Remote ischemic conditioning protects hearts against ischemia and reperfusion (I/R) injury. However, it is unclear whether ischemic conditioning of visceral organs such as the liver, the largest metabolic organ in the body, can produce cardioprotection. This is the first study to show the cardioprotective effect of remote liver ischemic conditioning in a rat model of myocardial I/R injury. We also, for the first time, demonstrated these protective properties are associated with glycogen synthase kinase-3β-dependent cell-survival signaling pathway.
Keywords: liver ischemic conditioning, myocardial ischemia and reperfusion injury, GSK-3β, cardioprotection
Abstract
Remote ischemic conditioning has been convincingly shown to render the myocardium resistant to a subsequent more severe sustained episode of ischemia. Compared with other organs, little is known regarding the effect of transient liver ischemic conditioning. We proposed the existence of cardioprotection induced by remote liver conditioning. Male Sprague-Dawley rats were divided into sham-operated control (no further hepatic intervention) and remote liver ischemic conditioning groups. For liver ischemic conditioning, three cycles of 5 min of liver ischemia-reperfusion stimuli were conducted before-(liver preconditioning), post-myocardial ischemia (liver postconditioning), or in combination of both (liver preconditioning + liver postconditioning). Rats were exposed to 45 min of left anterior descending coronary artery occlusion, followed by 3 h of reperfusion thereafter. ECG and hemodynamics were measured throughout the experiment. The coronary artery was reoccluded at the end of reperfusion for infarct size determination. Blood samples were taken for serum lactate dehydrogenase and creatine kinase-MB test. Heart tissues were taken for apoptosis measurements and Western blotting. Our data demonstrate that liver ischemic preconditioning, postconditioning, or a combination of both, offered strong cardioprotection, as evidenced by reduction in infarct size and cardiac tissue damage, recovery of cardiac function, and inhibition of apoptosis after ischemia-reperfusion. Moreover, liver ischemic conditioning increased cardiac (not hepatic) glycogen synthase kinase-3β (GSK-3β) phosphorylation. Accordingly, inhibition of GSK-3β mimicked the cardioprotective action of liver conditioning. These results demonstrate that remote liver ischemic conditioning protected the heart against ischemia and reperfusion injury via GSK-3β-dependent cell-survival signaling pathway.
NEW & NOTEWORTHY Remote ischemic conditioning protects hearts against ischemia and reperfusion (I/R) injury. However, it is unclear whether ischemic conditioning of visceral organs such as the liver, the largest metabolic organ in the body, can produce cardioprotection. This is the first study to show the cardioprotective effect of remote liver ischemic conditioning in a rat model of myocardial I/R injury. We also, for the first time, demonstrated these protective properties are associated with glycogen synthase kinase-3β-dependent cell-survival signaling pathway.
local cardiac preconditioning or postconditioning has been shown to have direct cardioprotective effects against myocardial ischemia and reperfusion (I/R) injury. Similarly, remote ischemic pre- or postconditioning is a phenomenon whereby brief episodes of short periods of I/R stimuli executed in distant tissues or organs may exert protective effects in the heart and render the myocardium more tolerant to a subsequent sustained episode of ischemia or reperfusion injury. Studies have shown that this remote cardioprotective trigger can be used in distant vascular beds (lower and upper limbs; cerebral, renal, mesenteric, intestinal, renal, and abdominal arteries) (18). However, it is unclear whether brief ischemic conditioning of visceral organs such as the liver, the largest metabolic organ in the body, can produce cardioprotection. A few reports have shown that liver preconditioning could protect remote organs, such as lung (27) or kidney (2), and prevent the incidence of arrhythmias in vivo (14) or ex vivo (24). Based on these previous studies, we, therefore, hypothesized that remote liver conditioning may also protect the heart against I/R injury in vivo. Furthermore, the timing of liver ischemic conditioning treatment, i.e., pre- vs. postconditioning, has not been evaluated. Thus the primary aim of this study was to evaluate whether liver ischemic conditioning protects the heart, using metrics including infarct size and myocardial damage serum markers.
The potential mechanisms involved in the cardioprotection afforded by remote ischemic conditioning are not known. It has been proposed that either endogenous neural or humoral agent(s) traveled from the remote organ to the heart, or production of anti-apoptosis and anti-inflammatory factors in the remote organ, mediated cardioprotection (10). Ultimately, the pro-survival reperfusion injury salvage kinase (RISK) pathway is activated in the conditioned heart (9). The glycogen synthase kinase-3β (GSK-3β) signaling molecules are key components of the prosurvival RISK pathway (31). Many studies have demonstrated that GSK-3β phosphorylation promotes survival of conditioned cardiac myocytes (11, 22, 31, 33). In addition, emerging evidence has indicated that the phosphorylation of GSK-3β at Ser9 results in the inhibition of GSK-3β activity, and that this inhibition of GSK-3β enhances cell survival and limits infarct size post-I/R injury (6). These findings let us hypothesize that remote liver ischemic conditioning protect the heart against I/R injury via inhibiting GSK-3β activity, thus promoting myocardial survival. Therefore, with the use of an in vivo rat model, this investigation aimed to determine whether remote liver ischemia conditions the heart against I/R injury, and whether this effect is associated with GSK-3β-dependent cell-survival signaling pathways.
MATERIAL AND METHODS
Animals.
The experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee of Sichuan University (Sichuan, China, approval no. 2015035A) and in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH publication, 8th edition, 2011). Male Sprague-Dawley rats (7–8 wk old, 250–300 g) purchased from Chengdu Dashuo Experimental Animal Research Center (Chengdu, China), were housed at 20–25°C under a 12:12-h light-dark cycle. Humidity was maintained at 60 ± 5% before experiments.
Experimental protocol.
Rats were anesthetized with pentobarbital sodium (50 mg/kg ip). After establishing adequate anesthesia, they were incubated and ventilated with a rodent ventilator (Taimeng, Chengdu, China). A standard limb lead II configuration electrocardiographic system was applied for heart rate and ST segment monitoring (Biolap 420F, Taimeng). The animals were then subjected to instrumentation for hemodynamics and surgical interventions (see below) under constant monitoring. Baseline measurement of the hemodynamics was conducted 10 min after the animals were stabilized.
