Abstract
Chronic kidney disease (CKD) patients have exercise intolerance associated with increased cardiovascular mortality. Previous studies demonstrate that blood pressure (BP) and sympathetic nerve responses to handgrip exercise are exaggerated in CKD. These patients also have decreased nitric oxide (NO) bioavailability and endothelial dysfunction, which could potentially lead to an impaired ability to vasodilate during exercise. We hypothesized that CKD patients have exaggerated BP responses during maximal whole body exercise and that endothelial dysfunction correlates with greater exercise pressor responses in these patients. Brachial artery flow-mediated dilation (FMD) was assessed before maximal treadmill exercise in 56 participants: 38 CKD (56.7 ± 1.2 yr old, 38 men) and 21 controls (52.8 ± 1.8 yr old, 20 men). During maximal treadmill exercise, the slope-of-rise in systolic BP (+10.32 vs. +7.75 mmHg/stage, P < 0.001), mean arterial pressure (+3.50 vs. +2.63 mmHg/stage, P = 0.004), and heart rate (+11.87 vs. +10.69 beats·min−1·stage−1, P = 0.031) was significantly greater in CKD compared with controls. Baseline FMD was significantly lower in CKD (2.76 ± 0.42% vs. 5.84 ± 0.97%, P = 0.008). Lower FMD values were significantly associated with a higher slope-of-rise in systolic BP (+11.05 vs. 8.71 mmHg/stage, P = 0.003) during exercise in CKD, as well as poorer exercise capacity measured as peak oxygen uptake (V̇o2peak; 19.47 ± 1.47 vs. 24.57 ± 1.51 ml·min−1·kg−1, P < 0.001). These findings demonstrate that low FMD in CKD correlates with augmented BP responses during exercise and lower V̇o2peak, suggesting that endothelial dysfunction may contribute to exaggerated exercise pressor responses and poor exercise capacity in CKD patients.
Keywords: chronic kidney disease, endothelial dysfunction, flow-mediated dilation, exercise pressor reflex, exercise
more than 31 million U.S. adults have chronic kidney disease (CKD) (25) and are at significantly increased risk for adverse cardiovascular events (26) and mortality (3). Exercise intolerance is a major feature of CKD and is associated with increased cardiovascular risk in these patients. Exercise capacity, measured as peak oxygen uptake (V̇o2peak), has been shown to be impaired in patients with CKD (8). Although poor exercise capacity is an independent risk factor for mortality in patients with reduced renal function (30), the mechanisms that link exercise intolerance and cardiovascular risk in CKD have yet to be fully elucidated.
One potential mechanism by which poor exercise tolerance may be linked to increased cardiovascular risk in CKD is via abnormal hemodynamic responses during physical activity. The normal physiological response to exercise includes increased heart rate (HR) and blood pressure (BP) to meet the increased metabolic demands of the working skeletal muscle. Blood pressure responses are mediated by a balance between vasoconstriction via reflex activation of the sympathetic nervous system (9, 27) and vasodilation within exercising skeletal muscle mediated largely by ischemic metabolites and nitric oxide (NO) (23). Our prior work has demonstrated that CKD and end-stage renal disease (ESRD) patients have exaggerated increases in BP during handgrip exercise due, in part, to overactivation of the sympathetic nervous system (11, 20). However, under baseline conditions, sympathetic nerve responses were equivocal between CKD patients and controls without kidney disease in the setting of exaggerated pressor responses, suggesting that impaired vasodilation may also contribute to the exaggerated exercise pressor response in CKD. Since CKD patients are known to have markedly reduced NO bioavailability leading to endothelial dysfunction (36), and NO is necessary to induce local vasodilation and oppose sympathetically mediated vasoconstriction during exercise (32), decreased NO bioavailability may be contributing to the augmented BP response by impairing exercise-induced vasodilation. Additionally, this reduced NO bioavailability may also contribute to exaggerated increases in sympathetic responses during exercise due to the lack of NO-mediated inhibition of central sympathetic nervous system activation (2, 24).
The goals of this study were to determine if the exaggerated BP responses observed during low- and moderate-intensity handgrip exercise also extend to whole body walking exercise and to more intense or maximal exercise in CKD. This study also sought to determine whether decreased endothelial function, measured as brachial artery flow-mediated dilation (FMD), correlates with exaggerated BP responses in CKD. We further tested the hypothesis that endothelial dysfunction correlates with poor exercise capacity, suggesting that decreased NO bioavailability could be one mechanism linking exercise intolerance and abnormal hemodynamic responses during physical activity.
MATERIALS AND METHODS
Study Population
Fifty-nine sedentary participants (38 CKD and 21 controls) were recruited and enrolled from outpatient clinics at the Atlanta Veterans Affairs (VA) Medical Center. All CKD participants had a confirmed diagnosis of either Stage 2 [estimated glomerular filtration rate (eGFR) of 60–89 ml·min−1·1.73 m−2 with concomitant urinary microalbumin:creatinine ratio of >30 mg/g] or Stage 3 (eGFR of 30–59 ml·min−1·1.73 m−2) CKD (10). CKD participants had at least a 3-mo history of stable renal function (≤10% fluctuation in eGFR) and stable antihypertensive medication regimen before enrollment. Exclusion criteria included type I and type II diabetes, use of antidiabetic medications, heart failure, vascular disease, uncontrolled hypertension (BP > 160/90 mmHg), symptomatic heart disease, anemia, or liver dysfunction.
