Abstract
Investigating enteric neuromuscular function poses specific challenges that are not encountered in other systems. The gut has a complex cellular composition, and methods to study diverse multicellular interactions during physiological gut functions have been limited. However, new technologies are emerging in optics, genetics, and bioengineering that greatly expand the capabilities to study integrative functions in the gut. In this mini-review, I discuss several areas where the application of these technologies could benefit ongoing efforts to understand enteric neuromuscular function. I specifically focus on technologies that can be applied to study specific cellular networks and the mechanisms that link activity to function.
the neural control of gut smooth muscle is a fundamental process that governs intestinal motility. Neural reflexes controlling motility are contained within the myenteric plexus of the enteric nervous system (ENS), and these intrinsic circuits are capable of coordinating basic gut functions in the absence of input from the brain or spinal cord. In this regard, the ENS can be viewed as the “sheriff” that enforces the “law of the intestine” described by Bayliss and Starling (5, 6). Some aspects of the neural control of gut motility are now well described such as the essential nature of the ENS and the physiology of the main populations of enteric neurons. What is much less clear is how homo- and heterotypic interactions between enteric neurons and the many other cell types involved form the basis of functional reflexes. For example, our understanding of the ENS at the network level is still relatively poor, and the roles of key nonneuronal cells such as interstitial cells of Cajal (ICC), PDGFRα+ cells, enterochromaffin cells, and enteric glia are still debated.
Addressing some of these outstanding questions requires the application of new technologies that will allow investigators to probe the functions of defined populations of cells in physiological settings. Fortunately, intense interest in functional networks in the brain has driven the development of new techniques to study the activity of large ensembles of cells and methods to manipulate cellular function that exhibit high spatial and temporal resolution. Many of these technologies are transferrable to gastrointestinal research, but their adoption has been relatively slow. The goal of this brief review is to introduce a handful of current technologies that would be useful in answering key questions in enteric neuroscience.
What Do We Know Now?
The basic enteric reflex that controls intestinal motility is likely the most well-studied aspect of the ENS to date. This has led to a detailed understanding of the neurochemical coding and electrophysiological properties of individual subtypes of enteric neurons in several species, and readers are referred to several excellent books (12, 50) and reviews (11, 13, 47) for details. Based on the information from individual cells, one can construct a simple model of the enteric motility reflex that resembles a spinal reflex arc. According to this model, mucosal stimuli activate the processes of afferent neurons located in the myenteric plexus. Afferent neuron depolarization excites both ascending and descending interneurons that, in turn, activate excitatory motor neurons above, and inhibitory motor neurons below, the point of stimulation. This model is very useful to illustrate the basic concept of the motility reflex, but we know now that the circuitry is actually much more complex and involves interactions with enterochromaffin cells at the level of the mucosa, processing within the plexus that involves multiple types of neurons and glia, and interactions with ICCs, PDGFRα+ cells, and smooth muscle cells that form the SIP (Smooth muscle, ICC, PDGFRα+) syncytium (39).
What Are the Questions, and What Tools Do We Need to Answer Them?
In general, we still have a very poor understanding of how enteric neurons function together as a network and how they interact with other cell types. New tools are needed to both investigate questions regarding network activity as well as address outstanding debates in the field regarding the roles of EC cells, ICCs, PDGFRα+ cells, and glia. Clarifying these issues will yield novel insight into how the ENS process signals and subsequently modulates effector tissues. Understanding these processes is an essential first step in the development of more effective therapies for motility disorders. In the following sections, I will highlight a few key broad questions and describe some of the available tools that would help to address these issues.
Who Is Active and When?
One of the major hurdles in understanding ENS function at the network level has been the lack of appropriate techniques to record from large ensembles of cells. Single cell electrophysiological recordings have formed the fundamentals of neurogastroenterology (24, 33, 48, 49), and the importance of this technique cannot be overemphasized. Electrophysiology gives fine resolution information about individual excitable cells at a level of detail that is still far superior to other methods. The types of information that can be acquired by this technique include passive and active membrane characteristics, ionic properties, synaptic properties, and levels of excitability. However, single cell electrophysiological recordings are extremely low throughput, they are difficulty to learn, and they involve random impalement of neurons, interstitial cells, or muscle. Likewise, using this technique to understand network dynamics is not feasible, and new approaches are needed. The introduction of calcium and voltage indicator dyes partially alleviated this issue by allowing investigators to simultaneously record the activity of many cells within a ganglion and even across several ganglia (32, 44). However, these techniques are not without their shortcomings. Dye loading can be temperamental, and bulk loading is not cell type specific. It also requires tissue dissection and is not reliable for in vivo recordings of network activity in the gut.
