Abstract
μ-Opioid receptors are distributed widely in the brain stem respiratory network, and opioids with selectivity for μ-type receptors slow in vivo respiratory rhythm in lowest effective doses. Several studies have reported μ-opioid receptor effects on the three-phase rhythm of respiratory neurons, but there are until now no reports of opioid effects on oscillatory activity within respiratory discharges. In this study, effects of the μ-opioid receptor agonist fentanyl on spike train discharge properties of several different types of rhythm-modulating medullary respiratory neuron discharges were analyzed. Doses of fentanyl that were just sufficient for prolongation of discharges and slowing of the three-phase respiratory rhythm also produced pronounced enhancement of spike train properties. Oscillation and burst patterns detected by autocorrelation measurements were greatly enhanced, and interspike intervals were prolonged. Spike train properties under control conditions and after fentanyl were uniform within each experiment, but varied considerably between experiments, which might be related to variability in acid-base balance in the brain stem extracellular fluid. Discharge threshold was shifted to more negative levels of membrane potential. The effects on threshold are postulated to result from opioid-mediated disinhibition and postsynaptic enhancement of N-methyl-d- aspartate receptor current. Lowering of firing threshold, enhancement of spike train oscillations and bursts and prolongation of discharges by lowest effective doses of fentanyl could represent compensatory adjustments in the brain stem respiratory network to override opioid blunting of CO2/pH chemosensitivity.
Keywords: respiratory neuron discharges, burst and oscillation patterns, opioid actions bursts and oscillations, opioid lowers respiratory neuron discharge threshold
neurons in the dorsolateral pons and ventral respiratory column (VRC) of the medulla exhibit a three-phase rhythm and modulate tidal volume in human and nonhuman mammals (38, 39, 44), and several studies have demonstrated how opiates affect VRC and pontine respiratory neuron rhythm and excitability (11, 16, 18, 20, 26, 29, 31, 32, 36, 48, 51). Low doses of opiates with μ- or δ-receptor affinity slow respiratory frequency, with lesser effects on depth of respiration in humans (9, 10, 14, 47) and cats (26, 28). Larger doses decrease tidal volume, increase airway resistance, reduce pulmonary compliance, and blunt respiratory responsiveness to hypercapnia and hypoxia (35). Opiates also produce a shift to the right in the alveolar ventilation- relationship, proportional to the dose given to anesthetized humans (35) and cats (25), indicating that they alter the dynamics of the negative feedback loop (24) between central nervous Pco2/pH chemoreceptors and lung-body tissue gas exchange.
It is not absolutely clear how μ-opioid receptor agonists disturb respiratory rhythm, although one likely mechanism has been advanced based on two studies in rats. A phenomenon known as quantal slowing of respiratory rhythm, characterized by skipped inspiratory neuron discharges in neonatal rat brain stem slices, is attributed to a failure of synaptic transmission from preinspiratory neurons to pre-Bötzinger complex neurons (32). Moreover, in juvenile rats in vivo, brain stem transection at the caudal end of the facial nucleus, which blocks Pre-I neuron synaptic transmission to pre-Bötzinger complex neurons, eliminates quantal breathing (21). The conclusion reached in the two studies is that opioid-induced quantal slowing of respiration is due to synaptic inhibition of Pre-I neurons or inspiratory motoneurons.
Another plausible mechanism that explains the relatively high sensitivity of the respiratory rhythm to μ-opioid receptor-mediated disturbances is that high-frequency oscillatory patterns within respiratory neuron discharges are especially targeted. This hypothesis agrees with the proposal that oscillations in synaptic activity are essential for controlling the timing and pattern or respiratory neuron discharges (12), and they also have significant consequences for the generation of respiratory muscle force (52).
It is conceivable, therefore, that lowest effective doses of μ-opioid receptor agonists are particularly effective in enhancing oscillatory discharge patterns in VRC neurons. Accordingly, the primary goal of the present investigation was to test this hypothesis by determining how lowest effective doses of a μ-opioid receptor agonist given intravenously affect oscillatory and burst properties in several types of medullary VRC neurons. This study in pentobarbital-anesthetized adult cats reveals that lowest effective intravenous doses of fentanyl, a highly selective μ-opioid receptor agonist, produce impressive increases in the intensity of burst and oscillatory patterns in VRC neurons, and they slow oscillation and burst frequency as well. In addition, μ-opioid agonist effects on oscillation patterns are accompanied by shifts in action potential threshold to more negative membrane potential levels, indicative of increased excitability.
Lowering of firing threshold, enhancement of spike train oscillations and bursts and prolongation of discharges in VRC neurons could represent compensatory adjustments in the brain stem respiratory network that override opioid blunting of CO2/pH chemosensitivity. The study also revealed considerable variability between experiments in burst and oscillatory patterns under baseline conditions and in response to fentanyl.
MATERIALS AND METHODS
Animals, surgical preparation, and procedures.
Previously unpublished findings reported here (26, 27) describe opioid effects on spike train oscillations and burst properties in VRC neurons. They were obtained from measurements made on 22 neurons, selected from a total of 113 VRC neurons that were analyzed in previous studies for other effects of fentanyl on neuron respiratory rhythm. Animals were purchased from a commercial breeding colony. Care and use of animals were in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, National Research Council, and approved by the University of Wisconsin Medical School Animal Care and Utilization Committee. Anesthesia was induced in cats weighing 3.0–5.7 kg with halothane in a chamber (5% halothane in oxygen, 5 l/min gas flow) followed by administration through a mask (2.5–3.5%, 2–3 l/min), and then pentobarbital sodium (Abbott Laboratories, North Chicago, IL) 30 mg/kg iv was given during gradual withdrawal of halothane, thereby maintaining anesthesia. During surgery and experimentation, supplemental intravenous doses of pentobarbital sodium (4–8 mg/kg iv) were given if systolic arterial pressure increased spontaneously, if breathing became irregular, or if discharges of phrenic nerve activity decreased in duration and increased in frequency or showed a tonic discharge component. Additional anesthetic was also given if shivering indicative of lightening of anesthesia occurred or if surgical procedures or pressure on a paw produced withdrawal reflexes. Animals were given atropine methyl nitrate (0.2 mg/kg ip; Sigma Chemical) to minimize airway secretions. A femoral artery and both femoral veins were cannulated for monitoring arterial pressure and administering glucose-Ringer solution and drugs. A cannula was inserted into the trachea below the larynx. Neuromuscular paralysis was achieved by intravenous administration of gallamine triethiodide (American Cyanamid, Pearl River, NY), 4–8 mg/kg initially followed by 4–8 mg/h. Animals were ventilated with oxygen-enriched air (65–75 vol% O2) through the tracheal cannula. The rate of ventilation, end-tidal CO2 (ETCO2), and inspired O2 were monitored continuously (NormocapOxy, Datex-Ohmeda, Madison, WI). ETCO2 was maintained within the range recorded during spontaneous breathing before neuromuscular blockade (32–38 Torr) by adjusting ventilatory rate and tidal volume. Temperature was measured rectally and maintained at 36–38°C by external heating. Pneumothorax was performed bilaterally to increase stability of recording from medullary respiratory neurons. Applying 1- to 2-cm H2O pressure to the expiratory outflow prevented atelectasis. Animals were rigidly mounted in a stereotaxic head holder and suspended by thoracic and lumbar spinal clamps. Phrenic nerves (C4–C5) and cervical vagus nerves were exposed bilaterally through a dorsal approach and sectioned, their central ends were desheathed, and then nerve trunks were mounted on bipolar silver hook electrodes and covered with a mixture of Vaseline and mineral oil. The head of the animal was ventroflexed to allow wide exposure of the dorsal surface of the medulla by occipital craniotomy. The dura and arachnoid membranes were reflected, and patches of pia membrane were removed to allow insertion of fine-tipped glass microelectrodes. A pressure foot was placed gently on the surface of the medulla over the site of microelectrode insertion. A cervical laminectomy (C2–C4) was performed, and the dura was cut and reflected for insertion of stimulating electrodes. After placement of electrodes, the spinal cord and other exposed tissue except for the medulla were coated with warm (37°C) Ringer solution containing 3% agar.
