Abstract
To maintain core body temperature in mammals, the normal central nervous system (CNS) thermoregulatory reflex networks produce an increase in brown adipose tissue (BAT) thermogenesis in response to skin cooling and an inhibition of the sympathetic outflow to BAT during skin rewarming. In contrast, these normal thermoregulatory reflexes appear to be inverted in hibernation/torpor; thermogenesis is inhibited during exposure to a cold environment, allowing dramatic reductions in core temperature and metabolism, and thermogenesis is activated during skin rewarming, contributing to a return of normal body temperature. Here, we describe two unrelated experimental paradigms in which rats, a nonhibernating/torpid species, exhibit a “thermoregulatory inversion,” which is characterized by an inhibition of BAT thermogenesis in response to skin cooling, and a switch in the gain of the skin cooling reflex transfer function from negative to positive values. Either transection of the neuraxis immediately rostral to the dorsomedial hypothalamus in anesthetized rats or activation of A1 adenosine receptors within the CNS of free-behaving rats produces a state of thermoregulatory inversion in which skin cooling inhibits BAT thermogenesis, leading to hypothermia, and skin warming activates BAT, supporting an increase in core temperature. These results reflect the existence of a novel neural circuit that mediates inverted thermoregulatory reflexes and suggests a pharmacological mechanism through which a deeply hypothermic state can be achieved in nonhibernating/torpid mammals, possibly including humans.
Keywords: brown adipose tissue, hibernation, hypothermia, thermogenesis, thermoregulation
autonomic thermoregulation contributes to the maintenance of a homeostatic body core temperature (TCORE) (6). The normal thermoregulatory reflex regulation of TCORE is achieved through a complex neural network (22, 28, 30). Peripheral thermal signals from cutaneous thermoreceptors are transmitted via the lateral parabrachial nucleus (lPBN) (33, 34) to be integrated with TCORE information in the preoptic area of the hypothalamus (POA). Such integration results in rebalancing of the inhibitory and excitatory inputs to medullary premotor neurons involved in the control of peripheral thermoeffectors, which are engaged to counteract environmental temperature challenges to TCORE. Specifically regarding thermogenesis in brown adipose tissue (BAT), the central nervous system (CNS) thermoregulatory network responds to skin cooling or to decreased TCORE by increasing BAT activation (27, 28) and to skin warming by inhibiting BAT thermogenesis. These responses are thought to result from respective increases and decreases in the activity of thermogenesis-promoting neurons in the dorsomedial hypothalamus (DMH) (26, 28, 32, 33, 35) that provide excitatory input to BAT sympathetic premotor neurons in the rostral raphe pallidus (rRPa) (7). In addition, because thermogenesis is energetically demanding, neurotransmission within the thermoregulatory network leading to activation of thermogenesis is strongly influenced by the CNS networks controlling energy balance and metabolism (19, 21, 28).
Prompted by the apparent failure of cold-defense reflexes in hibernating/torpid mammals, in which TCORE falls during cold exposure, and by the search for new approaches for the induction of therapeutic hypothermia (3), we sought to determine in this study whether evidence could be provided for a brain state, exhibited by a nonhibernating/nontorpid mammal, in which the normal cold- and warm-defensive reflex regulation of BAT thermogenesis was inverted. Here, we define this unique state of the CNS thermoregulatory network as “thermoregulatory inversion,” in which cold exposure results in an inhibition of BAT sympathetic nerve activity (SNA) and BAT thermogenesis and warm exposure stimulate BAT activity. We demonstrate that the phenomenon of thermoregulatory inversion can be induced by two different and unrelated experimental manipulations: 1) a transection of the neuraxis immediately rostral to the DMH (i.e., pre-DMH transection) that severs the inputs to the DMH from more rostral structures, including the POA, and 2) activation of central A1 adenosine receptors (A1AR), which we have shown to induce a deeply hypothermic state in free-behaving rats exposed to a cool ambient temperature (TAMB) (44).
METHODS
Animals.
Male Wistar rats (300–400 g; Charles River Laboratories) were maintained on a standard 12:12-h light-dark cycle (lights on at 0900) with ad libitum access to standard chow and water. Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (8th ed., National Research Council, National Academic Press, 2010), and protocols were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University.
Surgical and experimental procedures for experiments involving recording of BAT SNA.
Rats were anesthetized initially with 3% isoflurane in 100% O2 and transitioned to urethane (0.8 g/kg) and chloralose (80 mg/kg) following cannulation of a femoral artery and vein. Heart rate was derived from the femoral arterial pressure signal. Rats were positioned prone in a stereotaxic frame, with the incisor bar at −4 mm below interaural zero. A spinal clamp installed on the T10 vertebra was used to maintain the spine in a rigid and elevated position that provided 1) a constant and horizontal positioning of the caudal brain stem, 2) an optimal oil pool within which to record BAT SNA, and 3) a reduced potential for respiratory-related artifacts in the BAT SNA recording. Rats were paralyzed with d-tubocurarine (0.3 mg initial dose, 0.1 mg/h supplements) and artificially ventilated via a tracheal cannula with 100% O2 (60–70 cycles/min, tidal volume 3–3.5 ml). Small adjustments in minute ventilation were made to maintain basal mixed/expired CO2 levels between 3.0 and 4.5%. Thermocouples (Physitemp Instruments with Sable Systems International meter) were placed 1) on the shaved abdominal skin to measure skin temperature (TSKIN) beneath a water-perfused blanket wrapped around the rat’s trunk, 2) 6 cm into the rectum to measure TCORE, and 3) into the medial aspect of the left interscapular BAT (iBAT) pad to measure TBAT. TCORE was maintained at ~37°C by perfusing the water blanket with warm water. As required for specific experimental protocols, TSKIN and TCORE were adjusted by changing the temperature of the water perfusing the thermal blanket; cooling elicited a basal level of BAT SNA and warming inhibited BAT SNA via stimulation of skin thermal receptors.
