Abstract
Reactive oxygen species (ROS) play a profound role in cardiorespiratory function under normal physiological conditions and disease states. ROS can influence neuronal activity by altering various ion channels and transporters. Within the nucleus tractus solitarii (nTS), a vital brainstem area for cardiorespiratory control, hydrogen peroxide (H2O2) induces sustained hyperexcitability following an initial depression of neuronal activity. The mechanism(s) associated with the delayed hyperexcitability are unknown. Here we evaluate the effect(s) of H2O2 on cytosolic Ca2+ (via fura-2 imaging) and voltage-dependent calcium currents in dissociated rat nTS neurons. H2O2 perfusion (200 µM; 1 min) induced a delayed, slow, and moderate increase (~27%) in intracellular Ca2+ concentration ([Ca2+]i). The H2O2-mediated increase in [Ca2+]i prevailed during thapsigargin, excluding the endoplasmic reticulum as a Ca2+ source. The effect, however, was abolished by removal of extracellular Ca2+ or the addition of cadmium to the bath solution, suggesting voltage-gated Ca2+ channels (VGCCs) as targets for H2O2 modulation. Recording of the total voltage-dependent Ca2+ current confirmed H2O2 enhanced Ca2+ entry. Blocking VGCC L, N, and P/Q subtypes decreased the number of cells and their calcium currents that respond to H2O2. The number of responder cells to H2O2 also decreased in the presence of dithiothreitol, suggesting the actions of H2O2 were dependent on sulfhydryl oxidation. In summary, here, we have shown that H2O2 increases [Ca2+]i and its Ca2+ currents, which is dependent on multiple VGCCs likely by oxidation of sulfhydryl groups. These processes presumably contribute to the previously observed delayed hyperexcitability of nTS neurons in in vitro brainstem slices.
Keywords: ROS, autonomic nervous system, redox signaling, voltage-gated calcium channel
the nucleus tractus solitarii (nTS) in the brainstem is critical for cardiorespiratory regulation. It constitutes the first step for central integration and processing of peripheral visceral information (1). Many neuromodulators alter cellular activity within the nTS (30, 33, 43) before information is conveyed to other brain centers in the cardiorespiratory reflex axis. Reactive oxygen species (ROS) are recognized neuromodulators/second messengers that effectively participate in cardiorespiratory function. For example, NADPH oxidase-derived ROS play an important role in angiotensin II signaling within the nTS (55) and during chemoreflex activation in the rostral ventrolateral medulla (40). Also, antioxidants influence baroreflex control in conscious rats, implicating active involvement of ROS (17). However, ROS are also associated with dysfunction of cardiorespiratory regions in disease states like obstructive sleep apnea, heart failure, and hypertension (8, 12, 56).
Among the members of the ROS family, H2O2 exhibits many features important for neuromodulation and second messenger function. It is produced upon stimulation, has specific cellular targets, is relatively long lived, easily crosses membranes, and its actions are terminated by specific antioxidants (47). Its influence on cellular activity can occur through various different types of ion channels. For example, H2O2 excites rat hippocampal CA1 neurons by increasing currents through voltage-activated sodium channels (38). On the other hand, H2O2 may inhibit spike activity by increasing currents of ATP-sensitive K+ channels as in substantia nigra neurons (3) or by increasing intracellular Cl− through GABAA receptor-gated channels in the cortex and hippocampus (48). ROS may also alter Ca2+ homeostasis via channels situated on the neuronal membrane (34, 59) or on intracellular Ca2+ stores (16).
With regards to the nTS, in vivo microinjection studies showed that H2O2 elicits bradycardia and depressor responses (7), possibly via increased output of nTS neurons in the baroreflex pathway. Using the in vitro brainstem slice, we showed that H2O2 exerted a biphasic effect in nTS neurons (42). Initially, H2O2 inhibited neuronal activity by hyperpolarizing the membrane potential via increased current of barium-sensitive K+ channels. Upon H2O2 removal, a delayed and sustained hyperexcitability became apparent. This hyperexcitability was due to a return and further depolarization of the membrane potential above resting levels and a decreased action potential threshold voltage. However, the mechanism(s) underlying the hyperexcitability were unknown. Wang et al. (54) demonstrated an angiotensin II- and ROS-related increase of L-type Ca2+ current in the nTS. Thus, in this study, we focused specifically on the effects of H2O2 on cytosolic Ca2+ and its entry in primary dissociated nTS neurons using the fluorescent dye fura-2 and patch-clamp recordings. Our data show that H2O2 increases intracellular Ca2+ via multiple voltage-gated Ca2+ channels (VGCCs) in a time-delayed fashion.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Harlan; n = 54, aged 3–4 wk) were maintained in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited vivarium of the Dalton Cardiovascular Research Center. Rats were held on a 12-h day-night cycle at 22°C and 40% humidity with water and food available ad libitum. All experimental protocols were conducted in accordance with National Institutes of Health guidelines (Guide for the Care and Use of Laboratory Animals) and approved by the Animal Care and Use Committee of the University of Missouri.
Cellular Imaging
Primary nTS cell culture.
Similar to our previous studies (31), the brainstem of anesthetized rats (isoflurane; VetOne, Boise, ID) was quickly removed and chilled in ice-cold low calcium-high magnesium artificial cerebral spinal fluid (in mM: 124 NaCl, 3 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 d-glucose, 1 CaCl2, and 2 MgCl2, saturated with 95% O2-5% CO2, pH 7.4, ~300 mosM). Horizontal slices (~400 µm) containing the nTS were cut using a vibrating microtome (VT 1000S; Leica). Subsequently, the nTS was excised using a razor blade and placed in Hank’s balanced salt solution (HBSS; no calcium, no magnesium; GIBCO, Big Cabin, OK) with 10 U/ml activated papain (Worthington, Lakewood, NJ). The papain was activated with EDTA (0.27 mM), mercaptoethanol (0.017 mM), and cysteine-HCl (1.37 mM; Sigma, St. Louis, MO). After 30 min in the incubator (MCO-18AIC, Sanyo) at 37°C and 5% CO2, the papain-HBSS solution was replaced with ~1 ml Dulbecco's modified Eagle’s medium (DMEM; high glucose; GIBCO) containing 10% horse serum (GIBCO) to stop enzymatic activity. The tissue was then triturated using progressively smaller fire-polished glass pipettes until cells were visibly dispersed. Cells were concentrated (700 rpm for 5 min; Centrifuge 5804 R; Eppendorf), and the supernatant was replaced with ~0.3 ml Neurobasal-A (GIBCO) with added B-27 supplement (2%; GIBCO), GlutaMAX (1%; GIBCO), insulin-transferrin (1%; GIBCO), and penicillin streptomycin (1%; GIBCO). nTS cells were plated on poly-d-lysine (100 µg/ml) coated glass coverslips and allowed to settle and attach for 2 h in the incubator. Subsequently, dishes were flooded with Neurobasal-A as culture medium. Recordings were made on the next day.
Fura-2 calcium imaging.
One fura-2 AM vial (50 µg; ThermoFisher Scientific/Invitrogen, Eugene, OR) was reconstituted with 50 µl of 20% Pluronic F-127 in DMSO (Molecular Probes). Cells were loaded for 30 min with 0.05% fura-2 AM in Neurobasal-A on a shaker at 37°C and 5% CO2 in the dark. Then, cells were washed two times and incubated for another 30 min at room temperature with extracellular recording solution containing the following (in mM): 136 NaCl, 5.4 KCl, 0.33 NaH2PO4, 10 HEPES, 10 d-glucose, 1.8 CaCl2, and 1 MgCl2. The glass coverslips with fura-2-loaded nTS cells were placed in a superfusion chamber on an inverted microscope (IX71; Olympus) and superfused at a rate of 500–800 µl/min using a positive pressure regulator (AirPulse 7500; Fishman). Cells were visualized using a ×40 objective and a camera (Retiga Exi Fast 1394; QImaging). The fluorophore was excited at 340 and 380 nm with a Polychrome V monochromator (TILL Photonics) and visualized at ~510 nm. Images were acquired every 5–10 s using Micro-Manager software (Open Imaging).
Protocols and drugs.
Baseline periods were recorded for ≥2 min before changes were induced in cytosolic Ca2+ with 1-min perfusions of H2O2 (100–500 µM; certified ACS 30%; Fisher Scientific) or high K+ (55.4 mM; NaCl reduced to 86 mM). Based on our initial observations and the delayed responses to H2O2 in nTS slices (42), we allowed a ≥10-min window after H2O2 to monitor any Ca2+ responses. To examine the role of H2O2 during depolarization-induced increases in cytosolic Ca2+, cells were exposed to repetitive 1-min high K+ stimulation in the absence and presence of a 5-min perfusion of H2O2. Based on the Goldman-Hodgkin-Katz equation, perfusion with high K+ depolarizes the cell membrane potential by ~39 mV, assuming a physiological intracellular composition of the following (in mM): 15 Na+, 150 K+, and 10 Cl−, and permeability values relative to K+ of pK:pNa:pCl = 1:0.04:0.45 (23).