Nerve recording procedures and measurements.
To monitor respiratory network rhythm, phrenic nerves (C4–C5) were exposed bilaterally through a dorsal cervical approach and prepared for recording from their central ends with bipolar silver hook electrodes. Phrenic nerve activity (PNA) was amplified (×2,000–10,000; Grass Instruments, Quincy, MA), band-pass filtered (100–3,000 Hz), displayed on an oscilloscope (Tektronix Instruments, Beaverton, OR), and registered on magnetic tape (frequency response, DC-5 kHz; Vetron Technology, Rebersburg, PA) and on chart recorder paper (DC-10 kHz; Gould, Cleveland, OH) as raw discharges (neurograms) of compound action potential frequency. To obtain quantitative estimates of PNA, measurements included 1) the peak frequency of compound action potentials obtained from the moving average and 2) the durations of the inspiratory, postinspiratory, and expiratory phases of the cycle.
Intracellular recordings.
Neurons were impaled with sharp microelectrodes filled with 2 M potassium methylsulfate. DC electrode resistance in brain tissue was 60–80 MΩ. Membrane potential was recorded in either discontinuous single-electrode current-clamp mode (bandwidth DC-10 kHz, switching frequency 25 kHz; SEC-05XL amplifier, NPI, Tamm, Germany) or balanced bridge mode. In some experiments, input resistance was measured by injecting 60-ms negative-going constant current pulses through the microelectrode, and the resulting hyperpolarizing voltage drop across the cell membrane was recorded. The SEC-05XL microelectrode amplifier is equipped with circuits that provide rapid voltage settling during high-frequency switching between pulse injection and membrane potential recording to eliminate series electrode resistance and capacitance. Data sampling frequency in bridge or constant current recording modes ranged from 20 to 40 kHz.
Data monitoring.
PNA and neuron membrane potential were displayed on an oscilloscope (Tektronix Instruments, Beaverton, OR), registered on magnetic tape (DC-5 kHz; Vetron Technology, Rebersburg, PA), as well as on a computer-controlled data acquisition system (DC-10 kHz; PowerLab, AD Instruments, Castle Hill, Australia) and a paper chart recorder (Gould, Cleveland, OH; DC-10 kHz), and stored on compact discs (CDs). Arterial blood pressure and heart rate were also registered on the chart recorder.
Localization of VRC neurons.
Respiratory-rhythmic neurons in the VRC were searched for using the obex as a surface reference site and stereotaxic reference coordinates from Berman’s brain stem atlas of the cat (5). Positioning of microelectrodes was carried out under microscopic control.
Identification of bulbospinal neurons and vagal laryngeal motoneurons.
To determine if VRC neurons were bulbospinal, two concentric coaxial stimulating electrodes (model 158 SNEX-100, AM Systems, Everett, WA) were positioned bilaterally in the cervical reticulospinal tracts at the C3 level. Stimulation with single bilateral shocks (5–15 V, 0.1-ms pulse duration) antidromically activated bulbospinal neurons, as verified by collision with spontaneous action potentials. Vagal inspiratory laryngeal motoneurons were identified by antidromic activation with single shocks (1.5–3 V, 0.1 ms) applied to the central end of the ipsilateral cervical vagus nerve.
Selection of VRC neurons.
Neurons described in this study were found in the VRC at stereotaxic coordinate locations where a wide range of dose-related responses to fentanyl have been reported (25–28). Neurons were selected for analysis if intracellular recording was stable over test durations of 20 to 30 min and if membrane potential during the silent period was at least −60 mV.
Opiate injection.
Fentanyl citrate (Sigma-Aldrich Chemical, St. Louis, MO), dissolved in Ringer solution (40 μg/ml), was administered intravenously in increments of 1 μg/kg at 1-min intervals until lengthening of medullary neuron burst discharges occurred in parallel with prolongation of phrenic nerve inspiratory discharges.
Euthanasia.
Experiments were terminated by intravenous injection of pentobarbital sodium in sufficient quantity to produce permanent cardiac arrest.
Data analysis.
Post hoc measurements of data stored on CDs were made with PowerLab and IGOR Pro 6.36 software (WaveMetrics, Portland, OR). Neuromatic’s “Spike” electrophysiological software subroutine (Jason Rothman, University College London, UK) was used to measure the number of action potentials in a burst, average spike frequency and interspike intervals. IGOR software generated autocorrelograms, wave averages, and measurements of power spectral density. The autocorrelogram generated by IGOR is a continuous waveform, represented by:
Wave amplitude is represented in millivolts, and values at calibrated time intervals along the x-axis were computed (10 points = 1 ms). Autocorrelation scaling set the center point equal to zero so that the center count value was equal to 1.0. Mean values in the series were removed with a software option to eliminate the otherwise triangular configuration of the correlogram. A linear autocorrelation algorithm was used, and waves to the left of and including the refractory period peak were digitally deleted. To facilitate estimation of burst and oscillatory patterns, waveform smoothing was applied (6). This was done with an IGOR binomial (Gaussian) algorithm, in which control and fentanyl autocorrelograms were subjected to 1,000-pass smoothing.
Spike train patterns were analyzed with IGOR software. Autocorrelograms of individual burst and oscillation strengths were measured by using criteria adapted from Bingmer and colleagues (7). The initial and largest peak of the autocorrelogram that immediately follows the refractory period serves as the primary reference point. The oscillation that subsequently follows has a smaller peak, referred to as the side peak. Oscillations that reach a steady level of uniform intervals toward the end of the train serve as baseline, and three or more oscillations separated by pauses are termed bursts.
Averaging of autocorrelograms with an IGOR analysis algorithm and graphical superimposition of unaveraged individual autocorrelograms were used to determine if the patterns and intensities of bursting and oscillatory activity were consistent before and after fentanyl administration. Waves were deemed acceptable for quantitative scoring of bursts and oscillations and for figure presentation if they aligned temporally and in amplitude when superimposed, and if the superimposed records matched the corresponding wave average (see results, Fig. 1B).
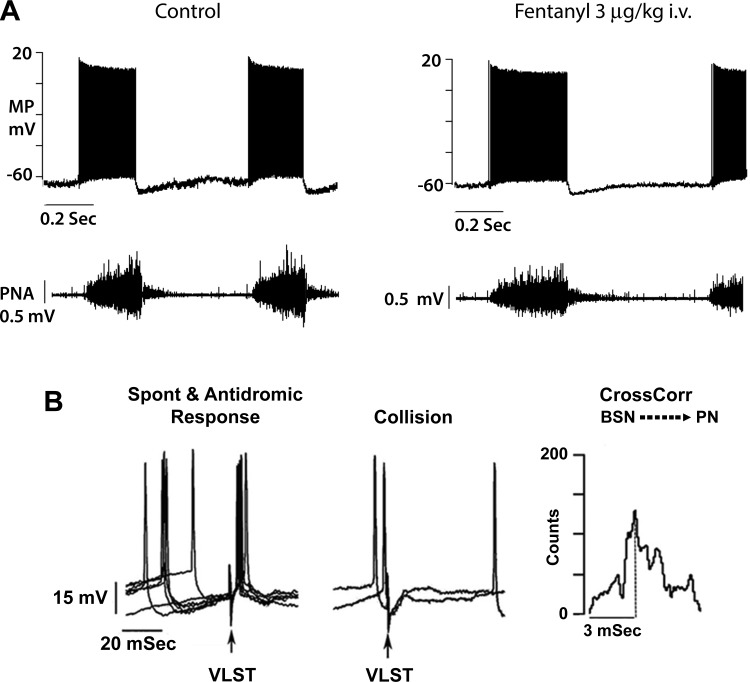
Fig. 1.