Postganglionic BAT SNA was recorded from the central cut end of a small nerve bundle dissected from the ventral surface of the right iBAT pad after the fat pad was divided along the midline and reflected laterally. Nerve activity was recorded with bipolar hook electrodes, filtered (1–300 Hz), and amplified (×20,000, Cyberamp 380; Axon Instruments). Evoked nerve potentials following electrical stimulation of the sympathetic premotor neurons in the rostral raphe pallidus (rRPa; relative to lambda: −3.0 mm posterior, 0.0 mm lateral, −9.2 mm ventral) verified the viability of the isolated BAT nerve.
Procedure for pre-DMH transection.
A cranial window (~4 × 4 mm) was made just behind bregma and centered on the sagittal suture. The dura mater was carefully dissected from the superior sagittal sinus and removed throughout the cranial window to allow transection of the brain without damage to the sinus or the major vessels converging to it. A transection knife (15 mm long, 2 mm wide, and 0.1 mm thick) was mounted vertically in a stereotaxic manipulator and positioned perpendicular and lateral to the sagittal sinus at −2.0 mm caudal to bregma and with the medial edge on the midline. To minimize bleeding during the transection procedure, the sagittal sinus was retracted laterally to allow the insertion of the knife. The knife was then lowered into the brain sequentially on the left and right sides of the superior sagittal sinus to a depth of ~10 mm, which avoided any rupture of the vessels in the circle of Willis. This transection procedure resulted in very little bleeding, appearing to be limited to small parenchymal vessels in the path of the transection knife. After each experiment, the brain was perfused with 4% formalin fixative, and the region of the transection was dissected to assess its position and extent.
Surgical procedure for experiments in awake, free-behaving rats.
Rats were anesthetized with 2% isoflurane in 100% O2 and instrumented for chronic recording of physiological variables, as described previously (44). Specifically for the results described here, rats were implanted with miniature thermistors (NTC surface mount thermistor, NB21L00223JBB; Farnell) for recording TBAT (from beneath the interscapular BAT) and TCORE (from the mediastinum). The TBAT probe failed in two of the six rats. A guide cannula (C315G-26 gauge; PlasticsOne) was stereotaxically positioned in the lateral ventricle for intracerebroventricular injection of drugs. Electrode wires were tunneled subcutaneously and connected to a 12-pin electronic connector that, along with the intracerebroventricular guide cannula, was secured to the skull with screws and dental acrylic. After the surgical procedure, rats were treated with buprenorphine (0.1 mg/kg) and penicillin G (40 kIU/kg) and hydrated with isotonic saline (5 ml sc). Each rat recovered for 7 days in a temperature-controlled recording chamber at an ambient temperature (TAMB) of 25°C, received daily meloxicam (1 mg/kg orally) for the first 5 days to reduce postsurgical inflammation and was acclimated to the recording tether for 2 days before the experimental recording sessions.
Intracerebroventricular drug injection procedure.
N6-cyclohexyladenosine (CHA; Sigma Aldrich) was dissolved in isotonic saline (vehicle) to a concentration of 1 mM. For intracerebroventricular injections, an internal cannula was connected to a 25-µl Hamilton syringe and positioned in the implanted guide cannula, and 10 µl of drug solution was injected into the lateral ventricle for 2 min. To perform the intracerebroventricular injection, the rat was disconnected from the recording apparatus and briefly removed from the recording chamber, the intracerebroventricular injection procedure was performed in <5 min with minimal stress to the rat, and the rat was immediately returned to the recording chamber for continued data acquisition. The absence of a change in TCORE following intracerebroventricular injections of vehicle during cold exposure was reported previously (44).
Data acquisition.
Physiological variables were digitized (Micro 1401 MKII; Cambridge Electronic Design) at the following rates: 1 kHz BAT SNA, 5 Hz TBAT, 5 Hz TCORE, 5 Hz TSKIN, 5 Hz TPAW, 200 Hz expired CO2, and 200 Hz AP. They were then recorded onto a computer hard drive for subsequent analysis (Spike 2, CED). A continuous measurement (4-s bins) of BAT SNA amplitude was calculated as the root mean square (square root of the total power in the 0.1- to 20-Hz band of the autospectrum) value of sequential 4-s segments of the BAT SNA signal.
Data and statistical analysis.
For analysis of the physiological variables in the pre-DMH transection experiment, the data were averaged into 30-s bins, and group data are reported as means ± SE. To account for slight differences in nerve recording characteristics among experiments, raw BAT SNA values in individual experiments were normalized to the minimum BAT SNA, recorded under warm conditions (i.e., TCORE >36°C), and expressed as percent of control value. To quantify the effects of skin cooling and skin warming treatments on BAT SNA or on TBAT, we calculated cooling response and warming response transfer function gain values. In experiments in which BAT SNA was recorded, these gain values were calculated by dividing the change in BAT SNA (i.e., the reflex output) by the change in TSKIN (i.e., the reflex input). For a normal cold-defense reflex, for instance, the reflex gain would be negative, since increases in BAT SNA are produced by decreases in TSKIN. In experiments in awake, free-behaving rats, these reflex gains were calculated as the change in TCORE (i.e., the reflex output) divided by the change in TAMB (i.e., the reflex input). Data were considered to be distributed normally if positive results were obtained by at least two of the following three normality tests: D’Agostino & Pearson omnibus, Kolmogorov-Smirnov test, and Shapiro-Wilk. Data are reported as means ± SE. All statistics were performed using Prism software (version 6; GraphPad Software). The statistical comparisons used the nonparametric one- or two-tailed Wilcoxon matched-pair test. Statistical results with P < 0.05 were considered significant.
RESULTS
A pre-DMH transection of the neuraxis produces thermoregulatory inversion.
