Abstract
Lymphatic vessels are vital for the trafficking of immune cells from the interstitium to draining lymph nodes during inflammation. Hypertension is associated with renal infiltration of activated immune cells and inflammation; however, it is unknown how renal lymphatic vessels change in hypertension. We hypothesized that renal macrophage infiltration and inflammation would cause increased lymphatic vessel density in hypertensive rats. Spontaneously hypertensive rats (SHR) that exhibit hypertension and renal injury (SHR-A3 strain) had significantly increased renal lymphatic vessel density and macrophages at 40 wk of age compared with Wistar-Kyoto (WKY) controls. SHR rats that exhibit hypertension but minimal renal injury (SHR-B2 strain) had significantly less renal lymphatic vessel density compared with WKY rats. The signals for lymphangiogenesis, VEGF-C and its receptor VEGF-R3, and proinflammatory cytokine genes increased significantly in the kidneys of SHR-A3 rats but not in SHR-B2 rats. Fischer 344 rats exhibit normal blood pressure but develop renal injury as they age. Kidneys from 24-mo- and/or 20-mo-old Fischer rats had significantly increased lymphatic vessel density, macrophage infiltration, VEGF-C and VEGF-R3 expression, and proinflammatory cytokine gene expression compared with 4-mo-old controls. These data together demonstrate that renal immune cell infiltration and inflammation cause lymphangiogenesis in hypertension- and aging-associated renal injury.
Keywords: hypertension, lymphatics, lymphatic endothelial cells, kidney, inflammation
essential hypertension, which affects one in three adults, is characterized by immune system activation. Several rat models of hypertension, including the spontaneously hypertensive rat (SHR), the Dahl salt-sensitive rat, and gestational hypertensive rats, exhibit inflammation, renal dysfunction, and increased numbers of macrophages, dendritic cells, and T cells in the kidney (5, 6, 8, 11, 18–20, 24, 25). Immunosuppression reduces the renal immune cell infiltration in these models, which, not only attenuates the inflammation-induced injury and dysfunction, but also lowers blood pressure (4, 5, 20, 25). Immunosuppressive drug therapy in patients with rheumatological disease is associated with decreased blood pressure (10). This suggests that depletion of activated immune cells in the kidney is a promising hypertension therapeutic target; however, immunosuppression is not a feasible therapy for the millions of patients with hypertension. It is possible that extracting activated immune cells from the kidney would be beneficial; unfortunately, little is known about activated immune cell trafficking out of the kidney during hypertension.
Lymphatic vessels play a vital role in transporting immune cells and antigens from the interstitium to draining lymph nodes where they encounter the adaptive immune system T and B cells and modulate the immune response. During inflammation, lymphatic vessels extract immune cells out of the interstitial space into the collecting lymphatic vessels via chemokines and chemokine receptor expression. In numerous chronic, progressive kidney diseases in both animals and humans, lymphangiogenesis has been reported to occur in both the cortex and medulla following the inflammation and injury (22). Lymphangiogenesis is mediated primarily by vascular endothelial growth factors C and D (VEGF-C, VEGF-D) acting on their receptor, VEGF-R3, and this does occur in the kidney (1, 12, 14). A previous study determined that transforming growth factor-β (TGF-β) can induce VEGF-C production by epithelial cells as well as monocyte-derived macrophages, leading to lymphangiogenesis (23). However, little is known about how lymphatics are altered in hypertension.
Previous papers have detailed how skin and cardiac lymphatics are altered in the context of hypertension. The lack of lymphangiogenesis in dermal interstitium was determined to lead to salt storage and salt-sensitive hypertension (17, 26). In both mice and rats, it was demonstrated that hypertension induced by 2 wk of high-salt feeding elicited lymphatic hyperplasia in the ear and hindlimb as well as increased contractions in the inguinal afferent and efferent lymphatic vessels (15). In the heart, induced lymphangiogenesis via systemic VEGF-C gene delivery was reported to decrease macrophage infiltration, detrimental left ventricular remodeling, and blood pressure during high-salt feeding in SHR rats, whereas inhibiting lymphangiogenesis resulted in increased macrophage infiltration, myocardial fibrosis, and blood pressure (27). It was not determined how these lymphangiogenesis manipulations administered intravenously affected renal lymphatics. Given the important role of activated immune cell infiltration into the kidney in the development of hypertension, we determined how renal lymphatic vessels are altered in the setting of hypertension and renal injury. We hypothesized that increased renal lymphatic vessel density would be associated with macrophage infiltration, inflammation, injury, and hypertension.
MATERIALS AND METHODS
Animals and treatments.