Lengthening of discharge and slowing of respiratory rhythm in an augmenting inspiratory (AugI) bulbospinal neuron by a threshold intravenous dose of fentanyl. A: in top traces, AugI neuron membrane potential (MP) and discharge properties; bottom traces, phrenic nerve activity (PNA). B: tests showing that the AugI neuron is bulbospinal. Left: superimposition of traces showing spontaneous action potentials preceding antidromic firing evoked by bilateral electric shocks (arrow) applied to the ventrolateral spinal tract (VLST). Middle: spontaneous action potentials arriving during the absolute refractory period annihilate action potential generation by antidromic VLST. Right: cross-correlation (CrossCorr) of AugI bulbospinal neuron (BSN) action potentials with phrenic nerve compound action potentials (PN). Plot shows the interval distribution of lag intervals between BSN and PN. Dotted line shows that the most frequent interval between BSN and PN is 3 ms.
The degree of variability in autocorrelation patterns was estimated from the coefficient of variation (CV) (40). Wave amplitude measurements at 1-ms intervals from beginning to end of a discharge were transferred to Excel (Excel Software, Henderson, NV). A mean value and its standard deviation were calculated for each discharge to derive CV.
In addition to autocorrelograms, power spectral density plots of action potential frequency in a neuron discharge were generated with IGOR software (segment length, 4096 points; Hahn window). Inter spike interval histograms (bin size, 10 ms) and an average value of action potential frequency in a discharge segment were generated by Spike (Neuromatic).
Statistics.
Discharge properties were analyzed with Excel version 2011 supplemented by QI Macros 2014 add-on software (KnowWare Software, Henderson, CO). Tests for statistical significance were made from paired t-tests. Differences between control and treatment values were considered significant if P < 0.05. Data sets were also analyzed for normality of distribution with StatPlus:mac Professional, 2009 (AnalystSoft, Walnut, CA) and IgorPro 6.3 (Wavemetrics, Lake Oswego, OR). With StatPlus and IgorPro 6.3, the following tests for normality of data distribution under control conditions and after fentanyl administration were made: Kolmogorov-Smirnov/Lilliefor Test; Shapiro-Wilk test; D’Agostino kurtosis and omnibus tests; and the Jarque-Bera statistic and its critical value.
RESULTS
Overview of neuron types and locations.
Five types of VRC neurons were analyzed for threshold effects of fentanyl (Table 1): bulbospinal augmenting inspiratory (AugI) neurons, bulbospinal augmenting expiratory (AugE) neurons, augmenting inspiratory laryngeal motoneurons (LMN), decrementing postinspiratory (Post-I) neurons designated as propriobulbar since they are not antidromically activated by spinal cord or vagus nerve stimulation, and augmenting propriobulbar preinspiratory (Pre-I) neurons. The AugI bulbospinal neurons, the most widely distributed of the VRC neurons in this study, were located 1–3 mm rostral to the obex, 3.4–3.8 lateral, 3.2–4.0 below the dorsal surface. AugE bulbospinal neurons were located caudal to the obex, clustered in a relatively localized region where bulbospinal neurons predominate in the cat (4). Another bulbospinal AugE neuron was located rostral to the obex, close to the pre-Bötzinger complex and nucleus ambiguus, where AugI laryngeal motoneurons are located (46).
Table 1.
Different types of ventral respiratory column respiratory neurons analyzed
| Neuron Type | Projection | Discharge Pattern | n |
|---|---|---|---|
| Inspiratory | Bulbospinal | Augmenting | 3 |
| Laryngeal inspiratory | Vagal motoneuron | Augmenting | 4 |
| Expiratory | Bulbospinal | Augmenting | 9 |
| Preinspiratory | Propriobulbar | Augmenting | 2 |
| Postinspiratory | Propriobulbar | Decrementing | 2 |
| Rostral VRC expiratory | Propriobulbar | Augmenting | 1 |
| Inspiratory | Propriobulbar | Plateau | 1 |
VCR, ventral respiratory column.
Responses to lowest effective doses of intravenous fentanyl.
Threshold doses of fentanyl (2–3 μg/kg) significantly increased discharge duration and slowed rhythm in all types of neurons except the propriobulbar Pre-I type. Figures 1–7 highlight effects of fentanyl on rhythm and discharge properties for a representative example of each type of VRC neuron.
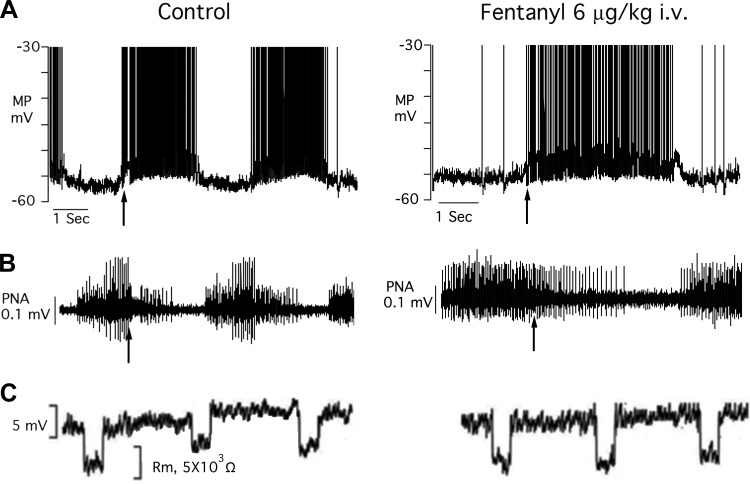
Fig. 7.
A and B: records from a propriobulbar postinspiratory neuron (MP) and phrenic nerve activity (PNA). Arrows denote the abrupt onset of the decrementing discharge, coincident with the termination of the inspiratory burst phase of PNA. The dose of fentanyl (6 μg/kg iv) is threshold for effects on the neuron. C: hyperpolarizing electrotonic potentials evoked during current-clamp recording to estimate cell membrane input resistance (Rm) of the Post-I neuron.
AugI bulbospinal neurons.
Figure 1A exhibits discharge lengthening of inspiratory discharges and PNA after administration of a threshold dose of fentanyl. Figure 1B presents evidence for the neuron being bulbospinal. Ventrolateral spinal cord stimulation evoked relatively constant-latency back firing of the neuron when spontaneous action potentials occurred 20–40 ms before the neuron’s refractory period. The cross-correlation measurement also supports identification of the neuron as bulbospinal. The correlogram is set up with neuron action potentials as the source and PNA as the target. The peak lag time between neuron action potentials and PNA was 3 ms. The assumption is that the delay represents axon conduction time over a distance of ~100 mm, from AugI neuron to the ventral horn of cervical spinal cord, and an oligosynaptic connection with the phrenic motoneurons.