Descending projections from the POA, including those to the DMH and the rRPa (31, 33–35, 42), are responsible for the cutaneous and core temperature-derived modulations of BAT thermogenesis during normal thermoregulatory reflex responses (31, 33–35, 42). Based on this fact, we hypothesized that eliminating the influence of POA neurons on the thermogenesis-promoting neurons in the DMH might reveal a heretofore unrecognized modulation of the BAT sympathoexcitatory neurons in the DMH that could support thermoregulatory inversion. To test this hypothesis, we severed the axonal connections between the POA and caudal brain structures with a pre-DMH transection (Fig. 1D) and determined the effect of skin cooling and skin warming on BAT SNA.
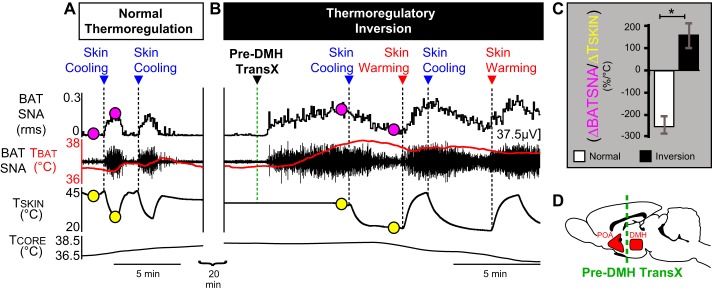
Fig. 1.
Predorsomedial hypothalamus (DMH) transection, which eliminates the rostral hypothalamic input from downstream brain structures, induces a dramatic thermoregulatory inversion. A: a naïve rat exhibiting normal thermoregulation in which skin cooling produces a strong and repeatable increase in brown adipose tissue (BAT) sympathetic nerve activity (SNA) and BAT temperature (TBAT; red trace), whereas skin rewarming inhibits BAT SNA and reduces TBAT. B: after a pre-DMH transection, the same rat exhibits thermoregulatory inversion, in which skin cooling produces strong and repeatable inhibitions of BAT SNA and falls in TBAT, whereas skin rewarming elicits prompt and repeatable increases in BAT SNA and TBAT. Circles indicate the 2 points corresponding to the zenith and nadir of TSKIN values in the recordings in the naïve rat (A) and after the pre-DMH transection (B) at which respective BAT SNA (pink circles) and TSKIN (yellow circles) values were taken to calculate the gain (C; ΔBAT SNA/ΔTSKIN) of the skin-cooling response transfer function during thermoregulatory reflex responses under conditions of normal and pre-DMH transection. The change in sign of the gain of the skin-cooling transfer function from negative values under conditions of normal thermoregulation (A) to positive values following pre-DMH transection (B) reflects the transition to a state of thermoregulatory inversion. D: schematic of a midline sagittal section of the rat brain illustrating the relative positions of the preoptic area (POA), the pre-DMH transection (dashed green line), and the DMH. TSKIN, skin temperature; TCORE, core temperature; rms, root mean square.
In naïve rats, the normal thermoregulatory response to skin cooling is a prompt increase in BAT SNA (ΔTSKIN = −6.0 ± 1.7°C from a baseline of 37.2 ± 1.0°C; ΔBAT SNA: +1,280.9 ± 249.8% of precooling control; n = 6, P = 0.0156; Fig. 1A) (32), resulting in a negative mean cooling response transfer function gain (ΔBAT SNA/ΔTSKIN) equal to −247.4 ± 39.4% control SNA/°C. Conversely, skin warming in a naïve rat produces a strong inhibition of BAT SNA (Fig. 1A), returning BAT SNA to the low levels consistently observed in warm rats.
In a warm rat with a low level of BAT SNA, a pre-DMH transection immediately increased BAT SNA and TBAT (TSKIN = 36.0 ± 0.4°C; ΔBAT SNA: +960.2 ± 463.1% of pretransection control; n = 5, P = 0.035; Fig. 1B), resulting in an increase in TBAT (Fig. 1B) of +0.5 ± 0.1°C (P = 0.03). We characterize an elevated BAT SNA in a rat with a warm TSKIN as an inverted thermoregulatory response to skin warming. Subsequent thermoregulatory responses to skin cooling (Fig. 1B) indicated that pre-DMH transections result in a sustained state of thermoregulatory inversion, rather than simply removing a tonic inhibitory influence on BAT SNA. Following pre-DMH transection, skin cooling consistently caused decreases in BAT SNA (ΔTSKIN = −6.7 ± 1.7°C from a baseline of 36.7 ± 1.0°C; ΔBAT SNA = −906.3 ± 403.5% of precooling BAT SNA; n = 6, P = 0.0156; Fig. 1B), resulting in a positive mean cooling-response transfer function gain (ΔBAT SNA/ΔTSKIN) equal to +157.7 ± 55.9% control SNA/°C (Fig. 1C). Subsequent skin-warming episodes again produced an inverted response in BAT, i.e., a prompt and significant increase in BAT SNA (Fig. 1B), resulting in a positive mean warming/response transfer function gain equal to +196.3 ± 38.6% control SNA/°C (Fig. 1C). The change in sign of the thermoregulatory response transfer functions from negative values in the naïve rat to positive values after pre-DMH transection (Fig. 1C) is the hallmark of a transition to a thermoregulatory inversion.
Thermoregulatory inversion governs TCORE during the torpor-like state induced by central activation of A1AR.
Central administration of the A1AR agonist CHA in free-behaving rats exposed to an ambient temperature (TAMB) of 15°C induces a torpor-like state characterized by a deep hypothermia (44) (Figs. 2A and 3A). To test the hypothesis that the hypothermic component of the torpor-like state induced by intracerebroventricular administration of CHA represents a thermoregulatory inversion, we determined the effects of skin cooling and rewarming (via changes in TAMB) on TCORE in free-behaving rats (n = 6) after an intracerebroventricular injection of CHA.
