Abstract
This study was proposed to compare the relative efficacy and tolerability of the second and third generation AEDs for refractory epilepsy. The 50% responder rate (RR) was selected as the efficacy outcome whereas the incidence of dizziness and somnolence were considered to evaluate the tolerability of AEDs. Odds ratio (OR) and their 95% credible interval (CrI) were obtained using a consistency model and surface under the cumulative ranking curve (SUCRA) value was calculated to rank AEDs. Topiramate appeared to be significantly more effective than placebo, eslicarbazepine acetate, perampanel, pregabalin, zonisamide, gabapentin and lamotrigine with respect to the 50% RR (all OR > 1). Patients who were managed by eslicarbazepine acetate, perampanel, oxcarbazepine, topiramate and pregabalin were more likely to suffer from dizziness compared to those who receive placebo (all OR > 1). Perampanel, topiramate and pregabalin were related to elevated risks of somnolence compared to placebo (all OR > 1). Moreover, topiramate ranked highest with respect to 50% RR (SUCRA = 0.968) whereas levetiracetam appeared to have balanced efficacy and tolerability (SUCRA = 0.769, 0.743, 0.604 and 0.659). In conclusion, topiramate was the most efficacious AED, while levetiracetam was able to provide patients with balanced efficacy and tolerability.
Introduction
Drug-resistant epilepsy (DRE), also called medically intractable epilepsy was defined as “a failure of adequate trials of two tolerated, appropriately chosen and used anticonvulsant drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom” according to the International League Against Epilepsy (ILAE)1 Approximately 1 million people in the United States continue suffering from seizures despite adequate treatment with anti-epileptic drugs (AEDs), accounting for 40% of patient amount2. DRE can be associated with developmental delay in infants and young children, and severe disability and morbidity in older children and adults, as well as a mortality rate 5–10 times that of the general population3. There is a prevalent assumption declaring that refractory epilepsy is developed from the onset, however, this assumption may not be very convincible and a large number of patients develop intractable epilepsy despite that they response well to AEDs in the early stages4.
AEDs are appropriate for patients with following features: at least two epileptic seizures; abnormal results obtained from imaging, neurological exam or electroencephalography (EEG); family histories of seizures5. The primary objective of using AEDs for epilepsy is to control unexpected seizures while minimizing the corresponding side effects resulted from AEDs. Compared with other options such as surgical procedures, implantable devices and dietary therapies, AEDs have several advantages including excellent oral absorption and bioavailability. Moreover, the second and third generation AEDs are of equivalent efficacy and safer than the older ones and have several significant advantages including reduced drug-drug interactions, less life-threatening adverse events and less negative impact on cognitive functions6. Despite of improved efficacy and tolerability, the second and third generation AEDs are still far away from the ideal ones which are able to fully control seizures without significant impact on the life quality of patients while being highly affordable7.
The choice of an appropriate AED is a matter with huge complexity and it is unlikely to predict whether a patient will have a favorable response to an AED merely based on clinical features or laboratory results8. Several factors should be taken into consideration for clinical decision-making: monotherapy or multidrug therapy; the corresponding side effects; drug-drug or drug-food interactions; the corresponding costs and availability; the ability of patients to endure and manage side effects. Since the number of AEDs approved by the FDA has been increased dramatically in the past 15 years, evaluating the available AEDs with different mechanisms are essential in clinical practices. Besides, the development of new drugs is costly and risky mainly due to the fact that we have incomplete knowledge about the resistance to AEDs9. Therefore, undertaking a system review and evidence synthesis may help clinicians and drug developers understand and improve various mechanisms of AEDs as well as the corresponding adverse effects which are often underestimated by clinicians.
Network meta-analysis (NMA) is an objective way of comparing alternative treatments where direct treatment comparisons have not been made by comparative effectiveness researches. In our review, we conducted a NMA of randomized control trials (RCTs) to evaluate twelve AEDs including eslicarbazepine acetate, levetiracetam, retigabine, tiagabine, vigabatrin, perampanel, oxcarbazepine, lamotrigine, topiramate, pregabalin, zonisamide and gabapentin with respect to their efficacy and tolerability for refractory epilepsy. Our paper aims to provide an up-to-date and comprehensive synthesis of direct and indirect evidence to guide clinical application of AEDs for DRE.
Material and Methods
Literature search
Firstly, we carried out a systematic review for our research topic by reviewing the following elements in the current literature: population, interventions, outcomes, inclusion criteria, data extraction, quality assessment and data analysis. Then, a thorough literature search was carried out in multiple sources including PubMed, Embase and Cochrane Library from inception to 11 March 2016. The entire literature search process was conducted by two independent reviewers and the corresponding results were reviewed by a third reviewer. A well-designed search strategy comprising of multiple keywords with respect to the above AEDs and their marketing names were input into online databases in order to retrieve relevant articles. Apart from that, we manually searched the corresponding reference lists of relevant articles and such an extensive search may increase precision and minimize the risk of small study effect, publication bias as well as selective reporting.
Inclusion criteria
The inclusion criteria were as follows: 1) the trial was RCT conducted on human subjects 2) the trial were focused on at least one AED including eslicarbazepine acetate, levetiracetam, retigabine, tiagabine, vigabatrin, perampanel, oxcarbazepine, lamotrigine, topiramate, pregabalin, zonisamide and gabapentin; 3) the diagnosis of epilepsy (partial seizure) was confirmed by brain CT scan, EEG, or MRI; 4) at least one efficacy or tolerability endpoint was assessed in the trial; 5) data can be extracted from studies to implement NMA. The primary outcome in our study is the 50% responder rate (RR) which is defined as the percentage of patients in the sample whose long term frequency of seizures was reduced by 50%. Endpoints with respect to tolerability included dizziness and somnolence which are commonly observed among patients. Screening of literatures was conducted by carefully reviewing their titles, abstracts as well as matching their contents with the corresponding selection criteria. Ineligible studies or duplicated studies were removed from the list prior to data extraction. Observational studies were also excluded from the eligible list.
Data extraction and synthesis
Each variable extracted from eligible studies was clearly defined earlier in order to accomplish our research purpose. A data extraction spreadsheet was used to extract relevant data from individual studies and this process was implemented by two independent reviewers. Data extraction results were compared and any disagreement was resolved by discussion. Missing data contained in the individual studies were not imputed in the data extraction spreadsheet. Data with respect to the same AED within the same study may be combined if they only differ in doses.
Statistical data analysis
Pairwise comparison between AEDs and placebo was visualized by forest plots and summary statistics such as odds ratios (ORs) together with their 95% confidence intervals (CIs) were used to assess the relative efficacy and tolerability of AEDs. Heterogeneity across individual studies was assessed by using the Cochran Q (Chi-squared) and Higgins I-squared statistics which quantified the percent of total variation due to between study heterogeneity10. Moreover, the implementation of NMA is based on the Bayesian Framework and non-informative prior probabilities were used in the Bayesian statistical approach. All statistical analysis and graphical procedures were conducted by R software (Version 3.2.4, The R Project for Statistical Computing) in conjunction with the GeMTC package. The random effect assumption was adopted in our NMA and the consistency model was generated from the GeMTC package. The competing AEDs were ranked based on their corresponding surface under the cumulative ranking curve (SUCRA) which indicates the cumulative probability that an AED is among the top n treatments and a cumulative ranking plots was created from R software in order to compliment the numerical summary of SUCRA. If there is clear evidence of inconsistency between direct evidence and indirect with respect to a specific comparison, then the consistency model was replaced by the inconsistency model in the GeMTC package.