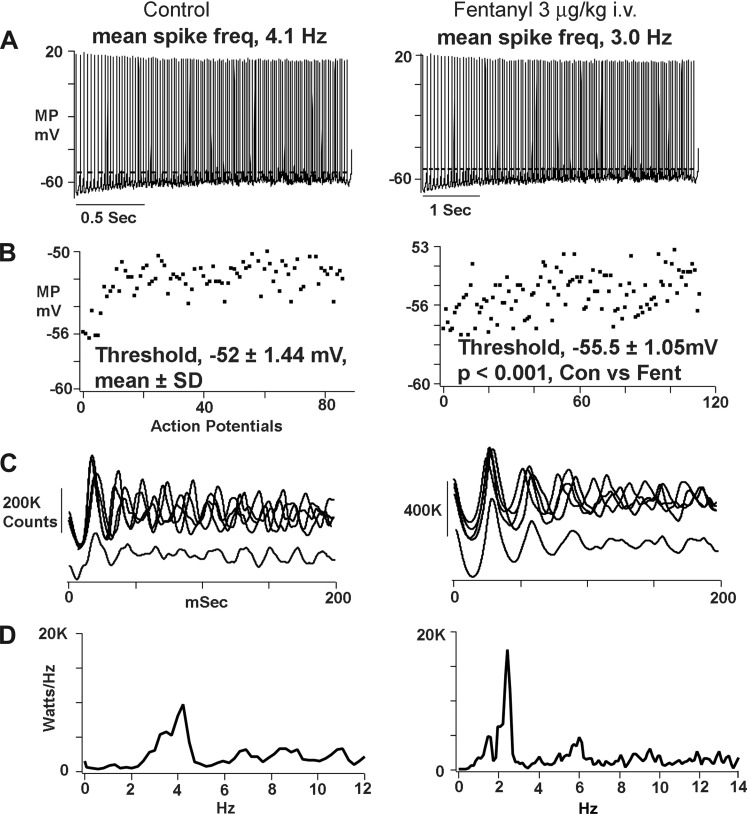
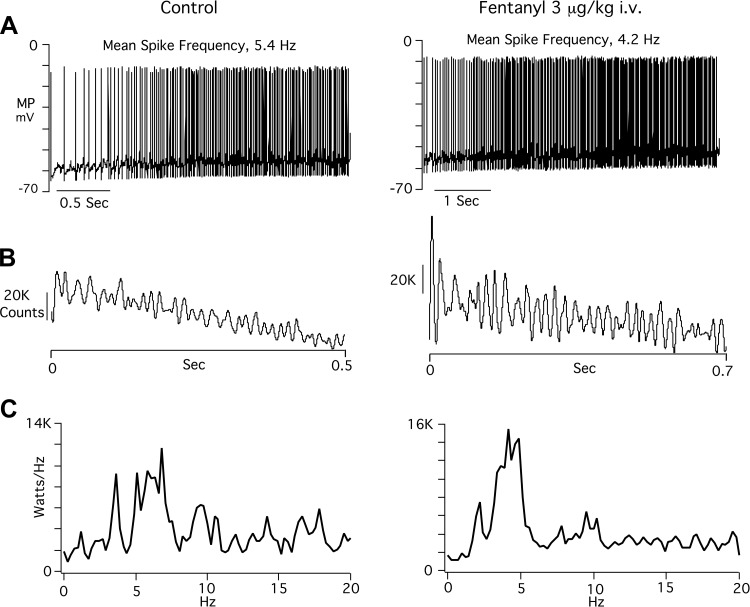
Figure 2 illustrates the effects of fentanyl on the bulbospinal neuron (BSN) shown in Fig. 1. Figure 2A shows inspiratory neuron discharges under control conditions and after fentanyl. Time scales are different because fentanyl increased discharge duration by 25%. The mean spike frequency detected by the spike subroutine of neuromatic was decreased by fentanyl, from 4.1 to 3.0 Hz. In addition, action potential threshold was reached at a more negative level of membrane potential. Figure 2B shows scattergram plots obtained by measuring the firing threshold of each action potential. The firing threshold after giving fentanyl (–55.5 ± 1.05 mV, mean ± SD, n = 117 action potentials) was significantly more negative than control (−52 ± 1.4 mV, n = 88, P < 0.001). Figure 2C displays superimposed autocorrelograms that were recorded at 5-min intervals during 30 min of recording, verifying the stability of recording. The autocorrelogram averages (Fig. 2C, bottom traces) show that fentanyl produced significant increases in oscillation and bursts magnitude (P < 0.01). Power spectral density measurements in Fig. 2D illustrate a shift in peak power from 3 to 2 Hz.
Fig. 2.
Records, taken from the AugI neuron shown in Fig. 1, showing effects of the threshold dose of fentanyl on spike train properties. A: single inspiratory phase neuron discharges before and after giving fentanyl. Time scales are different in left and right because discharge duration is considerably lengthened by fentanyl. Dashed line in each panel denotes the average action potential threshold under control conditions. B: scatter plots of MP threshold for all action potentials depicted in A. Each dot represents threshold value (ordinate) of a corresponding action potential. C: autocorrelation traces. Top traces: superimposed autocorrelations recorded at 5-min intervals. Bottom traces: averages derived from the superimposed autocorrelations. D: power spectral density [kilowatts/hertz plotted against frequency (Hz)].
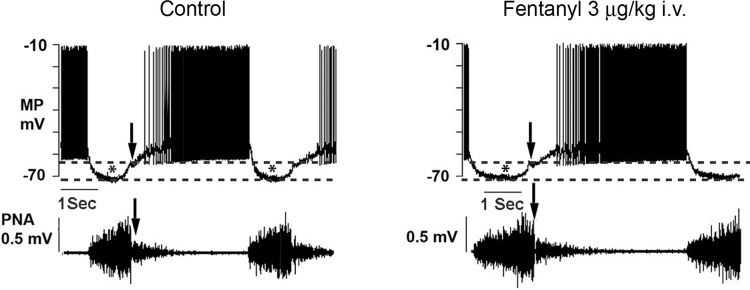
Figure 3 illustrates responses of an AugE BSN to fentanyl that are typical of the other eight neurons. In each panel, downward-directed arrows identify the onset of the postinspiratory phase in the neuron and PNA. Asterisks and dashed lines identify the onset, duration, and magnitude of the waves of inhibitory synaptic potentials, which coincide with phrenic nerve inspiratory phase discharges. This figure illustrates the typical, predominant lengthening of AugE BSN discharge by threshold doses of fentanyl, without appreciable change in discharge frequency.
Fig. 3.
Records of a bulbospinal augmenting expiratory neuron (AugE) discharge (MP) and phrenic nerve activity (PNA) under control conditions and in response to a threshold dose of fentanyl. Dashed lines denote the amplitudes of waves (*) of inhibitory synaptic potentials that coincide with phrenic nerve inspiratory phase discharges (PNA). Arrows denote the termination of the inspiratory phase.
Figure 4 illustrates discharge properties recorded from the AugE BSN shown in Fig. 3. Figure 4A illustrates that fentanyl slowed mean spike train frequency, from 5.4 to 4.2 Hz. Action potential threshold was not appreciably affected in this AugE neuron nor in the others. For all AugE neurons, firing threshold was −54.0 ± 3.89 mV, and fentanyl threshold was −54.9 ± 2.16 mV (P = 0.59). The autocorrelograms of Fig. 4B show that burst and oscillatory patterns were considerably increased in intensity by fentanyl in this neuron. However, the differences between control and fentanyl in all AugE neurons were not statistically different, owing to large standard deviations under control and fentanyl conditions. Fentanyl increased the mean burst score from 3.5 ± 2.06 to 6.0 ± 3, a 67% increase over control (P = 0.07). Oscillation score increased from 2.13 ± 0.92 to 2.98 ± 1.15, 40% increase (P = 0.12). In Fig. 4C, power spectral density plots show a shift to a lower peak frequency after fentanyl administration.
Fig. 4.
Records of the effects of a threshold dose of fentanyl on the AugE bulbospinal neuron illustrated in Fig. 3. A: single expiratory phase neuron discharges before and after giving fentanyl. B: autocorrelograms before and after giving fentanyl. C: power spectral density plots.
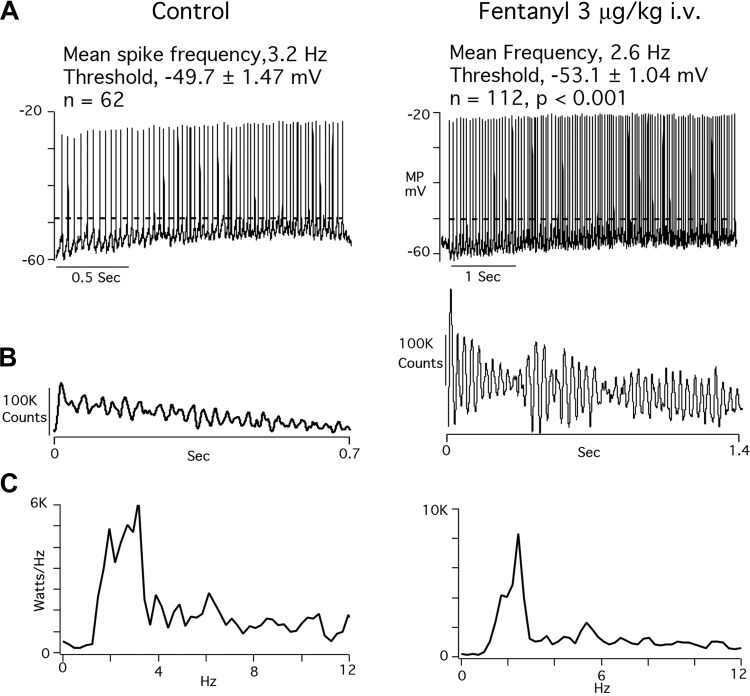
Figure 5 highlights effects of fentanyl on one of the four vagal laryngeal motoneurons. Figure 5A shows the typical lengthening of the inspiratory discharge in parallel with PNA. In addition, the expiratory wave of membrane depolarization in the control panel is abolished by fentanyl, but action potentials are generated at a more negative membrane potential during both the inspiratory and expiratory phases. Figure 5B shows superimposed antidromic evoked responses to cervical vagus nerve stimulation, and Fig. 5C shows the temporal properties of spontaneous firing and its temporal relationship to PNA.
