Abstract
Low-carbohydrate/high-fat (LCHF) diets are increasingly popular dietary interventions for body weight control and as treatment for different pathological conditions. However, the mechanisms of action are still poorly understood, in particular, in long-term administration. Besides liver, brain, and heart, skeletal muscle is one of the major organs involved in the regulation of physiological and pathophysiological ketosis. We assessed the role of the peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) in skeletal muscle of male wild-type control and PGC-1α muscle-specific knockout mice upon 12 wk of LCHF diet feeding. Interestingly, LCHF diet administration increased oxygen consumption in a muscle PGC-1α-dependent manner, concomitant with a blunted transcriptional induction of genes involved in fatty acid oxidation and impairment in exercise performance. These data reveal a new role for muscle PGC-1α in regulating the physiological adaptation to long-term LCHF diet administration.
Keywords: skeletal muscle, ketogenic diet, PGC-1α, exercise, ketone bodies
in recent years, ketogenic diets have emerged as potent therapeutic strategies for numerous diseases (27). In contrast to classical high-fat diets, ketogenic diets are characterized by a lower content of carbohydrates and proteins and will promote a dietary state reminiscent of fasting, diametrically opposite of the fed-like phenotype evoked by high-fat diets. Historically, low-carbohydrate/high-fat (LCHF) diets have been developed for and successfully used in the treatment of epilepsy, in particular, to reduce seizures in children who are nonresponders to pharmacological interventions (19). Increasing evidence has expanded the use of LCHF diets to metabolic disorders, such as obesity, cardiovascular diseases, or type 2 diabetes, but also to certain types of cancer (6, 7, 9, 12, 30, 37). LCHF diets induce a state known as ketosis, which also occurs physiologically after prolonged fasting periods, exercise, or other contexts of low-carbohydrate availability (20). Ketosis is characterized by the increased production of ketone bodies, such as β-hydroxybutyrate (β-OHB) and acetoacetate, in a process called ketogenesis in the liver (14). Circulating ketone bodies are then used by extrahepatic tissues as energy substrates in the Krebs cycle and oxidative phosphorylation, in particular, in the brain, skeletal, and heart muscles. The exact mechanisms by which LCHF diets exert their actions are still poorly understood. However, increased fatty acid oxidation (25, 34), mitochondrial biogenesis, and ATP production (8) have been proposed to be important pathways mediating the positive effects of ketogenic diets.
The peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) functions as an essential transcriptional coactivator for target genes in all of these metabolic processes (4). Furthermore, PGC-1α regulates ketolytic gene expression in skeletal muscle and thereby, potently affects systemic ketosis (33). Strikingly, high-muscle PGC-1α reduced postexercise ketosis in mice, as previously observed in trained vs. untrained individuals (1, 33), and thus constitutes a major regulator of ketone body homeostasis in exercise. Moreover, skeletal muscle emerges as the key tissue to modulate ketone body homeostasis actively and voluntarily. Importantly, the beneficial and detrimental effects of long-term administration of LCHF diets are still debated, and the compatibility with exercise training is unclear. Therefore, we now tested whether muscle PGC-1α, the regulatory nexus in endurance training, also contributes to the local and systemic effects of long-term LCHF diet feeding and thus evaluated whole-body homeostasis and skeletal muscle metabolism in wild-type control (CTRL) and PGC-1α muscle-specific knockout (mKO) mice fed an LCHF diet for 12 wk. Indeed, we demonstrate that PGC-1α in skeletal muscle is not only essential for basal ketolytic gene expression but also affects exercise performance and whole-body oxygen consumption (V̇o2) upon LCHF diet feeding. These findings reveal a new role for PGC-1α in systemic ketone body metabolism and shed new light onto the mechanisms through which LCHF diets exert their effects.
MATERIALS AND METHODS
Mice and diets.
Male mice, at the age of 15 wk, were housed in a conventional facility with a 12-h:12-h light-dark cycle, with free access to food and water. Experiments were performed in accordance with Swiss federal guidelines and were approved by the Kantonales Veterinäramt of Kanton Basel-Stadt, Switzerland. The C57BL/6 PGC-1α mKO mice used in this study were generated as described in Svensson et al. (33). A chow diet (AIN-93G; 7% fat, 58.5% carbohydrates, and 18% protein) and a ketogenic diet (XL75:XP10; 74.4% fat, 3% carbohydrates, and 9.9% protein) were purchased from Provimi Kliba AG (Kaiseraugst, Switzerland). After 12 wk of chow or LCHF diet feeding ad libitum, mice were not fed for 2 h in the morning and euthanized by carbon dioxide inhalation, and tissue samples were collected.
Body composition and indirect calorimetry.
Body weight was monitored weekly, and body composition was determined using an EchoMRI-100 analyzer (EchoMRI, Houston, TX) at the end of the treatment period.
Mice were placed in a Comprehensive Lab Animal Monitoring System (Columbus Instruments, Columbus, OH) to assess V̇o2, carbon dioxide production, the respiratory exchange ratio (RER), as well as food intake and spontaneous locomotion (number of breaks of infrared beams in x,y,z dimensions).
Exercise tests.
Animals were acclimatized to an open treadmill (Columbus Instruments) for 2 days before the start of the experiment, for 5 min at 0 m/min, followed by 5 min at 8 m/min and 5 min at 10 m/min, with an incline of 5°. The endurance exercise trial started at 5 m/min for 5 min with a 5° incline, followed by 8 m/min for 10 min. The speed of the treadmill was subsequently increased by 2 m/min every 15 min until exhaustion. Basal blood glucose and lactate levels were assessed in tail-vein blood before and after exercise. For indirect calorimetry assessments, mice were acclimatized to treadmill running, as described above. Mice were placed in a closed treadmill (Columbus Instruments), where they first sat for 5 min at 0 m/min at a 5° incline. Subsequently, the test started at 8 m/min for 5 min, and the speed was increased every 5 min for 2 m/min until exhaustion.
Blood analysis.