Results
Basic characteristics of included studies and patients
According to the prior inclusion criteria, a total of 32 studies involving 7,658 patients were included in our NMA11–42. Details of flow chart were shown in Figure S1. Of the 32 trials, 30 were placebo-controlled, one was active-controlled (Zonisamide vs. Pregabalin) and one was a three-arm study (Pregabalin vs. Lamotrigine vs. Placebo). Detailed characteristics of included studies such as authors, publication year, mean age, AEDs, dose, efficacy or tolerability endpoint are displayed in Table 1. The detailed direct and indirect comparison with respect to each endpoint was illustrated by the network plot (Fig. 1) in which the node size was proportional to the number of subjects involved in each AED and the thickness of connected lines between two interventions was proportional to the number of comparisons between these two interventions. Patients included in this NMA all suffered from drug-resistant epilepsy, together with previous AED medication history. Besides, the seizure types were complicated, including simple partial, complex partial, secondarily generalized.
Table 1.
Basic characteristics of included clinical trials.
| Reference | Mean age | Intervention (N) | Dose* | 50%RR | Treatment adverse events | |
|---|---|---|---|---|---|---|
| Dizziness | Somnolence | |||||
| Faught, E.21 | 36.9 | Eslicarbazepine acetate(136); Placebo(45) | 200–600 mg/d | Yes | Yes | Yes |
| Gil-Nagel, A.25 | 36.4; 37.7 | Eslicarbazepine acetate(165); Placebo(87) | 800, 1200 mg/d | Yes | Yes | Yes |
| French, J. A.24 | 36.1; 34.4 | Eslicarbazepine acetate(250); Placebo(136) | 8, 12 mg/d | Yes | Yes | Yes |
| French, J. A.23 | 36.3; 35.6 | Eslicarbazepine acetate(267); Placebo(121) | 8, 12 mg/d | Yes | Yes | Yes |
| Elger, C.19 | 39.1; 37.0 | Eslicarbazepine acetate(300); Placebo(102) | 400–1200 mg/d | Yes | Yes | Yes |
| Appleton, R.11 | 8.5; 8.4 | Gabapentin(119); Placebo(128) | 600–1800 mg/d | Yes | Yes | Yes |
| Wu, X. Y.38 | 32.7; 32.8 | Levetiracetam(102); Placebo(100) | 1000–3000 mg/d | Yes | Yes | Yes |
| Zhou, B.42 | 28.2; 31.3 | Levetiracetam(13); Placebo(11) | 750 mg/d | Yes | ||
| Ben-Menachem, E.15 | 37.0; 36.0 | Levetiracetam(181); Placebo(105) | 3000 mg/d | Yes | Yes | |
| Shorvon, S. D.34 | 37.0 | Levetiracetam(212); Placebo(112) | 1000, 2000 mg/d | Yes | Yes | Yes |
| Xiao, Z.39 | 32.8; 32.5 | Levetiracetam(28); Placebo(28) | 3000 mg/d | Yes | Yes | Yes |
| Tsai, J. J.37 | 32.8; 31.7 | Levetiracetam(47); Placebo(47) | 1000–2000 mg/d | Yes | Yes | Yes |
| Betts, T.16 | 37.5; 35 | Levetiracetam(80); Placebo(39) | 6000 mg/d | Yes | Yes | Yes |
| French, J. A.22 | 38.8; 39.1 | Oxcarbazepine(245); Placebo(121) | 1200, 2400 mg/d | Yes | Yes | Yes |
| Barcs, G.12 | 34.5, 34.3 | Oxcarbazepine(519); Placebo(173) | 600, 1200, 2400 mg/d | Yes | Yes | Yes |
| Krauss, G. L.27 | 34.0; 33.4 | Perampanel(521); Placebo(185) | 2, 4, 8 mg/d | Yes | Yes | Yes |
| Lee, B. I.28 | 33.3; 35.1 | Pregabalin(119); Placebo(59) | 150–600 mg/d | Yes | Yes | Yes |
| Baulac, M.13 | 39.8; 39.4; 39.1 | Pregabalin(140); Lamotrigine(152); Placebo(141) | 300, 600 mg/d; 300, 400 mg/d | Yes | ||
| Brodie, M. J.19 | 37.6; 37.7 | Retigabine(359); Placebo(179) | 600, 900 mg/d | Yes | Yes | Yes |
| Kalviainen, R.26 | 36.4; 36 | Tiagabine(77); Placebo(77) | 12–30 mg/d | Yes | Yes | Yes |
| Privitera, M.30 | 35.5 | Topiramate(143); Placebo(47) | 600–1000 mg/d | Yes | Yes | Yes |
| Yen, D. J.40 | 31.4; 32.0 | Topiramate(23); Placebo(23) | 300 mg/d | Yes | Yes | |
| Sharief, M.33 | 35.4; 32.6 | Topiramate(23); Placebo(24) | 400 mg/d | Yes | Yes | |
| Tassinari, C. A.36 | 32.9 | Topiramate(30); Placebo(30) | 600 mg/d | Yes | Yes | Yes |
| Zhang, L.41 | 72.6; 73.9 | Topiramate(46); Placebo(40) | 200 mg/d | Yes | Yes | |
| Ben-Menachem, E.15 | 34.1; 32.0 | Topiramate(81); Placebo(52) | 400–800 mg/d | Yes | ||
| Faught, E.20 | 35.8; 34.2 | Zonisamide(118); Placebo(85) | 100, 200, 400 mg/d | Yes | Yes | Yes |
| Brodie, M. J.18 | 35.3; 36.5 | Zonisamide(229); Placebo(120) | 100–500 mg/d | Yes | Yes | Yes |
| Lu, Y.29 | 36.83; 29.81 | Zonisamide(53); Placebo(51) | 300–400 mg/d | Yes | Yes** | Yes** |
| Taghdiri, M. M.35 | 6.2; 5.9 | Zonisamide(61); Pregabalin(60) | 2–12 mg/kg/d; 5–15 mg/kg/d | Yes | ||
| Schmidt, D.32 | 36.2; 33.4 | Zonisamide(71); Placebo(68) | 400–1200 mg/d | Yes | Yes | Yes |
| Sackellares, J. C.31 | 35.6; 36.4 | Zonisamide(78); Placebo(74) | 400–600 mg/d | Yes | ||
*Dose for placebo not specified; **Numbers of patients are 52 and 50 for Zonisamide and placebo respectively.
Figure 1.
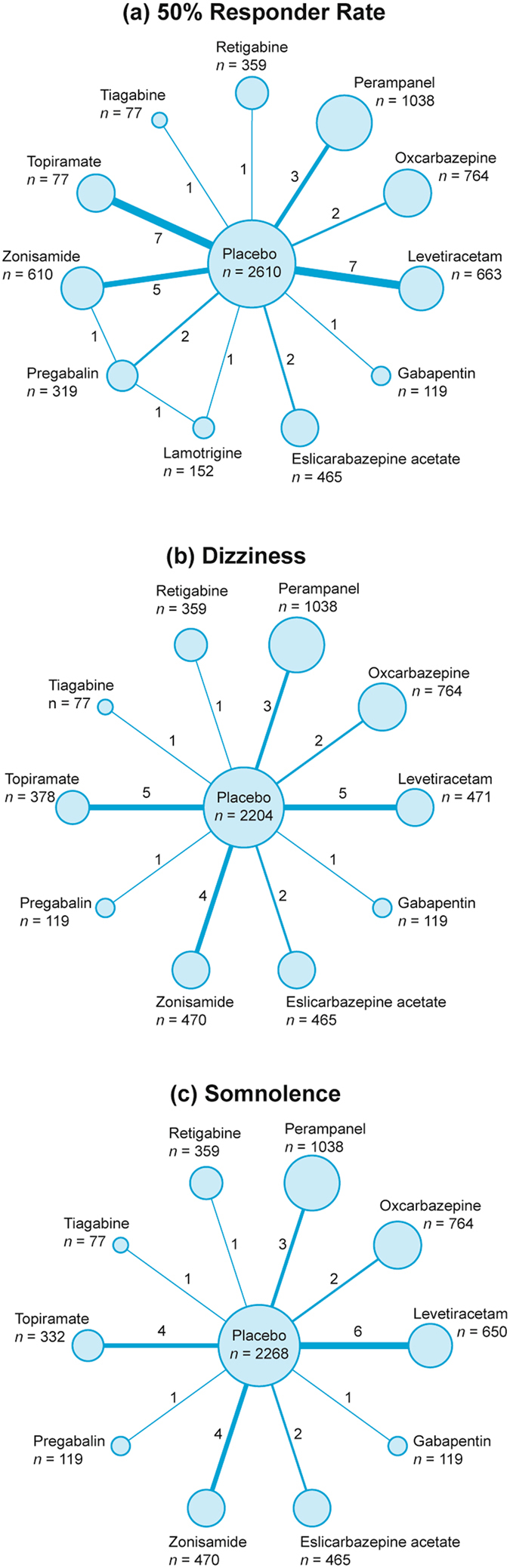
Network diagram for 50% responder rate, dizziness and somnolence.
