Abstract
Women with a history of preeclampsia (PE) have an increased risk to develop cardiovascular and renal diseases later in life, but the mechanisms underlying this effect are unknown. In rats, we assessed whether placental ischemia results in long-term effects on the maternal cardiovascular and renal systems using the reduced uterine perfusion pressure (RUPP) model for PE. Sprague-Dawley rats received either a Sham or RUPP operation at gestational day 14. The rats were followed for 8 wk after delivery (Sham n = 12, RUPP n = 21) at which time mean arterial pressure (MAP; conscious), 24-h albuminuria, glomerular filtration rate (GFR; transcutaneous, FITC-sinistrin), and cardiac function (Vevo 770 system) were assessed. Subsequently, all rats were euthanized for mesenteric artery vasorelaxation and histology of heart and kidney. At 8 wk after delivery, there was no difference in MAP and albuminuria. However, RUPP rats showed a significantly reduced GFR [2.61 ± 0.53 vs. 3.37 ± 0.74 ml/min; P = 0.01]. Ultrasound showed comparable cardiac structure, but RUPP rats had a lower left ventricular ejection fraction (62 ± 7 vs. 69 ± 10%; P = 0.04). Heart and kidney histology was not different between Sham or RUPP rats. Furthermore, there were no differences in endothelial-dependent or -independent vasorelaxation. We show that exposure to placental ischemia in rats is accompanied by functional disturbances in maternal renal and cardiac function 8 wk after a preeclamptic pregnancy. However, these changes were not dependent on differences in blood pressure, small artery vasorelaxation, or cardiac and renal structure at this time point postpartum.
Keywords: cardiac function, glomerular filtration rate, postpartum, placental ischemia, preeclampsia
preeclampsia (PE) is a pregnancy-specific disorder characterized by the development of de novo hypertension and proteinuria and/or other organ disturbances during the second half of pregnancy (33). Reduced uteroplacental perfusion is postulated to be the initiating event of PE, leading to placental ischemia and subsequent release of placental factors into the maternal circulation causing widespread endothelial dysfunction of the maternal vasculature (9). While symptoms of PE disappear soon after delivery of the placenta, these women appear to be at increased risk for future cardiovascular disease. Indeed, formerly preeclamptic women are reported to have a twofold increased risk for long-term cardiovascular disease (CVD) (4) and a 5- to 14-fold increased risk for end stage kidney disease (ESKD) (37, 38, 40).
An increased blood pressure has been reported in the first years after PE (17, 35), and epidemiologic studies suggest that formerly preeclamptic women develop hypertension 6–8 yr earlier compared with women having a history of normotensive pregnancy (11, 32). Cardiovascular disturbances in formerly preeclamptic women include endothelial dysfunction as measured by flow-mediated dilatation (39) and subclinical impairment of ventricular function, especially after early onset PE (<34 wk) (21, 22). So far, studies on glomerular filtration rate (GFR) and albuminuria after PE are sparse and include heterogeneous populations, with conflicting results (2, 20, 29). To what extent renal and cardiac hemodynamic abnormalities persist after PE or gradually develop during later life is unclear.
A current hypothesis is that cardiovascular disturbances observed after PE can be explained by risk factors that are already present before pregnancy (25). However, some human studies suggest that PE itself might contribute to this risk as common risk factors and familial factors could not fully explain the presence of cardiovascular disturbances in formerly preeclamptic women (7, 24, 36). Interpretation of these human studies is difficult due to confounding preexisting risk factors. Experimental models of PE might help us to determine whether exposure to PE affects long-term cardiovascular and renal function. Only a few studies have addressed this question in experimental models of PE. After both lipopolysaccharide (LPS) and soluble Fms-like tyrosine kinase-1 (sFlt-1) induced experimental PE, no changes in postpartum blood pressure were reported in rodents (5, 6, 34). However, rodents exposed to experimental PE were more sensitive to a second cardiovascular hit as compared with formerly normal pregnant rodents (23, 34). A limitation of these studies is that they used extrinsic stimuli to induce experimental PE, an approach not representative of placental ischemia. As yet, only one study investigated postpartum effects of exposure to placental ischemia in rats and reported reduced endothelial-dependent mesenteric artery relaxation at 3 mo postpartum (3).
In this study, we hypothesized that PE, as elicited by placental ischemia in rats, results in increased maternal blood pressure, impaired endothelial function and reduced renal and cardiac function postpartum. We utilized the reduced uterine perfusion pressure (RUPP) model of PE in rats, which is characterized by the development of hypertension, endothelial dysfunction, proteinuria and a reduced GFR during pregnancy initiated by the placental ischemia (1, 9, 16). Rats were randomized to receive either a Sham or RUPP procedure, and 8 wk after delivery, blood pressure, renal and cardiac function and structure, and small artery vasorelaxation were assessed.
METHODS
Experimental animals.