To circumvent these issues, the field is increasingly turning to genetically-encoded fluorescent indicators (Fig. 1). As with other branches of optogenetics, their use has become widespread over the past two decades, and their application for large-scale recordings of neural activity in the brain has been transformative for nervous system research. What makes this approach particularly useful is the ability to encode indicator expression in a cell type-specific manner and the fact that genetically encoded indicators typically have optical reporting properties that are superior to organic indicator dyes (26). Several different variants of fluorescent protein indicators have been developed for the detection of vesicle release (38), neurotransmitters (25), transmembrane voltage (23, 41), and intracellular calcium (14, 26), and a variety of transgenic mouse lines and viral delivery systems are readily available to drive expression. When combined with the appropriate microscopy technique, optical indicators enable investigators to evaluate the activity of thousands, if not millions, of cells simultaneously. For example, Yu Shin Kim and colleagues recently used mice that express a genetically encoded calcium indicator (GECI) in sensory neurons to simultaneously monitor the activity of over 1,600 neurons per dorsal root ganglion in vivo (21). Other groups have used similar techniques to simultaneously record from several thousand neurons in the brains of awake, behaving mice (40) and from all neurons within the brain of larval zebrafish (1).
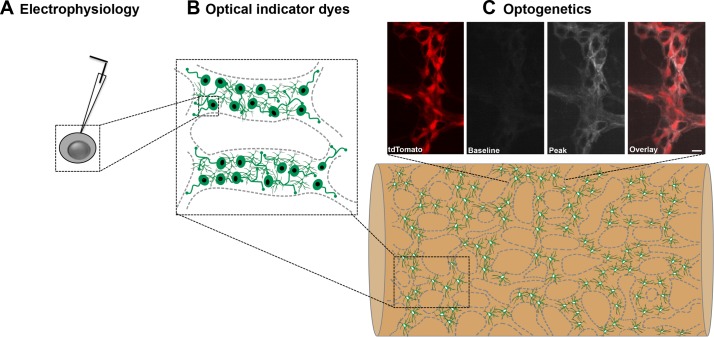
Fig. 1.
The progression of methods to study large networks of cells. A: traditional electrophysiological recordings provide fine detail information about single cells. B: optical imaging dyes can be used to study the activity of cells in one, or several, ganglia in isolated tissue preparations. C: optogenetic reporters can be expressed in specific cells and used to study network activity in intact tissue or in vivo. C, top: representative recordings from mice expressing the optogenetic reporter GCaMP5g (grayscale) and tdTomato (red) in enteric glial cells [from McClain and Gulbransen (30)]. Only one myenteric ganglion is shown here for clarity but the same technique could be used to study large areas of the ENS during physiological functions.
Adopting these techniques to study functional cellular networks in the gut is relatively straightforward, and several groups have already demonstrated the feasibility of using genetically encoded indicators to record the activity of enteric neurons (7, 17, 36), glia (7, 17, 30), and ICCs (3). GECIs are by far the most widely used method to date in the gut. This is largely driven by the fact that the GECI field is more mature compared with other genetically encoded indicators and because of the physiological importance of calcium as a second messenger in excitable cells. Studies using GECIs in the gut have taken advantage of several commercially available mouse lines that allow investigators to express GECIs, such as GCaMP3 (3, 7, 17) or GCaMP5 (30) in specific cells using a Cre-loxP system. Current work with these models has been mostly limited to investigating cellular responses in whole mount preparations of intestine, and the results are very promising (3, 7, 17, 30). Under these conditions, the specific cellular expression and large dynamic changes of the GECIs has eliminated the need to dissect tissue and has allowed investigators to study cells in a more physiological setting. Importantly, the success of these studies implies that similar techniques could be used in combination with advanced microscopy to study the activity of large networks of cells in an intact, or semi-intact, organ. The ultimate goal would be to record cellular network activity in vivo, and new results suggest that this may be a possibility in the near future. For example, Rakhilin et al. (36) recently succeeded in recording neuronal responses in vivo by using an optical window into the abdominal cavity of mice expressing GCaMP3 in enteric neurons. Only small patches of the ENS were imaged in this study, but its success is an important step toward observing functional networks in vivo. Interestingly, this study also employed graphene sensors along the serosal surface of the gut to record electrical activity in the ENS. How well these sensors specifically detect the electrical activity of neurons sandwiched between two electrically active smooth muscle coats is still debatable, so additional validation of this method would be beneficial. A more feasible approach might again be to study the electrical activity of enteric neurons using genetically encoded voltage indicators. The development of new genetically encoded voltage indicators is still rapidly evolving, and new mouse models are now available that permit the expression of advanced voltage indicators in a Cre-dependent manner (27). The superior cellular resolution and specificity that these model systems afford now make imaging with this class of indicators in intact tissue a real possibility.
