Abstract
Polyphenols, which include phenolic acids, flavonoids, stilbenes, and phenylethanoids, are generally known as useful antioxidants. Tyrosol, hydroxytyrosol, and salidroside are typical phenylethanoids. Phenylethanoids are found in plants such as olive, green tea, and Rhodiola and have various biological activities, including the prevention of cardiovascular diseases, cancer, and brain damage. We used Escherichia coli to synthesize three phenylethanoids, tyrosol, hydroxytyrosol, and salidroside. To synthesize tyrosol, the aromatic aldehyde synthase (AAS) was expressed in E. coli. Hydroxytyrosol was synthesized using E. coli harboring AAS and HpaBC, which encodes hydroxylase. In order to synthesize salidroside, 12 uridine diphosphate-dependent glycosyltransferases (UGTs) were screened and UGT85A1 was found to convert tyrosol to salidroside. Using E. coli harboring AAS and UGT85A1, salidroside was synthesized. Through the optimization of these three E. coli strains, we were able to synthesize 531 mg/L tyrosol, 208 mg/L hydroxytyrosol, and 288 mg/L salidroside, respectively.
Introduction
Many countries have traditional foods or medicines; olive oil in southern Europe and the Middle East and green tea from Asia are considered regional traditional foods. Olive oil and green tea contain many antioxidants due to presence of phenolic compounds. Antioxidant protects cells and tissues from oxidative injury1, which can cause Parkinson’s disease, Alzheimer’s disease, other forms of dementia, cancer, and heart disease. Historically, olive oil has been called a “miracle food” because it aids in digestion and combats skin cancer. Also, olive oil has been shown to effect on cardiovascular disease and certain types of cancers2. Two major ingredients in olive are tyrosol and hydroxytyrosol, both of which are considered bioactive ingredients having various biological activities. It has been proven that tyrosol can lower the risk of developing Alzheimer’s disease3. Salidroside, a glucoside of tyrosol, is one of the major ingredients of the medicinal herb, Rhodiola 4. Salidroside exhibits various biological activities, including nerve and brain cell protection, bone loss reduction and weight reduction5–8. Tyrosol, hydroxytyrosol, and salidroside belong to a group of plant phenolic compounds called phenylethanoids.
Tyrosol is synthesized from tyrosine. There are two possible biosynthesis pathways for tyrosol synthesis in plants. In the first proposed pathway, tyrosine is converted into tyramine by tyrosine decarboxylase (TDC). Subsequent oxidation and reduction of tyramine result in the formation of tyrosol9 (Fig. 1). However, growing evidence indicates that tyrosol is synthesized via tyramine, as TDC was identified in Rhodiola sachalinensis 10, 11. The carbon backbone of tyrosol is C6-C2. Plant phenolic compounds have been synthesized via cinnamic acid, which has a C6-C3 carbon backbone and is derived from phenylalanine. In order to synthesize other phenolic compounds such as flavonoids and stilbenes, malonyl-CoA serves as a carbon donor to transfer two carbons to hydroxycinnamic acid. The synthesis of C6-C1 phenolic compounds, such as benzoic acid relies on the coenzyme A-dependent β-oxidation of cinnamoyl-CoA11. These results support that tyrosol is synthesized through tyrosine decarboxylation.
Figure 1.
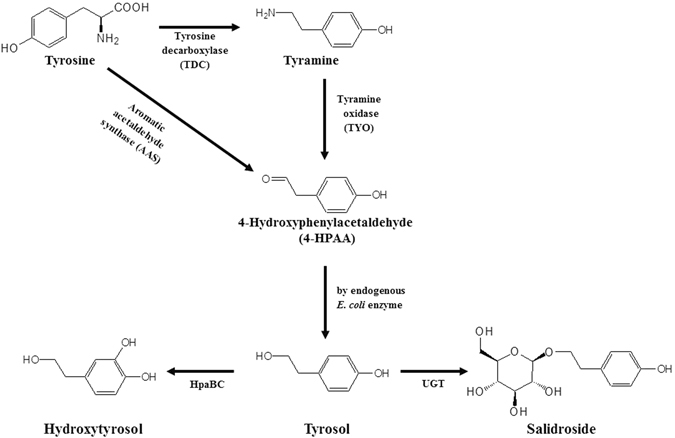
Scheme of biosynthesis of three phenylethanoids, tyrosol, hydroxytyrosol, and salidroside.
Salidroside is synthesized from tyrosol by a uridine diphosphate dependent glycosyltransferase (UGT). UGT73B6 from R. sachalinensis was found to be involved in the biosynthesis of salidroside although it also produces icariside D2 using tyrosol12, 13.
Escherichia coli has been widely used to synthesize diverse phytochemicals. Biological synthesis using microorganisms has advantages over enzymatic and chemical syntheses; it does not require expensive cofactors and it confers regioselectivity and stereoselectivity. Tyrosol, hydroxytyrosol, and salidroside have been previously synthesized in E. coli. There are two possible routes for the production of tyrosol in E. coli. In the first method using tyrosine as a substrate, TDC and TYO were introduced into an E. coli feaB (phenylacetaldehyde dehydrogenase) deletion strain14. FeaB competes for 4-hydroxyphenylacetaldehyde (4-HPAA) and produces 4-hydroxyphenylacetate instead of tyrosol. Therefore, deletion of feaB results in and increase in tyrosol production by approximately 43%. Using this E. coli strain, Satoh et al.14 were able to synthesize 69 mg/L tyrosol. Second route of tyrosol production in E. coli utilized aro10 from yeast encoding pyruvate decarboxylase that converts 4-hydroxyphenylpyruvate to 4-HPAA. Bai et al.13 used aro10 and other genes for the biosynthesis of tyrosine, in addition to several E. coli mutants to produce tyrosol. This group also introduced UGT into the tyrosine producing E. coli strain to synthesize salidroside13. Hydroxytyrosol was synthesized using tyrosine hydroxylase (TH), L-DOPA decarboxylase (DDC) and tyramine oxidase (TYO)14.