Study Design
After written informed consent was obtained, office BP, basic metabolic panel, and urinary microalbumin and creatinine levels were obtained. During a separate visit, brachial artery flow-mediated dilation (FMD) was obtained before maximal exercise testing. All measurements were obtained in a quiet, temperate (21°C) environment, after abstaining from food, caffeine, smoking, and alcohol for at least 12 h, and exercise for at least 24 h. A standard snack of 2 graham crackers and one small boxed juice was given after the FMD measurements and just before the exercise treadmill test. All participants reported having taken prescribed medications as normally directed. This study was approved by the Emory University Institutional Review Board and the Atlanta VA Medical Center Research and Development Committee.
Measurements and Procedures
Blood pressure.
Baseline BP was measured after 5 min of rest in a seated position with the arm supported at heart level using an appropriately sized cuff. Throughout the exercise treadmill test, BP was measured manually by a single investigator (JP). Mean arterial blood pressures were calculated as 2/3 diastolic BP + 1/3 systolic BP. Heart rate was monitored using continuous ECG during the exercise treadmill test.
Brachial artery flow-mediated dilation.
A forearm occlusion cuff was placed on participants while they were in a supine position. A 13-MHz high-resolution ultrasound transducer (Acuson Aspen) was used longitudinally 2–10 cm above the antecubital fossa to record brachial artery measurements. Baseline values were obtained by averaging diameter and blood velocity over three cardiac cycles measured via ECG gating to capture end-diastolic arterial diameters. The forearm cuff was inflated to suprasystolic levels (50 mmHg above systolic BP) using a rapid cuff inflator (D.E. Hokanson) for 5 min. Peak hyperemic blood velocity was measured by Doppler ultrasound during the first 10 s following cuff release. Diameter measurements were obtained 60 and 90 s following cuff release. FMD calculations were made using 60-s and 90-s measurements to determine peak diameter. Arterial diameters were measured and analyzed by a single investigator blinded to clinical status of the participant from digitized images utilizing customized software (Medical Imaging Applications). FMD is given as the percent change in artery diameter from baseline: (peak hyperemic diameter – baseline diameter)/baseline diameter. Shear rate at baseline and peak hyperemia was calculated as 4 × peak blood velocity/arterial diameter. FMD values were then normalized for peak hyperemic shear rate. This calculation of shear rate is consistent with our previous reports (19).
Maximal exercise treadmill test.
Participants underwent a modified Balke protocol, as previously described (5). Briefly, participants were allowed to warm up for 2 min using a treadmill (GE T2100 controlled via GE Case V6.5 software) set to a speed of 1.5 mph with the slope set to 0%. At the start of the test, the speed was increased to 2.0 mph. At 3 min, and every subsequent 3 min, the treadmill slope was increased by 3.5%. At the 18th minute, the speed was increased to 3.0 mph while the treadmill slope was decreased to 12.5%. The slope was again increased by 3.5% every 3 min until the participant achieved exhaustion. HR was monitored continuously with ECG. BP was manually measured by the same investigator (JP) at 1 min and 30 s into each stage; rate of perceived exertion (RPE) was reported at 1 min and 50 s into each stage. Expired O2, CO2, and ventilation were recorded every 30 s (Sensormedics VMax Spectra 29) during exercise to determine exercise capacity (V̇o2peak). The V̇o2peak was defined as the highest V̇o2 observed during maximal exercise testing, after achieving a plateau in V̇o2 (≤0.15 l/min increase in V̇o2 with increase in workload).
Statistics
Statistical analysis was performed using SAS 9.4 (SAS Institute). Baseline characteristics were compared using standard two-sided, independent, two-sample t-tests, where the F-test for equality of two variances was conducted to determine whether to treat the variances in two groups as being equal. The following linear mixed model was fitted to the BP and HR data:
In this model, Yij represents the BP or HR at stage j (j = 0, 1, 2, . . ., 10) for the ith subject. The variable Disease is an indicator variable taking the value 1 if the ith subject is a CKD participant, and 0 if a control. The inclusion of Disease allows a different intercept for CKD participants and controls. Stage models an overall linear growth trend of BP or HR, and Disease × Stage makes the slopes different between CKD participants and controls. Thus the test of whether β3 = 0 assessed whether the slopes-of-rise of these variables differ between two groups. This mixed model assumes a variance-covariance structure for εij arising from the same subject. εij from different subjects are assumed to be independent. Hypertension, age, race, and body mass index (BMI) were treated as fixed effects in the linear mixed model to adjust for potential confounding effect. To fit this model, we used PROC MIXED, which uses all available data, so that the data in later stage of exercise contribute to the estimation and hypothesis test concerning the slope. Unless otherwise reported, all results are reported as means ± SE.
RESULTS
Baseline Characteristics
Thirty-eight participants with CKD and 21 age-matched controls without kidney disease who met the eligibility criteria were enrolled in the study. Table 1 shows the baseline characteristics of the study population. Groups were well matched for age. The majority of participants in each group were male and African American. The CKD group had higher body weight and body mass index (BMI) than the control group (32.8 ± 0.7 vs. 29.0 ± 1 kg/m2, P = 0.002). The mean estimated glomerular filtration rate (eGFR) in the CKD group was significantly lower than the control group (50 ± 2 vs. 90 ± 3 ml·min−1·1.73 m−2, P < 0.001), as expected. There were no significant differences in random blood glucose levels between the groups; type I and type II diabetics were excluded from these studies, and no participants were treated with antidiabetic drugs. Baseline systolic (134 ± 2 vs. 124 ± 3 mmHg, P = 0.010) and diastolic BP (82 ± 2 vs. 75 ± 2 mmHg, P = 0.14) were significantly higher in the CKD group compared with controls. The majority of participants in both groups had hypertension, but a greater proportion of CKD patients vs. controls (76% vs. 100%, P < 0.05) had hypertension. A greater proportion of CKD patients were treated with angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers (ARBs), calcium channel blockers, and beta blockers compared with controls. There was no difference in the proportion of participants treated with diuretics, aldosterone receptor blockers, alpha blockers, hydralazine, or HMG CoA reductase inhibitors (statins), between the groups.