Blood glucose, lactate, and β-OHB were measured in tail-vein blood with a glucose meter (Accu-Chek; Roche, Mannheim, Germany), a Lactate Plus meter (Nova Biomedical, Waltham, MA), or a β-OHB meter (Precision Xtra; Abbott Laboratories, Abbott Park, IL). For plasma analysis, whole tail-vein blood was collected in Microvette tubes (Sarstedt, Nümbrecht, Germany) and centrifuged at 2,000 g for 5 min. Total cholesterol, aspartate transaminase (ASAT), and alanine transaminase (ALAT) levels were analyzed with a Cobas c 111 system (Roche Diagnostics AG, Rotkreuz, Switzerland). Nonesterified fatty acids (NEFAs) were measured in plasma using a NEFA kit, according to the manufacturer’s instructions (Wako Diagnostics, Richmond, VA).
Glycogen measurement.
Frozen tissue (10 mg) was homogenized in 200 µl water using a motorized pestle. To inactivate enzymes, samples were boiled at 95°C in a water bath for 10 min before centrifugation at 18,000 g. Supernatant was assayed for glycogen using a glycogen assay kit, according to the manufacturer’s instructions (Abcam, Cambridge, UK).
RNA extraction and qRT-PCR.
Frozen tissue was homogenized, and total RNA was extracted with TRIzol reagent (Thermo Fisher Scientific, Zug, Switzerland), according to the manufacturer’s protocol. cDNA synthesis was done using 1 µg total RNA. Semiquantitative real-time PCR (semi-qRT-PCR) analysis was performed with Fast SYBR Green Master Mix on a StepOnePlus Real-Time PCR System (both from Thermo Fisher Scientific, Waltham, MA). Relative expression levels for each gene of interest were calculated with the comparative threshold (ΔΔCt) method, using 18S rRNA as the normalization control. The primer sequences are listed in Table 1.
Table 1.
qRT-PCR primer list
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| 18S | AGTCCCTGCCCTTTGTACACA | CGATCCGAGGGCCTCACTA |
| Acadl | CCAGCTAATGCCTTACTTGGAGA | GCAATTAAGAGCCTTTCCTGTGG |
| Acadvl | GTAGCCTCCATCCGAAGCTC | CAGGCCCCCATTACTGATCC |
| Acat1 | GTGAAGGAAGTCTACATGGGCA | TGTGGTGCATGGAGTGGAAATA |
| Bdh1 | TTTGCTGGCTGTTTGATGAAGG | TTGAGCTGGATGGTTCTCAGTC |
| CD36 | GGCAAAGAACAGCAGCAAAAT | TGGCTAGATAACGAACTCTGTATGTGT |
| Cpt1b | ATCATGTATCGCCGCAAACT | CCATCTGGTAGGAGCACATGG |
| Cs | CCCAGGATACGGTCATGCA | GCAAACTCTCGCTGACAGGAA |
| ERRα | ACTGCAGAGTGTGTGGATGG | GCCCCCTCTTCATCTAGGAC |
| Glut 4 | GATGAGAAACGGAAGTTGGAGAGA | GCACCACTGCGATGATCAGA |
| HKII | AAAACCAAGTGCAGAAGGTTGAC | GAACCGCCTAGAAATCTCCAGAA |
| Mct1 | TGCAACGACCAGTGAAGTATCA | ACAACCACCAGCGATCATTACT |
| Oxct1 | CCCATACCCACTGAAAGACGAA | CTGGAGAAGAAAGAGGCTCCTG |
| Pdk4 | AAAATTTCCAGGCCAACCAA | CGAAGAGCATGTGGTGAAGGT |
| Pfkm | GGGGATCACCAATCTGTGTGT | ATCATTCAGCAAGTCGCTCCA |
| PGC-1α | AGCCGTGACCACTGACAACGAG | GCTGCATGGTTCTGAGTGCTAAG |
| PGC-1β | CCATGCTGTTGATGTTCCAC | GACGACTGACAGCACTTGGA |
| Pkm1 | CATTATCGTGCTCACCAAGTCTG | GATTTCGAGTCACGGCAATGATA |
| PPARα | ACAAGGCCTCAGGGTACCA | GCCGAAAGAAGCCCTTACAG |
| PPARδ | GCAAGCCCTTCAGTGACATCA | CCAGCGCATTGAACTTGACA |
| Sdhb | TGACGTCAGGAGCCAAAATGG | CCTCGACAGGCCTGAAACTG |
| Uqcrc2 | CCCATCTTGCTTTGCTGTCTG | AATAAAATCTCGAGAAGGACCCG |
Immunoblot analysis.
Tissues were homogenized in radioimmunoprecipitation assay buffer, and equal amounts of proteins were separated on SDS-polyacrylamide gels and transferred to a nitrocellulose membrane (Whatman; Sigma-Aldrich, St. Louis, MO). The proteins of interest were detected with the following antibodies: succinyl-CoA:3-ketoacid-CoA transferase 1 (OXCT1; ab105320; Abcam), acetyl-CoA acetyltransferase 1 (ACAT1; HPA004428; Sigma-Aldrich), eukaryotic elongation factor 2 (eEF2; 2332; Cell Signaling Technology, Danvers, MA), mitoprofile (MS604; Mitosciences, Eugene, OR), and polyclonal swine anti-rabbit Igs/horseradish peroxidase or polyclonal rabbit anti-mouse Igs/horseradish peroxidase, respectively (P0399 and P0260; Dako, Kyoto, Japan). Densitometric analysis of immunoblots was performed on six individual samples with ImageJ software (National Institutes of Health, Bethesda, MD); a representative selection from this group is presented in the respective figures.
Seahorse assay.
Total mitochondria were isolated from fresh quadriceps muscle using gradual centrifugation. Minced muscle was homogenized with a motorized pestle and centrifuged at 700 g for 10 min. Supernatant was recentrifuged at 10,500 g for 10 min to obtain a crude mitochondrial pellet. Equal amounts of protein were plated on a 96-well Seahorse plate, and mitochondrial respiration was measured using the Seahorse XF Cell Mito Stress Test kit (103015-100; Agilent Technologies, Santa Clara, CA) on an XFe96 Extracellular Flux Analyzer (Agilent Technologies). The assay buffer was supplemented with either 10 mM malate/10 mM pyruvate or 20 mM succinate/2 µM rotenone, respectively, to assess complex I or complex II activity. The amount of ADP used was 4 mM, and ATP production was estimated by subtracting ADP-induced V̇o2 rate (OCR) values from oligomycin-induced OCR values.
