodology, Box–Behnken design
Introduction
Biosurfactants have gained a considerable attention in recent years due to their potential uses in a broad range of application areas, including environmental remediation, agriculture, biofilm formation, quorum sensing, textile, pharmaceuticals, cosmetics, and the food, oil, and petrochemical industries (Banat et al. 2000; Kiran et al. 2016). They have superior characteristics, such as higher biodegradability, lower toxicity, unusual structural diversity, higher efficacy and greater stability towards temperature, pH, and salt concentration than many of their synthetic analogs (Banat et al. 2000; Lovaglio et al. 2011). Rhamnolipid, a glycolipid biosurfactant, possessing a hydrophobic fatty acid moiety and a hydrophilic moiety composed of one or two rhamnose, is one of the most commonly used surfactant mainly produced by Pseudomonas aeruginosa (Banat et al. 2000). The use of rhamnolipids in commercial applications has been limited due to low yield and high production costs. To cover the industrial aspects, it is essential to improve the efficiency of the bioprocess by increasing the yield while decreasing the cost of the production. Fermentative processes are highly affected by medium composition and cultivation conditions. Carbon and nitrogen sources of the culture medium can be considered as the major parameters. To achieve a cost-effective bioprocess, cheap sources should be selected.
The raw material costs (mainly carbon and nitrogen source) can account for up to 50% of the total production cost of biosurfactants (Mulligan and Gibbs 1993). The reuse of waste materials has gained great interest in terms of minimizing cost and elimination of environmental disposals. Used frying oils are generated during food preparation in large amounts and contribute to environmental pollution. A variety of waste oils from different industries (food and agro-based raw materials) have been used as alternative and cheap substrates (George and Jayachandran 2013; Henkel et al. 2012; Lan et al. 2015). Hence, the use of waste frying oil as the carbon source for rhamnolipid production will be both cost-effective and environmental-friendly.
Different nitrogen sources have also been used for the production of rhamnolipid. Feather, a waste by-product of industrial poultry-processing plants, was shown to be an effective nitrogen source supporting the microbial growth (Taskin and Kurbanoglu 2011). The presence of amino acids (especially isoleucine) and several micronutrients (e.g., K, Fe, P, Mg, S, and Ca salts) stimulates rhamnolipid production in P. aeruginosa (Lotfabad et al. 2009; Nawawi et al. 2010; Singh et al. 2013).
Another strategy to improve the efficiency of the bioprocess is the optimization of fermentation conditions. Unlike the traditional ‘one-factor at a time’ techniques, statistical optimization methods (e.g., full factorial design, response surface, etc.) take into account the interactions of variables in generating the optimum process response and are used extensively for media optimization (Haaland 1989). Full factorial design provides extensive information, but involves unfeasible complexity resulting from high number of experiments. Response surface methodology (RSM) decreases time and cost by providing fewer numbers of experiments compared to full factorial design. RSM has two types of designs, Box–Behnken design (BBD) and central composite design (CCD). Unlike CCD, BBD is generally recommended when performing non-sequential experiments, that is, when planning to perform the experiment once. BBD does not have axial points but CCD does; thus, in BBD, all design points will fall within the safe operating zone (Box and Behnken 1960).
The present work aimed to improve the yield of rhamnolipid production by P. aeruginosa OG1 in a cost-effective manner to promote the commercial use of microbial surfactants. Therefore, RSM was applied for the optimization of the production medium containing waste frying oil and chicken feather peptone (CFP) as the cheap carbon and nitrogen sources, respectively.
Materials and methods
Chemicals
The chemicals used throughout the study were analytical grade and purchased from Sigma-Aldrich (St Louis, MO, USA) and Difco (Detroit, MI, USA).
Microorganism
P. aeruginosa strain OG1 was used in this study. The strain was isolated from cockroaches living in pesticide-contaminated environments (Ozdal et al. 2016). The sequence of P. aeruginosa OG1 was deposited in GenBank with the accession number KC453990.
Growth conditions
The inoculum growth medium was tryptic soy broth and the shake flasks were incubated at 30 °C and 150 rpm for 24 h. Two mL of the inoculum was added to 50 mL production medium in 250 mL Erlenmeyer flasks.
