In this study, we showed that age did not intensify the atrophy response to unloading in rats, but rather that the degree of atrophy was highly variable across muscles, indicating that changes in protein synthesis and protein degradation occur in a muscle-specific manner. Our data emphasize the importance of studying muscles of varying fiber-type and physiological function at multiple time points to fully understand the molecular mechanisms responsible for disuse atrophy.
Keywords: aging, muscle atrophy, proteasome, miR-23a
Abstract
Disuse is a potent inducer of muscle atrophy, but the molecular mechanisms driving this loss of muscle mass are highly debated. In particular, the extent to which disuse triggers decreases in protein synthesis or increases in protein degradation, and whether these changes are uniform across muscles or influenced by age, is unclear. We aimed to determine the impact of disuse on protein synthesis and protein degradation in lower limb muscles of varied function and fiber type in adult and old rats. Alterations in protein synthesis and degradation were measured in the soleus, medial gastrocnemius, and tibialis anterior (TA) muscles of adult and old rats subjected to hindlimb unloading (HU) for 3, 7, or 14 days. Loss of muscle mass was progressive during the unloading period, but highly variable (−9 to −38%) across muscle types and between ages. Protein synthesis decreased significantly in all muscles, except for the old TA. Atrophy-associated gene expression was only loosely associated with protein degradation as muscle RING finger-1, muscle atrophy F-box (MAFbx), and Forkhead box O1 expression significantly increased in all muscles, but an increase in proteasome activity was only observed in the adult soleus. MAFbx protein levels were significantly higher in the old muscles compared with adult muscles, despite the old having higher expression of microRNA-23a. These results indicate that adult and old muscles respond similarly to HU, and the greatest loss in muscle mass occurs in predominantly slow-twitch extensor muscles due to a concomitant decrease in protein synthesis and increase in protein degradation.
NEW & NOTEWORTHY In this study, we showed that age did not intensify the atrophy response to unloading in rats, but rather that the degree of atrophy was highly variable across muscles, indicating that changes in protein synthesis and protein degradation occur in a muscle-specific manner. Our data emphasize the importance of studying muscles of varying fiber-type and physiological function at multiple time points to fully understand the molecular mechanisms responsible for disuse atrophy.
skeletal muscle mass is maintained through a balance between protein synthesis and protein degradation. When there is a shift in this balance, such that protein synthesis is greater than protein degradation, muscle growth, or hypertrophy, occurs. On the other hand, a loss of muscle mass, or muscle atrophy, occurs when protein degradation exceeds protein synthesis. In humans, one of the most common and effective strategies for building muscle mass is resistance exercise. Resistance exercise produces a significant increase in muscle protein synthesis (MPS) that outweighs any concomitant increase in muscle protein breakdown (MPB), resulting in a net protein balance that is more positive (i.e., less negative, in the absence of exogenous amino acids), shifting the exercised muscle toward anabolism (8, 36). Although questions remain regarding what exercise frequency, intensity, duration, and nutritional supplementation is best for optimal muscle growth, it is evident that the primary driver of muscle growth is increased protein synthesis. Conversely, the dominant mechanism that leads to a loss of muscle mass is less clear, partly because of the variable stressors that converge on a muscle atrophy phenotype.
Loss of muscle mass can be triggered through direct changes to the muscle (unloading, neural damage), as a consequence of disease (cancer cachexia, heart failure, diabetes, acquired immunodeficiency syndrome, etc.), or as a result of aging (sarcopenia). Since muscle mass decreases under these conditions, MPB must be greater than MPS, but is this the result of decreases in MPS, increases in MPB, or a combination of both? This question was recently debated, with respect to disuse-induced muscle atrophy, in a CrossTalk put forth by the Journal of Physiology (35, 37). Disuse atrophy occurs as the result of decreases in external loading and/or neural activation of the muscle and is evident during bed rest, limb immobilization, and spaceflight. In humans and rodents, disuse can result in a rapid decline in muscle mass (10, 17, 27) and has been suggested to play a role in the development of sarcopenia (55). In the human-based literature, the prevailing theory is that disuse atrophy is caused solely by reductions in protein synthesis (35). This theory is supported by numerous studies that have shown disuse to cause significant decreases in protein synthesis, with little or no change in markers of protein degradation (14, 18, 46). However, the present theory is based almost exclusively on data collected from one muscle, the vastus lateralis (VL). Given its peripheral location, broad surface area, and lack of major superficial nerves/blood vessels, the VL is the preferred choice for muscle biopsy studies. However, other muscles exhibit faster rates of disuse atrophy and have vastly different fiber-type compositions and activity patterns in locomotion (1, 7). Thus there is a need to study mechanisms controlling protein balance resulting from disuse in muscles other than the VL.
As a counter to the argument that decreases in MPS fully account for the loss in muscle mass with disuse, data from rodent studies suggest that increases in protein degradation contribute to the disuse-induced atrophy response (52, 60). While it has been suggested that rodents are a poor model for the study of human disuse atrophy due to inherent biological differences (39), recent data suggest that the response to disuse is actually quite similar between humans and rodents (38). There are many potential explanations for the discrepancies between the human and rodent studies; two of the most likely are 1) the duration and experimental model used to study disuse-induced muscle atrophy, and 2) the muscles and time points selected for analysis (31, 37). To determine the extent to which changes in protein synthesis and/or protein degradation contribute to muscle atrophy under conditions of disuse, we chose to use the hindlimb suspension model. Hindlimb suspension is a well-accepted model of microgravity and bed rest, conditions that result in unloading of the hindlimb muscles with minimal impact on neural activation of the muscles. In this study, we examined the impact of unloading on muscles of varying physiological functions (postural/phasic or extensors/flexors) and fiber-type composition in both adult and old Fischer 344-Brown Norway rats. A better understanding of how disuse affects specific muscles in both the short and long term will help determine whether changes in MPS or MPB pathways are critical to the atrophy process. Furthermore, this work will assist in the design of optimal treatment strategies to recover muscle mass, or prevent its loss, following disuse-induced atrophy.
METHODS
Ethical approval.
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
Animals.
Adult (9 mo) and old (29 mo) male Fischer 344-Brown Norway (F344BN) rats were obtained from the National Institute on Aging. Rats were allowed to acclimate to their environment for at least 1 wk before testing. The rats utilized in this study are from a larger study that specifically examined the recovery of hindlimb muscles from disuse (5).
Hindlimb unloading.
Unloading of the hindlimbs was achieved by tail suspension, as previously described (5, 49). Briefly, the rats were attached via a plastic bar to a swivel mounted at the top of the cage, allowing free 360° rotation. The rats were maintained in ~30° head-down tilt position with their hindlimbs unloaded for a period of 3, 7, or 14 days (n = 6–7/group). During the unloading period, rats had access to food and water ad libitum.
Muscle collection.
Following completion of each unloading time period, rats were weighed, anesthetized with 2% inhaled isoflurane for tissue removal, and then killed by removal of the heart. The soleus, medial gastrocnemius (MG), plantaris (PLN), tibialis anterior (TA), and extensor digitorum longus (EDL) muscles were removed from both hindlimbs, weighed, and then frozen in liquid nitrogen for biochemical analyses, with the exception of the right soleus and TA, which were pinned at resting length and flash frozen in liquid nitrogen cooled isopentane for histological analysis.
Fiber-type-specific cross-sectional area.
Serial cross sections (10 μm) were cut from the TA and soleus using a Leica CM 3050S cryostat. To determine fiber-type-specific cross-sectional area (CSA), TA and soleus muscle sections were fixed in cold acetone for 5 min at −20°C, followed by three 5-min washes with phosphate-buffered saline with 0.1% Tween 20. Sections were blocked in 5% normal goat serum in phosphate-buffered saline with 0.1% Tween 20 (blocking buffer) for 30 min at room temperature (RT) and then incubated in primary antibody overnight at 4°C; BA-F8 (myosin heavy chain slow type, Mm, IgG2B), SC-71 (myosin heavy chain 2A, Mm, IgG1), and BF-F3 (myosin heavy chain 2B, Mm, immunoglobulin M) were diluted 1:250 in blocking buffer. All anti-myosin heavy chain antibodies were purchased from the Developmental Studies Hybridoma Bank (Iowa City, IA). A polyclonal laminin antibody (1:500, Sigma catalog no. L9393) was included for the determination of CSA. After incubation in primary antibody, sections were incubated in secondary antibody for 30 min at RT and then coverslipped using ProLong Gold Antifade reagent (Life Technologies, catalog no. P36930). For simultaneous detection of multiple mouse primary antibodies, fluorescently conjugated goat-anti-mouse immunoglobulin-specific secondary antibodies were used (Alexa Fluor 350, 488, and 555, Life Technologies). Goat-anti-rabbit AlexaFluor 647 secondary was used to detect laminin. Slides were imaged using a Zeiss Axio Imager.M1 fluorescent microscope using the EC Plan-Neofluar ×10 objective and analyzed using Axiovision software. Fibers from five to six regions of a single section were analyzed per muscle, per animal.
Protein synthesis measurements.
The SUnSET (SUrface SEnsing of Translation) technique was used as previously described (57) to determine changes in MPS. Puromycin (EMD Millipore, catalog no. 540222) was dissolved in sterile saline and delivered by intraperitoneal injection (0.02 μmol/g body wt) 30 min before muscle collection. Puromycin-truncated peptides, reflecting the rate of global MPS, were analyzed by Western blot.
Western blotting.
Frozen TA, MG, and soleus muscles were homogenized in sucrose lysis buffer (50 mM Tris, pH 7.5, 250 mM sucrose, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 50 mM NaF) with added protease and phosphatase inhibitors (Pierce, catalog no. 88668). The supernatant was collected following centrifugation at 8,000 g for 10 min, and protein concentrations were determined using the Bradford method. Ten to twenty micrograms of protein were subjected to SDS-PAGE on 4–20% Criterion TGX stain-free gels (Bio-Rad, cat. 5678095) and transferred to polyvinylidene diflouride membrane (EMD Millipore, catalog no. IPVH00010). Membranes were blocked in 3% nonfat milk in Tris-buffered saline with 0.1% Tween 20 for 1 h and then probed with puromycin (1:500, EMD Millipore, catalog no. MABE343), muscle RING finger-1 (MuRF1) (1:1000, ECM Biosciences, catalog no. MM3161) or muscle atrophy F-box (MAFbx) (1:1000, ECM Biosciences, catalog no. AM3141) overnight at 4°C. Membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibodies at 1:5,000 for 1 h at RT. Immobilon Western chemiluminescent horseradish peroxidase substrate (EMD Millipore, catalog no. WBKLS0500) was then applied to the membranes before image acquisition. Image acquisition and band quantification were performed using the ChemiDoc MP System and Image Laboratory 5.0 software (Bio-Rad), respectively. Total protein staining of the membrane was used as the normalization control for all blots.