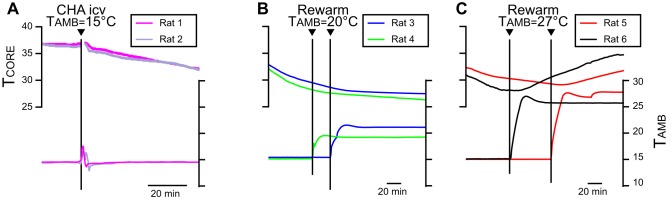
Fig. 2.
A: core body temperature (TCORE; top traces) and ambient temperature (TAMB; bottom traces) records from 2 awake, free-behaving rats during the onset of the hypothermia induced by intracerebroventricular (icv) administration of the A1 adenosine receptor (A1AR) agonist N6-cyclohexyladenosine (CHA) at a TAMB of 15°C. Note that TCORE declines under conditions of cold TAMB, consistent with an inhibition of thermogenesis. B: TCORE and TAMB records from 2 different intracerebroventricular CHA-treated rats during skin rewarming with a TAMB of 20°C. Note that TCORE continues to decline under conditions of a TAMB of 20°C. C: TCORE and TAMB records from 2 different intracerebroventricular CHA-treated rats during skin rewarming with a TAMB of 27°C. Note that TCORE increases under conditions of a TAMB of 27°C and that the rise in TCORE was time locked to the increase in TAMB.
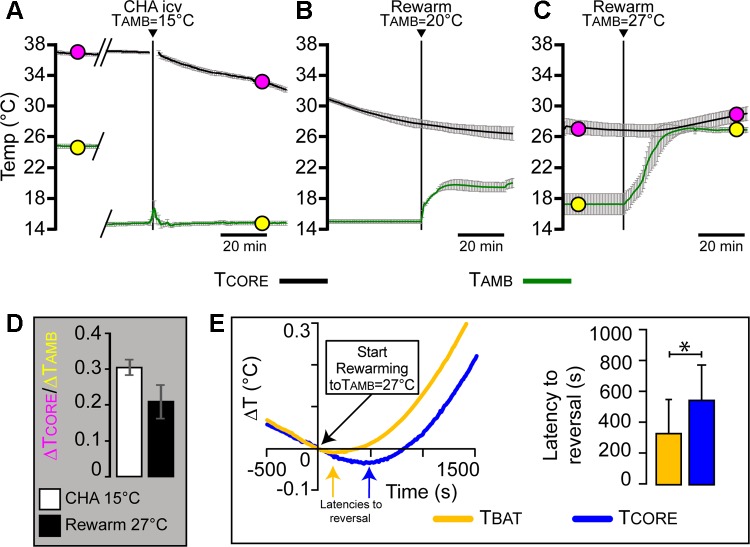
Fig. 3.
Activation of central A1 adenosine receptors (A1AR) induces a deeply hypothermic state characterized by a thermoregulatory inversion. A: time courses (1-min bins) of the mean ± SE body core temperature [(TCORE; top traces (black)] and ambient temperature [TAMB; bottom traces (green)] records from 6 awake, free-behaving rats during the onset of the hypothermia induced by the intracerebroventricular (icv) administration (arrowhead) of the A1AR agonist CHA after a reduction of TAMB to 15°C, which occurred during the trace breaks (diagonal lines). B: time courses of the mean ± SE TCORE (black) and TAMB (green) records from 3 free-behaving, intracerebroventricular, CHA-treated rats during a skin rewarming with a TAMB of 20°C. C: time courses of the mean ± SE TCORE (black) and TAMB (green) records from 6 free-behaving, intracerebroventricular, CHA-treated rats during a skin rewarming with a TAMB of 27°C. Filled circles (A and C) indicate the pairs of simultaneous TCORE (pink) and TAMB (yellow) values used to calculate the gain (ΔTCORE/ΔTAMB; D) of the skin cooling response (A) and skin warming response (C) transfer functions in CHA-treated rats. The positive sign of the gain of the thermoregulatory response transfer function is a hallmark of thermoregulatory inversion. E: representative example (left) of the time courses of the TBAT (yellow trace) and TCORE (blue trace) surrounding the time point (time 0) at which TAMB began to increase. The latency to the reversal of the TBAT (yellow arrow) was ~340 s shorter than that of the TCORE (blue arrow). The mean (n = 4) latency to the reversal of the decline in TBAT was significantly (*P < 0.05) shorter than that in TCORE, consistent with an activation of BAT thermogenesis that preceded the increase in TCORE.
CHA (1 mM, 10 µl icv) administration to rats in a TAMB of 15°C caused a slow and maintained decline in TCORE (Fig. 2A and 3A), from a baseline of 36.7 ± 0.2°C to a nadir of 31.8 ± 0.3°C (n = 6, P = 0.0313) during the first hour after intracerebroventricular injection of CHA. The finding that a reduction in TAMB elicited a fall in TCORE, resulting in a positively valued gain (ΔTCORE/ΔTAMB = +0.3 ± 0.02; Fig. 3D) for the skin-cooling response transfer function, is consistent with central CHA administration inducing a transition to a state of thermoregulatory inversion.
