Abstract
Lymphomas involving the mediastinum occur in a wide age range and represent heterogeneous histological subtypes with various clinical symptoms and complex radiological findings. However, studies that describe the clinical and radiological features of different subtypes among Chinese pediatric and adult patients are limited. We analyzed the clinical, radiological and pathological features of 31 pediatric lymphomas involving the mediastinum, and compared them to the features of 21 adult patients. Although several histological subtypes were identified in adults, pediatric patients presented with T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia (T-LBL/T-ALL) and classical Hodgkin lymphomas (CHL) in 24 and 7 cases, respectively. Compared to adults, pediatric patients were more likely to be male (P = 0.089) and showed a higher incidence of T-LBL/T-ALL (P = 0.001), prevalence of dyspnea (P = 0.001), frequency of stage IV tumors (P = 0.008), and ratio of tumor diameter to maximum transthoracic diameter (P = 0.015). T-LBL/T-ALL patients presented with a higher frequency with stage IV disease (P = 0.000 and P = 0.001), compression of the blood vessels (P = 0.005 and P = 0.017), and pleural effusions (P = 0.001, for both) than CHL and PMBL patients. Compared to adults, pediatric patients with mediastinal lymphomas presented with exclusive histological subtypes of T-LBL/T-ALL and CHL, which showed distinctive characteristics of histological distribution, clinical presentation and radiological assessments.
Introduction
Lymphomas account for approximately 12% of total mediastinal tumors and represent heterogeneous histological subtypes1. Lymphomas involving the mediastinum exhibit a wide age range, occurring in both pediatric and adult patients2.
T-cell lymphoblastic lymphoma (T-LBL)/T-cell acute lymphoblastic leukemia (T-ALL) occurs most frequently in childhood but is also observed in adults3. Classical Hodgkin lymphoma (CHL) peaks at 15–35 years of age and in late life, and it usually presents with cervical lymphadenopathy. CHL patients often have mediastinal masses4. Primary mediastinal large B-cell lymphoma (PMBL), which arises from thymic medullary B-cells, typically occurs in young adults5. Mucosa-associated lymphoid tissue (MALT) lymphoma, diffuse large B cell lymphoma (DLBCL), and mediastinal gray-zone lymphoma have also been reported in adults and elderly individuals6, 7.
The clinical and radiological presentations of lymphomas involving the mediastinum are complex and nonsepcific8, 9. The initial and differential diagnoses of different histological subtypes are confused by clinicians and radiologists in some cases. Moreover, some subtypes demonstrate similar morphological features, representing serious challenges for pathological diagnosis7, 10. The clinical and radiological findings are useful to determine the appropriate staging and management.
Previous studies have focused on a single histological subtype, such as T-LBL/T-ALL or PMBL. However, detailed descriptions of clinicopathological and clinicoradiological comparisons between these subtypes are rare. Moreover, few studies have analyzed the clinicopathological features of different subtypes of lymphomas involving the mediastinum in pediatric patients11–13. Studies clarifying the differences in histological distribution, clinical spectrum, and radiological features between pediatric and adult patients are limited.
In this study, we conducted a retrospective analysis of the clinical, radiological, morphological, and immunophenotypical features of lymphomas involving the mediastinum, and we further compared the differences between pediatric and adult patients.
Results
Morphological and immunohistochemical findings
T-LBL/T-ALL, CHL, PMBL, DLBCL, and MALT lymphomas were identified in 31, 12, 6, 2, and 1 cases, respectively. The immunohistochemical results are shown in Supplementary Tables 1 and 2.
T-LBL/T-ALL were composed of small to medium-sized lymphoblasts with sparse cytoplasm and irregular nuclei. Tumor cells were commonly positive for TDT (21/26, 80.8%), CD3 (29/31, 93.5%), and CD7 (25/27, 92.6%), and they were variably positive for CD5 (16/24, 66.7%), CD99 (14/19, 73.7%), CD2 (12/18, 66.7%), and CD1a (13/21, 61.9%).
CHLs consisted of 11 cases of nodular sclerosis CHL (NSCHL) and 1 case of lymphocyte-rich CHL. NSCHL morphologically characterized in collagen bands surrounding nodules and Reed-Stenberg cells. CHL tumor cells were positive for PAX5 (10/12, 83.3%), CD30 (12/12, 100%), CD15 (9/12, 75.0%), CD20 (4/12, 33.3%) and CD79a (2/9, 22.2%).
PMBLs showed medium to large cells with round or lobulated nuclei and abundant cytoplasm. PMBLs expressed B-cell markers including PAX5 (5/5, 100%), CD20 (5/6, 83.3%) and CD79a (4/5, 80.0%). PMBL tumor cells were frequently positive for CD30 (3/5, 60.0%) and CD23 (4/5, 80.0%).
Systemic DLBCLs involving the mediastinum demonstrated the same pathological features to nodular DLBCLs. The MALT lymphoma was composed of small lymphocytes and monocytoid cells, which were positive for B-cell markers with low Ki-67 expression.
Clinical characteristics
The clinical characteristics of the 52 patients are summarized in Table 1. The comparison between pediatric and adult patients is shown in Table 2, and the comparisons among the main histological subtypes are shown in Table 3.
Table 1.