RNA isolation and gene expression.
RNA was isolated using RNAzol RT reagent (Sigma, catalog no. R4533), according to the manufacturer’s instructions, and quantified by absorbance spectrophotometry. cDNA was synthesized using a reverse transcription kit (Life Technologies, catalog no. 4368814) from 1 μg of total RNA. Gene expression was analyzed by quantitative PCR (qPCR) using SYBR Green JumpStart Taq ReadyMix (Sigma, catalog no. S9194) on an ABI 7900HT thermocycler. Each sample was run in triplicate. Gene expression was normalized to tissue weight as previously described (24). The rat primer sequences used were as follows: MuRF1 forward, 5′-ACAACCTCTGCCGGAAGTGT-3′; MuRF1 reverse, 5′-CCGCGGTTGGTCCAGTAG-3′; MAFbx forward, 5′-CATCCTTATGCACGCTGGTC-3′; MAFbx reverse, 5′-GGTCTCCATTCGATACACCCA-3′; Forkhead box O1 (FOXO1) forward, 5′-ATTCGCCACAATCTGTCCCT-3′; FOXO1 reverse, 5′-TTTCTTAGCAGCCCGTCCTC-3′; Forkhead box O3a (FOXO3a) forward, 5′-TGAGGAAAGGGGAAATGGGC-3′; FOXO3a reverse, 5′-TGGGTTAGGAAGATGGCGTG-3′; histone deacetylase 4 (HDAC4) forward, 5′-AGAGGTGAAGATGAAGCTGCA-3′; HDAC4 reverse, 5′-GCTGTGTCTTCCCATACCAGT-3′; growth arrest and DNA damage-inducible 45α (Gadd45a) forward, 5′-GTCACTCCCCACGCTGATG-3′; Gadd45a reverse, 5′-TGCAAAGTCATCTCCTAGCCC-3′.
Quantification of microRNA-23a.
RNA was isolated using TRIzol reagent (Ambion, catalog no. 15596026), according to the manufacturer’s instructions, and quantified by absorbance spectrophotometry. One microgram of RNA was DNase treated (Invitrogen, catalog no. 18068-015) and then reverse transcribed using a Taqman MicroRNA Reverse Transcription Kit (Applied Biosystems, catalog no. 4366596) and microRNA-23a (miR-23a) specific primers (Applied Biosystems Taqman MicroRNA Assay ID 000399). Following reverse transcription, cDNA was preamplified using TaqMan PreAmp Mastermix (Applied Biosystems, catalog no. 4488593). Reverse transcription, preamplification, and qPCR were performed according to the manufacturer’s instructions as listed in Custom RT and Preamplification Pools protocol (Life Technologies protocol 4465407). qPCR was performed using TaqMan Universal MasterMix II, no UNG (Applied Biosystems, catalog no. 4440040) with 10-μl reaction volumes on an ABI 7900HT thermocycler. Each sample was run in triplicate. Gene expression was calculated using the delta, delta threshold cycle method (29) and normalized to tissue weight.
Proteasome activity.
20S and 26S proteasome activities were performed as previously described (5, 21). Proteasome activities were determined by adding substrates at 100 µM: Z-Leu-Leu-Glu-MCA (Peptide Institute, catalog no. 3179-v), Boc-Leu-Ser-Thr-Arg-AMC (Bachem, catalog no. I-1940), or succinyl-Leu-Leu-Val-Tyr-7-AMC (Bachem, catalog no. I-1395), for β1- (caspase-like), β2- (trypsin-like), and β5-subunits (chymotrypsin-like), respectively. Each assay was conducted in the absence and presence of the proteasome inhibitor bortezomib (Cell Signaling, catalog no. 2204) at a final concentration of 2 mM (β5) or 10 mM (β1 and β2). All proteasome assays were conducted using 10 µg of protein/well on a 96-well plate (Greiner Bio-One, catalog no. 655076), and each sample was loaded once on the plate to allow for all samples to be loaded on the same plate. The activity of the 20S and 26S proteasome was measured by calculating the difference between fluorescence units recorded with or without the inhibitor in the reaction medium. Fluorescence was measured using a Fluoroskan Ascent fluorometer (Thermo Electron, excitation wavelength, 390 nm; emission wavelength, 460 nm) at 15-min intervals for 2 h.
Cathepsin L activity.
Cathepsin L enzymatic activity was assayed by adding 100 µM Z-Phe-Arg-AMC (Calbiochem, catalog no. 03321501) to a reaction buffer containing 100 mM sodium acetate, pH 5.5, 1 mM EDTA, and 2 mM DTT in the absence and presence of 10 μM cathepsin L inhibitor I (Calbiochem, catalog no. 219421). Each assay was conducted using 34 µg of protein/well, and each sample was loaded once on a 96-well plate (Greiner Bio-One, catalog no. 655076) to allow for all samples to be loaded on the same plate. Fluorescence was measured at excitation and emission wavelengths of 390 nm and 460 nm as carried out for the proteasome assays. Activity was calculated by determining the difference between fluorescence units recorded with or without the inhibitor in the reaction medium.
Statistics.
Muscle weight data found in Table 1 are presented as means ± SD. All other results are presented as means ± SE. The difference in starting body weight between adult and old rats was analyzed by Student’s t-test, whereas the remaining data were analyzed by one- or two-way ANOVA (age × time) using GraphPad Prism 7 software (GraphPad Software). Tukey’s post hoc analysis was used to determine differences when interactions existed. Statistical significance was set at P < 0.05.
Table 1.
Effect of hindlimb unloading on muscle mass of adult and old rats
| Control | 3-day HU | 7-day HU | 14-day HU | %Loss from Control after 14 Days | |
|---|---|---|---|---|---|
| TA | |||||
| Adult | 0.779 ± 0.043 | 0.659 ± 0.066* | 0.659 ± 0.053* | 0.625 ± 0.046* | −19.8 |
| (1.895 ± 0.072) | (1.776 ± 0.062) | (1.799 ± 0.056) | (1.803 ± 0.074) | ||
| Old | 0.741 ± 0.059 | 0.671 ± 0.048 | 0.655 ± 0.037 | 0.630 ± 0.061* | −15.0 |
| (1.304 ± 0.163)# | (1.265 ± 0.078)# | (1.376 ± 0.067)# | (1.260 ± 0.070)# | ||
| EDL | |||||
| Adult | 0.191 ± 0.007 | 0.168 ± 0.018 | 0.169 ± 0.008 | 0.157 ± 0.010* | −17.7 |
| (0.460 ± 0.026) | (0.452 ± 0.013) | (0.462 ± 0.025) | (0.453 ± 0.015) | ||
| Old | 0.188 ± 0.019 | 0.174 ± 0.013 | 0.175 ± 0.010 | 0.172 ± 0.014 | −8.9 |
| (0.330 ± 0.037)# | (0.330 ± 0.037)# | (0.367 ± 0.021)# | (0.344 ± 0.028)# | ||
| Soleus | |||||
| Adult | 0.178 ± 0.013 | 0.166 ± 0.013 | 0.132 ± 0.007* | 0.111 ± 0.020* | −37.9 |
| (0.437 ± 0.028) | (0.447 ± 0.012) | (0.360 ± 0.013)* | (0.318 ± 0.045)* | ||
| Old | 0.167 ± 0.016 | 0.152 ± 0.013 | 0.140 ± 0.010* | 0.117 ± 0.010* | −29.8 |
| (0.293 ± 0.033)# | (0.286 ± 0.030)# | (0.294 ± 0.016)# | (0.235 ± 0.017)*,# | ||
| PLN | |||||
| Adult | 0.414 ± 0.041 | 0.365 ± 0.044 | 0.352 ± 0.022* | 0.306 ± 0.017* | −26.3 |
| (1.018 ± 0.096) | (0.981 ± 0.068) | (0.962 ± 0.047) | (0.883 ± 0.047)* | ||
| Old | 0.392 ± 0.016 | 0.334 ± 0.017* | 0.332 ± 0.027* | 0.309 ± 0.030* | −21.7 |
| (0.692 ± 0.098)# | (0.632 ± 0.069)# | (0.697 ± 0.058)# | (0.618 ± 0.027)# | ||
| MG | |||||
| Adult | 0.938 ± 0.061 | 0.790 ± 0.098* | 0.749 ± 0.049* | 0.643 ± 0.043* | −31.4 |
| (2.288 ± 0.129) | (2.122 ± 0.092) | (2.047 ± 0.082) | (1.870 ± 0.122)* | ||
| Old | 0.872 ± 0.064 | 0.759 ± 0.055* | 0.653 ± 0.021*,# | 0.625 ± 0.061* | −28.3 |
| (1.536 ± 0.205)# | (1.436 ± 0.145)# | (1.390 ± 0.092)# | (1.252 ± 0.068)*,# |
Muscle masses are means ± SD in g. In parentheses, muscle mass is normalized to body weight (mg wet mass/g body wt) and expressed as means ± SD; n = 6–7/group. HU, hindlimb unloading; TA, tibialis anterior; EDL, extensor digitorum longus; PLN, plantaris; MG, medial gastrocnemius.
P < 0.05 vs. age-matched control.
P < 0.05 vs. adult at same time point.
RESULTS
Loss of body weight and muscle mass following hindlimb unloading.