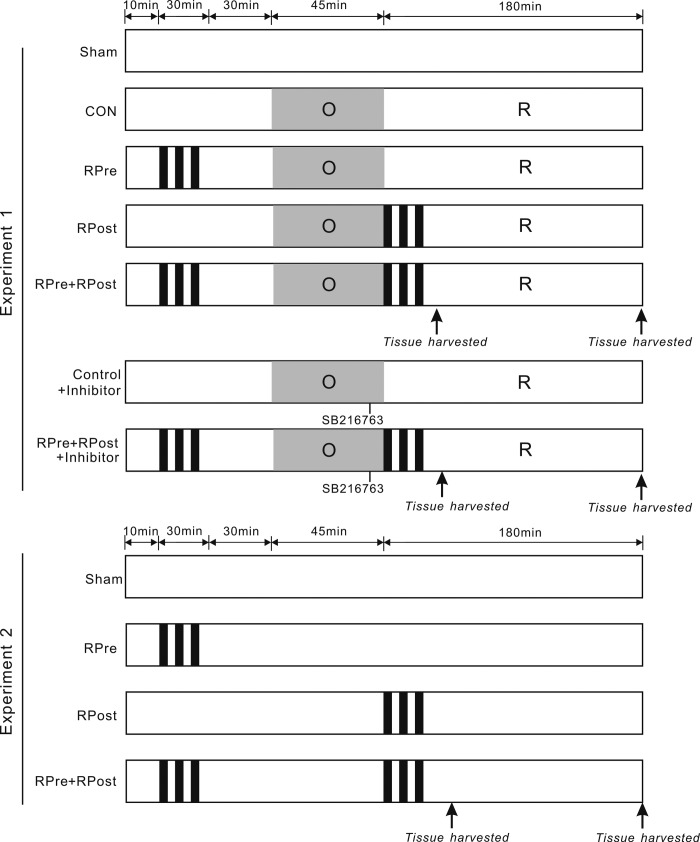
The experimental protocol is summarized in Fig. 1. Rats that died of anesthesia or during surgery, or did not reach 3 h of reperfusion stage, were excluded. There were two experimental series. Experiment 1 (a total of 115 rats, among which 10 died during the ligation period or during the reperfusion period because of acute heart failure) had an overall survival rate of 91.3%. Following the interventions, hearts were collected at 40 min for protein phosphorylation analysis and at 3 h for infarction and apoptosis image studies post-cardiac reperfusion. In experiment 2, only liver I/R stimuli protocol was executed without cardiac I/R to examine hepatic damage. A total of 40 rats were used. Liver sections and samples were collected at the same time points as those in experiment 1. Rats were randomly assigned to a sham-operated group (sham; hepatic arterial and venous trunk were exposed without intervention, chests were opened without coronary artery ligation), control group (CON; no further hepatic intervention), or remote liver ischemic conditioning groups of preconditioning (RPre), postconditioning (RPost), or a combination of preconditioning and postconditioning (RPre+RPost). In addition, two groups with inhibitor (CON + inhibitor and RPre+RPost + inhibitor) were included in experiment 1.
Fig. 1.
Experimental protocols. In experiment 1, all hearts were subjected to 45-min ligation of the left anterior descending coronary artery (LAD), followed by 3 h of reperfusion (R), except for the sham-operated ones. For remote liver ischemic preconditioning (RPre), three cycles of 5 min of liver ischemia with 5-min intermittent reperfusions were conducted before myocardial ischemia. For remote liver ischemic postconditioning (RPost), the above three cycles of liver I/R stimulus were induced after myocardial ischemia (at the onset of myocardial reperfusion). For the combination of remote liver ischemic preconditioning and postconditioning (RPre+RPost), the liver conditioning stimulus was induced before myocardial ischemia and at the onset of myocardial reperfusion. The GSK-3β inhibitor SB-216763 was applied 5 min before reperfusion. Thirty minutes of acute memory phase were allowed before being followed by a 45-min LAD occlusion and a subsequent 180 min of reperfusion (I/R). Arrows indicate the time points at which tissue samples were harvested. In experiment 2, hepatic stimuli were conducted without myocardial I/R intervention. Liver was taken at the same time points as in experiment 1.
Surgical procedures.
To produce liver conditioning, laparotomy was performed via a midline abdominal incision. The hepatic arterial and venous trunk, as well as the portal vein, were identified and isolated. Three cycles of 5 min of liver ischemia with 5-min intermittent reperfusions, i.e., clamping the vessel with a microvascular clip to induce ischemia (ischemia period), and releasing the clip to initiate reperfusion (reperfusion period), were conducted pre- (RPre) or post-myocardial ischemia (RPost) or a combination of both (RPre+RPost).
To produce cardiac I/R, a median thoracotomy was performed to expose the heart. Myocardial I/R was conducted by ligation of left anterior descending coronary artery (LAD) approximately halfway between the base and the apex for a period of 45 min, followed by release of the suture and reperfusion for 3 h. Successful coronary artery occlusion was verified by the presence of regional dyskinesia and epicardial cyanosis in the ischemic zone. Reperfusion was verified by visual observation of an epicardial hyperemic response. A heating blanket was used for body temperature maintenance. For the sham-operated animals, LAD was separated but not occluded. To determine if the cardioprotective effect of liver conditioning is associated with MAPKs signaling cascade, SB-216763 (0.6 mg/kg, GSK-3β inhibitor; Sigma-Aldrich, St. Louis, MO) was administrated 5 min before myocardial reperfusion via the femoral vein. For euthanasia, an overdose of pentobarbital sodium (200 mg/kg ip) was given at the end of the experiment.
Hemodynamic analyses.
A 20-G heparin-filled catheter (Spacelabs Medical, Redmond, WA) was inserted from the right carotid artery to the left ventricle and connected to a pressure transducer (Biolap 420F, Taimeng) connected for hemodynamic parameters measurement. Left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), and maximum rate of increase/decrease in left ventricular pressure (±dP/dtmax) were recorded throughout the experiment.
Infarct size determination.
At the end of the experiment, LAD was reoccluded and the heart was perfused with 1% Evan’s blue (Sigma-Aldrich). The left ventricular area at risk (AAR) was identified from the normal myocardium, which was stained blue. Hearts were then frozen at −20°C for 30 min before being cut into 2-mm-thick slices parallel to the atrioventricular groove. Heart slices were then incubated at 37°C with 1% triphenyltertrazolium chloride (Sigma-Aldrich) in 0.1 M phosphate buffer (pH 7.4) for 20 min. Infarcted tissue within the AAR area was stained white. After fixation in 4% paraformaldehyde for 24 h, the infarcted and the noninfarcted tissue were separated and weighed. Infarct size was expressed as a percentage of the AAR for each group.
Serum analyses.
At the end of cardiac reperfusion, whole blood was collected and centrifuged to obtain plasma (4,000 rpm for 10 min at 4°C). Serum levels of lactate dehydrogenase (LDH) and creatine kinase-MB (CK-MB) were quantified using a Mindray BS-120 Chemistry Analyzer (Mindray Medical, Shenzhen, China). All samples were measured in duplicate.
Tissue collection.
A parallel experiment was carried out for immunohistochemistry and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining. Left ventricular AAR regions were identified from normal area by the use of Evans blue dye. AAR areas were then separated, rinsed in saline, dried, embedded in 10% phosphate-buffered formalin, and cut into 5-μm sections parallel to the atrioventricular groove. Serial sections of transverse myocardial and liver slices were deparaffinized in xylene and isopropanol and then were mounted on glass slides for immunohistochemical analysis and apoptosis measurements.
Apoptosis measurements.
The TUNEL method was used for determination of apoptotic cells. The heart or liver sections were stained using the In Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN), according to the manufacturer’s instructions. TUNEL-positive nuclei were stained red, and TUNEL-negative nuclei were stained blue. More than 10 different microscopic fields per heart section were chosen at random and were evaluated blindly. The apoptotic index was calculated as a ratio of TUNEL-positive nuclei to the total nuclei population. Images were obtained and viewed using an inverted microscope (Olympus A/S, Ballerup, Denmark) and were analyzed with Image-pro plus (Media Cybernetics, Carlsbad, CA).
Immunohistochemistry.
Primary antibodies, including anti-Bcl-2 and anti-Bax antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), were used, followed by incubation with second antibodies (Santa Cruz Biotechnology) of either biotinylated goat anti-rabbit IgG (Bcl-2) or goat anti-mouse IgG (Bax). Cardiac tissues were stained with 3,3-diaminobenzene (Beijing Zhongshan Golden Bridge Biotechnology) solution, and all sections were counterstained with hematoxylin to visualize cell nuclei. Phosphate buffer solution was used as a negative control. Images were captured by an inverted microscope (CAST system, Olympus A/S) and analyzed with Image-pro plus software (Media Cybernetics). Ten views were randomly selected per slide, and positive expression was characterized as brown staining in the cytoplasm of cells. Statistical value was calculated by the ratio of optical density of area positively stained to mean optical density, i.e., positive expressive index.