Clinical presentations of 52 cases with lymphomas involving the mediastinum.
| Case no. | Age (y)/Gender | Histological subtype | Symptom | LN involvement | LDH (U/L) | Stage | Response/Follow-up (m) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cough | Dyspnea | Chest pain | Others | |||||||
| Pediatric patients | ||||||||||
| 1 | 4/F | T-LBL/T-ALL | Y | N | N | N | C | 896 | III | PD/UT |
| 2 | 5M | T-LBL/T-ALL | Y | N | Y | N | N | 563 | IV | CR/UT |
| 3 | 5/M | T-LBL/T-ALL | Y | Y | N | Fever | C | NA | IV | DOD (6) |
| 4 | 5/F | T-LBL/T-ALL | Y | Y | N | N | N | 766 | III | CR/UT |
| 5 | 6/M | T-LBL/T-ALL | N | Y | Y | Neck pain | C | 1102 | IV | DOD (4) |
| 6 | 6/M | T-LBL/T-ALL | Y | N | N | Fever | N | 398 | IV | PR/UT |
| 7 | 6/F | T-LBL/T-ALL | N | N | N | Neck enlargement | C and A | 326 | IV | CR/UT |
| 8 | 6/M | T-LBL/T-ALL | Y | Y | Y | N | C and A | 1882 | IV | DOD (5) |
| 9 | 7/M | T-LBL/T-ALL | Y | Y | N | N | Md | 635 | III | PR/UT |
| 10 | 7/M | T-LBL/T-ALL | Y | N | N | Fever | Md | NA | IV | CR/NR (10) |
| 11 | 7/F | T-LBL/T-ALL | N | N | N | Neck enlargement | C | 344 | IV | CR/NR (15) |
| 12 | 8/M | T-LBL/T-ALL | Y | Y | N | N | Md | 628 | IV | CR/NR (30) |
| 13 | 9/F | T-LBL/T-ALL | Y | Y | N | N | C | 419 | IV | CR/UT |
| 14 | 9/M | T-LBL/T-ALL | Y | N | N | N | C | 2289 | III | CR/UT |
| 15 | 11/M | T-LBL/T-ALL | Y | N | N | N | C | 271 | IV | DOD (7) |
| 16 | 11/M | T-LBL/T-ALL | Y | N | Y | N | N | 795 | IV | CR/UT |
| 17 | 11/M | T-LBL/T-ALL | Y | N | N | Fever | C, Ax and Ab | 285 | IV | PR/UT |
| 18 | 12/F | T-LBL/T-ALL | N | Y | Y | SVCS | C, Ax and Ab | 292 | IV | DOD (8) |
| 19 | 13/M | T-LBL/T-ALL | Y | Y | N | Fever | C, Ax, and Md | 759 | IV | PD/UT |
| 20 | 13/M | T-LBL/T-ALL | N | Y | N | Dysphagia | NA | 297 | III | CR/UT |
| 21 | 13/M | T-LBL/T-ALL | Y | N | N | Fever | C and Ab | 2403 | IV | CR/UT |
| 22 | 14/M | T-LBL/T-ALL | N | Y | N | N | C | NA | IV | CR/NR (32) |
| 23 | 18/M | T-LBL/T-ALL | Y | N | N | N | C, Ax and Ab | 987 | IV | CR/NR (12) |
| 24 | 18/F | T-LBL/T-ALL | Y | N | Y | Fever | C, Ax and Md | 386 | IV | DOD (7) |
| 25 | 4/M | CHL | N | N | N | Fever | Abdominal LV | 368 | III | CR/NR (14) |
| 26 | 5/M | CHL | Y | N | N | N | C, Ax and Md | 378 | II | CR/NR (24) |
| 27 | 5/F | CHL | N | N | N | N | C | 252 | III | PR/NR (14) |
| 28 | 7/M | CHL | Y | N | N | Fever | C | NA | II | CR/NR (31) |
| 29 | 10/F | CHL | Y | N | N | Fever | C and Md | NA | II | CR/NR (32) |
| 30 | 13/M | CHL | N | Y | Y | N | C and Md | 394 | III | PR/UT |
| 31 | 14/M | CHL | N | N | N | Neck enlargement | C, Ax and Md | 200 | II | CR/UT |
| Adult patients | ||||||||||
| 32 | 20/M | T-LBL/T-ALL | Y | N | Y | Fever | C | 227 | IV | CR/NR (10) |
| 33 | 20/M | T-LBL/T-ALL | N | N | N | N | C and Md | 264 | IV | PD/UT |
| 34 | 28/M | T-LBL/T-ALL | Y | N | N | N | C, Ax, and Ab | 2286 | IV | CR/UT |
| 35 | 31/M | T-LBL/T-ALL | Y | N | N | Fever, neck enlargement | C, Ax, Md and Ab | 189 | IV | PR/NR (32) |
| 36 | 34/M | T-LBL/T-ALL | Y | N | N | N | C | 972 | II | PD/UT |
| 37 | 42/M | T-LBL/T-ALL | N | N | Y | Neck enlargement | C, Md and I | 245 | III | CR/UT |
| 38 | 54/F | T-LBL/T-ALL | N | N | Y | Fever | C, Md and I | 233 | III | PD/Relapsed |
| 39 | 21/F | CHL | Y | N | N | Fever | C, M | 432 | III | CR/NR (16) |
| 40 | 22/F | CHL | N | N | N | N | C | NA | II | CR/NR (35) |
| 41 | 23/M | CHL | N | N | N | N | C and Md | 152 | II | CR/NR (20) |
| 42 | 25/F | CHL | N | N | N | N | C and M | 461 | III | PR/NR (20) |
| 43 | 34/F | CHL | N | N | N | N | C | NA | II | CR/NR (14) |
| 44 | 23/F | PMBL | N | N | Y | N | C | 225 | III | CR/NR (33) |
| 45 | 25/F | PMBL | Y | N | N | Neck enlargement | C and Md | 156 | II | PD/UT |
| 46 | 27/F | PMBL | N | N | Y | N | NA | NA | II | CR/NR (31) |
| 47 | 28/F | PMBL | N | N | Y | N | N | NA | I | CR/NR (24) |
| 48 | 36/M | PMBL | Y | N | N | N | C and Md | NA | II | CR/UT |
| 49 | 44/F | PMBL | N | N | Y | N | N | NA | I | CR/NR (30) |
| 50 | 52/F | MALT | N | N | Y | Fever | C and Md | 159 | II | PR/NR (23) |
| 51 | 34/M | DLBCL | Y | N | N | Fever | C | 356 | IV | PR/NR (29) |
| 52 | 64/M | DLBCL | Y | N | N | N | Md | NA | II | PR/NR (20) |
Abbreviations: Ab, abdominal; Ax, axillary; C, cervical; CHL, classical Hodgkin lymphoma; CR, complete remission; D, diffuse large B-cell lymphoma; DOD, dead of disease; F, female; LN, lymph node; I,inguinal; MALT, mucosa associated lymphoid tissue lymphoma; Md, mediastinal node; M, male; N, no; NA, not available; NSCHL, nodular sclerosis classical Hodgkin lymphoma; NR, no relapse; PMBL, primary mediastinal large B-cell lymphoma; PD, progressing disease; PR, partial remission; T-LBL, T-cell lymphoblastic lymphoma/T-cell lymphoblastic leukemia; UT, under treatment; Y, yes; SVCS, superior vena cava syndrome.