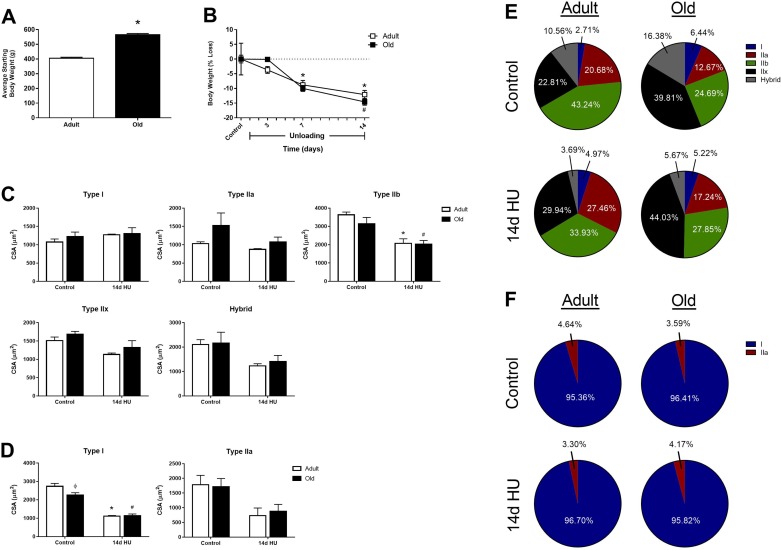
To determine the impact of age on disuse-induced muscle atrophy, adult and old F344BN rats underwent hindlimb unloading (HU) for 3, 7, or 14 days, and body weight, along with the wet weights of the TA, EDL, soleus, PLN, and MG, were compared between the two ages. Similar decreases in body weight were observed between the adult (12.2% loss) and old (14.6% loss) rats through 14 days of HU (Fig. 1A), despite the old rats being significantly heavier at the start of the unloading period (Fig. 1B). As previously noted, at baseline, muscles from old rats tended to be smaller than those of the adult rats, but were not significantly different (P > 0.05) (5). Three days of HU caused significant atrophy of both the adult and old MG (Table 1). By day 7, significant atrophy was found in all of the adult and old muscles, except the EDL. Excluding the old EDL, mass continued to drop in all hindlimb muscles for both age groups between days 7 and 14 of HU. Loss of mass was not uniform across muscles, with the ankle extensors (soleus, PLN, MG) atrophying to a greater extent than the ankle flexors (TA, EDL). When muscle mass was normalized to body weight, significant decreases in this ratio were found for the adult soleus, PLN, and MG, and for the old soleus and MG, after 14 days of HU (Table 1). Moreover, when muscle mass was calculated as a percent loss from control, adult rats lost a greater percentage of their hindlimb muscle mass after 2 wk of HU compared with the old rats (Table 1), suggesting that aging does not accelerate disuse-induced muscle loss.
Fig. 1.
Body weight and fiber-type-specific cross-sectional area (CSA) changes in adult and old rats in response to hindlimb unloading (HU). A: average starting body weight for adult (9 mo) and old (29 mo) male F344BN rats. B: loss of body weight in adult and old rats after 3, 7, and 14 days of HU. All values are expressed as percent loss from age-matched control animals for each HU time point. Changes in fiber-type-specific CSA were measured in the TA (C) and soleus (D) of adult (open bars) and old (solid bars) rats at baseline (control) and after 14 days of HU (n = 3–6/group). Values are means ± SE. *P < 0.05 vs. adult control. #P < 0.05 vs. old control. ϕP < 0.05 vs. adult at same time point. Fiber-type compositions of the adult and old rat TA (E) and soleus (F) were calculated at baseline (control) and after 14 days of HU. The no. of each fiber type was counted and expressed as a percentage of the total number of fibers.
Aging can have a profound impact on skeletal muscle, with loss of muscle fiber CSA occurring as early as 27 mo of age in some hindlimb muscles of the F344BN rats (30). In the present study, muscle wet weights tended to be lower in the old rats compared with the adult, suggesting subtle changes were occurring in the 29-mo-old muscles. Thus fiber-type-specific CSA measurements, along with calculations of muscle fiber composition, were made in the adult and old TA and soleus muscles at baseline and after 14 days of HU (Fig. 1, C–F). In the predominantly fast-twitch TA muscle, CSA was similar between the adult and old rats at baseline, although there was a trend for the type IIa fibers to be larger, and the type IIb fibers to be smaller, in the old (Fig. 1C). Following 14 days of HU, a reduction in type IIb fiber CSA was noted for both the adult and old rats, with no change in CSA detected for any of the other fiber types. In the predominantly slow-twitch soleus muscle, CSA was significantly reduced in the old type I fibers at baseline, and both the adult and old rats showed significant declines in type I fiber CSA after 14 days of HU (Fig. 1D). No major changes in fiber composition were observed in the adult or old soleus at baseline or after HU (Fig. 1F). However, in the TA, the old at baseline had fewer type IIb fibers and a greater percentage of type IIx and hybrid fibers compared with the adult TA (Fig. 1E). After 14 days of HU, the adult TA showed a decrease in the percentage of type IIb fibers and an increase in the percentage of type IIa and IIx fibers, while the old TA showed a decrease in the percentage of hybrid fibers and a similar increase in the percentage of type IIa and IIx fibers (Fig. 1E).
Changes in protein synthesis and protein degradation differ between adult hindlimb muscles in response to unloading.
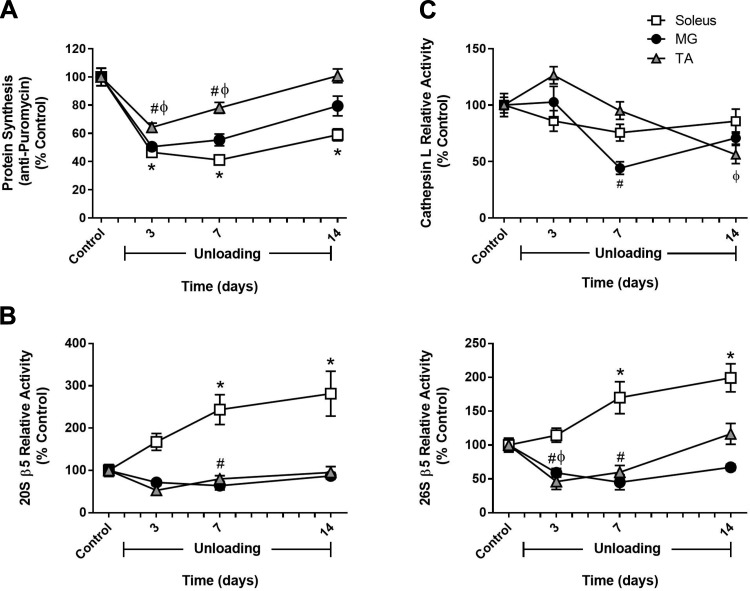
To determine whether distinct changes in protein synthesis and/or protein degradation could explain the disparity in disuse atrophy seen in the different hindlimb muscles, measurements of MPS and muscle proteolysis were made in the soleus, MG, and TA of adult rats at baseline (control) and after 3, 7, and 14 days of HU. As shown in Fig. 2A, after 3 days of HU, significant decreases in MPS were found in all three hindlimb muscles, and this decrease continued through day 7. By day 14, only the soleus continued to exhibit decreased MPS, whereas MPS had returned to control levels in both the MG and TA (Fig. 2A).
Fig. 2.
Comparison of changes in muscle protein synthesis and protein degradation between hindlimb muscles in response to unloading. Adult (9 mo) male rats underwent hindlimb unloading (HU) for 3, 7, or 14 days. Protein synthesis (A), 20S and 26S β5 proteasome subunit activity (B), and cathepsin L activity (C) were measured in the soleus (open squares), medial gastrocnemius (MG; solid circles), and tibialis anterior (TA; shaded triangles) at each unloading time point. Total protein determined by stain-free imaging of the PVDF membrane was used to normalize puromycin protein expression. All values are expressed as a percentage relative to the control group for each muscle (n = 5–6/muscle). Values are means ± SE. *P < 0.05 vs. control soleus. #P < 0.05 vs. control MG. ϕP < 0.05 vs. control TA.
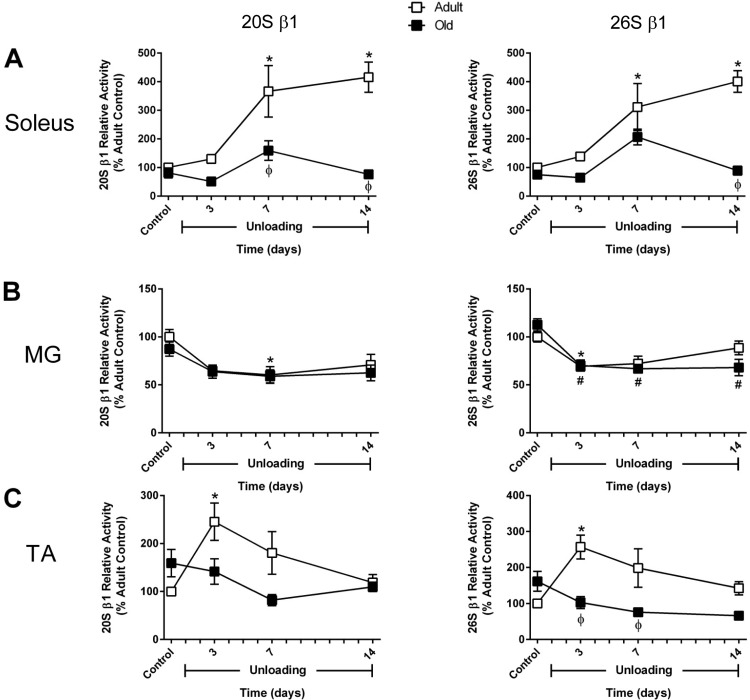
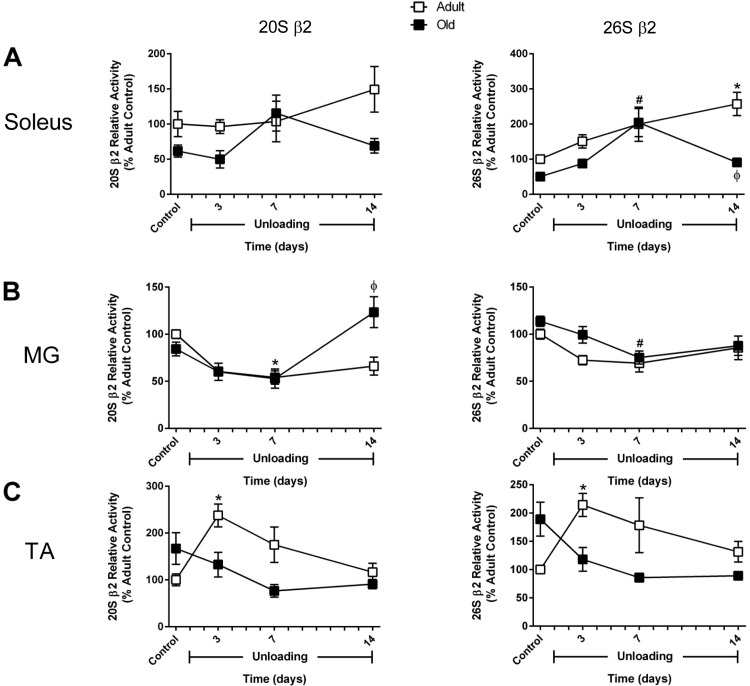
Protein degradation was also determined in the soleus, MG, and TA of adult rats by measuring the ATP-independent (20S) and ATP-dependent (26S) activities of the three proteolytic subunits (β1, β2, and β5) of the proteasome, along with the activity of the lysosomal enzyme cathepsin L. In general, significant increases in protein degradation occurred only in the adult soleus and TA. After 3 days of unloading, β1- and β2-subunit activity was significantly higher in the TA, but then returned to baseline by day 7 (see Figs. 5 and 6). In the soleus, significant increases in proteasome activity were observed at day 7 of unloading and remained elevated through day 14 (Fig. 2, B and C; see Figs. 5 and 6). In the adult MG, activity of these two proteolytic markers remained significantly lower or at control levels throughout the unloading period (Fig. 2, B and C; see Figs. 5 and 6).