All animal experiments were conducted in accordance with the National Institutes of Health (NIH, Bethesda, MD) Guide for the Care and Use of Laboratory Animals with all animal-use protocols approved by The University of Mississippi Medical Center’s Institutional Animal Care and Use Committee. Timed-pregnant Sprague-Dawley rats were purchased from Harlan Laboratories (barrier no 202A; Indianapolis, IN). Animals were maintained in the animal facility of the University of Mississippi Medical Center at a 12-12 h light-dark cycle. After operative procedures, rats were housed individually for 1 wk. During the postpartum experiments, animals were housed with two to three rats a cage. The rats had ad libitum access to Envigo 2020X (during pregnancy) and 8640 (postpartum) diets and tap water.
Study protocol.
At gestational day (gd) 14, Sprague-Dawley rats were randomized to two groups: Sham (n = 24) or RUPP (n = 38). The RUPP procedure involved placing silver clips on the lower abdominal aorta and branches of the ovarian arteries to induce placental ischemia (16). Total viable and reabsorbed fetuses were also recorded on gd 14 before clip placement. The Sham procedure involved only opening the abdomen and externalization of the uterus. At day 18 of gestation 24-h urine was collected and at gd 19 a subgroup of animals underwent invasive blood pressure measurement and arterial blood sampling. After delivery, all pups were removed within 12 h after delivery to avoid effects caused by differences in litter size and lactation. Mothers (n = 3) of which we had no evidence that they had given birth were included in the analysis since rats with nonsurviving pups are most probably exposed to high levels of placental ischemic factors and therefore stand a good chance to have a long-term phenotype, even when exposure time to high amounts was shorter, and because we could not be certain surviving pups indeed did not give birth as we checked the rats once in 12 h and fragile pups might have been eaten by the mother. Some of the rats had a follow-up of 8 wk postpartum, at which time we collected 24-h urine samples and measured GFR and cardiac function. On the following day, blood pressure was measured and the rats were euthanized for tissue collection. Third-order mesenteric arteries were used to assess vasorelaxation, as detailed below.
Mean arterial blood pressure.
On gd 19 (Sham n = 6; RUPP: n = 11) and at week 8 postpartum (Sham n = 11; RUPP n = 16), mean arterial blood pressure (MAP) and heart rates were measured (31). One to two days before the measurement, we inserted catheters under isoflurane anesthesia in the left carotid artery and exposed them at the nape (Butler Schein Animal Health, Dublin, OH). Catheters consisted of V/1 tubing attached to V/3 tubing (Scientific Commodities, Lake Havasu City, AZ). Approximately 2.5 cm of the V/3 end of the catheter was inserted into the carotid. Catheters were filled with sterile heparin-saline (300 mg/ml; Pfizer, New York City, NY) and stoppered with a sterile nail. On the day of measurement, rats were placed in restrainers and catheters were connected to pressure transducers (MLT0699; ADInstruments, Colorado Springs, CO) coupled to a computerized data acquisition system (PowerLab; ADInstruments). Before recording was started, animals acclimatized to restraint for ~1 h. Means of a 30-min recording of blood pressure were used for analysis.
Glomerular filtration rate.
At 8 wk postpartum, GFR was measured by a noninvasive transcutaneous clearance measurement, as developed by Mannheim Pharma & Diagnostics (Mannheim, Germany) (Sham n = 8; RUPP n = 15). One to two days before measurements, jugular catheters were implanted during the same operative time as the carotid surgeries above. On the day of measurement, rats were briefly anesthetized with isoflurane to remove hair from the nape and to extend the jugular catheter and place the USB device and battery at the nape using doubled-sided adhesive tape. The device plus battery was immobilized by a jacket (Kent Scientific, Torrington, CT). Rats recovered from anesthesia for 15 to 20 min followed by a bolus dose of 3 mg/100 g body wt FITC-sinistrin in 0.2 ml sterile irrigation saline (Baxter Healthcare, Deerfield, IL) via jugular catheter while rats moved freely in their cages. Determination of transdermally measured half time (t1/2) of FITC-sinistrin clearance was performed according to the one compartment model. GFR was calculated in units of milliliters per minute per 100 g [formula: GFR (ml·min−1·100 g body wt−1) = 31.26 (ml/100 g body wt)/t1/2(FITC-s)(min)] multiplied by the body weight (27).
Cardiac ultrasound.
Echocardiographic analysis was undertaken by an experienced echographer (B. Bakrania) blinded to the group allocation using a Vevo-770 high-resolution in vivo imaging system and RMV710B scan head for small rodents (VisualSonics, Toronto, ON, Canada) at 8 wk postpartum in Sham (n = 9) and RUPP (n = 19) rats. For the analysis, rats were anesthetized with ∼2% isoflurane administered in 2 l/min O2. During anesthesia, rats were placed on a heating pad (37.5°C) with rectal temperature measured continuously and electrocardiograms and heart rate monitored. A total of four cardiac views were obtained per rat: parasternal long-axis, parasternal short-axis, four-chamber apical, and suprasternal views (focused on the aorta). Body weight indexing was used for cardiac structural and functional parameters. Functional parameters were calculated using the Vevo 770 imaging software.
Mesenteric artery vasorelaxation.