Studies of plant aromatic amino acid decarboxylases (AAADs) revealed that some AAADs are bifunctional enzymes capable of catalyzing both decarboxylation and oxidation15–17. This family of AAADs is known as aromatic aldehyde synthases (AASs). AAS converts tyrosine into 4-HPAA, and then 4-HPAA can be converted into tyrosol in E. coli. In this report, we used plant AAS to synthesize tyrosol, hydroxytyrosol, and salidroside in E. coli. Through the metabolic engineering of E. coli, 531 mg/L tyrosol and 208 mg/L hydroxytyrosol were synthesized. Furthermore, in order to synthesize salidroside, we screened 12 UGTs from Arabidopsis thaliana. Using engineered E. coli harboring AAS and the identified UGT, we could synthesize 288 mg/L salidroside.
Results
Synthesis of tyrosol using AAS in E. coli
For the synthesis of tyrosol in E. coli, we constructed the pathway from tyrosine to tyrosol using AAS. AAS is a bifunctional enzyme that converts tyrosine into 4-HPAA oxidation15–17. In E. coli, 4-HPAA is converted to tyrosol by alcohol dehydrogenase(s)14. We tested three different AAS genes from A. thaliana, Petunia hybrid, and Petroselinum crispum. After induction of each protein, tyrosine (100 μM) was added to the culture. The culture filtrates from the E. coli strains harboring each AAS were analyzed using HPLC. E. coli harboring AAS from P. crispum produced approximately 12.6 mg/L tyrosol (Fig. 2). E. coli harboring AAS from A. thaliana did not produce tyrosol and E. coli harboring AAS from P. hybrid produced approximately 5.3 mg/L tyrosol. The structure of the reaction product in Fig. 1 was determined to be tyrosol using NMR. We decided to use the PcAAS for further experiments. We could not observe the reaction intermediates such as 4-HPAA, suggesting that AAS and the endogenous E. coli reductase, which converts 4-HPAA into tyrosol, were balanced in the production of tyrosol.
Figure 2.
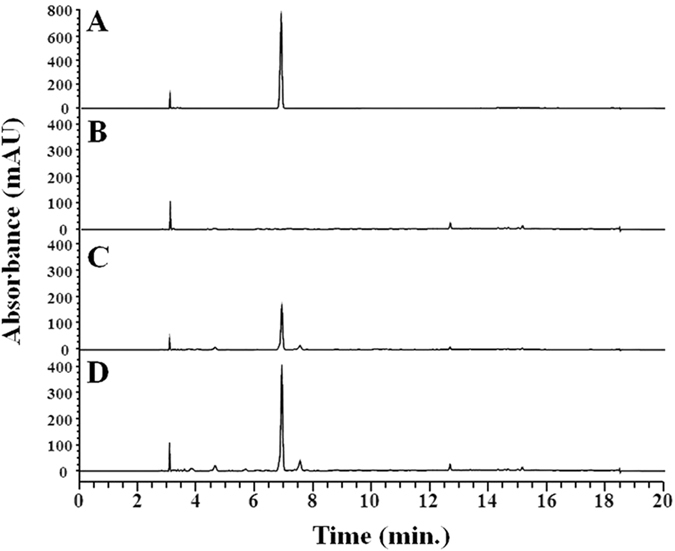
Production of tyrosol using E. coli harboring AAS. (A) standard tyrosol; (B) AAS from Arabidopsis thaliana; (C) AAS from Petunia hybrid; (D) AAS from Petroselinum crispum.
Tyrosol is synthesized from tyrosine. Therefore, it is likely that total tyrosine content is correlated with the final tyrosol yield. The reaction intermediate, the 4-HPAA, can be converted into 4-hydroxyphenylacetic acid by phenylacetaldehyde dehydrogenase (feaB), which competes with the E. coli alcohol dehydrogenase for 4-HPAA and results in reduction of the final yield of tyrosol. We used two mutants B-TP and B-TPF (Table 1). The strain B-TP produced more tyrosine than did the wild type due to the deletions of the transcriptional regulator, tyrR, which is inhibited by tyrosine, and pheA encoding a chorismate mutase/prephenate dehydratase that drives prephenate toward the biosynthesis of the phenylalanine instead of biosynthesis of tyrosine18, 19. The strain B-TPF contains deletions in feaB, tyrR, and pheA. FeaB encodes phenylacetaldehyde dehydrogenase, which converts 4-HPAA to 4-hydroxyphenylacetate (4-HPA). Deletion of this gene was expected to increase more 4-HPAA for tyrosol synthesis. As an alternative route for the tyrosol biosynthesis, we used tyrosine decarboxylase (TDC) from Papaver somniferum and tyrosine oxidase (TYO) from Micrococcus luteus. These two gene was transformed into E. coli BL21(DE3). Strain B-TY1 produced 138.9 mg/L of tyrosol, B-TY2 produced 188.1 mg/L, and B-TY3 produced 250.4 mg/L (Fig. 3). This result demonstrated that the contents of tyrosine and 4-HPAA are critical to the final yield of tyrosol. However, B-TY4, which had TDC and TYO, produced only 49.2 mg/L tyrosol.
