Abstract
The renal proximal tubules are a key functional component of the kidney and express the angiotensin precursor angiotensinogen; however, it is unclear the extent that tubular angiotensinogen reflects local synthesis or internalization. Therefore, the current study established the extent to which angiotensinogen is internalized by proximal tubules and the intracellular distribution. Proximal tubules were isolated from the kidney cortex of male sheep by enzymatic digestion and a discontinuous Percoll gradient. Tubules were incubated with radiolabeled 125I-angiotensinogen for 2 h at 37°C in serum/phenol-free DMEM/F12 media. Approximately 10% of exogenous 125I-angiotensinogen was internalized by sheep tubules. Subcellular fractionation revealed that 21 ± 4% of the internalized 125I-angiotensinogen associated with the mitochondrial fraction with additional labeling evident in the nucleus (60 ± 7%), endoplasmic reticulum (4 ± 0.5%), and cytosol (15 ± 4%; n = 4). Subsequent studies determined whether mitochondria directly internalized 125I-angiotensinogen using isolated mitochondria from renal cortex and human HK-2 proximal tubule cells. Sheep cortical and HK-2 mitochondria internalized 125I-angiotensinogen at a comparable rate of (33 ± 9 vs. 21 ± 10 pmol·min−1·mg protein−1; n = 3). Lastly, unlabeled angiotensinogen (100 nM) competed for 125I-angiotensinogen uptake to a greater extent than human albumin in HK-2 mitochondria (60 ± 2 vs. 16 ± 13%; P < 0.05, n = 3). Collectively, our data demonstrate angiotensinogen import and subsequent trafficking to the mitochondria in proximal tubules. We conclude that this pathway may constitute a source of the angiotensinogen precursor for the mitochondrial expression of angiotensin peptides.
Keywords: angiotensinogen, mitochondria, protein uptake, proximal tubules, renin-angiotensin system
the physiological relevance of local or tissue-based renin-angiotensin systems (RAS) vs. the circulating RAS remains equivocal (5, 16, 26). The kidney is clearly an important target of the RAS regarding the regulation of sodium, water, and blood pressure, as well as the development and progression of renal injury. The kidney expresses all angiotensin receptor isotypes [AT1, AT2, AT7/Mas, and AT4/insulin-regulated aminopeptidase (IRAP)] that may interact with circulating angiotensins [ANG II, ANG-(2–8) or ANG III, ANG-(1–7), and ANG-(3–8), or ANG IV]; however, the tubular epithelium also expresses RAS components to generate these peptides (16, 47). Binding of ANG II to the AT1 receptor (AT1R) stimulates internalization of the peptide receptor complex, which may further contribute to the tissue levels of ANG II (39, 48). The tubular epithelium synthesizes angiotensinogen and the protein is subsequently secreted apically into the tubular fluid (16), but certain segments of the proximal tubule also internalize the protein. Pohl et al. reported that knockdown of the protein transporter megalin attenuated the tubule expression of angiotensinogen (30). Subsequent studies by Matsusaka et al. demonstrate that knockdown of liver angiotensinogen abolished the proximal tubule content of both angiotensinogen and ANG II suggesting that the tubular uptake of the precursor contributes to local ANG II expression (22). Moreover, knockdown of kidney angiotensinogen failed to reduce the tubular expression of angiotensinogen or ANG II (22).
The pathways for angiotensinogen expression in the renal tubules may be important in regards to the extent that the precursor serves as a source for intracellular peptides to target receptors on various subcellular organelles (32). Several laboratories including our own find a high density of angiotensin receptors on isolated nuclei from the renal cortex and medulla (11, 13, 20, 27). Zhuo and colleagues report that enhanced intracellular levels of ANG II within the proximal tubules increased blood pressure and was associated with higher expression of the Na+/H+ exchanger (NHE3) (17, 19). These data support earlier findings that ANG II stimulates reactive oxygen species (ROS) and NHE3 mRNA expression on isolated renal nuclei; both responses were abolished by an AT1R antagonist (18, 28). We also found evidence for the AT7/MasR on isolated nuclei from the sheep kidney that was coupled to an increase in nitric oxide (NO) consistent with the signaling signature of the ANG-(1–7)-MasR axis in various cell types (12). In isolated mitochondria from mouse liver, Abadir et al. reported immunoreactive evidence for both AT1R and AT2R (1, 2). Functionally, the AT2R was coupled to an increase in mitochondrial NO (1). Moreover, mitochondria from older animals exhibited a lower AT2R to AT1R ratio and this reduced receptor ratio may contribute to mitochondrial dysfunction in aging (1). We recently demonstrated evidence for an ANG-(1–7)-AT7/MasR axis in mitochondria isolated from the sheep renal cortex (43). The sheep renal mitochondria expressed immunoreactive levels of ANG-(1–7) and ANG II, as well as evidence for [des-ANG I]-angiotensinogen (43). These studies support a potential role for various elements of the RAS to influence mitochondrial function (31). Since the mitochondrial genome does not code for angiotensinogen (or other RAS components), the cellular pathways for the potential localization of angiotensinogen to the mitochondria remain to be established. Therefore, the current study sought to elucidate the extent for uptake of angiotensinogen within isolated tubules from the sheep renal cortex and the subsequent trafficking of the protein to intracellular organelles.
METHODS
Animals.
Mixed breed sheep (obtained from a private local vendor) were delivered at term, farm raised, and weaned at 3 mo of age. Adult male sheep (10–12 mo of age) were anesthetized with ketamine and isoflurane and euthanized by exsanguination. The kidneys were removed immediately, and the renal cortex was dissected out on ice for immediate isolation of proximal tubules as previously described (42). All procedures were approved by the Wake Forest University School of Medicine Institutional Animal Care and Use Committee.
HK-2 cells.