How Does the Activation of Specific Cell Types Contribute?
Observing relevant forms of cellular activity with the methods above is a good starting point, but these observational methods must be complemented with tools to directly test the functional significance of the observed activity. These tools must be cell type specific, work through relevant mechanisms, and be appropriate for use in intact organs or, more ideally, in vivo. Luckily, a powerful toolkit of genetically encoded proteins has evolved in recent years that allows investigators to control the activity or functions of defined cells in their native environment. Collectively, these proteins are referred to as “actuators” for their ability to control cellular mechanisms. The most commonly used genetically encoded actuators are ion channels, pumps, or G protein-coupled receptors (GPCRs) (Fig. 2) that allow investigators to excite or inhibit the activity of cells of interest (10, 37). However, other classes of cellular actuators are available to control diverse cellular functions such as protein trafficking (20), gene expression (20), protein interactions (45), protein conformation (35) and cell motility (51). Genetically encoded actuators are broadly grouped according to the type of energy source used by the control signal. Thus actuators driven by light are referred to as optogenetic, whereas those driven by chemicals, magnetic fields, or radio waves are referred to as chemogenetic, magnetogenetic, and sonogenetic, respectively. However, the basic strategy with their use is the same: express an engineered receptor that signals through relevant endogenous transduction pathways, trigger its activation with biologically inert stimuli, and observe the effect on a defined function. The ultimate choice of which protein to express is dictated by two major considerations: 1) how well the exogenous protein controls relevant endogenous signaling in the cell type of interest and 2) the feasibility of the activation strategy.
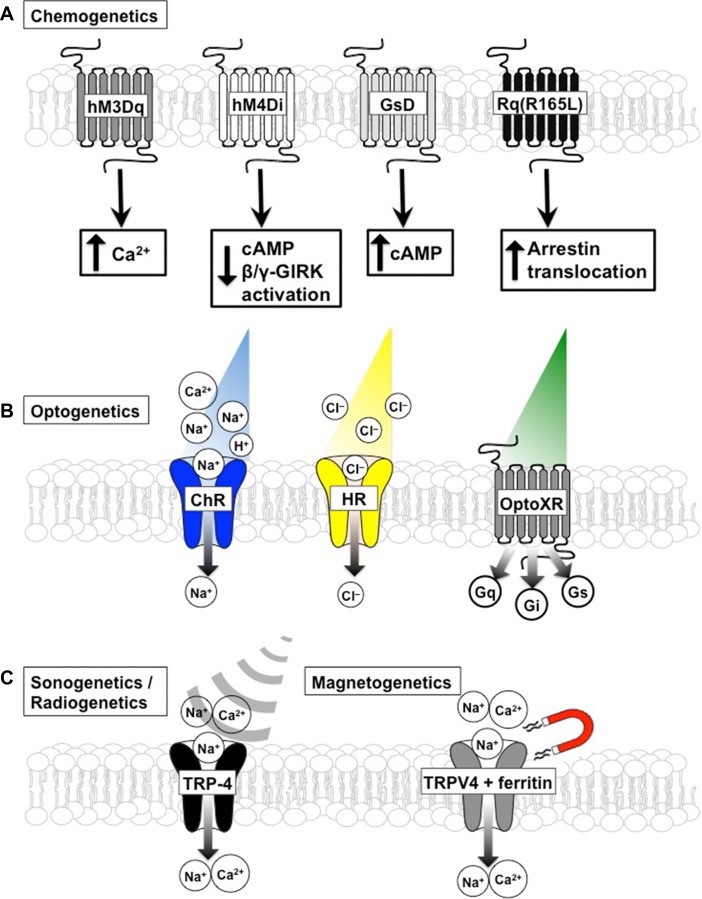
Fig. 2.