Preparations of chicken feather peptone (CFP) and waste frying oil
Chicken feathers were obtained from the Demircioglu Poultry Farm, Zonguldak, Turkey. Feathers were treated according to the method of Kurbanoglu (2004) (Fig. S1). Chicken feather (100 g) was hydrolyzed with HCl and H2SO4, and then neutralized with NaOH, KOH, Mg(OH)2, and Ca(OH)2. The main chemical composition showed that CFP had high protein (56/100 g), ash (42/100 g), nitrogen (8.8/100 g), and low fat (0.2/100 g) contents (Fig. S2). CFP contained the tested amino acids, except methionine and tryptophan, at varying concentrations, and was especially rich in alanine (3.72/100 g), leucine (5.2/100 g), glutamate (6.1/100 g), glycine (5.54/100 g), serine (4.1/100 g), and proline (8.2/100 g). CFP was rich in especially Ca, K, Mg, S, and Na because of the hydrolysis processes.
Waste frying oil, used as carbon source throughout the study, was obtained from sunflower oil. Sunflower oil was used five times for frying potatoes. The waste oil was filtered through Whatman No. 1 filter paper to separate any particles.
Biomass estimation
Ten mL of the sample was withdrawn from the culture flasks at 24 h interval for 8 days and centrifuged at 10,000 rpm for 20 min. The biomass was dried to a constant weight in an oven at 80 °C and the dry weight of the cells was calculated.
Rhamnolipid determination
The culture broth was centrifuged at 10,000 rpm for 12 min. Rhamnolipid concentration was estimated by the phenol–sulphuric acid method (Dubois et al. 1956). Rhamnolipid production was determined by the colorimetric assay at 480 nm using a Shimadzu UV–Vis spectrophotometer. A standard curve prepared using different concentrations of L-rhamnose was used to determine the rhamnolipid concentration.
Emulsification index (E24)
E24 of culture samples was determined by adding 2 mL of olive oil to the same amount of culture, mixing with a vortex mixer for 2 min, and leaving it to stand for 24 h. The E24 index is given as the percentage of the height of emulsified layer (mm) divided by the total height of the liquid column (mm).
Screening the carbon and nitrogen sources
Pseudomonas aeruginosa strain OG1 was cultivated in 250 mL Erlenmeyer flasks containing 50 mL medium. To determine the effect of carbon sources on rhamnolipid production, minimal salt medium (MSM) containing 4 g/L KH2PO4, 0.2 g/L MgSO4·7H2O, 0.1 g/L CaCl2, 5 g/L yeast extract, and 30 g/L of different carbon sources (i.e., corn oil, sunflower oil, olive oil, and waste frying sunflower oil) was used. To study the effect of nitrogen sources, yeast extract was replaced with CFP, tryptone, and bacto peptone. The pH of the medium was initially adjusted to 7.4 with 2.0 M NaOH. The cultures were incubated at 30 °C in an orbital shaker for 8 days.
Statistical optimization by response surface methodology using Box–Behnken design
BBD of RSM was used to evaluate variables. Three independent variables (Oil, CFP, and KH2PO4) were evaluated at three levels (−1, 0, +1) for the optimization of dependent variable (rhamnolipid production) by the experimental plan given in Table 1. The ranges for each factor were 30–70 g/L for oil, 5–11 g/L for CFP, and 1–7 g/L for KH2PO4. Minitab v.15 (Minitab Inc., State College, PA) was applied to analyze the experimental results. According to BBD, 30 experiments were performed. The result of each response variable was given as the average of two replicates in Table 1.
Table 1.