Statistical analysis.
Data are presented as means ± SE. The unpaired two-tailed Student’s t-test was used to determine differences between groups. Significance was set at P < 0.05, and significant differences between the genotypes (CTRL CHOW vs. mKO CHOW and CTRL LCHF vs. mKO LCHF diets) and between the conditions (CTRL CHOW vs. CTRL LCHF and mKO CHOW vs. mKO LCHF diets) were marked.
RESULTS
PGC-1α mKO mice fail to increase V̇o2 on an LCHF diet.
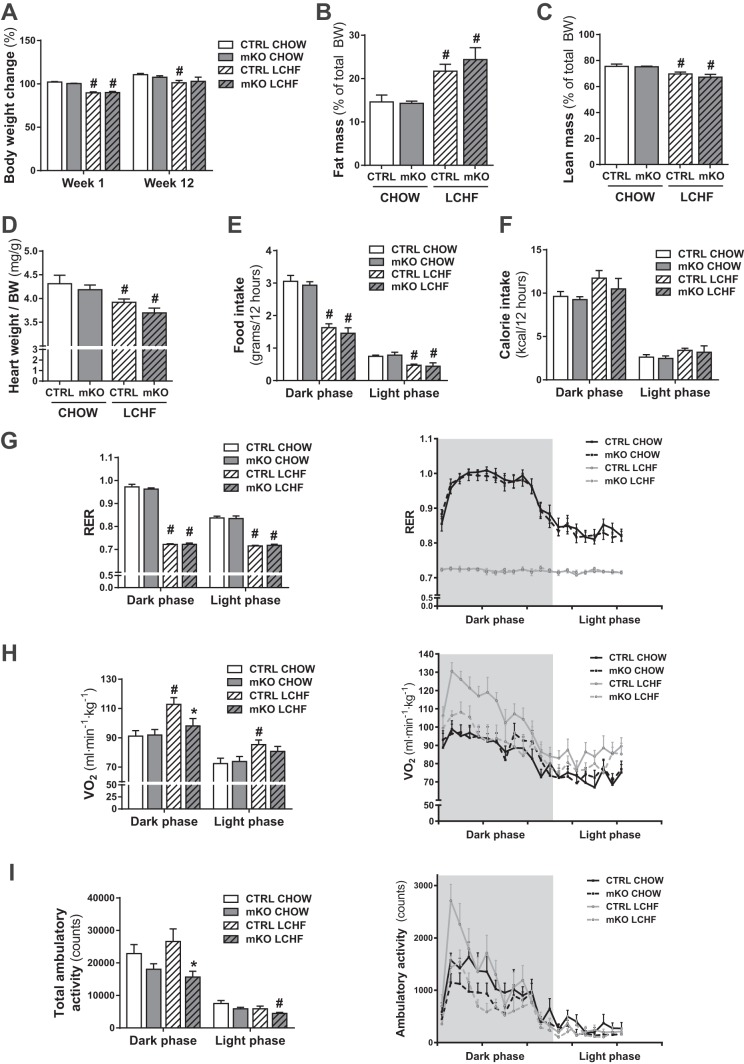
PGC-1α mKO and CTRL mice were fed a normal chow diet or an LCHF diet for 12 wk. Both LCHF diet-fed CTRL and PGC-1α mKO mice showed a reduction in body weight after 1 wk compared with the chow-fed cohorts (Fig. 1A). After 12 wk, only the LCHF diet-fed CTRL mice were significantly lighter than their chow-fed counterparts (Fig. 1A). At the end of the 12 wk of LCHF diet feeding, CTRL and PGC-1α mKO mice displayed a significant increase in fat mass (Fig. 1B), as well as reduced lean mass (Fig. 1C), compared with the chow-fed cohorts. This was reflected further in the relative decrease in heart weight (Fig. 1D) in LCHF diet-fed compared with chow-fed mice. LCHF diet feeding resulted in reduced food intake by weight (Fig. 1E) but importantly, not by caloric content (Fig. 1F). LCHF diet-fed mice showed a significant decrease in RER compared with chow-fed mice (Fig. 1G), which reflected the high-fat content of the LCHF diet. Interestingly, LCHF diet feeding increased the OCR only in CTRL mice, whereas PGC-1α mKO mice displayed no increase with LCHF diet feeding (Fig. 1H). LCHF diet-fed PGC-1α mKO mice also showed a significantly reduced ambulatory activity compared with LCHF diet-fed CTRL mice (Fig. 1I). These findings indicate that PGC-1α mKO mice exhibit a blunted adaptation to long-term LCHF diet feeding.
Fig. 1.
LCHF diet feeding increases fat mass and oxygen consumption (V̇o2) while lowering the respiratory exchange ratio (RER). A: body weight (BW) curve of mice with an initial weight of 28 g fed a chow or an LCHF diet for 1 or 12 wk (n = 13–16). B: fat mass in percent of total body weight measured by EchoMRI in mice fed a chow or LCHF diet for 12 wk (n = 7–8). C: lean mass in percent of total body weight measured by EchoMRI in mice fed a chow or LCHF diet for 12 wk (n = 7–8). D: relative heart weight of mice fed a chow or LCHF diet for 12 wk (n = 7–8). E: average food intake, measured over a 48-h period, in mice fed a chow or LCHF diet for 8 wk (n = 6–8). F–I: average calorie intake (F), RER (G), V̇o2 rate (H), and total ambulatory activity (I) measured by indirect calorimetry over a 48-h period in mice fed a chow or LCHF diet for 8 wk (n = 7–8). Error bars represent SE. *Significant differences between genotypes: chow-fed CTRL and mKO mice and LCHF diet-fed CTRL and mKO mice (P < 0.05), respectively; #significant differences between conditions: chow and LCHF diet-fed CTRL and chow and LCHF diet-fed mKO mice (P < 0.05), respectively.
PGC-1α mKO mice show a reduced induction of genes encoding proteins involved in fatty acid metabolism in skeletal muscle.