Table 1.
Plasmids, Escherichia coli strains, and primers used in this study.
| Plasmids or E. coli strain or Primers | Relevant properties or genetic marker | Source or reference | |
|---|---|---|---|
| Plasmids | |||
| pACYCDDuet | P15A ori, Cmr | Novagen | |
| pCDFDuet | CloDE13 ori, Strr | Novagen | |
| pGEX | f1 ori, Ampr | GE Healthcare | |
| Constructs | |||
| pC-PcAAS | pCDFDuet carrying aromatic aldehyde synthase (AAS) from Petroselinum crispum | This study | |
| pC-PcAAS-HpaBC | pCDFDuet carrying aromatic aldehyde synthase (AAS) from and P. crispum and HpaBC from Escherichia coli | This study | |
| pE-HpaBC | pETDuet carrying HpaBC from E. coli | This study | |
| pC-TDC-TYO | pCDFDuet carrying TDC from Papaver somniferum and TYO from Micrococcus luteus | This study | |
| Strains | |||
| BL21 (DE3) | F− ompT hsdS B(rB − mB −) gal dcm lon (DE3) | Novagen | |
| B-TP | BL21(DE3) ΔtyrR::FRT- ΔPheA::FRT-kan R -FRT | 36 | |
| B-TPF | BL21(DE3) ΔtyrR::FRT- ΔPheA:: ΔfeaB-FRT FRT-kan R -FRT | This study | |
| B-TY1 | BL21 (DE3) harboring pC-PcAAS | This study | |
| B-TY2 | B-TP harboring pC-PcAAS | This study | |
| B-TY3 | B-TPF harboring pC-PcAAS | This study | |
| B-TY4 | BL21 (DE3) harboring pC-TDC-TYO | This study | |
| B-SAL1 | BL21 (DE3) harboring pC-PcAAS and pG-AtUGT85A1 | This study | |
| B-SAL2 | B-TPF harboring pC-PcAAS and pG-AtUGT85A1 | This study | |
| B-HTY1 | BL21 (DE3) harboring pC-PcAAS-HpaBC | This study | |
| B-HTY2 | BL21 (DE3) harboring pC-PcAAS and pE-HpaBC | This study | |
| B-HTY3 | B-TPF harboring pC-PcAAS-HpaBC | This study | |
Figure 3.
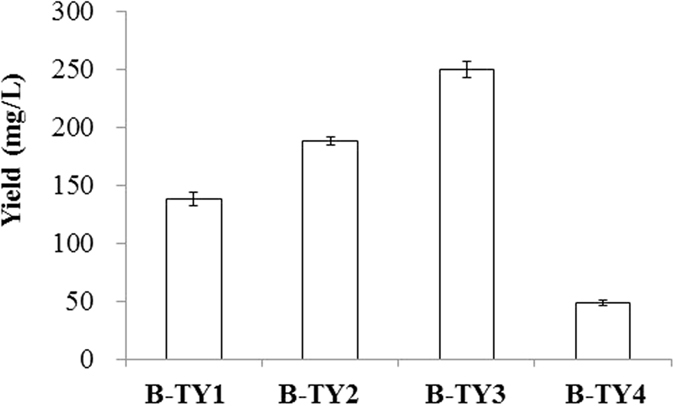
Effect of different E. coli strain on the production of tyrosol. B-TY1, wild type harboring AAS; B-TY2, tyrR and pheA deletion mutant harboring AAS; B-TY3, tyrR, pheA, and feaB deletion mutant harboring AAS; B-TY4, wild type harboring TDC and TYO.
Strain B-TY3 was used to synthesize tyrosol. The optimal culturing temperature and the initial cell concentration were determined. Tyrosol production at 30 °C in B-TY3 was better than that at 25 °C or 37 °C. The production at 25 °C and 37 °C was 57.7% and 20.4% of that at 30 °C, respectively. The initial cell concentration was tested at OD600 = 0.5, 1, 1.5, 2. 2.5, and 3. The production tyrosol at OD600 = 0.5 was highest, and production declined with increasing cell concentrations. Using the optimized incubation time and cell concentration of B-TY3, the production of tyrosol was monitored for 48 h. The production of tyrosol continued to increase until 36 h, at which 539.4 mg/L tyrosol was produced (Fig. 4). After 36 h, tyrosol production did not increase.
Figure 4.
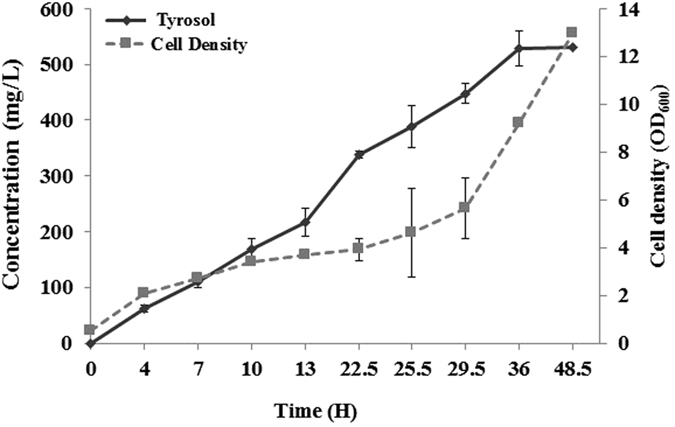
Production of tyrosol using strain B-TY3 after optimization of incubation time and cell concentration.