Box–Behnken design of three factors (oil, CFP, and KH2PO4) along with the response (rhamnolipid production) using response surface methodology
| Run | Oil (g/L) | CFP (g/L) | KH2PO4 (g/L) | Y: Rhamnolipid (g/L) |
|---|---|---|---|---|
| 1 | 30 | 11 | 4 | 9.15 |
| 2 | 50 | 8 | 4 | 13 |
| 3 | 50 | 8 | 4 | 12 |
| 4 | 50 | 11 | 1 | 7.4 |
| 5 | 70 | 8 | 7 | 8.4 |
| 6 | 70 | 11 | 4 | 9.2 |
| 7 | 50 | 11 | 7 | 8.8 |
| 8 | 70 | 5 | 4 | 7.1 |
| 9 | 30 | 5 | 4 | 6.4 |
| 10 | 50 | 5 | 7 | 4.8 |
| 11 | 70 | 5 | 4 | 7.1 |
| 12 | 30 | 8 | 1 | 6.6 |
| 13 | 50 | 8 | 4 | 12.6 |
| 14 | 30 | 8 | 7 | 7.4 |
| 15 | 70 | 8 | 7 | 8.4 |
A second-order polynomial model was fitted to the data to relate the rhamnolipid production of Y to the amount of oil, CFP, and KH2PO4. For a three-factor system, the quadratic model equation (Eq. 1) is as follows:
| 1 |
where Y is predicted response; X 1, X 2, X 3, independent variables; b 0, the intercept (the value of fixed response at the central point of the design); b 1, b 2, b 3, linear coefficients; b 11, b 22, b 33, the quadratic (squared) coefficients; and b 12, b 13, b 23, interaction coefficients.
MINITAB™ Statistical Software was used for the experimental design, statistical analysis of the data, response surface plots, and determination of the optimum values of independent variables. Significance level was considered as 95% (P < 0.05) in regression analysis.
Results and discussion
Selection of optimal carbon and nitrogen sources
In the present study, the use of low cost and waste substrates was emphasized for the cost-effective production of rhamnolipid biosurfactant by P. aeruginosa OG1. Therefore, different carbon and nitrogen sources were evaluated for rhamnolipid production. Results given in Table 2 show that waste frying oil was not the best carbon source; however, considering the economy of the process, it was preferred to be further used in optimization by RSM. Among the nitrogen sources, the use of CFP resulted in the highest rhamnolipid production and chosen for further use in optimization by RSM.
Table 2.
Effect of different carbon and nitrogen sources on the production of rhamnolipid by P. aeruginosa OG1
| Carbon sources (3%) | Rhamnolipid concentration (g/L) | Nitrogen sources (0.5%) | Rhamnolipid concentration (g/L) |
|---|---|---|---|
| Corn oil | 4.4 ± 0.50 | Yeast extract | 3.2 ± 0.40 |
| Sunflower oil | 4.0 ± 0.35 | CFP | 7.2 ± 0.71 |
| Olive oil | 3.8 ± 0.32 | Tryptone | 5.0 ± 0.44 |
| Waste frying sunflower oil | 3.2 ± 0.30 | Bacto peptone | 4.1 ± 0.36 |
Response surface methodology using Box–Behnken design
BBD of RSM was used for optimization of rhamnolipid production by P. aeruginosa OG1. The design of the variables (oil, CFP, and KH2PO4) and the corresponding responses (rhamnolipid yield) are presented in Table 1.
The values of regression coefficients calculated by ANOVA (analysis of variance) for rhamnolipid production are shown in Table 3a. Rhamnolipid production is best predicted by the model equation (Eq. 2):
| 2 |
where Y is predicted rhamnolipid production.
Table 3.