Schematic of various chemogenetic (A), optogenetic (B), sonogenetic and radiogenetic (C) approaches to control cellular activity and their signaling properties. A: 4 chemogenetic receptors shown are modified GPCRs that signal through canonical and noncanonical (arrestin) GPCR pathways. Receptors specifically activated by clozapine-n-oxide include hM3Dq, GsD and Rq(R165L), all modified M3 muscarinic receptors, and hM4Di, a modified M4 receptor. Not shown is the chemogenetic receptor KORD. KORD is a modified k-opioid receptor that is selectively activated by salvinorin B and signals through GPCR pathways. B: 3 examples of light-gated channels and receptors. Channelrhodopsin (ChR) is a light-gated cation channel that is used to drive cellular excitability. Halorhodopsin (HR) is a light-gated ion pump that is selective for chloride ions and is often used to silence excitable cells. OptoXRs are a family of light-gated GPCRs that couple to various intracellular signal transduction cascades. C: 2 examples of channels gated by sound waves (sonogenetics) or magnetic fields. TRP-4 is a mechanosensitive cation channel that is activated by low pressure ultrasonic sound. Magneto is a fusion protein between TRPV4 cation channels and ferritin. Magnetic fields physically pull this cation channel open.
The field of optogenetics has gained great fame in the “age of light” for the ability to integrate optics and genetic engineering to measure or manipulate the activity of cells (8, 10). This is a powerful technique to control the activity of neurons, in particular, because of the capacity for fine temporal control on the order of milliseconds. Optogenetic actuators have great potential for studies of enteric neuromuscular function, but their application has been limited to one published study thus far (42). In this study, Stamp et al. (42) used neural stem cells expressing channel rhodopsin to show that these cells can be grafted into the adult intestine, develop into mature neurons and functionally innervate the gut smooth muscle. Studying functional innervation with optogenetics ex vivo by measuring excitatory and inhibitory junction potentials as was done in this study is an effective approach that could be applied to study multiple different cell types in the gut. However, translating these types of studies to in vivo work is much more challenging. Some of the hurdles that have limited widespread use of optogenetics in the gut include the limited number of cell type specific promoters and technical issues with delivering light to the cells of interest in vivo. Unlike the brain where fiber optic cables can be implanted and fixed in place to deliver light, the gut is a dynamically moving organ, and implanting traditional fiber optics is not an option. One solution is to use implantable wireless optogenetic devices that could deliver light pulses to regions of the intestine (31). We recently piloted a variation of this technique and found that we could effectively control a small light source inside a synthetic fecal pellet (Fig. 3). This approach may work well in vivo to selectively modulate the activity of cells in the superficial layers of the gut epithelium such as EC cells. However, it has not been validated for use stimulating cells in deeper layers of the gut wall, and the light scattering properties of gut tissue will decrease the efficiency of short wavelength light used to gate the popular channel rhodopsins and halorhodopsins. Combining luminal devices that emit long wavelength light with red-shifted optogenetic channels may allow this technique to reach cells at the level of the myenteric plexus and smooth muscle coats, but this is still theoretical.
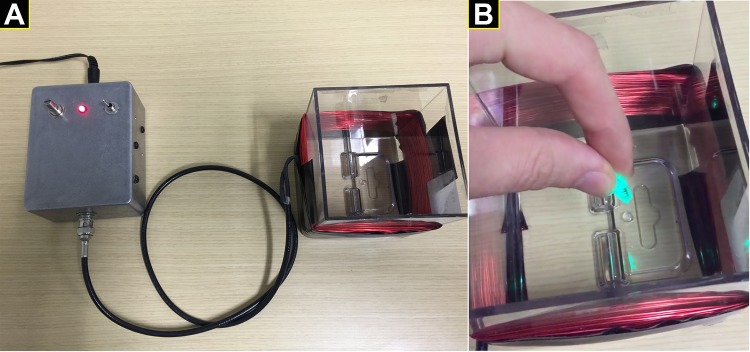
Fig. 3.