Western blotting analyses.
The left ventricular AAR region (including the infarct zone) isolated from the normal area was homogenized and prepared as previously described (12, 13, 15, 16). Briefly, the bicinchoninic acid method (Pierce, Rockford, IL) was adopted for protein concentration determination. Fifteen micrograms of protein were loaded per lane and resolved on a 10% SDS-PAGE gel before being transferred onto nitrocellulose membranes (VWR, Batavia, IL). Primary antibodies raised against phosphorylated GSK-3β (Ser9) (p-GSK-3β Ser9, rabbit, 1:1,000; Cell Signaling), total GSK-3β, and cleaved caspase-3 and caspase-3 (rabbit, 1:1,000; Cell Signaling) were used, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (Bio-Rad, Hercules, CA). An Amersham Imager 600 system (GE Healthcare, Little Chalfont, UK) was used for detection of signals. Band densities were analyzed by ImageJ Data Acquisition software (National Institutes of Health, Bethesda, MD). Phosphorylation signal densities were normalized to total protein signal densities. All samples were run in duplicate on separate gels.
Statistical analysis.
All values are expressed as means ± SE. Statistical analyses were performed using Graphpad Prism 5 software (NIH) or SPSS 13.0 software for Windows (SPSS Inc., Chicago, IL). The statistical test for hemodynamics over time was performed by two-way repeated-measures ANOVA. Other comparisons of two means (by an unpaired Student’s t-test), or several means (by one-way ANOVA) were performed, assuming statistical significance with P values < 0.05.
RESULTS
Liver ischemic conditioning reduced cardiac tissue damage against myocardial I/R injury.
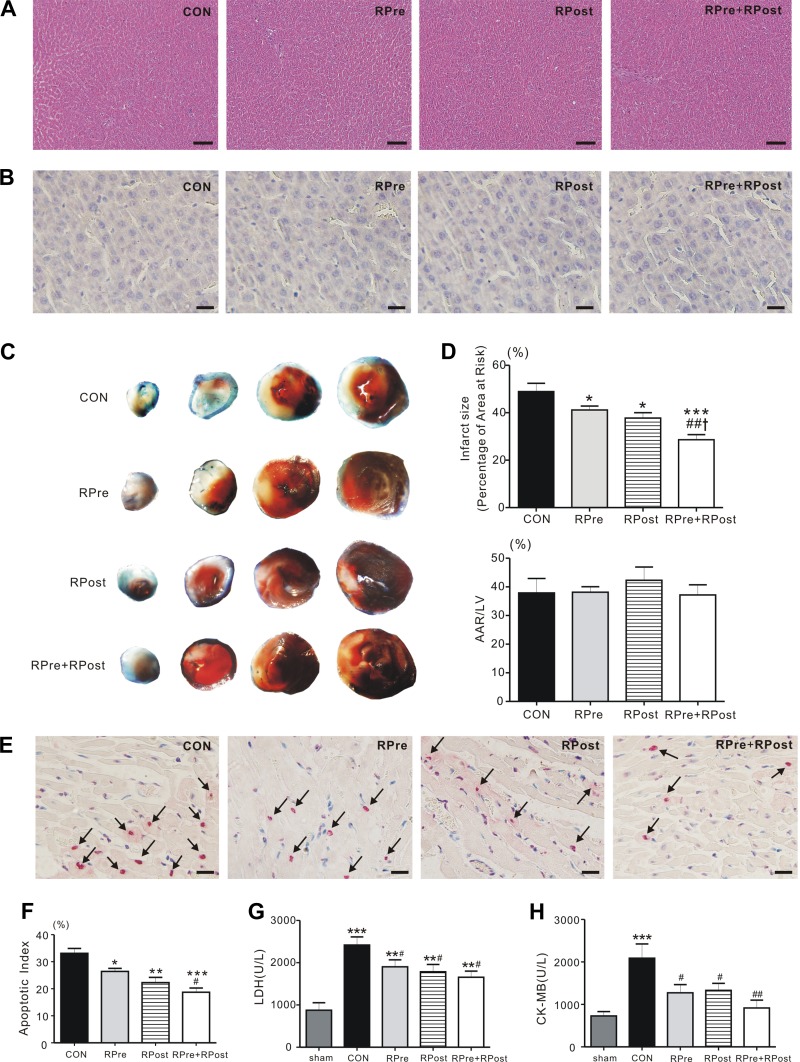
We first examined if the live conditioning protocol (i.e., 3 episodes of 5-min ischemia followed by reperfusion) produce hepatic injury. Hematoxylin and eosin (Fig. 2A) and TUNEL (Fig. 2B) staining on liver sections revealed that these hepatic I/R interventions did not induce hepatic cell death. No cytoplasmic vacuolation, edema, hemorrhage, or steatosis was found in liver samples. Thus our liver conditioning protocol did not cause harm to the liver. After liver conditioning, myocardial ischemia followed by reperfusion was carried out. The myocardial infarct size in RPre (41.2 ± 1.7%, P < 0.05), RPost (37.8 ± 2.2%, P < 0.05), and RPre+RPost rats (28.6 ± 2.1%, P < 0.001) was significantly lower than that of rats in the CON group (48.9 ± 3.5%) (Fig. 2, C and D). The ratio of the AAR to the left ventricle was similar among groups (P > 0.05, Fig. 2D). Moreover, the reduction in heart infarct size was greater in rats subjected to a combination of treatments, RPre+RPost, compared with rats subjected to only RPre (P < 0.01) or RPost (P < 0.05). To explore the effect of liver ischemic conditioning on apoptosis, TUNEL staining was applied on heart sections after 3 h of reperfusion. TUNEL-positive nuclei were prevalent in the left ventricular AAR. However, liver ischemic conditioning caused a marked reduction of apoptotic nuclei (RPre: 26.4 ± 1.1%, RPost: 22.3 ± 1.9%, RPre+RPost: 18.7 ± 1.6%) compared with the CON group (32.6 ± 1.6%; P < 0.05, P < 0.01, and P < 0.001, respectively). In addition, RPre+RPost treatment effectively decreased apoptosis compared with RPre alone, following cardiac reperfusion (P < 0.05, Fig. 2, E and F).
Fig. 2.
Liver conditioning ameliorated myocardial damage post reperfusion. A: hepatic ischemia and reperfusion cycles did not cause liver damage. Representative (of n = 5 rats/group) hematoxylin-and-eosin-stained micrographs of liver section are shown. Scale bars, 100 μm. B: representative images of terminal transferase dUTP nick-end labeling (TUNEL)-stained liver sections. Nuclei were counterstained with 3,3-diaminobenzene (n = 4–5). RPre, remote liver ischemic preconditioning; RPost, remote liver ischemic postconditioning; RPre+RPost, the combination of remote liver ischemic preconditioning and postconditioning. Scale bars, 10 μm. C: representative sections of triphenyltetrazolium chloride (TTC)-stained heart subjected to 45-min myocardial ischemia followed by 3-h reperfusion. CON, control; RPre, remote liver ischemia preconditioning; RPost, remote liver ischemia postconditioning; RPre+RPost, the combination of remote liver ischemia preconditioning with remote liver ischemic postconditioning. D: quantification of myocardial infarct size expressed as a percentage of left ventricular (LV) area at risk (AAR) (top) and AAR expressed as a percentage of LV area (bottom). Values are means ± SE; n = 5 each group. *P < 0.05 and ***P < 0.001 compared with CON; ##P < 0.01 compared with RPre; and †P < 0.05 compared with RPost (by one-way ANOVA). E: representative images of TUNEL-stained heart sections. Myocardial apoptosis was determined by measurement of TUNEL-positive cardiomyocyte nuclei in the AAR of myocardium obtained from liver-conditioned and CON rats after coronary artery reperfusion injury. TUNEL-positive (red) cardiomyocytes were identified as apoptotic cells. Positive cells were not detected in the nonischemic zone. Arrows denote TUNEL-positive nucleus. Scale bars = 10 μm. F: bar graph showing the TUNEL-positive nuclei expressed as a percentage of total nuclei in the heart AAR sections. Values are means ± SE; each group, n = 4–6. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with CON; #P < 0.05 compared with RPre (by one-way ANOVA). G: serum levels of lactate dehydrogenase (LDH) in rats subjected to 45 min of left anterior descending artery occlusion followed by 3 h of reperfusion. Values are means ± SE; n = 11–13 per group. **P < 0.01 and ***P < 0.001 vs. sham; #P < 0.05 vs. CON. H: post-reperfusion injury mean serum levels of creatine kinase MB (CK-MB) for CON and liver ischemic conditioned rats. Values are means ± SE; n = 7–12 per group. ***P < 0.001 vs. sham; #P < 0.05 and ##P < 0.01 vs. CON.