HK-2 cells derived from human proximal tubular cells were obtained from American Tissue Type Culture (Manassas, VA). The cells were incubated at 37°C under 5% CO2 humidified atmosphere and were routinely maintained in Dulbecco's modified Eagle’s medium (DMEM/F12, 4.5 g/l glucose, 25 mM HEPES, and l-glutamine) supplemented with 10% FBS, insulin-transferrin-selenium-cortisol, and 100 μg/ml penicillin/streptomycin (42). HK-2 cells were placed in serum-free DMEM/F12 media for 24 h before mitochondria isolation.
125I-angiotensinogen import.
Internalization of 125I-angiotensinogen was established in intact cortical tubules, cortical mitochondria, and mitochondria from HK-2 cells. Purified recombinant human angiotensinogen was purchased from Sino Biolgoical (Beijing, China) and iodinated utilizing the chloramine T method (6) and purified on a 30-kDa cut-off centrifugal filter (Microsep Advance Centrifugal Device; Pall). ANG I, ANG II, and ANG-(1–7) peptides were iodinated by the chloramine T method and purified by HPLC to a specific activity >2,000 Ci/mmol. Radiolabeled peptides are stable for up to 2 mo at 4°C. As shown in Fig. 1A, 125I-angiotensinogen (20 nM) was incubated with purified tubules (500 µg) for 2 h at 37°C in serum-free and phenol-free DMEM/F12. The import reaction was stopped by addition of 0.01% phosphoric acid and tubules were centrifuged at 16,000 g for 1 min. The supernatant was retained and the resulting pellet was washed twice with DMEM/F12 containing 0.01% Triton X-100 and 0.01% bovine serum albumin (BSA); the pellet was subsequently treated with 50 mM glycine-HCl (pH 3.0) for 5 min at 4°C to remove surface-bound or membrane-associated radioligand. Following glycine treatment, tubules were gently vortexed and centrifuged at 16,000 g for 1 min. This resultant pellet was considered the intracellular protein derived from the internalization of 125I-angiotensinogen. The supernatants were combined and considered the extracellular or non-internalized fraction. The distribution was expressed as a percentage (%) of the initial counts:
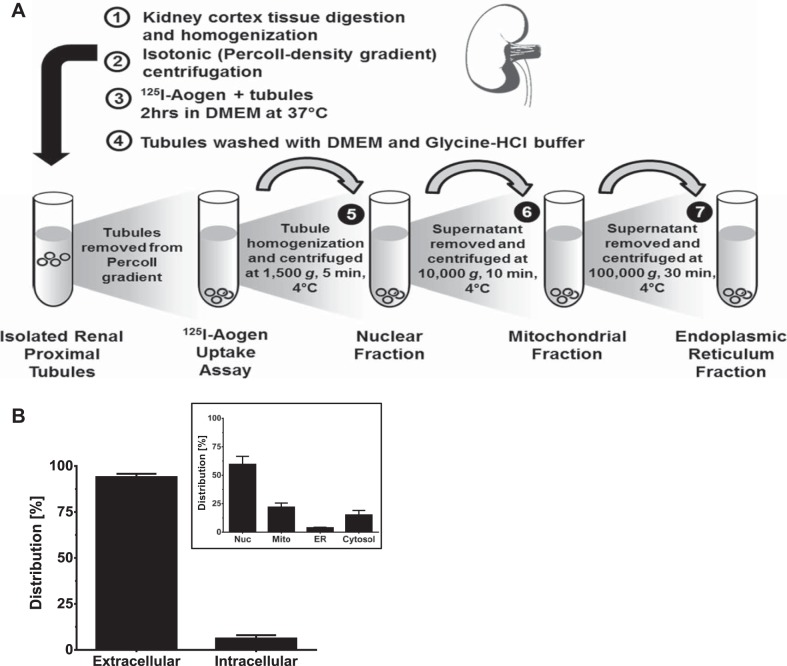
Fig. 1.
Uptake of 125I-angiotensinogen in isolated proximal tubules. A: schematic of the 125I-angiotensinogen uptake assay and subcellular fractionation. 125I-angiotensinogen (20 nM) was incubated with isolated sheep renal proximal tubules (500 µg protein) for 2 h at 37°C. B: distribution of extracellular and intracellular 125I-angiotensinogen. Data are mean ± SE expressed a %initial cpm; n = 4. Inset: subcellular fractionation revealed that 21 ± 4% of internalized 125I-angiotensinogen associated with the mitochondrial (Mito) fraction, with additional labeling in the nucleus (Nuc; 60 ± 7%), endoplasmic reticulum (ER, 4 ± 0.5%), and cytosol (15 ± 4%). Data are means ± SE expressed as the %total intracellular cpm; n = 4.
Subcellular fractionation.
Following the incubation with 125I-angiotensinogen, sheep tubules were centrifuged for 5 min at 1,500 g to wash and collect cells (Fig. 1A). The supernatant was removed and tubule pellet was resuspended in DMEM/F12. Samples were then homogenized with a 20-ml all-glass Dounce homogenizer (Kontes Glass, Vineland, NJ) for 1 min and centrifuged at 1,500 g for 5 min. The supernatant was saved and the pellet (nuclear fraction) was counted. The resulting supernatant was centrifuged at 10,000 g for 10 min. The pellet (mitochondrial fraction) was counted and the supernatant was centrifuged at 100,000 g for 30 min. The final pellet (endoplasmic reticulum fraction) and supernatant (cytosolic fraction) were counted. The distribution of 125I-angiotensinogen was expressed as percentage (%) of the total intracellular counts:
Isolation of mitochondria.