The following drugs were added to the extracellular solution in subsets of experiments: 1 µM thapsigargin (Sigma), 1 mM EDTA (Sigma) in extracellular solution with 0 Ca2+ (MgCl2 was increased to 2.8 mM in the perfusate to substitute the omitted CaCl2), 500 µM CdCl2 (Sigma), 5 µM nifedipine (Sigma), 0.1 µM ω-conotoxin GVIA (Sigma), 0.1 µM ω-agatoxin IVA (Sigma), and 2 mM dithiothreitol (Sigma). For the majority of protocols, cells were exposed at least 5 min to a particular drug before H2O2 perfusion. Longer thapsigargin (≥ 30 min) exposure was performed to achieve greater or near complete sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) block.
Electrophysiology
nTS cell dispersion.
Rats were anesthetized with isoflurane, and the brainstem was rapidly removed and placed in ice-cold low calcium PIPES-buffered solution (in mM; 115 NaCl, 5 KCl, 20 PIPES, 25 D-glucose, 0.1 CaCl2, and 5 MgCl2, pH 7.0 with NaOH, ~310 mosM) (27, 32). The brainstem was cut in ice-cold PIPES-buffered solution in the coronal plane into three ~360-μm sections spanning the caudal to medial nTS using a vibratome as above. The nTS sections were allowed to recover for 20 min at ~33°C in low-calcium PIPES buffer before enzyme incubation with 20 units of activated papain (as above) in PIPES for 30–35 min at ~33°C. Subsequently, slices were rinsed three times with enzyme-free PIPES buffer and maintained at room temperature (~22°C) in a continuously oxygenated jar with PIPES buffer. When cells were needed, a single brainstem slice was placed on a Sylgard covered petri dish and the nTS region was identified and removed using two pulled glass patch pipettes that served as microscalpels. The nTS was gently triturated in ~500 μl ice-cold PIPES containing 0.1% DNAase (ThermoFisher Scientific) and 0.5% bovine serum albumin (BSA; Sigma) using progressively smaller fire-polished glass pipettes. After trituration, ~250 μl each of the 500-μl neuron suspension were placed onto poly-d-lysine (100 μg/ml)-treated 15-mm coverslips that were seated in Warner Instruments RC-42LP incubation chambers (volume of 87 μl/mm). Neurons were allowed to settle and adhere to the coverslip for at least 20 min, after which superfusion of the cells began with a normal physiological solution (see below). All neurons were studied within 2 h of dispersion from a brainstem slice and within 8 h of removal from the rat.
Calcium current protocol.
Neurons were visualized on an Olympus IMT2 inverted microscope with phase optics. Recording electrodes were pulled from borosilicate glass (B150-86-7.5; Sutter Instruments, Novato, CA) via a horizontal pipette puller (Sutter Instruments P-97) and positioned using a hydraulic micromanipulator (MX6600R; Siskiyou). Signals were filtered at 2 kHz and acquired at 10 kHz using an AxoPatch 200B amplifier controlled by Clampex 10 software by a Digidata 1320 interface (Molecular Devices, Sunnyvale, CA).
Neurons were initially bathed in a normal physiological external solution consisting of the following (in mM): 137 NaCl, 3 KCl, 10 d-glucose, 10 HEPES, 2 CaCl2, and 1 MgCl2 (pH 7.4 with NaOH; ~315 mosM). To record calcium currents in whole cell mode, electrodes (3–6 MΩ) were filled with a solution containing the following (in mM): 124 CsCl, 11 EGTA, 1 CaCl2, 10 HEPES, 1 MgCl2, 4 MgATP, 0.2 NaGTP, 20 phosphocreatine, 0.02 leupeptin, and 35 U/ml phosphocreatine kinase (pH 7.2 with CsOH; ~300 mosM). ATP and the ATP-regenerating system in the pipette solution minimized “rundown” of currents (6). Following formation of a gigaseal and membrane rupture, the external solution was switched to one designed to enhance visualization of currents through VGCCs. Barium (Ba2+) replaced Ca2+ as the charge carrier in this modified external solution, which contained the following (in mM): 132 NaCl, 5 BaCl2, 10 tetraethylammonium chloride (TEA-Cl), 2 MgCl2, 10 HEPES, and 10 d-glucose (30, 45, 53). Tetrodotoxin (TTX; 1 μM) was added to block sodium currents.
To investigate total Ba2+ current through VGCCs, depolarizing voltage steps in 10-mV increments from −80 to +60 mV for 100 ms were applied to the neuron from a holding potential of −80 mV (30). This protocol generated a current-voltage (I–V) relationship. Alternatively, a single maximally (peak) evoked current (Ipeak) was evoked from −80 mV every 30 s. The voltage applied to evoke peak currents was determined by analysis of the I–V curve 3 min after cell membrane rupture and was between −10 and −20 mV (see I–V curves in results). Evoked Ba2+ currents were subsequently classified as Ca2+ currents [()]. H2O2 and/or calcium channel blockers were applied for 1 min. As in our imaging experiments, ω-conotoxin GIVA, ω-agatoxin IVA, nifedipine, or CdCl2 was either diluted in extracellular Ba2+ solution alone or with H2O2. In a subset of neurons not exposed to H2O2, we examined the relative proportion of L, N, and P/Q channels. In these protocols, Ipeak was recorded in the absence and then following subsequent addition of VGCC antagonists to the perfusate, followed by 100 μM CdCl2 to block the remaining current. Solutions were applied to the cell at a rate of 150 µl/min using a superfusion system with its outlet ~500 μm upstream from the cell. This system allowed switching of solutions that the cell was exposed to within a few seconds.
In seven neurons, we examined cellular resting potential as an indicator of general cell viability. For these recording, the pipette solution contained the following (in mM): 10 NaCl, 130 K+ gluconate, 11 EGTA, 1 CaCl2, 10 HEPES, 1 MgCl2, 2 MgATP, and 0.2 NaGTP (pH of 7.3, 295–300 mosM), and the neurons were bathed in normal physiological external solution detailed above. Membrane potential was −53.3 ± 3.2 mV in these dissociated neurons, comparable to our previous reports (42).
Immunocytochemistry
A subset of culture dishes with nTS cells was fixed for immunocytochemical analysis. The glass coverslips were exposed for 20 min to 2% paraformaldehyde (Fisher Scientific, Pittsburgh, PA) at room temperature and then washed three times with 0.01 M PBS and stored at 2–8°C. Following blocking using 10% normal donkey serum (Millipore, Temecula, CA) in 0.3% Triton-PBS, coverslips were incubated with primary antibody against neuronal nuclear antigen (NeuN; neuronal marker; mouse; 1:500; MAB377; Millipore) and the antioxidant catalase (rabbit; 1:300; ab1877; Abcam, Cambridge, MA) in 0.3% Triton-PBS and 1% normal donkey serum for 24 h. Then, coverslips were rinsed in 0.01 M PBS and incubated with the secondary antibodies Cy5-conjugated donkey anti-mouse IgG (1:200; 715–175–151; Jackson Immunoresearch, West Grove, PA) and Cy2-conjugated donkey anti-rabbit IgG (1:200; 711–225–152; Jackson Immunoresearch) in 0.3% Triton-PBS for 1 h in the dark. After being rinsed with 0.01 M PBS, glass coverslips were dried and mounted with ProLong Gold with 4′,6-diamidino-2-phenylindole (DAPI; nuclear marker; Invitrogen, Carlsbad, CA) on gelatin-coated slides. Fluorescence was visualized using an epifluorescent microscope (BX51; Olympus), a digital monochrome camera (ORCA-ER; Hamamatsu), a spinning disk confocal unit (Olympus), and appropriate filter sets and excitation wavelengths. Each fluorophore was imaged in the same focal plane, pseudocolored, and postprocessed using ImageJ (National Institutes of Health) to adjust contrast and brightness.
Data Analysis
For imaging experiments, postprocessing of images and calculation of the 340-to 380-nm excitation ratio were done with ImageJ (National Institutes of Health) and Excel software (Microsoft). We analyzed 517 cells and excluded those with fibroblast-like morphology or visibly moving cilia that originate from the ependymal lining of the fourth ventricle. Based on our initial experiments, we defined the following criteria to clearly identify responders to H2O2: 1) the Ca2+ response must have a clear peak with a rising and decaying phase; 2) the peak must occur within our 10-min window from the end of the stimulation; 3) no other peaks of similar or greater height should be observed before drug perfusion to separate potential random Ca2+ fluctuations from H2O2-induced responses; and 4) the peak amplitude must be greater than a threshold of 6.2% from baseline. The threshold was defined by the average maximal change (4.1 ± 0.01%) during vehicle perfusion (as time control) and the added 95% confidence interval. The baseline to measure peaks was taken directly before the response to minimize errors due to slowly rising or decaying levels of Ca2+ over the entire course of the protocol. The onset variance of Ca2+ responses between high K+ and H2O2 was calculated using Excel software. The fura-2 340/380 ratio was taken as an indicator of internal Ca2+ concentration ([Ca2+]i) (39, 51).