Table 1.
Baseline characteristics of study population
| Characteristic | Control (n = 21) | CKD (n = 38) | P Value |
|---|---|---|---|
| Age, yr | 52.8 ± 1.8 | 56.7 ± 1.2 | 0.076 |
| Sex, men/women | 20/1 | 38/0 | 0.356 |
| Race, n (%) | |||
| Black | 16 (76%) | 36 (95%) | 0.085 |
| White | 5 (23%) | 2 (5%) | 0.085 |
| Body weight, kg | 94 ± 3.7 | 106.4 ± 2.7 | 0.004 |
| Body mass index, kg/m2 | 29.0 ± 1.0 | 32.8 ± 0.8 | 0.004 |
| Estimated GFR, ml·min−1·1.73 m−2 | 89.5 ± 2.8 | 51.9 ± 2.2 | <0.001 |
| Stage II CKD, n | 9 | ||
| Stage III CKD, n | 29 | ||
| Baseline blood glucose, mg/dl | 91 ± 4 | 97 ± 3 | 0.404 |
| Hypertension, n (%) | 16 (76%) | 38 (100%) | 0.005 |
| Smokers, n (%) | 6 (29%) | 7 (18%) | 0.513 |
| Antihypertensive medications, n (%) | |||
| Calcium channel blockers | 7 (29%) | 23 (53%) | 0.029 |
| ACEI/ARB | 7 (29%) | 32 (74%) | <0.001 |
| Diuretics | 10 (42%) | 22 (51%) | 0.291 |
| β-Blockers | 0 (0%) | 13 (30%) | 0.002 |
| Aldosterone receptor blockers | 2 (8%) | 3 (7%) | 1.000 |
| α-Blockers | 3 (13%) | 5 (12%) | 1.000 |
| Hydralazine | 0 (0%) | 2 (5%) | 0.534 |
| Statins, n (%) | 7 (29%) | 13 (30%) | 0.557 |
| Baseline hemodynamics | |||
| Systolic blood pressure, mmHg | 124 ± 3 | 134 ± 2 | 0.010 |
| Diastolic blood pressure, mmHg | 75 ± 2 | 82 ± 2 | 0.014 |
| Mean arterial pressure, mmHg | 91 ± 2 | 99 ± 2 | 0.004 |
| Heart rate, beats/min | 65 ± 3 | 63 ± 2 | 0.709 |
| Maximal exercise testing | |||
| Maximum systolic blood pressure, mmHg | 176 ± 6 | 186 ± 5 | 0.197 |
| Maximum diastolic blood pressure, mmHg | 82 ± 2 | 88 ± 2 | 0.015 |
| Maximum mean arterial pressure, mmHg | 111 ± 3 | 118 ± 2 | 0.055 |
| Maximum heart rate, beats/min | 150 ± 4 | 135 ± 4 | 0.011 |
| Maximum rate of perceived exertion, RPE | 16 ± 1 | 16 ± 1 | 0.429 |
| Respiratory exchange ratio, RER | 1.02 ± 0.03 | 1.02 ± 0.02 | 0.943 |
| Time to V̇o2peak, min | 21.7 ± 1.6 | 18.0 ± 0.9 | 0.076 |
| V̇o2peak, ml·min−1·kg−1 | 30 ± 2 | 22 ± 1 | <0.001 |
Values are expressed as means ± SE. V̇o2peak, peak oxygen consumption. Significant P values are shown in bold.
Maximal Exercise Test
All study participants completed a maximum treadmill exercise test using the modified Balke protocol (5). There was no significant difference in the maximum systolic BP achieved during the exercise test between the groups (Table 1). Maximum diastolic BP during exercise testing was significantly higher in CKD patients compared with controls (88 ± 2 vs. 82 ± 2 mmHg, P = 0.015), and there was a trend toward higher maximum mean arterial pressure in CKD (111 ± 3 vs. 118 ± 2 mmHg, P = 0.055). Maximum heart rate was significantly lower in CKD vs. controls (150 ± 4 vs. 135 ± 4 beats/min, P = 0.011). There were no significant differences in maximum rate of perceived exertion (RPE) or respiratory exchange ratio (RER) between the groups. Exercise capacity measured as peak oxygen uptake during maximal treadmill exercise (V̇o2peak) was significantly lower in the CKD group compared with controls (22 ± 1 vs. 30 ± 2 ml·min−1·kg−1, P < 0.001).