Coefficient estimates in the regression model for rhamnolipid yield selected through variable selection (a) and ANOVA for rhamnolipid yield (b)
| a | ||||
|---|---|---|---|---|
| Variable | Coefficient | Standard error | t | p |
| Constant | 10.717 | 0.178 | 60.16 | 0.000* |
| Oil | 0.241 | 0.109 | 2.21 | 0.039* |
| CFP | 1.009 | 0.109 | 9.25 | 0.000* |
| KH2PO4 | 0.587 | 0.109 | 5.39 | 0.000* |
| Oil*Oil | −1.393 | 0.161 | −8.67 | 0.000* |
| CFP*CFP | −1.318 | 0.161 | −8.21 | 0.000* |
| KH2PO4*KH2PO4 | −2.111 | 0.161 | −13.15 | 0.000* |
| Oil*CFP | −0.131 | 0.154 | −0.85 | 0.405 |
| Oil*KH2PO4 | 0.288 | 0.154 | 1.86 | 0.077 |
| CFP*KH2PO4 | 0.213 | 0.154 | 1.38 | 0.184 |
| b | |||||
|---|---|---|---|---|---|
| Source | Degree of freedom | Sum of squares | Mean squares | F | p |
| Model | 9 | 76.5844 | 8.5094 | 44.70 | 0.000 |
| Linear | 3 | 22.7503 | 7.5834 | 39.84 | 0.000 |
| Square | 3 | 52.6737 | 17.5579 | 92.23 | 0.000 |
| Interaction | 3 | 1.1603 | 0.3868 | 2.03 | 0.142 |
| Residual Error | 20 | 3.8074 | 0.1904 | ||
| Lack-of-Fit | 3 | 0.7928 | 0.2643 | 1.49 | 0.253 |
| Pure Error | 17 | 3.0146 | 0.1773 | ||
| Total | 29 | 80.3917 | |||
S: 0.4363, R 2: 95.26%, R 2(pred): 90.45%, R 2 (adj): 93.13%
The magnitudes of the model coefficients given in Table 3a clearly indicated that the main factors had significant effects on rhamnolipid production with P values less than 0.05. CFP and KH2PO4 had more apparent effects on rhamnolipid production than oil. The three factors had negative quadratic effects (the magnitudes of oil and CFP effects are close to each other) which means that a better response is obtained at low levels of the variable. The interactive effect of oil*CFP was negative, while the oil*KH2PO4 and CFP*KH2PO4 had positive interactive effects (Table 3a). For the interactions, a positive value means that the response increases if both variables change to the same level, low or high (Martendal et al. 2007). On the other hand, all the interactive effects had higher p values (p > 0.05) and were statistically insignificant.
The ANOVA for the response surface model for rhamnolipid production was given in Table 3b. The statistical significance of the regression model was evaluated by F test. The results of F test showed that the model is highly significant (p: 0.000). Regression model equation adequately fits to the experimental data with a high R 2 value of 0.95 for rhamnolipid production. The pred R 2 value of 90.45 showed a good correlation with adj R 2 of 93.13 (Table 3b). This indicated a satisfactory agreement between the experimental and predicted values for rhamnolipid production.
The results of variance analysis shown in Table 3b indicated that the model had significant linear, and quadratic effects (p < 0.05) and insignificant interaction effect (p > 0.05) for rhamnolipid production. The results of variance analysis also revealed that the model was adequate in predicting rhamnolipid production (p: 0.253) due to insignificant lack-of-fit.
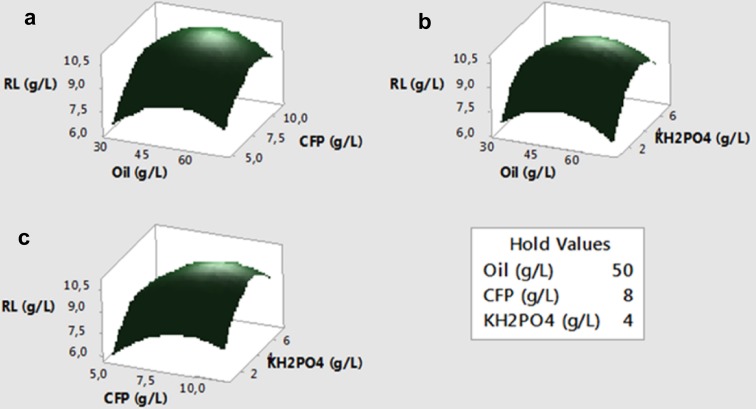
The three-dimensional response surfaces and contour plots based on the model equation were plotted in Figs. 1 and 2, respectively. Response surface plots explain the interaction of these variables and it is simple to find the optimum level of each variable for a maximum response. The peaks in the surface plots indicate that the optimum points were within the design limits. All surface plots showed that increasing the levels of each of the three variables was beneficial for rhamnolipid production up to a certain level. Both surface plots (Fig. 1) and contour plots (Fig. 2) showed that maximum rhamnolipid production of 13 g/L was obtained when the oil, CFP, and KH2PO4 concentrations reached slightly beyond the 50, 9, and 4.5 g/L, respectively. Subsequent increase either did not affect or caused adverse effect on responses. Especially for CFP, concentration greater than 9 g/L was inhibitory to rhamnolipid production which might be due to high salt concentration, some toxic materials, and the change of C/N ratio of culture medium (Kurbanoglu et al. 2015). The elliptical shape of the contour plots indicates a significant interaction, while circular shape indicates insignificant interaction (Gurkok et al. 2011). According to Fig. 2, circular shape of the contour plots for CFP*oil interaction shows quite negligible interaction which can also be seen from the p value (0.405) given in Table 3a. The contour plots for KH2PO4*oil (p: 0.077) and KH2PO4*CFP (p: 0.184) interactions are slightly elliptical; however, they are still negligible with p values higher than 0.05.