To establish that intracerebroventricular CHA induces a state of thermoregulatory inversion, we subsequently increased TAMB to test the effect of skin warming on TCORE. After ≥2 h of hypothermia (Table 1), rats were rewarmed by increasing the TAMB to a nominal TAMB of 27°C (actual mean TAMB: 26.9 ± 0.3°C; Figs. 2C and 3C). Rewarming with a nominal TAMB of 27°C produced increases in TCORE (26.7 ± 0.8°C before rewarming to 29.0 ± 0.9°C after 1 h of rewarming; n = 6, P = 0.031; Figs. 2C and 3C), thereby reversing the hypothermia induced by intracerebroventricular CHA. The increase in TCORE during rewarming resulted in a positively valued gain (ΔTCORE/ΔTAMB = +0.2 ± 0.04; Fig. 3D) for the skin-warming response transfer function, consistent with thermoregulatory inversion. Rewarming at TAMB at 27°C also induced a slow awakening from the torpor-like state. Throughout the rewarming, TAMB was always less than TCORE for each rat, and therefore, there was no temperature gradient that would support a passive heat transfer from the ambient air to the rat. TBAT declined in parallel with TCORE at a TAMB of 15°C, and these declines were reversed during the increase in TAMB to 27°C (Fig. 3E, left). In particular, the fall in TBAT was always reversed before that in TCORE (Fig. 3E, left). To compare the relative latencies to reversal, we measured for each rat the delay between the time point at which TAMB began to rise and the respective time points at which the TBAT and the TCORE began to rise (Fig. 3E, left). The mean latency to reversal for TBAT was significantly (P < 0.05) shorter than that for TCORE (Fig. 3E, right). This result is consistent with a role for increases in BAT thermogenesis in initiating and contributing to the rewarming-stimulated increases in TCORE (Figs. 2C and 3, C and E), similar to results implicating BAT activation in the recovery of TCORE following bouts of hibernation/torpor (11, 15, 36, 39). However, once TCORE begins to rise, we would expect a contribution to rising TCORE from a simultaneous increase in obligatory metabolic thermogenesis arising from the inefficiency of ATP catabolism as cellular temperatures, and thus their metabolic consumption of ATP, increase.
Table 1.
Time interval between intracerebroventricular injection of CHA and increase in TAMB to rewarm the skin
| RT164 | RT169 | RT172 | RT181 | RT390 | RT391 | |
|---|---|---|---|---|---|---|
| Interval (h:min) between CHA injection and increased TAMB = 20°C | 2:36 | 2:06 | 2:04 | |||
| Interval (h:min) between CHA injection and increased TAMB = 27°C | 3:10 | 5:49 | 5:26 | 5:52 | 7:08 | 2:07 |
CHA, N6-cyclohexyladenosine; TAMB, ambient temperature.
In three rats, the rewarming included a 30-min period during which TAMB was maintained at 19.5 ± 0.2°C (Figs. 2B and 3B). Unlike rewarming with a TAMB at 27°C, rewarming with a TAMB at 20°C did not reverse the decline in TCORE that followed the intracerebroventricular CHA treatment (Figs. 2B and 3B). We also observed that when rewarming with a TAMB of 27°C, the resulting increase in TCORE was independent of the time (Table 1) after CHA administration, at which the TAMB was increased to 27°C. Together, these results indicate that the increase in TCORE when TAMB was raised to 27°C was not simply due to a decline in the pharmacological effectiveness of the CHA treatment.
DISCUSSION
In the separate settings of a pre-DMH brain transection in anesthetized rats and of a pharmacological activation of central A1AR in free-behaving rats, we have discovered the unexpected phenomenon of thermoregulatory inversion. This represents a novel state of the central thermoregulatory network in which the normal thermoregulatory reflex responses of BAT thermogenesis to skin thermoreceptors are inverted; skin cooling inhibits BAT SNA and BAT thermogenesis, whereas skin warming facilitates BAT heat production. Although we have described thermoregulatory inversion in two model systems employing the nonphysiological maneuvers of brain transection and intracerebroventricular drug injection, the fact that inverted thermoregulatory reflex responses can be clearly and consistently observed in the rat indicates that the neuroanatomic and neurochemical substrates mediating such inverted thermoregulatory reflex responses, and thus responsible for the novel thermoregulatory state of thermoregulatory inversion, are present in the brains of nonhibernating/nontorpid mammals, perhaps including humans. Although our experiments do not elucidate the neural circuitry underlying thermoregulatory inversion, the demonstration of thermoregulatory inversion following a pre-DMH transection is consistent with the existence of heretofore unrecognized thermoregulatory reflex pathways in the region caudal to the transection level (~2.0 mm caudal to bregma), and through which cutaneous thermoreceptor signaling can evoke inverted cold- and warm-defense responses in BAT SNA and BAT thermogenesis, without the integration of cutaneous thermal stimuli by POA neurons (41). Because intracerebroventricular CHA treatment produces not only a thermoregulatory inversion but also a torpor-like state in free-behaving rats exposed to a cool TAMB (44), we speculate that establishing a state of thermoregulatory inversion within the central thermoregulatory network is an essential component of the induction of the hypothermia through which hibernating/torpid mammals reduce their metabolism and energy consumption to cope with cold environmental challenges.
Regarding the neural pathways underlying the normal cold-defensive activation and the warm-defensive inhibition of BAT thermogenesis, cutaneous thermal afferent pathways synapse in the dorsal horn (10) and in the lPBN (12, 32, 34), providing opportunities for spinal modulation of cutaneous thermal signals (9), as well as their parabrachial integration with other sensory modalities such as viscerosensory inputs to the nucleus of the tractus solitarii (13) and metabolic signals from the arcuate nucleus (5). However, the demonstration that cutaneous thermal signals are transmitted from the lPBN to the POA (34) and the existence of GABAergic neurons in POA that are intrinsically activated by the warming of their local environment (4, 18, 38, 41) and have projections to DMH (41) provides strong support for the view that the POA is the site at which cutaneous and core thermal signals are integrated to produce inhibitory output signals to DMH neurons that regulate the level of heat production in BAT, such that skin cooling drives a disinhibition of thermogenesis-promoting neurons in DMH, resulting in increased BAT activity.