Hemodynamic function.
Table 1 illustrates the time course of hemodynamics during the experiment. Under baseline conditions, there was no statistical difference in systemic hemodynamics among the groups before the coronary occlusion (P > 0.05). Reperfusion caused a reduction of systolic function, as indicated by LVSP and dP/dtmax, and depression of diastolic function as indicated by LVEDP and −dP/dtmax, compared with the respective baseline values (P < 0.05, P < 0.01, or P < 0.001) in CON, RPre, and RPost groups, respectively. Repeated two-way ANOVA showed a statistically significant difference among CON, RPre, RPost, and RPre+RPost groups in LVSP (P = 0.002), dP/dtmax (P = 0.044), and −dP/dtmax (P = 0.008) over the period of measurement. The recovery of cardiac function, including LVSP, dP/dtmax, and −dP/dtmax, was greater in the RPre+RPost hearts than in the CON group or in hearts treated with a single liver conditioning stimulus (P < 0.05 or P < 0.01). Significant interactions between group assignment and time were observed for LVSP (P = 0.002) and LVEDP (P = 0.024).
Table 1.
Hemodynamics during the experiment investigating the effect of liver conditioning on reperfusion injury
| Reperfusion |
||||
|---|---|---|---|---|
| Variable | Baseline | 1 h | 2 h | 3 h |
| LVSP, mmHg | ||||
| CON | 136 ± 4 | 116 ± 3*** | 104 ± 2*** | 94 ± 3*** |
| RPre | 138 ± 1 | 112 ± 3*** | 116 ± 2*** | 102 ± 3*** |
| RPost | 131 ± 2 | 114 ± 3** | 115 ± 5** | 109 ± 4**# |
| RPre+RPost | 136 ± 5 | 127 ± 4#†‡ | 127 ± 6## | 121 ± 5###†† |
| LVEDP, mmHg | ||||
| CON | −11 ± 2 | −4 ± 1*** | −1 ± 1*** | 4 ± 1*** |
| RPre | −17 ± 3 | −5 ± 4* | −3 ± 3* | 4 ± 2*** |
| RPost | −13 ± 2 | −3 ± 1** | −2 ± 2** | 2 ± 1*** |
| RPre+RPost | −12 ± 3 | −9 ± 2#† | −6 ± 4 | −2 ± 1 |
| dP/dtmax, mmHg/s | ||||
| CON | 4,593 ± 350 | 2,793 ± 211*** | 2,582 ± 244*** | 2,104 ± 208*** |
| RPre | 4,331 ± 447 | 2,955 ± 165** | 3,076 ± 128** | 2,703 ± 184** |
| RPost | 4,610 ± 111 | 3,354 ± 303* | 2,996 ± 545* | 2,779 ± 480* |
| RPre+RPost | 4,748 ± 274 | 3,991 ± 334 | 3,888 ± 478 | 3,547 ± 218## |
| −dP/dtmax, mmHg/s | ||||
| CON | −3,666 ± 262 | −2,794 ± 269* | −2,724 ± 252* | −1,880 ± 111** |
| RPre | −3,866 ± 84 | −2,853 ± 320** | −2,543 ± 300** | −2,021 ± 198*** |
| RPost | −4,465 ± 608 | −3,069 ± 288* | −2,893 ± 278* | −2,546 ± 220* |
| RPre+RPost | −4,065 ± 472 | −3,739 ± 151 | −3,537 ± 237 | −2,895 ± 232##† |
Values are means ± SE; n = 5–7 in each group. CON, control; RPre, remote liver ischemia preconditioning; RPost, remote liver ischemia postconditioning; RPre+RPost, the combination of remote liver ischemia preconditioning with remote liver ischemia postconditioning; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; ±dP/dtmax, maximum rate of increase/decrease in left ventricular pressure.
P < 0.05,
P < 0.01, and
P < 0.001 vs. baseline.
P < 0.05,
P < 0.01, and
P < 0.001 vs. CON.
P < 0.05 and
P < 0.01 vs. RPre.
P < 0.05 vs. RPost.
LDH and CK-MB release.
Significant differences were detected among the experimental groups in LDH and CK-MB activities (LDH: P < 0.0001; CK-MB: P = 0.005). Myocardial reperfusion caused a significant elevation of LDH level in CON, RPre, RPost, and RPre+RPost groups (all P < 0.01 vs. sham), with the highest in CON rats, indicating the protective effect of liver conditioning against myocardial reperfusion injury (RPre, RPost, and RPre+RPost vs. CON, P < 0.05, Fig. 2G). In addition, although serum CK-MB level increased significantly after I/R injury in the CON rats compared with the sham group (P < 0.001), significantly lower CK-MB level was observed in rats with liver ischemic conditioning (P < 0.05 or P < 0.01 vs. CON, Fig. 2H).
Liver ischemic conditioning decreases ventricular apoptosis after cardiac I/R injury.