Table 2.
Comparisons of histological subtypes, clinical features, and radiological findings between pediatric and adult patients.
| T, n = 52, no. (%) | P, n = 31, no. (%) | A, n = 21, no. (%) | P-value (P vs. A) | |
|---|---|---|---|---|
| Median age, years (range) | 13.0 (4–64) | 8.0 (4–18) | 28.0 (20–64) | |
| M:F | 32:20 | 22:9 | 10:11 | 0.089 |
| Histological subtype | ||||
| T-LBL/T-ALL | 31/52 (59.6) | 24/31 (77.4) | 7/21 (33.3) | 0.001 |
| CHL | 12/52 (23.1) | 7/31 (22.6) | 5/21 (23.8) | 0.918 |
| PMBL | 6/52 (11.5) | 0/31 (0) | 6/21 (28.6) | 0.003 |
| MALT | 1/52 (1.9) | 0/31 (0) | 1/21 (4.8) | 0.404 |
| DLBCL | 2/52 (3.8) | 0/31 (0) | 2/21 (9.5) | 0.158 |
| Clinical symptom | ||||
| Cough | 30/52 (57.7) | 21/31 (67.7) | 9/21 (42.9) | 0.075 |
| Dyspnea | 12/52 (23.1) | 12/31 (38.7) | 0/21 (0) | 0.001 |
| Chest pain | 15/52 (28.8) | 7/31 (22.6) | 8/21 (38.1) | 0.226 |
| Fever | 16/52 (30.8) | 10/31 (32.2) | 6/21 (28.6) | 0.777 |
| Clinical stage | ||||
| Stage I | 2/52 (3.8) | 0/31 (0) | 2/21 (9.5) | 0.158 |
| Stage II | 13/52 (25.0) | 4/31 (12.9) | 9/21 (42.9) | 0.034 |
| Stage III | 13/52 (25.0) | 8/31 (25.8) | 5/21 (23.8) | 0.870 |
| Stage IV | 24/52 (46.2) | 19/31 (61.3) | 5/21 (23.8) | 0.008 |
| Mortality rate | 6/52 (11.5) | 6/31 (19.4) | 0/21 (0) | 0.070 |
| Tumor size (cm) | 9.37 ± 3.24 | 9.52 ± 3.22 | 9.07 ± 3.42 | 0.700 |
| Ratio of TD to MTD | 0.47 ± 0.17 | 0.52 ± 0.17 | 0.38 ± 0.14 | 0.015 |
| Tumor compression or encasement | ||||
| Blood vessels encasement | 29/35 (82.9) | 21/23 (91.3) | 8/12 (66.7) | 0.151 |
| Pericardiaum | 23/35 (65.7) | 16/23 (69.6) | 7/12 (58.3) | 0.709 |
| Trachea | 19/35 (54.3) | 13/23 (56.5) | 6/12 (50.0) | 0.736 |
| Complication | ||||
| Pleural effussion | 23/35 (65.7) | 18/23 (78.3) | 5/12 (41.7) | 0.059 |
| Pericardial effusion | 14/35 (40.0) | 11/23 (47.8) | 3/12 (25.0) | 0.282 |
| Pneumonia | 17/35 (48.6) | 11/23 (47.8) | 6/12 (25.0) | 1.000 |
Abbreviations: T, total; P, pediatric patients; A, adult patients; TD, tumor diameter; MTD, maximum transthoracic diameter.
Table 3.
Comparisons of clinical features, and radiographic findings among different histological subtypes.
| Total, n = 52, no. (%) | T-LBL/T-ALL, n = 31, no. (%) | CHL, n = 12, No. (%) | PMBL, n = 6, No. (%) | DLBCL, n = 2, no. (%) | MALT, n = 1, no. (%) | P-value (T-LBL/T-ALL vs. CHL) | P-value (T-LBL/T-ALL vs. PMBL) | |
|---|---|---|---|---|---|---|---|---|
| Median age, years (range) | 13.0 (4–64) | 11.0 (4–54) | 13.5 (4–34) | 27.5 (23–44) | 34, 64 | 52 | ||
| M:F | 32:20 | 23:8 | 6:6 | 1:5 | 2:0 | 0:1 | 0.129 | 0.014 |
| Clinical symptom | ||||||||
| Cough | 30/52 (57.7) | 22/31 (71.0) | 4/12 (33.3) | 2/6 (33.3) | 2/2 (100) | 0/1 (0) | 0.055 | 0.157 |
| Dyspnea | 12/52 (23.1) | 11/31 (35.5) | 1/12 (8.3) | 0/6 (0) | 0/2 (0) | 0/1 (0) | 0.161 | 0.151 |
| Chest pain | 15/52 (28.8) | 9/31 (29.0) | 1/12 (8.3) | 4/6 (66.7) | 0/2 (0) | 1/1 (100) | 0.299 | 0.157 |
| Fever | 16/52 (30.8) | 10/31 (32.3) | 4/12 (33.3) | 0/6 (0) | 1/2 (50.0) | 1/1 (100) | 1.000 | 0.162 |
| Clinical stage | ||||||||
| Stage I | 2/52 (3.8) | 0/31 (0) | 0/12 (0) | 2/6 (33.3) | 0/2 (0) | 0/1 (0) | 0.023 | |
| Stage II | 13/52 (25.0) | 1/31 (3.2) | 7/12 (58.3) | 3/6 (50.0) | 1/2 (50.0) | 1/1 (100) | 0.001 | 0.010 |
| Stage III | 13/52 (25.0) | 7/31 (22.6) | 5/12 (41.7) | 1/6 (16.7) | 0/2 (0) | 0/1 (0) | 0.211 | 1.000 |
| Stage IV | 24/52 (46.2) | 23/31 (74.2) | 0/12 (0) | 0/6 (0) | 1/2 (50.0) | 0/1 (0) | 0.000 | 0.001 |
| Tumor size (cm) | 9.37 ± 3.24 | 10.31 ± 2.74 | 7.82 ± 4.77 | 6.60 ± 1.42 | 0.266 | 0.015 | ||
| Ratio of TD to MTD | 0.47 ± 0.17 | 0.52 ± 0.15 | 0.45 ± 0.24 | 0.28 ± 0.07 | 0.579 | 0.004 | ||
| Tumor compression or encasement | ||||||||
| Blood vessels | 29/35 (82.9) | 23/23 (100) | 3/6 (50.0) | 2/4 (50.0) | 1/2 (50.0) | 0.005 | 0.017 | |
| Pericardium | 23/35 (65.7) | 18/23 (78.3) | 3/6 (50.0) | 1/4 (25.0) | 1/2 (50.0) | 0.305 | 0.065 | |