Results of NMA with respect to efficacy
The relative efficacy of AEDs was evaluated by using the 50% RR which was defined as the proportion of patients whose long-term seizures had been reduced by at least 50%. As suggested by Table 2, several AEDs demonstrated their significant effectiveness in terms of reducing the incidence of long-term seizures: topiramate, perampanel, oxcarbazepine, levetiracetam, pregabalin and zonisamide compared with placebo (all OR > 1). Furthermore, topiramate (OR = 7.09, 95% CrI = 3.93–13.36) appeared to be far more effective than several AEDs including eslicarbazepine acetate, perampanel, pregabalin, zonisamide, gabapentin and lamotrigine (all OR > 1). Therefore, we suspected that topiramate might exhibit the highest efficacy compared to other AEDs with respect to seizure reduction (Table 2, Fig. 2).
Table 2.
NMA analysis results for 50% responder rate, dizziness and somnolence.
| Placebo | Esl. acetate | Levetiracetam | Retigabine | Tiagabine | Perampanel | Oxcarbazepine | Topiramate | Pregabalin | Zonisamide | Gabapentin | Lamotrigine | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50% RR | Placebo | 1.99 (0.83, 4.92) | 3.53 (2.12, 6.03) | 3.25 (0.99, 10.63) | 2.50 (0.54, 12.94) | 2.02 (1.01, 4.09) | 2.70 (1.16, 6.24) | 7.09 (3.93, 13.36) | 2.11 (1.01, 4.51) | 1.81 (1.06, 3.16) | 1.26 (0.37, 4.45) | 1.21 (0.40, 3.71) | |
| Eslicarbazepine acetate | 0.50 (0.20, 1.20) | 1.77 (0.64, 4.96) | 1.64 (0.37, 6.87) | 1.26 (0.21, 7.88) | 1.02 (0.33, 3.12) | 1.36 (0.40, 4.59) | 3.56 (1.23, 10.57) | 1.05 (0.34, 3.30) | 0.91 (0.33, 2.49) | 0.64 (0.14, 2.86) | 0.61 (0.14, 2.48) | ||
| Levetiracetam | 0.28 (0.17, 0.47) | 0.57 (0.20, 1.55) | 0.92 (0.25, 3.32) | 0.72 (0.14, 3.88) | 0.57 (0.24, 1.36) | 0.77 (0.29, 2.03) | 2.02 (0.89, 4.58) | 0.60 (0.24, 1.49) | 0.52 (0.24, 1.10) | 0.36 (0.09, 1.36) | 0.34 (0.10, 1.19) | ||
| Retigabine | 0.31 (0.09, 1.01) | 0.61 (0.15, 2.74) | 1.09 (0.30, 3.99) | 0.79 (0.11, 5.89) | 0.63 (0.16, 2.46) | 0.84 (0.20, 3.45) | 2.20 (0.60, 8.36) | 0.65 (0.16, 2.64) | 0.56 (0.15, 2.12) | 0.39 (0.07, 2.21) | 0.37 (0.07, 1.90) | ||
| Tiagabine | 0.40 (0.08, 1.85) | 0.79 (0.13, 4.67) | 1.39 (0.26, 7.12) | 1.27 (0.17, 9.02) | 0.80 (0.14, 4.31) | 1.06 (0.18, 6.13) | 2.83 (0.50, 14.79) | 0.83 (0.14, 4.63) | 0.72 (0.13, 3.74) | 0.50 (0.06, 3.70) | 0.47 (0.07, 3.22) | ||
| Perampanel | 0.49 (0.24, 0.99) | 0.98 (0.32, 3.01) | 1.75 (0.74, 4.24) | 1.60 (0.41, 6.37) | 1.24 (0.23, 7.31) | 1.34 (0.45, 3.87) | 3.50 (1.41, 9.08) | 1.03 (0.37, 2.95) | 0.89 (0.37, 2.18) | 0.63 (0.15, 2.58) | 0.60 (0.16, 2.25) | ||
| Oxcarbazepine | 0.37 (0.16, 0.87) | 0.74 (0.22, 2.50) | 1.30 (0.49, 3.51) | 1.20 (0.29, 4.93) | 0.94 (0.16, 5.71) | 0.75 (0.26, 2.23) | 2.63 (0.95, 7.49) | 0.78 (0.26, 2.47) | 0.67 (0.25, 1.83) | 0.47 (0.11, 2.08) | 0.45 (0.11, 1.83) | ||
| Topiramate | 0.14 (0.07, 0.25) | 0.28 (0.09, 0.81) | 0.50 (0.22, 1.12) | 0.46 (0.12, 1.67) | 0.35 (0.07, 2.00) | 0.29 (0.11, 0.71) | 0.38 (0.13, 1.05) | 0.30 (0.11, 0.76) | 0.26 (0.11, 0.59) | 0.18 (0.04, 0.72) | 0.17 (0.05, 0.60) | ||
| Pregabalin | 0.47 (0.22, 0.99) | 0.95 (0.30, 2.91) | 1.68 (0.67, 4.21) | 1.54 (0.38, 6.23) | 1.20 (0.22, 7.03) | 0.97 (0.34, 2.70) | 1.29 (0.40, 3.85) | 3.35 (1.31, 9.04) | 0.86 (0.37, 1.97) | 0.60 (0.14, 2.62) | 0.57 (0.19, 1.77) | ||
| Zonisamide | 0.55 (0.32, 0.94) | 1.10 (0.40, 3.05) | 1.94 (0.91, 4.13) | 1.78 (0.47, 6.61) | 1.40 (0.27, 7.71) | 1.12 (0.46, 2.74) | 1.49 (0.55, 4.03) | 3.91 (1.70, 8.94) | 1.16 (0.51, 2.67) | 0.70 (0.18, 2.75) | 0.67 (0.20, 2.23) | ||
| Gabapentin | 0.79 (0.22, 2.72) | 1.57 (0.35, 7.29) | 2.78 (0.73, 11.00) | 2.55 (0.45, 14.32) | 1.99 (0.27, 15.75) | 1.59 (0.39, 6.65) | 2.12 (0.48, 9.44) | 5.57 (1.39, 22.96) | 1.66 (0.38, 7.17) | 1.44 (0.36, 5.58) | 0.94 (0.18, 5.28) | ||
| Lamotrigine | 0.82 (0.27, 2.53) | 1.65 (0.40, 6.91) | 2.91 (0.84, 10.10) | 2.68 (0.53, 14.19) | 2.12 (0.31, 14.75) | 1.68 (0.44, 6.12) | 2.24 (0.55, 8.87) | 5.82 (1.66, 21.52) | 1.74 (0.57, 5.31) | 1.50 (0.45, 5.04) | 1.06 (0.19, 5.64) | ||
| Placebo | Esl. acetate | Levetiracetam | Retigabine | Tiagabine | Perampanel | Oxcarbazepine | Topiramate | Pregabalin | Zonisamide | Gabapentin | |||
| Dizziness | Placebo | 3.83 (1.21, 13.41) | 1.50 (0.68, 3.83) | 4.06 (0.93, 18.37) | 3.59 (0.72, 18.47) | 4.44 (1.93, 10.84) | 2.99 (1.02, 8.62) | 2.38 (1.07, 5.76) | 6.41 (1.24, 38.10) | 1.55 (0.64, 3.84) | 1.84 (0.17, 24.83) | ||
| Eslicarbazepine acetate | 0.26 (0.07, 0.82) | 0.40 (0.09, 1.75) | 1.07 (0.15, 6.92) | 0.92 (0.12, 7.15) | 1.16 (0.25, 4.93) | 0.79 (0.15, 3.72) | 0.62 (0.14, 2.67) | 1.67 (0.21, 13.95) | 0.40 (0.09, 1.80) | 0.48 (0.03, 7.98) | |||