Example of a low-cost, wireless device to power a light-emitting fecal pellet. A: device consists of a power supply and a resonant cavity where electromagnetic energy is supplied to the LED within the animal. B: synthetic fecal pellet containing the small LED is powered by resonance energy transfer and emits light when placed into the electromagnetic field. This device was conceived and developed by David Fried.
Other classes of genetically encoded actuators circumvent the issues of accessibility experienced by optogenetics and may be more easily adapted for in vivo work in the intestine. For example, chemogenetics is widely used in both the brain and the periphery to control and investigate cell signaling (2, 37). Chemogenetic receptors are engineered so that they become unresponsive to native ligands and gain the affinity for specific small molecules that would otherwise be biologically inert. These attributes are very attractive because they allow precise cellular control with limited off-target effects. The main drawback of this technique is that it lacks fine temporal control in vivo, but modulating cellular activity with drugs is a very relevant approach for translational medicine. Chemogenetics is also a particularly attractive technique to investigate non-neuronal cells where the time course of signaling is slower and mainly driven by GPCRs. For example, we recently used a chemogenetic approach to selectively modulate the activity of enteric glial cells in vivo and in vitro to investigate their roles in intestinal motility (28) and secretomotor function (15). Similar strategies could easily be used to investigate the roles of other key populations of cells such as PDGFRα+ cells or EC cells. Importantly, chemogenetic receptors selective for different ligands can be used in tandem to control multiple cell populations at once or multiple signal transduction processes in the same cell type. For example, chemogenetic receptors such as hM3Dq, hM3Di, GsD, and Rq(R165L) are specifically activated by the drug clozapine-n-oxide (CNO), whereas chemogenetic KORD receptors are controlled by salvinorin B (SALB) (43). However, investigators should be aware of the fact that biased agonism could affect the interpretation of data obtained with chemogenetic models. For example, some GPCRs are able to respond to different ligands by signaling through different pathways. Thus a small molecule may target one pathway but miss a different biologically relevant pathway for that given receptor.
Opto- and chemogenetics are extremely powerful tools but, as noted above, display several limitations such as invasive methods of stimulation or slow kinetics. Of course, the ideal actuator for work in the intestine would be one that could be controlled in a noninvasive manner and exhibit high temporal and spatial precision. This may seem like a tall order, but it is encouraging that these types of technologies are beginning to come of age. Of particular interest are developments that are occurring in genetically encoded actuators that are controlled by sonic waves (sonogenetics) or magnetic fields (magnetogenetics). For example, Ibsen and colleagues showed that they were able to drive specific behaviors in Caenorhabditis elegans with low-pressure ultrasonic sound by expressing the ultrasound-sensitive channel TRP-4 in certain neurons (18). Likewise, Wheeler and colleagues (46) recently synthesized a magnetically sensitive actuator by fusing ferritin to TRPV4 and were able to genetically encode its expression in subpopulations of neurons. They went on to show that neurons expressing TRPV4-ferritin could be remotely activated by magnetic fields in vivo and drive behaviors in mice and zebrafish. Neither technique has been applied to the intestine yet, but both are extremely well suited for this application.
What Are the Important Cellular Mechanisms?
Assuming one has now used the techniques above to observe cellular activity associated with a function and then selectively triggered cellular activity to drive the function, we are now able to begin asking questions regarding the mechanisms that link activity to function. The most straightforward way to do this is to use one of a growing number of techniques to silence specific cellular mechanisms. The most classic approach is with Cre-loxP genetics where genes of interest are deleted in specific cells. However, many cells compensate to the constitutive deletion of genes very well so the field is increasingly turning to inducible mouse models that are based on either the tet-operon/repressor bi-transgenic system or the estrogen receptor (ER) ligand-binding domain fused to Cre. Inducible transgenes such as CreERT allow for the induction of recombination in specific cells at defined time points by administering a drug; tamoxifen, in this case. However, the potential effects of the drug should not be taken lightly since some models use drugs that modify gut physiology. For example, tet-operon/repressor bi-transgenic systems (Tet-On, Tet-Off) utilize the antibiotic doxycycline to regulate transgene expression and antibiotics have major effects on the gut microbiota. In the future, investigators may consider turning to new photoactivatable Cre approaches that could eliminate the need for drugs and restrict Cre activation to the gut (45). The main benefit of inducible transgenic systems is that they avoid many of the complications that arise with traditional knockout models during development and allow more direct studies of functional mechanisms in adult animals. An excellent example of how this technology can be applied to the gut is the work of Klein and colleagues (22) who used inducible Cre-loxP systems to specifically study the role of ICCs in inhibitory neuromuscular transmission in the gut. The results from this study provided clear evidence that ICCs are critically involved in mediating the nitrergic component of inhibitory neuromuscular transmission in adult animals. Likewise, we have used similar strategies to selectively ablate connexin-43 hemichannels from enteric glial cells in adult animals to investigate the role of this glial signaling mechanism in motility (29), secretomotor function (15), and inflammatory processes (9).
