Abstract
This study was designed to determine the effect of active sensitization with ovalbumin (Ova) on cough responses to inhaled irritant gases in mice. Conscious mice moved freely in a recording chamber, while the pressure change in the chamber and audio and video signals of the mouse movements were recorded simultaneously to measure the frequencies of cough reflex (CR) and expiration reflex (ER). To further verify the accuracy of cough analysis, the intrapleural pressure was also recorded by a telemetry sensor surgically implanted in the intrapleural space in a subgroup of mice. During the irritant gas inhalation challenge, sulfur dioxide (SO2; 200 and 400 ppm) or ammonia (NH3; 0.1% and 0.2%) was drawn into the chamber at a constant flow rate for 8 min. Ova sensitization and sham sensitization with vehicle (Veh) were performed over a 25-day period in separate groups of mice. Our results showed that 1) both SO2 and NH3 inhalation challenges increased CR and ER frequencies in a concentration-dependent manner before Ova sensitization; 2) the baseline CR frequency was significantly elevated after Ova sensitization, accompanied by pronounced airway inflammation; and 3) Ova sensitization also markedly augmented the responses of CR and ER to both SO2 and NH3 inhalation challenges; in sharp contrast, the cough responses did not change after sham sensitization in the Veh group. In conclusion, Ova sensitization caused distinct and lingering increases in baseline cough frequency, and also intensified both CR and ER responses to inhaled irritant gases, which probably resulted from an allergic inflammation-induced hypersensitivity of airway sensory nerves.
Keywords: airway, inflammation, allergen cough, inhaled irritants
chronic airway inflammation induced by active sensitization with allergen significantly enhances the sensitivity of vagal bronchopulmonary C-fibers in Brown-Norway rats (22, 39, 40). These C-fibers represent a majority of the vagal afferents that innervate the entire respiratory tract and play an important role in regulating airway functions in animals and humans under both normal and disease conditions (8, 25, 30). These sensory nerve endings can be activated abruptly by irritant gases inhaled into the respiratory tract, which triggers important airway reflex responses such as cough, bronchoconstriction, and airway secretion; these reflex responses can expel and/or reduce the penetration of these harmful gases into deeper regions of the lung. On the other hand, hypersensitivity of these C-fiber afferents resulting from airway allergic inflammation (22, 34, 39) can induce cough hyperresponsiveness and excessive bronchoconstriction, which are common symptoms of allergic airway inflammation. Whether allergic inflammation alters the cough response to inhaled irritant gases in mice is not yet known.
Therefore, this study was designed to test the hypothesis that the cough responses to inhalation of sulfur dioxide (SO2) and ammonia (NH3) are elevated following induction of chronic airway inflammation by active sensitization with ovalbumin (Ova) in awake mice. SO2 and NH3 were chosen for this study because both are irritant gases that are present in the environment and can cause lung injury at high concentrations during occupational exposure or chemical accident (9, 10).
Both cough and expiration reflexes are airway-protective functions, and their regulatory mechanisms have been studied extensively in various animal species, such as guinea pigs, dogs, cats, and monkeys (29). Despite the vast and growing interest generated by the availability of various transgenic and knockout mouse models for physiological and pharmacological studies, the advances in our knowledge about the regulation of cough reflex (CR) and expiration reflex (ER) in this species have been relatively limited, in comparison with other animal species. In fact, questions have been raised as to whether the cough reflex is present in mice (1, 29). One of the reasons is probably related to the fact that cough is suppressed by anesthesia, whereas respiratory signals recorded in awake mice are inherently noisy, making it difficult to accurately analyze the CR and ER responses. Hence, another aim of this study was to characterize and differentiate the CR and ER responses to inhaled irritant gases in awake mice by recording the intrapleural pressure directly with the telemetry technique in a subgroup of these animals.
METHODS
Thirty adult male mice (C57BL6/J) were used in this study; their average age at the beginning of the study was 12.8 wk. The procedures described below were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health and were also approved by the University of Kentucky Institutional Animal Care & Use Committee.
Measurements of cough responses.
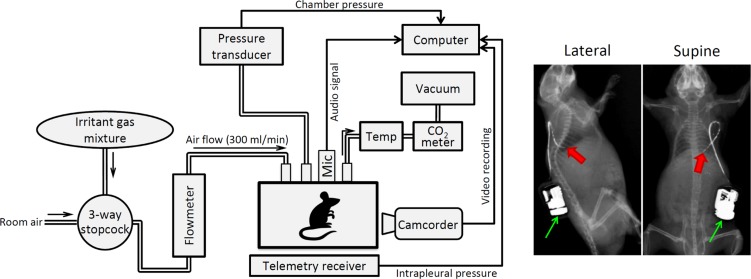
During the experiment, awake mice moved freely in a Plexiglas recording chamber (volume 160 ml). To prevent CO2 accumulation and hyperthermia, room air was drawn through the chamber at a constant flow rate (200 ml/min) by a negative pressure (Fig. 1). The CO2 concentration (CWE model CapStar-100, Ardmore, PA) and temperature (model TH-8; PhysiTemp, Clifton, NJ) of the outlet air were continuously monitored, which reached steady-state peaks of ~0.7% and ~25°C, respectively. The pressure in the chamber (Pcham) was recorded continuously by a pressure transducer (model TSD160A; Biopac, Goleta, CA); both audio and video signals of the mouse movements were also recorded simultaneously by a microphone (model Zm-Mic1; Zalman, Gyeonggi-do, South Korea) and a video camera (model C920; Logitech, Lausanne, Switzerland), respectively. These signals were used collectively for identifying and analyzing the cough frequency.
Fig. 1.
Left: schematic diagram of the experimental setup. Mic, microphone for recording audio signal; Temp, air temperature in the chamber measured by a thermometer. Right: X-ray pictures illustrating the positions of the telemetry sensor (red arrow) and transmitter (green arrow) implanted in the intrapleural space and under the lower back skin of a mouse, respectively. The telemetry catheter was painted with a radio opaque ink (Creative Materials, Ayer, MA) for identifying its position.