Calcium current data were analyzed using Clampfit (Molecular Devices) and Excel software (Microsoft). Across all dissociated nTS cells tested, cell capacitance was 7.77 ± 0.35 pF (n = 64), comparable to other isolated studies (4, 24, 44, 52). Acquired calcium currents were first normalized to the capacitance of the cell (pA/pF). Current-voltage (I–V) graphs were then expressed relative to the peak current of the control exposure as described by Strege et al. (49). Specifically, the peak inward current of the control trace was normalized to 1 using the equation Inorm = −1 · (I–V/Ipeak), where Inorm is normalized current, I–V is measured current at a given voltage, and Ipeak is maximum peak inward current of the control trace. Thus peaks of all other exposures (H2O2, channel antagonist, or wash) per cell were expressed as a fraction of 1. Peak currents evoked from a single voltage step were also normalized to the initial control exposure.
Statistical analyses were performed using SigmaPlot 13.0 (Systat Software) and GraphPad Prism 7 (GraphPad Software). Results were tested via t-test and one- or two-way repeated-measures ANOVA followed by Student-Newman-Keuls post hoc test unless otherwise stated in the text. Results are considered significantly different at P ≤ 0.05. Group data are presented as means ± SE.
RESULTS
To study the changes of [Ca2+]i in response to H2O2, we loaded primary dissociated nTS cells with the fluorescent dye fura-2 (Fig. 1A). Our cultures primarily consisted of neurons as identified using NeuN in paraformaldehyde-fixed cultures (Fig. 1B). These neurons colabeled to a variable degree with catalase, verifying antioxidant capabilities also found in vivo (42).
Fig. 1.
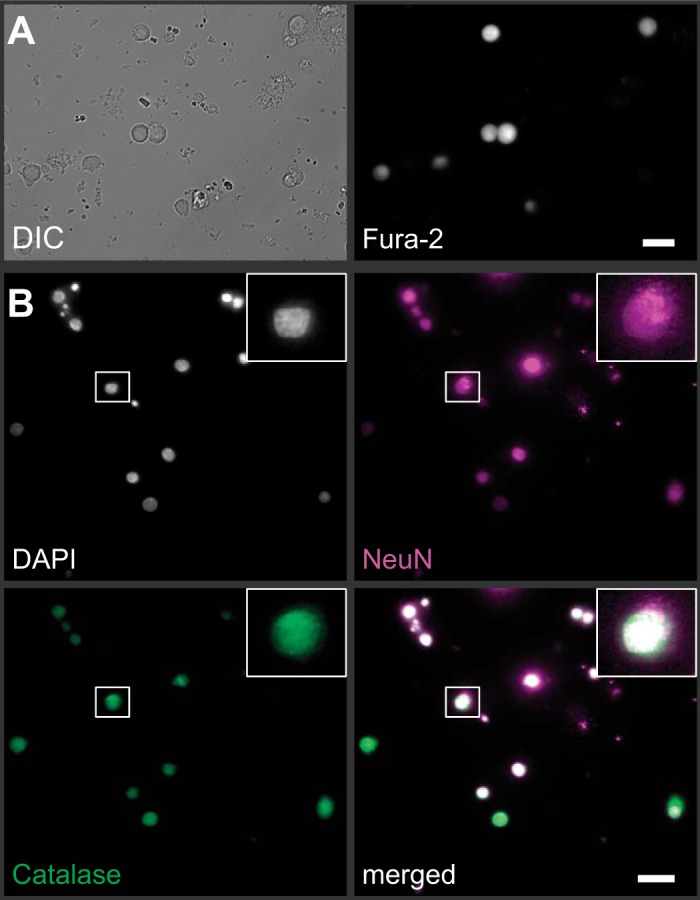
Primary dissociated cell culture and fura-2 imaging of nucleus tractus solitarii (nTS) neurons. A: example of nTS cells visualized using differential interference contrast (DIC; left) also show strong fura-2 fluorescence when excited with 380 nm (right). Scale bar = 20 µm. B: immunocytochemical verification on paraformaldehyde-fixed nTS cells that our cultures primarily consisted of neurons (NeuN, magenta) that colabeled with nuclei (DAPI, gray) and the antioxidant catalase (green). Scale bar = 20 µm. Inset: zoomed image of one nTS neuron.
H2O2 Causes a Delayed Increase of Cytosolic Ca2+
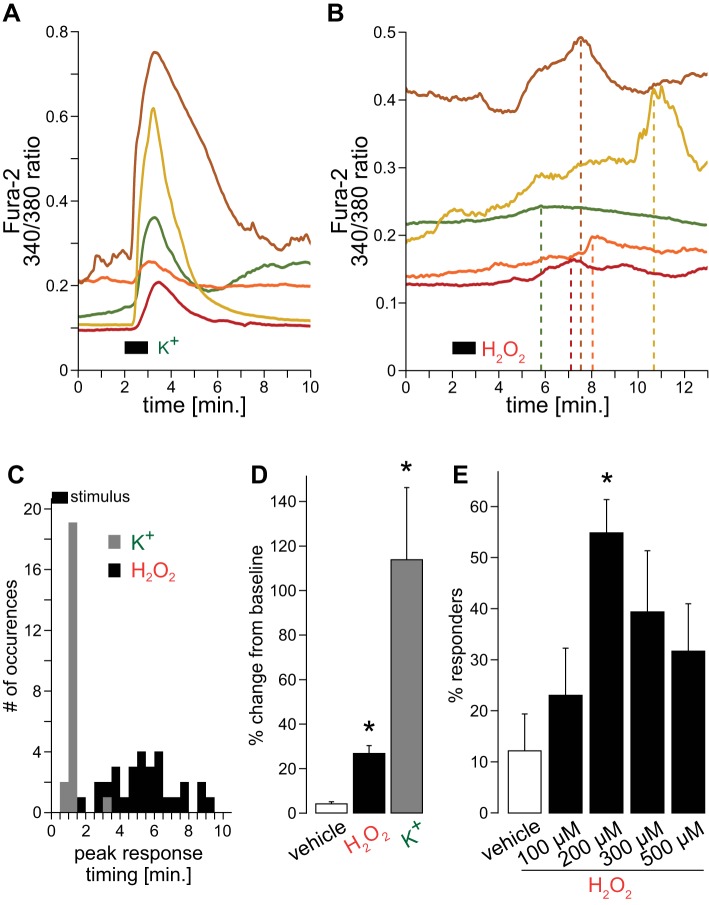
To evaluate the changes in [Ca2+]i, fura-2-loaded nTS neurons were exposed to an increased K+ concentration of the perfusate solution (55 mM) to induce cellular depolarization. Representative traces show a prompt increase in [Ca2+]i in response to 1 min of high K+, with a large amplitude and decay that varied among cells (Fig. 2A). In contrast, variably long latencies of intracellular Ca2+ peaks were observed when the cells were perfused with 200 µM H2O2 (Fig. 2B, dashed lines). The mean time to response peak was significantly shorter following high K+ (0.36 ± 0.11 min, n = 22) when compared with H2O2 (4.72 ± 0.37 min, n = 31; P < 0.001, t-test). The variance in peak response time is depicted in the response time histogram in Fig. 2C (bin = 30 s). Quantitatively, the variance in peak response time was significantly less in response to high K+ (0.26 ± 0.21, n = 22 vs. H2O2, 4.16 ± 1.01, n = 31; P < 0.01, t-test). Given this high variability and longer response time to H2O2 perfusion, for the remainder of the fura-2 study we used a 10-min window following the stimulus to allow detection of Ca2+ peaks in response to H2O2. However, the majority of responses occurred at 5 min following the stimulus.
Fig. 2.
Effects of H2O2 on cytosolic Ca2+ in nTS cells. A: typical raw traces of cytosolic Ca2+ levels (using fura-2) to 1 min of high K+ (55.4 mM). B: examples of cytosolic Ca2+ levels to 1 min H2O2 (200 µM). Note the variable timing of peak responses (dashed lines). C: peristimulus time histogram grouping response timings to high K+ (responders to 55.4 mM; n = 22) and H2O2 (responders to 200 µM; n = 31; 30-s bins are shown. D: change from baseline for vehicle (n = 21), H2O2 (n = 31), and high K+ (n = 22). *P ≤ 0.05 vs. vehicle by one-way ANOVA on ranks. E: percent responders to different H2O2 concentration. Total number of cells tested: vehicle = 21, 100 µM = 22, 200 µM = 58, 300 µM = 28, and 500 µM = 15. *P ≤ 0.05 vs. vehicle by one-way ANOVA.
Average changes from baseline are depicted in Fig. 2D. [Ca2+]i did not appreciably change during vehicle perfusion. Of those neurons that responded to H2O2 with more than a 6.2% increase from baseline (see materials and methods for details), [Ca2+]i augmented by 27%. In comparison, high K+ elevated Ca2+ by 114% from baseline indicating that H2O2 elicits only moderate responses in nTS neurons. The amount of increase between the various H2O2 concentrations was similar (~27% for 100–500 µM, data not shown), but the number of responders per concentration significantly differed (Fig. 2E). Most cells responded to 200 µM H2O2, which was used throughout the remainder of the study.