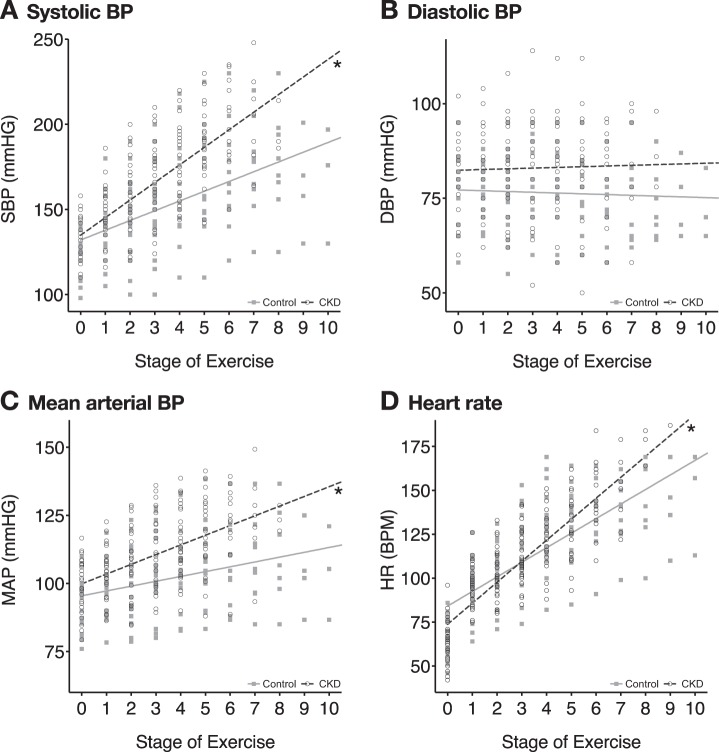
Cardiovascular responses for all participants during exercise testing are shown in Fig. 1. There was a significantly increased slope-of-rise in systolic BP (+10.32 vs. +7.75 mmHg/stage, P < 0.001; Fig. 1A), MAP (+3.50 vs. +2.63 mmHg/stage, P = 0.004; Fig. 1C), and HR (+11.87 vs. +10.69 beats·min−1·stage−1, P = 0.031; Fig. 1D) during maximal treadmill exercise in the CKD group compared with controls. These differences remained statistically significant after adjusting for body mass index, comorbid hypertension, age, race, and baseline blood pressure in the linear mixed models. Results were also similar between Stage II and Stage III CKD patients, and when Stage II CKD patients were removed from the analysis. The absolute values of systolic BP and MAP were significantly higher in the CKD group compared with the Control group starting at Stage 6 of exercise, which corresponded with an oxygen uptake of 20.6 ml·min−1·kg−1, or 93.5% of V̇o2peak. There was no significant change in diastolic BP over the course of testing in either group, and no difference in the slope-of-rise in diastolic BP between the groups (+11.87 vs. 10.69 mmHg/stage, P = 0.871; Fig. 1B).
Fig. 1.
Cardiovascular responses showing slopes-of-rise in systolic blood pressure (BP) (P < 0.001) (A), diastolic BP (P = 0.871) (B), mean arterial pressure (P = 0.004) (C), and heart rate (P = 0.031) (D) during maximal treadmill exercise testing in CKD (n = 38) vs. age-matched, non-CKD controls (n = 21). A linear mixed model was fitted to estimate the slope of an overall linear growth trend of BP and heart rate during maximal treadmill exercise in CKD and controls. *Significant difference in slope-of-rise vs. controls.
Flow-Mediated Dilation
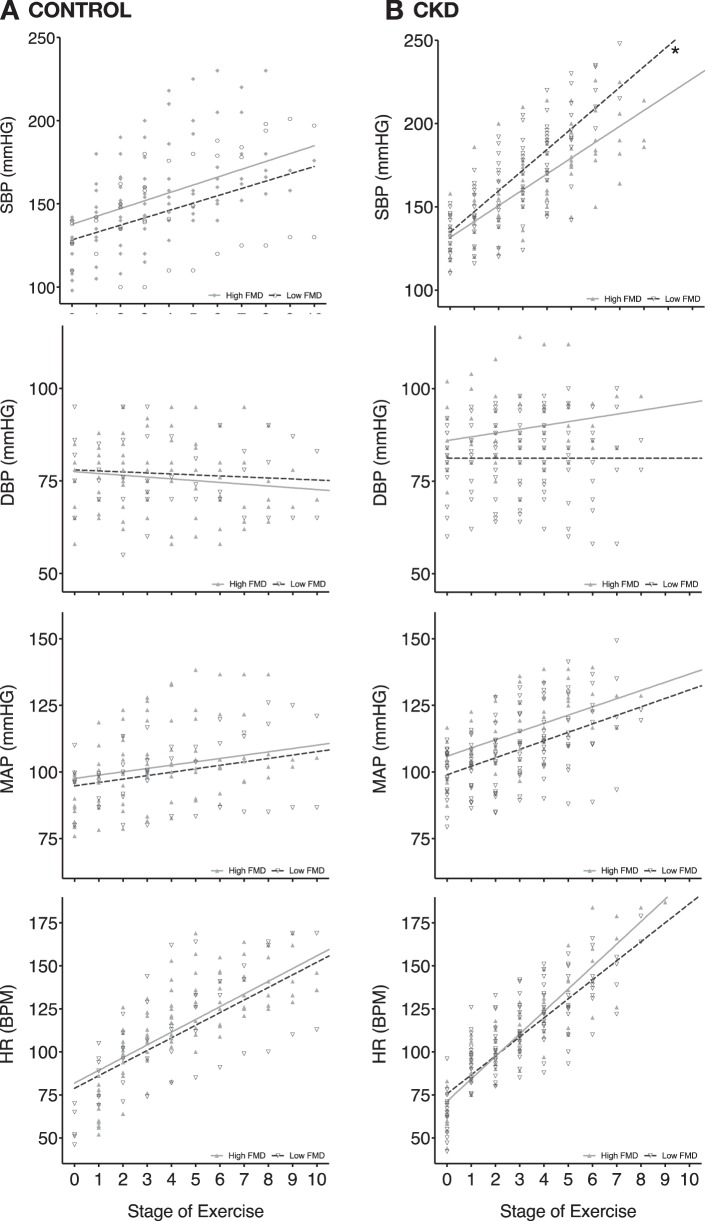
Brachial artery flow-mediated dilation (FMD) was measured to assess endothelial function as a measure of endogenous NO release due to shear stress. Raw values are shown in Table 2. Three controls and 7 CKD participants did not have FMD measurements obtained due to scheduling issues. Baseline FMD (P = 0.008) and FMD controlled for shear stress (P = 0.041) were significantly lower in CKD participants. To determine if endothelial dysfunction correlates with the BP response to maximal exercise, the CKD and control groups were each divided into high-FMD (equal to or higher than the common median FMD value of 2.95%) and low-FMD (lower than the common median FMD value) groups. Among the CKD patients, the low-FMD group had a significantly greater slope-of-rise in systolic BP (+11.05 vs. 8.71 mmHg/stage, P = 0.003; Fig. 2A) during maximal treadmill exercise compared with the high-FMD group. There were no significant differences between the high- and low-FMD groups in the slope-of-rise in diastolic BP (+0.17 vs. −0.14 mmHg/stage, P = 0.394; Fig. 2B), MAP (+3.02 vs. +3.58 mmHg/stage, P = 0.149; Fig. 2C), or HR (+11.51 vs. +11.74 beats·min−1·stage−1/stage, P = 0.748; Fig. 2D) during maximal treadmill exercise within the CKD group. Among the control group, there were no significant differences in the slope-of-rise in systolic BP, diastolic BP, MAP, or HR during maximal treadmill exercise between the high-FMD and low-FMD groups. Among the CKD group, the low-FMD group had significantly lower V̇o2peak measurements compared with the high-FMD group (P = 0.033; Fig. 3).