| Levetiracetam | 0.67 (0.26, 1.48) | 2.52 (0.57, 11.34) | 2.70 (0.46, 14.47) | 2.37 (0.35, 14.52) | 2.95 (0.82, 9.53) | 1.99 (0.48, 7.47) | 1.59 (0.45, 5.11) | 4.29 (0.62, 28.74) | 1.03 (0.29, 3.39) | 1.22 (0.09, 19.26) | |||
| Retigabine | 0.25 (0.05, 1.08) | 0.94 (0.14, 6.65) | 0.37 (0.07, 2.16) | 0.89 (0.09, 8.03) | 1.10 (0.20, 6.02) | 0.74 (0.12, 4.56) | 0.59 (0.11, 3.39) | 1.59 (0.17, 14.56) | 0.38 (0.06, 2.22) | 0.45 (0.03, 8.96) | |||
| Tiagabine | 0.28 (0.05, 1.40) | 1.08 (0.14, 8.49) | 0.42 (0.07, 2.82) | 1.12 (0.12, 10.54) | 1.24 (0.21, 7.78) | 0.83 (0.12, 5.77) | 0.66 (0.11, 4.15) | 1.80 (0.17, 19.34) | 0.44 (0.07, 2.74) | 0.52 (0.03, 10.09) | |||
| Perampanel | 0.23 (0.09, 0.52) | 0.86 (0.20, 4.04) | 0.34 (0.10, 1.22) | 0.91 (0.17, 5.10) | 0.81 (0.13, 4.80) | 0.68 (0.16, 2.58) | 0.54 (0.16, 1.81) | 1.43 (0.22, 10.09) | 0.35 (0.10, 1.20) | 0.42 (0.03, 5.94) | |||
| Oxcarbazepine | 0.33 (0.12, 0.98) | 1.27 (0.27, 6.78) | 0.50 (0.13, 2.08) | 1.34 (0.22, 8.64) | 1.21 (0.17, 8.52) | 1.48 (0.39, 6.14) | 0.80 (0.21, 3.14) | 2.15 (0.31, 17.43) | 0.52 (0.13, 2.08) | 0.63 (0.04, 9.33) | |||
| Topiramate | 0.42 (0.17, 0.94) | 1.62 (0.37, 7.01) | 0.63 (0.20, 2.24) | 1.71 (0.29, 9.26) | 1.52 (0.24, 9.29) | 1.86 (0.55, 6.22) | 1.25 (0.32, 4.82) | 2.69 (0.41, 18.32) | 0.65 (0.19, 2.23) | 0.76 (0.06, 11.64) | |||
| Pregabalin | 0.16 (0.03, 0.81) | 0.60 (0.07, 4.78) | 0.23 (0.03, 1.61) | 0.63 (0.07, 5.95) | 0.56 (0.05, 5.86) | 0.70 (0.10, 4.52) | 0.46 (0.06, 3.24) | 0.37 (0.05, 2.45) | 0.24 (0.03, 1.57) | 0.29 (0.01, 6.88) | |||
| Zonisamide | 0.64 (0.26, 1.56) | 2.48 (0.56, 11.70) | 0.97 (0.29, 3.50) | 2.63 (0.45, 15.48) | 2.29 (0.36, 14.88) | 2.85 (0.83, 10.14) | 1.91 (0.48, 7.71) | 1.53 (0.45, 5.24) | 4.09 (0.64, 30.12) | 1.16 (0.09, 19.23) | |||
| Gabapentin | 0.54 (0.04, 6.03) | 2.10 (0.13, 31.45) | 0.82 (0.05, 11.06) | 2.23 (0.11, 39.58) | 1.94 (0.10, 35.04) | 2.41 (0.17, 31.79) | 1.60 (0.11, 22.91) | 1.32 (0.09, 16.93) | 3.49 (0.15, 74.27) | 0.86 (0.05, 10.62) | |||
| Somnolence | Placebo | 2.38 (0.81, 8.33) | 1.63 (0.90, 3.09) | 2.37 (0.63, 8.74) | 0.90 (0.20, 3.86) | 2.40 (1.13, 5.48) | 2.15 (0.84, 5.36) | 2.86 (1.26, 6.54) | 5.99 (1.12, 39.13) | 1.55 (0.70, 3.40) | 2.02 (0.41, 9.99) | ||
| Eslicarbazepine acetate | 0.42 (0.12, 1.23) | 0.67 (0.17, 2.35) | 1.00 (0.16, 5.13) | 0.37 (0.05, 2.21) | 1.01 (0.24, 3.90) | 0.88 (0.18, 3.71) | 1.18 (0.28, 4.73) | 2.52 (0.31, 21.05) | 0.65 (0.14, 2.46) | 0.85 (0.10, 5.44) | |||
| Levetiracetam | 0.61 (0.32, 1.12) | 1.48 (0.43, 5.74) | 1.44 (0.35, 6.21) | 0.56 (0.11, 2.70) | 1.48 (0.55, 4.05) | 1.30 (0.41, 3.89) | 1.74 (0.61, 4.82) | 3.69 (0.61, 26.51) | 0.95 (0.34, 2.55) | 1.23 (0.23, 6.60) | |||
| Retigabine | 0.42 (0.11, 1.59) | 1.00 (0.19, 6.34) | 0.70 (0.16, 2.87) | 0.38 (0.05, 2.61) | 1.01 (0.23, 4.75) | 0.91 (0.18, 4.41) | 1.20 (0.25, 5.68) | 2.55 (0.30, 25.17) | 0.65 (0.14, 2.96) | 0.85 (0.11, 6.39) | |||
| Tiagabine | 1.11 (0.26, 4.91) | 2.68 (0.45, 18.55) | 1.80 (0.37, 9.38) | 2.64 (0.38, 19.08) | 2.67 (0.51, 14.97) | 2.37 (0.42, 13.52) | 3.14 (0.58, 16.76) | 6.65 (0.73, 70.06) | 1.71 (0.33, 9.44) | 2.25 (0.25, 19.36) | |||
| Perampanel | 0.42 (0.18, 0.89) | 0.99 (0.26, 4.15) | 0.68 (0.25, 1.83) | 0.99 (0.21, 4.30) | 0.37 (0.07, 1.95) | 0.89 (0.25, 2.89) | 1.18 (0.36, 3.68) | 2.47 (0.38, 19.09) | 0.64 (0.21, 1.89) | 0.84 (0.14, 4.69) | |||
| Oxcarbazepine | 0.46 (0.19, 1.20) | 1.13 (0.27, 5.68) | 0.77 (0.26, 2.43) | 1.10 (0.23, 5.67) | 0.42 (0.07, 2.37) | 1.13 (0.35, 3.98) | 1.32 (0.39, 4.73) | 2.83 (0.41, 22.82) | 0.72 (0.22, 2.46) | 0.96 (0.15, 5.85) | |||
| Topiramate | 0.35 (0.15, 0.80) | 0.85 (0.21, 3.60) | 0.57 (0.21, 1.65) | 0.83 (0.18, 3.97) | 0.32 (0.06, 1.72) | 0.85 (0.27, 2.76) | 0.75 (0.21, 2.54) | 2.09 (0.32, 16.51) | 0.55 (0.17, 1.73) | 0.71 (0.11, 4.22) | |||
| Pregabalin | 0.17 (0.03, 0.89) | 0.40 (0.05, 3.18) | 0.27 (0.04, 1.64) | 0.39 (0.04, 3.31) | 0.15 (0.01, 1.37) | 0.41 (0.05, 2.64) | 0.35 (0.04, 2.41) | 0.48 (0.06, 3.12) | 0.25 (0.03, 1.66) | 0.34 (0.03, 3.14) | |||
| Zonisamide | 0.64 (0.29, 1.44) | 1.54 (0.41, 6.91) | 1.05 (0.39, 2.98) | 1.54 (0.34, 7.09) | 0.58 (0.11, 3.00) | 1.56 (0.53, 4.87) | 1.38 (0.41, 4.60) | 1.82 (0.58, 5.91) | 3.92 (0.60, 30.10) | 1.32 (0.21, 7.50) | |||
| Gabapentin | 0.49 (0.10, 2.42) | 1.18 (0.18, 9.62) | 0.81 (0.15, 4.43) | 1.17 (0.16, 8.97) | 0.44 (0.05, 4.01) | 1.19 (0.21, 7.22) | 1.04 (0.17, 6.75) | 1.41 (0.24, 8.82) | 2.98 (0.32, 36.22) | 0.76 (0.13, 4.67) |
Figure 2.