Frozen kidney samples and paraffin-embedded blocks from WKY/SHR rats and Fischer 344 rats were provided by Drs. Doris and Parrish, respectively. Each group and time point consisted of a frozen kidney sample and a paraffin-embedded block of kidney from each animal, and the number of animals in each group were as follows: WKY-18 wk = 3, WKY-40 wk = 3, SHR-B2-18 wk = 3, SHR-B2-40 wk = 3, SHR-A3-18 wk = 3, SHR-A3-40 wk = 3, Fischer-4 mo = 5, Fischer-16 mo = 4, Fischer-20 mo = 4, Fischer-24 mo = 4, Fischer-24 mo-calorie restricted = 4. The animals and treatments have been described in their respective publications (4, 7). The use of animals was approved by the IACUC of the respective institution and all procedures adhered to APS’s Guiding Principles in the Care and Use of Vertebrate Animals and Training.
Lymphatic vessel immunofluorescence.
Paraffin-embedded blocks were cut into 5-μm sections using a microtome. Unstained slides were deparaffinized and rehydrated according to the following: 4 min of xylene twice, 3 min of 50% xylene and EtOH, 3 min of EtOH, 3 min of 95% EtOH and water, 3 min of 70% EtOH and water, 3 min of 50% EtOH and water, and 2 min of water. Following deparaffinization and rehydration, slides were permeabilized with TBS-Triton and rinsed with PBS-Tween. Slides were then blocked with Aquablock and left overnight at 4° with the following primary antibodies: podoplanin 1:100 (R&D Systems), CD68 (KP1) 1:100 (Santa Cruz Biotechnology), and LYVE-1 1:100 (R&D Systems). Washes were performed with TBS-Tween, and the following secondary antibodies were added for 1 h: donkey anti-goat 1:100 (Life Technologies), goat anti-mouse 1:100 (Life Technologies), and donkey anti-sheep IgG Northern Lights 1:100 (R&D Systems). All slides were viewed on an Olympus Bx-41 equipped with an Olympus Q5 camera, and images were taken using the Olympus cellSens software. Quantification of lymphatic vessel density was performed by counting vascular structures (rounded with a lumen) that were positive for fluorescent staining. Three different people agreed on the fields of view (FOVs), which were predetermined, and then all three people counted vessel-like structures (rounded with a lumen similar to those described previously) (12, 14). The total numbers from all three FOVs were averaged between the three investigators and then compared between groups. To normalize the FOVs and eliminate any area bias, the renal artery was identified as the first landmark and centered in FOV1. The number of lymphatic vessels around the renal artery was counted by all three people. Then the FOV was moved directly up until the last part of the tissue section was at the top of FOV2 so that this contained one area of the renal cortex. After the counting by all three investigators, the FOV was moved back down to FOV1 with the renal artery centered; then the FOV was moved to the right until the edge of the tissue was on the right edge of the FOV, and then the lymphatic structures were counted in the renal cortex of this FOV3. If tissue did not completely fill FOV2 or FOV3, the FOV was moved back toward FOV1 until the entire FOV was full of tissue.
Immunoblotting.
Frozen kidneys were placed into liquid nitrogen and crushed with a mortar and pestle. The samples were then treated with RIPA lysis and extraction buffer and centrifuged at 14,000 revolution/min for 10 min. Protein concentration was determined by the Bradford assay using bovine serum albumin as the standard and measured with a spectrometer. SDS sample buffer was added, and protein samples were heated to 100°C for 10 min. Renal homogenates (50 μg for VEGF-C and 50 μg for VEGF-R3) were separated by electrophoresis on Novex 4–12% Bis-Tris Gels (Life Technologies) and then transferred to Immobilon-FL PVDF membranes (Millipore) overnight at 4°C. Western blot analyses were performed using the following primary antibodies: VEGF-C 1:500 (Santa Cruz Biotechnology), VEGF-R3 1:500 (Santa Cruz Biotechnology), and β-actin 1:5,000 (Sigma). Secondary antibodies consisted of anti-mouse and anti-rabbit IgGs conjugated to Alexa-Flour 680 or IR800Dye (LI-COR Biosciences). The bands were identified using infrared visualization (Odyssey System, LI-COR Biosciences), and densitometry was performed using the Odyssey software.
Quantitative real-time PCR.