Fig. 5.
Changes in activity of the 20S and 26S β1 proteasome subunit in adult and old rats during hindlimb unloading (HU). Proteolytic activity of the β1-subunit of the 20S (ATP-independent) and 26S (ATP-dependent) proteasome was measured in the soleus (A), medial gastrocnemius (MG; B), and tibialis anterior (TA; C) muscles of adult (9 mo, □) and old (29 mo, ■) rats after 3, 7, and 14 days of HU. Data are expressed as a percentage relative to the activity of the adult control group (n = 4–6/group). Values are means ± SE. *P < 0.05 vs. adult control. #P < 0.05 vs. old control. ϕP < 0.05 vs. adult at same time point.
Fig. 6.
Changes in activity of the 20S and 26S β2 proteasome subunit in adult and old rats during hindlimb unloading (HU). Proteolytic activity of the β2-subunit of the 20S (ATP-independent) and 26S (ATP-dependent) proteasome was measured in the soleus (A), medial gastrocnemius (MG; B), and tibialis anterior (TA; C) muscles of adult (9 mo, □) and old (29 mo, ■) rats after 3, 7, and 14 days of HU. Data are expressed as a percentage relative to the activity of the adult control group (n = 4–6/group). Values are means ± SE. *P < 0.05 vs. adult control. #P < 0.05 vs. old control. ϕP < 0.05 vs. adult at same time point.
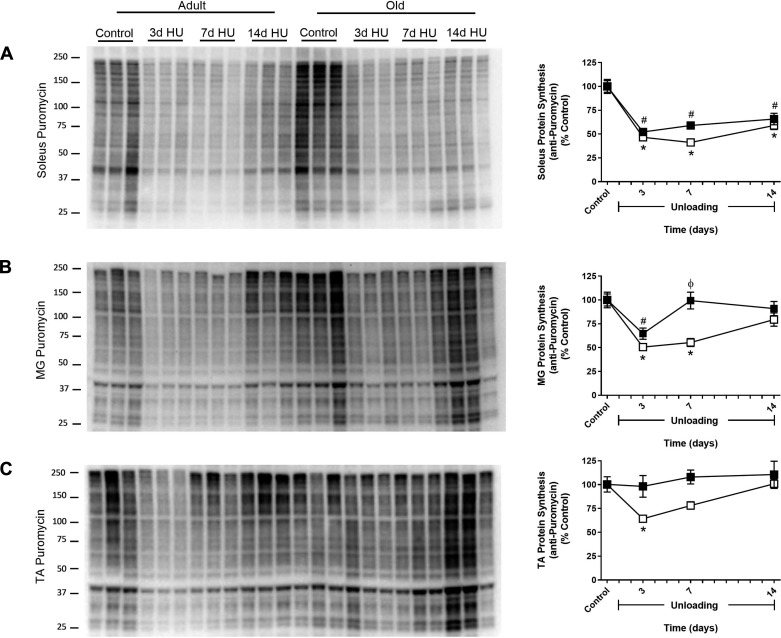
Adult rats show larger decreases in protein synthesis than old rats in response to HU.
In Fig. 2, we demonstrated that the pattern of change in MPS differed between the soleus, MG, and TA throughout the 14-day HU period in adult rats. To determine whether aging influenced the MPS response to HU, the same muscles from the old rats were analyzed after 3, 7, and 14 days of HU. In control animals, MPS was not statistically different between the two ages (data not shown). As was observed in the adult rats, MPS in the soleus and MG of the old rats decreased significantly after 3 days of HU. Surprisingly, a drop in MPS was not observed in the old TA, i.e., MPS was similar to control levels at all time points (Fig. 3C). MPS returned to control levels by 7 days of HU in the old MG, but remained significantly lower in the old soleus through the 14 days of unloading (Fig. 3, A and B).
Fig. 3.
Effect of hindlimb unloading (HU) on muscle protein synthesis in adult and old rats. Protein synthesis was measured in the soleus (A), medial gastrocnemius (MG; B), and tibialis anterior (TA; C) muscles of adult (9 mo, □) and old (29 mo, ■) rats after 3, 7, and 14 days of HU. Total protein, determined by stain-free imaging of the PVDF membrane, was used to normalize protein expression. Puromycin values are expressed as a percentage of each age-matched control group (n = 5–7/group). Values are means ± SE. *P < 0.05 vs. adult control. #P < 0.05 vs. old control. ϕP < 0.05 vs. adult at same time point.
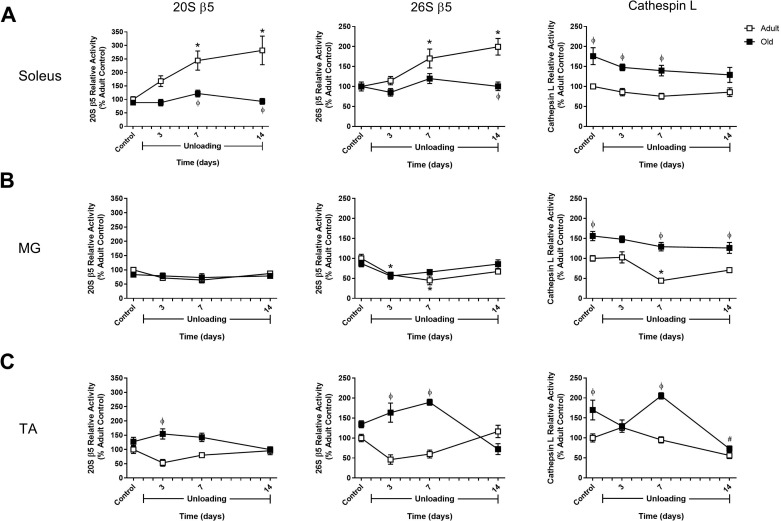
HU did not increase activity of the proteasome or cathepsin L enzyme activity in old rats.
Activity of the 20S and 26S proteolytic subunits of the proteasome, along with cathepsin L activity, was measured in the old soleus, MG, and TA at baseline (control) and after 3, 7, and 14 days of HU. In the old soleus, the only significant increase in proteasome activity was found for the 26S β2-subunit at day 7 of HU (Figs. 4–6). In the old MG and TA muscles, proteasome activity was not increased at any of the HU time points analyzed (Figs. 4–6). However, compared with the adult, the TA of the old rats was found to have significantly higher β5 activity during the first week of HU (Fig. 4C). No change in cathepsin L activity was found in response to HU, but all three old muscles were found to have higher baseline activity and higher activity at day 7 of HU compared with the adult rats at the same time point (Fig. 4).
Fig. 4.
Proteasome and cathepsin L activity changes in adult and old rats during hindlimb unloading (HU). Proteolytic activity of the β5-subunits of the 20S (ATP-independent) and 26S (ATP-dependent) proteasome and cathepsin L activity were measured in the soleus (A), medial gastrocnemius (MG; B), and tibialis anterior (TA; C) muscles of adult (9 mo, □) and old (29 mo, ■) rats after 3, 7, and 14 days of HU. Data are expressed as a percentage relative to the activity of the adult control group (n = 4–6/group). Values are means ± SE. *P < 0.05 vs. adult control. #P < 0.05 vs. old control. ϕP < 0.05 vs. adult at same time point.
HU-induced changes in protein degradation activity are not explained by changes in expression of atrophy-related genes.
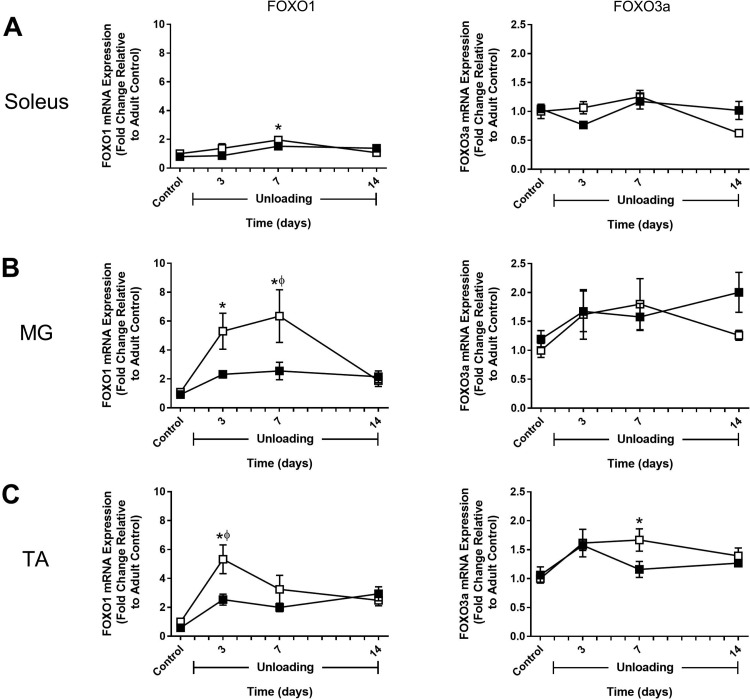
Thus far, comparison of the soleus, MG, and TA has shown that activation of protein degradation pathways mainly occurred in the adult soleus and TA in response to 2 wk of HU. To determine whether these findings could be explained by muscle- and/or age-specific differences in the expression of atrophy-associated genes, expression of MuRF1, MAFbx, FOXO1, FOXO3, HDAC4, and Gadd45a was examined in both adult and old rats at baseline (control) and after 3, 7, and 14 days of HU (Fig. 7–9). Although the pattern of expression varied widely across muscles and between ages, it is clear that induction of these atrophy markers was not limited to the adult muscles, which had the greatest increases in protein degradation.