| Trachea | 19/35 (54.3) | 14/23 (60.9) | 2/6 33.3) | 1/4 (25.0) | 2/2 (100) | 0.364 | 0.294 | |
| Complication | ||||||||
| Pleural effusion | 23/35 (65.7) | 21/23 (91.3) | 1/6 (16.7) | 0/4 (0) | 1/2 (50.0) | 0.001 | 0.001 | |
| Pericardial effusion | 14/35 (40.0) | 13/23 (56.5) | 1/6 (16.7) | 0/4 (0) | 0/2 (0) | 0.169 | 0.098 | |
| Pneumonia | 17/35 (48.6) | 14/23 (60.9) | 1/6 (16.7) | 0/4 (0) | 2/2 (100) | 0.080 | 0.041 | |
Histological subtypes and age groups
The 52 patients, ranging from 4 to 64 years (median 13 years), were classified as 31 pediatric patients (from 4 to 18 years, median 8 years) and 21 adult patients (from 20 to 64 years, median 28 years). The pediatric patients presented with T-LBL/T-ALL and CHL in 24 and 7 cases, respectively. The adult patients presented with T-LBL/T-ALL, PMBL, CHL, DLBCL, and MALT lymphoma in 7, 6, 5, 2, and 1 cases, respectively. Pediatric patients had a significantly higher prevalence of T-LBL/T-ALL (24/31 and 77.4% vs. 7/21 and 33.3%; P = 0.001) and lower incidence of PMBL (0/31 and 0% vs. 6/21 and 28.6%; P = 0.003) than adult patients.
The ages of patients with T-LBL/T-ALL, CHL, and PMBL ranged from 4 to 54 years (median, 11 years), 4 to 34 years (median, 13.5 years), and 23 to 44 years (median, 27.5 years), respectively. The two DLBCL patients were 34 and 64 years old, and the MALT patient was 52 years old. T-LBL/T-ALL and CHL occurred in both pediatric and young adult patients, whereas PMBL exclusively occurred in young adults.
Gender tendencies and age groups
Pediatric patients were more likely to be male predilection with a M:F ratio of 22:9, as compared with adults of 10:11 (P = 0.089). Adult patients with T-LBL/T-ALL showed a male predominance with a M:F ratio of 6:1, compared to pediatric patients of 17:7. The tendency of male predominance in T-LBL/T-ALL patients seemed to be increased with age. Patients with PMBL and CHL presented with female predominance with the M:F ratios of 1:5 and 6:6 respectively. Patients with T-LBL/T-ALL showed a higher male predominance than patients with PMBL (P = 0.014).
Initial presentations and age groups
Five patients had been identified during physical examination without symptoms. Forty-seven patients exhibited various symptoms, including cough (30/52, 57.7%), chest pain (15/52, 28.8%), fever (16/52, 30.8%), dyspnea (12/52, 23.1%), and neck enlargement (6/52, 11.5%). Cough was the most common symptom in T-LBL/T-ALL patients (22/31, 71.0%) and chest pain was often presented in PMBL patients (4/6, 66.7%). Pediatric patients showed higher frequency of dyspnea than adults (12/31 and 38.7% vs. 0/21 and 0%; P = 0.001).
Clinical stages and age groups
Stages I, II, III, and IV were present in 0 (0%), 4 (12.9%), 8 (25.8%), and 19 (61.3%) pediatric patients, respectively, as well as in 2 (9.5%), 9 (42.9%), 5 (23.8%), and 5 (23.8%) adult patients, respectively. Compared to adults, pediatric patients more frequently presented stage IV disease (19/31 and 61.3% vs. 5/21 and 23.8%; P = 0.008) and less frequently presented II disease (4/31 and 12.9% vs. 9/21 and 42.9%; P = 0.034). T-LBL/T-ALL patients presented higher frequency with stage IV disease than CHL (P = 0.000) and PMBL (P = 0.001) patients, and less frequency with stage II presentation than CHL (P = 0.001) and PMBL (P = 0.010) patients.
Clinical follow-up
Six pediatric patients with T-LBL/T-ALL died due to progression of the disease during the process of therapy, 22 patients were still under treatment, and the other 24 patients were followed up from 10 to 35 months (mean, 23.5 months).
Radiological assessments
Radiological data of 50 cases were available, including computed tomography (CT), fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT), and magnetic resonance imaging (MRI) in 50, 21, and 1 cases, respectively. Radiological data of 35 cases were available at initial diagnosis, and the radiological features are summarized in Table 4.
Table 4.