Mitochondria were isolated from fresh sheep renal cortex by a discontinuous Percoll gradient exactly as described (43). For isolation of HK-2 mitochondria, cells in 100 mm dishes were washed with PBS, collected in DMEM/F12 and centrifuged (1,500 g, 5 min, 4°C). Cells were briefly frozen at −80°C for 5 min and subsequently thawed by resuspension in DMEM/F12 media and homogenized using a 20-ml all-glass Dounce homogenizer. The cell homogenate was centrifuged (1,500 g, 5 min, 4°C), and the supernatant fraction was centrifuged at 10,000 g, for 10 min at 4°C to obtain the mitochondrial-enriched pellet. Internalization of 125I-angiotensinogen (20 nM) was determined in purified mitochondria (~200 µg) for 1 h at 37°C in DMEM/F12 media (Fig. 2). Internalized 125I-angiotensinogen was characterized by autoradiography following SDS-PAGE separation of the sheep cortex and HK-2 mitochondria (100 µg) and exposure to GeneMate Blue Autoradiography film for 18 h at room temperature. 125I-angiotensinogen uptake was also determined in the presence of an import inhibitor, the metallopeptidase inhibitor o-phenanthroline (O-PHEN; 10 µM) or the mitochondrial uncoupling agents carbonyl cyanide 3-chlorophenylhydrazone (CCC; 10 µM) and valinomycin (Val, 10 µM).
Fig. 2.
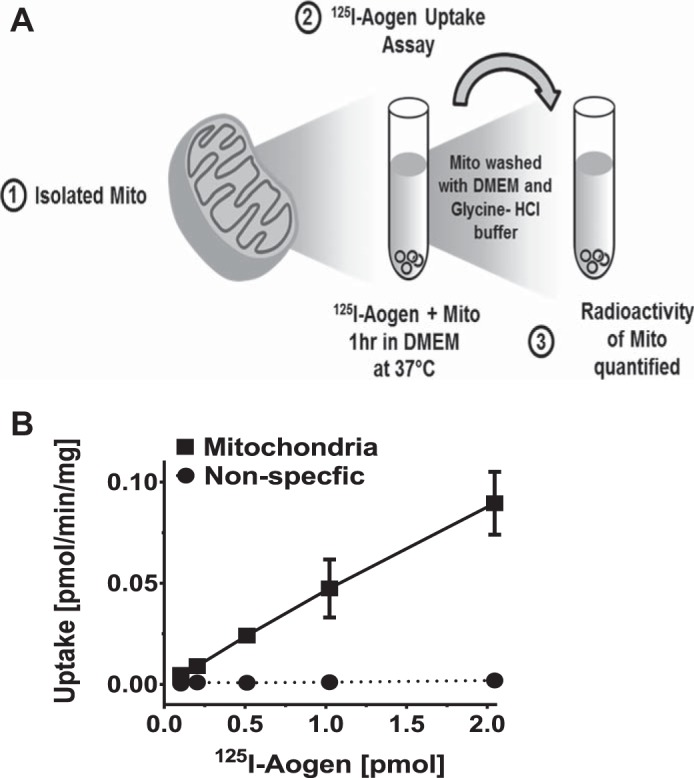
Uptake of 125I-angiotensinogen in sheep cortical mitochondria. A: schematic of mitochondrial 125I-angiotensinogen uptake assay. B: increasing concentrations of 125I-angiotensinogen result in a linear increase in mitochondrial uptake of 125I-angiotensinogen. Nonspecific binding or uptake of 125I-angiotensinogen where bovine serum albumin (BSA) was substituted for mitochondria in the uptake assay is included. Data are means ± SE; n = 3.
Submitochondrial membrane fractionation.
To characterize outer membrane import of 125I-angiotensinogen, isolated mitochondria were treated with the nonionic detergent digitonin to disrupt the outer membrane (35). Sheep cortex and HK-2 mitochondria were solubilized in 5 mg/ml digitonin (Sigma) in serum/phenol free DMEM/F12 and subjected to constant shaking at room temperature and aliquots were removed at 1, 5, and 15 min. The reaction was terminated by the addition and subsequent washing (2×) with cold DMEM/F12 to remove residual detergent and centrifuged at 16,000 g for 1 min. The resulting sheep and HK-2 mitochondria (20 μg protein) were subjected to SDS-PAGE for immunoblot analysis of the outer membrane protein voltage-dependent anion channel (VDAC). Treated mitochondria were fractionated using 10% polyacrylamide gels for 1 h at 120 V in Tris-glycine SDS and transferred to a polyvinylidene difluoride (PVDF) membrane. Blots were blocked with 5% Bio-Rad Dry Milk (Bio-Rad, Hercules, CA) and Tris-buffered saline (TBS) with Tween (0.05%) and probed overnight at 4°C with a primary antibody against VDAC (1:1,000; Lot #3; Cell Signaling).
Statistical analysis.
All measurements are expressed as means ± SE. Differences between two sample means were compared using Student’s t test analysis while differences between multiple groups were analyzed by one-way ANOVA and Newman-Keuls multiple comparison analysis. Statistical analyses were performed and figures were constructed with GraphPad Prism V (GraphPad Software, San Diego, CA). A P < 0.05 was required for statistical significance.
RESULTS
Angiotensinogen internalization by renal tubules.
To establish the extent of angiotensinogen internalization, 125I-angiotensinogen was incubated with freshly isolated proximal tubules at 37°C in serum/phenol free DMEM/F12 (Fig. 1A). Following a 2-h incubation, ~10% of the initial 125I-angiotensinogen was internalized (Fig. 1B). Subcellular fractionation of the proximal tubules revealed that 22% of the internalized 125I-angiotensinogen associated with the mitochondrial fraction (Mito), with additional labeling evident in the nucleus (Nuc; 59%), the endoplasmic reticulum (ER; 4%), and cytosol (15%) (Fig. 1B, inset).
Angiotensinogen import in sheep and human renal mitochondria.