Total RNA from RNALater-stabilized kidneys was extracted using an RNeasy Mini Isolation Kit (Qiagen), and RNA yield and purity were determined using a NanoDrop spectrophotometer. cDNA was synthesized using the RT2 First Strand Kit (Qiagen) in which 0.5 μg of total RNA was used for reverse transcription for each sample. Per the manufacturer’s protocol, SABiosciences RT2 SYBR Green ROX qPCR Mastermix, nuclease-free water, and primers (10 μM) were used in creating assay-specific premixes. Specific rat primers were obtained from PrimerBank using NCBI Gene IDs and were as follows: Rpl19 forward 5′ ACAGAAATGGCATCAAGAAACCC 3′, reverse 5′ TCTTGTTGTGCTTCTTGGCAAA 3′; Lyve1 forward 5′ AGGCTGAAGTTTAGGTGCACGAGA 3′, reverse 5′ GAGCCAACAGTGGCTTGCTTCTTT 3′; Gmcsf forward 5′ GACCATGATAGCCAGCCACT 3′, reverse 5′ TTCCAGCAGTCAAAAGGGATA 3′; Mcp1 forward 5′ GCTGCTACTCATTCACTGGCAA 3′, reverse 5′ TGCTGCTGGTGATTCTCTTGTA 3′; Ifng forward 5′ CAGCTGTCACCAGATCTAGCC 3′, reverse 5′ ATTGGCACACTCTCTACCCCAG 3′; and Il6 forward 5′ TCCTACCCCAACTTCCAATGCTC 3′, reverse 5′ TTGGATGGTCTTGGTCCTTAGCC 3′. Amplification was performed in a 25-μl reaction volume. Reactions were carried out in a 96-well optical reaction plate using an Agilent AriaMx Real-Time PCR Detection System. Thermal conditions proceeded with an initial 10-min incubation at 95°C followed by 40 cycles of amplification: 95°C for 15 s, 55°C for 30 s, and lastly 72°C for 30 s. Dissociation curves for each primer were verified to have single peaks. Fold changes in gene expression were calculated using the −ΔΔCt method. The expression of target genes was normalized by comparing the raw Ct values of the samples to those of the reference gene, Rpl19.
Statistical analyses.
Results are presented as means ± SE. For multiple comparisons, an ANOVA was performed followed by the Student's-Newman-Keuls post hoc test. The significance level was set at 0.05. All analyses were performed using SigmaStat software.
RESULTS
Increased renal lymphatic vessel density in hypertensive SHR-A3 rats but not SHR-B2 rats.
Renal lymphatic vessel density, quantitated by three observers counting tubular, lumen-containing structures with positive staining for the lymphatic endothelial cell marker podoplanin, was decreased significantly in kidneys from male SHR-B2 rats at 18 wk of age, whereas there was no difference in male SHR-A3 rats compared with male WKY rats (Fig. 1, A, C, and E). At 40 wk of age, SHR-A3 rats had significantly increased renal lymphatic vessel density compared with WKY controls, whereas SHR-B2 rats continued to have significantly less renal lymphatic vessel density (Fig. 1, B, D, and F). The quantification is provided in Fig. 1M. In support of the increased renal lymphatic vessel density in SHR-A3 rats at 40 wk of age, renal mRNA expression of the lymphatic endothelial cell marker lymphatic vessel endothelium hyaluronan receptor 1 (LYVE-1) was also increased significantly (Fig. 1N). Monocytes and macrophages, evident by positive CD68 staining, were increased in kidneys from SHR-A3 rats at 40 wk of age but not in SHR-B2 rats, consistent with previous reports (Fig. 1, G–L) (3, 4).
Fig. 1.
Podoplanin-positive lymphatic vessels in kidneys from young and aged WKY, SHR-B2, and SHR-A3 rats. A–F: representative images exhibit red podoplanin staining of lymphatic vessels in kidneys from 18-wk-old and 40-wk-old WKY, SHR-B2, and SHR-A3 rats. G–L: images show the same red podoplanin staining designated by white arrows but with an overlay containing the macrophage marker CD68 (green) designated by pink arrows and the nucleus stain DAPI (blue). Magnification is ×40, and the scale bar = 50 µm. M: renal lymphatic vessel density, determined by the average number of lumen-containing lymphatic vessels from 3 fields of view (FOVs) counted by 3 investigators, is decreased significantly in SHR-B2 rats compared with WKY rats, whereas SHR-A3 rats had significantly increased renal lymphatic vessel density at 40 wk of age. N: Lyve1 mRNA expression, determined by real-time qPCR, is increased significantly in SHR-A3 rats at 40 wk of age. *P < 0.05. The numbers of animals in each group were as follows: WKY-18 wk = 3, WKY-40 wk = 3, SHR-B2-18 wk = 3, SHR-B2-40 wk = 3, SHR-A3-18 wk = 3, SHR-A3-40 wk = 3.