Table 2.
Values for brachial artery flow-mediated dilation
| Control (n = 18) | CKD (n = 31) | P Value | |
|---|---|---|---|
| Baseline arterial diameter, mm | 4.00 ± 0.12 | 4.43 ± 0.08 | 0.003 |
| Baseline blood velocity, m/s | 0.94 ± 0.06 | 0.76 ± 0.02 | 0.002 |
| Baseline shear rate, s−1 × 103 | 0.97 ± 0.07 | 0.70 ± 0.03 | <0.001 |
| Hyperemic arterial diameter, mm | 4.23 ± 0.14 | 4.54 ± 0.08 | 0.041 |
| Hyperemic blood velocity, m/s | 1.76 ± 0.06 | 1.41 ± 0.04 | <0.001 |
| Hyperemic shear rate, s−1 × 103 | 1.71 ± 0.10 | 1.26 ± 0.05 | <0.001 |
| FMD, % | 5.84 ± 0.97 | 2.76 ± 0.42 | 0.001 |
| FMDshear, %/(s−1 × 103) | 3.72 ± 0.62 | 2.23 ± 0.35 | 0.043 |
Values are expressed as means ± SE. FMD %, flow-mediated dilation as % change from baseline diameter. Normalized FMD, FMDshear, was adjusted for peak hyperemic shear rate.
Fig. 2.
Both control (A) and CKD (B) groups were divided along the common median FMD value (2.95%) into low-FMD vs. high-FMD groups. A: during maximal treadmill exercise testing, there were no significant differences in the slope-of-rise in systolic blood pressure (SBP), diastolic BP (DBP), mean arterial pressure (MAP), and heart rate (HR) between the low-FMD (n = 7) and high-FMD (n = 11) groups within the controls. B: during maximal treadmill exercise testing, there was a significantly greater slope-of-rise in SBP (P = 0.003), but not DBP, MAP, or HR between the low-FMD (n = 20) and high-FMD (n = 11) groups within CKD patients. A linear mixed model was fitted to estimate the slope of an overall linear growth trend of BP and HR during maximal treadmill exercise for the low-FMD vs. high-FMD subgroups, within the CKD and control groups. *Significant difference in slope-of-rise between low-FMD and high-FMD groups within the control and CKD groups.
Fig. 3.
Peak oxygen uptake (V̇o2peak) during maximal exercise testing for control (n = 21) vs. CKD (n = 38) groups (P < 0.001) (A); low-FMD (n = 7) vs. high-FMD (n = 11) groups divided along the common median FMD value of 2.95% (P = 0.241) within the control group (B); and low-FMD (n = 20) vs. high-FMD (n = 11) groups divided along the common median FMD value within the CKD group (P = 0.033) (C). Two-sided, independent 2-sample t-tests were performed to compare mean values between groups. *Significant difference between groups.
DISCUSSION
CKD patients have poor exercise tolerance. Multiple studies have demonstrated that both CKD and ESRD patients have significantly poorer exercise capacity measured as V̇o2peak (8, 31). In addition to these objective measures, patients with renal dysfunction consistently report marked reductions in physical capacity that negatively impact quality of life (22). The current study demonstrates that CKD patients with mild to moderate renal dysfunction (CKD stages II and III) have significantly impaired V̇o2peak compared with age-matched controls, suggesting that reduced physical capacity begins early in the course of renal disease. These findings are clinically relevant because decreased exercise capacity not only impacts quality of life, but is also associated with a significantly increased risk for cardiovascular disease and mortality (8).