Fig. 1.
Response surface plots of interactions between CFP and oil (a), KH2PO4 and oil (b), and KH2PO4 and CFP (c) on rhamnolipid (RL) production by P. aeruginosa OG1
Fig. 2.
Contour plots of interactions between CFP and oil (a), KH2PO4 and oil (b), and KH2PO4 and CFP (c) on rhamnolipid (RL) production by P. aeruginosa OG1
Validation of the model
The optimal values of the variables were found as 52 g/L oil, 9.2 g/L CFP, and 4.5 g/L KH2PO4 by the use of “response optimizer” facility of Minitab v15. Eight days of cultivation were performed in the optimized medium in triplicate. Figure 3 is the time course graph showing the relations between rhamnolipid production, biomass generation, and E24% in the optimized medium. A satisfactory correlation between the predicted and measured rhamnolipid yield was achieved. The maximum rhamnolipid production reached 13.31 g/L in the stationary phase, which was higher than the corresponding predicted value of 10 g/L. This validation experiment proved that the model was accurate and reliable. Compared to the control cultivations, a twofold increase in rhamnolipid production was obtained as the result of optimizing culture conditions using inexpensive waste frying oil and CFP.
Fig. 3.
Rhamnolipid production, growth, and %EI24 during cultivation of P. aeruginosa OG1 in 100 mL medium containing 52 g/L oil, 9.2 g/L CFP, and 4.5 g/L KH2PO4 at 30 °C, 150 rpm
The use of microbial surfactants in commercial applications instead of synthetic ones, which is restricted due to high cost and low productivity, could be encouraged by the use of cheap substrates and optimized conditions. The reuse of waste materials, which is common in literature (George and Jayachandran 2013; Lan et al. 2015), provided both cost-effective and environmental-friendly alternative to cover the industrial aspects for large-scale rhamnolipid production. Improvement of rhamnolipid production by optimization has also been reported recently in different studies (Lotfabad et al. 2009; Nawawi et al. 2010; Kumar et al. 2015; Ma et al. 2016).
In the present study, the optimization of rhamnolipid biosurfactant production by P. aeruginosa OG1 on low cost substrates is reported. The effects of waste frying oil, CFP, and KH2PO4 concentrations on rhamnolipid production were investigated by RSM using BBD. RSM proved to be an effective and a reliable optimization tool for the improvement of rhamnolipid yield. In the optimized medium containing 52 g/L oil, 9.2 g/L CFP, and 4.5 g/L KH2PO4, rhamnolipid yield reached up to 13.31 g/L, which is twofold higher than the yield obtained from control cultivations.
Different species were reported as rhamnolipid producers. Dubeau et al. (2009) obtained only 0.419 and 1.473 g/L rhamnolipid production with Burkholderia thailandensis using glycerol and canola oil, respectively. Costa et al. (2011) obtained 0.555 and 1 g/L rhamnolipid yields with B. glumae using glycerol and canola oil as the carbon sources, respectively. Onwosi and Odibo (2012) obtained 5.46 g/L rhamnolipid yield with Pseudomonas nitroreducens using glucose and sodium nitrate. Compared to other species, P. aeruginosa strains still maintain their leading position in rhamnolipid production. The rhamnolipid yield achieved in the present study with P. aeruginosa OG1 (13.31 g/L) using waste frying oil and CFP at optimal concentrations was much greater than the yields obtained in several previous studies. A wide range of rhamnolipid production yield by Pseudomonas species using vegetable oils and/or their wastes as carbon source has been reported. P. aeruginosa produced rhamnolipid when grown on water-insoluble substrates such as coconut oil (2.26 g/L) (George and Jayachandran 2013), Mesua ferrea seed oil (6.95 g/L) (Singh et al. 2013), soy bean oil (12 g/L) (Abbasi et al. 2012), waste cooking oil (13.93 g/L) (Lan et al. 2015), waste frying oil (6.6 g/L) (Luo et al. 2013), and waste frying oil (24.61 g/L) (Zhu et al. 2007). The rhamnolipid yield achieved in the present study with P. aeruginosa OG1 (13.31 g/L) using waste frying oil and CFP at optimal concentrations was much greater than the yields obtained in several previous studies.