H2O2 Increases Baseline Ca2+
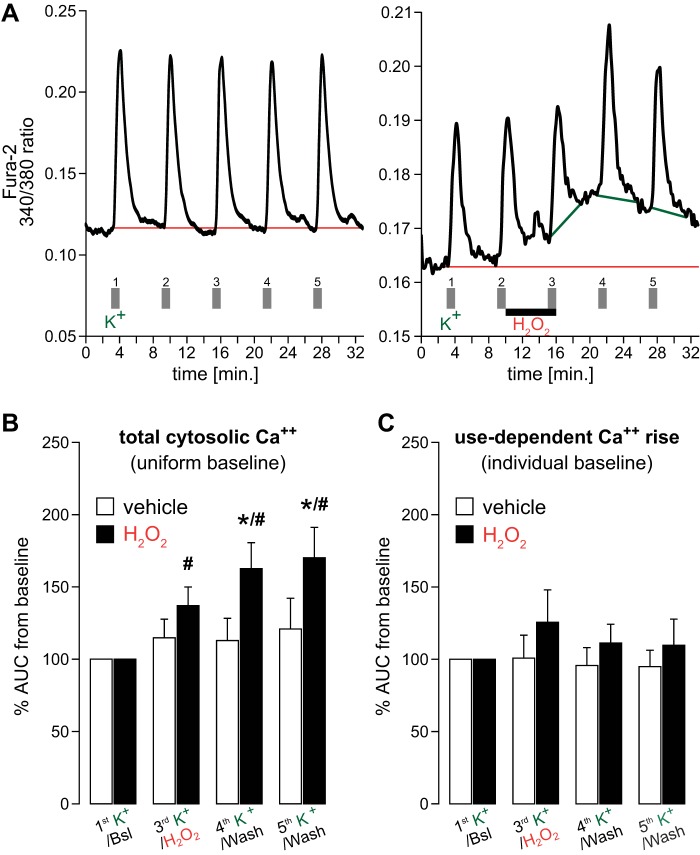
To determine whether H2O2 increases [Ca2+]i during cellular depolarization, we measured the H2O2-dependent increase in Ca2+ during and following repetitive high K+ stimulation (1-min episodes). Repeated stimulation with high K+ in the absence of H2O2 (i.e., vehicle treatment) elicited a reliable and repeatable depolarization, which recovered between stimuli as seen by the Ca2+ response (Fig. 3A, left). Following H2O2, [Ca2+]i rose, yet this was seen primarily in the baseline response (Fig. 3A, right). Ca2+ responses to high K+ were additive to the increased baseline Ca2+ level due to H2O2. These data suggest that H2O2 used in this study did not elicit maximal Ca2+ responses in nTS cells and constitutes a rather moderate stimulus.
Fig. 3.
Ca2+ responses to H2O2 during membrane depolarization using high K+. A: example traces for intracellular Ca2+ responses to high K+ (5 stimulations) in the absence (left) and presence of 5 min H2O2 (right). Uniform (red lines; aligned to lowest Ca2+ level after the first K+ response) or individual (green lines on the right; 5.5-min window aligned to beginning of rise) baselines were used to access the area under the curve (AUC) for quantification of cytosolic Ca2+. B and C: averaged data depicting total content of Ca2+ using AUC for vehicle (n = 14) and H2O2 (n = 21) to a uniform baseline (B) or to individual baselines (C). *P ≤ 0.05 vs. vehicle; #P ≤ 0.05 vs. own baseline by two-way repeated-measures (RM)-ANOVA.
We quantified the changes in Ca2+, and the influence of 200 µM H2O2, by measuring the area under the curve (AUC) of the K+ response. This was accomplished using a 5.5-min analysis window aligned to the beginning of the Ca2+ rise for each K+-induced response. The length of the analysis window ensured a return of [Ca2+]i to baseline values and excluded any responses to subsequent perfusions with high K+. We initially examined the Ca2+ response from a single uniform baseline (Fig. 3B; red lines in Fig. 3A), defined as the lowest Ca2+ level following the first K+ stimulation. Such a baseline accommodated the diverse kinetics of Ca2+ removal (i.e., the time for [Ca2+]i to return to baseline levels) and evaluated the overall increase in Ca2+ from the initial baseline. In contrast to vehicle, H2O2 significantly increased the AUC, and this effect progressively continued into our washout periods (Fig. 3B).
We also measured the K+ response (i.e., the AUC) and the influence of H2O2 from individual baselines for each cell depolarization (green lines in Fig. 3A, right). Individual baseline analysis provided access to any H2O2-provoked changes in depolarization-induced Ca2+ currents while excluding changes of baseline Ca2+ level. The averaged group data show that responses to high K+ were similar in the presence and absence of H2O2, as well as in vehicle (Fig. 3C). These data indicate that H2O2 does not increase K+-evoked intracellular Ca2+ but rather provides an elevated baseline that is additive to depolarizations by high K+.
H2O2 Increases [Ca2+]i Through Extracellular Sources
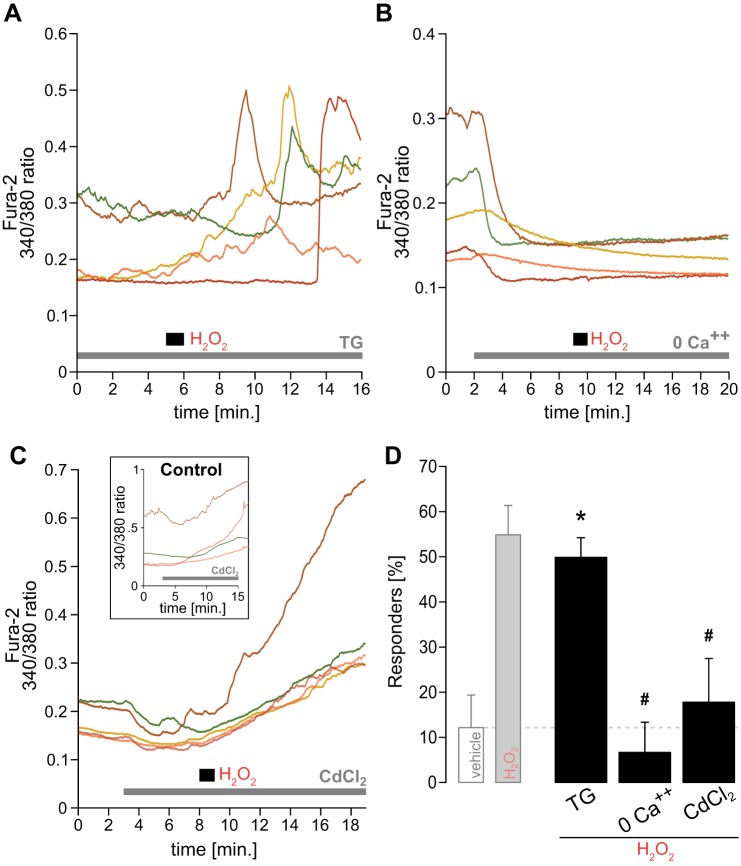
Cytosolic Ca2+ may increase due to its release from major intracellular stores such as the endoplasmic reticulum, or it may enter from the extracellular fluid. To identify the Ca2+ source we blocked several potential pathways and examined fura-2 fluorescence before and following H2O2. We initially used thapsigargin (TG; 1 µM), a potent inhibitor of the endoplasmic reticulum Ca2+-ATPase, to explore the role of this internal store. With TG in the perfusate, the typical delayed Ca2+ increase to H2O2 was still present (Fig. 4A). The latency of mean response peaks following H2O2 during TG (5.05 ± 0.47 min, n = 26) and its variance (5.41 ± 1.37, n = 26) were comparable to responses to H2O2 in the absence of TG. Furthermore, also the percentage of H2O2 responders during TG was similar to that responding to H2O2 without TG (Fig. 4D). These data indicate that the endoplasmic reticulum is not responsible for the H2O2-induced Ca2+ rise.
Fig. 4.
H2O2 increases intracellular Ca2+ concentration ([Ca2+]i) via extracellular sources. A: raw traces of intracellular Ca2+ to H2O2 in the presence of thapsigargin (1 µM; TG), an inhibitor of the endoplasmic reticulum Ca2+-ATPase. All examples shown stem from the same experiment with simultaneous H2O2 exposure. Note the high variance in peak response timing to H2O2. B: traces showing the typical lack of response to H2O2 in a zero Ca2+ perfusate with added EDTA (1 mM). C: Ca2+ levels to H2O2 in the presence of the voltage-gated calcium channel (VGCC) blocker CdCl2 (500 µM). Note the lack of H2O2-typical Ca2+ responses. Inset: examples of CdCl2-dependent rise of Ca2+ in the absence of H2O2. D: percentage of responders to H2O2 in the presence of TG (n = 51), zero Ca2+/EDTA (n = 21), and CdCl2 (n = 17), compared with vehicle and plain H2O2 (displayed in light gray since the same data is shown in Fig. 2E). *P ≤ 0.05 vs. vehicle; #P ≤ 0.05 vs. H2O2 by one-way ANOVA.
To further explore whether Ca2+ enters nTS cells from the extracellular space, we removed extracellular Ca2+. Removing extracellular Ca2+ using a zero Ca2+ perfusate with the Ca2+ chelator EDTA (1 mM) reduced cellular fura-2 fluorescence and completely ablated responses to H2O2 (Fig. 4B). The percentage of responders strongly declined and was significantly reduced compared with H2O2 alone (Fig. 4D). Thus H2O2 triggers Ca2+ entry in nTS cells across the cell membrane.