After euthanasia, third-order mesenteric arteries were collected in 5 ml PSS containing the following (concentration in mmol/l): 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 11.1 dextrose. Within 1 h after collection, the mesenteric arteries were cleaned of perivascular adipose tissue for vascular function studies. In short, vascular rings (1 per rat) of ~2.5 mm in length were mounted on a wire myograph (model 620M; Danish Myo Technology, Aarhus, Denmark) containing 5 ml PSS after which 4 mN of preload was placed on the rings. The integrity of the arterial rings (1 per rat) was tested with a bolus dose of phenylephrine (Phe) to produce vasoconstriction followed by a bolus of acetylcholine (ACh) to produce vasorelaxation. Arterial segments were washed, equilibrated for 15 min, and constricted with Phe (~10 μmol/l), and cumulative concentration-response curves were generated to ACh [1E-18M to 3E7M] (Sigma) and to sodium nitroprusside (SNP) [1E-10 to 3E-5] (Sigma). ACh response curves (Sham: n = 8; RUPP n = 8) and SNP response curves (Sham: n = 6; RUPP n = 9) were generated using the same ring.
Quantification of sFlt-1 and albuminuria.
ELISA kits were used to measure plasma sFlt-1 (MVR100 R&D Systems with rat sFlt-1 cross reactivity) at gd 19 (Sham n = 9; RUPP n = 4) and 8 wk postpartum rats (Sham n = 12; RUPP n = 21). Urinary albumin concentration (mg/ml) was determined using the Exocell Nephrat kit (Philadelphia, PA) and multiplied by the total amount (ml) of urine excreted during 24 h at gd 19 and 8 wk postpartum.
Histology.
Harvested kidneys and hearts were fixed in 10% formalin, embedded in paraffin, and sectioned. Kidney sections were stained with Periodic acid-Schiff and scored for glomerular and tubular-interstitial damage (14). To measure glomerular area, ×40 original magnification images were acquired of 25 glomeruli. The surface of each glomerulus was outlined and total area in pixels was measured with ImageJ (NIH). Glomerular endothelial cells were stained with JG12 (mouse anti-JG12, BMS1104, 1:200; Bender Medsystems, Vienna, Austria). The endothelial (JG12+) area in 25 glomeruli per kidney was determined using Adobe Photoshop CS5 Extended, version 12.0 × 32 (Adobe Systems, San Jose, CA). The JG12+ area was corrected for glomerular area. Cardiac sections were stained with Sirius Red (SR) for collagen I and III content. Photographs were taken of 20 fields using a polarization filter, and percentage area fibrosis was calculated. Histological analysis of SR-stained sections was performed using Adobe Photoshop (Adobe Systems) and ImageJ (NIH). A technician blinded to the group allocation scored all the histology.
Statistics.
Data were graphed and statistics were performed using GraphPad Prism version 6.0 and SPPS 22, presented as means ± SD or median (25–75 percentile), and tested with a Student’s t-test or Mann-Whitney U-test, respectively. Mesenteric reactivity data were analyzed with a two-way ANOVA for repeated measures. Area under the curve (AUC; arbitrary units) and logEC50 were calculated with GraphPad Prism and subsequently tested with a Student’s t-test.
RESULTS
Pregnancy characteristics.
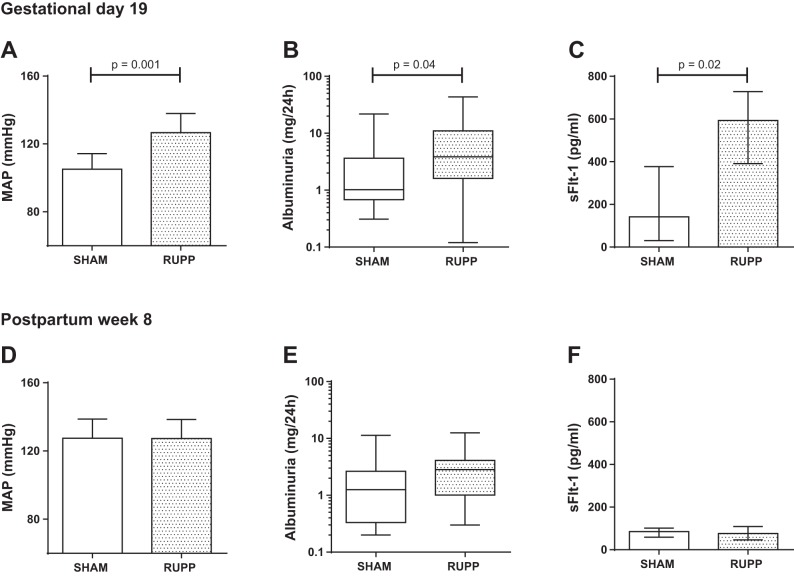
The pregnancy characteristics of the Sham and RUPP rats are presented in Table 1. The mean weight gain of the RUPP rats during pregnancy was significantly lower compared with the Sham rats indicating successful RUPP procedure. Placental ischemia elicited a PE-like maternal phenotype with high blood pressure, albuminuria, and increased plasma sFlt-1 (Fig. 1, A–C). Litter size was significantly reduced but mean weight of the pups at birth was not affected by the RUPP procedure.
Table 1.