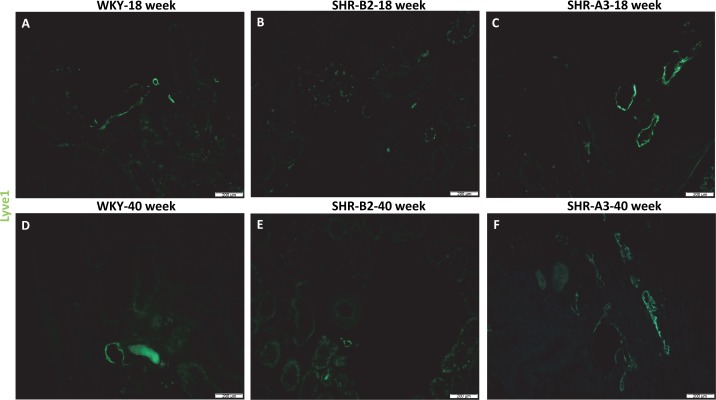
To confirm that podoplanin-positive vascular structures were indeed lymphatic vessels, serial kidney sections were stained for LYVE-1. Although the detailed quantification that was performed in Fig. 1 for podoplanin staining was not replicated for LYVE-1 because the staining was almost identical, the same trend was observed, as there was increased LYVE-1-positive vessel density in SHR-A3 rats at 40 wk of age compared with WKY controls (Fig. 2, F vs. D); SHR-B2 had decreased LYVE-1-positive vessel density compared with WKY controls at both 18 and 40 wk of age (Fig. 2, B and E, vs. Fig. 2, A and D), confirming the podoplanin data.
Fig. 2.
LYVE1-positive lymphatic vessels in kidneys from young and aged WKY, SHR-B2, and SHR-A3 rats. A–F: representative images show green LYVE-1 staining of lymphatic vessels in kidneys from 18-wk-old and 40-wk-old WKY, SHR-B2, and SHR-A3 rats. Similar to Fig. 1, subjectively there are reduced numbers of LYVE-1+ lymphatic vessels in SHR-B2 rats but increased size and numbers in SHR-A3 rats at 18 wk and 40 wk of age. Magnification is ×10, and the scale bar = 200 µm. The numbers of animals in each group were as follows: WKY-18 wk = 3, WKY-40 wk = 3, SHR-B2-18 wk = 3, SHR-B2-40 wk = 3, SHR-A3-18 wk = 3, SHR-A3-40 wk = 3.
Increased renal expression of lymphangiogenesis markers and proinflammatory cytokines in SHR-A3 rats.
We next measured protein expression of the lymphangiogenesis signal VEGF-C and its receptor VEGF-R3 in kidneys from WKY, SHR-B2, and SHR-A3 rats at 18 and 40 wk of age. Kidneys from SHR-A3 rats at 18 wk of age had significantly increased levels of both VEGF-C (Fig. 3, A and B) and VEGF-R3 (Fig. 3, C and D) compared with both WKY and SHR-B2 rats. There were no differences in VEGF-C or VEGF-R3 expression between SHR-B2 and WKY controls at any age (Fig. 3, A and B, as well as Fig. 3, C and D, respectively).
Fig. 3.
Lymphangiogenic protein levels in whole kidneys from WKY, SHR-B2, and SHR-A3 rats. Vascular endothelial growth factor C (VEGF-C) (A) and its receptor VEGF-R3 (C), involved in lymphangiogenesis, are increased significantly in kidneys from SHR-A3 rats at 18 wk of age (*P < 0.05 vs. WKY-18 wk). B and D: actin was used as a loading control, and densitometry was quantitated as a ratio of protein/actin and expressed as a percentage of WKY-18 wk controls on each immunoblot and then averaged. The numbers of animals in each group were as follows: WKY-18 wk = 3, WKY-40 wk = 3, SHR-B2-18 wk = 3, SHR-B2-40 wk = 3, SHR-A3-18 wk = 3, SHR-A3-40 wk = 3.
To confirm the proinflammatory state of the kidneys in SHR-A3 rats, which is absent in SHR-B2 rats (3, 4), we measured mRNA expression of several proinflammatory cytokines. Kidneys from SHR-A3 rats at both 18 and 40 wk of age had significantly increased mRNA levels of granulocyte macrophage colony-stimulating factor (GM-CSF; Fig. 4A), monocyte chemoattractant protein-1 (MCP-1; Fig. 4B), interferon-γ (IFN-γ; Fig. 4C), and interleukin 6 (IL-6; Fig. 4D) compared with WKY controls. There were no differences in mRNA expression of proinflammatory cytokines between SHR-B2 and WKY controls at any age (Fig. 4, A–D).
Fig. 4.
mRNA expression levels of proinflammatory cytokines in whole kidneys from WKY, SHR-B2, and SHR-A3 rats at 18 and 40 wk of age. Gmcsf (A), Mcp1 (B), Ifng (C), and Il6 (D) mRNA expression levels were increased significantly in kidneys from SHR-A3 rats at both 18 and 40 wk of age (*P < 0.05 vs. WKY-18 wk). The numbers of animals in each group were as follows: WKY-18 wk = 3, WKY-40 wk = 3, SHR-B2-18 wk = 3, SHR-B2-40 wk = 3, SHR-A3-18 wk = 3, SHR-A3-40 wk = 3.