Breathing is inherently unstable in awake free-moving mice, which can alter the respiratory signals and affect its analysis. To further verify the accuracy of the cough analysis, the intrapleural pressure (Pip) was measured directly in 44.4% (8/18) of the animals studied for cough: the tip of a telemetry sensor (model PA-C10; Data Sciences International, St. Paul, MN; marked by the red arrow in Fig. 1) and the transmitter (green arrow) with an attached catheter were surgically implanted in the intrapleural space and under the lower back skin, respectively, under anesthesia by isoflurane inhalation. Two to three weeks were allowed for recovery from the surgery before any measurement or experiment was performed in the animal.
Inhalation challenges of irritant gases.
During the inhalation challenge, a gas mixture of SO2 or NH3 was drawn into the chamber (replacing room air) at the same flow rate (200 ml/min) for 8 min (Fig. 1). Two concentrations of SO2 (200 and 400 ppm) and NH3 (0.1 and 0.2%) balanced in air were tested in each animal; SO2 (2500 ppm) and NH3 (100%) contained in gas cylinders (Scott-Gross, Lexington, KY) were diluted by air to the desired concentrations in each experiment. The mouse was placed in the recording chamber for >30 min of adaptation period before the beginning of the experiment. All signals were recorded for 5 min before, 8 min during, and 5 min after each inhalation challenge. At least 30 min was allowed for a full recovery between two consecutive challenges.
Ova sensitization.
Mice were randomly divided into two groups. One group (Ova group; n = 18) was actively sensitized by intraperitoneal injection of 10 µg Ova/1 mg aluminum hydroxide (Imject Alum, Thermo Scientific, Rockford, IL) in 0.3 ml of sterile saline suspension on day 1 and day 11. Beginning on day 21, the mice were treated with Ova aerosol, 30 min daily for five consecutive days. Aerosols were generated from Ova/saline solution (wt/vol: 1%) by an ultrasonic nebulizer (model no. 100; Devilbiss, Port Washington, NY). During the treatment, the awake mouse was placed in a plastic restrainer and breathed aerosol through a nose cone connected directly to the aerosol reservoir. The other group (Veh group; n = 12) of mice received the intraperitoneal injections and aerosol inhalations of the vehicles following the identical procedures.
Airway inflammatory cell, mediator, and cytokine analyses.
To determine the severity of airway inflammation induced by Ova sensitization, bronchoalveolar lavage fluid (BALF) was obtained in both Ova and Veh groups of mice (n = 6 in each group) at 3–5 h after the last inhalation exposure to Ova or Veh aerosol on day 25 (no cough measurement was made in these mice). The procedures have been described in detail in our previous study (26). Briefly, the BALF samples were collected and centrifuged at 1,500 rpm, 4°C for 10 min. The pelleted cells were treated with 0.24-ml Tris-buffered ammonium chloride solution (pH 7.2) to lyse red blood cells, and the remaining cells were washed with 1.2-ml PBS supplemented with Hanks’ buffer solution with 20% FBS. Differential leukocyte counts were then performed on cytospin slides (by University of Kentucky Clinical Laboratory) after Wright-Giemsa staining using standard morphologic criteria. A minimum of 500 leukocytes were counted by two different individuals, and the data were then averaged.
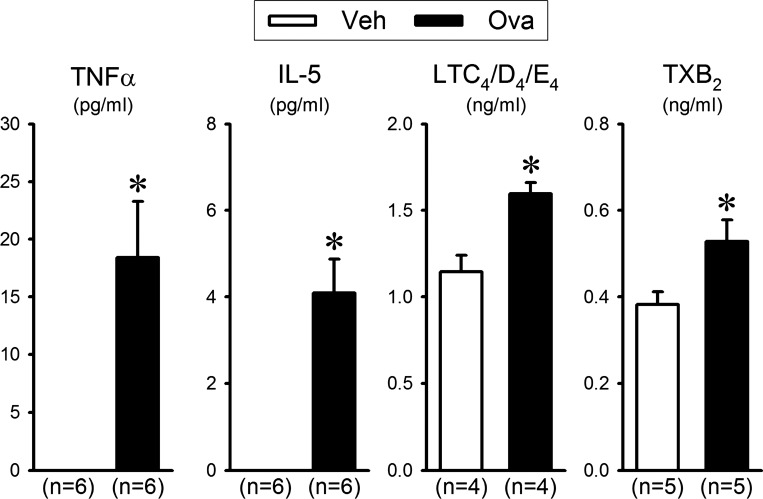
To compare the inflammatory mediators and cytokines released in the BALF, supernatants were transferred to other tubes for ELISA of inflammatory mediators and cytokines, including leukotrienes (LTs) C4/D4/E4, thromboxane B2 (TXB2), IL-5, and tumor necrosis factor-α (TNF-α). All assays of the BALF collected from Ova and Veh groups were performed at the same time to avoid any possible experimental error and variability. Only these inflammatory mediators and cytokines were selected for measurements in this study because they are known to be upregulated during allergic inflammation (7, 16), and previous reports have shown that they can cause a stimulatory or sensitizing effect on airway sensory nerves (17, 21, 26, 27, 31).
Experimental protocols and data analysis.
Experiments were initiated after the mice had acclimatized to the chamber and experimental protocols daily for a week. Cough responses to SO2 and NH3 inhalation challenges during control (before Ova sensitization or Veh treatment) were studied on three separate days in each animal, and the control data were calculated as the average of the three days. Identical protocols were then carried out at the following time points: 1 (3–5 h), 2, 3, and 10 days following the last inhalation exposure to Ova or Veh.
The signals (pressures, audio, and video) recorded in each inhalation challenge were played back and analyzed; frequencies of CR and ER were calculated as the total numbers of CR and ER elicited in each minute for the entire duration of each testing condition. These numbers were independently determined by two individuals, and the data from their determinations were averaged.
For each inhalation challenge, the CR and ER frequencies were analyzed for the 5-min baseline, 8-min challenge, and 5-min recovery in each animal, and the data were then pooled for all animals in that group.
Statistical analysis.
Data were analyzed with the one-way or two-way repeated-measures ANOVA. For example, in the study of how Ova sensitization affected the cough frequency response to inhaled SO2, one factor was the treatment effect of Ova, and the other factor was the effect of inhalation challenge. When either one-way or two-way ANOVA showed a significant positive interaction, pairwise comparisons were made with a post hoc analysis (Fisher’s least significant difference). The only exception is in the BALF cell/mediator/cytokine analyses, where the paired t-test was used to evaluate the difference. A value of P < 0.05 was considered significant. Data are reported as means ± SE.