We previously reported that mitochondria isolated from the sheep cortex expressed renin and [des-ANG I]-angiotensinogen (43). Based on 125I-angiotensinogen within the mitochondrial fraction following tubular uptake, we determined whether isolated mitochondria directly internalize angiotensinogen. Purified mitochondria obtained from the Percoll density fractionation were incubated with increasing concentrations of 125I-angiotensinogen. The mitochondria were then washed twice in uptake buffer, once in the low pH glycine-HCl buffer, and the resultant mitochondrial pellet counted (Fig. 2A). As shown in Fig. 2B, mitochondrial uptake of 125I-angiotensinogen increased in a linear fashion with higher concentrations of 125I-angiotensinogen. As a negative control, nonspecific binding or uptake of 125I-angiotensinogen was negligible when the mitochondrial fraction was substituted with bovine serum albumin (BSA) at a comparable protein concentration (Fig. 2B).
We then assessed the rate of 125I-angiotensinogen internalization in isolated mitochondria from HK-2 cells, a well-characterized human proximal tubule cell line (36). The isolated human mitochondria internalized 125I-angiotensinogen at a similar rate to the sheep mitochondria (Fig. 3A). The 125I-angiotensinogen taken up in both sheep cortical and human mitochondria was subsequently analyzed by SDS-gel autoradiography. The autoradiograph demonstrates a single protein band corresponding to the molecular weight of angiotensinogen at 55 kDa (Fig. 3A, inset). Moreover, unlabeled angiotensinogen (100 nM) competed for 125I-angiotensinogen uptake to a significantly greater extent than human serum albumin (HSA) in HK-2 mitochondria (Fig. 3B).
Fig. 3.
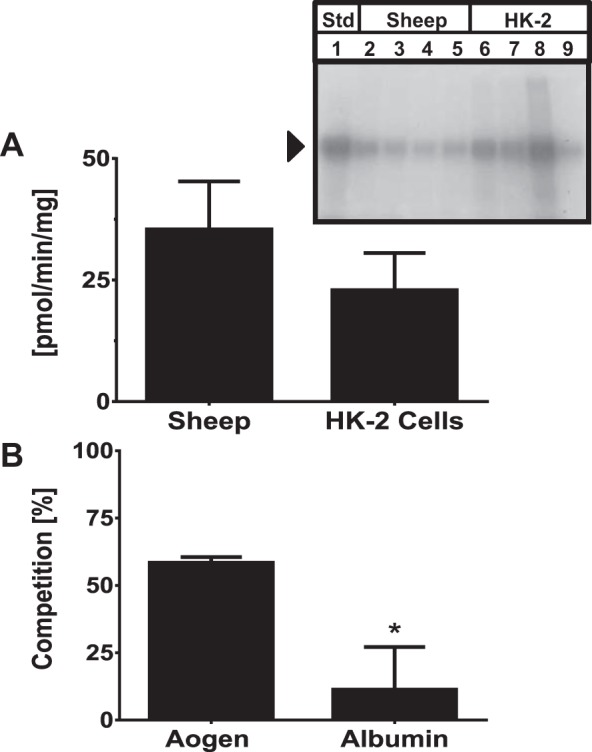
Rate of 125I-angiotensinogen uptake is comparable between sheep and human mitochondria. A: sheep cortical mitochondria and human HK-2 mitochondria internalized 125I-angiotensinogen at a comparable rate of (33 ± 9 vs. 21 ± 10 pmol·min−1·mg protein−1; n = 3). Inset: Purified sheep cortical (lanes 2–5) and HK-2 (lanes 6–9) mitochondria were fractionated by 10% PAGE-SDS and exposed to autoradiography film. A single protein band of 55 kDa corresponding to the molecular weight of the 125I-angiotensinogen standard (Std, lane 1) was detected. B: unlabeled angiotensinogen (100nM) competed for 125I-angiotensinogen uptake to a greater extent than human serum albumin (HAS; 100 nM) in the HK-2 mitochondria (60 ± 2 vs. 16 ± 13%). Data are means ± SE expressed as the %control uptake; P < 0.05; n = 3.
Next, we evaluated whether angiotensin peptides are imported within mitochondria at a comparable rate to angiotensinogen. ANG I, ANG II, and ANG-(1–7) were iodinated and purified as previously described (7) and then incubated with the isolated mitochondria from the human HK-2 cells. In this set of studies, the HK-2 mitochondria internalized 125I-angiotensinogen at a rate of 5.6 ± 1.1 pmol·min−1·mg protein−1; however, the rate of uptake for angiotensin peptides was minimal (<100 fold vs. angiotensinogen; Table 1).
Table 1.
Angiotensinogen and angiotensin peptide uptake in human HK-2 mitochondria
| Radioligand | Uptake Rate, pmol·min−1·mg protein−1 |
|---|---|
| 125I-angiotensinogen | 5.8 ± 1.1 |
| 125I-angiotensin I | 0.02 ± 0.004* |
| 125I-angiotensin II | 0.01 ± 0.001* |
| 125I-angiotensin-(1–7) | 0.04 ± 0.01* |
Values are means ± SE; n = 4. Initial angiotensinogen concentration of 20 nM. Initial angiotensin peptide concentration of 3 nM. Incubation for 1 h at 37°C.
P < 0.001 vs. angiotensinogen.
Characterization of angiotensinogen import.
Mitochondrial processing peptidases (MPPs) are metallopeptidases that actively cleave mitochondrial targeting signals to direct import and sorting of proteins into the mitochondrial subcompartments (4, 10, 38). Therefore, we assessed whether the metallopeptidase inhibitor O-PHEN that blocks MMP activity would attenuate uptake of angiotensinogen in sheep and human mitochondria. As shown in Fig. 4, preincubation of the mitochondria with 10 µM O-PHEN did not reduce uptake of 125I-angiotensinogen. Protein transport across mitochondrial membranes is also mediated by membrane potential (4). However, addition of the uncoupling agents CCCP and valinomycin (VAL) or their combination did not inhibit 125I-angiotensinogen uptake in either the sheep or human mitochondria (Fig. 4).
Fig. 4.