Transgenic models have historically been the mainstay method to address mechanisms in intact systems. However, transgenics are not without their drawbacks that include being largely limited to mouse models, being cost and time intensive, displaying variable effectiveness, and often experiencing undesirable side effects of transgene expression. Some of these issues can now be avoided by using new, more flexible methods of genome editing such as the CRISPR (clustered regularly interspersed short palindromic repeats)-Cas9 system (4, 16, 19). Since its conception in 2012 (19), this method has revolutionized genomic engineering and has made genome editing possible in basically all living organisms. This system takes advantage of a bacterial adaptive immune defense mechanism that involves directing an endonuclease (Cas9) to specific DNA sites with a single guide RNA molecule, creating DNA cleavage and subsequently inserting mutations using error-prone DNA repair mechanisms (16). This technology is now widely used, and the methods are commercially available. Genomic editing with CRISPR-Cas9 has massive potential to address mechanisms in gut physiology and pathophysiology in diverse model systems. However, the field is still developing, and many questions remain about the specificity and efficacy of this technology (34). Despite these issues, CRISPR-Cas9 will likely be an extremely powerful tool to expand mechanistic studies in the intestine.
CONCLUSIONS
Expanding the diversity of techniques used to investigate enteric neuromuscular function has the potential to provide new insight into the mechanisms that control gut motility. The techniques discussed above represent only a small, but important, subset of the cutting edge approaches that are now becoming available. These techniques are widely used in neuroscience and have proven to be powerful tools to study functional networks and cellular mechanisms. Many of these techniques are well suited for work in the intestine. However, others will require adaptation to meet the specific needs of the unique environment of the gut. Combining several different technologies at once has even more potential experimental power. These are exciting prospects that will likely play a key role in generating a sophisticated, integrative understanding of enteric neuromuscular function.
GRANTS
B. D. Gulbransen receives research support from the National Institutes of Health (R01-DK-103723), the Crohn’s and Colitis Foundation of America (Senior Research Award) and the Department of Defense (GW150178).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.D.G. conceived and designed research, prepared figures, and drafted, edited, revised, and approved final version of manuscript.
ACKNOWLEDGMENTS
I thank Dr. John Wiley for the invitation to present this topic at the 2016 Federation of Neurogastroenterology and Motility Joint International Meeting and Dr. Nigel Bunnett for the opportunity to prepare the presentation as a mini-review.