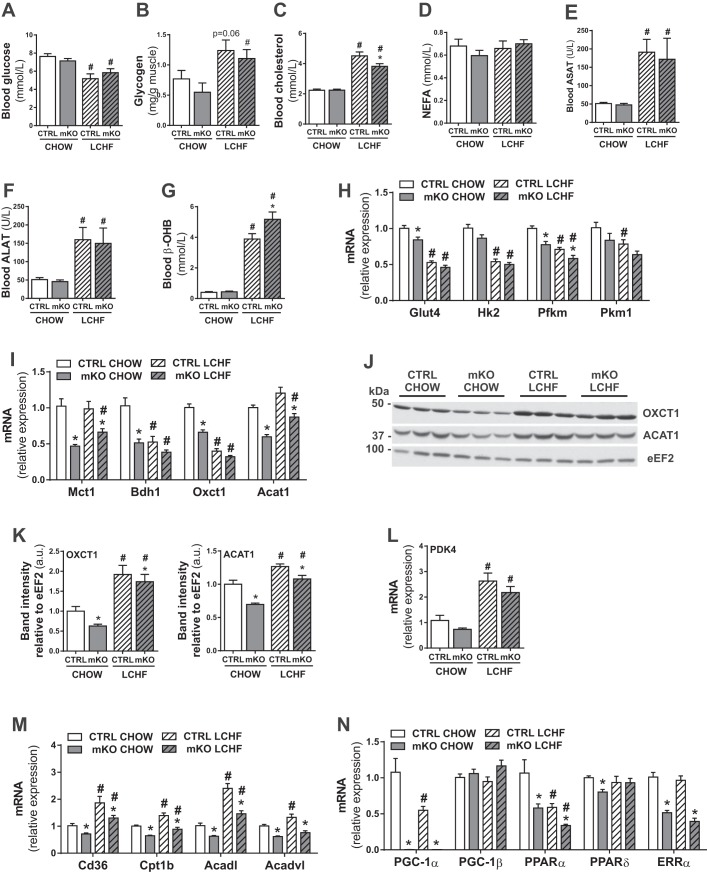
LCHF diets affect both glucose and cholesterol metabolism (6, 7, 9, 12, 30, 37). In our study, LCHF diet feeding led to reduced circulating glucose levels and increased muscle glycogen content in CTRL and PGC-1α mKO mice (Fig. 2, A and B) compared with the chow-fed counterparts. Circulating cholesterol levels were increased in both genotypes (Fig. 2C). However, blood cholesterol was significantly lower in LCHF diet-fed PGC-1α mKO mice compared with LCHF diet-fed CTRL mice (Fig. 2C). Circulating NEFAs were not different between the groups (Fig. 2D). In line with previous studies (15, 17), significantly increased circulating levels of ASAT and ALAT were observed in LCHF diet-fed CTRL and PGC-1α mKO mice (Fig. 2, E and F), indicative of liver stress caused by LCHF diet feeding. Furthermore, LCHF diet feeding elevated circulating β-OHB levels in both cohorts, even though PGC-1α mKO mice depicted a significant hyperketonemia compared with CTRL mice (Fig. 2G), similar to our previous observations (33). Next, we assessed the impact of LCHF diet feeding on metabolic pathways in skeletal muscle of CTRL and PGC-1α mKO mice. In line with the reduced circulating glucose levels with LCHF diet feeding, there was a significant reduction in the expression of genes involved in glucose uptake [glucose transporter 4 (Glut4)] and glycolysis [hexokinase II (HKII); muscle phosphofructokinase (Pfkm); pyruvate kinase muscle 1 (Pkm1)] in skeletal muscle from LCHF diet-fed CTRL and PGC-1α mKO mice (Fig. 2H). Surprisingly, the transcription of ketolytic genes [3-OHB dehydrogenase type 1 (Bdh1) and Oxct1] was reduced significantly upon LCHF diet feeding (Fig. 2I). In stark contrast, protein levels of OXCT1 and ACAT1 were increased significantly (Fig. 2, J and K). The transcript levels of Glut4, Pfkm, monocarboxylate-transporter 1 (Mct1), Bdh1, Oxct1, and Acat1 (Fig. 2, H and I) were lower in PGC-1α mKO mice, even when compared with LCHF diet-fed CTRL animals. The increased levels of pyruvate dehydrogenase lipoamide kinase isozyme 4 (Pdk4) with LCHF diet feeding (Fig. 2L) and various genes encoding proteins involved in fatty acid uptake [cluster of differentiation 36 (Cd36)] and oxidation [carnitine palmitoyltransferase 1b (Cpt1b); CoA dehydrogenase long chain (Acadl); CoA dehydrogenase very long chain (Acadvl)] indicate a substrate shift toward fatty acid metabolism in CTRL mice (Fig. 2M). Importantly, the induction of these genes was blunted in PGC-1α mKO mice (Fig. 2M). Interestingly, despite the central role of PGC-1α and peroxisome proliferator-activated receptor α (PPARα) for the transcriptional control of fatty acid metabolism in skeletal muscle (35), gene expression of both of these regulators was reduced in muscle with LCHF diet feeding (Fig. 2N). Furthermore, the expression levels of PGC-1β, PPARδ, and estrogen-related receptor α (ERRα) were not changed upon LCHF diet feeding, but transcript levels of PPARδ and ERRα were reduced significantly in PGC-1α mKO mice (Fig. 2N).
Fig. 2.
LCHF diet-fed mice show a PGC-1α-dependent switch from glucose to fatty acid oxidation in skeletal muscle. A: plasma glucose levels of mice fed a chow or LCHF diet for 12 wk (n = 7–8). B: relative glycogen levels in gastrocnemius muscle of mice fed a chow or LCHF diet for 12 wk (n = 6–8). C–G: plasma total cholesterol (C), nonesterified fatty acids (NEFA; D), ASAT (E), ALAT (F), and β-hydroxybutyrate (β-OHB; G) levels of mice fed a chow or LCHF diet for 12 wk (n = 7–9). H and I: gene expression in gastrocnemius muscle relative to 18S of genes involved in glucose metabolism (H) and ketolysis (I; n = 6–8). Hk2, HKII. J and K: representative immunoblots (J) and protein levels (K) of OXCT1 and ACAT1 in gastrocnemius muscle relative to eukaryotic elongation factor 2 (eEF2; n = 6). L–N: gene expression in gastrocnemius muscle relative to 18S of PDK4 (L) and genes involved in fatty acid uptake and oxidation (M) and transcriptional regulation (N; n = 6–8). Error bars represent SE. *Significant differences between genotypes: chow-fed CTRL and mKO mice and LCHF diet-fed CTRL and mKO mice (P < 0.05), respectively; #significant differences between conditions: chow and LCHF diet-fed CTRL and chow and LCHF diet-fed mKO mice (P < 0.05), respectively.