To identify the site of entry, we used the general blocker CdCl2 (500 µM) to block VGCCs. In control experiments where nTS neurons were exposed to 500 µM CdCl2 alone, an initial decrease was followed by a steady rise in fluorescence (Fig. 4C, inset). This effect was concentration independent since it also occurred using a fivefold lower concentration of CdCl2 (100 µM, data not shown). Apart from this CdCl2-induced rise in fluorescence, H2O2 did not further increase [Ca2+]i greater than threshold (Fig. 4C), as opposed to the previously observed responses shown in Figs. 2B and 4A. With CdCl2 the number of responders to H2O2 significantly decreased (Fig. 4D).
Protein Oxidation by H2O2 Contributes to Ca2+ Entry
H2O2 is known to exert its actions via oxidizing exposed sulfhydryl groups in other tissues (20). To identify whether the same is true for nTS neurons, we used the reducing agent dithiothreitol (DTT; 2 mM) to retain sulfhydryl groups in their reduced state. Under DTT perfusion, the percentage of neurons responding with elevated [Ca2+]i to H2O2 greatly decreased (16.8 ± 7.0%, P ≤ 0.05, n = 39, one-way ANOVA) to a value similar to vehicle perfusion and significantly different from H2O2 alone. These data suggest that the H2O2-induced increase in [Ca2+]i involves oxidative modulation of sulfhydryl groups.
H2O2 Causes a Delayed Enhancement of Calcium Currents
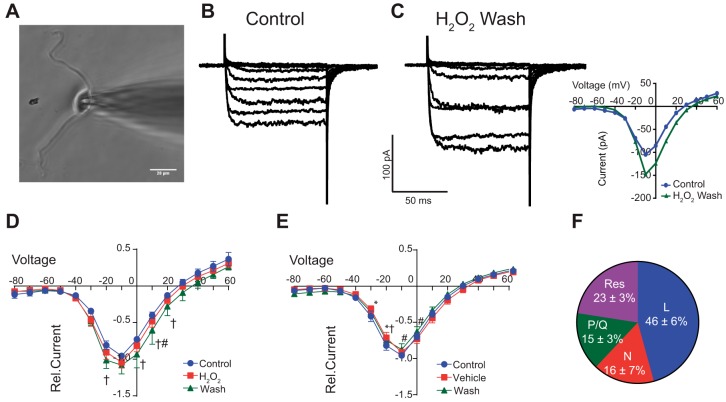
We next examined the contribution of H2O2 to modulate inward Ca2+ current () via VGCC. nTS neurons were isolated and depolarizing voltage steps were applied for 100 ms from −80 to +60 mV to evoke inward currents via VGCC (Fig. 5, A and B). Ba2+ served as the charge carrier and the currents evoked were classified as Ca2+ currents []. Step depolarization from −80 mV evoked that was activated at −40 mV with maximum currents observed at approximately −10 mV (Fig. 5B). Focal H2O2 did not have an immediate effect on (Fig. 5D, n = 12). However, and similar to our fura-2 experiments, removal (washout) of H2O2 significantly elevated (Fig. 5, C and D, inset). The small shift in reversal potential upon H2O2 washout was not significant from control (P = 0.376, paired t-test). Peak currents (Ipeak) increased with H2O2 wash by 20.7 ± 13.4% (P = 0.03 vs. control) and, following an additional 3-min washout period, returned to near baseline (3.1 ± 11.2%, P > 0.05 vs. control, ANOVA with Dunns multiple comparison, n = 11). Conversely, application of vehicle solution (instead of H2O2) did not increase during its application or washout periods (Fig. 5E, n = 8). Also, peak currents during vehicle wash did not significantly change over time. Together, these data confirm that the increase in was due to H2O2 exposure rather than “run-up” of the current.
Fig. 5.
H2O2 enhances calcium currents upon its removal. A: photomicrograph of nTS neuron with attached patch electrode. Scale bar = 20 μm. B and C: inward calcium currents () under control and following H2O2 removal in a single neuron. Note the increase in currents in the wash. Currents shown were evoked between −80 and +10 mV. C, inset: current-voltage (I-V) relationship of raw traces shown in B and C. Note the increase in currents from control (blue) with H2O2 wash (green). D: current voltage (I–V) plots for during control, H2O2, and H2O2 removal (i.e., wash). Currents are presented relative (Rel.) to peak control currents. Note the increase in currents in the wash period between −20 and +20 mV (green line); n = 12. E: I–V plots for vehicle (time) control experiments. Note the lack of increase in currents across the various time points; n = 8. F: relative proportion of L-, N-, and P/Q- subtype VGCC channels in nTS neurons; n = 7. Res, residual current that is blocked by Cd2+. *P ≤ 0.05, Veh/H2O2 vs. control; #P ≤ 0.05, Veh/H2O2 vs. wash; †P ≤ 0.05, wash vs. control by two-way RM-ANOVA.
In a separate set of neurons (n = 7), we sequentially added VGCC subtype blockers to identify their contribution to Ipeak (see materials and methods for details). These experiments showed that the total calcium currents in our dissociated nTS neurons were comprised of several high-voltage VGCCs, L, N, and P/Q, as well as a residual current that was blocked by 100 μM CdCl2 (Fig. 5F).
H2O2 Triggers Ca2+ Entry via Modulation of VGCCs
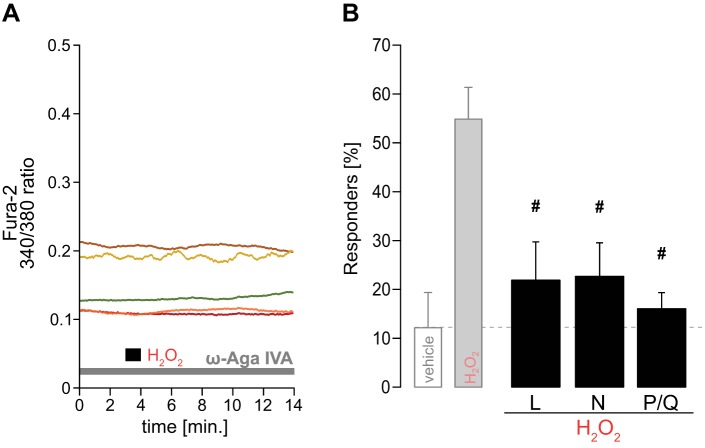
The ablation of H2O2-induced [Ca2+]i increases with removal of external Ca2+ or application of CdCl2 suggests the involvement of one or more membrane bound calcium channels. To elucidate the subtype of VGCCs involved, we repeated our fura-2 imaging with H2O2 stimulation in the presence of multiple antagonists to specifically block L-type (5 µM nifedipine), N-type (0.1 µM ω-conotoxin GVIA), and P/Q-type (0.1 µM ω-agatoxin IVA) VGCCs. Raw traces in Fig. 6A exemplify the absence of response to H2O2 after VGCC block (here: P/Q subtype). The group data show that individually blocking VGCC subtypes significantly reduced the number of responders to H2O2 (Fig. 6B).
Fig. 6.
Cytosolic Ca2+ rise by H2O2 via VGCCs. A: exemplary raw traces for H2O2 during P/Q-type VGCC blocker [0.1 µM ω-agatoxin (ω-aga) IVA]. Note the absent response to H2O2. B: averaged responders to H2O2 during different VGCC subtype-specific blockers: L type (5 µM nifedipine; n = 26), N type (0.1 µM ω-conotoxin GVIA; n = 27), and P/Q type (n = 38). Light gray bars are from data shown in Fig. 2E. #P ≤ 0.05 vs. H2O2 by one-way ANOVA.
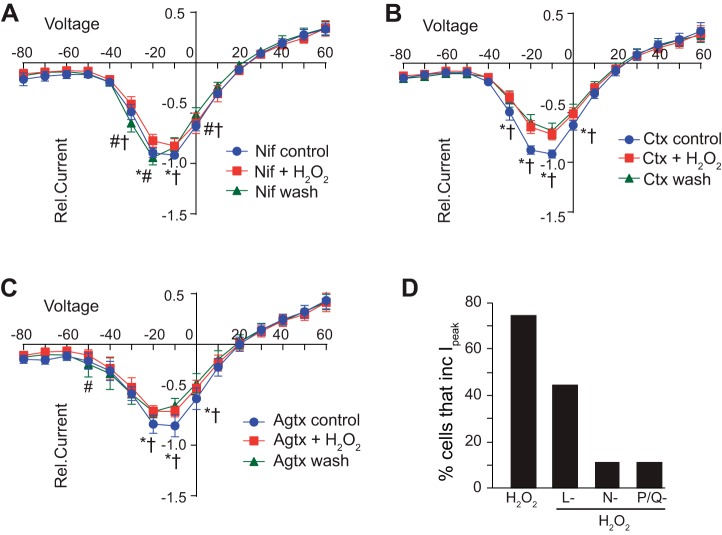
We next examined the contribution of VGCCs to H2O2-induced changes in inward . As in our fura-2 imaging experiments, after characterization of initial properties, L, N, or P/Q channels were blocked (1 min, control) followed by H2O2 (1 min) in VGCC antagonists. H2O2 was then removed and currents were again examined after 3–4 min in VGCC blocker (i.e., wash). As quantified, in the presence of nifedipine (Fig. 7A, n = 9), ω-conontoxin (Fig. 7B, n = 9), or ω-agatoxin (Fig. 7C, n = 9), the H2O2-induced increase in was eliminated. Similar to our fura-2 imaging data, the majority of nTS neurons responded to H2O2 wash with an increase in (9/12, Fig. 7D). Conversely, in the presence of an individual blocker only a small number of neurons increase peak to H2O2 (nifedipine 4/9; ω-conontoxin 1/9; and ω-agatoxin 1/9). To examine the impact of residual calcium channels (presumable R and/or T type) in these responses, we simultaneously blocked L, N, and P/Q channels with a cocktail of nifedipine, ω-conontoxin, and ω-agatoxin and examined the influence of H2O2 on . H2O2 did not increase Ipeak in the presence of all three blockers (−10.1 ± 2.6 pA/pF vs. in H2O2, −7.7 ± 2.3 pA/pF, P = 0.19, paired t-test, n = 4). H2O2 also did not augment during L, N, and P/Q blockade with 100 μM CdCl2 (Ipeak, VGCC block, 1.2 ± 0.4 pA/pF vs. H2O2 in VGCC block, 1.3 ± 0.6 pA/pF, P = 0.53, paired t-test, n = 6). Taken together, these data suggest H2O2 induces calcium entry in nTS neurons through modulation of multiple VGCCs.