Increased renal lymphatic vessel density in aged Fischer 344 rats.
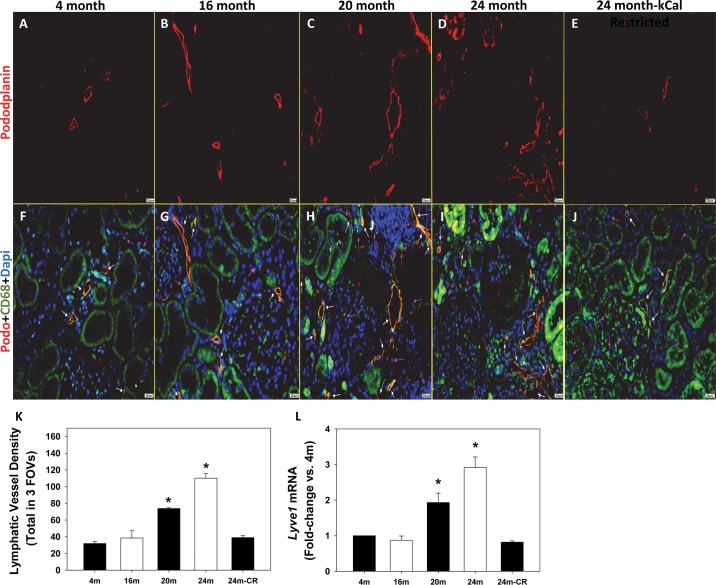
We next obtained kidneys from aged male Fischer 344 rats to determine whether renal lymphangiogenesis occurs in rats that develop age-related nephropathy but not hypertension. Kidneys from 20-mo-old and 24-mo-old Fischer rats had significantly increased podoplanin-positive lymphatic vessel density compared with 4-mo-old controls (Fig. 5, C and D, vs. 5A). There were no differences in renal podoplanin-positive lymphatic vessel density at 16 mo of age compared with 4-mo-old controls (Fig. 5, B vs. A), and calorie restriction, which prevents the renal inflammation and injury as described previously (7), also prevented the increase in lymphatic vessel density in 24-mo-old Fischer rats (Fig. 5E). The quantification is provided in Fig. 5K. Lyve1 mRNA expression was also increased significantly in 20-mo-old and 24-mo-old Fischer rats (Fig. 5L). Monocyte and macrophage CD68 stainings were confirmed to be increased in kidneys from 20-mo-old and 24-mo-old Fischer rats (Fig. 5, F–J), consistent with a previous report (7). Again, although not quantitated like the podoplanin staining, the same trends were observed in serial sections stained for LYVE-1 (Fig. 6, A–E).
Fig. 5.
Podoplanin-positive lymphatic vessels in kidneys from Fischer rats at 4, 16, 20, and 24 mo of age without or with calorie restriction (CR). A–E: representative images exhibit red podoplanin staining of lymphatic vessels in kidneys from Fischer rats. F–J: images show the same red podoplanin staining designated by white arrows but with an overlay containing the macrophage marker CD68 (green) designated by pink arrows and the nucleus stain DAPI (blue). Magnification is ×40, and the scale bar = 20 µm. K: renal lymphatic vessel density, determined by the average number of lumen-containing lymphatic vessels from 3 FOVs counted by 3 investigators, is increased significantly in 20-mo-old and 24-mo-old Fischer rats compared with 4-mo-old Fischer rats. L: Lyve1 mRNA expression, determined by real-time qPCR, is increased significantly in 20-mo-old and 24-mo-old Fischer rats. *P < 0.05. The numbers of animals in each group were as follows: Fischer-4 mo = 5, Fischer-16 mo = 4, Fischer-20 mo = 4, Fischer-24 mo = 4, Fischer-24 mo-calorie restricted = 4.
Fig. 6.
LYVE1-positive lymphatic vessels in kidneys from Fischer rats at 4, 16, 20, and 24 mo of age without or with CR. A–E: representative images show green LYVE-1 staining of lymphatic vessels in kidneys from Fischer rats. Similar to Fig. 5, subjectively there are increased numbers of LYVE-1+ lymphatic vessels in 20-mo-old and 24-mo-old Fischer rats compared with 4-mo-old controls. Magnification is ×10, and the scale bar = 50 µm. The numbers of animals in each group were as follows: Fischer-4 mo = 5, Fischer-16 mo = 4, Fischer-20 mo = 4, Fischer-24 mo = 4, Fischer-24 mo-calorie restricted = 4.
Increased renal expression of lymphangiogenesis markers and proinflammatory cytokines in aged Fischer 344 rats.
Kidneys from 20-mo-old Fischer rats had significantly increased levels of both VEGF-C (Fig. 7, A and B) and VEGF-R3 (Fig. 7, C and D) compared with 4-mo-old controls. No other time points were significantly different from 4-mo-old controls for either VEGF-C or VEGF-R3 (Fig. 7, A and B, as well as Fig. 7, C and D, respectively).