REFERENCES
- 1.Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Methods 10: 413–420, 2013. doi: 10.1038/nmeth.2434. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63: 27–39, 2009. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker SA, Drumm BT, Saur D, Hennig GW, Ward SM, Sanders KM. Spontaneous Ca(2+) transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine. J Physiol 594: 3317–3338, 2016. doi: 10.1113/JP271699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrangou R. RNA events. Cas9 targeting and the CRISPR revolution. Science 344: 707–708, 2014. doi: 10.1126/science.1252964. [DOI] [PubMed] [Google Scholar]
- 5.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol 24: 99–143, 1899. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayliss WM, Starling EH. The movements and the innervation of the large intestine. J Physiol 26: 107–118, 1900. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boesmans W, Martens MA, Weltens N, Hao MM, Tack J, Cirillo C, Vanden Berghe P. Imaging neuron-glia interactions in the enteric nervous system. Front Cell Neurosci 7: 183, 2013. doi: 10.3389/fncel.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268, 2005. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 9.Brown IAM, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2: 77–91, 2016. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci 18: 1213–1225, 2015. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst 81: 87–96, 2000. doi: 10.1016/S0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 12.Furness JB. The Enteric Nervous System. Malden, MA: Wiley, 2008. [Google Scholar]
- 13.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286–294, 2012. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 14.Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron 73: 862–885, 2012. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Grubišić V, Gulbransen BD. Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J Physiol. 8 January 2017. doi: 10.1113/JP273492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haeussler M, Concordet JP. Genome editing with CRISPR-Cas9: can it get any better? J Genet Genomics 43: 239–250, 2016. doi: 10.1016/j.jgg.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hennig GW, Gould TW, Koh SD, Corrigan RD, Heredia DJ, Shonnard MC, Smith TK. Use of genetically encoded calcium indicators (GECIs) combined with advanced motion tracking techniques to examine the behavior of neurons and glia in the enteric nervous system of the intact murine colon. Front Cell Neurosci 9: 436, 2015. doi: 10.3389/fncel.2015.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibsen S, Tong A, Schutt C, Esener S, Chalasani SH. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat Commun 6: 8264, 2015. doi: 10.1038/ncomms9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821, 2012. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaberniuk AA, Shemetov AA, Verkhusha VV. A bacterial phytochrome-based optogenetic system controllable with near-infrared light. Nat Methods 13: 591–597, 2016. doi: 10.1038/nmeth.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, Saijilafu, Young L, He S, LaVinka PC, Zhou F, Bergles D, Hanani M, Guan Y, Spray DC, Dong X. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron 91: 1085-1096, 2016. doi: 10.1016/j.neuron.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R, Allescher H-D, Vanderwinden J-M, Hofmann F, Schemann M, Rad R, Storr MA, Schmid RM, Schneider G, Saur D. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat Commun 4: 1630, 2013. doi: 10.1038/ncomms2626. [DOI] [PubMed] [Google Scholar]
- 23.Knöpfel T, Gallero-Salas Y, Song C. Genetically encoded voltage indicators for large scale cortical imaging come of age. Curr Opin Chem Biol 27: 75–83, 2015. doi: 10.1016/j.cbpa.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 24.LePard KJ, Galligan JJ. Analysis of fast synaptic pathways in myenteric plexus of guinea pig ileum. Am J Physiol 276: G529–G538, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Liang R, Broussard GJ, Tian L. Imaging chemical neurotransmission with genetically encoded fluorescent sensors. ACS Chem Neurosci 6: 84–93, 2015. doi: 10.1021/cn500280k. [DOI] [PubMed] [Google Scholar]