Rat characteristics during pregnancy
| Sham (n = 24) | RUPP (n = 38) | P Value | |
|---|---|---|---|
| Day 14 | |||
| Maternal weight, g | 282 ± 11 | 286 ± 9 | 0.126 |
| Number of fetuses | 13.5 ± 1.4 | 13.5 ± 2.2 | 0.934 |
| Number of reabsorptions | 1 (0.3–3.0) | 1 (0.3–2) | 0.672 |
| Day 19 | |||
| Weight gain, g | 61.6 ± 10.2 | 31.1 ± 16.1 | <0.001* |
| Day of birth | |||
| Number of pups | 10.5 (8.5–12.0) | 4 (1.75–8.25) | <0.001* |
| Weight of pups, g | 5.9 ± 0.5 | 5.6 ± 0.6 | 0.107 |
Data are means ± SD or medians (25–75 percentile). RUPP, reduced uterine perfusion pressure. *P < 0.05.
Fig. 1.
Mean arterial pressure (MAP), 24-h albuminuria, and plasma soluble Fms-like tyrosine kinase-1 (sFlt-1) and 24-h albuminuria at gestational day 19 (A–C) and 8 wk postpartum (D–F) in Sham (white bars) and reduced uterine perfusion pressure (RUPP, gray bars) rats. MAP and sFlt-1 are expressed as means ± SD and albuminuria as median (25–75 percentile).
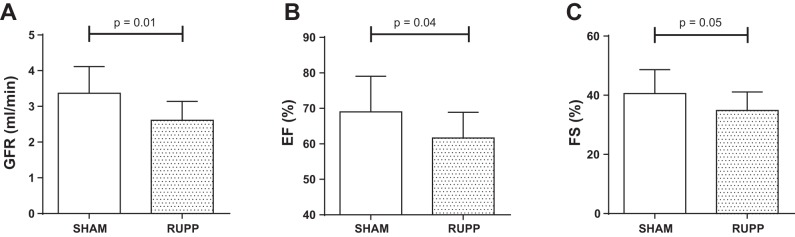
Postpartum blood pressure and GFR after placental ischemia.
At 8 wk postpartum, RUPP rats showed a significant reduction in GFR compared with Sham (Fig. 2A). No differences in mean arterial blood pressure were observed between groups (Fig. 1D). Furthermore, both systolic and diastolic blood pressures were comparable between groups (systolic blood pressure: Sham 142 ± 11 vs. RUPP 143 ± 12 mmHg and diastolic blood pressure: Sham 110 ± 12 vs. RUPP 111 ± 12 mmHg) and heart rate did not significantly differ between groups during the blood pressure recordings (Sham 376 ± 27 vs. RUPP 406 ± 40 beats/min). Albuminuria was not different between groups (Fig. 1E) and plasma sFlt-1 had returned to normal values at 8 wk postpartum (Fig. 1F). RUPP and Sham rats had comparable body weights at termination (Sham 270 ± 13 vs. RUPP 266 ± 14 g).
Fig. 2.
Glomerular filtration rate (GFR; A), ejection fraction (EF; B), and fractional shortening (FS; C) at 8 wk postpartum in Sham (white bars) and RUPP (gray bars) rats. Data are expressed as means ± SD.
Postpartum cardiac function and structure after placental ischemia.
Cardiac echography revealed that RUPP rats had a significantly lower left ventricular ejection fraction at 8 wk postpartum compared with Sham rats (Fig. 2B). In line with this, we observed a trend toward a reduced fractional shortening (Fig. 2C). Structural features, cardiac output, and diastolic function parameters did not differ between groups (Table 2).
Table 2.
In vivo structural and functional cardiac parameters measured by echocardiography in Sham and RUPP rats at 8 wk postpartum
| Sham (n = 9)* | RUPP (n = 19)* | P Value | |
|---|---|---|---|
| Heart weight, g | 0.85 ± 0.07 | 0.89 ± 0.07 | 0.185 |
| Structural | |||
| LV mass, mg | 789 ± 166 | 812 ± 141 | 0.707 |
| LV mass/body weight, mg/g | 2.9 ± 0.7 | 3.0 ± 0.5 | 0.646 |
| LVPWd, mm | 1.7 ± 0.5 | 1.7 ± 0.4 | 0.886 |
| LVPWs, mm | 2.6 ± 0.6 | 2.3 ± 0.5 | 0.274 |
| LVAWd, mm | 1.5 ± 0.3 | 1.6 ± 0.3 | 0.408 |
| LVAWs, mm | 2.5 ± 0.2 | 2.5 ± 0.3 | 0.741 |
| LVIDd, mm | 7.25 ± 0.64 | 7.12 ± 0.61 | 0.624 |
| LVIDs, mm | 4.34 ± 0.86 | 4.63 ± 0.54 | 0.278 |
| LV VOLd, μl | 278.8 ± 51.9 | 273.6 ± 36.7 | 0.761 |
| LV VOLs, μl | 89.3 ± 38.3 | 100.9 ± 26.0 | 0.355 |
| Functional | |||
| HR, beats/min | 337 ± 19 | 342 ± 23 | 0.613 |
| CO, ml | 63.7 ± 8.9 | 59.1 ± 11.1 | 0.300 |
| Mean aortic velocity, mm/s | 743 ± 167 | 982 ± 364 | 0.115 |
| Peak aortic velocity, mm/s | 1338 ± 332 | 1735 ± 593 | 0.115 |
| Peak aortic gradient | 7.6 ± 3.6 | 10.5 ± 5.8 | 0.235 |
| MV E, mm/s | 904 (741–927) | 861 (602–912) | 0.667 |
| MV A, mm/s | 659 (604–807) | 653 (508–737) | 1.000 |
| MV E/A | 1.3 ± 0.2 | 1.3 ± 0.1 | 0.957 |
| IVRT, ms | 19.2 ± 5.3 | 22.0 ± 6.3 | 0.318 |
| IVCT, ms | 21.1 ± 7.2 | 23.9 ± 7.3 | 0.415 |
Data are means ± SD or medians (25–75 percentile). LV, left ventricle; LVPWd and LVPWs, left ventricular posterior wall during end diastole and end systole; LVAWd and LVAWs, left ventricular anterior wall during end diastole and end systole; LVIDd and LVIDs, left ventricular internal diameter end diastole and end systole; LV VOLd and LV VOLs, left ventricular volume during diastole and systole HR, heart rate; CO, cardiac output; MV E, peak mitral valve (MV) early filling; MV A, peak MV active filling; MV E/A, MV early-to-active filling ratio; IVRT, isovolumetric relaxation time; IVCT, isovolumetric contraction time
Total number of rats in each group, all parameters were successful in at least 70% of the animals.