Fig. 7.
Lymphangiogenic protein levels in whole kidneys from Fischer rats at 4, 16, 20, and 24 mo of age without or with CR. VEGF-C (A) and its receptor VEGF-R3 (C) are increased significantly in kidneys from Fischer rats at 20 mo of age (*P < 0.05 vs. 4-mo-old rats). B and D: actin was used as a loading control, and densitometry was quantitated as a ratio of protein/actin and expressed as a percentage of Fischer 4-mo-old controls on each immunoblot and then averaged. The numbers of animals in each group were as follows: Fischer-4 mo = 5, Fischer-16 mo = 4, Fischer-20 mo = 4, Fischer-24 mo = 4, Fischer-24 mo-calorie restricted = 4.
Similar to the SHR-A3 data, mRNA expression levels of GM-CSF (Fig. 8A) and MCP-1 (Fig. 8B) were increased significantly in 20-mo-old and 24-mo-old Fischer rats compared with 4-mo-old controls. IFN-γ mRNA levels were increased significantly at 16 mo, 20 mo, and 24 mo of age (Fig. 8C), whereas IL-6 mRNA expression was only increased significantly at 20 mo of age (Fig. 8D).
Fig. 8.
mRNA expression levels of proinflammatory cytokines in whole kidneys from Fischer rats at 4, 16, 20, and 24 mo of age without or with CR. Gmcsf (A) and Mcp1 (B) mRNA expression levels were increased significantly in kidneys from Fischer rats at both 20 and 24 mo of age (*P < 0.05 vs. Fischer 4-mo-old controls). Ifng expression levels (C) were increased significantly in kidneys from Fischer rats at 16, 20, and 24 mo of age, whereas renal Il6 expression levels (D) were increased significantly only at 20 mo of age (*P < 0.05 vs. 4 mo of age). The numbers of animals in each group were as follows: Fischer-4 mo = 5, Fischer-16 mo = 4, Fischer-20 mo = 4, Fischer-24 mo = 4, Fischer-24 mo-calorie restricted = 4.
DISCUSSION
The major findings of the present study are that 1) SHR-A3 rats that develop hypertension and renal injury experience renal macrophage infiltration and increased lymphatic vessel density, 2) SHR-B2 rats that are hypertensive but resistant to renal injury do not have renal macrophage infiltration and increased lymphatic vessel density, and 3) aged Fischer rats develop renal inflammation and injury but not hypertension and demonstrate renal macrophage infiltration and increased lymphatic vessel density.
The differences between the renal injury-prone SHR-A3 and the injury-resistant SHR-B2 strains were reported to relate to immunity and inflammation (4). The lack of immune cell infiltration despite the hypertension in SHR-B2 rats is likely the root cause for the lack of lymphangiogenesis and even a decrease in renal lymphatic vessel density. CD68+ macrophages, which are known to produce VEGF-C, whereas T and B cells do not, were evident at high levels in the kidneys from SHR-A3 and Fischer rats but absent in the kidneys from SHR-B2 rats. This is consistent with the renoprotective and antihypertensive effects of mycophenolate mofetil, which reduces proinflammatory CD68+ macrophage infiltration into the kidney and ameliorates renal injury (2, 4, 13, 20). Together these data suggest that improving the efficiency of lymphatic vessels in removing activated immune cells during inflammation and hypertension should be renoprotective and possibly lower blood pressure.
Renal lymphangiogenesis occurs as a response to interstitial immune cell and fluid accumulation over time, as it is not evident in acute kidney injury/nephritis (9, 29). Renal lymphangiogenesis has been reported to occur in numerous chronic kidney diseases, including diabetic nephropathy, lupus nephritis, antineutrophil cytoplasmic antibody-related glomerulonephritis, tubulointerstitial nephritis, and IgA nephropathy (9, 21). However, lymphangiogenesis may not always be beneficial, as the inflammatory milieu has been reported to inhibit lymphatic vessel contractions (16). Dysfunctional lymphangiogenesis or ligation of renal lymphatics is sufficient to induce proteinuria, tubulointerstitial fibrosis, and mesangial expansion within weeks (28). Nonetheless, a study demonstrated that increasing VEGF-C levels in SHR rats given a high-salt diet lowered blood pressure, and conversely inhibiting VEGF-R3 signaling increased blood pressure (27). As stated by Seeger et al. (22), the functional role of the newly formed lymphatic vessels likely depends on the timing of the inflammation and tissue injury with lymphangiogenesis being beneficial to help remove activated immune cells but detrimental when they further enable immune activation in the lymph node, resulting in augmentation of interstitial inflammation. Studies are presently underway to determine whether reducing or augmenting lymphatic vessels in the kidney during hypertension is beneficial or detrimental. We believe that the formation of new lymphatic vessels in the kidney in response to a hypertensive, inflammatory setting is a physiological response to both the local inflammation and provides additional exit routes for interstitial immune cells in an effort to restore the tissue back to health. However, this expansion is beneficial only when the lymphatics are operating efficiently. If we can improve lymphatic-mediated immune cell exfiltration from the kidney either by improving lymphatic efficiency or augmenting their numbers, then we would expect to see a reduction in blood pressure.