Radiological characteristics of 52 cases with lymphomas involving the mediastinum.
| *Case no. | Histological subtype | Location | Size (cm) | TD (cm)/MTD (cm) (ratio) | Compression or encasement | Complication | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood vessels | Pericardiaum | Trachea | Pleural effusion | Pericardial effusion | Pneumonia | |||||
| Pediatric patients | ||||||||||
| 1 | T-LBL/T-ALL | Anterior M | 11.3 | 11.3/17.2 (0.657) | Y | Y | Y | Y | Y | N |
| 2 | T-LBL/T-ALL | Anterior M | 8.8 | 8.8/17.9 (0.492) | Y | Y | Y | Y | Y | Y |
| 3 | T-LBL/T-ALL | Anterior-superior M | 11.6 | 11.6/16.3 (0.712) | Y | Y | Y | Y | Y | Y |
| 4 | T-LBL/T-ALL | Anterior and middle M | 14.1 | 14.1/16.5 (0.856) | Y | Y | Y | Y | N | Y |
| 5 | T-LBL/T-ALL | Anterior M | 13.5 | 13.5/18.5 (0.730) | Y | Y | N | Y | Y | Y |
| 6 | T-LBL/T-ALL | Anterior M | 8.6 | 8.6/17.7 (0.486) | Y | Y | Y | Y | Y | N |
| 7 | T-LBL/T-ALL | Anterior-superior M | 8.0 | 8.0/16.9 (0.473) | Y | N | Y | Y | Y | N |
| 8 | T-LBL/T-ALL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 9 | T-LBL/T-ALL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 10 | T-LBL/T-ALL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 11 | T-LBL/T-ALL | Anterior M | 6.0 | NA | Y | N | N | N | N | N |
| 12 | T-LBL/T-ALL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 13 | T-LBL/T-ALL | Anterior-superior M | 6.0 | 6.0/18.9 (0.317) | Y | Y | Y | Y | N | Y |
| 14 | T-LBL/T-ALL | Anterior and middle M | 12.0 | 12.0/19.4 (0.619) | Y | Y | Y | Y | Y | Y |
| 15 | T-LBL/T-ALL | Anterior-superior M | 8.9 | 8.9/23.4 (0.380) | Y | Y | N | Y | N | Y |
| 16 | T-LBL/T-ALL | Anterior M | 9.8 | 9.8/21.8 (0.450) | Y | Y | N | Y | Y | N |
| 17 | T-LBL/T-ALL | Anterior M | 13.5 | 13.5/20.8 (0.649) | Y | Y | Y | Y | N | N |
| 18 | T-LBL/T-ALL | Anterior-superior M | 5.7 | 5.7/15.1 (0.377) | Y | N | N | Y | N | Y |
| 19 | T-LBL/T-ALL | Anterior M | 9.3 | 9.3/23.6 (0.394) | Y | Y | N | Y | Y | Y |
| 20 | T-LBL/T-ALL | Anterior-superior M | 6.1 | 6.1/23.8 (0.256) | Y | N | Y | N | N | N |
| 21 | T-LBL/T-ALL | Superior M | 10.7 | 10.7/22.2 (0.482) | Y | N | N | Y | N | N |
| 22 | T-LBL/T-ALL | Anterior and middle M | 12.7 | 12.7/21.1 (0.602) | Y | Y | Y | Y | Y | Y |
| 23 | T-LBL/T-ALL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 24 | T-LBL/T-ALL | Anterior M | 12.0 | 12.0/24.6 (0.488) | Y | Y | N | Y | N | N |
| 25 | CHL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 26 | CHL | Anterior M | 4.0 | 4.0/16.7 (0.239) | N | N | N | N | N | N |
| 27 | CHL | Anterior-superior M | 10.0 | 10.0/14.6 (0.685) | Y | Y | Y | N | N | N |
| 28 | CHL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 29 | CHL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 30 | CHL | Anterior-superior M | 13.4 | 13.4/20.1 (0.658) | Y | Y | Y | Y | Y | Y |
| 31 | CHL | Anterior-superior M | 3.0 | NA | N | N | N | N | N | N |
| Adult patients | ||||||||||
| 32 | T-LBL/T-ALL | Anterior M | 12.0 | 12.0/24.9 (0.482) | Y | Y | Y | Y | Y | Y |
| 33 | T-LBL/T-ALL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 34 | T-LBL/T-ALL | Anterior M | 11.9 | 11.9/23.4 (0.508) | Y | Y | N | Y | N | Y |
| 35 | T-LBL/T-ALL | Anterior-superior M | 9.8 | 9.8/22.9 (0.428) | Y | Y | Y | Y | Y | Y |
| 36 | T-LBL/T-ALL | Anterior M | 14.8 | 14.8/24.2 (0.612) | Y | Y | Y | Y | Y | Y |
| 37 | T-LBL/T-ALL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 38 | T-LBL/T-ALL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 39 | CHL | Anterior-superior M | 3.8 | 3.8/24.3 (0.165) | N | N | N | N | N | N |
| 40 | CHL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 41 | CHL | Anterior M | 12.7 | 12.7/24.3 (0.523) | Y | Y | N | N | N | N |
| 42 | CHL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 43 | CHL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 44 | PMBL | Anterior M | 4.5 | 4.5/24.2 (0.186) | N | N | N | N | N | N |
| 45 | PMBL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 46 | PMBL | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 47 | PMBL | Anterior M | 7.4 | 7.4/22.1 (0.335) | Y | Y | N | N | N | N |
| 48 | PMBL | Anterior-superior M | 7.0 | 7.0/26.9 (0.260) | N | N | N | N | N | N |
| 49 | PMBL | Anterior M | 7.5 | 7.5/21.8 (0.344) | Y | N | Y | N | N | N |
| 50 | MALT | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 51 | DLBCL | Anterior M | 10.6 | 10.6/25.2 (0.421) | Y | Y | Y | N | N | Y |
| 52 | DLBCL | Middle M | 6.8 | 6.8/25.6 (0.266) | N | N | Y | Y | N | Y |
Abbreviations: M, mediastinum; NA, not available; N, no; Y, yes.
*Case numbers are identical with the patient number used in Table 1.
The anterior or both the anterior and the superior mediastinum was the most common location of mediastinal lymphomas (31/35, 88.6%), followed by both the anterior and middle mediastinum (3/35, 8.6%), and the middle mediastinum (1/35, 2.8%).