Fig. 7.
VGCC block attenuated H2O2 increases in . Relative current-voltage (I–V) plots demonstrating that block of L (A)-, N (B)-, or P/Q (C)-type calcium channels attenuates H2O2-induced increases in . H2O2 was applied in the presence of VGCC block. Data are plotted relative to control (blocker alone) for each cell; n = 9 each. D: number of cells that increase peak currents within the I–V plot during H2O2 and H2O2 in the presence of L-, N-, and P/Q-type blockers. Nif, nifedipine; Ctx, conotoxin; Agtx, agatoxin. *P ≤ 0.05, H2O2 vs. control; # P ≤ 0.05, H2O2 vs. wash; †P ≤ 0.05, wash vs. control by two-way RM ANOVA.
DISCUSSION
In the present study, we show that short exposure of nTS neurons to H2O2 elicits a moderate and reversible increase in cytosolic Ca2+ ([Ca2+]i). This rise in [Ca2+]i affects baseline levels independent of cell depolarization. The response to H2O2 occurs within 10 min following stimulation and its timing is highly variable among cells. Eliminating the contribution of endoplasmic reticulum stores to [Ca2+]i changes did not alter H2O2-induced Ca2+ entry. On the other hand, removing extracellular Ca2+ or blocking general Ca2+ channels with CdCl2 prevented responses to H2O2. This increase on [Ca2+]i is most likely dependent on sulfhydryl oxidation. Elevation of [Ca2+]i occurs via multiple subtypes of VGCCs as shown by fura-2 imaging and patch-clamp electrophysiology.
The nTS is the first central synapse for integration and processing of several viscerosensory inputs before its relay to other central nuclei. This neurophysiological profile is reflected in the heterogeneous nature of its neuronal population due to well-known input and output connections, as well as its numerous interneurons. Our cultures likely represent this heterogeneity. While we do not know whether the neurons we recorded or imaged receive sensory information from baro-, chemo-, or other inputs, nor do we know their projections, it is likely that each neuron has its own distinct response to ROS and ionic channel composition and density. In addition, while our fura-2 imaging represents spontaneous influx of Ca2+ (under physiological conditions) due to ROS, our studies under patch-clamp depolarization-induced Ca2+ currents () represent the maximal changes in Ca2+ entry and allow the biophysical cell properties to be evaluated. While they cannot be directly compared due to these distinct differences, each may lend insight into the other on the modulatory role of H2O2.
We have previously shown in nTS slices that micromolar concentrations of H2O2 induce delayed hyperexcitability in postsynaptic nTS neurons (42). Here we examined the influence of H2O2 on [Ca2+]i as a potential contributor to these excitatory responses. This was tested in dissociated nTS neurons to eliminate the influence of synaptic activity and to examine the direct impact of H2O2 on cellular Ca2+. H2O2 increased [Ca2+]i with the greatest percentage of responders occurring at 200 µM H2O2. Higher concentration of H2O2 did not increase the number of responders or the magnitude of the response, which could possibly be attributed to cytotoxicity and/or inactivation of the Ca2+ channels. The augmentation of [Ca2+]i was relatively modest (27% across all concentrations using Ca2+ imaging) and readily reversed. A similar ~21% increase to 200 µM H2O2 was observed with the Ca2+ currents of nTS cells in our patch-clamp analysis and in [Ca2+]i of rat hippocampal cells (16). The H2O2 concentrations examined encompass those expected during endogenous ROS production (i.e., 15–150 µM H2O2) considering the predicted 10- to 100-fold decrease with cell entry (47). Given the moderate and reversible response, our data suggest that H2O2 did not produce permanent oxidative damage but rather modulated Ca2+ via one or more mechanisms.
In contrast to the rapid, time-invariant, and robust increase in [Ca2+]i to K+ depolarization, H2O2 produced effects that varied greatly in their onset among cells. This variability is consistent with our previous study and others (16, 20, 26, 42) that demonstrated H2O2 induces functional cellular responses of up to 6 min following its application. Several possibilities may contribute to the delayed response in nTS neurons. For instance, we have shown that H2O2 initially hyperpolarizes the membrane potential of nTS cells by increasing barium-sensitive potassium currents (42). This effect is reversible with removal of H2O2 and may thus contribute to the longer response latency of [Ca2+]i, which may differ among cells. Another possibility is that that concentration or location of endogenous antioxidants protecting ROS-sensitive targets may vary among cells. We have shown that the antioxidant catalase is widely distributed in nTS neurons in a dense perinuclear network (42); a similar expression was seen in dissociated nTS neurons. Responses may then be delayed when target sites of H2O2 are particularly close to ROS-scavenging antioxidants. Another possibility for increased variability is the site of modulation for the Ca2+ increase. If the effect of exogenous H2O2 requires passage through the cellular membrane to reach intracellular targets, it is likely to require a longer onset for responses. We have previously shown that H2O2 exerts its effects via modulation of intracellular sites (42).
Several potential sites by which H2O2 affects Ca2+ were examined. The endoplasmic reticulum is a major intracellular Ca2+ store in neurons and may contribute to [Ca2+]i rises upon ROS exposure. Thapsigargin is the prototypical inhibitor of SERCA pumps that control release and reuptake of Ca2+ in the endoplasmic reticulum (37). When oxidized, SERCA pumps are inhibited and augment [Ca2+]i (11). In the present study, thapsigargin did not prevent the H2O2-induced augmentation of Ca2+, suggesting that intracellular endoplasmic reticulum stores did not substantially contribute to its elevation. However, depletion of intracellular stores by thapsigargin may also induce extracellular calcium entry via activation of store-operated (capacitive) channels, and H2O2 may contribute to this effect (19). In the absence of extracellular Ca2+, H2O2-induced responses were eliminated and similar to vehicle. Exogenous cadmium, a nonspecific VGCC blocker, also effectively eliminated H2O2-induced Ca2+ rises, suggesting the involvement of VGCCs in the Ca2+ influx across the membrane. Unexpectedly, CdCl2 alone induced a steady rise in fura-2 fluorescence independent from our H2O2 stimulation. This increase may be due to Cd2+ augmenting [Ca2+]i by block of membrane-bound Ca2+-ATPase or Na+-Ca2+ exchange (28). Cadmium is also able to increase ROS by depleting intracellular antioxidants (15) to elevate intracellular Ca2+, as shown in other cell cultures (57). Alternatively, Cd2+ is able to directly bind to fura-2 and increase the fluorescent signal (22, 41), which may contribute in the present study. Taken together, these data indicate H2O2 enhances [Ca2+]i via influx across the plasma membrane and not intracellular stores.
nTS neurons express the high-voltage-activated L, N, and P/Q VGCCs (2, 14). Direct exogenous application of H2O2 to isolated nTS neurons augments L-type Ca2+ currents (54). Likewise, NADPH oxidase-derived ROS potentiate L-type Ca2+ currents in nTS neurons during angiotensin II signaling (55). Our electrophysiological recordings further support the idea that H2O2 enhances . Interestingly, block of a single VGCC attenuated H2O2-induced responders as well and the increase in . This was not anticipated as while one VGCC was blocked (e.g., L-type), the other channels should remain functional and thus continue to provide a H2O2 response. While we do not know the exact mechanism by which this did not occur, several possibilities may exist. For instance, the antagonists used may not specifically block the subtype desired. This possibility is unlikely as we used concentrations similar to other studies (13, 18, 30, 35), the neurons were only exposed to a given antagonist for a short period, and each blocker when given sequentially provided a proportional block (Fig 5F). Another possibility is that each neuron likely expresses a unique proportion of VGCCs on its membrane, with its threshold for activation determined by the membrane potential and intracellular signals. Thus, in the neurons examined, the influence of a particular VGCC blocker on H2O2 responses may not be equally distributed across channels or neurons. The modest 27% increase in fura-2 fluorescence and 21% increase in by H2O2 is likely spread across multiple subtypes, which is dependent on their expression pattern on individual neurons. In addition, the H2O2 increase in fluorescence does not occur in every neuron at the concentrations we tested; this may be due, in part, to our conservative threshold in our fura-2 analysis. H2O2 may induce a response less than this threshold after an individual VGCC blocker. Nevertheless, although not significant, the continued number of responders (fura-2, Fig 6) and increases in peak current (Fig 7D) in the presence of VGCC blockers suggest that other channels are responding to H2O2. Additional future studies are required to completely delineate these possible mechanisms.