Fig. 5.
A: control responses and effects of a threshold dose of fentanyl on an AugI laryngeal motoneuron and on phrenic nerve activity. B: superimposed antidromic responses of the neuron evoked by electrical pulses applied to the central end of the sectioned vagus nerve. C: spontaneous action potentials (MP) coinciding with phrenic nerve activity (PNA).
Figure 6, taken from the same vagal laryngeal motoneuron shown in Fig. 5, illustrates several noteworthy fentanyl effects. Figure 6A illustrates that mean action potential frequency is reduced, and mean action potential threshold is shifted to a more negative level of membrane intensities of burst and oscillation patterns, and Fig. 6C power spectral density plots affirm the shift to lower spike frequencies. Values for fentanyl effects on the four neurons are the following: increased burst intensities, from 301 1.76 ± 0.33 to 2.09 ± 0.19 (19% increase); increased oscillation intensities, from 5.30E+04 ± 36,825 to 8.70E+04 ± 66,125 (64% increase); and more negative action potential threshold, from −52.6 ± 8.7 to −55.3 ± 7.8 (5% change).
Fig. 6.
Effects of a threshold dose of fentanyl on spike train properties of the AugI laryngeal motoneuron depicted in Fig. 5. A: fentanyl lengthens the inspiratory phase laryngeal neuron discharge and slows action potential frequency. Dashed lines emphasize the more negative discharge threshold after giving fentanyl. Thresholds of all action potentials were measured to derive the mean frequencies and standard deviations of control and fentanyl-affected discharges. B: autocorrelograms that illustrate pronounced increases in oscillation and burst patterns after fentanyl administration. C: power spectral density plots. Under control conditions, power peaks are evident at 2–4 Hz, whereas after giving fentanyl, a single, more prominent peak is evident at 2 Hz.
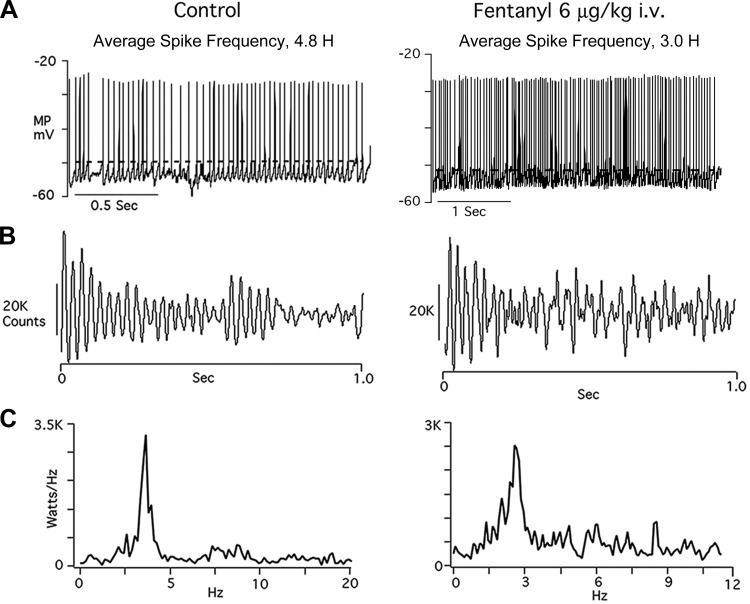
Figure 7 illustrates effects of fentanyl on one of two propriobulbar postinspiratory neurons. In both neurons, higher doses of fentanyl were required to prolong neuron discharge and slow respiratory rhythm. The upward-directed arrows in Fig. 7, A and B, call attention to the abrupt, decrementing discharge that normally coincides with off-switching of the phrenic nerve inspiratory burst under control basal firing (38) and the earlier onset relative to PNA when fentanyl is given. Figure 7C shows that cell input resistance is not significantly altered, indicating that the site of action of fentanyl on these neurons is not postsynapt.
Figure 8 further illustrates spike train features of the Post-I neuron shown in Fig. 7. Fentanyl is seen to slow average action potential frequency, from 4.8 to 3.0 Hz (Fig. 8A). Burst activity is depressed in the later part of the spike train (Fig. 8B), and peak power at 3 Hz is reduced.
Fig. 8.
Records of spike train discharge properties from the Post-I neuron illustrated in Fig. 7. Fentanyl slows average spike frequency and lowers action potential threshold (A). There is no significant effect on oscillation intensity, but burst patterns are depressed (B) and peak power at 3 Hz is reduced (C).
Higher doses of fentanyl were required to affect preinspiratory neuron discharges (not shown). In experiments performed on two Pre-I neurons, doses of 5 and 6 μg/kg, respectively, abolished action potentials that coincided with the phrenic nerve expiratory silent period and prolonged the duration of inspiratory phase discharges. Oscillation patterns were not affected significantly, whereas bursts were depressed.
Lowering of membrane potential threshold for action potential generation.
In 14 of the 22 VRC neurons, fentanyl shifted membrane potential threshold to more negative levels of membrane potential, from −53.1 ± 1.12 to −56.4 ± 1.25 mV (means ± SD, P < 0.001). Firing threshold was not changed in the AugE bulbospinal neurons of the caudal VRC or in the propriobulbar neurons.
Statistical analysis of response variability.
Lowest effective doses of fentanyl produced striking changes in five of seven spike train properties in the 22 VRC neurons. Differences between control and fentanyl responses ranged from 9.3 to 31.4%, as shown in Figs. 9 and 10. Nonetheless, across experiments, differences between control and fentanyl means were not statistically significant because there was considerable variability in spike train properties, with large standard deviation from the means both under control conditions and in the responses to fentanyl.
Fig. 9.
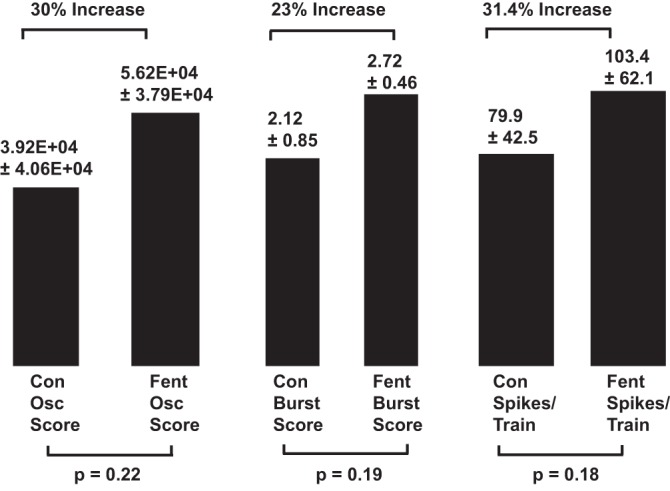
Histograms summarizing the effects of threshold doses of fentanyl (Fent) on oscillation (Osc) intensity, burst intensity, and action potentials/train based on pooled data from all ventral respiratory column (VRC) neurons (n = 22). Mean and standard error values for the control (Con) and fentanyl measurements are positioned above each histogram, along with changes produced by fentanyl. Significance of differences between control and fentanyl means is entered below each bar.
Fig. 10.