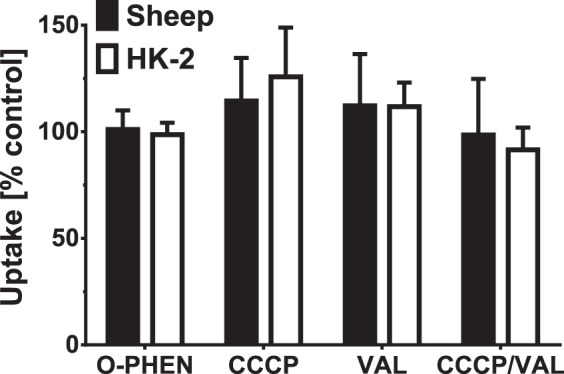
Characterization of 125I-angiotensinogen import. Isolated HK-2 and sheep cortical mitochondria were exposed to the mitochondrial processing peptidase inhibitor o-phenanthroline (O-PHEN; 10 µM) or the mitochondrial membrane uncoupling agents carbonyl cyanide 3-chlorophenylhydrazone (CCCP; 10 µM), valinomycin (VAL; 10 µM), and their combination (CCCP/VAL). OPHEN, CCCP, or VAL did not inhibit 125I-angiotensinogen import. Data are means ± SE expressed as the %control uptake; P > 0.05, n = 3.
Angiotensinogen import is dependent on the outer mitochondrial membrane.
The outer mitochondrial membrane harbors numerous protein transporters that facilitate import of cytosolic proteins into mitochondrial compartments that include translocases of the outer membrane (TOM complexes) (44–46). The receptor domain of TOM complexes faces the cytosolic compartment and initiates the recognition and subsequent transport of mitochondrial protein precursors (44–46). To establish whether angiotensinogen uptake is dependent on outer membrane transport, sheep cortical and HK-2 mitochondria were treated with the non-ionic detergent digitonin to remove the TOM complexes. We confirmed that digitonin removed the outer mitochondrial membrane (OM) as evident by the disappearance of the immunoreactive band for the OM protein VDAC from both sheep cortex (Fig. 5A) and HK-2 cell mitochondria (Fig. 5B). Digitonin treatment for 15 min significantly reduced but did not abolish 125I-angiotensinogen uptake in the sheep cortical and HK-2 mitochondria (Fig. 5C).
Fig. 5.
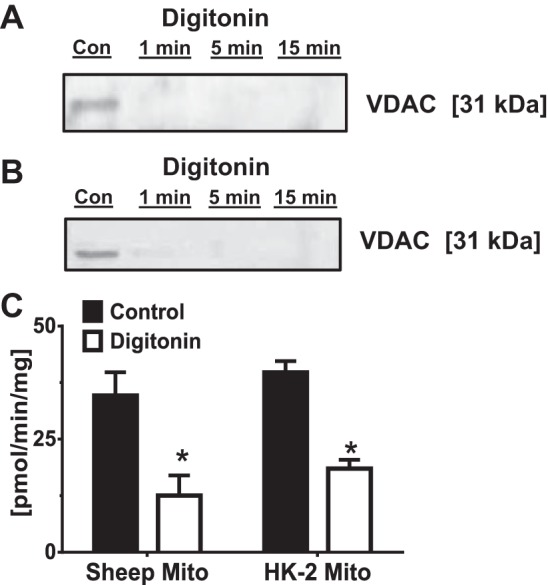
Uptake of 125I-angiotensinogen is dependent on the outer mitochondrial membrane. A and B: purified sheep cortical (A) and HK-2 (B) mitochondria were exposed to the nonionic detergent digitonin (5 mg/ml) for various times to remove the outer mitochondrial membrane. Following the digitonin treatment, mitochondrial preparations were subjected to 10% SDS gel fractionation and immunoblotting with an antibody to VDAC. Digitonin treatment depleted the outer membrane marker VDAC in both sheep and human HK-2 mitochondria. C: digitonin treatment at 15 min significantly reduced 125I-angiotensinogen uptake. Data are means ± SE; *P < 0.05 vs. control; n = 3.
DISCUSSION
We previously reported that mitochondria isolated from sheep renal cortex expressed elements of a local RAS including renin, [des-ANG I]-angiotensinogen, ANG II, and ANG-(1–7), as well as the AT7/Mas receptor (43). Although these data support earlier studies by Abadir and colleagues on the evidence of a mitochondrial RAS, the mechanisms for the intracellular expression of RAS components in the mitochondria and other subcellular compartments are not well established (3, 33, 43). Moreover, there remains debate regarding the extent that local angiotensinogen synthesis vs. internalization of the protein contributes to ANG II content in the proximal tubules (21, 22). Therefore, the present studies assessed the extent that proximal tubules take up angiotensinogen and the intracellular distribution of the internalized protein. We find that angiotensinogen import actively occurs within isolated sheep proximal tubules and the internalized protein traffics predominantly to the nuclear and mitochondrial fractions. Moreover, we show that both sheep cortical and human mitochondria directly internalize angiotensinogen. The uncoupling of mitochondria or treatment with an MMP inhibitor failed to reduce uptake of the precursor protein, indicating that the translocation of angiotensinogen may be facilitated via a non-motor or energy-independent mechanism (38).