Abnormal physiological adjustments during exercise can contribute to both exercise intolerance and increased cardiovascular risk (7, 28). We previously demonstrated that CKD and ESRD patients exhibit exaggerated increases in blood pressure compared with controls during both moderate-intensity isometric and low-intensity rhythmic handgrip exercise (17, 20). In addition, exaggerated pressor responses to exercise in chronic heart failure patients have been shown to correlate with increased mortality risk (21) and could contribute to increased cardiovascular risk in CKD patients. To our knowledge, the current results are the first to demonstrate that CKD patients also have an exaggerated increase in the slope-of-rise in BP and HR during whole body maximal treadmill exercise. This finding remained statistically significant after controlling for hypertension and baseline BP, suggesting that augmented BP responses during exercise in CKD are independent of hypertension. Taken together, the current findings, in combination with previous studies, suggest that CKD patients may have higher hemodynamic reactivity while engaging in activities of daily living, such as low-intensity activities and handgrip maneuvers, as well as while walking, and during more strenuous physical activity. Thus exaggerated exercise pressor responses may contribute to increased cardiovascular risk in CKD and increase the risk of adverse events during both exercise and activities of daily living in CKD patients.
The mechanisms underlying the exaggerated increase in BP during physical activity in CKD patients are not fully understood. Our prior work suggests that overactivation of the sympathetic nervous system contributes, in part, to the exaggerated exercise pressor response (17, 18). When the augmented BP responses were equalized pharmacologically in CKD patients to controls, thereby equalizing the influence of the arterial baroreflexes, sympathetic nerve responses were found to be significantly augmented in CKD patients during exercise. However, under baseline conditions, when the exercise pressor response was augmented in CKD patients, there was no significant difference in the sympathetic nerve responses between the groups, suggesting that other factors besides heightened sympathetic nervous system activation also contribute to the exaggerated exercise pressor response in CKD. Additional mechanisms could include increased action of other vasoconstrictors such as endothelin, or impairment in generation of vasodilators such as NO.
During exercise, vasodilatory substances are generated in exercising skeletal muscle to oppose sympathetically mediated vasoconstriction and thereby preserve conductance to exercising skeletal muscle, as well as prevent exaggerated increases in BP during exercise. One major factor underlying exercise hyperemia is NO (15). Patients with CKD have decreased NO bioavailability leading to endothelial dysfunction due to a variety of factors such as chronic inflammation and accumulation of uremic toxins such as asymmetric dimethylarginine (ADMA) (14). In the current study, we observed that patients with mild to moderate CKD have significantly lower brachial artery FMD compared with hypertensive controls. CKD is characterized by decreased NO bioavailability and endothelial dysfunction which contribute to increased cardiovascular risk (35) and could impair exercise hyperemia. We demonstrate that CKD patients with low FMD display a greater slope-of-rise in systolic BP compared with those with higher FMD, suggesting that decreased NO bioavailability may contribute to the exaggerated exercise pressor response in CKD (4).
Interestingly, we also observed that among the CKD group, low FMD is associated with decreased exercise capacity measured as V̇o2peak, suggesting that endothelial dysfunction may also correlate with exercise intolerance in CKD patients. Although not directly tested, reduced NO bioavailability may contribute to a reduction in the capacity to preserve or increase blood flow to exercising skeletal muscle, thereby contributing to reduced exercise tolerance. Animal studies have shown that inhibition of NO synthase reduced blood flow to exercising skeletal muscles during high-speed treadmill exercise, suggesting that NO plays a major role in regulating muscle blood flow during exercise (15). Decreased NO bioavailability reflected by low FMD could similarly contribute to abnormalities in exercising muscle blood flow in CKD patients and contribute to exercise intolerance. Our finding that low FMD correlates with decreased exercise capacity in CKD appears to be a novel finding and could have clinical implications. Therapeutic interventions targeting endothelial dysfunction in CKD may have the potential to improve both hemodynamic responses during physical activity, as well as exercise tolerance, and should be tested in future studies.
Whether exercise training improves augmented exercise pressor responses in CKD observed in the current study should be tested in future studies. Some prior studies have shown that exercise training improves a number of health measures in CKD such as exercise capacity, physical functioning, arterial stiffness, inflammation, and BP (8, 12, 22, 29, 33). Whether exercise training improves endothelial function in CKD patients is less clear. Several prior experimental studies in animal models of CKD have shown significant improvement in endothelial function with exercise training (13, 29), whereas a recent human study in CKD Stage III–IV patients showed that exercise training improved V̇o2peak without alterations in endothelial function or arterial stiffness (34). Others have shown that exercise training improved exercise capacity without improving vascular stiffness in humans with CKD (6), and improved insulin resistance without improving kidney function (16) in CKD patients. Future studies investigating the effects of exercise intervention on endothelial function and exercise-induced hypertension in CKD, as well as long-term effects on cardiovascular risk and progression of renal disease, are warranted.