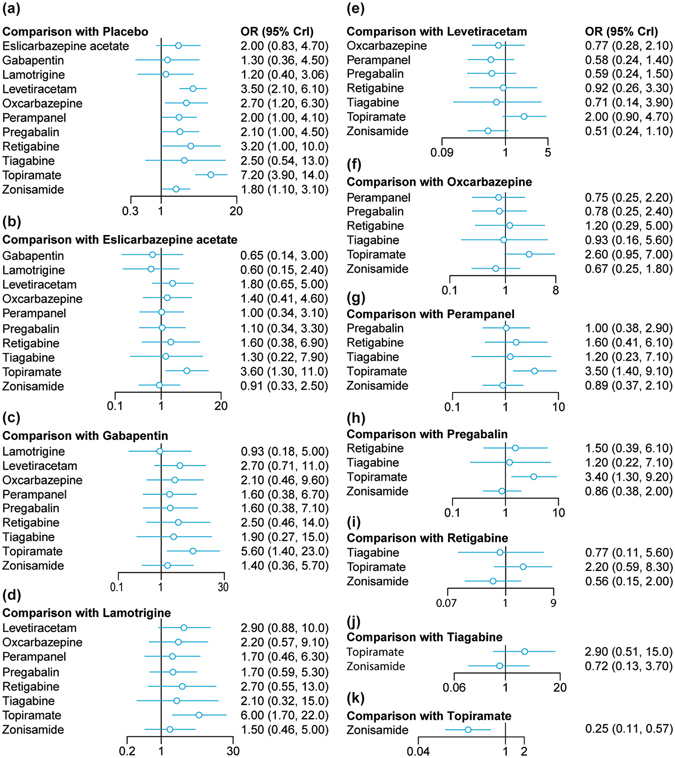
Forest plots for 50% responder rate.
Results of NMA with respect to adverse events
The incidence of three major adverse events including dizziness and somnolence were used to assess the tolerability of AEDs. As illustrated by Table 2 and Fig. 3, patients who were treated by eslicarbazepine acetate, perampanel, oxcarbazepine, topiramate and pregabalin were more likely to suffer from dizziness compared to those who receive placebo (all OR > 1). However, there was no significant difference in the risk of dizziness between any of the AEDs. Apart from that, patients who received perampanel, topiramate and pregabalin were at higher risks of suffering from somnolence compared to those who received placebo (all OR > 1; Table 2, Fig. 4).
Figure 3.
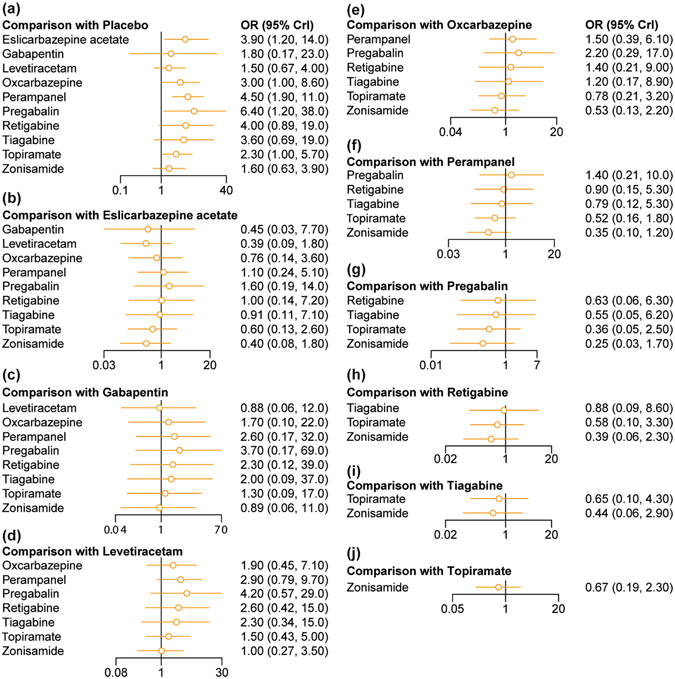
Forest plots for dizziness.
Figure 4.
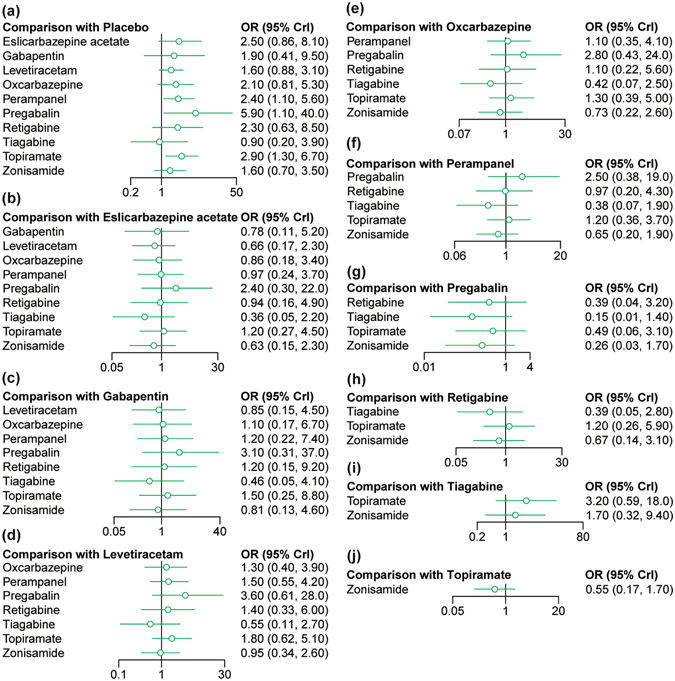
Forest plots for somnolence.
Ranks of AEDs by using SUCRA values
One distinctive advantage of NMA based on the Bayesian Framework is its ability to rank the corresponding interventions by using their corresponding SUCRA values (Fig. 5). A higher SUCRA value in efficacy index provides evidence that it outperforms others, while in adverse effect a higher SUCRA value suggests a low probability of the occurrence of side effect. Topiramate (SUCRA = 0.968), levetiracetam (SUCRA = 0.769) and retigabine (SUCRA = 0.693) appeared to have the highest, second and third SUCRA values except for placebo with respect to 50% RR. However, lamotrigine appeared to be the least efficacious AED due to its lowest SUCRA value (SUCRA = 0.220) with respect to 50% RR. Also, levetiracetam (SUCRA = 0.743) and zonisamide (SUCRA = 0.735) appeared to be more tolerable than other AEDs with respect to the incidence of dizziness. Apart from that, tiagabine (SUCRA = 0.822) and zonisamide (SUCRA = 0.636) appeared to be more favorable than others with respect to somnolence. In general, topiramate appeared to be the most effective AED for managing seizures and levetiracetam had a balanced efficacy and tolerability in comparison to other AEDs since its corresponding SUCRA values of different endpoints all ranks relatively high (Table 3).
Figure 5.