As a conclusion, an appreciable rhamnolipid production yield was achieved with the use of statistical optimization using RSM. Hence, a cost-effective, environmentally friendly, and highly productive process is achieved for rhamnolipid production.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Ataturk University.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest associated with the present research.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0774-x) contains supplementary material, which is available to authorized users.
References
- Abbasi H, Hamedi MM, Lotfabad TB, Zahiri HS, Sharafi H, Masoomi F, Noghabi KA. Biosurfactant-producing bacterium, Pseudomonas aeruginosa MA01 isolated from spoiled apples: physicochemical and structural characteristics of isolated biosurfactant. J Biosci Bioeng. 2012;113(2):211–219. doi: 10.1016/j.jbiosc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Banat IM, Makkar RS, Cameotra SS. Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol. 2000;53(5):495–508. doi: 10.1007/s002530051648. [DOI] [PubMed] [Google Scholar]
- Box GEP, Behnken DW. Some new three level design for the study of quantitative variables. Technometrics. 1960;2:455–575. doi: 10.1080/00401706.1960.10489912. [DOI] [Google Scholar]
- Costa SG, Déziel E, Lépine F. Characterization of rhamnolipid production by Burkholderia glumae. Lett Appl Microbiol. 2011;53(6):620–627. doi: 10.1111/j.1472-765X.2011.03154.x. [DOI] [PubMed] [Google Scholar]
- Dubeau D, Déziel E, Woods DE, Lépine F. Burkholderia thailandensis harbors two identical rhl gene clusters responsible for the biosynthesis of rhamnolipids. BMC Microbiol. 2009;9:263. doi: 10.1186/1471-2180-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PAT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- George S, Jayachandran K. Production and characterization of rhamnolipid biosurfactant from waste frying coconut oil using a novel Pseudomonas aeruginosa D. J Appl Microbiol. 2013;114(2):373–383. doi: 10.1111/jam.12069. [DOI] [PubMed] [Google Scholar]
- Gurkok S, Cekmecelioglu D, Ogel ZB. Optimization of culture conditions for Aspergillus sojae expressing an Aspergillus fumigatus α-galactosidase. Bioresour Technol. 2011;102:4925–4929. doi: 10.1016/j.biortech.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Haaland PD. Experimantal design in biotechnology. Newyork: Marcel Dekker; 1989. [Google Scholar]
- Henkel M, Müller MM, Kügler JH, Lovaglio RB, Contiero J, Syldatk C, Hausmann R. Rhamnolipids as biosurfactants from renewable resources: concepts for next-generation rhamnolipid production. Process Biochem. 2012;47(8):1207–1219. doi: 10.1016/j.procbio.2012.04.018. [DOI] [Google Scholar]
- Kiran GS, Ninawe AS, Lipton AN, Pandian V, Selvin J. Rhamnolipid biosurfactants: evolutionary implications, applications and future prospects from untapped marine resource. Crit Rev Biotechnol. 2016;36(3):399–415. doi: 10.3109/07388551.2014.979758. [DOI] [PubMed] [Google Scholar]
- Kumar AP, Janardhan A, Radha S, Viswanath B, Narasimha G. Statistical approach to optimize production of biosurfactant by Pseudomonas aeruginosa 2297. 3 Biotech. 2015;5(1):71–79. doi: 10.1007/s13205-014-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurbanoglu EB. Enhancement of lactic acid production with ram horn peptone by Lactobacillus casei. World J Microbiol Biotechnol. 2004;20(1):37–42. doi: 10.1023/B:WIBI.0000013289.89034.47. [DOI] [Google Scholar]