Postpartum mesenteric artery vasorelaxation after placental ischemia.
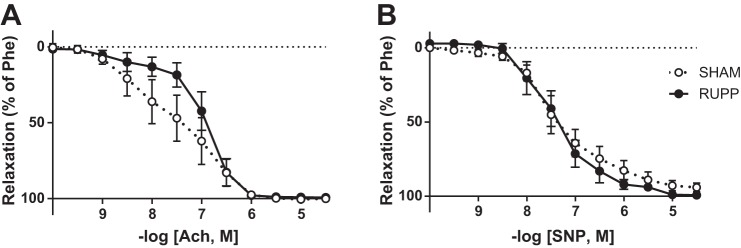
Figure 3 shows the relaxation curves of the third-order mesenteric arteries in response to ACh (Fig. 3A) and SNP (Fig. 3B) in the Sham and RUPP rats at 8 wk postpartum. There were no significant differences at any of the concentrations for endothelial-dependent (ACh) or -independent (SNP) vasorelaxation between the groups (two-way ANOVA: ACh P = 0.254; SNP P = 0.758), although we did observe a trend toward a reduction in endothelial dependent vasorelaxation. Neither the AUC and logEC50 of the ACh relaxation curves (AUC: Sham 250 ± 31 vs. RUPP 291 ± 18; P = 0. 274 and logEC50: Sham 7.5 ± 0.3 vs. RUPP 6.9 ± 0.14 –log [Ach, M]; P = 0.130) nor the AUC and logEC50 of the SNP relaxation curves (AUC: Sham 289 ± 24 vs. RUPP 281 ± 22 –log [SNP, M]; P = 0.803 and logEC50: Sham 7.3 ± 0.2 vs. RUPP 7.37 ± 0.2; P = 0.825) differed between the groups.
Fig. 3.
ACh relaxation curve (A) and SNP relaxation curve after incubation with nitro-l-arginine methyl ester (l-NAME.B) of mesenteric arteries at 8 wk postpartum in Sham (white dots) and RUPP (black dots) rats. Data are expressed as means ± SD.
Postpartum morphology of kidneys and hearts after placental ischemia.
At 8 wk postpartum no differences in kidney weight were observed between groups (Table 3). At this time point histological evaluation of the kidney also did not reveal any differences in glomerular and tubular-interstitial damage. In addition, mean glomerular area and the percentage of JG12 positive area within the glomerulus were similar. No fibrosis in the heart was visible in any rat after staining with SR.
Table 3.
Macroscopic and microscopic features of kidney in Sham and RUPP rats at 8 wk postpartum
| Sham (n = 12) | RUPP (n = 21) | P Value | |
|---|---|---|---|
| Left kidney weight, g | 0.72 ± 0.08 | 0.70 ± 0.04 | 0.443 |
| Right kidney weight, g | 0.73 ± 0.07 | 0.75 ± 0.06 | 0.526 |
| Glomerular area, pixels × 103 | 243 ± 27 | 241 ± 28 | 0.885 |
| Glomerular damage, %glomeruli | |||
| None | 77.1 ± 7.5 | 77.1 ± 6.3 | 0.921 |
| Partial | 22.7 ± 7.4 | 22.1 ± 6.5 | |
| Total | 0.2 ± 0.6 | 0.2. ± 0.6 | |
| Tubulo-interstitial infiltrate score | 0.03 (0.00–0.06) | 0.00 (0.00–0.06) | 0.765 |
| Tubulo-interstitial fibrosis score | 0.11 (0.05–0.15) | 0.10 (0.00–0.17) | 0.765 |
| Tubulo-interstitial atrophy score | 0.06 (0.00–0.12) | 0.06 (0.00–0.10) | 0.866 |
| JG12+, %glomerular area | 23 ± 4 | 25 ± 7 | 0.518 |
Data are means ± SD or medians (25–75 percentile).