Consistent with this model (26) of the reflex circuit for the cold-defensive activation of BAT, we found that a pre-DMH transection produced an increase in BAT SNA and TBAT (Fig. 1B), potentially resulting from severing the axons of the GABAergic, warm-sensitive neurons in the POA that project to the DMH (41). Indeed, similar increases in TBAT and TCORE following a similar brain transection immediately caudal to the POA were reported earlier (8). However, if the sole effect of a pre-DMH transection was to eliminate the warm skin-driven GABAergic inhibition of BAT sympathoexcitatory neurons in the DMH, similar to injections of the GABAA receptor antagonist bicuculline into the DMH (7), the expectation would have been that subsequent skin cooling would have no effect on the elevated level of BAT SNA following the pre-DMH transection. Remarkably, skin cooling following a pre-DMH transection inhibited BAT SNA (Fig. 1B), thereby yielding a positively valued cooling reflex gain. Subsequent skin rewarming activated BAT SNA, again yielding a positively valued reflex gain, and these reflex responses were repeatable (Fig. 1B) and consistent among rats (Fig. 1C). These observations provide indirect evidence suggesting that simply eliminating the GABAergic input to DMH is not sufficient to produce thermoregulatory inversion. Together, these results reveal the existence of a novel thermoregulatory paradigm, thermoregulatory inversion, which reflects a brain state that could produce global energy conservation, employing the inhibition of thermogenic energy consumption in the cold as a strategy to lower TCORE and thereby reduce cellular metabolism.
Our finding that a pre-DMH transection results in a state of thermoregulatory inversion suggests that the neural circuitry mediating the resulting skin cooling-evoked inhibition of BAT SNA and skin warming-evoked activation of BAT SNA resides in the brain regions at or caudal to the level of the DMH. As described above, the cutaneous thermoreceptor afferents driving normal thermoregulatory reflex responses synapse in the dorsal horn and then in the lPBN, and both of these regions are caudal to the level of the pre-DMH transection. On the efferent side of the normal thermoregulatory reflexes, the activity of neurons in the DMH that project to BAT sympathetic premotor neurons in rRPa is increased during normal cold-defensive activation of BAT thermogenesis (32) and thought to be decreased during normal warm-defensive inhibition of BAT activity (33). With the expectation that the same cutaneous thermal afferents mediate the thermoregulatory inverted reflex responses in BAT SNA following pre-DMH transection, we speculate that these inverted responses would employ heretofore unrecognized connections between thermal afferent signaling in the dorsal horn and/or lPBN and BAT sympathoexcitatory efferent neurons in the DMH and/or the rRPa and/or the spinal intermediolateral nucleus. Future experiments will delineate the pathways for reflex responses under conditions of thermoregulatory inversion.
Because activation of local A1AR inhibits POA neurons (29), and a pre-DMH transection eliminates descending axonal projections from POA to DMH, our two models for generating a state of thermoregulatory inversion may have the common aspect of excluding the influence of POA neurons from the neural network mediating thermoregulatory responses. Consistent with this possibility, inverted thermoregulatory responses may also occur during the transition from non-rapid eye movement (NREM) sleep to REM sleep, a condition in which POA thermosensitive neurons are inhibited (1, 37). In animals exposed to a low ambient temperature, the entrance into REM sleep is accompanied by a paradoxical inhibition of thermogenesis that can lead to hypothermia (2, 37). Additionally, the POA responses to thermal stimuli, which are normally active during wakefulness and NREM sleep, are abolished during REM sleep (1, 14, 23, 24). Although more specific experiments are required, these observations and our current results are consistent with the possibility that the REM sleep state includes an inhibition of the activity of a population of thermoregulatory neurons in the POA that results in the induction of a state of thermoregulatory inversion.
The present results provide strong albeit indirect support for our hypothesis that the induction and maintenance of the hypothermia characteristic of natural hibernation/torpor is an active, neurochemically driven process that is achieved through the natural induction of a state of thermoregulatory inversion. First, the thermoregulatory inversion following a pre-DMH transection, in which cooling elicits an inhibition of facultative thermogenesis, is a state of the central thermoregulatory network that would be conducive to producing hypothermia in an environment in which TAMB is less than TCORE. Under these TAMB conditions, heat will leave the body and TCORE will fall, with the rate of the reduction in TCORE dependent on the level of cutaneous vasoconstriction, on the difference between TAMB and TCORE and on the level of obligatory thermogenesis. This would be a positive feedback process, since obligatory thermogenesis will be reduced as cellular metabolism is reduced and TCORE falls. Second, we demonstrate here that intracerebroventricular administration of the A1AR agonist CHA produces a state of the central thermoregulatory network that is deeply hypothermic and has the characteristics of thermoregulatory inversion. Intracerebroventricular CHA administration to rats in a cold TAMB also induces a behavioral state in this nonhibernating/torpid species with many of the characteristics of hibernation/torpor (43, 44). Third, central A1AR administration induces a hibernation/torpor-like state in naturally hibernating/torpid species (25, 29) and plays a prominent role in the natural induction of hibernation/torpor (16, 17, 40). Thus, although our observations are consistent with the potential for thermoregulatory inversion to be a paradigm-shifting approach to understanding the neural mechanism underlying the descent of hibernating/torpid mammals into a deep hypothermia, such a conclusion must await detailed studies of the central thermoregulatory network in naturally hibernating/torpid species.
Overall, the close parallels between the state of thermoregulatory inversion induced by either pre-DMH transection or activation of central A1AR in nonhibernating/torpid species and the hypothermia of the naturally occurring hibernation/torpor state suggest not only that thermoregulatory inversion plays a role in the induction of the deeply hypothermic state of hibernation but also that a “hibernation/torpor neuronal circuit” responsible for thermoregulatory inversion exists in the brains of nonhibernating/torpid species, possibly including humans. However, the current results do not address the perplexing question of why, if the circuitry for thermoregulatory inversion exists in the brains of nonhibernating/torpid species, only hibernating/torpid species can access this energy-conserving behavioral paradigm naturally. It may be, for instance, that the levels of an endogenous A1AR ligand are never sufficiently elevated in the required brain regions of nonhibernating/nontorpid mammals to provoke a transition to the state of thermoregulatory inversion that induces entrance into the hibernating/torpid behavioral state with its accompanying deep hypothermia. Nonetheless, our results demonstrate clearly that the neural circuitry required for the inverted skin thermoreceptor-evoked responses in BAT SNA must exist in the brain regions at the level of or caudal to the DMH of nonhibernating/torpid species. Further characterization of the neural circuitry underlying thermoregulatory inversion and of pharmacological approaches to induce this thermoregulatory state will be relevant for the induction of therapeutic hypothermia (43, 45, 46) and potentially for a better understanding of hypometabolic diseases such as obesity, characterized by reduced energy consumption in the cold (20, 47).