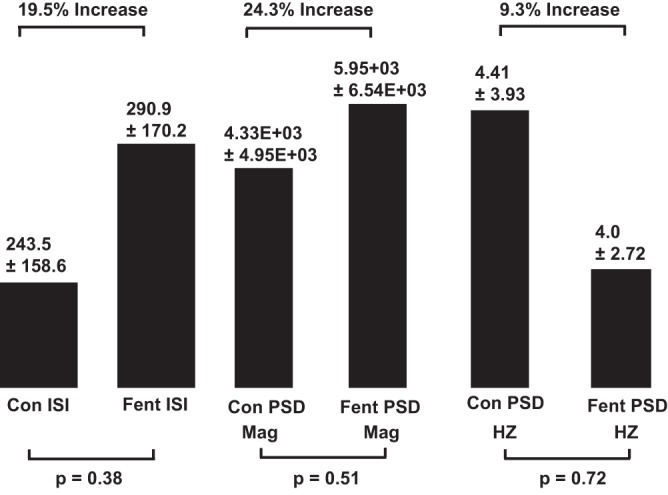
Histogram summarization of the effects of threshold fentanyl doses on interspike intervals (ISI), power spectral density (PSD), magnitude (Mag), and peak frequency (Hz) on all VRC neurons. Refer to the Fig. 9 legend for details.
Statistical tests to determine if nonnormal data distribution might have been a critical factor revealed varying degrees of skewedness and kurtosis as well as outliers in data distributions. However, departures from normal distribution do not by themselves account for the large differences in spike train properties across experiments, because some data sets were normally distributed yet had a high degree of variability such that differences between control and treatment means were not statistically significant. For example, interspike interval (ISI) values were normally distributed, as shown by Kolmogorov, Shapiro-Wilk, D’Agostino, and Jarque-Bera tests. Under control conditions, ISI was 243 ± 158.6 ms, means ± SD. The fentanyl ISI was 290.9 ± 170.2 ms (19.5% increase), and the P value for significance of difference was 0.38.
Effect of fentanyl on discharge CV and its relation to firing threshold.
Neurons that discharge in the midst of a noise-driven environment frequently exhibit discharges during spontaneous firing that are characterized by high conductance states and large fluctuations in membrane potential. Under such circumstances, action potential discharge probability is characteristic of a Poisson process. Action potentials occur randomly and CV approach unity (40, 47). In addition, membrane potential is close to spike threshold.
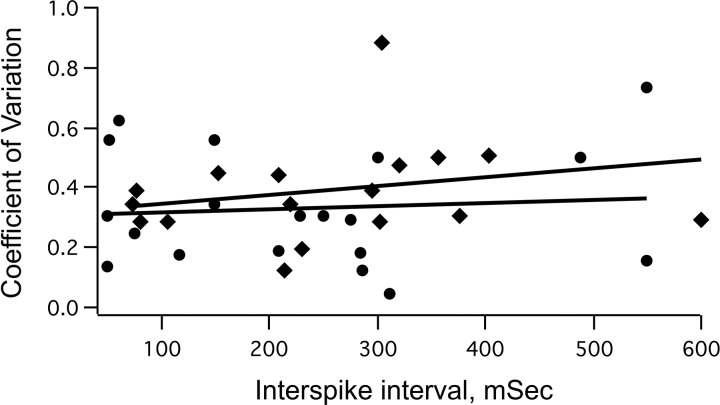
Large membrane potential fluctuations and high conductance state are typical features of medullary respiratory neurons in vivo (39). Therefore, to ascertain whether an increase in discharge variability in VRC neurons might have contributed to the lower firing threshold of rhythmic burst discharges after giving fentanyl, CV was calculated from control and treatment discharges. In Fig. 11, CV values calculated for the 22 VRC neurons are plotted against the interspike interval to evaluate discharge variability. CV increased only moderately, from 0.33 ± 0.19 (means ± SD) under control conditions to 0.40 ± 0.20 after fentanyl administration. If neuron discharges follow random Poisson probability, they typically have a steep upward slope in such plots, and peak values of CV are close to 1.0. However, in the present study, control and fentanyl best-fit lines of average pass through 0.3–0.4, and the coefficient of regression (r2) is 0.05 for control and 0.1 for fentanyl. Evidently, fentanyl does not lower firing threshold by increasing discharge variability to promote larger membrane potential fluctuations and increased excitability.
Fig. 11.
Coefficients of variation plotted against interspike intervals for all VRC neurons (n = 22). Circles with the line of best fit are taken from control measurements before fentanyl administration. Diamonds with the best-fit line are from fentanyl measurements made after injecting threshold doses of fentanyl.
DISCUSSION
Methodological considerations.
One advantage of the intracellular analysis used in the present study is that effects of lowest effective doses of fentanyl on the whole spectrum of membrane potential changes are revealed: subthreshold events to spike train discharge properties. This approach has uncovered two particularly noteworthy effects of fentanyl on the 22 VRC neurons analyzed in this study. First, considerable increases in burst and oscillation magnitudes accompany spike train lengthening in most of the neurons. Second, excitability is enhanced in most of the VRC neurons, as evidenced by shifting of action potential threshold to more negative levels. The changes in spike train properties and threshold are evidently mediated by fentanyl via presynaptic sites and mechanism of action, or at spatially remote dendritic regions of VRC neurons, because membrane input resistance and spike shape properties were not affected by fentanyl in this study and in other published studies from this laboratory (25, 28).
Animals in this study were anesthetized with pentobarbital, which has direct GABA-mimetic neuronal effects on CNS neurons in vitro (50). Although a previous study from this laboratory found no differences between pentobarbital-anesthetized and unanesthetized, midcollicular decerebrate cats in the slowing of respiratory rhythm by fentanyl (25), pentobarbital anesthesia might have altered opioid effects on oscillatory and burst activities in the present study.
Overview of previous studies.
Fast oscillatory activity was detected in vivo in VRC neurons of adult cats by Cohen et al. (8, 19), Mitchell and Herbert (33), Mellen et al. (32), and more recently in neonatal rat medullary inspiratory neurons in vitro (12, 13, 45). The latter study (45) demonstrated a critical contribution of inhibitory amino acid neurotransmission to synchronization of oscillatory activity. Analysis of hypoglossal nerve oscillatory activity in neonatal brain stem slice preparations revealed that GABAergic and glycinergic transmission contributes to synchronization of discharges. In fact, the time course of synaptic GABAergic currents is a determinant of oscillation frequency. From their in vivo investigation in anesthetized cats, Mitchell and Herbert (33) postulated that inspiratory VRC neurons receive, in the form of high-frequency oscillations (HFOs), a highly synchronized excitatory synaptic input from other sites in the pontomedullary complex, whereas HFOs in expiratory neurons are derived from medullary inspiratory neurons with connections to inhibitory interneurons. Huang et al. (19) pointed out significant temporal differences in inspiratory, expiratory, and laryngeal motoneuron HFOs, which they attributed, as did Mitchell and Herbert, to input connectivity differences. Parkis et al. (34) and Funk et al. (13) focused attention on the functional importance of HFOs in VRC neurons to respiratory motoneuron performance, contending that by controlling the timing of action potentials, HFOs increase efficiency of respiratory muscle contraction.
Compelling evidence obtained from dynamic feedback clamp studies (34) led to three principal conclusions concerning the functional importance of oscillations in the VRC: 1) oscillations constrain mechanisms by which neuromodulators affect respiratory motoneuron activity, 2) oscillations influence the development of muscle force in respiratory muscles such as the diaphragm, and 3) oscillations synchronize phrenic motoneuron discharges, enhancing force transmission to the diaphragm.
Brain stem sites and mechanisms of action.
Because threshold doses of fentanyl slow rhythm in all categories of VRC neurons, it is assumed that widespread sites and mechanisms of opiate action in the brain stem respiratory network are involved, including the pre-Bötzinger complex, the parafacial region, dorsolateral pons, CNS chemoreceptor regions including the retrotrapezoid region and medullary raphe nuclei, and neurons of the solitary nucleus where axons terminals from the dorsolateral pons terminate (35), as well as afferent synaptic inputs from chest wall afferents. In the present investigation, effects of fentanyl on lung-evoked pulmonary reflexes seem unlikely to be contributory since the vagus nerves were transected bilaterally distal to the nodose ganglia. A likely mechanism of action involves delayed off-switching by phase-terminating early-inspiratory, post-inspiratory and late-inspiratory VRC neurons (6, 38, 39). These off-switch neurons are probably affected by fentanyl presynaptically (27, 28).
Functional impact on intracellular signaling.