Synthesis of salidroside in E. coli
We attempted to synthesize salidroside in E. coli. Salidroside is 2-(4-hydroxyphenyl) ethyl β-D-glucopyranoside. In order to synthesize salidroside from tyrosol, a uridine-dependent glycosyltransferase (UGT), which transfers glucose from UDP-glucose to an acceptor molecule such as tyrosol, was needed. We screened 12 UGTs from A. thaliana to identify a UGT that synthesizes salidroside from tyrosol. These 12 UGTs are known to transfer a glucose group from UDP-glucose to small compounds such as hydroxycinnaamtes like p-coumaric acid, caffeic acid, and ferulic acid20–23 and monoterpenoids like geraniol and perillyl alcohol24. We transformed E. coli with each UGT and each transformant was supplemented with tyrosol. E. coli strains harboring AtUGT73C5, AtUGT73C6, or AtUGT85A1, yeilded a product that had the same retention time as a standard of salidroside (Fig. 1S). Among these, AtUGT85A1 produced more salidroside than did the others. Therefore, we used AtUGT85A1 for salidroside synthesis.
To synthesize salidroside from glucose, we transformed E. coli with both PcAAS and AtUGT84A1. The resulting transformant, B-SAL1, was used for the production of salidroside. The analysis of the B-SAL1 culture filtrate using HPLC revealed a peak with the same retention time as salidroside (Fig. 5). The tyrosol was not observed, indicating that tyrosol was converted into salidroside as soon as it was produced. The structure of the reaction product was determined to be salidroside by NMR.
Figure 5.
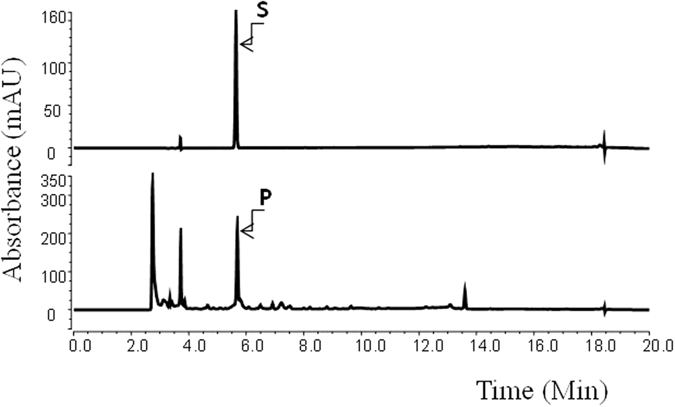
Production of salidroside using strain B-SAL1. S, standard salidroside; P, reaction product.
The synthesis of tyrosol was higher in strain B-TPF. We used B-TPF to synthesize salidroside. The wild type strain (B-SAL1 in Table 1) produced approximately 54.8 mg/L salidroside while the B-TPF strain (B-SAL2 in Table 1) produced 165.8 mg/L, approximately 3-fold more. The optimized reaction time and the initial cell concentration using B-SAL2 were determined to be 25 °C at OD600 = 5. Using the optimized incubation temperature and cell concentration, the synthesis of salidroside using B-SAL2 was monitored. Until 8.5 h, both tyrosol and salidroside had accumulated, and after which, the accumulation of saldroside continued to increase until 48 h, at which approximately 287.9 mg/L salidroside was synthesized (Fig. 6). But, tyrosol was converted into salidroside immediately after it was formed. Tyrosol was not observed at the end of the reaction.
Figure 6.
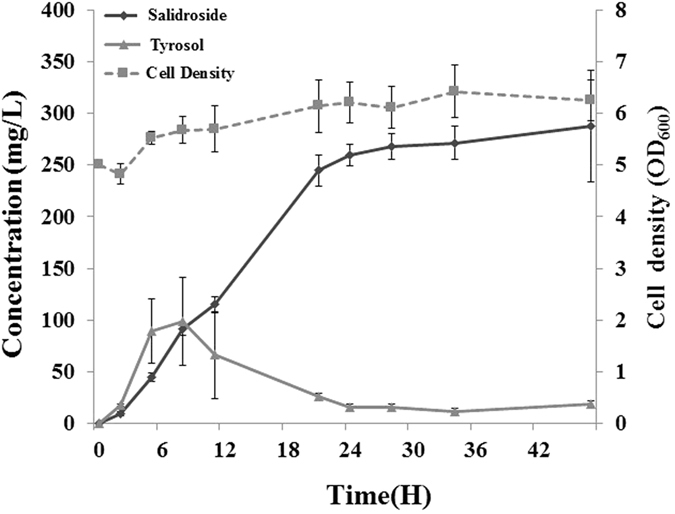
Production of salidroside using strain B-SAL2 after optimization of incubation time and cell concentration.