RESULTS
Breathing pattern was usually unstable in awake, free-moving mouse. The change in Pcham reflected the change of volume in the chamber: during inspiration in quiet breathing, the air inhaled into the lung expanded its volume due to an increase in the temperature (from room temperature to body temperature), causing the Pcham to increase and reach a peak at the end of each inspiration; likewise, Pcham continued to decrease during expiration until the lung volume reached the functional residual capacity (e.g., Fig. 2A). These changes in Pcham were synchronized with the changes of Pip measured directly by the telemetry sensor (Fig. 2A).
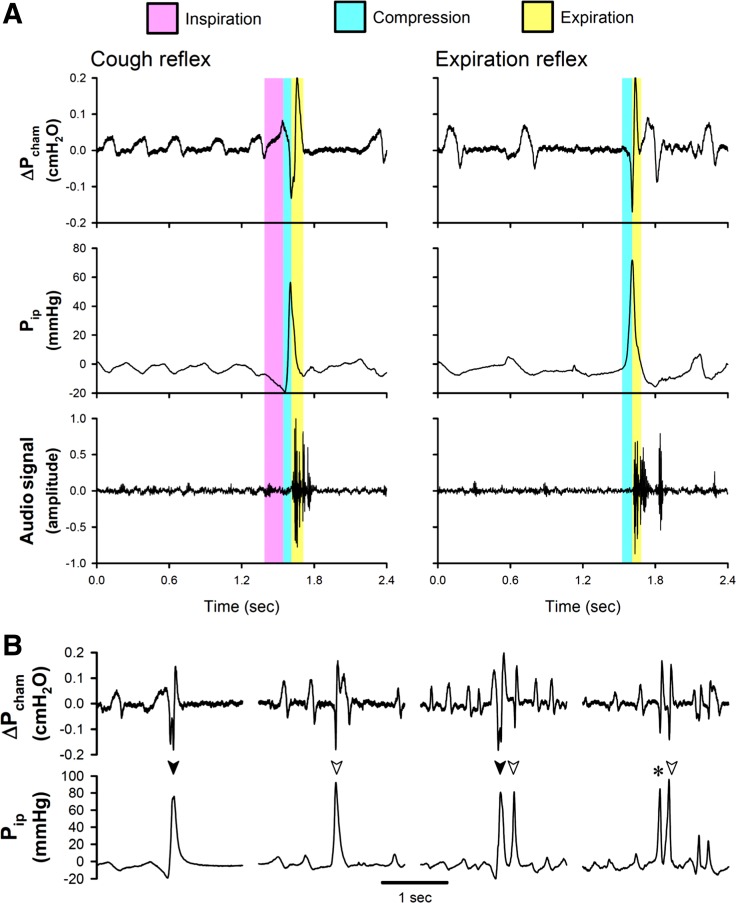
Fig. 2.
A: representative experimental records, illustrating the characteristic features of pressure changes and the audio signal generated by a single cough (cough reflex, CR; left) and a single expiration reflex (ER; right). B: additional experimental records (from left to right): a CR (solid arrowhead), an ER (open arrowhead), a cough epoch consisting of a combination of CR and ER, and a cough epoch of CR/ER and ER. CR/ER (asterisk) depicts the cough response that could not be distinguished between CR and ER. Pcham, pressure inside the chamber; Pip, intrapleural pressure recorded via the telemetry sensor in awake mice.
Each cough was characterized by three distinct but tightly connected phases in the change of Pcham; it was initiated by an inspiratory effort (an increase in Pcham) followed by a chest compression (depicted by a sharp reverse and decrease in Pcham), and then a forced expiration (a sharp increase in Pcham) coinciding with a cough sound relatively weaker than that in larger animals (e.g., dogs and cats) and a jerking movement of the head (representative audio/video recordings are submitted with this article as Supplemental Videos S1 and S2). All of these changes in Pcham were closely correlated with the synchronous changes in Pip (e.g., Fig. 2A). In addition, ER, similar to that described by other investigators (6, 36, 38), were also detected in awake mice during inhalation challenge with irritant gases. A typical ER consisted of only two phases: a chest compression, with no clear or significant preceding inspiratory effort, followed by a forced expiration synchronized with a cough sound, which could also be clearly identified by the Pip signal (Fig. 2A). During both CR and ER, the peak positive Pip generated by chest compression usually far exceeded 30 mmHg, and the corresponding ΔPcham dropped more than 0.1 cmH2O (e.g., Fig. 2, A and B); during chest compression, the peak Pip reached 60.1 ± 4.1 mmHg during CR (n = 63) and 42.1 ± 3.1 mmHg during ER (n = 77; P < 0.05), whereas the duration of chest compression was not different: 84.1 ± 5.1 ms during CR and 88.1 ± 6.1 ms during ER. In this study, ~20% of the responses could not be easily distinguished between CR and ER (e.g., in Fig. 2B), and they were categorized as CR/ER in our data analysis.
The recorded video and audio signals generated by the mouse were also useful when they were used in conjunction with the changes in Pcham, but they were not reliable in general and were frequently inaccurate when they were used alone in detecting and identifying coughs in the awake mice.