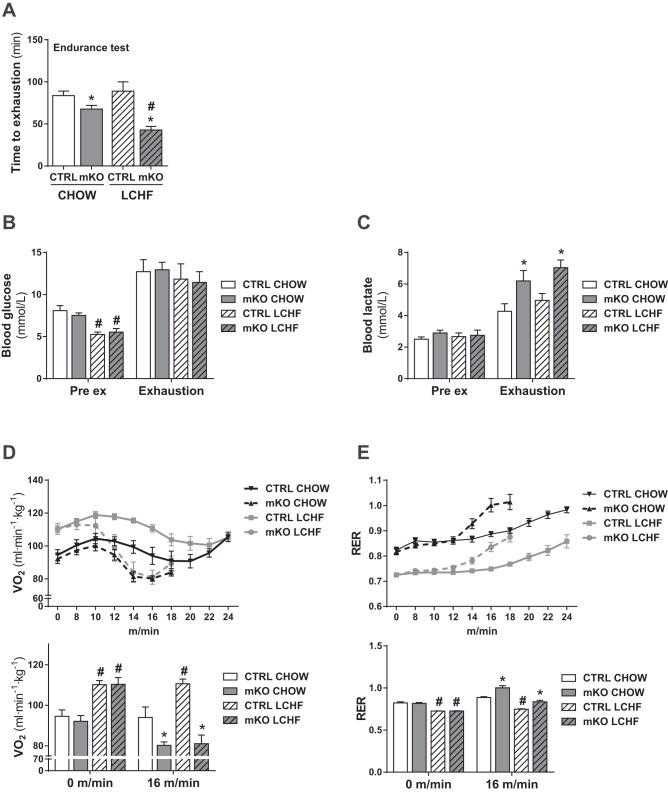
LCHF diet feeding leads to impaired exercise performance, specifically in PGC-1α mKO mice.
Since LCHF diet-fed PGC-1α mKO mice showed a blunted induction of fatty acid metabolism in skeletal muscle, we were interested if this would affect exercise performance and substrate use during endurance exercise. In line with previous findings (16), PGC-1α mKO mice displayed reduced endurance exercise performance compared with CTRL mice (Fig. 3A). LCHF diet feeding did not affect the endurance capacity of CTRL mice (Fig. 3A). Strikingly however, this diet specifically impaired the exercise performance of PGC-1α mKO mice (Fig. 3A). This phenotype was not associated with any impairment in the ability of PGC-1α mKO mice to increase circulating glucose levels with exercise (Fig. 3B). Moreover, whereas PGC-1α mKO mice showed elevated blood lactate levels upon exhaustion, as previously published (32), this effect was compared between chow-fed and LCHF diet-fed PGC-1α mKO mice (Fig. 3C). In closed treadmills, LCHF diet-fed mice displayed elevated V̇o2 during the exercise compared with chow-fed mice (Fig. 3D). CTRL mice were able to maintain this elevated V̇o2 during the entire exercise period, except for the last time point of measurement (Fig. 3D). In contrast, V̇o2 levels dropped rapidly in PGC-1α mKO animals as exercise intensity increased (Fig. 3D). Similarly, PGC-1α mKO animals could not maintain the low RER observed in LCHF diet-fed CTRL mice and displayed an earlier shift to carbohydrate metabolism, indicated by the sharp increase in RER (Fig. 3E). These differences were, however, diet independent, since chow-fed mKO mice also performed significantly worse than their CTRL littermates. Collectively, these findings suggest that LCHF diet-fed PGC-1α mKO mice have difficulties in keeping up with the increased energy demand in endurance exercise and are unable to properly cope with the metabolic changes elicited by LCHF diet feeding, in particular, in exercise.
Fig. 3.
PGC-1α in skeletal muscle is essential to maintain adequate energy levels during exercise upon LCHF diet feeding. A: endurance exercise test of mice fed a chow or LCHF diet for 10 wk (n = 7–8). B and C: blood glucose (B) and lactate (C) levels before (Pre ex) and after (Exhaustion) exhaustive endurance exercise test of mice fed a chow or LCHF diet for 10 wk (n = 7–8). D and E: average oxygen consumption rate (V̇o2; D) and respiratory exchange ratio (RER; E) measured by indirect calorimetry in a closed treadmill of mice fed a chow or LCHF diet for 11 wk and corresponding bar graphs (n = 6–8). Error bars represent SE. *Significant differences between genotypes: chow-fed CTRL and mKO mice and LCHF diet-fed CTRL and mKO mice (P < 0.05), respectively; #significant differences between conditions: chow and LCHF diet-fed CTRL and chow and LCHF diet-fed mKO mice (P < 0.05), respectively.
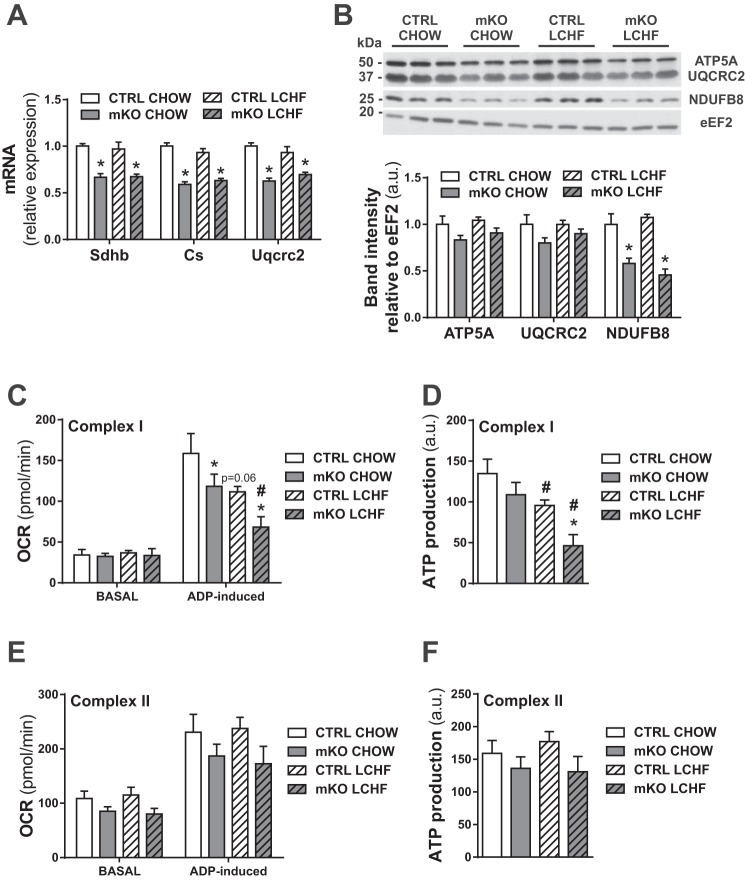
LCHF diet feeding does not lead to increased mitochondrial biogenesis or ATP levels in skeletal muscle.