Perspectives and Significance
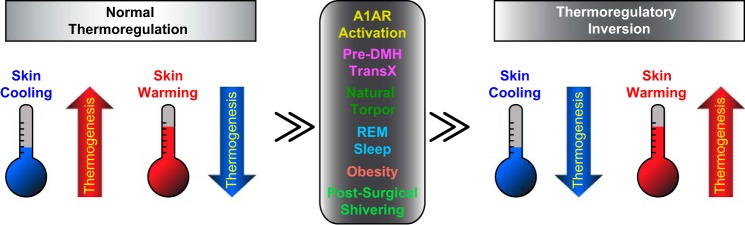
Our discovery of the novel thermoregulatory state of thermoregulatory inversion, demonstrated in the two experimental paradigms of a pre-DMH transection and activation of central A1AR (Fig. 4), opens a new field of thermoregulatory research into the induction of hypothermic, hypometabolic, energy-conserving behavioral states in nonhibernating/torpid species. Thermoregulatory inversion could explain the basis for the induction of hypothermia and hypometabolism of the hibernating/torpid state (Fig. 4) and provide new insights that will lead to the discovery of novel pharmacological approaches to induce thermoregulatory inversion in nonhibernating/torpid species, including humans. Induction of therapeutic hypothermia is beneficial for improving outcomes in ischemic pathologies, perinatal hypoxic encephalopathy, and COX inhibitor-resistant fevers (43, 45, 46). Additionally, one could hypothesize that thermoregulatory inversion, perhaps in competition with normal thermoregulatory responses, could occur in hypometabolic diseases such as obesity (Fig. 4), in which BAT thermogenesis is not properly activated in the cold (20, 47), or in clinical conditions such as postsurgical shivering (Fig. 4), in which thermogenesis is strongly activated in a warm environment. Maintaining a state of thermoregulatory inversion could also contribute to the reduction in human body temperature and metabolism required for prolonged space travel.
Fig. 4.
Graphic representing potential triggers and conditions in which “normal thermoregulation” may be shifted to “thermoregulatory inversion.” A1AR, A1 adenosine receptor; Pre-DMH TransX, transection of the neuraxis immediately rostral to the dorsomedial hypothalamus.
GRANTS
This research was supported by a Collins Medical Trust Grant (to D. Tupone) and by National Institutes of Health Grants NS-040987 (to S. F. Morrison), NS-091066 (to S. F. Morrison), MH-097037 (to G. Cano), and HL-113180 (to G. Cano).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.T. and S.F.M. conceived and designed research; D.T. and S.F.M. performed experiments; D.T. and S.F.M. analyzed data; D.T., G.C., and S.F.M. interpreted results of experiments; D.T. prepared figures; D.T. drafted manuscript; D.T., G.C., and S.F.M. edited and revised manuscript; D.T., G.C., and S.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Rubing Xing for histological processing and Carol Pengshung for contributions to data analysis.
REFERENCES
- 1.Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am J Physiol Regul Integr Comp Physiol 269: R1240–R1249, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Alföldi P, Rubicsek G, Cserni G, Obál F Jr. Brain and core temperatures and peripheral vasomotion during sleep and wakefulness at various ambient temperatures in the rat. Pflugers Arch 417: 336–341, 1990. doi: 10.1007/BF00371001. [DOI] [PubMed] [Google Scholar]
- 3.Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, Garami A, Bautista D, Gavva NR, Romanovsky AA. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci 32: 2086–2099, 2012. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol 48: 639–654, 1986. doi: 10.1146/annurev.ph.48.030186.003231. [DOI] [PubMed] [Google Scholar]
- 5.Campos CA, Bowen AJ, Schwartz MW, Palmiter RD. Parabrachial CGRP neurons control meal termination. Cell Metab 23: 811–820, 2016. doi: 10.1016/j.cmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 7.Cao WH, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology 51: 426–437, 2006. doi: 10.1016/j.neuropharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol 512: 883–892, 1998. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science 265: 252–255, 1994. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- 10.Craig AD, Krout K, Andrew D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neurophysiol 86: 1459–1480, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Dark J. Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr 25: 469–497, 2005. doi: 10.1146/annurev.nutr.25.050304.092514. [DOI] [PubMed] [Google Scholar]
- 12.Geerling JC, Kim M, Mahoney CE, Abbott SB, Agostinelli LJ, Garfield AS, Krashes MJ, Lowell BB, Scammell TE. Genetic identity of thermosensory relay neurons in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 310: R41–R54, 2016. doi: 10.1152/ajpregu.00094.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geerling JC, Stein MK, Miller RL, Shin JW, Gray PA, Loewy AD. FoxP2 expression defines dorsolateral pontine neurons activated by sodium deprivation. Brain Res 1375: 19–27, 2011. doi: 10.1016/j.brainres.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glotzbach SF, Heller HC. Changes in the thermal characteristics of hypothalamic neurons during sleep and wakefulness. Brain Res 309: 17–26, 1984. doi: 10.1016/0006-8993(84)91006-0. [DOI] [PubMed] [Google Scholar]
- 15.Hindle AG, Martin SL. Intrinsic circannual regulation of brown adipose tissue form and function in tune with hibernation. Am J Physiol Endocrinol Metab 306: E284–E299, 2014. doi: 10.1152/ajpendo.00431.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iliff BW, Swoap SJ. Central adenosine receptor signaling is necessary for daily torpor in mice. Am J Physiol Regul Integr Comp Physiol 303: R477–R484, 2012. doi: 10.1152/ajpregu.00081.2012. [DOI] [PubMed] [Google Scholar]