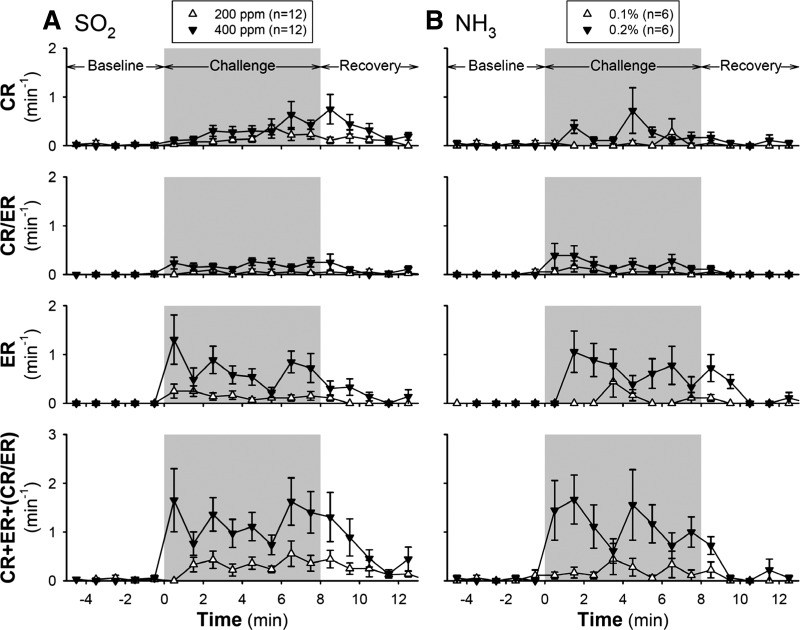
During the control (before Ova or Veh treatment) condition, both SO2 and NH3 evoked CRs in a concentration-dependent manner; only very mild responses to the low concentrations (200 ppm of SO2 and 0.1% of NH3) were detected (Figs. 3 and 4). The ER frequency abruptly increased in the first minute after exposure to 400 ppm of SO2 and remained elevated above the baseline during the 8 min of challenge, whereas the CR frequency increased progressively during the SO2 challenge (Fig. 3). Both CR and ER frequencies gradually declined toward the baseline during the 5-min recovery (Fig. 3). The peak magnitude of these responses varied between animals but was relatively reproducible in the same animal; the group data showed no significant difference (P > 0.05) in the CR, ER, and CR/ER responses to SO2 challenge between the three days of control experiments. There was no difference in the control responses to SO2 (either 200 or 400 ppm) between Ova and Veh groups.
Fig. 3.
Changes in frequencies of CR and ER in response to inhalation challenges of SO2 (A) and NH3 (B) during control (before ovalbumin sensitization or vehicle treatment). CR/ER represented the response that could not be distinguished between CR and ER. Responses were recorded for 5 min before (baseline), 8 min during (gray shaded areas), and 5 min after the inhalation challenge (recovery). Responses to both low and high concentrations of each of the irritant gases (SO2: 200 ppm and 400 ppm; NH3: 0.1% and 0.2%) were tested, and at least 30 min elapsed between two consecutive challenges. Each test was repeated on three consecutive days in each mouse, and the data were averaged. Each data point represents the mean ± SE of all the animals tested.
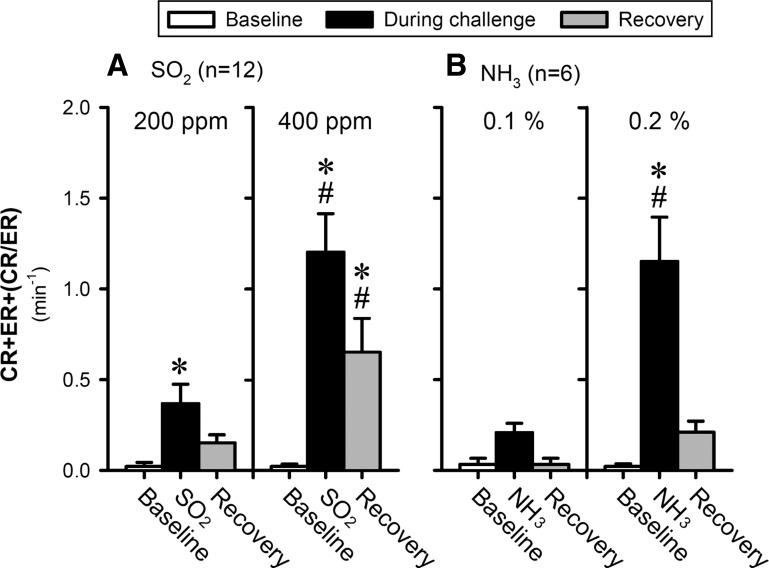
Fig. 4.
Group data of total cough responses (CR+ER+CR/ER) to inhalation challenges of SO2 (A) and NH3 (B) during control (before ovalbumin sensitization or vehicle treatment). Data (means ± SE) represent the group responses averaged over the 5-, 8- and 5-min durations of baseline, during challenge, and recovery, respectively; for details, see legend of Fig. 3. *Significantly (P < 0.05) different from the baseline data. #Significantly (P < 0.05) different from the responses to the low-concentration challenge.
A pronounced airway inflammation was induced by the Ova sensitization and challenge. The percentages of both eosinophils and neutrophils in the BALF collected from Ova-sensitized mice (n = 6) at 3–5 h after the last Ova aerosol challenge (the same time that cough responses were tested in all other animals) were ~26.7 (P < 0.05)- and ~18.7 (P < 0.05)-fold higher than that collected from Veh-treated mice (n = 6), respectively (Table 1). In addition, ELISA results showed that the levels of inflammatory mediators LTC4/D4/E4 and TXB2, and also the proinflammatory cytokines TNF-α and IL-5 in the BALF of the Ova-sensitized mice were significantly higher than that of the Veh mice (Fig. 5).
Table 1.
Effect of ovalbumin sensitization on differential cell counts of leukocytes in bronchoalveolar lavage fluid of mice
| Eosinophils, % | Neutrophils, % | Basophils, % | Lymphocytes, % | Monocytes, % | |
|---|---|---|---|---|---|
| Control (n = 6) | 0.22 ± 0.16 | 0.48 ± 0.22 | 0.03 ± 0.03 | 10.55 ± 0.76 | 88.71 ± 0.52 |
| Sensitized (n = 6) | 5.95 ± 0.59* | 8.89 ± 2.19* | 0.12 ± 0.07 | 18.62 ± 1.41* | 66.46 ± 2.32* |
Data are expressed as means ± SE, using the Studentʼs t-test.
Significantly different from control group (P < 0.01).
Fig. 5.
Effect of ovalbumin (Ova) sensitization on inflammatory mediators and cytokines in the bronchoalveolar lavage fluid (BALF) collected from the vehicle (Veh)-treated group (open bars) and Ova-sensitized group (solid bars). BALF was collected at 3–5 h after the last exposure to Ova or Veh aerosol. TNF-α, tumor necrosis factor-α; IL-5, interleukins 5; LTC4/D4/E4, leukotrienes C4/D4/E4; TXB2, thromboxane B2. Each data point represents the mean ± SE (n = the number of mice studied). Data were analyzed by the Studentʼs t-test. *Significantly different from the Veh group (P < 0.05). Concentrations of TNF-α and IL-5 were not detectable in the Veh group.