With regard to burst and oscillatory patterns, Kaneoke and Vitek (23), propose that oscillations reflect discharge activity of several nearby synaptically coupled neurons that convey a coded message to a neuron, whereas bursting is an output signal. The functional consequences of bursting may be to augment neurotransmitter or neuromodulator release at downstream synapses. For example, burst firing is postulated to increase dopamine release from substantia nigra neurons (15) and norepinephrine release from hypothalamic paraventricular neurons (48a). If those interpretations apply to the spike train properties of VRC neurons, then it might be surmised that threshold doses of μ-opioid receptor agonists such as fentanyl enhance oscillatory activity set up by nearby clusters of interneurons that receive synaptic input from various brain stem sources. Burst patterns, which presumably convey motor commands, are also more robust, more prolonged, and have slower temporal properties when lowest effective doses of fentanyl are given. There is a potential advantage to this combination of effects. Limited, progressive respiratory slowing following low-dose fentanyl administration will cause gradual hypercapnia and respiratory acidosis that contributes to the maintenance of respiration through increased, compensatory, respiratory neural network drive (17, 35).
Variability of fentanyl effects on spike train properties.
In all experiments, end-tidal CO2 (ETCO2) was maintained within a range that maintained robust respiratory neuron discharges. Across experiments, ETCO2 levels ranged from 32 to 38 Torr. On the basis of other studies, differences in ETCO2 could explain why spike train properties were highly variable across experiments. In tests made on anesthetized cats, appreciable variability in action potential frequency occurred in medullary bulbospinal expiratory neurons when was varied between 20 to 40 Torr (3). In anesthetized rats, hypercapnia significantly increases variability in spike train patterns of genioglossus motor units (41), and in humans CO2-dependent ventilatory drive also varies considerably between subjects (49).
Lowering of action potential threshold by μ-opioid receptor activation.
On first consideration, it seems paradoxical that μ-opioid receptor agonists increase excitability in VRC neurons, because they increase cell permeability to several types of hyperpolarizing K+ channels and decrease depolarizing Na+ channel and Ca+ channel permeability (2). However, increased neuron excitability by fentanyl is not without precedent. The highly selective μ-opioid receptor agonist DAMGO increases excitability postsynaptically in some types of striatal neurons (30). Current pulse-evoked action potential threshold is lowered, rheobase current is decreased, and cell input resistance increases. At the same time, spontaneous inhibitory postsynaptic current and excitatory postsynaptic current frequencies are reduced. Periaqueductal neurons in the slice preparation are similarly affected by DAMGO (53). Although membrane potential is hyperpolarized and excitatory postsynaptic potentials are suppressed, stimulus threshold is nonetheless reduced. Suggested mechanisms include removal of postsynaptic Na+-channel inactivation and presynaptic block of GABAergic inputs. In cultured dorsal horn neurons, intrinsic excitability is increased by opioid enhancement of N-methyl-d-aspartate (NMDA) current (53). NMDA receptors are constitutively bound to the COOH terminus of μ-opioid receptors by the HINT1 protein. Agonist binding to the μ-opioid receptor breaks the connection and increases sensitivity of the NNMDA receptors to both glutamate and glycine, and when more of the former than the latter is present, NMDA current is enhanced. An opioid receptor-mediated postsynaptic effect on NMDA receptors in the VRC is also plausible because in the pre-Bötzinger complex in vitro, NMDA receptors have excitatory postsynaptic effects on respiratory drive (32).
Presynaptic effects might also have contributed to the lowering of VRC discharge threshold by μ-opioid receptor agonists increasing excitability through presynaptic disinhibition: for example, Area CA1 of the hippocampus (31), dopamine-containing regions of the ventral tegmental area (22) and the dentate gyrus of the hippocampus (1). Since medullary respiratory neurons in vivo receive a continual barrage of glutamatergic excitatory postsynaptic potentials, glycinergic excitatory postsynaptic potentials (IPSPs) and GABAergic IPSPs throughout the respiratory cycle (37, 39, 43), presynaptic depression of IPSPs by fentanyl would further increase excitatory synaptic drive on VRC neurons.
Perspectives and Significance
This investigation demonstrates that μ-opioid receptor activation by fentanyl, given in doses that selectively slow respiratory rhythm, produces notable increases in burst and oscillatory activities, prolongation of discharges, and increased excitability in VRC neurons. This constellation of respiratory neural network effects could be the consequence of opioid blunting of CO2/pH and Po2 chemosensitivity, leading to compensatory brain stem neural network responses and respiratory motor output patterns that increase respiratory muscle efficiency and performance (51) to allay hypoxia and hypercapnia. The use of an intact respiratory neural network has permitted expression of effects emanating, most likely, from various regions of the VRC as well as pontine respiratory regions, and intracellular recording has provided more precise, detailed information about ways in which different types of VRC neurons process oscillatory and burst signaling.
GRANTS
Support for this study was provided by National Heart, Lung, and Blood Institute Grant HL-65526 (to P. Lalley) and by funding from the Institute for Cardiovascular and Metabolic Disease, UNTHSC.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.M.L. conceived and designed research; P.M.L. performed experiments; P.M.L. analyzed data; P.M.L. and S.W.M. interpreted results of experiments; P.M.L. and S.W.M. prepared figures; P.M.L. and S.W.M. drafted manuscript; P.M.L. and S.W.M. edited and revised manuscript; P.M.L. and S.W.M. approved final version of manuscript.
REFERENCES
- 1.Akaishi T, Saito H, Ito Y, Ishige K, Ikegaya Y. Morphine augments excitatory synaptic transmission in the dentate gyrus through GABAergic disinhibition. Neurosci Res 38: 357–363, 2000. doi: 10.1016/S0168-0102(00)00177-2. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115: 1363–1381, 2011. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bainton CR, Kirkwood PA. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J Physiol 296: 291–314, 1979. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballantyne D, Richter DW. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol 370: 433–456, 1986. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berman A. The Brain Stem of the Cat: A Cytoarchitectonic Atlas with Stereotaxic Coordinates. Madison, WI: Univ. of Wisconsin Press, 1968. [Google Scholar]
- 6.Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75: 1–45, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Bingmer M, Schiemann J, Roeper J, Schneider G. Measuring burstiness and regularity in oscillatory spike trains. J Neurosci Methods 201: 426–437, 2011. doi: 10.1016/j.jneumeth.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MI, See WR, Christakos CN, Sica AL. High-frequency and medium-frequency components of different inspiratory nerve discharges and their modification by various inputs. Brain Res 417: 148–152, 1987. doi: 10.1016/0006-8993(87)90190-9. [DOI] [PubMed] [Google Scholar]
- 9.Drummond GB. Comparison of decreases in ventilation caused by enflurane and fentanyl during anaesthesia. Br J Anaesth 55: 825–835, 1983. doi: 10.1093/bja/55.9.825. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson LM, Drummond GB. Acute effects of fentanyl on breathing pattern in anaesthetized subjects. Br J Anaesth 96: 384–390, 2006. doi: 10.1093/bja/ael011. [DOI] [PubMed] [Google Scholar]