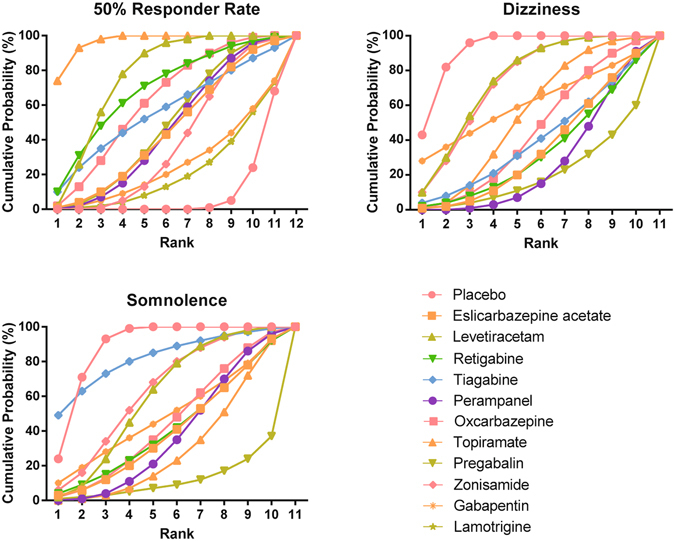
Cumulative probability diagram for 50% responder rate, dizziness, somnolence and headache.
Table 3.
SUCRA results for 50% responder rate, dizziness and somnolence.
| Treatment | 50%RR | Dizziness | Somnolence |
|---|---|---|---|
| Placebo | 0.089 | 0.921 | 0.887 |
| Eslicarbazepine acetate | 0.458 | 0.344 | 0.401 |
| Levetiracetam | 0.769 | 0.743 | 0.604 |
| Retigabine | 0.693 | 0.328 | 0.413 |
| Tiagabine | 0.566 | 0.393 | 0.822 |
| Perampanel | 0.465 | 0.264 | 0.376 |
| Oxcarbazepine | 0.628 | 0.446 | 0.450 |
| Topiramate | 0.968 | 0.543 | 0.298 |
| Pregabalin | 0.489 | 0.199 | 0.117 |
| Zonisamide | 0.392 | 0.735 | 0.636 |
| Gabapentin | 0.261 | 0.605 | 0.489 |
| Lamotrigine | 0.220 | — | — |
Discussion
Results from NMA indicated that topiramate might be more efficacious than other AEDs with respect to long-term seizures control. Meanwhile, DRE patients who were treated by levetiracetam, tiagabine or pregabalin were at lower risks of dizziness or somnolence. Apart from that, levetiracetam demonstrated the best combination of long-term efficacy and tolerability for DRE patients.
Topiramate is a new generation antiepileptic drug registered and introduced in 1995. It is usually applied in clinical practices as a monotherapy or polytherapy for managing DRE epilepsy43. Previous literature has indicated that topiramate has been widely used and seems to be far more effective than conventional anticonvulsants for its multiple effects on receptors and ion channels44–46. First of all, topiramate is able to block sodium-channel and may inhibit the process of synaptic conductance which is responsible for the transmission of epileptiform discharges47. Another key action of topiramate is its capability to impede excitatory glutamatergic transmission and thereby terminating seizure discharges44. Other actions of topiramate include the inhibition of calcium channels with high voltage activation as well as the inhibition of carbonic anhydrase activity that is linked with pH modulation48, 49. Furthermore, topiramate combined with conventional agents is likely to trigger an additional blockade of the sodium channel since it potentially interacts with other anticonvulsants to produce effects on protein binding50. Topiramate is characterized by linear pharmacokinetics, low protein binding and few active metabolites which result in fast absorption and oral bioavailability46. Preliminary data from long-term follow-up study also indicates that the efficacy of topiramate is more sustained in patients who experience localization-related epilepsy than in those who experience generalized epilepsy. All the above advantages of topiramate may explain its superiority over other AEDs with respect to the management of long-term seizures.
With respect to adverse effects, serious adverse effects associated with topiramate are rare but clinicians still have to be familiar with several mild to moderate adverse effects since these adverse effects particular for cognitive problems may result in the treatment discontinuation51, 52. Results from our NMA also suggested that epilepsy patients treated by topiramate were associated with significant increases in the risks of dizziness and somnolence compared to those who received placebo. However, cognitive adverse effects are not inevitable and tolerability of topiramate can be improved by setting low initial doses of 25 mg/day with slow titration53, 54.
Levetiracetam is another new AED for DRE. Our study has indicated that levetiracetam is the second most efficacious AED for control of long-term seizures and appears to have balanced efficacy and tolerability. A head-to-head comparison between levetiracetam and topiramate reveals that levetiracetam exhibits significantly higher retention rate and less side effects with equivalent efficacy compared with topiramate55. The above trend is strongly consistent with our NMA which indicated that topiramate was more efficacious than levetiracetam whereas the latter appeared to be far more tolerable with respect to the adverse events of dizziness and somnolence. Levetiracetam exhibits its unique antiepileptic mechanism by inhibiting high-voltage-activated Ca2+ currents in hippocampal neurones. Like topiramate, levetiracetam exhibits an excellent pharmacokinetic profile which is featured by rapid absorption through oral administration, linear pharmacokinetics, predominantly renal excretion and insignificant drug interactions56. These desirable properties enable levetiracetam to be suitable for treating epilepsy in children who usually require AEDs with high safety and tolerability profiles57. As suggested by animal studies, levetiracetam does not exhibit anticonvulsant activity against maximal electroshock seizures, while fully kindled seizures as well as the development of kindling can be effectively attenuated by levetiracetam57. Therefore, it may be useful in controlling seizures among patients who are susceptible to posttraumatic epilepsy58.
With respect to adverse effects, a study conducted by Neyens et al. indicated that levetiracetam as an adjunct AED did not have significant impairment on cognitive functions of chronic epilepsy patients who were treated by standard AEDs59. However, the use of levetiracetam may cause several behavioral adverse effects such as hostility and nervousness in children as suggested by previous trials60. Although levetiracetam is more tolerable than other AEDs, a cross-sectional study reveals that significantly lower bone mineral density (BMD) was presented in patients who are treated by levetiracetam and thereby suggesting an unfavorable effect of levetiracetam on bone health61. Therefore, levetiracetam should be selected with caution especially for infants and children. The recommended starting dose of levetiracetam for adults is 1000 mg/day, while the corresponding starting dose should be reduced to 250 mg at bedtime for those with higher risk of psychiatric adverse effects60. As mentioned earlier in our study, identifying factors that can be used to predict response to a specific AED such as levetiracetam can be very challenging and ongoing researches should be devoted to overcoming this challenge.
Our study also provided evidence that lamotrigine was relatively less efficacious with respect to the 50% RR in comparison to other AEDs. Lamotrigine is considered as a good initial monotherapy option for epilepsy patients, while this suggestion is not supported by our analysis potentially due to the fact that only one study comparing pregabalin, lamotrigine and placebo was incorporated in our analysis. Therefore, the lack of evidence and comparisons may conceal the true effectiveness of lamotrigine which has been demonstrated by retrospective studies. For instance, a retrospective study conducted by Arif et al. indicates that lamotrigine exhibits the highest retention rate (79%) as well as the highest seizure-free rate (54%) over a period of 12 months62. However, this retrospective study was carried out in older adults with epilepsy and hence the corresponding results may not be generalized to other populations. Besides that, the tolerability of lamotrigine with respect to dizziness and somnolence cannot be assessed by our NMA due to the lack of evidence. Thus, extensive systematic review should be conducted in the future in order to ascertain the relative efficacy and tolerability of lamotrigine.
Several limitations of our study should also be acknowledged. Firstly, our analysis is merely based on randomized trials and may produce inconsistent results as compared to those obtained from retrospective studies. Most of included RCTs were placebo-controlled experiment, lead to a lack of evidence for direct comparison between different treatments. The results of comparison between different treatments came from indirect evidence mostly hence it was hard to assess the consistency of the results. Besides, the selection of endpoints with respect to the efficacy and tolerability of AEDs may differ from those of other studies. For instance, one popular approach to evaluate the long term performance of AEDs is to assess their corresponding retention rates which reflect the efficacy, safety as well as the willingness of patients to continue treatment simultaneously. However, we are unable to cope with studies in which the retention rate is considered as the primary endpoint since it is unfeasible to interchange between the retention rate and 50% responder rate. Furthermore, randomized trials included in our study may be carried out in different populations and varied medication compliance or adherence may have significant influence on the long-term efficacy.