While H2O2 augmented basal [Ca2+]i, and our studies show a prominent role of VGCCs in this response, there was only a small, nonsignificant increase in the [Ca2+]i (fura-2) response during concurrent K+-induced depolarization. The H2O2-induced augmentation in [Ca2+]i rather seemed independent and additive to K+-induced increases in [Ca2+]i. As noted above, depolarization with high K+ in the absence of H2O2 induced a robust increase in [Ca2+]i. The kinetics and time course of these increases were comparable to previous studies in isolated nTS neurons (21, 46), although with the observed time course fura-2 acting as a Ca2+ buffer cannot be completely ruled out (5). Although we did not isolate the influence of each Ca2+ channel subtype in our imaging studies, our results suggest minimal ROS effects during more pronounced K+-induced Ca2+ entry. This may be due to reduced Ca2+ drive during K+-induced elevation of [Ca2+]i, which would subsequently minimize the influx produced by H2O2. Alternatively, H2O2 may differentially affect VGCC subtype currents, perhaps having opposite and offsetting effects. However, given that all VGCC antagonists tested in the present study reduced ROS effects on [Ca2+]i and of VGCC subtypes, this mechanism is less likely. Additional membrane sources or ion channels activated by depolarization may also temper H2O2-mediated Ca2+ responses. For instance, activation of voltage-gated K+ channels may limit membrane depolarization and thus may not further increase Ca2+ influx. Washout of K+ and the resulting repolarization may permit prolonged VGCC activation as the membrane voltage may be within the range for its activation and thus induce the observed increases in baseline Ca2+. This may especially be prevalent in intact cells. The lack of changes in immediately during H2O2 in our patch-clamp studies is consistent with the fura-2 data suggesting minimal initial influence of ROS but rather a prolonged effect that is observed during the wash period.
Exposed cysteine residues (sulfhydryl groups) are primary targets for oxidation by ROS (10), and Ca2+ currents are directly altered when associated with the channel itself (9). We demonstrated the necessity of sulfhydryl oxidation in the augmentation of [Ca2+]i after H2O2 in nTS cells. Specifically, in the presence of the reducing-agent DTT, H2O2 did not augment [Ca2+]i. Our results are consistent with H2O2 augmenting Ca2+ influx via sulfhydryl oxidation of P/Q channels resulting in facilitation of channel opening (34). Also, oxidative modulation by H2O2 increases cardiac L-type current that is effectively blocked in the presence of DTT (50). Ca2+ channel activity can also increase due to direct glutathionylation by oxidized glutathione (25), an antioxidant for H2O2. Glutathione, in turn, is oxidized at its exposed sulfhydryl groups (36).
While our data suggest that H2O2 alters Ca2+ entry via oxidation and modulation of membrane-bound VGCC channels, other possibilities may exist. H2O2 may inhibit Ca2+-ATPase and Na+-Ca2+ exchanger pumps on the plasma membrane to elevate [Ca2+]i (29, 58). However, our electrophysiological data clearly show that H2O2 enhances across the membrane via VGCCs, although both mechanisms are not necessarily exclusive. Regardless of the mechanism, our studies demonstrate that H2O2 enhances [Ca2+]i through modulation of one or more ion channel functions. Ongoing experiments may help to elucidate these possibilities in the near future, as well as to identify the phenotype of nTS neurons and the extent to which they are sensitive to H2O2.
In summary, we have shown that physiological concentrations of H2O2 activate VGCCs via oxidation of sulfhydryl groups resulting in raised [Ca2+]i and in the majority of nTS cells. This increase in Ca2+ occurs in a delayed fashion and likely explains the delayed hyperexcitability of nTS neurons in response to H2O2 (42). The H2O2-induced increased Ca2+ in nTS neurons likely influences physiological responses and may constitute the underlying mechanisms for the bradycardia and hypotension observed with H2O2 microinjections in the nTS (7). Since ROS are readily produced under normal physiological conditions within the nTS (54), these data widen our understanding of the modulatory actions H2O2 has in this critical cardiorespiratory brainstem region. It may also be part of the central processes involved in diseases associated with excess ROS along the cardiorespiratory neuroaxis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-098602 (to D. D. Kline) and HL-128454 (to D. D. Kline) and American Heart Association Grant 12POST11670002 (to T. D. Ostrowski).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.D.O., L.P.-P., and D.D.K. conceived and designed the research; T.D.O., H.A.D., L.P.-P., and D.D.K. performed experiments; T.D.O., H.A.D., L.P.-P., and D.D.K. analyzed data; T.D.O., L.P.-P., and D.D.K. interpreted results of experiments; T.D.O. and D.D.K. prepared figures; T.D.O. and D.D.K. drafted manuscript; T.D.O., L.P.-P., and D.D.K. edited and revised manuscript; T.D.O., H.A.D., L.P.-P., and D.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Cathy Kovarik for helpful suggestions on cell culture and Dr. Eileen Hasser for helpful comments on the manuscript.
REFERENCES
- 1.Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 2.Aoki Y, Yamada E, Endoh T, Suzuki T. Multiple actions of extracellular ATP and adenosine on calcium currents mediated by various purinoceptors in neurons of nucleus tractus solitarius. Neurosci Res 50: 245–255, 2004. doi: 10.1016/j.neures.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Avshalumov MV, Chen BT, Koós T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci 25: 4222–4231, 2005. doi: 10.1523/JNEUROSCI.4701-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belugin S, Mifflin S. Transient voltage-dependent potassium currents are reduced in NTS neurons isolated from renal wrap hypertensive rats. J Neurophysiol 94: 3849–3859, 2005. doi: 10.1152/jn.00573.2005. [DOI] [PubMed] [Google Scholar]
- 5.Berlin JR, Konishi M. Ca2+ transients in cardiac myocytes measured with high and low affinity Ca2+ indicators. Biophys J 65: 1632–1647, 1993. doi: 10.1016/S0006-3495(93)81211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown AM, Schwindt PC, Crill WE. Voltage dependence and activation kinetics of pharmacologically defined components of the high-threshold calcium current in rat neocortical neurons. J Neurophysiol 70: 1530–1543, 1993. https://www.ncbi.nlm.nih.gov/pubmed/7506757. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso LM, Colombari DS, Menani JV, Toney GM, Chianca DA Jr, Colombari E. Cardiovascular responses to hydrogen peroxide into the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 297: R462–R469, 2009. doi: 10.1152/ajpregu.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan SH, Chan JY. Brain stem oxidative stress and its associated signaling in the regulation of sympathetic vasomotor tone. J Appl Physiol (1985) 113: 1921–1928, 2012. doi: 10.1152/japplphysiol.00610.2012. [DOI] [PubMed] [Google Scholar]
- 9.Chiamvimonvat N, O’Rourke B, Kamp TJ, Kallen RG, Hofmann F, Flockerzi V, Marban E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ Res 76: 325–334, 1995. doi: 10.1161/01.RES.76.3.325. [DOI] [PubMed] [Google Scholar]
- 10.Chung HS, Wang SB, Venkatraman V, Murray CI, Van Eyk JE. Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. Circ Res 112: 382–392, 2013. doi: 10.1161/CIRCRESAHA.112.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csordás G, Hajnóczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta 1787: 1352–1362, 2009. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumitrascu R, Heitmann J, Seeger W, Weissmann N, Schulz R. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid Med Cell Longev 2013: 234631, 2013. doi: 10.1155/2013/234631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endoh T. Pharmacological characterization of inhibitory effects of postsynaptic opioid and cannabinoid receptors on calcium currents in neonatal rat nucleus tractus solitarius. Br J Pharmacol 147: 391–401, 2006. doi: 10.1038/sj.bjp.0706623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endoh T, Sato D, Wada Y, Shibukawa Y, Ishihara K, Hashimoto S, Yoshinari M, Matsuzaka K, Tazaki M, Inoue T. Galanin inhibits calcium channels via Galpha(i)-protein mediated by GalR1 in rat nucleus tractus solitarius. Brain Res 1229: 37–46, 2008. doi: 10.1016/j.brainres.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 15.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1: 529–539, 2001. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 16.Gerich FJ, Funke F, Hildebrandt B, Fasshauer M, Müller M. H(2)O(2)-mediated modulation of cytosolic signaling and organelle function in rat hippocampus. Pflugers Arch 458: 937–952, 2009. doi: 10.1007/s00424-009-0672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giusti MF, Sato MA, Cardoso LM, Braga VA, Colombari E. Central antioxidant therapy inhibits parasympathetic baroreflex control in conscious rats. Neurosci Lett 489: 115–118, 2011. doi: 10.1016/j.neulet.2010.11.077. [DOI] [PubMed] [Google Scholar]