Synthesis of hydroxytyrosol in E. coli
Hydroxytyrosol can also be synthesized from tyrosol by hydroxylation. We tested the HpaBC gene, encoding 4-hydroxyphenylacetate 3-hydroxylase, from E. coli 25 and Sam5 from Saccharothrix espanaensis 26 in order to convert tyrosol into hydroxytyrosol. HpaBC and Sam5 were used to modify other phenolic compounds such as p-coumaric acid, tyrosine, and flavonoids27–29. The HpaBC or Sam5 genes were coexpressed with PcAAS in E. coli, and each transformant was tested for the production of hydroxytyrosol. The transformant harboring HpaBC and PcAAS produced more hydroxytyrosol than that harboring both Sam5 and PcAAS (data not shown). Therefore, we used HpaBC for the synthesis of hydroxytyrosol.
We made two constructs and independently transformed them into E. coli, independently. The strain B-HTY1 harbored a single construct containing both PcAAS and HpaBC and the strain B-HTY2 harbored two separate constructs, one with PcAAS and the other with HpaBC. We compared the production of hydroxytyrosol. HPLC analysis of culture filtrates from both strains revealed a new peak which had a different retention time with tyrosol. NMR analysis of this peak revealed that the reaction product was hydroxytyrosol. B-HTY1 produced 80.3 mg/L of hydroxytyrol but B-HTY2 produced only 16.6 mg/L, indicating that the strain harboring the single construct produced more tyrosol.
We used strain B-TPF to produce hydroxytyrsol because this strain produced more tyrosol than E. coli BL21(DE3). The strain B-HTY3 produced 116.7 mg/L tyrosol, more than B-HTY1 (80.3 mg/L) did. Using B-THY3, the optimized initial cell concentration and the incubation temperature were determined to be OD600 = 1.0 and 25 °C, respectively. Using the optimized cell concentration and the incubation time, we monitored the production of hydroxytyrosol. As shown in Fig. 7, production of hydroxytyrosol was initially observed after 3 h, and production continued to increase until 30 h, after which 208 mg/L hydroxytyrosol was synthesized. Tyrosol was observed until 5 h at less than 5 mg/L, indicating that tyrosol was converted into hydroxytyrosol as soon as it was produced.
Figure 7.
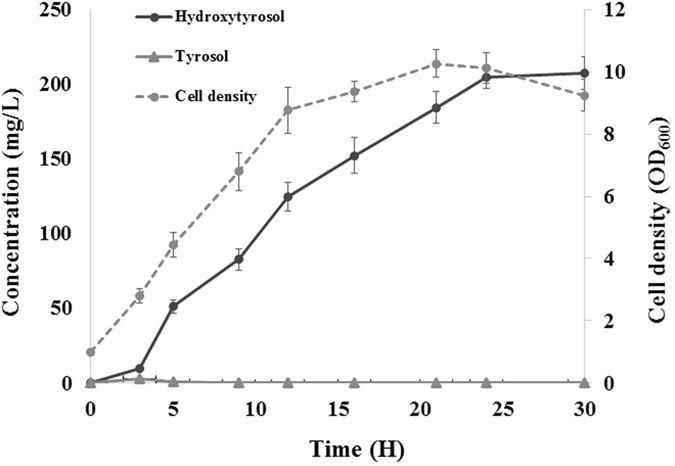
Production of hydroxytyrosol using strain B-THY3 after optimization of incubation time and cell concentration.
Discussion
Tyrosol has been synthesized in E. coli using TDC and TYO 14 or using Aro10 from yeast13. Hydroxytyrosol was also synthesized using TH, DDC and TYO 30 and salidroside was synthesized using Aro10 and UGT73B6 12. The protein encoded by Aro10 uses 4-hydroxypyruvate as a substrate to synthesize 4-HPAA, which undergoes further reduction to make tyrosol. Aro10 does not use tyrosine but 4-hydroxypyruvate as an intermediate for the synthesis of tyrosine. Therefore, they not only used E. coli mutants but also overexpressed several genes of the shikimate pathway of E. coli. AAS, which exhibits both tyrosine decarboxylase and deaminase activity, converts tyrosine into 4-HPAA and then the endogenous E. coli reductase converts it into tyrosol. AAS replaces the activity of both TDC and TYO, and is therefore, an efficient way to synthesize tyrosol from tyrosine in E. coli. This is the first report that AAS from plants could synthesize tyrosol as well as hydroxytyrosol and salidroside. We were able to synthesize 531 mg/L tyrosol without overexpressing other genes to increase the content of tyrosine in the cell.
For the synthesis of salidroside, the selection of UGT was critical. We screened 12 UGTs from A. thaliana and found AtUGT85A1 as the best UGT for the synthesis of salidroside. AtUGT85A1 is involved in the regulation of the plant hormone trans-zeatin through O-glucosylation31. It was surprising that AtUGT85A1 also could glucosylate tyrosol to salidroside. Some plant UGTs exhibited substrate promiscuity32. Therefore, AtUGT85A1 was capable of utilize diverse sugar acceptors including trans-zeatin, tyrosol, hydroxytyrosol, and 4-hydroxy benzoic acid (data not shown). However, unlike UGT73B6 from Rhodiola 13, which was previously used for the synthesis of salidroside, AtUGT85A1 exhibited regioselectivity; it did not transfer glucose to the phenolic hydroxyl group to produce icariside. Therefore, the final yield of salidroside was 288 mg/L, which was much higher than that of previous report12.