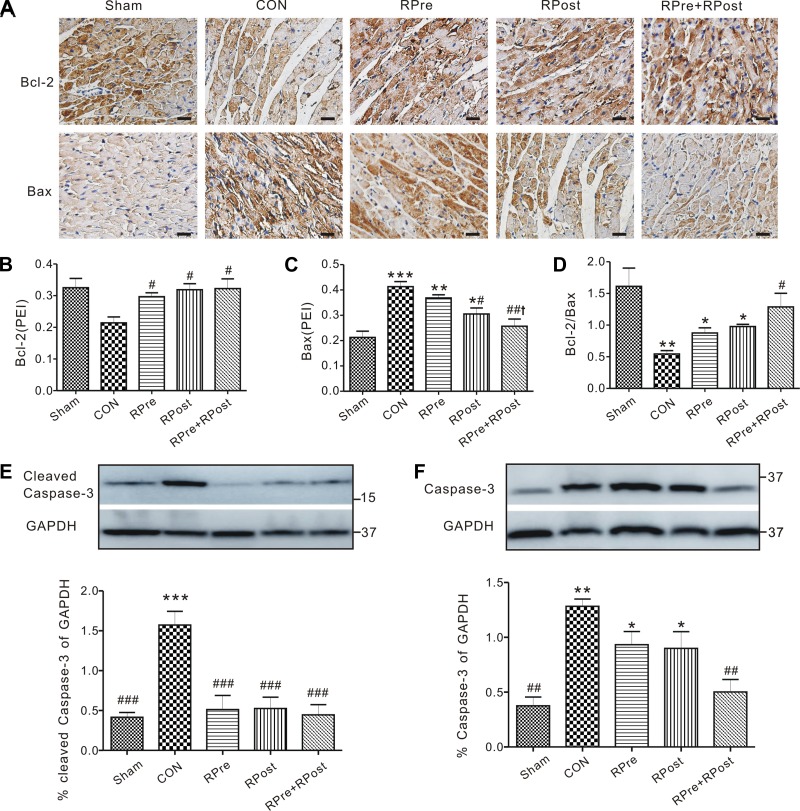
Immunohistochemistry indicated the expression of Bcl-2 and Bax proteins in cardiomyocytes of rats. As shown in Fig. 3, A–C, liver ischemic conditioning significantly upregulated the expression of Bcl-2 protein (P < 0.05, all vs. CON) and downregulated the expression of Bax proteins (P < 0.05, RPost vs. CON or P < 0.01, RPre+RPost vs. CON, Fig. 3C). Importantly, there was a dramatic protective effect of the combined treatment of RPre+RPost against reperfusion injury, as reflected by a greater Bcl-2-to-Bax ratio than that of the CON group (P < 0.05, Fig. 3D). Expression of cleaved caspase-3 and total caspase-3 protein in the myocardium was further determined by Western blotting (Fig. 3, E and F), Compared with the sham-operated group, reperfusion injury caused significant elevation of cleaved caspase-3 (Fig. 3E) or caspase-3 (Fig. 3F) protein expression (P < 0.01 or P < 0.001); however, this elevation was further attenuated with liver conditioning treatments (P < 0.001 vs. CON).
Fig. 3.
Myocardial apoptotic protein expression. A: representative immunostainings of Bcl-2 and Bax protein in the cytoplasm of the left ventricular myocytes isolated after 3 h of reperfusion. CON, control; RPre, remote liver ischemia preconditioning; RPost, remote liver ischemia postconditioning; RPre+RPost, the combination of remote liver ischemia preconditioning with remote liver ischemic postconditioning. Densitometric analysis of Bcl-2 (B), Bax (C), and Bcl-2/Bax (D) protein expression in rat left ventricles post-I/R is shown. Values are means ± SE; n = 4–6 per group. PEI, positive expressive index. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. sham; #P < 0.05 and ##P < 0.01 vs. CON; †P < 0.05 vs. RPre. Representative Western blots (top) and quantification (bottom) of cleaved caspase-3 (E) and caspase-3 (F) protein band density (normalized to GAPDH) in sham, CON, and liver-conditioned rat left ventricles are shown. Values are means ± SE; n = 4–5 per group. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. sham; ##P < 0.01 and ###P < 0.001 vs. CON.
Liver ischemic conditioning increases ventricular GSK-3β phosphorylation post-myocardial I/R.
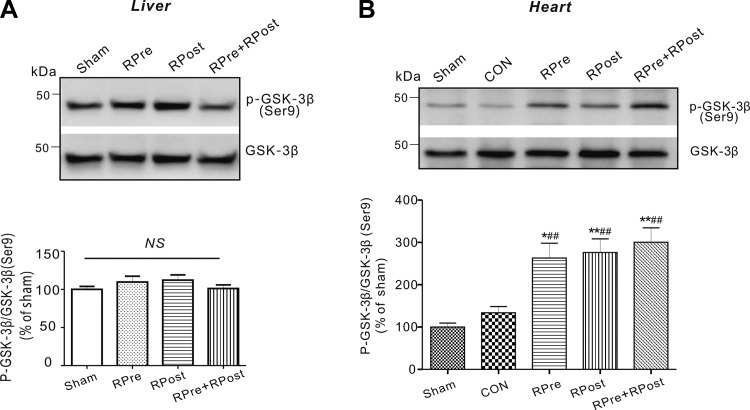
Importantly, remote organ ischemic conditioning stimulus could exert potent cardioprotection via activation of cell survival signaling pathways, such as GSK-3β signaling molecules. We determined levels of GSK-3β in all groups. Liver conditioning alone did not alter local liver GSK-3β phosphorylation (Fig. 4A). However, the ratio of phosphorylated (p) to total (t) GSK-3β in liver-conditioned hearts was almost double that of CON hearts post-cardiac I/R (P < 0.01, Fig. 4B), while total GSK-3β protein levels were similar between CON and liver-conditioned ischemic ventricles. We did not see any difference in cardiac GSK-3β phosphorylation levels among liver conditioning groups (P > 0.05) after cardiac I/R injury.
Fig. 4.
Liver ischemic conditioning stimulates ventricular GSK-3β phosphorylation. Representative immunoblots (top) and densitometric analysis (bottom) of phosphorylated GSK-3β (Ser9) (p-GSK-3β) and total GSK-3β in rat livers (A) and ventricles (B) are shown. Sham animals did not undergo liver stimulus. Sham, sham-operated group; RPre, remote liver ischemic preconditioning; RPost, remote liver ischemic postconditioning; RPre+RPost, the combination of remote liver ischemic preconditioning and postconditioning. All band densities were normalized to sham group. Values are means ± SE; n = 5 in each group. NS, no significant difference. *P < 0.05 and **P < 0.01 vs. sham; ##P < 0.01 vs. CON (by one-way ANOVA).
Inhibition of GSK-3β mimics the cardioprotective action of liver conditioning.
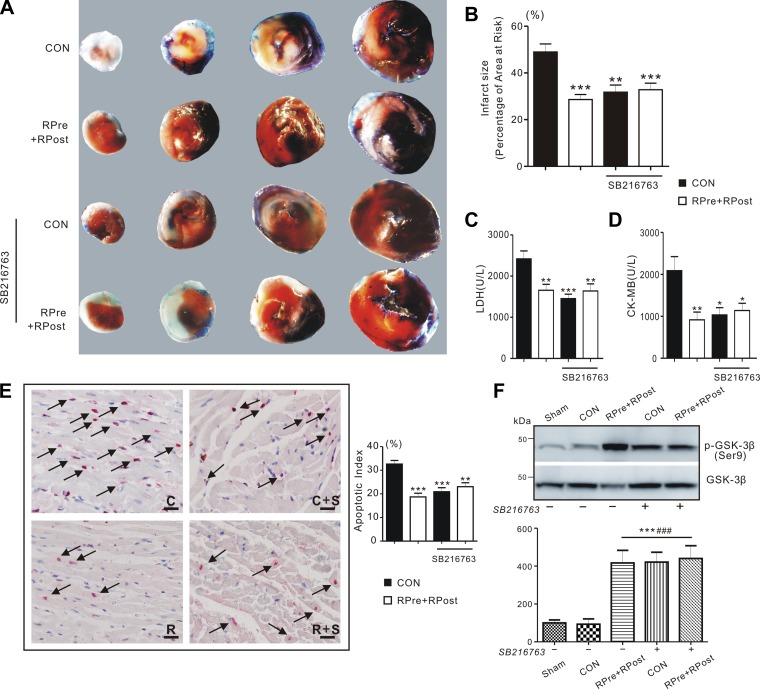
Inhibition of GSK-3β has been reported to exert cardioprotection against reperfusion injury and reduce infarct size (31). To investigate whether GSK-3β pathway plays a role in the liver ischemic conditioning-induced cardioprotection in our experiments, we administrated GSK-3β inhibitor SB-216763 after the LAD occlusion 5 min before commence of reperfusion. We selected the RPre+RPost group to administrate SB-216763, since this group exhibited the most potent liver conditioning effect. Consistent with previous studies, inhibition of GSK-3β mimicked the effects of liver ischemic conditioning: the inhibitor-treated CON group had similar reduction in heart infarct size as the RPre+RPost-treated group. However, inhibition of GSK-3β in RPre+RPost did not produce additional infarct size reduction compared with this observed in RPre+RPost alone (Fig. 5, A and B). Meanwhile, SB-216763 decreased serum concentration of LDH and CK-MB after I/R injury, to levels similar to that of liver-conditioned rats without inhibitors (P < 0.05, P < 0.01, and P < 0.001 vs. CON, Fig. 5, C and D). Consistently, inhibitor-treated CON hearts significantly reduced the number of TUNEL-positive stained apoptotic nuclei in the AAR compared with nontreated CON (P < 0.01 or P < 0.001). GSK-3β inhibition did not further enhance the anti-apoptotic activity in RPre+RPost hearts compared with RPre+RPost alone (P > 0.05, Fig. 5E). Pharmacological inhibition of GSK-3β also mimicked the effects of liver conditioning on ventricular GSK-3β phosphorylation (Fig. 5F). SB-216763 did not further increase GSK-3β phosphorylation or cardioprotection in liver-conditioned rats. However, SB-216763 induced similar degrees of GSK-3β phosphorylation and cardioprotection in CON rats as in liver-conditioned rats, supporting the vital role of GSK-3β phosphorylation (inactivation) in liver conditioning-induced cardioprotection.