Ketogenic diet feeding has been proposed to increase mitochondrial biogenesis and ATP levels in the context of neurological diseases (8). Thus to test whether LCHF diet feeding also leads to an induction of mitochondrial biogenesis in skeletal muscle, we measured the levels of mitochondrial gene expression [mitochondrial succinate dehydrogenase iron-sulfur subunit (Sdhb); citrate synthase (Cs); mitochondrial cytochrome b-c1 complex subunit 2 (Uqcrc2)], as well as mitochondrial proteins (ATP synthase 5 alpha; UQCRC2; mitochondrial NADH dehydrogenase 1 beta subcomplex subunit 8; Fig. 4, A and B). As expected, PGC-1α mKO mice exhibited reduced mitochondrial gene expression and protein content (22, 23). However, in contrast to studies in neurological tissues (8), LCHF diet feeding did not lead to increased mitochondrial transcript or protein levels in skeletal muscle (Fig. 4, A and B). Furthermore, mitochondria isolated from quadriceps muscles of LCHF diet-fed mice showed a drop in ADP-induced complex I respiration and concomitant complex I ATP production (Fig. 4, C and D), whereas complex II respiration was not affected by LCHF diet feeding (Fig. 4, E and F).
Fig. 4.
LCHF diet feeding does not affect mitochondrial biogenesis and lowers ATP production in skeletal muscle. A: gene expression in gastrocnemius muscle relative to 18S of genes involved in mitochondrial homeostasis (n = 6–8). B: protein levels of different mitochondrial chain complexes in gastrocnemius muscle relative to eukaryotic elongation factor 2 (eEF2) and representative immunoblots (n = 6). ATP5A, ATP synthase 5 alpha; NDUFB8, mitochondrial NADH dehydrogenase 1 beta subcomplex subunit 8. C and D: complex I-induced V̇o2 rate (OCR; C) and estimated ATP production (D) of isolated mitochondria from quadriceps muscle (n = 4–6). E and F: complex II-induced OCR (E) and estimated ATP production (F) of isolated mitochondria from quadriceps muscle (n = 4–6). Error bars represent SE. *Significant differences between genotypes: chow-fed CTRL and mKO mice and LCHF diet-fed CTRL and mKO mice (P < 0.05), respectively; #significant differences between conditions: chow and LCHF diet-fed CTRL and chow and LCHF diet-fed mKO mice (P < 0.05), respectively.
DISCUSSION
Besides physical activity, dietary interventions are a mainstay of prevention and therapy of many diseases. LCHF diets have been increasingly studied in the past decades due to their therapeutic potential, not only in the treatment of epilepsy and other brain-related disorders but also in other pathologies that are associated with peripheral organs (27). Endogenous ketone body levels are, in part, controlled by hepatic ketogenesis. Dietary ketosis is, however, largely determined by the ketone body metabolism in the brain, heart, and skeletal muscle. Of these three main consumers, only skeletal muscle can be directly and voluntarily affected, and indeed, training can reduce postexercise ketosis (1). Moreover, we have demonstrated previously that muscle PGC-1α can modulate systemic ketosis in numerous acute physiological and pathophysiological contexts (33). Here, we show that muscle PGC-1α likewise contributes to the local and systemic adaptations of long-term LCHF diet feeding. In particular, LCHF diet-induced V̇o2 was severely blunted in PGC-1α mKO mice. Even more dramatic, LCHF diet-fed PGC-1α mKO mice displayed a marked impairment in running performance already at moderate exercise intensities, and the initial increased OCR quickly dropped to the same level as that of chow-fed PGC-1α mKO mice. In contrast, LCHF diet-fed CTRL mice were able to run the same amount of time as their chow-fed counterparts, despite their reduced lean mass, assuming that the efficiency of consuming energy from fats is higher upon LCHF diet feeding, as suggested by the study of Paoli et al. (25). The analysis of skeletal muscle samples revealed that transcript levels of genes involved in fatty acid uptake and oxidation were elevated in LCHF diet-fed CTRL mice, whereas the upregulation of these genes in PGC-1α mKO animals was blunted. It is conceivable that the difference in OCRs between LCHF diet-fed PGC-1α mKO and CTRL mice is, in part, due to this reduced induction of the respective genes in skeletal muscle in PGC-1α mKO animals. Thus PGC-1α seems to participate in the LCHF diet-controlled metabolic switch from glucose to ketone body and fatty acid use. Furthermore, the decrease in activity levels in LCHF diet-fed mKO mice could also contribute to the reduced V̇o2. Thus muscle PGC-1α might thereby influence whole-body metabolism in LCHF diet feeding.
Given the important role of PGC-1α in systemic ketone body metabolism (33) and exercise (28), these findings raise questions about the compatibility of LCHF diets and training. Studies, so far, have been inconclusive as to whether LCHF diets improve or hinder training adaptations (24). For example, in the recent study of Zajac et al. (38), maximal V̇o2 and the lactate threshold were increased significantly in off-road cyclists treated with an LCHF diet. In competitive gymnasts, LCHF diets do not negatively impact explosive and strength performance, only when an adequate amount of protein is provided (26). Thus administration of LCHF diets might differ in endurance compared with resistance training, since LCHF diet feeding induces a “fasting-like” state that could hinder the buildup of muscle mass. The inherent problems of the LCHF diet could be circumvented by direct administration of ketone bodies, e.g., in the form of transesterified β-OHB precursor metabolites without the massive acid/salt load associated with intake of β-OHB in acid or salt form (11). The nutritional ketosis elicited by such metabolites promoted an improvement in endurance performance in cyclists, even in the presence of normal muscle glycogen, elevated insulin levels, or coadministrated carbohydrates (11).