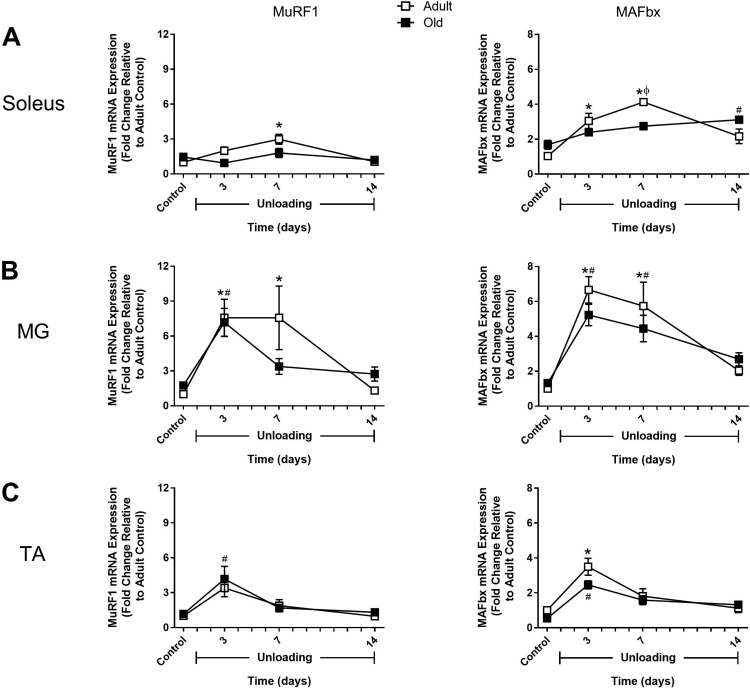
Fig. 7.
Changes in MuRF1 and MAFbx expression in response to hindlimb unloading (HU) in adult and old rats. mRNA expression of MuRF1 and MAFbx was assessed by quantitative PCR in the soleus (A), medial gastrocnemius (MG; B), and tibialis anterior (TA; C) muscles of adult (9 mo, □) and old (29 mo, ■) rats after 3, 7, and 14 days of HU. Gene expression was normalized to tissue weight. Data are expressed as a fold change relative to the adult control group (n = 5–6/group). Values are means ± SE. *P < 0.05 vs. adult control. #P < 0.05 vs. old control. ϕP < 0.05 vs. adult at same time point.
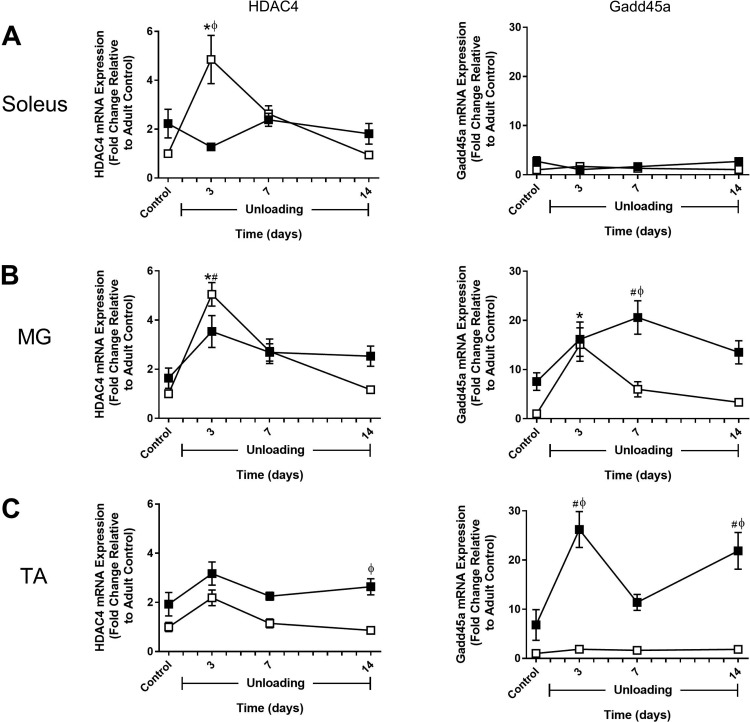
Fig. 9.
Effect of hindlimb unloading (HU) on HDAC4 and Gadd45a expression in adult and old rats. mRNA expression of HDAC4 and Gadd45a was assessed by quantitative PCR in the soleus (A), medial gastrocnemius (MG; B), and tibialis anterior (TA; C) muscles of adult (9 mo, □) and old (29 mo, ■) rats after 3, 7, and 14 days of HU. Gene expression was normalized to tissue weight. Data are expressed as a fold change relative to the adult control group (n = 5–6/group). Values are means ± SE. *P < 0.05 vs. adult control. #P < 0.05 vs. old control. ϕP < 0.05 vs. adult at same time point.
In adult rats, MuRF1 and MAFbx expression significantly increased in all three muscles in response to HU. In the soleus and MG, expression remained elevated through 7 days of HU and then returned to baseline by day 14 (Fig. 7, A and B). In the TA, expression peaked at 3 days and then dropped rapidly to baseline by day 7 (Fig. 7C). FOXO1 and FOXO3a expression patterns differed significantly in response to unloading. For FOXO1, changes in expression during the unloading period resembled those of MuRF1 (Fig. 8). In contrast, FOXO3a showed little change in expression in response to HU, with the only significant increase occurring in the adult TA after 7 days of HU (Fig. 8C). HDAC4 expression in the adult rats was very responsive to unloading and peaked in all muscles at 3 days of HU, with the greatest change occurring in the soleus and MG (Fig. 9). Conversely, Gadd45a expression changed very little in response to unloading, except for in the MG where there was a significant increase after 3 days of HU (Fig. 9).
Fig. 8.
Expression changes in FOXO1 and FOXO3a during hindlimb unloading (HU) in adult and old rats. mRNA expression of FOXO1 and FOXO3a was assessed by quantitative PCR in the soleus (A), medial gastrocnemius (MG; B), and tibialis anterior (TA; C) muscles of adult (9 mo, □) and old (29 mo, ■) rats after 3, 7, and 14 days of HU. Gene expression was normalized to tissue weight. Data are expressed as a fold change relative to the adult control group (n = 5–6/group). Values are means ± SE. *P < 0.05 vs. adult control. #P < 0.05 vs. old control. ϕP < 0.05 vs. adult at same time point.
In old rats, changes in MuRF1 and MAFbx expression in response to HU generally resembled what was observed in the adult rats (Fig. 7). The major difference was in the soleus, where changes in MuRF1 expression were blunted and changes in MAFbx expression increased gradually over the unloading period and remained elevated at day 14 of HU (Fig. 7A). While MuRF1 and MAFbx did respond to unloading in the old rats, FOXO1 and FOXO3a expression did not change significantly following unloading (Fig. 8). HDAC4 expression significantly increased in the MG after 3 days of HU, similar to the adult rats, but no change in expression of this gene was found in the soleus or TA during the unloading period (Fig. 9). Interestingly, in the old rats, Gadd45a expression tended to start higher and increased significantly in the MG and TA during the unloading period (Fig. 9, B and C). Furthermore, Gadd45a remained elevated in the old TA after 14 days of HU (Fig. 9C), which differed from the majority of the other gene targets in that expression typically returned to control levels by day 14. Overall, these data suggest that disuse does trigger early increases in the expression of atrophy-associated genes, but that mRNA abundance was not a good indicator of increased protein degradation as measured by proteasome subunit activities and cathepsin L activity.
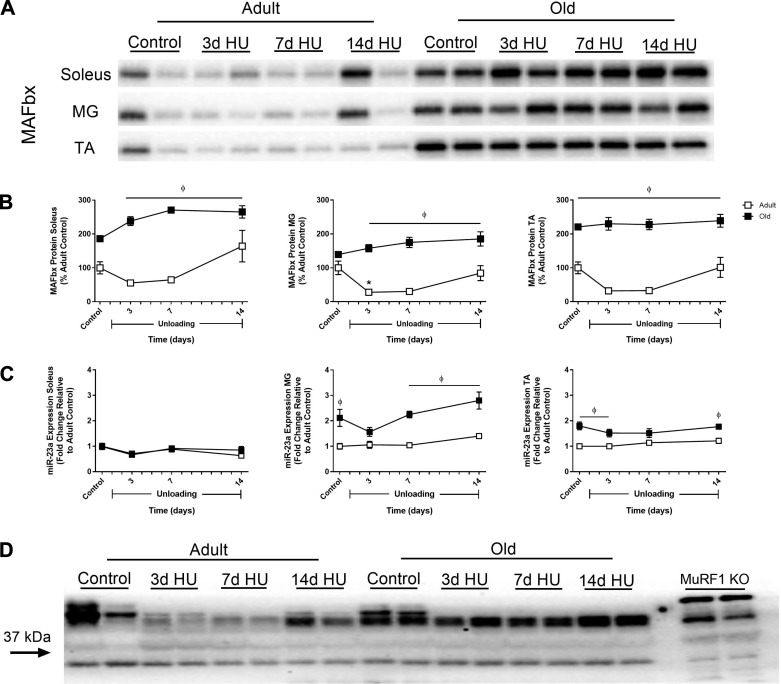
MAFbx protein levels do not match mRNA expression data and do not explain changes in protein degradation in response to HU.
Although the changes in MuRF1 and MAFbx gene expression could not account for the changes in protein degradation observed in response to HU, it is possible that MuRF1 and MAFbx protein levels are better associated with protein degradation activity. Thus an attempt was made to quantify MuRF1 and MAFbx protein levels in the adult and old soleus, MG, and TA muscles after 3, 7, and 14 days of HU. Surprisingly, MAFbx protein levels were not found to increase in any of the adult or old muscles at any point during the unloading period (Fig. 10, A and B). In the adult rats, MAFbx protein tended to decrease through the first 7 days of HU, but was only observed to be significantly decreased at day 3 of HU in the MG. While MAFbx did not change in the old rats, protein levels in all three muscle were significantly higher than adult rats at 3, 7, and 14 days of HU. As shown in Fig. 10D, the antibody for MuRF1 was not valid, since bands were visible in the lanes containing samples from MuRF1 knockout mice; thus MuRF1 protein levels could not be quantified.