- 18.Glaum SR, Miller RJ. Presynaptic metabotropic glutamate receptors modulate omega-conotoxin-GVIA-insensitive calcium channels in the rat medulla. Neuropharmacology 34: 953–964, 1995. doi: 10.1016/0028-3908(95)00076-I. https://www.ncbi.nlm.nih.gov/pubmed/8532176. [DOI] [PubMed] [Google Scholar]
- 19.Grupe M, Myers G, Penner R, Fleig A. Activation of store-operated I(CRAC) by hydrogen peroxide. Cell Calcium 48: 1–9, 2010. doi: 10.1016/j.ceca.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan SM, Redzic ZB, Alshuaib WB. Hydrogen peroxide-induced reduction of delayed rectifier potassium current in hippocampal neurons involves oxidation of sulfhydryl groups. Brain Res 1520: 61–69, 2013. doi: 10.1016/j.brainres.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Hay M, Edwards GL, Lindsley K, Murphy S, Sharma RV, Bhalla RC, Johnson AK. Increases in cytosolic Ca2+ in rat area postrema/mNTS neurons produced by angiotensin II and arginine-vasopressin. Neurosci Lett 151: 121–125, 1993. doi: 10.1016/0304-3940(93)90001-2. [DOI] [PubMed] [Google Scholar]
- 22.Hinkle PM, Shanshala ED II, Nelson EJ. Measurement of intracellular cadmium with fluorescent dyes. Further evidence for the role of calcium channels in cadmium uptake. J Biol Chem 267: 25553–25559, 1992. https://www.ncbi.nlm.nih.gov/pubmed/1281160. [PubMed] [Google Scholar]
- 23.Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol 108: 37–77, 1949. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwahori Y, Ikegaya Y, Matsuki N. Hyperpolarization-activated current I(h) in nucleus of solitary tract neurons: regional difference in serotonergic modulation. Jpn J Pharmacol 88: 459–462, 2002. doi: 10.1254/jjp.88.459. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone VP, Hool LC. Glutathionylation of the L-type Ca2+ channel in oxidative stress-induced pathology of the heart. Int J Mol Sci 15: 19203–19225, 2014. doi: 10.3390/ijms151019203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamsler A, Segal M. Hydrogen peroxide modulation of synaptic plasticity. J Neurosci 23: 269–276, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kay AR, Wong RK. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods 16: 227–238, 1986. doi: 10.1016/0165-0270(86)90040-3. https://www.ncbi.nlm.nih.gov/pubmed/3523050. [DOI] [PubMed] [Google Scholar]
- 28.Van Kerkhove E, Pennemans V, Swennen Q. Cadmium and transport of ions and substances across cell membranes and epithelia. Biometals 23: 823–855, 2010. doi: 10.1007/s10534-010-9357-6. [DOI] [PubMed] [Google Scholar]
- 29.Kip SN, Strehler EE. Rapid downregulation of NCX and PMCA in hippocampal neurons following H2O2 oxidative stress. Ann NY Acad Sci 1099: 436–439, 2007. doi: 10.1196/annals.1387.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kline DD, Hendricks G, Hermann G, Rogers RC, Kunze DL. Dopamine inhibits N-type channels in visceral afferents to reduce synaptic transmitter release under normoxic and chronic intermittent hypoxic conditions. J Neurophysiol 101: 2270–2278, 2009. doi: 10.1152/jn.91304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kline DD, King TL, Austgen JR, Heesch CM, Hasser EM. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience 167: 510–527, 2010. doi: 10.1016/j.neuroscience.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuehl-Kovarik MC, Partin KM, Magnusson KR. Acute dissociation for analyses of NMDA receptor function in cortical neurons during aging. J Neurosci Methods 129: 11–17, 2003. doi: 10.1016/S0165-0270(03)00196-1. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Prog Neurobiol 48: 21–53, 1996. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 34.Li A, Ségui J, Heinemann SH, Hoshi T. Oxidation regulates cloned neuronal voltage-dependent Ca2+ channels expressed in Xenopus oocytes. J Neurosci 18: 6740–6747, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li DP, Chen SR. Nitric oxide stimulates glutamatergic synaptic inputs to baroreceptor neurons through potentiation of Cav2.2-mediated Ca(2+) currents. Neurosci Lett 567: 57–62, 2014. doi: 10.1016/j.neulet.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012: 736837, 2012. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem 266: 17067–17071, 1991. [PubMed] [Google Scholar]
- 38.Meng Z, Nie A. Effects of hydrogen peroxide on sodium current in acutely isolated rat hippocampal CA1 neurons. Toxicol Lett 147: 45–52, 2004. doi: 10.1016/j.toxlet.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Monteith GR. Seeing is believing: recent trends in the measurement of Ca2+ in subcellular domains and intracellular organelles. Immunol Cell Biol 78: 403–407, 2000. doi: 10.1046/j.1440-1711.2000.00920.x. [DOI] [PubMed] [Google Scholar]
- 40.Nunes FC, Ribeiro TP, França-Silva MS, Medeiros IA, Braga VA. Superoxide scavenging in the rostral ventrolateral medulla blunts the pressor response to peripheral chemoreflex activation. Brain Res 1351: 141–149, 2010. doi: 10.1016/j.brainres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Ohkubo M, Miyamoto A, Shiraishi M. Heavy metal chelator TPEN attenuates fura-2 fluorescence changes induced by cadmium, mercury and methylmercury. J Vet Med Sci 78: 761–767, 2016. doi: 10.1292/jvms.15-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostrowski TD, Hasser EM, Heesch CM, Kline DD. H2O2 induces delayed hyperexcitability in nucleus tractus solitarii neurons. Neuroscience 262: 53–69, 2014. doi: 10.1016/j.neuroscience.2013.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostrowski TD, Ostrowski D, Hasser EM, Kline DD. Depressed GABA and glutamate synaptic signaling by 5-HT1A receptors in the nucleus tractus solitarii and their role in cardiorespiratory function. J Neurophysiol 111: 2493–2504, 2014. doi: 10.1152/jn.00764.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Paula PM, Tolstykh G, Mifflin S. Chronic intermittent hypoxia alters NMDA and AMPA-evoked currents in NTS neurons receiving carotid body chemoreceptor inputs. Am J Physiol Regul Integr Comp Physiol 292: R2259–R2265, 2007. doi: 10.1152/ajpregu.00760.2006. [DOI] [PubMed] [Google Scholar]
- 45.Polo-Parada L, Pilar G. Kappa- and mu-opioids reverse the somatostatin inhibition of Ca2+ currents in ciliary and dorsal root ganglion neurons. J Neurosci 19: 5213–5227, 1999. https://www.ncbi.nlm.nih.gov/pubmed/10377333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhim H, Miller RJ. Opioid receptors modulate diverse types of calcium channels in the nucleus tractus solitarius of the rat. J Neurosci 14: 7608–7615, 1994. https://www.ncbi.nlm.nih.gov/pubmed/7996199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice ME. H2O2: a dynamic neuromodulator. Neuroscientist 17: 389–406, 2011. doi: 10.1177/1073858411404531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sah R, Galeffi F, Ahrens R, Jordan G, Schwartz-Bloom RD. Modulation of the GABA(A)-gated chloride channel by reactive oxygen species. J Neurochem 80: 383–391, 2002. doi: 10.1046/j.0022-3042.2001.00706.x. [DOI] [PubMed] [Google Scholar]
- 49.Strege PR, Bernard CE, Ou Y, Gibbons SJ, Farrugia G. Effect of mibefradil on sodium and calcium currents. Am J Physiol Gastrointest Liver Physiol 289: G249–G253, 2005. doi: 10.1152/ajpgi.00022.2005. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y, Xu J, Minobe E, Shimoara S, Hao L, Kameyama M. Regulation of the Cav1.2 cardiac channel by redox via modulation of CaM interaction with the channel. J Pharmacol Sci 128: 137–143, 2015. doi: 10.1016/j.jphs.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev 79: 1089–1125, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Tolstykh G, Belugin S, Tolstykh O, Mifflin S. Responses to GABA(A) receptor activation are altered in NTS neurons isolated from renal-wrap hypertensive rats. Hypertension 42: 732–736, 2003. doi: 10.1161/01.HYP.0000084371.17927.02. [DOI] [PubMed] [Google Scholar]
- 53.Tolstykh G, de Paula PM, Mifflin S. Voltage-dependent calcium currents are enhanced in nucleus of the solitary tract neurons isolated from renal wrap hypertensive rats. Hypertension 49: 1163–1169, 2007. doi: 10.1161/HYPERTENSIONAHA.106.084004. [DOI] [PubMed] [Google Scholar]
- 54.Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension 48: 482–489, 2006. doi: 10.1161/01.HYP.0000236647.55200.07. [DOI] [PubMed] [Google Scholar]
- 55.Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci 24: 5516–5524, 2004. doi: 10.1523/JNEUROSCI.1176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Zhang SXL, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol 174: 307–316, 2010. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu B, Chen S, Luo Y, Chen Z, Liu L, Zhou H, Chen W, Shen T, Han X, Chen L, Huang S. Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS One 6: e19052, 2011. doi: 10.1371/journal.pone.0019052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaidi A. Plasma membrane Ca-ATPases: targets of oxidative stress in brain aging and neurodegeneration. World J Biol Chem 1: 271–280, 2010. doi: 10.4331/wjbc.v1.i9.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension 45: 717–723, 2005. doi: 10.1161/01.HYP.0000153463.22621.5e. [DOI] [PubMed] [Google Scholar]