We synthesized tyrosol using E. coli harboring TDC and TYO and compared the final yield to that of E. coli harboring AAS. The yield of tyrosol using TDC and TYO (49.2 mg/L) was lower than that using AAS (138.9 mg/L). Therefore, we used AAS gene to synthesize tyrosol. When hydroxytyrosol was fed to E. coli harboring AtUGT85A1, most of hydroxytyrosol was converted into hydroxysalidroside. This indicated that AtUGT85A1 could use hydroxytyrosol as a substrate. However, when hydroxysalidroside was synthesized using B-HTY3 harboring AtUGT85A1, only a small amount of hydroxysalidroside was synthesized and more hydroxytyrosol remained. Taken together, it appears that the reaction intermediate(s) inhibits the glycosyltransferase reaction. In particular, the byproduct from the reaction of oxygenase (during salidroside synthesis: TYO; during hyroxysalidroside synthesis: HpaBC) appears to inhibit the UGT acitivity. On the other hand, production of salidroside using Aro10 and UGT73B6 yielded only 56.9 mg/L salidroside while 764.6 mg/L tyrosol remained13. Therefore, selection of UGT having high activity as well as the presence of a UGT inhibitor, was critical to the final yield of salidroside.
Materials and Methods
Constructs and E. coli strains
The AAS genes from A. thaliana, P. hybrid, and P. crispum were cloned using reverse transcription polymerase chain reaction (RT-PCR). RNA was isolated from parsley purchased from a local market using the Plant Total RNA Isolation Kit (Qiagen, Velno, Netherlands). cDNA was synthesized as previously described before33. Primers for cloning AAS were synthesized based on the published parsley AAS sequnence (GenBank accession number: M96070.1): 5′-ATGGATCCGATGGGCTCCATCGATAATCTT-3′ (BamHI site is underlined.) and 5′-ATGCGGCCGCTTATGATAATACTTCCACGA-3′ (NotI site is underlined.); A. thaniana AAS gene (AT2G20340.1) 5′-ATGGATCCGATGGAAAATGGAAGCGGGAAG-3′ (BamHI site is underlined.) and 5′-ATGCGGCCGCTTACTTGTGAAGCAAGTAAG-3′ (NotI site is underlined.): P. hybrid AAS gene (GenBank accession number: DQ243784.1) 5′-ATGAATTCGATGGATACTATCAAAATCAACCCA-3′ (EcoRI site is underlined.) and 5′-ATGCGGCCGCCTACGCATTCAGCATCATAGTT-3′ (NotI site is underlined.). Each AAS gene was subcloned into the corresponding sites of the pCDF-Duet1 vector.
Tyrosine decarboxylase (TDC from Papaver somniferum; GenBank U08598.1), and tyrosine oxidase (TYO from Micrococcus luteus; GenBank AB010716.1) were synthesized after codon optimization using the published nucleotide sequences (Figs 2S and 3S). TDC was subcloned into BamHI/HindIII site of pCDF-Duet1 vector, and the resulting construct was pC-TDC. TYO was introduced into the second cloning site (NdeI/XhoI) of pC-TDC and the resulting constructs was called pC-TDC-TYO.
Twelve UGTs (AtUGT71C1 [At2g29750], AtUGT71C2 [At2g29740], AtUGT72B1 [At4g01070], AtUGT72E2 [At5g66690], AtUGT73C1[At2g36750], AtUGT73C3 [At2g36780], AtUGT73C5 [At2g36800], AtUGT73C6 [At2g36790], AtUGT76D1 [At2g26480], AtUGT76E2 [At5g59590], AtUGT76E12 [At3g46660], AtUGT85A1 [At1g22400]) from A. thaliana were subcloned into the pGEX 5X-3 vector.
The list of constructs and E. coli strains used in this study can be found in Table 1.
Production of tyrosol, hydroxytyrosol, and salidroside in E. coli
For the synthesis of tyrosol, hydroxytyrosol, and salidroside, E. coli was grown in LB medium containing 50 μg/mL appropriate antibiotics at 37 °C for 18 hr. The seed culture was inoculated into a fresh LB medium containing antibiotics and incubated at 37 °C until OD600 = 1.0. Cells were harvested by centrifugation, washed once with M9 medium. For the initial screening of AAS gene, the cell was resuspended with M9 containing 2% glucose, 50 μg/mL antibiotics, 1 mM IPTG, and 100 μM. For the synthesis of tyrosol, hydroxytyrosol, and salidroside, cells were resuspended with M9 containing 2% glucose, 50 μg/mL antibiotics, 1 mM IPTG, and 0.1% yeast extract. The cell density was adjusted to OD600 = 1.0. The resulting culture was incubated at 30 °C for 24 h. To detect tyrosol and hydroxytyrosol production, the culture was extracted with ethylacetate and the organic layer was collected after centrifugation and evaporated to dryness. The remaining reaction product was dissolved with dimethyl sulfoxide (DMSO) and analyzed by Thermos high performance liquid chromatography (HPLC). To examine the production of salidroside, the culture was boiled for 3 min and then centrifuged. The supernatant was filtered using 0.45 μm syringe filter (Millipore, Billerica, MA, USA) and analyzed by HPLC.
To analyze the formation of tyrosol, hydroxytyrosol, and salidroside by HPLC, the mobile phase was composed of water (solution A) and acetonitrile (solution B), which were combined with 0.1% formic acid. The elution program was as follows: the proportion of solution B was gradually increased from 10% to 40% over 8 min, increased to 90% over 4 min, and then maintained over 3 min. Finally, the proportion of solution B was rapidly decreased to 10% and maintained at that composition for 5 min. The flow rate wass 1 ml/min.