Fig. 10.
Changes in MAFbx protein and miR-23a expression in response to hindlimb unloading (HU) in adult and old rats. Representative Western blots (A) and quantification (B) of MAFbx protein levels in the soleus, medial gastrocnemius (MG), and tibialis anterior (TA) of adult (9 mo, □) and old (29 mo, ■) rats after 3, 7, and 14 days of HU. Total protein, determined by stain-free imaging of the PVDF membrane, was used to normalize protein expression. Data are expressed as a percentage relative to the adult control group (n = 5–6/group). Values are means ± SE. *P < 0.05 vs. adult control. ϕP < 0.05 vs. adult at same time point. C: miR-23a expression was assessed by quantitative PCR in the soleus, MG, and TA of adult (□) and old (■) rats after 3, 7, and 14 days of HU. Gene expression was normalized to tissue weight. Data are expressed as a fold change relative to the adult control group (n = 6/group). ϕP < 0.05 vs. adult at same time point. D: representative Western blot of MuRF1 in the MG muscle of adult and old rats after 3, 7, and 14 days of HU. The Western blot also includes two MG muscle samples from MuRF1 knockout (KO) mice, indicating that the antibody does not detect MuRF1 protein. According to the manufacturer, MuRF1 is 38 kDa.
The finding that MAFbx protein levels were significantly higher in the old rats compared with the adult rats was unexpected, since MAFbx mRNA expression in the old was never significantly higher than the adult at baseline or at any HU time point. Moreover, a large disconnect appears to exist between MAFbx mRNA and MAFbx protein levels in both age groups, as MAFbx expression was upregulated in response to unloading in both the adult and old rats, but this did not translate to an increase in MAFbx protein. To determine whether this disconnect could be explained by differential expression of miR-23a, a microRNA that has been reported to translationally suppress both MuRF1 and MAFbx (53), miR-23a expression was measured in the adult and old soleus, MG, and TA muscles after 3, 7, and 14 days of HU. As shown in Fig. 10C, HU did not influence miR-23a expression in the adult or old muscles, but expression in the old MG and TA was significantly higher at baseline and after 14 days of HU compared with the adult muscles. Based on these findings, miR-23a does not appear to be suppressing the translation of MAFbx in response to unloading, given that the higher expression in the old MG and TA did not result in decreased protein levels. In addition, these data suggest that MAFbx protein levels are also a poor indicator of muscle protein degradation activity.
DISCUSSION
Disuse is known to cause significant muscle atrophy, but whether this is due primarily to decreases in protein synthesis, increases in protein degradation, or both remains a topic of debate (35, 37). This debate is fueled by varying results within the literature, where the outcome measures and subsequent data interpretation may be heavily influenced by the organism studied (e.g., human vs. rodent), the disuse model (bed rest vs. immobilization vs. casting vs. tail suspension), the period of disuse (short term vs. long term), and the muscle(s) analyzed. In general, the majority of studies that have looked at disuse over longer periods of time (≥14 days) conclude that loss of muscle mass is driven by decreases in protein synthesis, since the markers of protein degradation examined (MuRF1, MAFbx, FOXO1) in these studies are typically found to be no different from baseline at later time points (9, 14). While this may be true, it does not rule out the possibility that changes in protein degradation occurred earlier within the disuse period and thus played a role in the atrophy response. Indeed, in human and rodent studies that have looked at short periods of disuse (≤7 days), both static and dynamic markers of protein degradation have been found to significantly increase (19, 22, 23, 47).
Disuse atrophy: protein synthesis vs. protein degradation.
Data from this study highlight that disuse atrophy is a complex process that involves changes in both protein synthesis and protein degradation; importantly, changes in these two processes are highly dependent on the muscle under study, the time points used for data collection, and the age of the animal. Furthermore, the data suggests that caution should be taken when using increased expression of atrophy-associated genes, such as MuRF1, MAFbx, FOXO1, and FOXO3a, as an indicator of increased protein degradation. In response to HU, increased expression of these genes was not always matched by an increase in protein degradation, at least as measured by proteasome and cathepsin L activities.
In mature, weight-stable, adult rats, 2 wk of HU causes significant atrophy of the major muscle groups controlling ankle extension (soleus, PLN, and MG) and flexion (TA and EDL); however, the percent atrophy is highly variable across muscles. Similar to previous reports (15, 44), greater atrophy is observed in the ankle extensors compared with the ankle flexors. The disparity in muscle loss is likely related to the change in load, relative to the loading history of each muscle, experienced by each muscle during the unloading period. The ankle extensors, especially the soleus, are postural muscles that are recruited to a greater extent during normal weight-bearing activity and thus experience a larger decrease in loading as a result of HU. In addition, fiber composition of the muscle may play a role in the atrophy response to HU, as unloading has been shown to induce greater atrophy of rodent muscles that are composed of predominantly slow motor units (48). In extensor muscles of mixed fiber type, such as the gastrocnemius, type I fibers atrophy to a greater extent than type II fibers (20). As our laboratory and others (16, 40) have shown, the soleus is composed primarily of type I fibers, and these fibers were found to significantly atrophy after 14 days of HU. Interestingly, the type IIb fibers, and not the type I fibers, in the adult TA were found to atrophy in response to HU, although it is important to note that type I fibers make up the smallest percentage of fibers in the TA.
Mechanistically, our data suggest that unloading triggers a rapid decrease in MPS in all muscles, and that the drop in MPS is congruent with the relative change in loading. After 3 days of HU, the percent decrease in MPS was much larger in the soleus and MG compared with the TA. This trend of MPS being lower in the ankle extensors was maintained throughout the 14-day unloading period, such that, at any time point, MPS in the soleus < MG < TA, which related nicely with the percent atrophy of the muscle (soleus > MG > TA). With respect to protein breakdown, we observed increases in the activities of all three proteasome subunits only in the soleus. The finding that protein synthesis was still reduced and markers of protein breakdown were still increased in the soleus at day 14 helps to explain why this muscle showed the greatest amount of atrophy in response to unloading, and why this muscle continues to atrophy under extended disuse periods (up to 21 days) (10). These data reveal that increases in protein breakdown do not appear to occur in all muscles subjected to unloading, but rather appear to occur primarily in predominantly slow oxidative postural muscles, such as the soleus. Moreover, increases in protein degradation pathways were associated with greater loss of muscle mass (adult vs. old, soleus vs. other muscles). In this study, global protein breakdown was not measured directly, but instead was measured by quantifying the activities of specific proteolytic enzymes that are known to be primary drivers of the breakdown of skeletal muscle proteins. Specifically, the ubiquitin-proteasome system (UPS) is considered to be the main degradation pathway in skeletal muscle and is responsible for the breakdown of myofibrillar proteins (2, 43). Previous studies have shown a direct relationship between increases in the UPS and increases in protein breakdown under conditions that induce muscle atrophy (6, 13). In addition to the UPS, the lysosomal proteases, cathepsins B, H, and L, have been shown to contribute to protein breakdown under atrophy conditions (25, 50).
Aging and disuse atrophy.
Aging results in a progressive loss of muscle mass and strength, which can be accelerated by periods of disuse. However, the cause of the accelerated muscle loss is likely not due to age-related differences in muscle mass loss during the period of disuse, but rather is due to impairments in recovery (5). As we and others have shown, aged rats and older humans lose similar, if not less, muscle mass than their younger counterparts during disuse (26, 45, 58). In this study, no significant differences in hindlimb muscle mass were found between adult and old rats after 14 days of HU (Table 1). Similar to the adult rats, 2 wk of HU caused greater muscle loss in the old ankle extensor muscles compared with the ankle flexor muscles, and similar decreases in the CSA of specific fiber types were observed in the adult and old TA and soleus muscles. However, given that the old rats started with slightly smaller muscles, the percent atrophy of each of the hindlimb muscles at the end of the unloading period was actually less than that observed for the adult rats. Interestingly, a less drastic shift in fiber composition was observed for the old TA after HU. Unlike the adult TA, the percentage of type IIb fibers remained constant, whereas the percentage of type IIx and IIa fibers was modestly increased, which was likely caused by a reduction in the percentage of hybrid fibers.
In this study, we investigated whether changes in protein synthesis and protein degradation pathways differed in response to unloading with age. In old rats, the decrease in MPS in the soleus was similar to that observed in the adult soleus, but the drop in MPS in the old MG and TA was not as great or as prolonged as in the adult rats. In humans, MPS decreases with disuse, irrespective of age (18, 28); however, whether the magnitude of suppression is similar between young and old individuals, and whether differences in MPS exist between muscles, is unclear. With respect to protein breakdown, we generally found that pathways involved in protein degradation (i.e., the proteasome and lysosome) were activated to a lesser extent in muscles from old compared with adult in response to unloading. Overall, the data reveal that the response to unloading is dampened in old muscles with less of a change in both protein synthesis and degradation (at least with respect to the UPS and lysosomal system).
Atrophy-associated genes and protein degradation.
In many studies, the expression of various atrophy-associated genes, or “atrogenes,” is used as a surrogate for protein degradation, especially the expression of those genes involved in the ubiquitin proteasome pathway (59). In this study, we examined the relationship between the expression of the four most commonly investigated atrophy-associated genes, i.e., MuRF1, MAFbx/atrogin-1, FOXO1, and FOXO3a, with proteasome activity. In adult rats, increases in MuRF1 and MAFbx expression were found in the soleus, MG, and TA within the first 7 days of HU; however, UPS activity only increased in the soleus and TA. Similarly, increases in MuRF1 and MAFbx expression were observed in the old MG and TA, but the MG did not exhibit an increase in UPS activity. Previous studies have also found increases in MuRF1 and MAFbx gene expression without concomitant increases in proteasome activities in rodents following dexamethasone treatment and alcohol intoxication (3, 51). Conversely, we have shown that, in the functional overload model, which induces rapid hypertrophy, a progressive and large increase in proteasome activity occurs during the first 7 days of overload with only a transient increase in MuRF1 and MAFbx (day 1 of overload) (4). All of these data suggest that caution should be taken when using MuRF1 and MAFbx expression as a proxy for increased protein degradation. While MuRF1 and MAFbx may be consistently expressed across models of muscle atrophy (11), changes in their expression do not always translate to changes in proteasome activity. In addition, it is important to note that MuRF1 and MAFbx expression increase rapidly with HU, and in every muscle except the old soleus, expression returns to control levels by day 14. Thus it is not surprising that increases in MuRF1 and MAFbx expression are not observed in muscles that have undergone extended periods of disuse (>14 days). The induction of these two genes with disuse is rapid and short lived, and, therefore, the timing of the tissue sampling following the onset of disuse is very important in the interpretation and conclusions that are drawn about data related to expression of these two ubiquitin ligases.