The tumor diameters (TD) were variable, ranging from 3 to 14.8 cm (mean, 9.4 cm), with pediatric patient tumor masses ranging from 3 to 14.1 cm (mean, 9.5 cm) and adult patient tumor masses ranging from 3.8 to 14.8 cm (mean, 9.1 cm). Bulky masses were more frequently revealed in T-LBL/T-ALL (mean, 10.3 cm) than in CHL (mean, 7.8 cm) and PMBL (mean, 6.6 cm) cases. The ratios of TD to maximum transthoracic diameter (MTD) in patients with T-LBL/T-ALL were higher than those of PMBL patients (0.52 ± 0.15 vs. 0.28 ± 0.07; P = 0.004). The ratios of TD to MTD in pediatric patients were significantly higher than those of adult patients (0.52 ± 0.17 vs. 0.38 ± 0.14; P = 0.015).
As shown in Fig. 1 and Table 4, tumor masses variably compressed or encased the blood vessels, pericardium, and trachea in 29 (29/35, 82.9%), 23 (23/35, 65.7%), and 19 (19/35, 54.3%) cases, respectively. T-LBL/T-ALL patients images exhibited greater tendency for compression of the blood vessels than CHL (P = 0.005) and PMBL (P = 0.017) patient.
Figure 1.
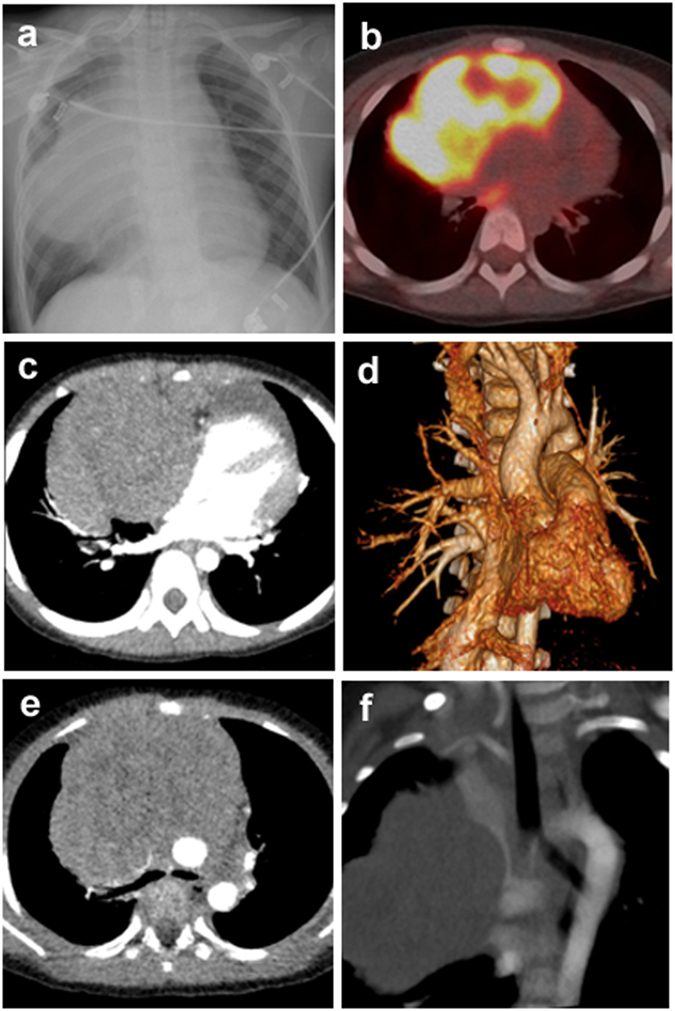
Radiological characteristics of T-LBL/T-ALL involving the mediastinum, case 1. (a) Chest X-ray image shows a large mass in the mediastinum. (b) PET-CT image shows increased FDG activity in the mediastinum. (c) Axial contrast-enhanced CT scan shows the compression of pericardiaum. (d) Three-dimensional volume rendered image of contrast-enhanced CT exhibits the compression of pericardiaum. (e) Axial contrast-enhanced CT scan shows vascular compression and tracheal encasement of the mass. (f) Maximal intensity projection of contrast-enhanced CT reveals the encasement of superior vena cava.
Complications of lymphomas involving the mediastinum commonly included pleural effusion (23/35, 65.7%), pericardium effusion (14/35, 40.0%), and pneumonia (17/35, 48.6%). T-LBL/T-ALL patients presented with a higher frequency of pleural effusions than CHL and PMBL patients, (P = 0.001) for both, and pneumonia than PMBL patients (P = 0.041). Pediatric patients were likely higher prevalence of pleural effusion than adult patients (18/23 and 78.3% vs. 5/12 and 41.7%; P = 0.059).
Discussion
Lymphomas involving the mediastinum occur in a wide age range and demonstrate various clinical symptoms and radiological findings. However, the clinicopathological and radiological differences between pediatric and adult patients have not been clearly defined in previous reports1, 10.
As reported previously, each histological subtype exhibited distinct clinicopathological features3–6, 14. However, comprehensive clinicopathological comparison among these subtypes is lacking in previous studies. In this study, pediatric patients exclusively presented with T-LBL/T-ALL and CHL, in contrast to adult patients, who presented with complex subtypes of T-LBL/T-ALL, CHL, PMBL, DLBCL, and MALT lymphomas. We further investigated the comparisons among different histological subtypes.
T-LBL/T-ALL is a highly aggressive neoplasm of lymphoblasts of T-cell origin. Due to the rapid growth of tumor cells, it is common for T-LBL/T-ALL patients to present with an advanced stage and unfavorable outcome as reported previously15, 16. In our results, T-LBL/T-ALL patients showed more frequent stage IV presentation than CHL and PMBL patients. Imaging of T-LBL/T-ALL patients commonly showed bulky masses, blood vessels compression, and pleural effusion.
PMBL is a common histological subtype in adult patients. PMBL patients are reported to have favorable clinical outcomes compared to patients with systemic DLBCL involving the mediastinum17, 18. In our study, PMBL occurred in young adults, who presented with a higher frequency of localized clinical stage than patients with T-LBL/T-ALL.