Conclusions
In conclusion, we conducted a methodologically and statistically rigorous analysis of second and third generation AEDs and indicated that topiramate appears to be the most efficacious AED and levetiracetam demonstrates balanced effectiveness and tolerability. Since the main objective of treating epilepsy with AEDs is to control seizure without significant side effects, our review may provide guidance for clinical decision-making and optimizes resource allocation. Also, the valid prediction of responses to AEDs should be proposed as the next step in this research area.
Electronic supplementary material
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Shandong Province (ZR2011HM023 to G. L.), the Science and Technology Project of Higher Education of Shandong Province (J10LF01 to G. L.), the Development of Medical Science, the Technology project of Shandong Province (2011HZ011 to G. L.), The key Project of Natural Science Foundation of Tianjin, China (17JCZDJC35700 to C.J.) and Found of Wenzhou Health Bureau (2013B28 to G.D.C).
Author Contributions
C.J.Z., R.H.J. and G.Y.L. performed the research; M.J.S., C.C. and G.D.C. analysed and interpreted the data; H.J.T., J.L., R.X. and D.G.J. designed the research study and wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Chuanjun Zhuo and Ronghuan Jiang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02525-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rong Xue, Email: yyc_dr@yeah.net.
Deguo Jiang, Email: fengsqing@126.com.
References
- 1.Kwan P, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 2.Kobau R, et al. Epilepsy surveillance among adults–19 States, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ. 2008;57:1–20. [PubMed] [Google Scholar]
- 3.Sperling MR, Barshow S, Nei M, Asadi-Pooya AA. A reappraisal of mortality after epilepsy surgery. Neurology. 2016;86:1938–1944. doi: 10.1212/WNL.0000000000002700. [DOI] [PubMed] [Google Scholar]
- 4.Sillanpaa M, Schmidt D. Natural history of treated childhood-onset epilepsy: prospective, long-term population-based study. Brain. 2006;129:617–624. doi: 10.1093/brain/awh726. [DOI] [PubMed] [Google Scholar]
- 5.Berg AT. Risk of recurrence after a first unprovoked seizure. Epilepsia. 2008;49(Suppl 1):13–18. doi: 10.1111/j.1528-1167.2008.01444.x. [DOI] [PubMed] [Google Scholar]
- 6.Mula M. Third generation antiepileptic drug monotherapies in adults with epilepsy. Expert Rev Neurother. 2016;16:1–6. doi: 10.1080/14737175.2016.1195264. [DOI] [PubMed] [Google Scholar]
- 7.Trinka E. Ideal characteristics of an antiepileptic drug: how do these impact treatment decisions for individual patients? Acta Neurol Scand Suppl. 2012;126:10–18. doi: 10.1111/ane.12015. [DOI] [PubMed] [Google Scholar]
- 8.Privitera M. Current challenges in the management of epilepsy. Am J Manag Care. 2011;17(Suppl 7):S195–203. [PubMed] [Google Scholar]
- 9.Perucca E, French J, Bialer M. Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol. 2007;6:793–804. doi: 10.1016/S1474-4422(07)70215-6. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Appleton R, et al. Gabapentin as add-on therapy in children with refractory partial seizures: a 12-week, multicentre, double-blind, placebo-controlled study. Gabapentin Paediatric Study Group. Epilepsia. 1999;40:1147–1154. doi: 10.1111/j.1528-1157.1999.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 12.Barcs G, et al. Oxcarbazepine placebo-controlled, dose-ranging trial in refractory partial epilepsy. Epilepsia. 2000;41:1597–1607. doi: 10.1111/j.1499-1654.2000.001597.x. [DOI] [PubMed] [Google Scholar]
- 13.Baulac M, et al. A comparison of pregabalin, lamotrigine, and placebo as adjunctive therapy in patients with refractory partial-onset seizures. Epilepsy Res. 2010;91:10–19. doi: 10.1016/j.eplepsyres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Menachem E. Clinical efficacy of topiramate as add-on therapy in refractory partial epilepsy: the European experience. Epilepsia. 1997;38(Suppl 1):S28–30. doi: 10.1111/j.1528-1157.1997.tb04514.x. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Menachem E, Falter U. Efficacy and tolerability of levetiracetam 3000 mg/d in patients with refractory partial seizures: a multicenter, double-blind, responder-selected study evaluating monotherapy. European Levetiracetam Study Group. Epilepsia. 2000;41:1276–1283. doi: 10.1111/j.1528-1157.2000.tb04605.x. [DOI] [PubMed] [Google Scholar]
- 16.Betts T, Waegemans T, Crawford P. A multicentre, double-blind, randomized, parallel group study to evaluate the tolerability and efficacy of two oral doses of levetiracetam, 2000 mg daily and 4000 mg daily, without titration in patients with refractory epilepsy. Seizure. 2000;9:80–87. doi: 10.1053/seiz.2000.0380. [DOI] [PubMed] [Google Scholar]
- 17.Brodie MJ, et al. Dose-dependent safety and efficacy of zonisamide: a randomized, double-blind, placebo-controlled study in patients with refractory partial seizures. Epilepsia. 2005;46:31–41. doi: 10.1111/j.0013-9580.2005.14704.x. [DOI] [PubMed] [Google Scholar]
- 18.Brodie MJ, et al. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology. 2010;75:1817–1824. doi: 10.1212/WNL.0b013e3181fd6170. [DOI] [PubMed] [Google Scholar]
- 19.Elger C, et al. Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures: a randomized, double-blind, placebo-controlled, parallel-group phase III study. Epilepsia. 2009;50:454–463. doi: 10.1111/j.1528-1167.2008.01946.x. [DOI] [PubMed] [Google Scholar]
- 20.Faught E, Ayala R, Montouris GG, Leppik IE. Randomized controlled trial of zonisamide for the treatment of refractory partial-onset seizures. Neurology. 2001;57:1774–1779. doi: 10.1212/WNL.57.10.1774. [DOI] [PubMed] [Google Scholar]
- 21.Faught E, et al. Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 200-, 400-, and 600-mg daily dosages. Topiramate YD Study Group. Neurology. 1996;46:1684–1690. doi: 10.1212/WNL.46.6.1684. [DOI] [PubMed] [Google Scholar]
- 22.French JA, Baroldi P, Brittain ST, Johnson JK. Efficacy and safety of extended-release oxcarbazepine (Oxtellar XR) as adjunctive therapy in patients with refractory partial-onset seizures: a randomized controlled trial. Acta Neurol Scand. 2014;129:143–153. doi: 10.1111/ane.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.French JA, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012;79:589–596. doi: 10.1212/WNL.0b013e3182635735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.French JA, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: Results of randomized global phase III study 305. Epilepsia. 2013;54:117–125. doi: 10.1111/j.1528-1167.2012.03638.x. [DOI] [PubMed] [Google Scholar]
- 25.Gil-Nagel A, et al. Efficacy and safety of 800 and 1200 mg eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures. Acta Neurol Scand. 2009;120:281–287. doi: 10.1111/j.1600-0404.2009.01218.x. [DOI] [PubMed] [Google Scholar]
- 26.Kalviainen R, et al. A double-blind, placebo-controlled trial of tiagabine given three-times daily as add-on therapy for refractory partial seizures. Northern European Tiagabine Study Group. Epilepsy Res. 1998;30:31–40. doi: 10.1016/S0920-1211(97)00082-X. [DOI] [PubMed] [Google Scholar]
- 27.Krauss GL, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78:1408–1415. doi: 10.1212/WNL.0b013e318254473a. [DOI] [PubMed] [Google Scholar]