Recent evidence has suggested that protein degradation mediated by the UPS and autophagy is regulated through the class O family of Forkhead transcription factors, particularly FOXO1 and FOXO3a (33, 61). FOXO1 and FOXO3a can bind to the promoter region of MuRF1 and MAFbx to induce expression of these genes (42, 54) and can increase the expression of various autophagy-related genes (32, 61). In response to HU, FOXO1 expression increased in the adult muscles, but not in the muscles of the old. In contrast to FOXO1, FOXO3a expression changed only slightly over the 14 days of unloading. Interestingly, the quick and transient increase in FOXO1 was similar to that found for MuRF1 and MAFbx. Thus, under disuse conditions, increased FOXO expression can be an indicator of muscle atrophy, but does not necessarily signify increases in proteasome or cathepsin L activity. Moreover, whereas FOXO1 and FOXO3a expression are often used interchangeably, the present data suggest that the regulation of these two genes is disconnected during unloading and can vary across muscles and atrophy conditions.
HDAC4 and Gadd45a have been identified as regulators of disuse atrophy caused by loss of neural input (12, 34); however, little is known about how expression of these genes change under disuse conditions with intact innervation. Comparison of the expression profiles of these two genes revealed that HDAC4 was generally more responsive to changes in load than Gadd45a in the adult rats. However, neither gene exhibited an expression pattern that closely correlated with changes in protein degradation. It should be noted that Gadd45a expression tended to be higher at baseline in the old rats and significantly increased in the old MG and TA at day 3 of HU. Indeed, the higher baseline expression of Gadd45a in the old control animals may indicate inactivity or denervation, which is associated with the aging process (41). Increased Gadd45a expression was observed after 3 days of HU in the adult MG, but quickly returned to control levels, while Gadd45a was still significantly elevated in the old MG and TA through 14 days. The pattern of Gadd45a expression in the old suggests that disuse may exacerbate age-related neuromuscular junction instability, which could play a role in the inability of aged muscle to recover mass upon reloading (5). Overall, HU-induced changes in HDAC4 and Gadd45a expression, and the distinct differences in Gadd45a expression between adult and old muscles, highlight the need to better understand how these genes regulate the neuromuscular junction, and how the neuromuscular junction influences disuse-induced muscle atrophy.
MAFbx protein and protein degradation.
At the mRNA level, MuRF1 and MAFbx are excellent markers of muscle atrophy, but it is unclear whether gene induction is paralleled by an increase in protein abundance. Moreover, while we caution the use of increased gene expression as evidence of increased protein degradation, it may be that MuRF1 and MAFbx protein levels are better associated with activity of degradation pathways. The limited data on changes in MuRF1 and MAFbx proteins under atrophy conditions may be partly related to the quality and specificity of the antibodies currently available. In testing various MuRF1 and MAFbx antibodies using muscle samples from MuRF1 and MAFbx knockout mice, we were only able to validate the MAFbx antibody used in this study. However, based on our findings, the induction of MAFbx in the adult and old rats after 3 days of HU was not matched by an increase in MAFbx protein at that same time point or at the later 7- and 14-day time points. If protein levels were indicative of proteasome activity, then proteasome activity should be increased in the soleus, MG, and TA of the old rats at baseline and in response to unloading; however, this was not the case. Thus MAFbx expression, either at the mRNA or protein level, does not appear to be directly linked with HU-induced changes in proteasome activity.
The finding that MAFbx protein levels did not change in either the adult or old rats during HU was unexpected, given the large induction in mRNA expression, especially in the MG. This discrepancy between mRNA and protein levels suggested to us that MAFbx may undergo posttranslational modification by microRNAs to inhibit its translation. MicroRNAs are small (~22 nucleotides) noncoding RNAs that can suppress translation by binding to the 3′ untranslated region of certain genes (56). Wada et al. (53) have recently shown that miR-23a can suppress both MuRF1 and MAFbx expression, and miR-23a transgenic mice exhibit attenuated muscle atrophy in response to glucocorticoid treatment. Thus we measured miR-23a expression in the adult and old muscles to determine whether differential expression of this microRNA between the two ages could explain the difference in protein levels. Surprisingly, miR-23a expression was not altered in response to HU and was found to be significantly higher in the old muscles compared with the adult. Consequently, it does not appear that miR-23a is influencing MAFbx translation in response to unloading, but this does not rule out the possibility that other microRNAs may be targeting MAFbx, and/or that MAFbx may undergo other posttranslational modifications to inhibit its translation under disuse atrophy conditions.
Summary.
In summary, our data show that disuse atrophy is a complex process that can involve changes in both protein synthesis and degradation, depending on the muscle type. In general, decreases in protein synthesis are apparent at some point during the unloading in all muscles; however, the magnitude and duration of the decrease are muscle specific. These data emphasize the importance of muscle type (both functionally and in relation to fiber-type composition) in determining the response to disuse. Care must be taken not to extend the results from one muscle to all other muscles. For example, the argument that disuse-induced atrophy in humans is primarily the result of a decrease in protein synthesis comes almost exclusively from biopsies taken from the VL muscle. If we had analyzed only the rat MG muscle, an extensor muscle of similar fiber-type composition and use as the VL, we would have arrived at a similar conclusion. However, examination of the soleus revealed that protein degradation is induced with disuse, contributing to greater atrophy. Our data also highlight the importance of the timing of sample collection in the interpretation of muscle atrophy. A single data point at the start or end of a period of disuse rarely provides a complete picture of the changes occurring in a specific muscle. Our data stress the need for sampling at multiple time points, especially when attempting to understand mechanisms of action. Finally, our data clearly demonstrate that the expression of atrophy-associated genes, such as MuRF1, MAFbx, FOXO1, and FOXO3a, is not a good surrogate for protein degradation. Whereas the expression of MuRF1, MAFbx, and FOXO1 clearly increases with disuse atrophy in multiple muscles, similar increases in proteasome subunit activity or cathepsin L activity did not occur. Our findings support the need for more comprehensive studies that focus on multiple muscles at multiple time points to fully understand the molecular mechanisms underlying disuse atrophy. This information is crucial for the development of effective interventions for treating disuse-induced muscle atrophy.
GRANTS
This work was funded by Veteran Affairs RR&D Merit Grant 1I01RX000673-01A1 (S. C. Bodine), National Institute on Aging award no. R01AG045375 (K. Baar), and a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship (D. W. D. West).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.M.B., D.W.W, K.B., and S.C.B. conceived and designed research; L.M.B., D.W.W., and S.C.B. performed experiments; L.M.B., D.W.W., A.G.M., G.R.M., K.B., and S.C.B. analyzed data; L.M.B., D.W.W., A.G.M., G.R.M., K.B., and S.C.B. interpreted results of experiments; L.M.B. prepared figures; L.M.B., D.W.W., K.B., and S.C.B. drafted manuscript; L.M.B., D.W.W., K.B., and S.C.B. edited and revised manuscript; L.M.B., A.G.M., G.R.M., K.B., and S.C.B. approved final version of manuscript.