The structure of reaction product was determined using nuclear magnetic resonance (NMR) spectroscopy34, 35 (NMR spectra was provided in the Figs 4S, 5S, and 6S). The NMR data were as follows; Tyrosol, 1 H NMR (400 MHz, CDCl3): δ 7.10 (d, J = 8.3 Hz, 2 H), 6.78 (d, J = 8.3 Hz, 2 H), 3.83 (t, J = 6.5 Hz, 2 H), 2.80 (t, J = 6.5 Hz, 2 H).
Salidroside, 1H NMR (400 MHz, DMSO-d 6) δ 7.04 (d, J = 8.3 Hz, 2 H), 6.67 (d, J = 8.3 Hz, 2 H), 4.17 (d, J = 8.5 Hz, 1 H), 3.87 (dd, J = 16.0, 8.6 Hz, 1 H), 3.67 (d, J = 11.4 Hz, 1 H), 3.56 (dd, J = 16.0, 8.8 Hz, 1 H), 3.44 (dd, J = 11.4, 5.4 Hz, 1 H), 3.15 (t, J = 8.5 Hz, 1 H), 3.07 (m, 1 H), 3.06 (d, J = 8.5 Hz, 1 H), 2.96 (t, J = 8.5 Hz, 1 H), 2.73 (m, 2 H); 13C NMR (100 MHz, DMSO-d 6) δ 155.6, 129.7, 128.6, 115.0, 102.8, 76.8, 76.7, 73.4, 70.0, 69.9, 61.0, 34.8.
Hydroxytyrosol, 1H NMR (400 MHz, MeOD): δ 6.68 (d, J = 8.0 Hz, 1 H), 6.66 (d, J = 1.9 Hz, 1 H), 6.53 (dd, J = 8.0, 2.0 Hz, 1 H), 3.68 (t, J = 7.2 Hz, 2 H), 2.66 (t, J = 7.2 Hz, 2 H).
Electronic supplementary material
Acknowledgements
This work was supported by a grant (NRF-2016R1A2B4014057), and Priority Research Centers Program (2009-0093824) through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology, Republic of Korea.
Author Contributions
J.H.A. designed and initiated the experiments. D.C. and S.Y.K. conducted experiments. D.C., S.Y.K., and J.H.A. analyzed data and wrote manuscripts. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Daeun Chung and So Yeon Kim contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02042-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stark AH, Madar Z. Olive oil as a functional food: epidemiology and nutritional approaches. Nutr. Rev. 2002;60:170–176. doi: 10.1301/002966402320243250. [DOI] [PubMed] [Google Scholar]
- 3.St-Laurent-Thibault C, Arseneault M, Longpré F, Ramassamy C. Tyrosol and hydroxytyrosol, two main components of olive oil, protect N2a cells against amyloid-β-induced toxicity. Involvement of the NF-κB signaling. Curr. Alzheimer. Res. 2011;8:543–551. doi: 10.2174/156720511796391845. [DOI] [PubMed] [Google Scholar]
- 4.Yousef GG, et al. Comparative phytochemical characterization of three Rhodiola species. Phytochemistry. 2006;67:2380–2391. doi: 10.1016/j.phytochem.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Cifani C, et al. Effect of salidroside, active principle of Rhodiola rosea extract, on binge eating. Physiol. Behav. 2010;101:555–562. doi: 10.1016/j.physbeh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo DR, Occhiuto F, Spadaro F, Circosta C. Rhodiola rosea extract protects human cortical neurons against glutamate and hydrogen peroxide-induced cell death through reduction in the accumulation of intracellular calcium. Phytother, Res. 2012;26:878–83. doi: 10.1002/ptr.3662. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, et al. Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem. Int. 2010;57:547–555. doi: 10.1016/j.neuint.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JK, et al. Protection by salidroside against bone loss via inhibition of oxidative stress and bone-resorbing mediators. PLoS One. 2013;8:e57251. doi: 10.1371/journal.pone.0057251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma LQ, et al. Effects of overexpression of endogenous phenylalanine ammonia-lyase (PALrs1) on accumulation of salidroside in Rhodiola sachalinensis. Plant Biol. (Stuttg) 2008;10:323–333. doi: 10.1111/j.1438-8677.2007.00024.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JX, et al. A tyrosine decarboxylase catalyzes the initial reaction of the salidroside biosynthesis pathway in Rhodiola sachalinensis. Plant Cell Rep. 2011;30:1443–1453. doi: 10.1007/s00299-011-1053-7. [DOI] [PubMed] [Google Scholar]
- 11.Qualley AV, et al. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc. Natl. Acad. Sci. USA. 2012;109:16383–16388. doi: 10.1073/pnas.1211001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma LQ, et al. Molecular cloning and overexpression of a novel UDP-glucosyltransferase elevating salidroside levels in Rhodiola sachalinensis. Plant Cell Rep. 2007;26:989–999. doi: 10.1007/s00299-007-0317-8. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, et al. Production of salidroside in metabolically engineered Escherichia coli. Sci. Rep. 2014;4:6640. doi: 10.1038/srep06640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh Y, et al. Engineering of a tyrosol-producing pathway, utilizing simple sugar and the central metabolic tyrosine, in Escherichia coli. J. Agric. Food. Chem. 2012;60:979–984. doi: 10.1021/jf203256f. [DOI] [PubMed] [Google Scholar]
- 15.Kaminaga Y, et al. Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation. J. Biol. Chem. 2006;281:23357–23366. doi: 10.1074/jbc.M602708200. [DOI] [PubMed] [Google Scholar]