The “Atkins diet,” a particular form of the LCHF diet, has popularized LCHF diet interventions for weight loss (3). However, despite the widespread use of the Atkins and related diets, the molecular mechanisms and potential detrimental effects are still largely unknown. Indeed, in our study, LCHF diet-fed mice displayed some negative effects on whole-body metabolism. Even though LCHF diet feeding led to an initial weight loss after 1 wk of treatment, which has also been shown in other rodent studies (5, 17, 18), the difference in body weight after 12 wk of LCHF diet feeding was only minor. Second, LCHF diet-fed mice displayed an increase in fat mass and a concomitant decrease in lean mass (10, 36). Even more alarmingly, LCHF diet-fed animals showed increased circulating levels of cholesterol, ASAT, and ALAT, indicative of dyslipidemia, and a certain degree of liver stress, in line with other studies in mice and humans (13, 21, 31, 39). In fact, long-term administration of LCHF diets in rodents, in most cases, leads to the development of hepatic steatosis and nonalcoholic fatty liver disease (29). Thus even though the effect of such diets on hepatic lipid levels in humans is less clear, caution is advised, in particular, in patients with nonalcoholic fatty liver disease (2). It is possible that administration of transesterified ketone body precursor metabolites could act therapeutically without the potential side effects of an LCHF diet (11). Furthermore, whereas the reason for the reduced cholesterol levels in LCHF diet-fed mKO compared with CTRL mice is unclear, this change might be a consequence of the hyperketonemia in mKO animals. Thus our previous (33) and present findings would suggest that physical activity and thereby elevation of muscle PGC-1α are important adjuvant interventions to manage the pathological consequences of ketosis.
In the brain, the therapeutic effect of LCHF diets on seizures and other pathologies has been linked to increased mitochondrial biogenesis or ATP levels (8). Surprisingly, even though the elevated V̇o2 and the lower RER values of LCHF diet-fed mice indicate an overall increase in oxidative metabolism, we did not find any change in mitochondrial gene expression and protein levels in skeletal muscle. Intriguingly, ATP production in isolated mitochondria from LCHF diet-fed mice was even lower than in chow-fed mice. Thus the observed increase in oxidative metabolism upon LCHF diet feeding is most likely due to the availability of energy substrates, which are mainly ketone bodies and other kind of fats. Furthermore, these data indicate that LCHF diet feeding predominantly acts on fatty acid oxidation rather than on mitochondrial biogenesis or ATP production in skeletal muscle. Moreover, a recent study in mitochondrial myopathy patients showed short-term adverse and long-term beneficial effects of LCHF diet feeding on skeletal muscle health. Acute treatment of patients with a modified Atkins diet resulted in muscle damage, especially in ragged-red fibers, indicating that nutrition can modify mitochondrial disease progression (1). Surprisingly, in the 2.5-yr follow-up study, patients showed improvements in muscle strength, suggesting that the initial fiber degeneration promoted subsequent fiber regeneration, resulting in increased muscle force. Thus care must be taken when administering LCHF diets to patients with mitochondrial-associated diseases and in evaluating responses to short-term treatment.
Taken together, our results clearly demonstrate that PGC-1α in skeletal muscle is essential for maintaining sufficient energy levels during prolonged muscle contractions, especially when carbohydrate availability is low, with important implications for whole-body metabolism and energy homeostasis. Finally, it is important to note that even though an LCHF diet induces beneficial health effects by increasing systemic oxidative metabolism, such interventions also exert potentially detrimental effects, including increasing total blood cholesterol levels—a known risk factor for cardiovascular diseases—or impaired liver function. Therefore, future studies should aim at elucidating the potential of non-LCHF diet-based interventions to modulate ketone body levels, such as nutritional ketosis. Alternatively, physiological, e.g., by adjuvant physical activity, or pharmacological modulation of muscle PGC-1α should be considered to mitigate the unwanted side effects of such interventions.
GRANTS
This project was funded by the Swiss National Science Foundation, European Research Council (ERC) Consolidator Grant 616830-MUSCLE_NET, Swiss Cancer Research Grant KFS-3733-08-2015, Swiss Society for Research on Muscle Diseases (SSEM), SystemsX.ch, “Novartis Stiftung für Medizinisch-Biologische Forschung,” and University of Basel.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.S. conceived and designed research; S.S., K.S., and B.C. performed experiments; S.S., K.S., B.C., and C.H. analyzed data; S.S., K.S., B.C., and C.H. interpreted results of experiments; S.S. and K.S. prepared figures; S.S. and C.H. drafted manuscript; S.S. and C.H. edited and revised manuscript; S.S., K.S., B.C., and C.H. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of K. Svensson: Dept. of Orthopaedic Surgery, Univ. of California San Diego School of Medicine, La Jolla, CA 92037.