REFERENCES
- 1.Akima H, Kuno S, Suzuki Y, Gunji A, and Fukunaga T. Effects of 20 days of bed rest on physiological cross-sectional area of human thigh and leg muscles evaluated by magnetic resonance imaging. J Gravit Physiol 4: S15–S21, 1997. [PubMed] [Google Scholar]
- 2.Attaix D, Ventadour S, Codran A, Béchet D, Taillandier D, Combaret L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem 41: 173–186, 2005. doi: 10.1042/bse0410173. [DOI] [PubMed] [Google Scholar]
- 3.Baehr LM, Furlow JD, Bodine SC. Muscle sparing in muscle RING finger 1 null mice: response to synthetic glucocorticoids. J Physiol 589: 4759–4776, 2011. doi: 10.1113/jphysiol.2011.212845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baehr LM, Tunzi M, Bodine SC. Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression. Front Physiol 5: 69, 2014. doi: 10.3389/fphys.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baehr LM, West DW, Marcotte G, Marshall AG, De Sousa LG, Baar K, Bodine SC. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) 8: 127–146, 2016. doi: 10.18632/aging.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreiro E, Puig-Vilanova E, Marin-Corral J, Chacón-Cabrera A, Salazar-Degracia A, Mateu X, Puente-Maestu L, García-Arumí E, Andreu AL, Molina L. Therapeutic approaches in mitochondrial dysfunction, proteolysis, and structural alterations of diaphragm and gastrocnemius in rats with chronic heart failure. J Cell Physiol 231: 1495–1513, 2016. doi: 10.1002/jcp.25241. [DOI] [PubMed] [Google Scholar]
- 7.Belavý DL, Miokovic T, Armbrecht G, Richardson CA, Rittweger J, Felsenberg D. Differential atrophy of the lower-limb musculature during prolonged bed-rest. Eur J Appl Physiol 107: 489–499, 2009. doi: 10.1007/s00421-009-1136-0. [DOI] [PubMed] [Google Scholar]
- 8.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268: E514–E520, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Blottner D, Bosutti A, Degens H, Schiffl G, Gutsmann M, Buehlmeier J, Rittweger J, Ganse B, Heer M, Salanova M. Whey protein plus bicarbonate supplement has little effects on structural atrophy and proteolysis marker immunopatterns in skeletal muscle disuse during 21 days of bed rest. J Musculoskelet Neuronal Interact 14: 432–444, 2014. [PubMed] [Google Scholar]
- 10.Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol 45: 2200–2208, 2013. doi: 10.1016/j.biocel.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodine SC, Latres E, Baumhueter S, Lai VK-M, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan Z-Q, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 12.Bongers KS, Fox DK, Ebert SM, Kunkel SD, Dyle MC, Bullard SA, Dierdorff JM, Adams CM. Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4. Am J Physiol Endocrinol Metab 305: E907–E915, 2013. doi: 10.1152/ajpendo.00380.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhary P, Suryakumar G, Prasad R, Singh SN, Ali S, Ilavazhagan G. Chronic hypobaric hypoxia mediated skeletal muscle atrophy: role of ubiquitin-proteasome pathway and calpains. Mol Cell Biochem 364: 101–113, 2012. doi: 10.1007/s11010-011-1210-x. [DOI] [PubMed] [Google Scholar]
- 14.De Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol 585: 241–251, 2007. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deschenes MR, Britt AA, Chandler WC. A comparison of the effects of unloading in young adult and aged skeletal muscle. Med Sci Sports Exerc 33: 1477–1483, 2001. doi: 10.1097/00005768-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Desplanches D, Mayet MH, Sempore B, Flandrois R. Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol (1985) 63: 558–563, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Dirks ML, Wall BT, Snijders T, Ottenbros CLP, Verdijk LB, van Loon LJC. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol (Oxf) 210: 628–641, 2014. doi: 10.1111/apha.12200. [DOI] [PubMed] [Google Scholar]
- 18.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627–E633, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira R, Vitorino R, Neuparth MJ, Appell HJ, Duarte JA, Amado F. Proteolysis activation and proteome alterations in murine skeletal muscle submitted to 1 week of hindlimb suspension. Eur J Appl Physiol 107: 553–563, 2009. doi: 10.1007/s00421-009-1151-1. [DOI] [PubMed] [Google Scholar]
- 20.Gardetto PR, Schluter JM, Fitts RH. Contractile function of single muscle fibers after hindlimb suspension. J Appl Physiol (1985) 66: 2739–2749, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Gomes AV, Zong C, Edmondson RD, Li X, Stefani E, Zhang J, Jones RC, Thyparambil S, Wang G-W, Qiao X, Bardag-Gorce F, Ping P. Mapping the murine cardiac 26S proteasome complexes. Circ Res 99: 362–371, 2006. doi: 10.1161/01.RES.0000237386.98506.f7. [DOI] [PubMed] [Google Scholar]
- 22.Gustafsson T, Osterlund T, Flanagan JN, von Waldén F, Trappe TA, Linnehan RM, Tesch PA. Effects of 3 days unloading on molecular regulators of muscle size in humans. J Appl Physiol (1985) 109: 721–727, 2010. doi: 10.1152/japplphysiol.00110.2009. [DOI] [PubMed] [Google Scholar]
- 23.Hanson AM, Harrison BC, Young MH, Stodieck LS, Ferguson VL. Longitudinal characterization of functional, morphologic, and biochemical adaptations in mouse skeletal muscle with hindlimb suspension. Muscle Nerve 48: 393–402, 2013. doi: 10.1002/mus.23753. [DOI] [PubMed] [Google Scholar]
- 24.Heinemeier KM, Olesen JL, Haddad F, Schjerling P, Baldwin KM, Kjaer M. Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J Appl Physiol (1985) 106: 178–186, 2009. doi: 10.1152/japplphysiol.91092.2008. [DOI] [PubMed] [Google Scholar]
- 25.Helliwell TR, Wilkinson A, Griffiths RD, McClelland P, Palmer TE, Bone JM. Muscle fibre atrophy in critically ill patients is associated with the loss of myosin filaments and the presence of lysosomal enzymes and ubiquitin. Neuropathol Appl Neurobiol 24: 507–517, 1998. doi: 10.1046/j.1365-2990.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 26.Hwee DT, Bodine SC. Age-related deficit in load-induced skeletal muscle growth. J Gerontol A Biol Sci Med Sci 64A: 618–628, 2009. doi: 10.1093/gerona/glp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasper CE, Maxwell LC, White TP. Alterations in skeletal muscle related to short-term impaired physical mobility. Res Nurs Health 19: 133–142, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 28.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 297: 1769–1774, 2007. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Lushaj EB, Johnson JK, McKenzie D, Aiken JM. Sarcopenia accelerates at advanced ages in Fisher 344xBrown Norway rats. J Gerontol A Biol Sci Med Sci 63: 921–927, 2008. doi: 10.1093/gerona/63.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malavaki CJ, Sakkas GK, Mitrou GI, Kalyva A, Stefanidis I, Myburgh KH, Karatzaferi C. Skeletal muscle atrophy: disease-induced mechanisms may mask disuse atrophy. J Muscle Res Cell Motil 36: 405–421, 2015. doi: 10.1007/s10974-015-9439-8. [DOI] [PubMed] [Google Scholar]
- 32.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Milan G, Romanello V, Pescatore F, Armani A, Paik J-H, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL, Blaauw B, DePinho RA, Sandri M. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun 6: 6670, 2015. doi: 10.1038/ncomms7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moresi V, Williams AH, Meadows E, Flynn JM, Potthoff MJ, McAnally J, Shelton JM, Backs J, Klein WH, Richardson JA, Bassel-Duby R, Olson EN. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 143: 35–45, 2010. doi: 10.1016/j.cell.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips SM, McGlory C. CrossTalk proposal: The dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J Physiol 592: 5341–5343, 2014. doi: 10.1113/jphysiol.2014.273615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Reid MB, Judge AR, Bodine SC. CrossTalk opposing view: The dominant mechanism causing disuse muscle atrophy is proteolysis. J Physiol 592: 5345–5347, 2014. doi: 10.1113/jphysiol.2014.279406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reid MB, Judge AR, Bodine SC. Rebuttal from Michael B. Reid, Andrew R. Judge and Sue C. Bodine. J Physiol 592: 5351, 2014. doi: 10.1113/jphysiol.2014.284398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rennie MJ, Selby A, Atherton P, Smith K, Kumar V, Glover EL, Philips SM. Facts, noise and wishful thinking: muscle protein turnover in aging and human disuse atrophy. Scand J Med Sci Sports 20: 5–9, 2010. doi: 10.1111/j.1600-0838.2009.00967.x. [DOI] [PubMed] [Google Scholar]
- 40.Riley DA, Slocum GR, Bain JL, Sedlak FR, Sowa TE, Mellender JW. Rat hindlimb unloading: soleus histochemistry, ultrastructure, and electromyography. J Appl Physiol (1985) 69: 58–66, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One 7: e29082, 2012. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz AL, Ciechanover A. Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu Rev Pharmacol Toxicol 49: 73–96, 2009. doi: 10.1146/annurev.pharmtox.051208.165340. [DOI] [PubMed] [Google Scholar]
- 44.Steffen JM, Fell RD, Geoghegan TE, Ringel LC, Musacchia XJ. Age effects on rat hindlimb muscle atrophy during suspension unloading. J Appl Physiol (1985) 68: 927–931, 1990. [DOI] [PubMed] [Google Scholar]
- 45.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985) 107: 1172–1180, 2009. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 46.Symons TB, Sheffield-Moore M, Chinkes DL, Ferrando AA, Paddon-Jones D. Artificial gravity maintains skeletal muscle protein synthesis during 21 days of simulated microgravity. J Appl Physiol (1985) 107: 34–38, 2009. doi: 10.1152/japplphysiol.91137.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA. Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol (1985) 105: 902–906, 2008. doi: 10.1152/japplphysiol.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol (1985) 68: 1–12, 1990. [DOI] [PubMed] [Google Scholar]
- 49.Thomason DB, Herrick RE, Surdyka D, Baldwin KM. Time course of soleus muscle myosin expression during hindlimb suspension and recovery. J Appl Physiol (1985) 63: 130–137, 1987. [DOI] [PubMed] [Google Scholar]
- 50.Tsujinaka T, Ebisui C, Fujita J, Kishibuchi M, Morimoto T, Ogawa A, Katsume A, Ohsugi Y, Kominami E, Monden M. Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin-6 transgenic mouse. Biochem Biophys Res Commun 207: 168–174, 1995. doi: 10.1006/bbrc.1995.1168. [DOI] [PubMed] [Google Scholar]
- 51.Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R1777–R1789, 2008. doi: 10.1152/ajpregu.00056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vazeille E, Codran A, Claustre A, Averous J, Listrat A, Béchet D, Taillandier D, Dardevet D, Attaix D, Combaret L. The ubiquitin-proteasome and the mitochondria-associated apoptotic pathways are sequentially downregulated during recovery after immobilization-induced muscle atrophy. Am J Physiol Endocrinol Metab 295: E1181–E1190, 2008. doi: 10.1152/ajpendo.90532.2008. [DOI] [PubMed] [Google Scholar]
- 53.Wada S, Kato Y, Okutsu M, Miyaki S, Suzuki K, Yan Z, Schiaffino S, Asahara H, Ushida T, Akimoto T. Translational suppression of atrophic regulators by microRNA-23a integrates resistance to skeletal muscle atrophy. J Biol Chem 286: 38456–38465, 2011. doi: 10.1074/jbc.M111.271270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waddell DS, Baehr LM, van den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, Bodine SC. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab 295: E785–E797, 2008. doi: 10.1152/ajpendo.00646.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wall BT, Dirks ML, van Loon LJC. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev 12: 898–906, 2013. doi: 10.1016/j.arr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Wang XH. MicroRNA in myogenesis and muscle atrophy. Curr Opin Clin Nutr Metab Care 16: 258–266, 2013. doi: 10.1097/MCO.0b013e32835f81b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West DW, Baehr LM, Marcotte GR, Chason CM, Tolento L, Gomes AV, Bodine SC, Baar K. Acute resistance exercise activates rapamycin-sensitive and -insensitive mechanisms that control translational activity and capacity in skeletal muscle. J Physiol 594: 453–468, 2016. doi: 10.1113/JP271365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White JR, Confides AL, Moore-Reed S, Hoch JM, Dupont-Versteegden EE. Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways. Exp Gerontol 64: 17–32, 2015. doi: 10.1016/j.exger.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winbanks CE, Murphy KT, Bernardo BC, Qian H, Liu Y, Sepulveda PV, Beyer C, Hagg A, Thomson RE, Chen JL, Walton KL, Loveland KL, McMullen JR, Rodgers BD, Harrison CA, Lynch GS, Gregorevic P. Smad7 gene delivery prevents muscle wasting associated with cancer cachexia in mice. Sci Transl Med 8: 348ra98, 2016. doi: 10.1126/scitranslmed.aac4976. [DOI] [PubMed] [Google Scholar]
- 60.Wu C-L, Kandarian SC, Jackman RW. Identification of genes that elicit disuse muscle atrophy via the transcription factors p50 and Bcl-3. PLoS One 6: e16171, 2011. doi: 10.1371/journal.pone.0016171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6: 472–483, 2007. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]