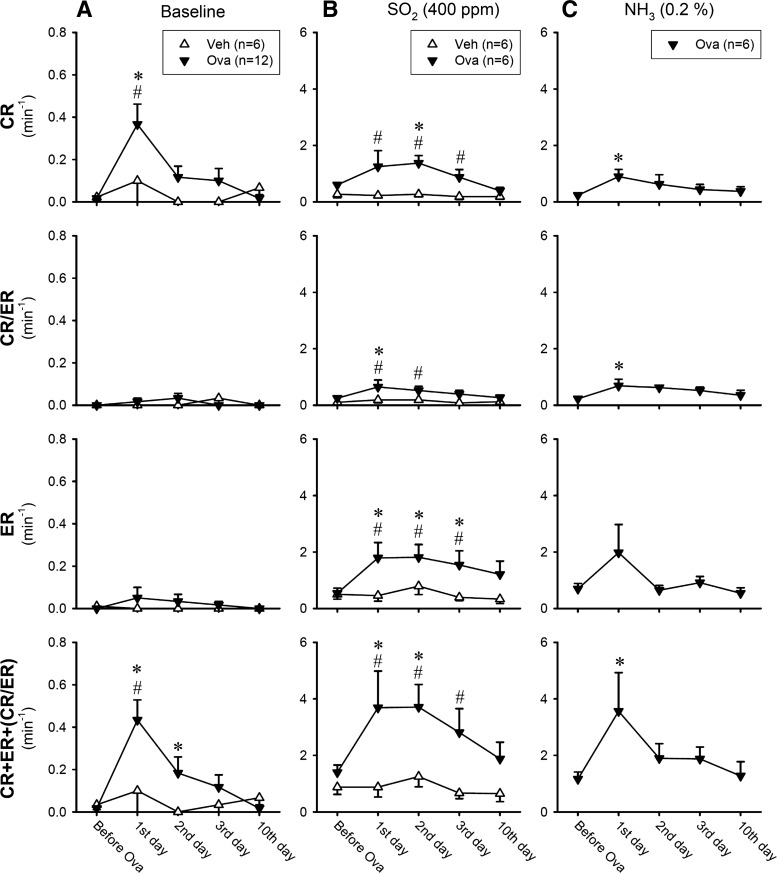
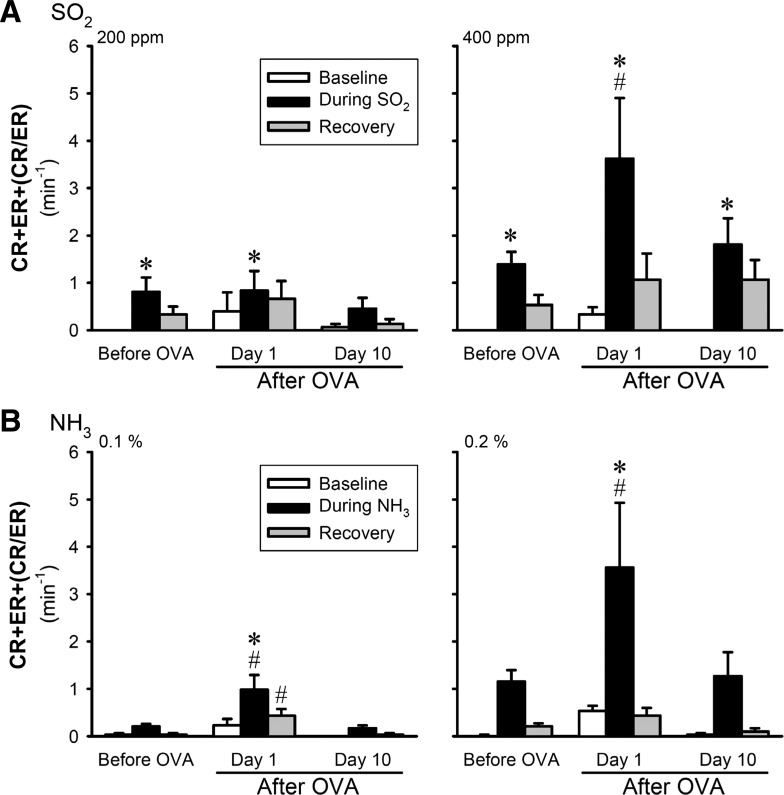
In 3–5 h after the last Ova aerosol inhalation, a distinct increase in the baseline (before the SO2 challenge) CR frequency was found in the Ova-sensitized mice (Fig. 6A, top); in comparison, there was no significant change in the baseline ER and CR/ER frequencies (Fig. 6A, middle). The total cough frequency (CR+ER+CR/ER) at baseline remained elevated for >24 h, and then gradually declined toward control. In contrast, no change in the baseline CR, ER, or CR/ER frequency was detected in the Veh group (Fig. 6A). The responses of CR, ER and CR/ER to SO2 challenge at low concentration (200 ppm) did not increase significantly after Ova sensitization (Fig. 7A). However, the total cough response (CR+ER+CR/ER) to SO2 challenge at high concentration (400 ppm) was elevated to >200% of the control (pre-Ova) responses in the Ova group (Fig. 6B); and both CR and ER increased in a similar pattern. The enhanced cough sensitivity persisted for >48 h after the last exposure to Ova aerosol (Fig. 6B). In sharp contrast, there was no difference in CR, ER, or CR/ER responses to SO2 (400 ppm) between before and after the Veh treatment in the Veh group (Fig. 6B).
Fig. 6.
Effects of Ova sensitization and Veh treatment on the frequencies of cough reflex (CR) and expiration reflex (ER) in response to inhalation challenges of SO2 and NH3 at different time points after the treatment. CR/ER represented the response that could not be distinguished between CR and ER. A: baseline cough frequency averaged over the 5-min duration immediately before the irritant gas inhalation challenge. B and C: cough responses to SO2 (400 ppm) and NH3 (0.2%) inhalation challenges, respectively, averaged over the 8-min duration. In A, n = 12 in the Ova-sensitized group and n = 6 in the Veh-treated group; this difference is due to the fact that the response to NH3 was not tested in the Veh group (C). Each data point represents the mean ± SE. *Significantly (P < 0.05) different from the data obtained before Ova sensitization or Veh treatment. #Significantly (P < 0.05) different from the corresponding data point in the Veh group.
Fig. 7.