We note several limitations of our study. First, our FMD data were collected at 60- and 90-s post cuff release. We must acknowledge that without continuous measurements, there may be an added degree of error in the measurement as the peak diameter could occur outside of the observed time points. However, the technique was uniformly applied across all subjects, and measurements were made by a single investigator with expertise in performing FMD. Second, the majority of controls (76%) and all CKD patients (100%) had hypertension controlled via antihypertensive medications. Although we excluded most comorbid conditions, including cardiovascular disease and diabetes, we were not able to exclude hypertensive patients, since the prevalence of hypertension among CKD patients is 85% or greater (1). To control for this, we enrolled primarily hypertensive controls without kidney disease, and also performed a linear mixed statistical model that included hypertension as a covariate. Third, because we studied relatively healthy CKD patients without comorbidities to isolate the effect of renal dysfunction, our study population may not be typical of the general CKD population that often has comorbid conditions including diabetes, heart failure, and vascular disease. Fourth, antihypertensive medications were not discontinued for participation in the study since controlled BP was required for undergoing maximal exercise testing. The majority of both groups were treated with antihypertensive medications, but a greater proportion of CKD patients were treated with calcium channel blockers, ACE-I/ARBs, and beta blockers, and so cannot exclude a differential effect of antihypertensive medications on hemodynamic responses during exercise. However, the CKD group had significantly augmented BP and HR responses during treadmill exercise despite being treated with a greater number of antihypertensive medications. Fifth, food records were not collected to ensure similar caloric and macronutrient intake between the groups that can impact blood pressure and FMD. However, all participants in both groups were fasting for 12 h before FMD and exercise testing. Finally, the study was conducted primarily in African-American men. As such, our results may not be generalizable to women and other racial or ethnic groups.
In conclusion, this study demonstrates that CKD patients have a significantly exaggerated slope-of-rise in BP and HR during whole body maximal exercise, similar to that previously shown in low-level and moderate handgrip exercise. We also show that among CKD patients, low FMD, reflective of decreased NO bioavailability, correlates with this exaggerated BP response, suggesting that endothelial dysfunction may contribute to the exaggerated pressor response. Additionally, low FMD in CKD patients is associated with decreased exercise capacity, suggesting that endothelial dysfunction may be one factor linking exercise intolerance and cardiovascular risk in this population. These findings encourage further studies examining whether interventions to improve NO bioavailability may ameliorate exaggerated exercise pressor responses, improve exercise tolerance, and ultimately improve cardiovascular risk in CKD.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants K23 HL-098744 (to J. Park) and R01-HL-135183 (to J. Park); National Institutes of Health Training Grants T32-DK-00756 (to J. Sands) and K12-GM-000680 (to D. Eaton); Satellite Health Care, a not-for-profit renal care provider; the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Studies Center, Decatur, Georgia; the Atlanta Research and Education Foundation; and NIH Grant UL- RR-025008 from the Clinical and Translational Science Award program, National Center for Research Resources.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.A.Q. and J.P. conceived and designed research; E.C.M., S.S., and J.P. performed experiments; R.M.D., P.L., and J.P. analyzed data; R.M.D., A.A.Q., and J.P. interpreted results of experiments; R.M.D. prepared figures; R.M.D. drafted manuscript; R.M.D., P.L., A.A.Q., S.S., and J.P. edited and revised manuscript; R.M.D., P.L., E.C.M., A.A.Q., S.S., and J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We give special thanks to D. DaCosta, M. Jefferson, and D. Gardner Morison for expert technical assistance.
A preliminary version of these findings was presented as an abstract at the 2016 Experimental Biology Meeting in San Diego, California.
REFERENCES
- 1.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 115: 291–297, 2003. doi: 10.1016/S0002-9343(03)00366-8. [DOI] [PubMed] [Google Scholar]
- 2.Bergamaschi CT, Campos RR, Lopes OU. Rostral ventrolateral medulla: A source of sympathetic activation in rats subjected to long-term treatment with L-NAME. Hypertension 34: 744–747, 1999. doi: 10.1161/01.HYP.34.4.744. [DOI] [PubMed] [Google Scholar]
- 3.Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375: 2073–2081, 2010. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- 5.Hanson P. Clinical exercise testing. In: Sports Medicine, edited by Strauss R. Philadelphia, PA: Saunders, 1984, p. 13–40. [Google Scholar]
- 6.Headley S, Germain M, Wood R, Joubert J, Milch C, Evans E, Poindexter A, Cornelius A, Brewer B, Pescatello LS, Parker B. Short-term aerobic exercise and vascular function in CKD stage 3: a randomized controlled trial. Am J Kidney Dis 64: 222–229, 2014. doi: 10.1053/j.ajkd.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jae SY, Fernhall B, Heffernan KS, Kang M, Lee M-K, Choi YH, Hong KP, Ahn ES, Park WH. Exaggerated blood pressure response to exercise is associated with carotid atherosclerosis in apparently healthy men. J Hypertens 24: 881–887, 2006. doi: 10.1097/01.hjh.0000222758.54111.e2. [DOI] [PubMed] [Google Scholar]