- Kurbanoglu EB, Ozdal M, Ozdal OG, Algur OF. Enhanced production of prodigiosin by Serratia marcescens MO-1 using ram horn peptone. Braz J Microbiol. 2015;46(2):631–637. doi: 10.1590/S1517-838246246220131143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan G, Fan Q, Liu Y, Chen C, Li G, Liu Y, Yin X. Rhamnolipid production from waste cooking oil using Pseudomonas SWP-4. Biochem Eng J. 2015;101:44–54. doi: 10.1016/j.bej.2015.05.001. [DOI] [Google Scholar]
- Lotfabad TB, Shourian M, Roostaazad R, Najafabadi AR, Adelzadeh MR, Noghabi KA. An efficient biosurfactant-producing bacterium Pseudomonas aeruginosa MR01, isolated from oil excavation areas in south of Iran. Colloid Surface B. 2009;69(2):183–193. doi: 10.1016/j.colsurfb.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Lovaglio RB, dos Santos FJ, Jafelicci M, Contiero J. Rhamnolipid emulsifying activity and emulsion stability: pH rules. Colloids Surf B Biointerfaces. 2011;85(2):301–305. doi: 10.1016/j.colsurfb.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Luo Z, Yuan XZ, Zhong H, Zeng GM, Liu ZF, Ma XL, Zhu YY. Optimizing rhamnolipid production by Pseudomonas aeruginosa ATCC 9027 grown on waste frying oil using response surface method and batch-fed fermentation. J Cent South Univ. 2013;20(4):1015–1021. doi: 10.1007/s11771-013-1578-8. [DOI] [Google Scholar]
- Ma KY, Sun MY, Dong W, He CQ, Chen FL, Ma YL. Effects of nutrition optimization strategy on rhamnolipid production in a Pseudomonas aeruginosa strain DN1 for bioremediation of crude oil. Biocatal Agric Biotechnol. 2016;6:144–151. [Google Scholar]
- Martendal E, Budziak D, Carasek E. Application of fractional factorial experimental and Box-Behnken designs for optimization of single-drop microextraction of 2, 4, 6-trichloroanisole and 2, 4, 6-tribromoanisole from wine samples. J Chromatogr A. 2007;1148(2):131–136. doi: 10.1016/j.chroma.2007.02.079. [DOI] [PubMed] [Google Scholar]
- Mulligan CN, Gibbs BF (1993) Factors influencing the economics of biosurfactants. In: Kosaric N (ed) Biosurfactants. Production, properties and applications. Surfactant Science Series, vol 48. Dekker, New York, pp 329–371
- Nawawi WMFW, Jamal P, Alam MZ. Utilization of sludge palm oil as a novel substrate for biosurfactant production. Bioresource Technol. 2010;101(23):9241–9247. doi: 10.1016/j.biortech.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Onwosi CO, Odibo FJC. Effects of carbon and nitrogen sources on rhamnolipid biosurfactant production by Pseudomonas nitroreducens isolated from soil. World J Microbiol Biotechnol. 2012;28:937. doi: 10.1007/s11274-011-0891-3. [DOI] [PubMed] [Google Scholar]
- Ozdal M, Ozdal OG, Algur OF. Isolation and characterization of α-endosulfan degrading bacteria from the microflora of cockroaches. Pol J Microbiol. 2016;65(1):63–68. doi: 10.5604/17331331.1197325. [DOI] [PubMed] [Google Scholar]
- Singh SP, Bharali P, Konwar BK. Optimization of nutrient requirements and culture conditions for the production of rhamnolipid from Pseudomonas aeruginosa (MTCC 7815) using Mesua ferrea seed oil. Indian J Microbiol. 2013;53(4):467–476. doi: 10.1007/s12088-013-0403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskin M, Kurbanoglu EB. Evaluation of waste chicken feathers as peptone source for bacterial growth. J Appl Microbiol. 2011;111(4):826–834. doi: 10.1111/j.1365-2672.2011.05103.x. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Gan JJ, Zhang GL, Yao B, Zhu WJ, Meng Q. Reuse of waste frying oil for production of rhamnolipids using Pseudomonas aeruginosa zju. u1M. J Zhejiang Univ Sci A. 2007;8(9):1514–1520. doi: 10.1631/jzus.2007.A1514. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.