Potentiating effects of Ova sensitization on the total cough responses (CR+ER+CR/ER) to inhalation challenges of both low and high concentrations of SO2 (A) and NH3 (B) in awake mice. Data (means ± SE) represent the group responses averaged over the 5-, 8-, and 5-min durations of baseline, during challenge and recovery, respectively. *Significantly (P < 0.05) different from the baseline data (open bars). #Significantly (P < 0.05) different from the corresponding data obtained before Ova sensitization.
A potentiating effect of Ova sensitization similar to that on the SO2 response was also found in the total cough response (CR+ER+CR/ER) to NH3 challenges at both low and high concentrations (Figs. 6C and 7B) in a separate group of Ova-sensitized mice. The cough responses to the NH3 challenges were not tested in Veh-treated mice.
DISCUSSION
In this study, we have characterized the cough reflex and expiration reflex elicited by inhaled irritant gases in awake mice. Furthermore, we have demonstrated that active sensitization with Ova increased the baseline cough frequency and markedly intensified the responses of cough and expiration reflexes to SO2 and NH3 inhalation challenges (Figs. 6 and 7). In sharp contrast, the sham sensitization with vehicle did not cause any change in the cough frequency and sensitivity to these inhaled irritant gases (Fig. 6).
Both SO2 and NH3 are colorless gases with a pungent odor and common irritant gases to human respiratory tract. SO2 in the environmental air is derived primarily from the burning of fossil fuels (coal, oil) and can reach a high concentration in work places such as power plants or from copper smelting (10). NH3 is generated by nature as part of the nitrogen cycle in the environment and is produced in large quantity for industrial use (e.g., agriculture fertilizers or household cleaning products) (9). Both SO2 and NH3 can cause lung injury and other harmful health effects to humans at high concentrations or during sustained exposure in the case of chemical accident or unregulated occupational conditions (9, 10). Sensory receptors that detect these irritant gases in the respiratory tract play an important role in performing the first-line pulmonary defense function by eliciting cough and expiration reflexes, which expel and limit the further inhalation of these harmful gases and protect the rest of the body.
In this study, both SO2 and NH3 inhalation challenges increased the frequencies of CR and ER in a concentration-dependent manner (Figs. 3 and 4). The lower concentrations of SO2 (200 ppm) and NH3 (0.1%) used in this study were only slightly above the threshold for triggering CR and ER in mice. The cough reflex elicited by inhaled SO2 or NH3 is probably elicited by activation of C-fibers and/or rapidly adapting receptors (RARs) located in the airways and lung (4, 17, 25) and/or tracheal cough receptors (3). Indeed, using a single-fiber recording technique, we have observed that SO2 or NH3 inhaled at the concentrations similar to that used in this study stimulated both of these afferents in anesthetized mice (unpublished data).
The expiration reflex is known to be elicited by stimulation of sensory endings located in the upper airways, particularly in the larynx and upper trachea (33, 36, 38). In this study, both Pcham and the Pip signal recorded by telemetry showed that the ER started with a forced expiration without a preceding inspiratory effort (e.g., Fig. 2), which is consistent with the observations reported by previous investigators in other species (33, 36, 38). During the inhalation challenge with SO2 or NH3 in awake mice, CR and ER occurred frequently in combination and rapid succession; two of these examples are shown in Fig. 2B. In these cough epochs, the second “cough” is usually an ER, identified on the basis of our criteria: forced expiration without a clear preceding inspiration. Whether the absence of inspiratory effort was due to the rapid and brisk onset of the succeeding “cough” in the epoch cannot be determined in this study. In any case, this may have accounted for the relatively higher ER frequency than the CR frequency during the SO2 or NH3 inhalation challenge at control (before the Ova sensitization; Fig. 3). Because both CR and ER are airway defense responses against inhaled irritants, they were pooled for data analysis in the evaluation of the overall cough responses to irritant gas inhalations in this study (Figs. 4 and 7).
We cannot determine the relative contributions of the different types of vagal afferents in the Ova sensitization-induced cough hyperresponsiveness in this study, but a possible involvement of certain key factors should be considered. SO2 can form sulfuric acid and activate transient receptor potential vanilloid type 1 (TRPV1) and acid-sensing ion channels (5, 14); both of these channels are abundantly expressed in the bronchopulmonary C-fibers (25). NH3 has been shown to stimulate both TRPV1 and transient receptor potential ankyrine type 1 (TRPA1) receptors (11). Both TRPV1 and TRPA1 receptors are ligand-gated nonselective cation channels (32) and are known to play important roles in the inflammation-induced hypersensitivity of vagal bronchopulmonary afferents (25, 30). Recent studies have demonstrated a striking synergistic effect in pulmonary sensory nerves when TRPA1 and TRPV1 were activated simultaneously, which may play an important role in the regulation of airway reflex functions, such as cough during airway inflammatory reaction (18, 24, 28).
A number of the pathophysiological features of human allergic asthma have been previously described in mice actively sensitized with Ova using the same protocol as in the present study (7). For example, acute inhalation challenge of Ova aerosol triggered both early and late phases of bronchoconstriction in Ova-sensitized mice after 30 min and 3–6 h, respectively, demonstrating the airway hyperreactivity to the antigen challenge (7). The early-phase bronchoconstriction is mediated primarily by mast cell-derived mediators, and the more sustained late-phase response is characterized by airway obstruction and inflammatory reaction, resulting from the action of various endogenous inflammatory mediators and cytokines released from the resident inflammatory cells (e.g., mast cells and macrophages), as well as the recruited inflammatory cells (neutrophils and eosinophils) (2). Furthermore, the bronchomotor responses to methacholine challenges were also markedly elevated in Ova-sensitized mice, indicating the airway hyperresponsiveness to nonspecific bronchoactive challenge (7). Hence, these airway responses in Ova-sensitized mice closely resemble the clinical features and pattern of airway hyperresponsiveness triggered by inhaled antigens in patients with allergic asthma (13, 35, 37). In this study, the elevated baseline cough frequency and enhanced cough responses to inhaled irritant gases were observed at 3–5 h after the last Ova aerosol challenge in sensitized mice (Fig. 6A), coinciding with the time of late-phase airway hyperresponsiveness (7).