Fig. 5.
The protective effect of pharmacological inhibitors on I/R injury. A: representative sections of TTC-stained heart subjected to 45-min myocardial ischemia followed by 3-h reperfusion. CON, control; RPre+RPost, the combination of remote liver ischemia preconditioning with remote liver ischemic postconditioning. B: quantification of myocardial infarct size expressed as a percentage of left ventricular area at risk. Values are means ± SE; n = 4–5 each group. Values for CON and RPre+RPost rats are repeated from Fig. 2C for comparison. **P < 0.01 and ***P < 0.001 compared with CON (by one-way ANOVA). C: mean serum levels of LDH of CON and liver ischemic conditioned rats after I/R injury with or without SB-216763 or U-0126. Values are means ± SE; n = 7–13. Values for CON and RPre+RPost rats are repeated from Fig. 2G for comparison. **P < 0.01 and ***P < 0.001 compared with CON (by one-way ANOVA). D: mean serum levels of CK-MB of CON and liver ischemic conditioned rats after I/R injury with or without SB-216763 or U-0126. Values are means ± SE; n = 7–12. Values for CON and RPre+RPost rats are repeated from Fig. 2H for comparison. *P < 0.05 and **P < 0.01 compared with CON (by one-way ANOVA). E, left: apoptotic nuclei detected by TUNEL technique in the area of myocardium at risk. Arrow indicates TUNEL-positive nuclei (red). Scale bars, 10 µm. C, control; R, the combination of remote liver ischemia preconditioning with remote liver ischemic postconditioning; S, SB-216763. Right: graph showing the averaged percentage of TUNEL-positive cells in the ischemic regions of LVs. Values are means ± SE; n = 4–6, each group. Values for CON and RPre+RPost rats are repeated from Fig. 2, E and F for comparison. **P < 0.01 and ***P < 0.001 compared with CON (by one-way ANOVA). F: representative Western blots (top) and densitometric analysis (bottom) of phosphorylated GSK-3β (Ser9) (p-GSK-3β) and total GSK-3β in rat ventricles with (+) or without (−) SB-216763 after 180 min of cardiac I/R injury. Sham, sham-operated group; RPre, remote liver ischemic preconditioning; RPost, remote liver ischemic postconditioning; RPre+RPost, the combination of remote liver ischemic preconditioning and postconditioning. All band densities were normalized to sham group. Values are means ± SE; n = 5 in each group. ***P < 0.001 vs. sham; ###P < 0.001 vs. CON (by one-way ANOVA).
The effect of inhibitor on hemodynamics was also investigated. At baseline, there were no differences among groups in the hemodynamic parameters before intervention. Remote liver conditioning with and without SB-216763 significantly improved hemodynamics in LVSP (P = 0.001), dP/dtmax (P = 0.019), and −dP/dtmax (P = 0.005), compared with CON over the period of measurement. We also observed significant group × time interactions for LVSP (P = 0.004) and dP/dtmax (P = 0.045). SB-216763 similarly improved hemodynamics in both CON and RPre+RPost-treated groups (P > 0.05). Thus inhibition of GSK-3β activity in CON resulted in similar hemodynamic enhancement as in RPre+RPost-treated hearts without inhibitor, i.e., enhanced cardiac function compared with non-inhibitor-treated CON hearts (Table 2).
Table 2.
The effect of pharmacological inhibitors on hemodynamics
| Reperfusion |
||||
|---|---|---|---|---|
| Variable | Baseline | 1 h | 2 h | 3 h |
| LVSP, mmHg | ||||
| CON | 136 ± 4 | 116 ± 3*** | 104 ± 2*** | 94 ± 3*** |
| RPre+RPost | 136 ± 5 | 127 ± 4 | 127 ± 6### | 121 ± 5### |
| CON+SB | 132 ± 3 | 125 ± 4 | 122 ± 2*## | 119 ± 1*### |
| RPre+Rpost+SB | 131 ± 3 | 124 ± 2 | 119 ± 2**## | 114 ± 2***### |
| LVEDP, mmHg | ||||
| CON | −11 ± 2 | −4 ± 1*** | −1 ± 1*** | 4 ± 1*** |
| RPre+RPost | −12 ± 3 | −9 ± 2 | −6 ± 4 | −2 ± 1 |
| CON+SB | −15 ± 2 | −8 ± 2* | −4 ± 1** | −3 ± 3** |
| RPre+Rpost+SB | −15 ± 2 | −10 ± 1* | −5 ± 1*** | −3 ± 2*** |
| dP/dtmax, mmHg/s | ||||
| CON | 4,593 ± 350 | 2,793 ± 211*** | 2,582 ± 244*** | 2,104 ± 208*** |
| RPre+RPost | 4,748 ± 274 | 3,991 ± 334# | 3,888 ± 478# | 3,548 ± 218### |
| CON+SB | 4,784 ± 209 | 3,874 ± 174**# | 3,569 ± 164*** | 3,201 ± 76***## |
| RPre+Rpost+SB | 4,712 ± 137 | 3,519 ± 346* | 3,289 ± 333* | 3,408 ± 379*## |
| −dP/dtmax, mmHg/s | ||||
| CON | −3,666 ± 262 | −2,794 ± 269* | −2,724 ± 252* | −1,880 ± 111** |
| RPre+RPost | −4,065 ± 472 | −3,739 ± 151 | −3,537 ± 237 | −2,896 ± 232## |
| CON+SB | −4,140 ± 137 | −3,378 ± 221* | −3,137 ± 277** | −2,529 ± 167***# |
| RPre+Rpost+SB | −4,267 ± 312 | −3,724 ± 191 | −3,476 ± 193 | −2,626 ± 202***# |
Values are means ± SE; n = 5–7 in each group. CON, control; RPre+RPost, the combination of remote liver ischemia preconditioning with remote liver ischemia postconditioning; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; ±dP/dtmax, maximum rate of increase/decrease in left ventricular pressure. SB, SB-216763. Values for control and RPre+RPost rats are repeated from Table 1 for comparison.
P < 0.05,
P < 0.01, and
P < 0.001 vs. baseline.
P < 0.05,
P < 0.01, and
P < 0.001 vs. CON.