Although the presence of a local RAS within kidney tubule cells is well established, the mechanisms by which RAS components are transported within tubules and traffic to intracellular sites are still equivocal. Due to its high molecular size (50–60 kDa), angiotensinogen should not undergo glomerular filtration; however, some degree of filtered angiotensinogen may be the source of the protein precursor within the tubules and contribute to the cellular levels of ANG II (22, 30, 37). Indeed, Roksnoer et al. report that 95% of the filtered angiotensinogen is reabsorbed by the proximal tubules (34). Angiotensinogen is internalized by the protein transporter megalin and developmental knockdown of megalin result in reduced tubular levels of angiotensinogen (30, 37). Liver-specific knockout of angiotensinogen markedly lowered the renal content of both the precursor protein and ANG II, implicating liver angiotensinogen as the primary source of renal angiotensinogen (22). In contrast, renal-specific angiotensinogen knockout mice expressed renal angiotensinogen that did not differ from wild-type mice (22). Megalin is likely the predominant mechanism to bind and internalize angiotensinogen; however, Pan et al. demonstrated specific angiotensinogen binding sites on the extracellular surface of human proximal tubules (25). The Pan study revealed a high affinity binding site for radiolabeled-angiotensinogen (Kd = 2 nM) that was displaced by angiotensinogen but not ANG II or the AT1 receptor antagonist losartan (29). Moreover, the cell surface angiotensinogen-receptor complex underwent internalization, although the intracellular distribution of this complex within the tubules was not established (29). Subcellular fractionation of angiotensinogen uptake in this study revealed accumulation in the nucleus (60%), mitochondria (20%), and ER (4%), suggesting organelle-specific mechanisms of the protein’s import. Given the intracellular expression of RAS enzymes within compartments such as the nucleus and mitochondria (8, 40), the organelle sorting of angiotensinogen may potentially contribute to ANG peptide tone; however, additional studies are required to address the extent of angiotensinogen processing in the mitochondria of both sheep and HK-2 proximal tubule cells.
We did not specifically assess the presence of high affinity angiotensinogen receptors on the cell surface of the sheep proximal tubules or HK-2 cells and cannot exclude that an angiotensinogen receptor may contribute in part to uptake of the protein by proximal tubules at this time. As to the mitochondrial uptake of angiotensinogen, to our knowledge, megalin expression on the mitochondria has not been reported, nor does the mitochondrial genome contain the megalin sequence. We utilized a single concentration of angiotensinogen at 100 nM that competed for ~60% of angiotensinogen uptake, but we cannot derive an IC50 value for mitochondrial internalization. It would appear that the angiotensinogen binding sites described by Pan and colleagues exhibit a higher affinity for angiotensinogen than the uptake in the mitochondria. This alludes that the uptake site in mitochondria is more distinct and may constitute a novel endocytic pathway for angiotensinogen import; however, further studies are clearly required to fully characterize this mitochondrial transport site.
Mitochondria contain numerous protein transporters and the precise mechanisms for the uptake of angiotensinogen or other RAS components are not clear at the present time. The mitochondrial outer membrane contains a multisubunit complex responsible for the specific recognition and translocation of precursor proteins within mitochondria (23). The TOM complex consists of three receptor proteins: Tom20, Tom22, and Tom70, the channel protein Tom40, and several small Tom proteins (23). Digitonin treatment is a standard approach to remove the outer membrane components of the mitochondria (35). Digitonin treatment of mitochondria significantly reduced but did not abolish the uptake of angiotensinogen.
The TOM complex faces the cytosol and has been extensively studied with respect to mitochondrial protein transport. However, various components of the TOM complex may confer ligand-specific affinity for protein import (29). Typically, mitochondrial proteins selected for import may feature basic amphipathic extensions that interact with acidic domains of TOM proteins; Kato et al. recently demonstrated that the Tom70 receptor component of the TOM complex was essential for the mitochondrial import of the PTEN-induced kinase 1 (PINK1) (14). Utilizing a radiolabeled form of the PINK1 protein and a cell-free system, these investigators demonstrated that the protein is targeted to the mitochondria. Moreover, the import of PINK1 was dependent on the mitochondrial membrane potential, but was not attenuated by the peptidase inhibitor O-PHEN (14). In our study, angiotensinogen was similarly radiolabeled and both cell and cell-free mitochondrial import were observed. The significant reduction in angiotensinogen import by digitonin treatment suggests that angiotensinogen import mechanisms may be present; however, elucidation of the potential TOM complex components involved remains the focus of future studies.
The remaining import activity following digitonin treatment may potentially reflect intra-mitochondrial transport proteins on the inner membrane to facilitate translocation of angiotensinogen into the mitochondrial matrix. Once proteins transport through the outer mitochondrial membrane, protein access to the mitochondrial matrix is governed by translocases on the inner membrane of mitochondria (TIMs) (10, 14). The mitochondrial inner membrane is considered to be the energy-producing membrane within the cell. The electrochemical proton gradient generated by the electron transport chain of the mitochondrial matrix drives ATP synthase and promotes the proton-motive force needed by translocases to control the import of ions and proteins (24). However, van der Laan et al. presented evidence for a motor-free mitochondrial translocase that is capable of facilitating the integration of preproteins into submitochondrial compartments (38). We find that the uncoupling agents CCCP and Valinomycin failed to inhibit angiotensinogen import in intact mitochondria. The mechanism of angiotensinogen transport across the outer membrane and potential retainment by the mitochondrial matrix require additional studies, although these processes may reflect non-energy-dependent mechanisms (38).
Perspectives and Significance
The mechanisms that contribute to the intracellular expression of the biologically active angiotensin peptides ANG II and ANG-(1–7) within the kidney remain equivocal. The present study provides biochemical evidence that the precursor protein angiotensinogen is internalized by the proximal tubules and localizes to the nucleus and mitochondria. Angiotensin receptors are expressed on these organelles and are coupled to the stimulation of NO, ROS, and calcium (1, 2, 15). The uptake and trafficking of angiotensinogen in the renal epithelium may provide an intracellular source for the receptor ligands within the nucleus and mitochondria. Alternatively, demonstration of direct uptake of angiotensinogen by the mitochondria may portend for biological actions of the precursor protein that may be distinct or independent from ANG II or ANG-(1–7) (21). Indeed, Corvol and colleagues demonstrate that both angiotensinogen and [des-ANG I]-angiotensinogen exhibit anti-angiogenic properties that were not blocked by AT1R antagonists, as well as attenuated tumor growth of hepatocarcinoma in transgenic mice (41). Elucidation of the possible direct effects of angiotensinogen vs. those of ANG II or ANG- (1–7) on mitochondrial function within the kidney awaits additional studies.