DISCUSSION
In this study, exposure to placental ischemia elicited by the RUPP was accompanied by alterations in cardiac and renal function in the postpartum period characterized by a reduced GFR and reduced left ventricular ejection at 8 wk postpartum. The observed changes could not be explained by concurrent increases in blood pressure, vascular reactivity, and histological damage.
This appears to be the first study to show that the maternal renal and cardiac function in the postpartum period is affected in an animal model for PE, induced by placenta ischemia. One study assessed postpartum effects of exposure to placental ischemia in RUPP rats but only reported on vascular function showing reduced endothelial dependent mesenteric artery relaxation at 3 mo postpartum (3). Other studies addressing the question of post-PE cardiovascular function mainly looked at blood pressure and proteinuria and were performed in models for experimental PE based on the administration of very high levels of single molecules that are associated with the development of PE. These models have the limitation that they only mimic some of the consequences of the placental ischemia syndrome without placental ischemia, the primary causal event. In rats exposed to LPS-induced PE, no baseline differences were observed in blood pressure and proteinuria at 9 wk postpartum. Similarly, no differences in blood pressure were found 6 mo after sFlt-1-induced PE (6, 34). In line with this, we did not observe a difference in blood pressure postpartum after placental ischemia at 8 wk postpartum in this study. However, our assessment of more sensitive parameters for cardiovascular function revealed slightly compromised function of heart and kidney after exposure to placental ischemia.
The presence of reduced renal and cardiac function 8 wk after exposure to placental ischemia implies that placental factors released during pregnancy could play a role in the development of cardiovascular and renal disease in the long term. In the RUPP model, it was shown that placental ischemia induces several functional and structural abnormalities in heart and kidney during pregnancy including a reduction in GFR by ~40% (1), a reduced cardiac index (28), and fibrosis in the heart (10). In our study, there was no evidence of histological damage postpartum, which suggests resolution of myocardial fibrosis after discontinuation of exposure to placental ischemia. We could also not identify signs of glomerular damage by assessing the intraglomerular endothelium with JG12 staining (19). Moreover, the absence of differences in glomerular area suggests that at 8 wk postpartum there was no glomerular stress or loss of nephrons (8, 12). Therefore, the functional alterations might be either the result a slower recovery of function compared with the histological changes or ongoing disturbances in regulatory systems. Slow recovery of some initial disturbances observed after PE is reported in humans, such as for proteinuria which resolves in almost all patients within 2-yr time period (2). On the other hand, the report of Brennan et al. (3) on vascular dysfunction after RUPP pregnancy suggests ongoing or even permanent target organ damage and/or dysregulation as they show that reduced mesenteric artery relaxation at 3 mo, but not 1 mo postpartum following RUPP-pregnancy.
Functional disturbances that might lead to ongoing impairment of postpartum cardiovascular and renal function after placental ischemia derived from human are increased sensitivity of renin angiotensin-aldosterone system (RAAS) and increased sensitivity of blood pressure to salt (13, 15, 18, 26, 30), but this has not yet been investigated in this animal model. Previous animal studies do suggest that the risk of cardiovascular dysfunction after PE might mainly result from enhanced responsiveness to a second hit administered after apparent resolution of PE. Pruthi et al. (23) showed increased smooth muscle cell proliferation and increased fibrosis after carotid damage in the sFlt-1 mouse model, and van de Graaf et al. (34) showed an increased hypertensive response after angiotensin II infusion in the pregnant LPS rat model. Future studies should elucidate whether there are specific factors that aggravate the observed target organ dysfunction after placental ischemia as this might uncover important targets for secondary prevention to reduce the cardiovascular burden in formerly preeclamptic women. In addition, aging studies are required to reveal whether the observed mild disturbances in cardiac and renal function lead to clinical features of hypertension and cardiovascular disease in the long term.
While the strength of this study is the observation of subclinical damage after placental ischemia in an animal model without confounding by preexisting factors, the use of the RUPP model in healthy rats can also be viewed as a limitation. First, PE might elicit different long-term sequelae in response to placental ischemia/PE in subjects with a predisposition for cardiovascular disease compared with the healthy baseline of SD rats used for the RUPP procedure (25). Additionally, in RUPP rats the clips remain in situ during the postpartum period, which theoretically could influence long-term cardiovascular function. The persistent effect of clips is unlikely to be an issue because of the limited flow postpartum through the ovarian artery. Furthermore, it is unlikely that aorta flow is increased in view of the stable maternal weight. Finally, the aorta clip is not likely to influence GFR and blood pressure values since the clip is placed on the lower abdominal aorta (caudal to the kidney arteries) and previous studies showed that in the nonpregnant state an abdominal clip of this size did not influence blood pressure (1). Another limitation of this study is that we cannot predict whether decreased GFR and ejection fraction, which were measured once in a terminal setting, will indeed contribute to the increased risk for cardiovascular and renal disease in later life. In general, it remains to be investigated whether a finding in an animal model for PE reflects the human situation.
Perspectives and Significance
In summary, we show alterations in heart and kidney function in the postpartum period after exposure to placental ischemia in an animal model for PE. These alterations occurred in the absence of differences in blood pressure, vascular reactivity, and histological damage. Our results suggest that exposure to placental ischemia during pregnancy affects the long-term cardiovascular health status of the mother.