- 26.Lin MZ, Schnitzer MJ. Genetically encoded indicators of neuronal activity. Nat Neurosci 19: 1142–1153, 2016. doi: 10.1038/nn.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lou S, Adam Y, Weinstein EN, Williams E, Williams K, Parot V, Kavokine N, Liberles S, Madisen L, Zeng H, Cohen AE. Genetically targeted all-optical electrophysiology with a transgenic Cre-dependent optopatch mouse. J Neurosci 36: 11059–11073, 2016. doi: 10.1523/JNEUROSCI.1582-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClain JL, Fried DE, Gulbransen BD. Agonist-evoked Ca(2+) signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol 1: 631–645, 2015. doi: 10.1016/j.jcmgh.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClain JL, Grubišić V, Fried D, Gomez-Suarez RA, Leinninger GM, Sévigny J, Parpura V, Gulbransen BD. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146: 497–507, 2014. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClain JL, Gulbransen BD. The acute inhibition of enteric glial metabolism with fluoroacetate alters calcium signaling, hemichannel function, and the expression of key proteins. J Neurophysiol 117: 365–375, 2017. doi: 10.1152/jn.00507.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery KL, Yeh AJ, Ho JS, Tsao V, Mohan Iyer S, Grosenick L, Ferenczi EA, Tanabe Y, Deisseroth K, Delp SL, Poon ASY. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat Methods 12: 969–974, 2015. doi: 10.1038/nmeth.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neunlist M, Peters S, Schemann M. Multisite optical recording of excitability in the enteric nervous system. Neurogastroenterol Motil 11: 393–402, 1999. doi: 10.1046/j.1365-2982.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- 33.North RA. Electrophysiology of the enteric nervous system. Neuroscience 7: 315–325, 1982. doi: 10.1016/0306-4522(82)90269-X. [DOI] [PubMed] [Google Scholar]
- 34.Peng R, Lin G, Li J. Potential pitfalls of CRISPR/Cas9-mediated genome editing. FEBS J 283: 1218–1231, 2016. doi: 10.1111/febs.13586. [DOI] [PubMed] [Google Scholar]
- 35.Pudasaini A, El-Arab KK, Zoltowski BD. LOV-based optogenetic devices: light-driven modules to impart photoregulated control of cellular signaling. Front Mol Biosci 2: 18, 2015. doi: 10.3389/fmolb.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakhilin N, Barth B, Choi J, Muñoz NL, Kulkarni S, Jones JS, Small DM, Cheng Y-T, Cao Y, LaVinka C, Kan E, Dong X, Spencer M, Pasricha P, Nishimura N, Shen X. Simultaneous optical and electrical in vivo analysis of the enteric nervous system. Nat Commun 7: 11800, 2016. doi: 10.1038/ncomms11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roth BL. DREADDs for Neuroscientists. Neuron 89: 683–694, 2016. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Y, Rosendale M, Campbell RE, Perrais D. pHuji, a pH-sensitive red fluorescent protein for imaging of exo- and endocytosis. J Cell Biol 207: 419–432, 2014. doi: 10.1083/jcb.201404107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith TK, Koh SD. A model of the enteric neural circuitry underlying the generation of rhythmic motor patterns in the colon: the role of serotonin. Am J Physiol Gastrointest Liver Physiol 312: G1–G14, 2017. doi: 10.1152/ajpgi.00337.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sofroniew NJ, Flickinger D, King J, Svoboda K. A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging. eLife 5: e14472, 2016. doi: 10.7554/eLife.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.St-Pierre F, Chavarha M, Lin MZ. Designs and sensing mechanisms of genetically encoded fluorescent voltage indicators. Curr Opin Chem Biol 27: 31–38, 2015. doi: 10.1016/j.cbpa.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamp LA, Gwynne RM, Foong JPP, Lomax AE, Hao MM, Kaplan DI, Reid CA, Petrou S, Allen AM, Bornstein JC, Young HM. Optogenetic demonstration of functional innervation of mouse colon by neurons derived from transplanted neural cells. Gastroenterology S0016-5085(17)30034-3, 2017. doi: 10.1053/j.gastro.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci 37: 387–407, 2014. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- 44.Tack J, Smith TK. Calcium imaging of gut activity. Neurogastroenterol Motil 16, Suppl 1: 86–95, 2004. doi: 10.1111/j.1743-3150.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 45.Taslimi A, Zoltowski B, Miranda JG, Pathak GP, Hughes RM, Tucker CL. Optimized second-generation CRY2-CIB dimerizers and photoactivatable Cre recombinase. Nat Chem Biol 12: 425–430, 2016. doi: 10.1038/nchembio.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wheeler MA, Smith CJ, Ottolini M, Barker BS, Purohit AM, Grippo RM, Gaykema RP, Spano AJ, Beenhakker MP, Kucenas S, Patel MK, Deppmann CD, Güler AD. Genetically targeted magnetic control of the nervous system. Nat Neurosci 19: 756–761, 2016. doi: 10.1038/nn.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood JD, Alpers DH, Andrews PL. Fundamentals of neurogastroenterology. Gut 45, Suppl 2: II6–II16, 1999. doi: 10.1136/gut.45.2008.ii6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood JD. Electrical activity from single neurons in Auerbach’s plexus. Am J Physiol 219: 159–169, 1970. [DOI] [PubMed] [Google Scholar]
- 49.Wood JD. Enteric neurophysiology. Am J Physiol 247: G585–G598, 1984. [DOI] [PubMed] [Google Scholar]
- 50.Wood JD. Enteric Nervous System: The Brain-In-The-Gut. Colloquium Series on Integrated Systems Physiology: From Molecule to Function Williston, VT: Morgan and Claypool, 2011. doi: 10.4199/C00039ED1V01Y201107ISP026. [DOI] [Google Scholar]
- 51.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461: 104–108, 2009. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]