CHL occurs in both pediatric and adult patients4. As shown in our study, the most common histological subtype was NSCHL. Previous reports have shown that NSCHL generally presents with localized disease and a better prognosis than other subtypes of CHL19. Some patients have morphological features overlapping between NSCHL and PMBL, such as mediastinal gray-zone lymphoma, which makes pathological diagnoses challenging20. Our results showed that an immunohistochemical profile of positivity for CD15 and CD30 was helpful for the diagnosis of CHL. In addition, distinguishing PMBL from CHL could also be based on the value of CD23, which has been confirmed previously21.
Thymic MALT lymphomas are quite rare and occur most frequently in Asian females from 40 to 60 years of age. They often show an indolent course and localized disease22. Thymic MALT lymphomas are distinct from MALT lymphomas of other sites in several ways, including gene abnormalities and geographic distribution23. In the current study, the MALT lymphoma patient was a 52-year-old female who presented with stage II. In our study, 2 cases of systemic DLBCL with secondary mediastinal involvement were identified. Despite the pathological similarities to PMBL, DLBCL exhibited more aggressive clinical features and a poor outcome.
Although the discrimination of histological subtypes of lymphomas may be the most important reason for the clinical and radiological features, age-related differences of tumor biology, host characteristics, or treatment protocols also contribute to different outcomes between pediatric and adult patients. For example, pediatric type follicular lymphoma is considered to be a separate entity which differs from usual adult follicular lymphoma in clinical and pathological features24, 25.
Age is an important factor for lymphoma. Non-Hodgkin lymphoma (NHL) in pediatric patients present biological and epidemiological peculiarities that if better understood could help optimize their outcome. In order to investigate the influence of age on biology of lymphomas, we have compared pediatric and adult lymphomas in the same anatomic sties. As we reported recently, B-cell lymphomas involving the Waldeyer’s ring had distinctive clinicopathological characteristics in pediatric patients compared to adult counterparts. A subset of cases belonged to the new entity of IRF4/MUM1 positive lymphoma26, 27.
Of note, for appropriate management of pediatric and adult lymphomas, different staging systems have been used. The original Ann Arbor staging system28 and the updated Lugano classification29 were designed without input from the pediatric NHL disease entities. The Murphy Classification30 and the revised Pediatric Non-Hodgkin Lymphoma Staging system (IPNHLSS)31 facilitated more precise staging for children and adolescents with NHL. However, few studies compared pediatric and adult patients with mediastinal NHL by the different staging systems. We assessed the clinical stages of pediatric and adult T-LBL/T-ALL patients using the revised IPNHLSS and the Lugano classification, respectively.
Despite of a population over 1300 million in China, to date, English information on mediastinal lymphomas of Chinese populations is limited. We described 52 cases of lymphomas involving the mediastinum, which exhibited a wide age range, heterogeneous histological subtypes, various clinical presentations and complex radiological manifestation. Compared to adult lymphomas, pediatric lymphomas presented distinctive histological subtypes, clinical behaviors, and radiological features. The limitation of this study is the relatively small number of cases, and further studies are needed for more detailed understanding.
Accordingly, this is the first multidisciplinary English report comprehensively compared the differences between Chinese pediatric and adult patients with mediastinal lymphomas and may provide new insight into the understanding of mediastinal lymphoma using different staging systems.
Materials and Methods
Case selection
Cases were obtained from a single Chinese institution: Shanghai Xinhua Hospital. A total of 506 patients with the mediastinal mass were reviewed between November 2011 and December 2016. Among these cases, 52 cases of lymphomas involving the mediastinum were identified and included in this study. The pathological diagnoses were originally established according to the criteria of the 2008 World Health Organization classification. All experimental protocols were approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine and performed in accordance with the approved guidelines of the institution. The appropriate informed consent was obtained from all subjects under the institutional review board-approved protocol.
Age grouping, clinical information, and staging
The patients were arbitrarily defined as 2 groups, including pediatric group under 19 years of age and adult group over 19 years of age. The clinical data, including the age, gender, date of initial diagnosis, symptoms, clinical stage, serum lactate dehydrogenase level, medical records, and surgical records, were reviewed. The disease stages of CHL and adult NHL patients were assessed according to the Lugano Classification29 and pediatric NHL patients to the IPNHLSS31.
Radiological modalities
The patients were identified by imaging assessments including chest X-ray, CT, MRI, or PET-CT.
Histological and immunohistochemistry analyses
Formalin-fixed paraffin-embedded tissues were stained with hematoxylin and eosin at the initial diagnosis. Immunohistochemistry was performed using the following panel of monoclonal and polyclonal antibodies: CD20 (clone L26, DAKO, Glostrup, Denmark); CD79a (clone 1.10E + 04, Leica Biosystems, Wetzlar, Germany); PAX5 (clone R1, DAKO); CD10 (clone 56C6, Leica Biosystems); BCL6 (clone P1F6, DAKO); MUM1 (clone MUM1p, DAKO); Ki-67 (clone MIB-1, DAKO); Terminal deoxynucleotidyl transferase (TDT, clone SP150, DAKO); CD2 (clone AB75, DAKO); CD3 (clone LN10, Leica Biosystems); CD4 (clone SP35, DAKO); CD7 (DAKO); CD8 (clone SP16, DAKO); CD1a (clone SP157, DAKO); CD34 (clone QBEnd/10, DAKO); CD43 (clone DF-T1, DAKO); CD99 (clone HO36-1.1, DAKO); CD117 (clone C-KIT, DAKO); CD45 (clone PAN-LCAL, DAKO); CD33 (clone WM-54, DAKO); MPO (clone SP72, DAKO); CD15 (clone C3D-1, DAKO); CD30 (clone Ber-H2, DAKO); CD23 (clone SP23, DAKO); and Epithelial membrane antigen (EMA, clone GP1.4, Leica Biosystems).
Statistical analysis
Statistical analyses were performed with SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA). Chi-squared and Fisher’s exact tests were used to determine correlation in the frequencies between groups. The TD to MTD were summarized using the mean and standard deviation. Two tailed independent samples t-test were used to assess statistical significance in comparisons between the pediatric and adult patients.
Electronic supplementary material
Acknowledgements
We acknowledge the members of the pathology department of Xinhua Hospital for their skillful technical assistance.