REFERENCES
- 1.Ahola S, Auranen M, Isohanni P, Niemisalo S, Urho N, Buzkova J, Velagapudi V, Lundbom N, Hakkarainen A, Muurinen T, Piirilä P, Pietiläinen KH, Suomalainen A. Modified Atkins diet induces subacute selective ragged-red-fiber lysis in mitochondrial myopathy patients. EMBO Mol Med 8: 1234–1247, 2016. doi: 10.15252/emmm.201606592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asrih M, Jornayvaz FR. Diets and nonalcoholic fatty liver disease: the good and the bad. Clin Nutr 33: 186–190, 2014. doi: 10.1016/j.clnu.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Atkins RC. Dr. Atkins’ Diet Revolution: The High Calorie Way to Stay Thin Forever. New York: D. McKay, 1972. [Google Scholar]
- 4.Austin S, St-Pierre J. PGC1α and mitochondrial metabolism—emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci 125: 4963–4971, 2012. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- 5.Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab 297: E1197–E1204, 2009. doi: 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackburn GL, Phillips JC, Morreale S. Physician’s guide to popular low-carbohydrate weight-loss diets. Cleve Clin J Med 68: 761–764, 2001. doi: 10.3949/ccjm.68.9.761. [DOI] [PubMed] [Google Scholar]
- 7.Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 142: 403–411, 2005. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- 8.Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 48: 43–58, 2007. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 9.Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab 88: 1617–1623, 2003. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 10.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr 26: 1–22, 2006. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 11.Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray AJ, Stubbs B, West J, McLure SW, King MT, Dodd MS, Holloway C, Neubauer S, Drawer S, Veech RL, Griffin JL, Clarke K. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab 24: 256–268, 2016. doi: 10.1016/j.cmet.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Dashti HM, Al-Zaid NS, Mathew TC, Al-Mousawi M, Talib H, Asfar SK, Behbahani AI. Long term effects of ketogenic diet in obese subjects with high cholesterol level. Mol Cell Biochem 286: 1–9, 2006. doi: 10.1007/s11010-005-9001-x. [DOI] [PubMed] [Google Scholar]
- 13.Ellenbroek JH, van Dijck L, Töns HA, Rabelink TJ, Carlotti F, Ballieux BE, de Koning EJ. Long-term ketogenic diet causes glucose intolerance and reduced β- and α-cell mass but no weight loss in mice. Am J Physiol Endocrinol Metab 306: E552–E558, 2014. doi: 10.1152/ajpendo.00453.2013. [DOI] [PubMed] [Google Scholar]
- 14.Fukao T, Lopaschuk GD, Mitchell GA. Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids 70: 243–251, 2004. doi: 10.1016/j.plefa.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, Ezenwajiaku N, Cotter DG, Brunt EM, Crawford PA. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol 300: G956–G967, 2011. doi: 10.1152/ajpgi.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem 282: 30014–30021, 2007. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 17.Jornayvaz FR, Jurczak MJ, Lee HY, Birkenfeld AL, Frederick DW, Zhang D, Zhang XM, Samuel VT, Shulman GI. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab 299: E808–E815, 2010. doi: 10.1152/ajpendo.00361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab 292: E1724–E1739, 2007. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- 19.Kessler SK, Neal EG, Camfield CS, Kossoff EH. Dietary therapies for epilepsy: future research. Epilepsy Behav 22: 17–22, 2011. doi: 10.1016/j.yebeh.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krebs HA. The regulation of the release of ketone bodies by the liver. Adv Enzyme Regul 4: 339–354, 1966. doi: 10.1016/0065-2571(66)90027-6. [DOI] [PubMed] [Google Scholar]
- 21.Kwiterovich PO Jr, Vining EP, Pyzik P, Skolasky R Jr, Freeman JM. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA 290: 912–920, 2003. doi: 10.1001/jama.290.7.912. [DOI] [PubMed] [Google Scholar]
- 22.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3: e101, 2005. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119: 121–135, 2004. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Paoli A, Bianco A, Grimaldi KA. The ketogenic diet and sport: a possible marriage? Exerc Sport Sci Rev 43: 153–162, 2015. doi: 10.1249/JES.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 25.Paoli A, Grimaldi K, Bianco A, Lodi A, Cenci L, Parmagnani A. Medium term effects of a ketogenic diet and a Mediterranean diet on resting energy expenditure and respiratory ratio. BMC Proc 6, Suppl 3: P37, 2012. doi: 10.1186/1753-6561-6-S3-P37. [DOI] [Google Scholar]
- 26.Paoli A, Grimaldi K, D’Agostino D, Cenci L, Moro T, Bianco A, Palma A. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J Int Soc Sports Nutr 9: 34, 2012. doi: 10.1186/1550-2783-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr 67: 789–796, 2013. doi: 10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 546: 851–858, 2003. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schugar RC, Crawford PA. Low-carbohydrate ketogenic diets, glucose homeostasis, and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care 15: 374–380, 2012. doi: 10.1097/MCO.0b013e3283547157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Blüher M, Stumvoll M, Stampfer MJ; Dietary Intervention Randomized Controlled Trial (DIRECT) Group . Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 359: 229–241, 2008. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 31.Sirven J, Whedon B, Caplan D, Liporace J, Glosser D, O’Dwyer J, Sperling MR. The ketogenic diet for intractable epilepsy in adults: preliminary results. Epilepsia 40: 1721–1726, 1999. doi: 10.1111/j.1528-1157.1999.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 32.Summermatter S, Santos G, Pérez-Schindler J, Handschin C. Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci USA 110: 8738–8743, 2013. doi: 10.1073/pnas.1212976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svensson K, Albert V, Cardel B, Salatino S, Handschin C. Skeletal muscle PGC-1α modulates systemic ketone body homeostasis and ameliorates diabetic hyperketonemia in mice. FASEB J 30: 1976–1986, 2016. doi: 10.1096/fj.201500128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tagliabue A, Bertoli S, Trentani C, Borrelli P, Veggiotti P. Effects of the ketogenic diet on nutritional status, resting energy expenditure, and substrate oxidation in patients with medically refractory epilepsy: a 6-month prospective observational study. Clin Nutr 31: 246–249, 2012. doi: 10.1016/j.clnu.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20: 1868–1876, 2000. doi: 10.1128/MCB.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veldhorst MA, Westerterp-Plantenga MS, Westerterp KR. Gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet. Am J Clin Nutr 90: 519–526, 2009. doi: 10.3945/ajcn.2009.27834. [DOI] [PubMed] [Google Scholar]
- 37.Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ, Kraemer WJ, Bibus DM, Fernandez ML, Feinman RD. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 44: 297–309, 2009. doi: 10.1007/s11745-008-3274-2. [DOI] [PubMed] [Google Scholar]
- 38.Zajac A, Poprzecki S, Maszczyk A, Czuba M, Michalczyk M, Zydek G. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Nutrients 6: 2493–2508, 2014. doi: 10.3390/nu6072493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Qin J, Zhao Y, Shi J, Lan R, Gan Y, Ren H, Zhu B, Qian M, Du B. Long-term ketogenic diet contributes to glycemic control but promotes lipid accumulation and hepatic steatosis in type 2 diabetic mice. Nutr Res 36: 349–358, 2016. doi: 10.1016/j.nutres.2015.12.002. [DOI] [PubMed] [Google Scholar]