GRANTS
Research reported in this publication was supported by the Dutch Kidney Foundation (15OKK65 to NDP and KJPB 11.026 to A. T. Lely); National Institute of General Medical Sciences Grant P20-GM-104357; National Heart, Lung, and Blood Institute Grants P01-HL-051971, R01-HL-108618, and HL36279; and American Heart Association Grant AHA-13POST16240000.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.D.P., J.P.G., and A.T.L. conceived and designed research; N.D.P., F.T.S., B.B., and Z.K.Z. performed experiments; N.D.P. analyzed data; N.D.P., J.A.J., J.P.G., and A.T.L. interpreted results of experiments; N.D.P. prepared figures; N.D.P. drafted manuscript; N.D.P., J.A.J., F.T.S., Z.K.Z., A.F., M.C.V., J.P.G., and A.T.L. approved final version of manuscript; J.A.J., F.T.S., A.F., M.C.V., J.P.G., and A.T.L. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank M. Arany, K. Cockrell, K. den Ouden, R. C. Kleisen, and E. van Veen for technical support during the experiments.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Heart Association.
REFERENCES
- 1.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. doi: 10.1161/01.HYP.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 2.Berks D, Steegers EA, Molas M, Visser W. Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol 114: 1307–1314, 2009. doi: 10.1097/AOG.0b013e3181c14e3e. [DOI] [PubMed] [Google Scholar]
- 3.Brennan L, Morton JS, Quon A, Davidge ST. Postpartum vascular dysfunction in the reduced uteroplacental perfusion model of preeclampsia. PLoS One 11: e0162487, 2016. doi: 10.1371/journal.pone.0162487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol 28: 1–19, 2013. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 5.Bytautiene E, Bulayeva N, Bhat G, Li L, Rosenblatt KP, Saade GR. Long-term alterations in maternal plasma proteome after sFlt1-induced preeclampsia in mice. Am J Obstet Gynecol 208: 388.e1–388.e10, 2013. doi: 10.1016/j.ajog.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bytautiene E, Lu F, Tamayo EH, Hankins GD, Longo M, Kublickiene K, Saade GR. Long-term maternal cardiovascular function in a mouse model of sFlt-1-induced preeclampsia. Am J Physiol Heart Circ Physiol 298: H189–H193, 2010. doi: 10.1152/ajpheart.00792.2009. [DOI] [PubMed] [Google Scholar]
- 7.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA 285: 1607–1612, 2001. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 8.Fogo A, Ichikawa I. Evidence for a pathogenic linkage between glomerular hypertrophy and sclerosis. Am J Kidney Dis 17: 666–669, 1991. doi: 10.1016/S0272-6386(12)80347-7. [DOI] [PubMed] [Google Scholar]
- 9.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension 38: 718–722, 2001. doi: 10.1161/01.HYP.38.3.718. [DOI] [PubMed] [Google Scholar]
- 10.Gutkowska J, Granger JP, Lamarca BB, Danalache BA, Wang D, Jankowski M. Changes in cardiac structure in hypertension produced by placental ischemia in pregnant rats: effect of tumor necrosis factor blockade. J Hypertens 29: 1203–1212, 2011. doi: 10.1097/HJH.0b013e3283468392. [DOI] [PubMed] [Google Scholar]
- 11.Heida KY, Franx A, van Rijn BB, Eijkemans MJ, Boer JM, Verschuren MW, Oudijk MA, Bots ML, van der Schouw YT. Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension 66: 1116–1122, 2015. 10.1161/HYPERTENSIONAHA.115.06005. [DOI] [PubMed] [Google Scholar]
- 12.Hoy WE, Bertram JF, Denton RD, Zimanyi M, Samuel T, Hughson MD. Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens 17: 258–265, 2008. doi: 10.1097/MNH.0b013e3282f9b1a5. [DOI] [PubMed] [Google Scholar]
- 13.Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, Markovic N, Thadhani R, Luft FC, Dechend R. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension 49: 612–617, 2007. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 14.Joles JA, van Goor H, Koomans HA. Estrogen induces glomerulosclerosis in analbuminemic rats. Kidney Int 53: 862–868, 1998. doi: 10.1111/j.1523-1755.1998.00825.x. [DOI] [PubMed] [Google Scholar]
- 15.Kvehaugen AS, Dechend R, Ramstad HB, Troisi R, Fugelseth D, Staff AC. Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension 58: 63–69, 2011. doi: 10.1161/HYPERTENSIONAHA.111.172387. [DOI] [PubMed] [Google Scholar]
- 16.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 303: H1–H8, 2012. doi: 10.1152/ajpheart.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangos GJ, Spaan JJ, Pirabhahar S, Brown MA. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens 30: 351–358, 2012. doi: 10.1097/HJH.0b013e32834e5ac7. [DOI] [PubMed] [Google Scholar]
- 18.Martillotti G, Ditisheim A, Burnier M, Wagner G, Boulvain M, Irion O, Pechère-Bertschi A. Increased salt sensitivity of ambulatory blood pressure in women with a history of severe preeclampsia. Hypertension 62: 802–808, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01916. [DOI] [PubMed] [Google Scholar]