DISCUSSION
To summarize, our data demonstrate that liver ischemic conditioning offers strong cardioprotection, as seen by significant reduction in infarct size, restoration of cardiac function, inhibition of apoptosis, and alleviation of cardiac tissue damage after myocardial reperfusion injury. Moreover, these data suggest that this cardioprotection is associated with GSK-3β-dependent cell-survival signaling pathway.
Remote ischemic conditioning (7), brief episode of regional ischemia followed by reperfusion in remote organs or limbs (other than hearts), has been convincingly shown to render the myocardium resistant to a subsequent sustained episode of ischemia. Preclinical studies have demonstrated that the short cycles of I/R stimulus imposed on limbs or distant organs attenuated the extent of myocardial injury (30). These are effective when conducted before myocardial ischemia [remote ischemic preconditioning (29)], or during cardiac reperfusion [remote ischemic postconditioning (1)]. Compared with other organs, little is known regarding the liver ischemic conditioning, which may have other beneficial effects, such as reducing inflammation or promoting hepatic regeneration (28). Several studies clearly demonstrated that liver preconditioning decreased the incidence of arrhythmias in vivo (14) and ex vivo (24). Here, we further demonstrated the existence of remote liver conditioning-induced cardioprotection. We showed that either liver ischemic preconditioning (RPre), postconditioning (RPost), or a combination of both treatments (RPre+RPost) attenuated myocardial damage post-reperfusion insult in vivo. Moreover, we found that the RPre+RPost combination decreased infarct size to a larger degree than RPre or RPost alone, which was in agreement with studies on limb conditioning when both strategies were used in combination for pulmonary protection in cardiac surgery patients with CPB (21).
Although numerous animal experiments and clinical trials in patients with acute ST-elevation myocardial infarction or percutaneous coronary interventions confirmed the beneficial effect of remote ischemic conditioning (3, 30), recent reports revealed that it failed to improve clinical outcomes following cardiac surgery (8, 20). The apparent discrepancy may result from different study designs, protocols, and primary end point used, which can produce considerable variability in outcomes. Importantly, these negative results were derived from clinical trials of cardiac surgery, during which numerous inherent cardioprotective regimens were used, such as anesthetics, cardioplegia, or hypothermia; thus their influence on the interpretation of available data cannot be ruled out.
It is clear that anesthetics have strong cardioprotective effects, including volatile anesthetics and propofol, whose presence during the surgery may largely conceal the existing protection effect of endogenous ischemic conditioning (16, 18, 35). Therefore, we chose sodium pentobarbital as the anesthetic to avoid any potential ambiguity. Pentobarbital may have effects on cardiac function preservation, depending on various surgery strategies or species; for example, one study showed that it could alter cardiac hemodynamics in mice (34). In contrast, no infarct size limitation was found in rabbits (5). One could argue that the use of pentobarbital may potentially eliminate the cardiac protective phenomenon observed in the present study; however, we and others (19) showed that remote ischemic stimulus conferred powerful cardioprotection compared with CON hearts in the presence of identical sodium pentobarbital in each group. Thus it is unlikely that pentobarbital would generate differences in parameters measured among groups.
The mechanisms of liver ischemic conditioning-induced cardiac protection have not been completely defined. Apoptosis plays a crucial role in the pathogenesis of myocardial I/R injury. It is triggered by apoptotic signals and is dominated by numbers of regulating genes during reperfusion injury (17). Although liver conditioning-induced cardioprotection against I/R injury occurs concomitantly with increases in Bcl-2 protein expression and decreases in Bax protein expression, thus resulting in an increased Bcl-2-to-Bax ratio, it appears that these liver ischemic stimuli failed to alter cleaved or total caspase-3 proteins expression. As such, the mechanisms of remote liver conditioning contrast to those of local preconditioning or postconditioning, for which there is substantial evidence showing that caspase-3 plays a key role in mediating apoptotic cell death in the final degradation phase (11).
Multiple signaling pathways were previously shown to participate in infarct reduction by remote conditioning (18). We demonstrated in the present study that GSK-3β, a key component of the RISK pathway, is involved and functionally significant in producing myocardial protection induced by liver ischemic conditioning in a rat model of I/R injury. GSK-3β regulates multiple biological events. Inactivation of GSK-3β through the phosphorylation at Ser9 sites leads to the inhibition of GSK-3β activity (4). Recent studies have shown that local ischemic conditioning or remote ischemic conditioning-induced GSK-3β phosphorylation and inactivation was cardioprotective (32, 33). Its phosphorylation increased the threshold of mitochondrial permeability transition pore opening, which promoted survival of cardiac myocytes (26). Our present results confirm and expand previous findings by demonstrating that liver ischemic treatment before or post-myocardial ischemia dramatically increased phosphorylation of ventricular GSK-3β (Ser9) post-reperfusion compared with nontreated rats (CON).
Furthermore, pharmacological inhibition of GSK activity increases GSK phosphorylation and is, therefore, cardioprotective in terms of reducing infarct size or alleviating myocardial damage. It has been reported that the pharmacological GSK-3β inhibition (via Ser9 phosphorylation) could prevent mitochondrial permeability transition pore opening, thus shifting myocytes toward survival (22, 25). It is not surprising that, in the present study, we found infarct size reduction on administration of GSK inhibitor SB-216763. However, while all of these findings strongly suggest the favorable role of GSK inhibition in the myocardium, few contradictory studies showed that inhibition of GSK-3β failed to minimize infarction, or GSK-3β (Ser9) phosphorylation level did not change after reperfusion (23). This controversial debate may reflect species differences or experimental protocol variability.
We acknowledge several limitations of this study. First, we used only one liver ischemic intervention protocol, i.e., three cycles of 5-min ischemia/5-min reperfusion. Thus it remains unknown whether other liver stimuli strategies can produce cardioprotection. Second, we did not use all three liver ischemic conditioned hearts for immunohistochemical analysis. Rather, we used only RPre+RPost-treated hearts, to prove the concept that liver conditioning can inhibit apoptosis in the myocardium. Thus it remains unknown whether RPre or RPost alone can affect apoptotic protein expression after I/R injury. Third, in the present study, we clearly showed that liver conditioning significantly reduced apoptotic index in ventricles compared with matched control ones. However, the current TUNEL technique is not capable of differentiating cell types; therefore, other cells infiltrated into the myocardium during I/R injury may possibly contribute to the population of apoptotic cells. In addition, the mechanisms by which remote liver conditioning enhance cytoprotective actions seem to be related to MAPK signaling pathways, since we found liver ischemic treatment elicited activation of RISKs, such as GSK-3β; large-scale identification and characterization of each signaling molecule, as well as their interactions, would be of great interest in future research on this topic.
In conclusion, liver conditioning exerts strong cardioprotective effects, as seen by reduction in infarct size and recovery of cardiac performance. This liver conditioning-mediated cardioprotection is associated with GSK-3β-dependent signaling pathways.
GRANTS
This study was supported by grant no. 81670300 (to Z. Hu) from the National Natural Science Foundation of China. G. W. Abbott was supported by US National Heart, Lung, and Blood Institute Grant HL-079275.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.Y., G.W.A., W.D.G., J.L., C.L., and Z.H. conceived and designed research; S.Y. and Z.H. performed experiments; S.Y., J.L., C.L., and Z.H. analyzed data; S.Y., G.W.A., W.D.G., J.L., C.L., and Z.H. interpreted results of experiments; S.Y., G.W.A., and Z.H. prepared figures; S.Y., G.W.A., W.D.G., J.L., C.L., and Z.H. approved final version of manuscript; G.W.A., W.D.G., and Z.H. edited and revised manuscript; Z.H. drafted manuscript.