GRANTS
B. A. Wilson is supported by an American Heart Association (AHA) Predoctoral Fellowship Grant 15PRE25120007. N. Cruz-Diaz is supported by a fellowship from the PRIME Institutional Research and Academic Career Development Award K12-GM-102773. Additional support for this study was provided by National Institute of Child Health and Human Development Grants HD-047584, HD-017644, and HD-084227; AHA 14GRNT20480131; the Groskert Heart Fund; the Wake Forest Venture Fund; and the Farley-Hudson Foundation (Jacksonville, NC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.A.W., N.C.-D., and Y.S. performed experiments; B.A.W. and M.C.C. analyzed data; B.A.W. and M.C.C. interpreted results of experiments; B.A.W. and M.C.C. prepared figures; B.A.W. and M.C.C. drafted manuscript; B.A.W. and M.C.C. edited and revised manuscript; B.A.W., N.C.-D., Y.S., J.C.R., T.M.G., and M.C.C. approved final version of manuscript.
ACKNOWLEDGMENTS
This work represents partial fulfillment of the requirements for the degree of Doctorate of Philosophy in the Department of Molecular Medicine and Translational Sciences at Wake Forest University School of Medicine for Bryan A. Wilson. The authors gratefully acknowledge Eric LeSaine for technical and surgical support.
REFERENCES
- 1.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108: 14849–14854, 2011. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abadir PM, Walston JD, Carey RM. Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides 38: 437–445, 2012. doi: 10.1016/j.peptides.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzayadneh EM, Chappell MC. Nuclear expression of renin-angiotensin system components in NRK-52E renal epithelial cells. J Renin Angiotensin Aldosterone Syst 16: 1135–1148, 2015. doi: 10.1177/1470320313515039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böhni PC, Daum G, Schatz G. Import of proteins into mitochondria. Partial purification of a matrix-located protease involved in cleavage of mitochondrial precursor polypeptides. J Biol Chem 258: 4937–4943, 1983. [PubMed] [Google Scholar]
- 5.Campbell DJ. Clinical relevance of local renin angiotensin systems. Front Endocrinol (Lausanne) 5: 113, 2014. doi: 10.3389/fendo.2014.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol 310: H137–H152, 2016. doi: 10.1152/ajpheart.00618.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chappell MC, Jacobsen DW, Tallant EA. Characterization of angiotensin II receptor subtypes in pancreatic acinar AR42J cells. Peptides 16: 741–747, 1995. doi: 10.1016/0196-9781(95)00044-K. [DOI] [PubMed] [Google Scholar]
- 8.Camargo de Andrade MC, Di Marco GS, de Paulo Castro Teixeira V, Mortara RA, Sabatini RA, Pesquero JB, Boim MA, Carmona AK, Schor N, Casarini DE. Expression and localization of N-domain ANG I-converting enzymes in mesangial cells in culture from spontaneously hypertensive rats. Am J Physiol Renal Physiol 290: F364–F375, 2006. doi: 10.1152/ajprenal.00110.2005. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Villalobos RA, Satou R, Katsurada A, Kobori H, Hammond TG, Navar LG. Megalin mediates the uptake of angiotensin II in proximal tubule cells (Abstract). FASEB J 21: A1245, 2007. [Google Scholar]
- 10.Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep 13: 378–385, 2012. doi: 10.1038/embor.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol 302: R518–R530, 2012. doi: 10.1152/ajpregu.00525.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin-(1-7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol 299: F983–F990, 2010. doi: 10.1152/ajprenal.00371.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imig JD, Navar GL, Zou L-X, O’Reilly KC, Allen PL, Kaysen JH, Hammond TG, Navar LG. Renal endosomes contain angiotensin peptides, converting enzyme, and AT1A receptors. Am J Physiol Renal Physiol 277: F303–F311, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Kato H, Lu Q, Rapaport D, Kozjak-Pavlovic V. Tom70 is essential for PINK1 import into mitochondria. PLoS One 8: e58435, 2013. doi: 10.1371/journal.pone.0058435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW, Ihm CG, Moon JY. Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells. PLoS One 7: e39739, 2012. doi: 10.1371/journal.pone.0039739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 17.Li XC, Cook JL, Rubera I, Tauc M, Zhang F, Zhuo JL. Intrarenal transfer of an intracellular fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am J Physiol Renal Physiol 300: F1076–F1088, 2011. doi: 10.1152/ajprenal.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XC, Hopfer U, Zhuo JL. Novel signaling mechanisms of intracellular angiotensin II-induced NHE3 expression and activation in mouse proximal tubule cells. Am J Physiol Renal Physiol 303: F1617–F1628, 2012. doi: 10.1152/ajprenal.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li XC, Zhuo JL. Proximal tubule-dominant transfer of AT1a receptors induces blood pressure responses to intracellular angiotensin II in AT1a receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 304: R588–R598, 2013. doi: 10.1152/ajpregu.00338.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Licea H, Walters MR, Navar LG. Renal nuclear angiotensin II receptors in normal and hypertensive rats. Acta Physiol Hung 89: 427–438, 2002. doi: 10.1556/APhysiol.89.2002.4.3. [DOI] [PubMed] [Google Scholar]
- 21.Lu H, Cassis LA, Vander Kooi CW, Daugherty A. Structure and functions of angiotensinogen. Hypertens Res 39: 492-500, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol 23: 1181–1189, 2012. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Model K, Prinz T, Ruiz T, Radermacher M, Krimmer T, Kühlbrandt W, Pfanner N, Meisinger C. Protein translocase of the outer mitochondrial membrane: role of import receptors in the structural organization of the TOM complex. J Mol Biol 316: 657–666, 2002. doi: 10.1006/jmbi.2001.5365. [DOI] [PubMed] [Google Scholar]