- 19.Matsui K, Nagy-Bojarsky K, Laakkonen P, Krieger S, Mechtler K, Uchida S, Geleff S, Kang DH, Johnson RJ, Kerjaschki D. Lymphatic microvessels in the rat remnant kidney model of renal fibrosis: aminopeptidase p and podoplanin are discriminatory markers for endothelial cells of blood and lymphatic vessels. J Am Soc Nephrol 14: 1981–1989, 2003. doi: 10.1097/01.ASN.0000076078.50889.43. [DOI] [PubMed] [Google Scholar]
- 20.McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis 55: 1026–1039, 2010. doi: 10.1053/j.ajkd.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension 58: 709–715, 2011. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 22.Orabona R, Vizzardi E, Sciatti E, Bonadei I, Valcamonico A, Metra M, Frusca T. Insights into cardiac alterations after pre-eclampsia: an echocardiographic study. Ultrasound Obstet Gynecol, 49: 124–133, 2016. doi: 10.1002/uog.15983. [DOI] [PubMed] [Google Scholar]
- 23.Pruthi D, Khankin EV, Blanton RM, Aronovitz M, Burke SD, McCurley A, Karumanchi SA, Jaffe IZ. Exposure to experimental preeclampsia in mice enhances the vascular response to future injury. Hypertension 65: 863–870, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation 122: 579–584, 2010. doi: 10.1161/CIRCULATIONAHA.110.943407. [DOI] [PubMed] [Google Scholar]
- 25.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ 325: 157–160, 2002. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension 55: 1239–1245, 2010. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schock-Kusch D, Sadick M, Henninger N, Kraenzlin B, Claus G, Kloetzer HM, Weiss C, Pill J, Gretz N. Transcutaneous measurement of glomerular filtration rate using FITC-sinistrin in rats. Nephrol Dial Transplant 24: 2997–3001, 2009. doi: 10.1093/ndt/gfp225. [DOI] [PubMed] [Google Scholar]
- 28.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol 293: H2080–H2084, 2007. doi: 10.1152/ajpheart.00667.2007. [DOI] [PubMed] [Google Scholar]
- 29.Spaan JJ, Ekhart T, Spaanderman ME, Peeters LL. Reduced renal function after preeclampsia does not result from accelerated age-dependent renal function loss. Acta Obstet Gynecol Scand 89: 1202–1205, 2010. doi: 10.3109/00016349.2010.484043. [DOI] [PubMed] [Google Scholar]
- 30.Spaanderman ME, Ekhart TH, de Leeuw PW, Peeters LL. Angiotensin II sensitivity in nonpregnant formerly preeclamptic women and healthy parous controls. J Soc Gynecol Investig 11: 416–422, 2004. doi: 10.1016/j.jsgi.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Spradley FT, Palei AC, Granger JP. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Physiol Rep 1: e00081, 2013. doi: 10.1002/phy2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki H, Watanabe Y, Arima H, Kobayashi K, Ohno Y, Kanno Y. Short- and long-term prognosis of blood pressure and kidney disease in women with a past history of preeclampsia. Clin Exp Nephrol 12: 102–109, 2008. doi: 10.1007/s10157-007-0018-1. [DOI] [PubMed] [Google Scholar]
- 33.Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, Zeeman GG, Brown MA. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens 4: 97–104, 2014. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 34.van der Graaf AM, Toering T, Van der Wiel MK, Navis G, Buikema H, Lely AT, Faas MM. Angiotensin II sensitivity after preeclampsia in human and rat (Abstract). Reprod Sci 21: 402A, 2014. [Google Scholar]
- 35.van Rijn BB, Nijdam ME, Bruinse HW, Roest M, Uiterwaal CS, Grobbee DE, Bots ML, Franx A. Cardiovascular disease risk factors in women with a history of early-onset preeclampsia. Obstet Gynecol 121: 1040–1048, 2013. doi: 10.1097/AOG.0b013e31828ea3b5. [DOI] [PubMed] [Google Scholar]
- 36.Vikse BE, Irgens LM, Karumanchi SA, Thadhani R, Reisæter AV, Skjærven R. Familial factors in the association between preeclampsia and later ESRD. Clin J Am Soc Nephrol 7: 1819–1826, 2012. doi: 10.2215/CJN.01820212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800–809, 2008. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 38.Wang IK, Muo CH, Chang YC, Liang CC, Chang CT, Lin SY, Yen TH, Chuang FR, Chen PC, Huang CC, Wen CP, Sung FC, Morisky DE. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ 185: 207–213, 2013. doi: 10.1503/cmaj.120230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weissgerber TL, Milic NM, Milin-Lazovic JS, Garovic VD. Impaired flow-mediated dilation before, during, and after preeclampsia: a systematic review and meta-analysis. Hypertension 67: 415–423, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu CC, Chen SH, Ho CH, Liang FW, Chu CC, Wang HY, Lu YH. End-stage renal disease after hypertensive disorders in pregnancy. Am J Obstet Gynecol 210: 147.e1–147.e8, 2014. doi: 10.1016/j.ajog.2013.09.027. [DOI] [PubMed] [Google Scholar]