REFERENCES
- 1.Aimo A, Borrelli C, Giannoni A, Pastormerlo LE, Barison A, Mirizzi G, Emdin M, Passino C. Cardioprotection by remote ischemic conditioning: mechanisms and clinical evidences. World J Cardiol 7: 621–632, 2015. doi: 10.4330/wjc.v7.i10.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ateș E, Genç E, Erkasap N, Erkasap S, Akman S, Firat P, Emre S, Kiper H. Renal protection by brief liver ischemia in rats. Transplantation 74: 1247–1251, 2002. doi: 10.1097/00007890-200211150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sørensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375: 727–734, 2010. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 4.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol 2: 769–776, 2001. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 5.Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology 86: 699–709, 1997. doi: 10.1097/00000542-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci 64: 1930–1944, 2007. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation 94: 2193–2200, 1996. doi: 10.1161/01.CIR.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 8.Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM; ERICCA Trial Investigators . Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 373: 1408–1417, 2015. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 9.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: united at reperfusion. Pharmacol Ther 116: 173–191, 2007. doi: 10.1016/j.pharmthera.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 79: 377–386, 2008. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- 11.Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol 8: 619–629, 2011. doi: 10.1038/nrcardio.2011.85. [DOI] [PubMed] [Google Scholar]
- 12.Hu Z, Crump SM, Anand M, Kant R, Levi R, Abbott GW. Kcne3 deletion initiates extracardiac arrhythmogenesis in mice. FASEB J 28: 935–945, 2014. doi: 10.1096/fj.13-241828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Z, Crump SM, Zhang P, Abbott GW. Kcne2 deletion attenuates acute post-ischaemia/reperfusion myocardial infarction. Cardiovasc Res 110: 227–237, 2016. doi: 10.1093/cvr/cvw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Z, Hu S, Yang S, Chen M, Zhang P, Liu J, Abbott GW. Remote liver ischemic preconditioning protects against sudden cardiac death via an ERK/GSK-3β-dependent mechanism. PLoS One 11: e0165123, 2016. doi: 10.1371/journal.pone.0165123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Z, Kant R, Anand M, King EC, Krogh-Madsen T, Christini DJ, Abbott GW. Kcne2 deletion creates a multisystem syndrome predisposing to sudden cardiac death. Circ Cardiovasc Genet 7: 33–42, 2014. doi: 10.1161/CIRCGENETICS.113.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu ZY, Luo NF, Liu J. The protective effects of emulsified isoflurane on myocardial ischemia and reperfusion injury in rats. Can J Anaesth 56: 115–125, 2009. doi: 10.1007/s12630-008-9016-3. [DOI] [PubMed] [Google Scholar]
- 17.Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem 20: 1–22, 2007. doi: 10.1159/000103747. [DOI] [PubMed] [Google Scholar]
- 18.Kloner RA. Remote ischemic conditioning: its benefits and limitations. J Cardiovasc Pharmacol Ther 21: 219–221, 2016. doi: 10.1177/1074248415618816. [DOI] [PubMed] [Google Scholar]
- 19.Mastitskaya S, Marina N, Gourine A, Gilbey MP, Spyer KM, Teschemacher AG, Kasparov S, Trapp S, Ackland GL, Gourine AV. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res 95: 487–494, 2012. doi: 10.1093/cvr/cvs212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Böning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schön J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K; RIP Heart Study Collaborators . A Multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med 373: 1397–1407, 2015. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 21.Min JJ, Bae JY, Kim TK, Kim JH, Hwang HY, Kim KH, Ahn H, Oh AY, Bahk JH, Hong DM, Jeon Y. Pulmonary protective effects of remote ischaemic preconditioning with postconditioning in patients undergoing cardiac surgery involving cardiopulmonary bypass: a substudy of the remote ischaemic preconditioning with postconditioning outcome trial. Heart Lung Circ 25: 484–492, 2016. doi: 10.1016/j.hlc.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Miura T, Miki T. GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ J 73: 1184–1192, 2009. doi: 10.1253/circj.CJ-09-0284. [DOI] [PubMed] [Google Scholar]
- 23.Nishino Y, Webb IG, Davidson SM, Ahmed AI, Clark JE, Jacquet S, Shah AM, Miura T, Yellon DM, Avkiran M, Marber MS. Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ Res 103: 307–314, 2008. doi: 10.1161/CIRCRESAHA.107.169953. [DOI] [PubMed] [Google Scholar]
- 24.Noorbakhsh MF, Arab HA, Kazerani HR. Liver ischemia preconditions the heart against ischemia-reperfusion arrhythmias. Iran J Basic Med Sci 18: 80–88, 2015. [PMC free article] [PubMed] [Google Scholar]
- 25.Obame FN, Plin-Mercier C, Assaly R, Zini R, Dubois-Randé JL, Berdeaux A, Morin D. Cardioprotective effect of morphine and a blocker of glycogen synthase kinase 3 beta, SB216763 [3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione], via inhibition of the mitochondrial permeability transition pore. J Pharmacol Exp Ther 326: 252–258, 2008. doi: 10.1124/jpet.108.138008. [DOI] [PubMed] [Google Scholar]
- 26.Onishi A, Miyamae M, Inoue H, Kaneda K, Okusa C, Inamura Y, Shiomi M, Koshinuma S, Momota Y, Figueredo VM. Sevoflurane confers additive cardioprotection to ethanol preconditioning associated with enhanced phosphorylation of glycogen synthase kinase-3β and inhibition of mitochondrial permeability transition pore opening. J Cardiothorac Vasc Anesth 27: 916–924, 2013. doi: 10.1053/j.jvca.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Peralta C, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. Protective effect of liver ischemic preconditioning on liver and lung injury induced by hepatic ischemia-reperfusion in the rat. Hepatology 30: 1481–1489, 1999. doi: 10.1002/hep.510300622. [DOI] [PubMed] [Google Scholar]
- 28.Peralta C, Serafin A, Fernández-Zabalegui L, Wu ZY, Roselló-Catafau J. Liver ischemic preconditioning: a new strategy for the prevention of ischemia-reperfusion injury. Transplant Proc 35: 1800–1802, 2003. doi: 10.1016/S0041-1345(03)00571-2. [DOI] [PubMed] [Google Scholar]
- 29.Przyklenk K, Whittaker P. Remote ischemic preconditioning: current knowledge, unresolved questions, and future priorities. J Cardiovasc Pharmacol Ther 16: 255–259, 2011. doi: 10.1177/1074248411409040. [DOI] [PubMed] [Google Scholar]
- 30.Randhawa PK, Bali A, Jaggi AS. RIPC for multiorgan salvage in clinical settings: evolution of concept, evidences and mechanisms. Eur J Pharmacol 746: 317–332, 2015. doi: 10.1016/j.ejphar.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev 90: 1507–1546, 2010. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamareille S, Mateus V, Ghaboura N, Jeanneteau J, Croué A, Henrion D, Furber A, Prunier F. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol 106: 1329–1339, 2011. doi: 10.1007/s00395-011-0210-z. [DOI] [PubMed] [Google Scholar]
- 33.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase–dependent pathway is cardioprotective. Circ Res 90: 377–379, 2002. doi: 10.1161/01.RES.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 34.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol Heart Circ Physiol 277: H1967–H1974, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Zaugg M, Lucchinetti E, Behmanesh S, Clanachan AS. Anesthetic cardioprotection in clinical practice from proof-of-concept to clinical applications. Curr Pharm Des 20: 5706–5726, 2014. doi: 10.2174/1381612820666140204120829. [DOI] [PubMed] [Google Scholar]