- 16.Torrens-Spence M, et al. Biochemical evaluation of a parsley tyrosine decarboxylase results in a novel 4-hydroxyphenylacetaldehyde synthase enzyme. Biochem. Biophys. Res. Commun. 2012;418:211–216. doi: 10.1016/j.bbrc.2011.12.124. [DOI] [PubMed] [Google Scholar]
- 17.Torrens-Spence M, et al. Biochemical evaluation of the decarboxylation-deamination activities of plant aromatic amino acid decarboxylase. J. Biol. Chem. 2013;288:2376–2387. doi: 10.1074/jbc.M112.401752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lütke-Eversloh T, Stephanopoulos G. L-Tyrosine production by deregulated strains of Escherichia coli. Appl. Microbiol. Biotechnol. 2007;75:103–110. doi: 10.1007/s00253-006-0792-9. [DOI] [PubMed] [Google Scholar]
- 19.Juminaga D, et al. Modular engineering of L-tyrosine production in Escherichia coli. Appl. Environ. Microbiol. 2012;78:89–98. doi: 10.1128/AEM.06017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim EK, et al. Identification of glucosyltransferase genes involved in sinapate metabolism and lignin synthesis in Arabidopsis. J. Biol. Chem. 2001;276:4344–4349. doi: 10.1074/jbc.M007263200. [DOI] [PubMed] [Google Scholar]
- 21.Lim E-K, et al. The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and benzoates. J. Biol. Chem. 2002;277:586–592. doi: 10.1074/jbc.M109287200. [DOI] [PubMed] [Google Scholar]
- 22.Lim E-K, Higgins GS, Li Y, Bowles DJ. Regioselectivity of glucosylation of caffeic acid by a UDP-glucose:glucosyltransferase is maintained in planta. Biochem. J. 2003;373:987–992. doi: 10.1042/bj20021453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim E-K, Jackson RG, Bowles DJ. Identification and characterization of Araabidopsis glycosyltransferase capable of glucosylating coniferyl aldehyde and sinapyl aldehyde. FEBS Letters. 2005;579:2802–2806. doi: 10.1016/j.febslet.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Caputi L, Lim E-K, Bowles DJ. Discovery of new biocatalysts for the glycosylation of terpenoid scaffolds. Chem, Eur. J. 2008;14:6656–6662. doi: 10.1002/chem.200800548. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Sandvik ER. Chrarcterization of 4-hydroxyphenylacetate 3-hydroxylase (HpaB) of Escherichia coli as a reduced flavin adenine dinucleotide-utilizing monoxygenase. App. Environ. Microbiol. 2000;66:481–486. doi: 10.1128/AEM.66.5.2052-2056.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berner M, et al. Genes and enzymes involved in caffeic acid biosynthesis in the actinomycete Saccharothix espanaensis. J. Bacteriol. 2006;188:2666–2673. doi: 10.1128/JB.188.7.2666-2673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y, Yan Y. Biosynthesis of caffeic acid in Escherichia coli using its endogenous hydroxylase complex. Microbial. Cell Fact. 2012;11:42. doi: 10.1186/1475-2859-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H, Kim B-G, Ahn J-H. Production of bioactive hydroxyflavones by using monooxygenase from. Saccharothrix espanaensis. J. Biotech. 2014;176:11–17. doi: 10.1016/j.jbiotec.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Jones JA, et al. Optimization of naringenin and p-coumaric acid hydroxylation using the native E. coli hydroxylase complex, HpaBC. Biotechnol Prog. 2016;32:21–25. doi: 10.1002/btpr.2185. [DOI] [PubMed] [Google Scholar]
- 30.Satoh Y, et al. Engineering of L-tyrosine oxidation in Escherichia coli and microbial production of hydroxytyrosol. Met. Eng. 2012;14:603–610. doi: 10.1016/j.ymben.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Jin SH, et al. Overexpression of glucosyltransferase UGT85A1 influences trans-zeatin homeostasis and trans-zeatin responses likely through O-glucosylation. Planta. 2013;237:991–999. doi: 10.1007/s00425-012-1818-4. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, et al. Comparing the acceptor promiscuity of a Rosa hybrida glucosyltransferase RhGT1 and an engineered microbial glucosyltransferase OleD(PSA) toward a small flavonoid library. Carbohydr, Res. 2013;368:73–77. doi: 10.1016/j.carres.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Kim BG, et al. Cloning and expression of the isoflavone synthase gene (IFS-Tp) from. Trifolium pratense. Mol. Cells. 2003;15:301–306. [PubMed] [Google Scholar]
- 34.Yoon J-A, et al. Production of a novel quercetin glycoside through metabolic engineering of Escherichia coli. Appl. Env. Microbiol. 2012;78:4256–4262. doi: 10.1128/AEM.00275-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim BG, Kim HJ, Ahn J-H. Production of bioactive flavonoid rhamnosides by expression of plant genes in Escherichia coli. J. Agri. Food Chem. 2012;60:11143–11148. doi: 10.1021/jf302123c. [DOI] [PubMed] [Google Scholar]
- 36.Kim MJ, Kim B-G, Ahn J-H. Biosynthesis of bioactive O-methylated flavonoids in Escherichia coli. Appl. Microbiol. Biot. 2013;97:7195–7204. doi: 10.1007/s00253-013-5020-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