- 28.Lee BI, et al. Pregabalin add-on therapy using a flexible, optimized dose schedule in refractory partial epilepsies: a double-blind, randomized, placebo-controlled, multicenter trial. Epilepsia. 2009;50:464–474. doi: 10.1111/j.1528-1167.2008.01954.x. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y, et al. Efficacy and safety of adjunctive zonisamide in adult patients with refractory partial-onset epilepsy: a randomized, double-blind, placebo-controlled trial. Clin Drug Investig. 2011;31:221–229. doi: 10.2165/11539750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Privitera M, et al. Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 600-, 800-, and 1,000-mg daily dosages. Topiramate YE Study Group. Neurology. 1996;46:1678–1683. doi: 10.1212/WNL.46.6.1678. [DOI] [PubMed] [Google Scholar]
- 31.Sackellares JC, et al. Randomized, controlled clinical trial of zonisamide as adjunctive treatment for refractory partial seizures. Epilepsia. 2004;45:610–617. doi: 10.1111/j.0013-9580.2004.11403.x. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt D, et al. Zonisamide for add-on treatment of refractory partial epilepsy: a European double-blind trial. Epilepsy Res. 1993;15:67–73. doi: 10.1016/0920-1211(93)90011-U. [DOI] [PubMed] [Google Scholar]
- 33.Sharief M, et al. Double-blind, placebo-controlled study of topiramate in patients with refractory partial epilepsy. Epilepsy Res. 1996;25:217–224. doi: 10.1016/S0920-1211(96)00029-0. [DOI] [PubMed] [Google Scholar]
- 34.Shorvon SD, et al. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group. Epilepsia. 2000;41:1179–1186. doi: 10.1111/j.1528-1157.2000.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 35.Taghdiri MM, et al. Comparative efficacy of zonisamide and pregabalin as an adjunctive therapy in children with refractory epilepsy. Iranian Journal of Child Neurology. 2015;9:49–55. [PMC free article] [PubMed] [Google Scholar]
- 36.Tassinari CA, et al. Double-blind, placebo-controlled trial of topiramate (600 mg daily) for the treatment of refractory partial epilepsy. Epilepsia. 1996;37:763–768. doi: 10.1111/j.1528-1157.1996.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 37.Tsai JJ, et al. Efficacy and safety of levetiracetam (up to 2000 mg/day) in Taiwanese patients with refractory partial seizures: a multicenter, randomized, double-blind, placebo-controlled study. Epilepsia. 2006;47:72–81. doi: 10.1111/j.1528-1167.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 38.Wu XY, et al. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in Chinese patients with refractory partial-onset seizures. Epilepsia. 2009;50:398–405. doi: 10.1111/j.1528-1167.2008.01729.x. [DOI] [PubMed] [Google Scholar]
- 39.Xiao Z, et al. Efficacy and safety of levetiracetam (3,000 mg/Day) as an adjunctive therapy in Chinese patients with refractory partial seizures. Eur Neurol. 2009;61:233–239. doi: 10.1159/000197109. [DOI] [PubMed] [Google Scholar]
- 40.Yen DJ, et al. A double-blind, placebo-controlled study of topiramate in adult patients with refractory partial epilepsy. Epilepsia. 2000;41:1162–1166. doi: 10.1111/j.1528-1157.2000.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, et al. Topiramate as an adjunctive treatment for refractory partial epilepsy in the elderly. J Int Med Res. 2011;39:408–415. doi: 10.1177/147323001103900208. [DOI] [PubMed] [Google Scholar]
- 42.Zhou B, et al. Effects of levetiracetam as an add-on therapy on cognitive function and quality of life in patients with refractory partial seizures. Epilepsy Behav. 2008;12:305–310. doi: 10.1016/j.yebeh.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Rudakova IG. [Pharmacotherapy of epilepsy: the use of topiramate in initial and additional treatment] Zh Nevrol Psikhiatr Im S S Korsakova. 2012;112:58–64. [PubMed] [Google Scholar]
- 44.Towne AR, et al. The use of topiramate in refractory status epilepticus. Neurology. 2003;60:332–334. doi: 10.1212/01.WNL.0000042783.86439.27. [DOI] [PubMed] [Google Scholar]
- 45.Watemberg N, et al. Clinical experience with open-label topiramate use in infants younger than 2 years of age. J Child Neurol. 2003;18:258–262. doi: 10.1177/08830738030180040901. [DOI] [PubMed] [Google Scholar]
- 46.Stojanova V, Rossetti AO. Oral topiramate as an add-on treatment for refractory status epilepticus. Acta Neurol Scand. 2012;125:e7–e11. doi: 10.1111/j.1600-0404.2011.01562.x. [DOI] [PubMed] [Google Scholar]
- 47.DeLorenzo RJ, Sombati S, Coulter DA. Effects of topiramate on sustained repetitive firing and spontaneous recurrent seizure discharges in cultured hippocampal neurons. Epilepsia. 2000;41(Suppl 1):S40–44. doi: 10.1111/j.1528-1157.2000.tb06048.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Velumian AA, Jones OT, Carlen PL. Modulation of high-voltage-activated calcium channels in dentate granule cells by topiramate. Epilepsia. 2000;41(Suppl 1):S52–60. doi: 10.1111/j.1528-1157.2000.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 49.Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia. 2000;41(Suppl 1):S35–39. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- 50.Garnett WR. Clinical pharmacology of topiramate: a review. Epilepsia. 2000;41(Suppl 1):S61–65. doi: 10.1111/j.1528-1157.2000.tb02174.x. [DOI] [PubMed] [Google Scholar]
- 51.Tatum WOt, et al. Postmarketing experience with topiramate and cognition. Epilepsia. 2001;42:1134–1140. doi: 10.1046/j.1528-1157.2001.41700.x. [DOI] [PubMed] [Google Scholar]
- 52.Bootsma HP, et al. Topiramate in clinical practice: long-term experience in patients with refractory epilepsy referred to a tertiary epilepsy center. Epilepsy Behav. 2004;5:380–387. doi: 10.1016/j.yebeh.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Dodson WE, et al. Topiramate titration to response: analysis of individualized therapy study (TRAITS) Ann Pharmacother. 2003;37:615–620. doi: 10.1345/aph.1C133. [DOI] [PubMed] [Google Scholar]
- 54.Faught E. Topiramate in the treatment of partial and generalized epilepsy. Neuropsychiatr Dis Treat. 2007;3:811–821. doi: 10.2147/NDT.S512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bootsma HP, et al. Long-term effects of levetiracetam and topiramate in clinical practice: A head-to-head comparison. Seizure. 2008;17:19–26. doi: 10.1016/j.seizure.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 56.Patsalos PN. Pharmacokinetic profile of levetiracetam: toward ideal characteristics. Pharmacol Ther. 2000;85:77–85. doi: 10.1016/S0163-7258(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 57.Pellock JM, et al. Pharmacokinetic study of levetiracetam in children. Epilepsia. 2001;42:1574–1579. doi: 10.1046/j.1528-1157.2001.41300.x. [DOI] [PubMed] [Google Scholar]
- 58.Loscher W, Honack D, Rundfeldt C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther. 1998;284:474–479. [PubMed] [Google Scholar]
- 59.Neyens LG, Alpherts WC, Aldenkamp AP. Cognitive effects of a new pyrrolidine derivative (levetiracetam) in patients with epilepsy. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:411–419. doi: 10.1016/0278-5846(95)00022-N. [DOI] [PubMed] [Google Scholar]
- 60.Abou-Khalil B. Levetiracetam in the treatment of epilepsy. Neuropsychiatr Dis Treat. 2008;4:507–523. doi: 10.2147/NDT.S2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beniczky SA, Viken J, Jensen LT, Andersen NB. Bone mineral density in adult patients treated with various antiepileptic drugs. Seizure. 2012;21:471–472. doi: 10.1016/j.seizure.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Arif H, et al. Comparative effectiveness of 10 antiepileptic drugs in older adults with epilepsy. Arch Neurol. 2010;67:408–415. doi: 10.1001/archneurol.2010.49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