- 11.Fone KC, Wilson H. The effects of alfentanil and selected narcotic analgesics on the rate of action potential discharge of medullary respiratory neurones in anaesthetized rats. Br J Pharmacol 89: 67–76, 1986. doi: 10.1111/j.1476-5381.1986.tb11121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funk GD, Parkis MA. High frequency oscillations in respiratory networks: functionally significant or phenomenological? Respir Physiol Neurobiol 131: 101–120, 2002. doi: 10.1016/S1569-9048(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 13.Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol 70: 1497–1515, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Goodman NW, Vanner RG, Wade JA. Effects of incremental doses of alfentanil and propofol on the breathing of anaesthetized patients. Br J Anaesth 63: 548–553, 1989. doi: 10.1093/bja/63.5.548. [DOI] [PubMed] [Google Scholar]
- 15.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science 286: 1566–1568, 1999. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross JB. When you breathe IN you inspire, when you DON’T breathe, you...expire: new insights regarding opioid-induced ventilatory depression. Anesthesiology 99: 767–770, 2003. doi: 10.1097/00000542-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Haji A, Yamazaki H, Ohi Y, Takeda R. Distribution of μ receptors in the ventral respiratory group neurons; immunohistochemical and pharmacological studies in decerebrate cats. Neurosci Lett 351: 37–40, 2003. doi: 10.1016/S0304-3940(03)00951-0. [DOI] [PubMed] [Google Scholar]
- 19.Huang WX, Cohen MI, Yu Q, See WR, He Q. High-frequency oscillations in membrane potentials of medullary inspiratory and expiratory neurons (including laryngeal motoneurons). J Neurophysiol 76: 1405–1412, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Hurlé MA, Dierssen MM, Flórez J. Mechanism of the respiratory action of pentobarbital at the medullary and pontine levels. Eur J Pharmacol 125: 225–232, 1986. doi: 10.1016/0014-2999(86)90031-2. [DOI] [PubMed] [Google Scholar]
- 21.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570: 407–420, 2006. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12: 483–488, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods 68: 211–223, 1996. doi: 10.1016/0165-0270(96)00081-7. [DOI] [PubMed] [Google Scholar]
- 24.Khoo MC. Determinants of ventilatory instability and variability. Respir Physiol 122: 167–182, 2000. doi: 10.1016/S0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 25.Lalley PM. Dopamine1 receptor agonists reverse opioid respiratory network depression, increase CO2 reactivity. Respir Physiol Neurobiol 139: 247–262, 2004. doi: 10.1016/j.resp.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Lalley PM. μ-Opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol 285: R1287–R1304, 2003. doi: 10.1152/ajpregu.00199.2003. [DOI] [PubMed] [Google Scholar]
- 27.Lalley PM. Opiate slowing of feline respiratory rhythm and effects on putative medullary phase-regulating neurons. Am J Physiol Regul Integr Comp Physiol 290: R1387–R1396, 2006. doi: 10.1152/ajpregu.00530.2005. [DOI] [PubMed] [Google Scholar]
- 28.Lalley P. Opioid actions on the bulbar respiratory network: consequences for breathing. In: Advances in Medicine and Biology, edited by Berhardt LV. New York: Nova Biomedical, 2012, vol. 50, p. 67–87. [Google Scholar]
- 29.Lalley PM. Opioidergic and dopaminergic modulation of respiration. Respir Physiol Neurobiol 164: 160–167, 2008. doi: 10.1016/j.resp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma YY, Cepeda C, Chatta P, Franklin L, Evans CJ, Levine MS. Regional and cell-type-specific effects of DAMGO on striatal D1 and D2 dopamine receptor-expressing medium-sized spiny neurons. ASN Neuro 4: 4453–4469, 2012. doi: 10.1042/AN20110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McQuiston AR, Saggau P. μ-Opioid receptors facilitate the propagation of excitatory activity in rat hippocampal area CA1 by disinhibition of all anatomical layers. J Neurophysiol 90: 1936–1948, 2003. doi: 10.1152/jn.01150.2002. [DOI] [PubMed] [Google Scholar]
- 32.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37: 821–826, 2003. doi: 10.1016/S0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell RA, Herbert DA. Synchronized high frequency synaptic potentials in medullary respiratory neurons. Brain Res 75: 350–355, 1974. doi: 10.1016/0006-8993(74)90760-4. [DOI] [PubMed] [Google Scholar]
- 34.Parkis MA, Feldman JL, Robinson DM, Funk GD. Oscillations in endogenous inputs to neurons affect excitability and signal processing. J Neurosci 23: 8152–8158, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pattinson KT. Opioids and the control of respiration. Br J Anaesth 100: 747–758, 2008. doi: 10.1093/bja/aen094. [DOI] [PubMed] [Google Scholar]
- 36.Prkic I, Mustapic S, Radocaj T, Stucke AG, Stuth EA, Hopp FA, Dean C, Zuperku EJ. Pontine μ-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. J Neurophysiol 108: 2430–2441, 2012. doi: 10.1152/jn.00185.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramirez JM, Telgkamp P, Elsen FP, Quellmalz UJ, Richter DW. Respiratory rhythm generation in mammals: synaptic and membrane properties. Respir Physiol 110: 71–85, 1997. doi: 10.1016/S0034-5687(97)00074-1. [DOI] [PubMed] [Google Scholar]
- 38.Richter D. Neural regulation of respiration: rhythmogenesis, and afferent control. In: Comprehensive Human Physiology, edited by Gregor R and Windhorst U. Heidelberg, Germany: Springer, 1996, p. 2079–2095. [Google Scholar]
- 39.Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology (Bethesda) 29: 58–71, 2014. doi: 10.1152/physiol.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudolph M, Destexhe A. The discharge variability of neocortical neurons during high-conductance states. Neuroscience 119: 855–873, 2003. doi: 10.1016/S0306-4522(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 41.Sahn SA, Zwillich CW, Dick N, McCullough RE, Lakshminarayan S, Weil JV. Variability of ventilatory responses to hypoxia and hypercapnia. J Appl Physiol 43: 1019–1025, 1977. [DOI] [PubMed] [Google Scholar]
- 43.Schmid K, Foutz AS, Denavit-Saubié M. Inhibitions mediated by glycine and GABAA receptors shape the discharge pattern of bulbar respiratory neurons. Brain Res 710: 150–160, 1996. doi: 10.1016/0006-8993(95)01380-6. [DOI] [PubMed] [Google Scholar]
- 44.Schwarzacher SW, Rüb U, Deller T. Neuroanatomical characteristics of the human pre-Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain 134: 24–35, 2011. doi: 10.1093/brain/awq327. [DOI] [PubMed] [Google Scholar]
- 45.Sebe JY, van Brederode JF, Berger AJ. Inhibitory synaptic transmission governs inspiratory motoneuron synchronization. J Neurophysiol 96: 391–403, 2006. doi: 10.1152/jn.00086.2006. [DOI] [PubMed] [Google Scholar]
- 46.Shiba K, Satoh I, Kobayashi N, Hayashi F. Multifunctional laryngeal motoneurons: an intracellular study in the cat. J Neurosci 19: 2717–2727, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Softky WR, Koch C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J Neurosci 13: 334–350, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stucke AG, Miller JR, Prkic I, Zuperku EJ, Hopp FA, Stuth EA. Opioid-induced respiratory depression is only partially mediated by the preBötzinger complex in young and adult rabbits in vivo. Anesthesiology 122: 1288–1298, 2015. doi: 10.1097/ALN.0000000000000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48a.Suaud-Chagny MF, Mermet C, Gonon F. Electrically evoked noradrenaline release in the rat hypothalamic paraventricular nucleus studied by in vivo electrochemistry: characterization and facilitation by increasing the stimulation frequency. Neuroscience 34: 411–422, 1990. doi: 10.1016/0306-4522(90)90150-3. [DOI] [PubMed] [Google Scholar]
- 49.van Lunteren E, Dick TE. Heterogeneity within geniohyoid motor unit subpopulations in firing patterns during breathing. Respir Physiol 124: 23–33, 2001. doi: 10.1016/S0034-5687(00)00182-1. [DOI] [PubMed] [Google Scholar]
- 50.Yakushiji T, Oyama Y, Akaike N. Comparative study on barbiturates using isolated single neurons: GABA-mimetic action and augmentatory action on GABA response. Brain Res 488: 357–360, 1989. doi: 10.1016/0006-8993(89)90730-0. [DOI] [PubMed] [Google Scholar]
- 51.Yeadon M, Kitchen I. Opioids and respiration. Prog Neurobiol 33: 1–16, 1989. doi: 10.1016/0301-0082(89)90033-6. [DOI] [PubMed] [Google Scholar]
- 52.Younes M, Riddle W. A model for the relation between respiratory neural and mechanical outputs. I. Theory. J Appl Physiol 51: 963–978, 1981. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Yang HL, Song JJ, Chen M, Dong Y, Lai B, Yu YG, Ma L, Zheng P. DAMGO depresses inhibitory synaptic transmission via different downstream pathways of μ opioid receptors in ventral tegmental area and periaqueductal gray. Neuroscience 301: 144–154, 2015. doi: 10.1016/j.neuroscience.2015.05.077. [DOI] [PubMed] [Google Scholar]