- 24.Palmieri F, Agrimi G, Blanco E, Castegna A, Di Noia MA, Iacobazzi V, Lasorsa FM, Marobbio CM, Palmieri L, Scarcia P, Todisco S, Vozza A, Walker J. Identification of mitochondrial carriers in Saccharomyces cerevisiae by transport assay of reconstituted recombinant proteins. Biochim Biophys Acta 1757: 1249–1262, 2006. doi: 10.1016/j.bbabio.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Pan N, Luo J, Kaiser SJ, Frome WL, Dart RA, Tewksbury DA. Specific receptor for angiotensinogen on human renal cells. Clin Chim Acta 373: 32–36, 2006. doi: 10.1016/j.cca.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 27.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 28.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun 384: 149–154, 2009. doi: 10.1016/j.bbrc.2009.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry AJ, Hulett JM, Likić VA, Lithgow T, Gooley PR. Convergent evolution of receptors for protein import into mitochondria. Curr Biol 16: 221–229, 2006. doi: 10.1016/j.cub.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem 285: 41935–41946, 2010. doi: 10.1074/jbc.M110.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Re RN, Cook JL. The mitochondrial component of intracrine action. Am J Physiol Heart Circ Physiol 299: H577–H583, 2010. doi: 10.1152/ajpheart.00421.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Re RN, Cook JL. Noncanonical intracrine action. J Am Soc Hypertens 5: 435–448, 2011. doi: 10.1016/j.jash.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Richoux JP, Cordonnier JL, Bouhnik J, Clauser E, Corvol P, Menard J, Grignon G. Immunocytochemical localization of angiotensinogen in rat liver and kidney. Cell Tissue Res 233: 439–451, 1983. doi: 10.1007/BF00238309. [DOI] [PubMed] [Google Scholar]
- 34.Roksnoer LC, Heijnen BF, Nakano D, Peti-Peterdi J, Walsh SB, Garrelds IM, van Gool JM, Zietse R, Struijker-Boudier HA, Hoorn EJ, Danser AH. On the origin of urinary renin: a translational approach. Hypertension 67: 927–933, 2016. doi: 10.1161/HYPERTENSIONAHA.115.07012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnaitman C, Erwin VG, Greenawalt JW. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol 32: 719–735, 1967. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shalamanova L, Wilkinson MC, McArdle F, Jackson MJ, Rustom R. Characterisation of the expression of the renin-angiotensin system in primary and immortalised human renal proximal tubular cells. Nephron, Exp Nephrol 116: e53–e61, 2010. doi: 10.1159/000318176. [DOI] [PubMed] [Google Scholar]
- 37.Tojo A, Kinugasa S, Fujita T, Wilcox CS. A local renal renin-angiotensin system activation via renal uptake of prorenin and angiotensinogen in diabetic rats. Diabetes Metab Syndr Obes 9: 1–10, 2016. doi: 10.2147/DMSO.S91245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Laan M, Meinecke M, Dudek J, Hutu DP, Lind M, Perschil I, Guiard B, Wagner R, Pfanner N, Rehling P. Motor-free mitochondrial presequence translocase drives membrane integration of preproteins. Nat Cell Biol 9: 1152–1159, 2007. doi: 10.1038/ncb1635. [DOI] [PubMed] [Google Scholar]
- 39.van Kats JP, van Meegen JR, Verdouw PD, Duncker DJ, Schalekamp MA, Danser AH. Subcellular localization of angiotensin II in kidney and adrenal. J Hypertens 19, Suppl: 583–589, 2001. doi: 10.1097/00004872-200103001-00010. [DOI] [PubMed] [Google Scholar]
- 40.Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol 286: F1039–F1045, 2004. doi: 10.1152/ajprenal.00371.2003. [DOI] [PubMed] [Google Scholar]
- 41.Vincent F, Bonnin P, Clemessy M, Contrerès J-O, Lamandé N, Gasc J-M, Vilar J, Hainaud P, Tobelem G, Corvol P, Dupuy E. Angiotensinogen delays angiogenesis and tumor growth of hepatocarcinoma in transgenic mice. Cancer Res 69: 2853–2860, 2009. doi: 10.1158/0008-5472.CAN-08-2484. [DOI] [PubMed] [Google Scholar]
- 42.Wilson BA, Cruz-Diaz N, Marshall AC, Pirro NT, Su Y, Gwathmey TM, Rose JC, Chappell MC. An angiotensin-(1-7) peptidase in the kidney cortex, proximal tubules, and human HK-2 epithelial cells that is distinct from insulin-degrading enzyme. Am J Physiol Renal Physiol 308: F594–F601, 2015. doi: 10.1152/ajprenal.00609.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson BA, Nautiyal M, Gwathmey TM, Rose JC, Chappell MC. Evidence for a mitochondrial angiotensin-(1–7) system in the kidney. Am J Physiol Renal Physiol 310: 2015, 2015. doi: 10.1152/ajprenal.00479.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto H, Fukui K, Takahashi H, Kitamura S, Shiota T, Terao K, Uchida M, Esaki M, Nishikawa S, Yoshihisa T, Yamano K, Endo T. Roles of Tom70 in import of presequence-containing mitochondrial proteins. J Biol Chem 284: 31635–31646, 2009. doi: 10.1074/jbc.M109.041756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamano K, Yatsukawa Y, Esaki M, Hobbs AEA, Jensen RE, Endo T. Tom20 and Tom22 share the common signal recognition pathway in mitochondrial protein import. J Biol Chem 283: 3799–3807, 2008. doi: 10.1074/jbc.M708339200. [DOI] [PubMed] [Google Scholar]
- 46.Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112: 41–50, 2003. doi: 10.1016/S0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- 47.Zhuo JL, Li XC. New insights and perspectives on intrarenal renin-angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides 32: 1551–1565, 2011. doi: 10.1016/j.peptides.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhuo JL, Li XC. Novel roles of intracrine angiotensin II and signalling mechanisms in kidney cells. J Renin Angiotensin Aldosterone Syst 8: 23–33, 2007. doi: 10.3317/jraas.2007.003. [DOI] [PMC free article] [PubMed] [Google Scholar]