At approximately the same time that cough responses were measured, as described above, the percentages of both eosinophils and neutrophils in the BALF of another group of Ova-sensitized mice were elevated to >15-fold higher than those in the Veh mice in this study (Table 1), revealing an infiltration of inflammatory cells in the airways. Eosinophils and neutrophils are primary inflammatory cells known to release a wide range of potent bronchoactive substances (e.g., cationic proteins, lipoxygenase and cyclooxygenase metabolites of arachidonic acid). Indeed, inflammatory mediators (LTC4/D4/E4 and TXB2) and proinflammatory cytokines (IL-5 and TNF-α) were also significantly elevated in the BALF of Ova-sensitized mice in this study (Fig. 5). Many of these mediators and cytokines are known to activate or sensitize bronchopulmonary C-fibers; for example, TXA2, the precursor of TXB2, is a potent stimulator of bronchopulmonary C-fibers (21, 27). LTC4 can also stimulate the C-fiber afferents and trigger the release of tachykinins in isolated trachea/bronchus of guinea pigs (31). Although TNF-α does not activate these airway sensory receptors directly, it can markedly enhance their sensitivity (19, 26). Moreover, eosinophil granule-derived cation proteins, such as major basic proteins, can exert potent stimulatory and sensitizing effects on bronchopulmonary C-fibers (15, 23). All of this evidence seems to suggest that an increase in the C-fiber sensitivity is probably involved in the increased cough responses to inhaled irritant gases in Ova-sensitized mice. This notion is in agreement with the finding that the cough sensitivity to inhalation of aerosolized capsaicin, a selective and potent chemical activator of the TRPV1 receptors expressed in C-fiber sensory neurons, is enhanced in asthmatic patients (12). In addition, in Ova-sensitized rats, capsaicin sensitivity was detected in a large percentage of the vagal myelinated afferents, including RARs, which normally do not respond to capsaicin (39). Furthermore, immunohistochemical evidence shows that sensitization and challenge with Ova significantly upregulated the expression of TRPV1 in bronchopulmonary neurons in rat nodose ganglia, mainly in neurofilament-positive (myelinated) neurons (39).
Previous investigators have questioned whether the cough reflex is present in the mouse (1, 29). This uncertainty is probably related to the technical difficulties in making direct and accurate measurements of the physiological variables characterizing the cough action (e.g., tracheal pressure, expiratory flow rate, and muscle EMG) in awake mice (6, 20, 29), due to their small body size and high breathing rate at rest (>250 breaths/min). In this study, we have used the telemetry technique to measure directly the change in intrapleural pressure in a subgroup of mice. Our results have yielded definitive evidence, illustrating the actions of both cough and expiration reflexes in awake mice that are consistent with those described in other species (3, 36, 38). Certain limitations of the experimental approach used in this study should be noted; for example, the telemetry method requires surgical implantation and care in each animal, as well as the cost for purchase of the equipment, which may be prohibitive for a large-scale study. Nonetheless, our results have shown that collective measurements of the change in the chamber pressure together with the audio and video signals can also accurately and reliably determine the cough frequency in awake mice, as verified by the simultaneous recording of the telemetry signal in this study.
In conclusion, this study has demonstrated that Ova sensitization caused distinct increases in both baseline cough frequency and cough responses to inhaled irritant gases in awake mice. The cough hyperresponsiveness probably resulted from an allergic inflammation-induced hypersensitivity of airway sensory nerves. Further investigations are required to uncover the signaling pathways and mechanisms involved.
Perspectives and Significance
This study demonstrated that airway allergic inflammation increased the baseline cough frequency and intensified the cough responses to inhaled irritant gases, such as SO2 and NH3, in awake mice. The direct recording of intrapleural pressure using the telemetry technology in a subgroup of mice has yielded the definitive evidence and further characterized the actions of cough and expiration reflex in this species. This murine model may serve as a useful tool for the future studies of the underlying mechanisms of cough hyperresponsiveness associated with airway inflammation and for testing potential therapeutic interventions.
GRANTS
This study was supported in part by National Institutes of Health Grants AI-123832, HL-96914, and UL1TR-001998.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.Z., R.-L.L., J.H., and L.-Y.L. performed experiments; C.Z., R.-L.L., J.H., and L.-Y.L. analyzed data; C.Z., R.-L.L., J.H., M.K., and L.-Y.L. interpreted results of experiments; C.Z., R.-L.L., M.K., and L.-Y.L. prepared figures; C.Z., R.-L.L., J.H., M.K., and L.-Y.L. drafted manuscript; C.Z., R.-L.L., J.H., M.K., and L.-Y.L. edited and revised manuscript; C.Z., R.-L.L., J.H., M.K., and L.-Y.L. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Matthew Ganslaw at Creative Materials, Inc. (Ayer, MA) for the generous gift of radio opaque paint used for identifying the telemetry catheter, and Ashami Athukorala, Reyno Tapia, and Tanya Seward for their technical assistance.