- 8.Johansen KL, Painter P. Exercise in individuals with CKD. Am J Kidney Dis 59: 126–134, 2012. doi: 10.1053/j.ajkd.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 11.Lin AM, Liao P, Millson EC, Quyyumi AA, Park J. Tetrahydrobiopterin ameliorates the exaggerated exercise pressor response in patients with chronic kidney disease: a randomized controlled trial. Am J Physiol Renal Physiol 310: F1016–F1025, 2016. doi: 10.1152/ajprenal.00527.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopes AA, Lantz B, Morgenstern H, Wang M, Bieber BA, Gillespie BW, Li Y, Painter P, Jacobson SH, Rayner HC, Mapes DL, Vanholder RC, Hasegawa T, Robinson BM, Pisoni RL. Associations of self-reported physical activity types and levels with quality of life, depression symptoms, and mortality in hemodialysis patients: the DOPPS. Clin J Am Soc Nephrol 9: 1702–1712, 2014. doi: 10.2215/CJN.12371213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martens CR, Kuczmarski JM, Kim J, Guers JJ, Harris MB, Lennon-Edwards S, Edwards DG. Voluntary wheel running augments aortic l-arginine transport and endothelial function in rats with chronic kidney disease. Am J Physiol Renal Physiol 307: F418–F426, 2014. doi: 10.1152/ajprenal.00014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Małyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta 411: 1412–1420, 2010. doi: 10.1016/j.cca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Musch TI, McAllister RM, Symons JD, Stebbins CL, Hirai T, Hageman KS, Poole DC. Effects of nitric oxide synthase inhibition on vascular conductance during high speed treadmill exercise in rats. Exp Physiol 86: 749–757, 2001. doi: 10.1111/j.1469-445X.2001.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 16.Navaneethan SD, Fealy CE, Scelsi AC, Arrigain S, Malin SK, Kirwan JP. A trial of lifestyle modification on cardiopulmonary, inflammatory, and metabolic effects among obese with chronic kidney disease. Am J Nephrol 42: 274–281, 2015. doi: 10.1159/000441155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J, Campese VM, Middlekauff HR. Exercise pressor reflex in humans with end-stage renal disease. Am J Physiol Regul Integr Comp Physiol 295: R1188–R1194, 2008. doi: 10.1152/ajpregu.90473.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J, Campese VM, Nobakht N, Middlekauff HR. Differential distribution of muscle and skin sympathetic nerve activity in patients with end-stage renal disease. J Appl Physiol (1985) 105: 1873–1876, 2008. doi: 10.1152/japplphysiol.90849.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J, Liao P, Sher S, Lyles RH, Deveaux DD, Quyyumi AA. Tetrahydrobiopterin lowers muscle sympathetic nerve activity and improves augmentation index in patients with chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 308: R208–R218, 2015. doi: 10.1152/ajpregu.00409.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J, Quyyumi AA, Middlekauff HR. Exercise pressor response and arterial baroreflex unloading during exercise in chronic kidney disease. J Appl Physiol (1985) 114: 538–549, 2013. doi: 10.1152/japplphysiol.01037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJS, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation 104: 2324–2330, 2001. doi: 10.1161/hc4401.098491. [DOI] [PubMed] [Google Scholar]
- 22.Rus RR, Ponikvar R, Kenda RB, Buturović-Ponikvar J. Effect of local physical training on the forearm arteries and veins in patients with end-stage renal disease. Blood Purif 21: 389–394, 2003. doi: 10.1159/000073441. [DOI] [PubMed] [Google Scholar]
- 23.Saltin B. Exercise hyperaemia: magnitude and aspects on regulation in humans. J Physiol 583: 819–823, 2007. doi: 10.1113/jphysiol.2007.136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sander M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension 33: 937–942, 1999. doi: 10.1161/01.HYP.33.4.937. [DOI] [PubMed] [Google Scholar]
- 25.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LYC, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JLT, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O’Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 67, Suppl 1: S1–S305, 2016. doi: 10.1053/j.ajkd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention . Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 42: 1050–1065, 2003. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 27.Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev 19: 313–349, 1991. doi: 10.1249/00003677-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Sharabi Y, Ben-Cnaan R, Hanin A, Martonovitch G, Grossman E. The significance of hypertensive response to exercise as a predictor of hypertension and cardiovascular disease. J Hum Hypertens 15: 353–356, 2001. doi: 10.1038/sj.jhh.1001157. [DOI] [PubMed] [Google Scholar]
- 29.Shelkovnikov S, Summers SM, Elahimehr R, Adams G, Purdy RE, Vaziri ND. Effect of exercise training on aortic tone in chronic renal insufficiency. Am J Hypertens 21: 564–569, 2008. doi: 10.1038/ajh.2008.24. [DOI] [PubMed] [Google Scholar]
- 30.Sietsema KE, Amato A, Adler SG, Brass EP. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int 65: 719–724, 2004. doi: 10.1111/j.1523-1755.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 31.Sietsema KE, Hiatt WR, Esler A, Adler S, Amato A, Brass EP. Clinical and demographic predictors of exercise capacity in end-stage renal disease. Am J Kidney Dis 39: 76–85, 2002. doi: 10.1053/ajkd.2002.29884. [DOI] [PubMed] [Google Scholar]
- 32.Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Craenenbroeck AH, Van Craenenbroeck EM, Kouidi E, Vrints CJ, Couttenye MM, Conraads VM. Vascular effects of exercise training in CKD: current evidence and pathophysiological mechanisms. Clin J Am Soc Nephrol 9: 1305–1318, 2014. doi: 10.2215/CJN.13031213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Craenenbroeck AH, Van Craenenbroeck EM, Van Ackeren K, Vrints CJ, Conraads VM, Verpooten GA, Kouidi E, Couttenye MM. Effect of moderate aerobic exercise training on endothelial function and arterial stiffness in CKD Stages 3–4: a randomized controlled trial. Am J Kidney Dis 66: 285–296, 2015. doi: 10.1053/j.ajkd.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz MI, Stenvinkel P, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Çakar M, Altun B, Yenicesu M, Carrero JJ. Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant 26: 3537–3543, 2011. doi: 10.1093/ndt/gfr081. [DOI] [PubMed] [Google Scholar]
- 36.Zoccali C. Traditional and emerging cardiovascular and renal risk factors: an epidemiologic perspective. Kidney Int 70: 26–33, 2006. doi: 10.1038/sj.ki.5000417. [DOI] [PubMed] [Google Scholar]