It is tempting to conclude that renal lymphangiogenesis is blood pressure independent and only dependent on macrophage infiltration and inflammation based on the present data from SHR-B2 and Fischer rats; however, these strains are resistant to the development of renal injury and hypertension, respectively. The more likely scenario in experimental hypertension in control animals and in patients with hypertension is that subclinical renal inflammation and injury lead to lymphangiogenesis, overt renal dysfunction, and hypertension, which may propagate each other. This is evident in most chronic nephropathies, as they are progressive and are associated with the development or amplification of hypertension.
In conclusion, this study demonstrates that increased renal lymphatic vessel density is associated with CD68+ macrophage infiltration, inflammation, injury, and hypertension in rats. A limitation of the study was that we were not able to determine definitively the number and de novo state of individual lymphatic vessels characteristic of lymphangiogenesis. Also, whether renal lymphangiogenesis occurs in other rat models of hypertension (i.e., Dahl salt-sensitive rats, angiotensin II-infused rats, DOCA-salt-treated rats, gestational hypertensive rats, etc.) needs to be examined and compared with the present findings in this genetic model of hypertension. Last, the functional role of these newly formed lymphatic vessels needs to be determined as well as whether increased removal of activated immune cells from the kidney can be protective and lower blood pressure.
GRANTS
This study was supported by National Institutes of Health Grants RO1 AG034154 to A. Parrish and NIH RO1 DK069632 to P. Doris.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
SCK, LEP, KRH, KMB, CALG performed the experiments and helped write the manuscript; JMR, ARP, PAD, BMM provided the tissue samples and reagents, consulted on data analysis, and helped write the manuscript.
ACKNOWLEDGMENTS
The authors thank the personnel of Dr. A. R. Parrish’s laboratory and Dr. P. A. Doris’s laboratory for producing the respective tissue used in the present studies.
REFERENCES
- 1.Alitalo K. The lymphatic vasculature in disease. Nat Med 17: 1371–1380, 2011. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 2.Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus 14, Suppl 1: s2–s8, 2005. doi: 10.1191/0961203305LU2109OA. [DOI] [PubMed] [Google Scholar]
- 3.Bell R, Herring SM, Gokul N, Monita M, Grove ML, Boerwinkle E, Doris PA. High-resolution identity by descent mapping uncovers the genetic basis for blood pressure differences between spontaneously hypertensive rat lines. Circ Cardiovasc Genet 4: 223–231, 2011. doi: 10.1161/CIRCGENETICS.110.958934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun MC, Herring SM, Gokul N, Monita M, Bell R, Zhu Y, Gonzalez-Garay ML, Wenderfer SE, Doris PA. Hypertensive renal injury is associated with gene variation affecting immune signaling. Circ Cardiovasc Genet 7: 903–910, 2014. doi: 10.1161/CIRCGENETICS.114.000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goulopoulou S, Matsumoto T, Bomfim GF, Webb RC. Toll-like receptor 9 activation: A novel mechanism linking placenta-derived mitochondrial DNA and vascular dysfunction in pre-eclampsia. Clin Sci (Lond) 123: 429–435, 2012. doi: 10.1042/CS20120130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunz-Borgmann EA, Nichols LA, Wiedmeyer CE, Spagnoli S, Trzeciakowski JP, Parrish AR. Structural equation modeling identifies markers of damage and function in the aging male Fischer 344 rat. Mech Ageing Dev 156: 55–62, 2016. doi: 10.1016/j.mad.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 311: F555–F561, 2016. doi: 10.1152/ajprenal.00594.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heller F, Lindenmeyer MT, Cohen CD, Brandt U, Draganovici D, Fischereder M, Kretzler M, Anders HJ, Sitter T, Mosberger I, Kerjaschki D, Regele H, Schlöndorff D, Segerer S. The contribution of B cells to renal interstitial inflammation. Am J Pathol 170: 457–468, 2007. doi: 10.2353/ajpath.2007.060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17, Suppl 3: S218–S225, 2006. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 11.Huang B, Cheng Y, Usa K, Liu Y, Baker MA, Mattson DL, He Y, Wang N, Liang M. Renal tumor necrosis factor α contributes to hypertension in dahl salt-sensitive rats. Sci Rep 6: 21960, 2016. doi: 10.1038/srep21960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa Y, Akasaka Y, Kiguchi H, Akishima-Fukasawa Y, Hasegawa T, Ito K, Kimura-Matsumoto M, Ishiguro S, Morita H, Sato S, Soh S, Ishii T. The human renal lymphatics under normal and pathological conditions. Histopathology 49: 265–273, 2006. doi: 10.1111/j.1365-2559.2006.02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang S, Tang Q, Rong R, Tang L, Xu M, Lu J, Jia Y, Ooi Y, Hou J, Guo J, Yang B, Zhu T. Mycophenolate mofetil inhibits macrophage infiltration and kidney fibrosis in long-term ischemia-reperfusion injury. Eur J Pharmacol 688: 56–61, 2012. doi: 10.1016/j.ejphar.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, Laakkonen P, Petrova T, Langer B, Raab I. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol 15: 603–612, 2004. doi: 10.1097/01.ASN.0000113316.52371.2E. [DOI] [PubMed] [Google Scholar]
- 15.Kwon S, Agollah GD, Chan W, Sevick-Muraca EM. Altered lymphatic function and architecture in salt-induced hypertension assessed by near-infrared fluorescence imaging. J Biomed Opt 17: 080504–1, 2012. doi: 10.1117/1.JBO.17.8.080504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang Q, Ju Y, Chen Y, Wang W, Li J, Zhang L, Xu H, Wood RW, Schwarz EM, Boyce BF, Wang Y, Xing L. Lymphatic endothelial cells efferent to inflamed joints produce iNOS and inhibit lymphatic vessel contraction and drainage in TNF-induced arthritis in mice. Arthritis Res Ther 18: 62, 2016. doi: 10.1186/s13075-016-0963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15: 545–552, 2009. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 18.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez-Iturbe B, Quiroz Y, Ferrebuz A, Parra G, Vaziri ND. Evolution of renal interstitial inflammation and NF-kappaB activation in spontaneously hypertensive rats. Am J Nephrol 24: 587–594, 2004. doi: 10.1159/000082313. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chávez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 282: F191–F201, 2002. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto I, Ito Y, Mizuno M, Suzuki Y, Sawai A, Tanaka A, Maruyama S, Takei Y, Yuzawa Y, Matsuo S. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int 75: 828–838, 2009. doi: 10.1038/ki.2008.661. [DOI] [PubMed] [Google Scholar]
- 22.Seeger H, Bonani M, Segerer S. The role of lymphatics in renal inflammation. Nephrol Dial Transplant 27: 2634–2641, 2012. doi: 10.1093/ndt/gfs140. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Ito Y, Mizuno M, Kinashi H, Sawai A, Noda Y, Mizuno T, Shimizu H, Fujita Y, Matsui K, Maruyama S, Imai E, Matsuo S, Takei Y. Transforming growth factor-β induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int 81: 865–879, 2012. doi: 10.1038/ki.2011.464. [DOI] [PubMed] [Google Scholar]
- 24.Tinsley JH, Chiasson VL, Mahajan A, Young KJ, Mitchell BM. Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens 22: 1314–1319, 2009. doi: 10.1038/ajh.2009.185. [DOI] [PubMed] [Google Scholar]
- 25.Tinsley JH, Chiasson VL, South S, Mahajan A, Mitchell BM. Immunosuppression improves blood pressure and endothelial function in a rat model of pregnancy-induced hypertension. Am J Hypertens 22: 1107–1114, 2009. doi: 10.1038/ajh.2009.125. [DOI] [PubMed] [Google Scholar]
- 26.Wiig H, Schröder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Müller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest 123: 2803–2815, 2013. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang GH, Zhou X, Ji WJ, Zeng S, Dong Y, Tian L, Bi Y, Guo ZZ, Gao F, Chen H, Jiang TM, Li YM. Overexpression of VEGF-C attenuates chronic high salt intake-induced left ventricular maladaptive remodeling in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 306: H598–H609, 2014. doi: 10.1152/ajpheart.00585.2013. [DOI] [PubMed] [Google Scholar]
- 28.Zhang T, Guan G, Liu G, Sun J, Chen B, Li X, Hou X, Wang H. Disturbance of lymph circulation develops renal fibrosis in rats with or without contralateral nephrectomy. Nephrology (Carlton) 13: 128–138, 2008. doi: 10.1111/j.1440-1797.2007.00851.x. [DOI] [PubMed] [Google Scholar]
- 29.Zimmer JK, Dahdal S, Mühlfeld C, Bergmann IP, Gugger M, Huynh-Do U. Lymphangiogenesis is upregulated in kidneys of patients with multiple myeloma. Anat Rec (Hoboken) 293: 1497–1505, 2010. doi: 10.1002/ar.21189. [DOI] [PubMed] [Google Scholar]