REFERENCES
- 1.Belvisi MG, Bolser DC. Summary: animal models for cough. Pulm Pharmacol Ther 15: 249–250, 2002. doi: 10.1006/pupt.2002.0349. [DOI] [PubMed] [Google Scholar]
- 2.Busse WW, Lemanske RF Jr. Asthma. N Engl J Med 344: 350–362, 2001. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 3.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557: 543–558, 2004. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol 152: 223–242, 2006. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Lai K, Lomask JM, Jiang B, Zhong N. Detection of mouse cough based on sound monitoring and respiratory airflow waveforms. PLoS One 8: e59263, 2013. doi: 10.1371/journal.pone.0059263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cieslewicz G, Tomkinson A, Adler A, Duez C, Schwarze J, Takeda K, Larson KA, Lee JJ, Irvin CG, Gelfand EW. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J Clin Invest 104: 301–308, 1999. doi: 10.1172/JCI7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Department of Health and Human Services. Public Health Statement: Ammonia (CAS#: 7664-41-7). Atlanta, GA: Agency For Toxic Substances And Disease Registry, 2004. https://www.atsdr.cdc.gov/toxprofiles/tp126-c1-b.pdf. [Google Scholar]
- 10.Department of Health and Human Services. Public Health Statement: Sulfur Dioxide (CAS#: 7446-09-5). Atlanta, GA: Agency For Toxic Substances And Disease Registry, 1998. https://www.atsdr.cdc.gov/ToxProfiles/tp116-c1-b.pdf. [Google Scholar]
- 11.Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, Patapoutian A. TRPV1 is activated by both acidic and basic pH. J Neurosci 29: 153–158, 2009. doi: 10.1523/JNEUROSCI.4901-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty MJ, Mister R, Pearson MG, Calverley PM. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax 55: 643–649, 2000. doi: 10.1136/thorax.55.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauvreau GM, El-Gammal AI, O’Byrne PM. Allergen-induced airway responses. Eur Respir J 46: 819–831, 2015. doi: 10.1183/13993003.00536-2015. [DOI] [PubMed] [Google Scholar]
- 14.Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 291: L58–L65, 2006. doi: 10.1152/ajplung.00517.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu Q, Wiggers ME, Gleich GJ, Lee LY. Sensitization of isolated rat vagal pulmonary sensory neurons by eosinophil-derived cationic proteins. Am J Physiol Lung Cell Mol Physiol 294: L544–L552, 2008. doi: 10.1152/ajplung.00271.2007. [DOI] [PubMed] [Google Scholar]
- 16.Henderson WR Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med 165: 108–116, 2002. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 17.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127: 113–124, 2001. doi: 10.1016/S0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- 18.Hsu CC, Lee LY. Role of calcium ions in the positive interaction between TRPA1 and TRPV1 channels in bronchopulmonary sensory neurons. J Appl Physiol (1985) 118: 1533–1543, 2015. doi: 10.1152/japplphysiol.00043.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, Gu Q, Lin RL, Kryscio R, Lee LY. Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor-alpha in rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 299: L483–L492, 2010. doi: 10.1152/ajplung.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwata T, Ito I, Niimi A, Ikegami K, Marumo S, Tanabe N, Nakaji H, Kanemitsu Y, Matsumoto H, Kamei J, Setou M, Mishima M. Mechanical Stimulation by Postnasal Drip Evokes Cough. PLoS One 10: e0141823, 2015. doi: 10.1371/journal.pone.0141823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karla W, Shams H, Orr JA, Scheid P. Effects of the thromboxane A2 mimetic, U46,619, on pulmonary vagal afferents in the cat. Respir Physiol 87: 383–396, 1992. doi: 10.1016/0034-5687(92)90019-S. [DOI] [PubMed] [Google Scholar]
- 22.Kuo YL, Lai CJ. Ovalbumin sensitizes vagal pulmonary C-fiber afferents in Brown Norway rats. J Appl Physiol (1985) 105: 611–620, 2008. doi: 10.1152/japplphysiol.01099.2007. [DOI] [PubMed] [Google Scholar]
- 23.Lee LY, Gu Q, Gleich GJ. Effects of human eosinophil granule-derived cationic proteins on C-fiber afferents in the rat lung. J Appl Physiol (1985) 91: 1318–1326, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Lee LY, Hsu CC, Lin YJ, Lin RL, Khosravi M. Interaction between TRPA1 and TRPV1: Synergy on pulmonary sensory nerves. Pulm Pharmacol Ther 35: 87–93, 2015. doi: 10.1016/j.pupt.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol 4: 287–324, 2014. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- 26.Lin RL, Lin YJ, Geer MJ, Kryscio R, Lee LY. Pulmonary chemoreflex responses are potentiated by tumor necrosis factor-alpha in mice. J Appl Physiol (1985) 114: 1536–1543, 2013. doi: 10.1152/japplphysiol.01301.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin S, Li H, Xu L, Moldoveanu B, Guardiola J, Yu J. Arachidonic acid products in airway nociceptor activation during acute lung injury. Exp Physiol 96: 966–976, 2011. doi: 10.1113/expphysiol.2011.058263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YJ, Lin RL, Ruan T, Khosravi M, Lee LY. A synergistic effect of simultaneous TRPA1 and TRPV1 activations on vagal pulmonary C-fiber afferents. J Appl Physiol (1985) 118: 273–281, 2015. doi: 10.1152/japplphysiol.00805.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackenzie AJ, Spina D, Page CP. Models used in the development of antitussive drugs. Drug Discov Today Dis Models 1: 297–302, 2004. doi: 10.1016/j.ddmod.2004.10.009. [DOI] [Google Scholar]
- 30.Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol Rev 96: 975–1024, 2016. doi: 10.1152/physrev.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAlexander MA, Myers AC, Undem BJ. Inhibition of 5-lipoxygenase diminishes neurally evoked tachykinergic contraction of guinea pig isolated airway. J Pharmacol Exp Ther 285: 602–607, 1998. [PubMed] [Google Scholar]
- 32.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 33.Poliacek I, Rose MJ, Corrie LW, Wang C, Jakus J, Barani H, Stransky A, Polacek H, Halasova E, Bolser DC. Short reflex expirations (expiration reflexes) induced by mechanical stimulation of the trachea in anesthetized cats. Cough 4: 1, 2008. doi: 10.1186/1745-9974-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riccio MM, Myers AC, Undem BJ. Immunomodulation of afferent neurons in guinea-pig isolated airway. J Physiol 491: 499–509, 1996. doi: 10.1113/jphysiol.1996.sp021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spahn JD. Asthma biomarkers in sputum. Immunol Allergy Clin North Am 27: 607–622, 2007. doi: 10.1016/j.iac.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Tatar M, Hanacek J, Widdicombe J. The expiration reflex from the trachea and bronchi. Eur Respir J 31: 385–390, 2008. doi: 10.1183/09031936.00063507. [DOI] [PubMed] [Google Scholar]
- 37.Waserman S, Olivenstein R, Renzi P, Xu LJ, Martin JG. The relationship between late asthmatic responses and antigen-specific immunoglobulin. J Allergy Clin Immunol 90: 661–669, 1992. doi: 10.1016/0091-6749(92)90140-W. [DOI] [PubMed] [Google Scholar]
- 38.Widdicombe J, Fontana G. Cough: what’s in a name? Eur Respir J 28: 10–15, 2006. doi: 10.1183/09031936.06.00096905. [DOI] [PubMed] [Google Scholar]
- 39.Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol 586: 5771–5786, 2008. doi: 10.1113/jphysiol.2008.161042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang G, Lin RL, Wiggers ME, Lee LY. Sensitizing effects of chronic exposure and acute inhalation of ovalbumin aerosol on pulmonary C fibers in rats. J Appl Physiol (1985) 105: 128–138, 2008. doi: 10.1152/japplphysiol.01367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.