Author Contributions
L.C. and T.L. participated in the design of the study and drafted the manuscript; M.W., H.F., and F.H. collected the clinical data and performed the statistical analysis. L.C. and T.L. critically revised the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02720-1
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Takeda S, et al. Clinical spectrum of primary mediastinal tumors: a comparison of adult and pediatric populations at a single Japanese institution. J Surg Oncol. 2003;83:24–30. doi: 10.1002/jso.10231. [DOI] [PubMed] [Google Scholar]
- 2.Temes R, et al. Primary Mediastinal Malignancies in Children: Report of 22 Patients and Comparison to 197 Adults. Oncologist. 2000;5:179–184. doi: 10.1634/theoncologist.5-3-179. [DOI] [PubMed] [Google Scholar]
- 3.Cortelazzo S, Ponzoni M, Ferreri AJ, Hoelzer D. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. 2011;79:330–343. doi: 10.1016/j.critrevonc.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Mauch PM, et al. Patterns of presentation of Hodgkin disease. Implications for etiology and pathogenesis. Cancer. 1993;71:2062–2071. doi: 10.1002/1097-0142(19930315)71:6<2062::AID-CNCR2820710622>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Steidl C, Gascoyne RD. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood. 2011;118:2659–2669. doi: 10.1182/blood-2011-05-326538. [DOI] [PubMed] [Google Scholar]
- 6.Weissferdt A, Moran CA. Primary MALT-type lymphoma of the thymus: a clinicopathological and immunohistochemical study of six cases. Lung. 2011;189:461–466. doi: 10.1007/s00408-011-9335-y. [DOI] [PubMed] [Google Scholar]
- 7.Wilson WH, et al. A prospective study of mediastinal gray-zone lymphoma. Blood. 2014;124:1563–1569. doi: 10.1182/blood-2014-03-564906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest. 2005;128:2893–2909. doi: 10.1378/chest.128.4.2893. [DOI] [PubMed] [Google Scholar]
- 9.Liu T, et al. Mediastinal lesions across the age spectrum: a clinicopathological comparison between pediatric and adult patients. Oncotarget. 2017;Apr:18. doi: 10.18632/oncotarget.17201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savage KJ, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102:3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 11.Allan BJ, et al. An analysis of 73 cases of pediatric malignant tumors of the thymus. J Surg Res. 2013;184:397–403. doi: 10.1016/j.jss.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Gun F, et al. Mediastinal masses in children: experience with 120 cases. Pediatr Hematol Oncol. 2012;29(2):141–147. doi: 10.3109/08880018.2011.646385. [DOI] [PubMed] [Google Scholar]
- 13.Patel JL, et al. The immunophenotype of T-lymphoblastic lymphoma in children and adolescents: a Children’s Oncology Group report. Br J Haematol. 2012;159:454–461. doi: 10.1111/bjh.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitolo U, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v91–v102. doi: 10.1093/annonc/mdw175. [DOI] [PubMed] [Google Scholar]
- 15.Le Gouill S, et al. Adult lymphoblastic lymphoma: a retrospective analysis of 92 patients under 61 years included in the LNH87/93 trials. Leukemia. 2003;17:2220–2224. doi: 10.1038/sj.leu.2403095. [DOI] [PubMed] [Google Scholar]
- 16.Coustan-Smith E, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abou-Elella AA, et al. Primary mediastinal large B-cell lymphoma: a clinicopathologic study of 43 patients from the Nebraska Lymphoma Study Group. J Clin Oncol. 1999;17:784–790. doi: 10.1200/JCO.1999.17.3.784. [DOI] [PubMed] [Google Scholar]
- 18.Rosenwald A, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allemani, C., Sant, M., De Angelis, R., Marcos-Gragera, R. & Coebergh, J. W. EUROCARE Working Group. Hodgkin disease survival in Europe and the U.S.: prognostic significance of morphologic groups. Cancer. 107, 352–360 (2006). [DOI] [PubMed]
- 20.Gualco G, Natkunam Y, Bacchi CE. The spectrum of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma: a description of 10 cases. Mod Pathol. 2012;25:661–674. doi: 10.1038/modpathol.2011.200. [DOI] [PubMed] [Google Scholar]
- 21.Salama ME, Rajan Mariappan M, Inamdar K, Tripp SR, Perkins SL. The value of CD23 expression as an additional marker in distinguishing mediastinal (thymic) large B-cell lymphoma from Hodgkin lymphoma. Int J Surg Pathol. 2010;18:121–128. doi: 10.1177/1066896909331994. [DOI] [PubMed] [Google Scholar]
- 22.Lorsbach RB, Pinkus GS, Shahsafaei A, Dorfman DM. Primary marginal zone lymphoma of the thymus. Am J Clin Pathol. 2000;113:784–791. doi: 10.1309/H7V2-G9L4-GR9G-8GK0. [DOI] [PubMed] [Google Scholar]
- 23.Inagaki H, et al. Primary thymic extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue type exhibits distinctive clinicopathological and molecular features. Am J Pathol. 2002;160:1435–1443. doi: 10.1016/S0002-9440(10)62569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, et al. Follicular lymphomas in children and young adults: a comparison of the pediatric variant with usual follicular lymphoma. Am J Surg Pathol. 2013;37:33–43. doi: 10.1097/PAS.0b013e31826b9b57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swerdlow SH, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Al-Kzayer LF, Liu Y, Liu T. B-cell lymphomas involving Waldeyer’s ring characterized by distinctive clinical and histopathological features: a comparison of pediatric to adult patients. Oncotarget. 2017 doi: 10.18632/oncotarget.14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, et al. IFR4/MUM1-positive lymphoma in Waldeyer ring with co-expression of CD5 and CD10. Pediatr Blood Cancer. 2017;64:311–314. doi: 10.1002/pbc.26236. [DOI] [PubMed] [Google Scholar]
- 28.Lister TA, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 29.Cheson BD, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–339. [PubMed] [Google Scholar]
- 31.Rosolen A, et al. Revised International Pediatric Non-Hodgkin Lymphoma Staging System. J Clin Oncol. 2015;33:2112–2118. doi: 10.1200/JCO.2014.59.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