- 17.Jinka TR, Tøien Ø, Drew KL. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. J Neurosci 31: 10752–10758, 2011. doi: 10.1523/JNEUROSCI.1240-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundius EG, Sanchez-Alavez M, Ghochani Y, Klaus J, Tabarean IV. Histamine influences body temperature by acting at H1 and H3 receptors on distinct populations of preoptic neurons. J Neurosci 30: 4369–4381, 2010. doi: 10.1523/JNEUROSCI.0378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madden CJ. Glucoprivation in the ventrolateral medulla decreases brown adipose tissue sympathetic nerve activity by decreasing the activity of neurons in raphe pallidus. Am J Physiol Regul Integr Comp Physiol 302: R224–R232, 2012. doi: 10.1152/ajpregu.00449.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madden CJ, Morrison SF. A high-fat diet impairs cooling-evoked brown adipose tissue activation via a vagal afferent mechanism. Am J Physiol Endocrinol Metab 311: E287–E292, 2016. doi: 10.1152/ajpendo.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol 566: 559–573, 2005. doi: 10.1113/jphysiol.2005.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAllen RM, Tanaka M, Ootsuka Y, McKinley MJ. Multiple thermoregulatory effectors with independent central controls. Eur J Appl Physiol 109: 27–33, 2010. doi: 10.1007/s00421-009-1295-z. [DOI] [PubMed] [Google Scholar]
- 23.McGinty D, Szymusiak R. Brain structures and mechanisms involved in the generation of NREM sleep: focus on the preoptic hypothalamus. Sleep Med Rev 5: 323–342, 2001. doi: 10.1053/smrv.2001.0170. [DOI] [PubMed] [Google Scholar]
- 24.McGinty D, Szymusiak R. Hypothalamic regulation of sleep and arousal. Front Biosci 8: s1074–s1083, 2003. doi: 10.2741/1159. [DOI] [PubMed] [Google Scholar]
- 25.Miyazawa S, Shimizu Y, Shiina T, Hirayama H, Morita H, Takewaki T. Central A1-receptor activation associated with onset of torpor protects the heart against low temperature in the Syrian hamster. Am J Physiol Regul Integr Comp Physiol 295: R991–R996, 2008. doi: 10.1152/ajpregu.00142.2008. [DOI] [PubMed] [Google Scholar]
- 26.Morrison SF. Central control of body temperature. F1000 Res 5: 880, 2016. doi: 10.12688/f1000research.7958.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 3: 00005, 2012. doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 19: 741–756, 2014. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muzzi M, Blasi F, Masi A, Coppi E, Traini C, Felici R, Pittelli M, Cavone L, Pugliese AM, Moroni F, Chiarugi A. Neurological basis of AMP-dependent thermoregulation and its relevance to central and peripheral hyperthermia. J Cereb Blood Flow Metab 33: 183–190, 2013. doi: 10.1038/jcbfm.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol 301: R1207–R1228, 2011. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. J Physiol 589: 3641–3658, 2011. doi: 10.1113/jphysiol.2011.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 292: R127–R136, 2007. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA 107: 8848–8853, 2010. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 11: 62–71, 2008. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience 161: 614–620, 2009. doi: 10.1016/j.neuroscience.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oelkrug R, Heldmaier G, Meyer CW. Torpor patterns, arousal rates, and temporal organization of torpor entry in wildtype and UCP1-ablated mice. J Comp Physiol B 181: 137–145, 2011. doi: 10.1007/s00360-010-0503-9. [DOI] [PubMed] [Google Scholar]
- 37.Parmeggiani PL. Thermoregulation and sleep. Front Biosci 8: s557–s567, 2003. doi: 10.2741/1054. [DOI] [PubMed] [Google Scholar]
- 38.Song K, Wang H, Kamm GB, Pohle J, Reis FC, Heppenstall P, Wende H, Siemens J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353: 1393–1398, 2016. doi: 10.1126/science.aaf7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swoap SJ, Weinshenker D. Norepinephrine controls both torpor initiation and emergence via distinct mechanisms in the mouse. PLoS One 3: e4038, 2008. doi: 10.1371/journal.pone.0004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamura Y, Shintani M, Nakamura A, Monden M, Shiomi H. Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain Res 1045: 88–96, 2005. doi: 10.1016/j.brainres.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 41.Tan CL, Cooke EK, Leib DE, Lin YC, Daly GE, Zimmerman CA, Knight ZA. Warm-sensitive neurons that control body temperature. Cell 167: 47–59.e15, 2016. doi: 10.1016/j.cell.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka M, McKinley MJ, McAllen RM. Preoptic-raphé connections for thermoregulatory vasomotor control. J Neurosci 31: 5078–5088, 2011. doi: 10.1523/JNEUROSCI.6433-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tupone D, Cetas JS, Morrison SF. Hibernation, hypothermia and a possible therapeutic “shifted homeostasis” induced by central activation of A1 adenosine receptor (A1AR). Nihon Shinkei Seishin Yakurigaku Zasshi 36: 51–54, 2016. [PMC free article] [PubMed] [Google Scholar]
- 44.Tupone D, Madden CJ, Morrison SF. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci 33: 14512–14525, 2013. doi: 10.1523/JNEUROSCI.1980-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tupone D, Madden CJ, Morrison SF. Highlights in basic autonomic neurosciences: central adenosine A1 receptor - the key to a hypometabolic state and therapeutic hypothermia? Auton Neurosci 176: 1–2, 2013. doi: 10.1016/j.autneu.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tupone D, Morrison S. Hypothermia, torpor and the fundamental importance of understanding the central control of thermoregulation. Temperature (Austin) 1: 89–91, 2014. doi: 10.4161/temp.29916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]