Abstract
Acute amino acid (AA) infusion increases AA oxidation rates in normal late gestation fetal sheep. Because the fetal oxygen consumption rate does not change with increased AA oxidation, we hypothesized that AA infusion would suppress glucose oxidation pathways and that the additional carbon supply from AA would activate hepatic glucose production. To test this, late gestation fetal sheep were infused intravenously for 3 h with saline or exogenous AA (AA). Glucose tracer metabolic studies were performed and skeletal muscle and liver tissues samples were collected. AA infusion increased fetal arterial plasma branched chain AA, cortisol, and glucagon concentrations. Fetal glucose utilization rates were similar between basal and AA periods, yet the fraction of glucose oxidized and the glucose oxidation rate were decreased by 40% in the AA period. AA infusion increased expression of PDK4, an inhibitor of glucose oxidation, nearly twofold in muscle and liver. In liver, AA infusion tended to increase PCK1 gluconeogenic gene and PCK1 correlated with plasma cortisol concentrations. AA infusion also increased liver mRNA expression of the lactate transporter gene (MCT1), protein expression of GLUT2 and LDHA, and phosphorylation of AMPK, 4EBP1, and S6 proteins. In isolated fetal hepatocytes, AA supplementation increased glucose production and PCK1, LDHA, and MCT1 gene expression. These results demonstrate that AA infusion into fetal sheep competitively suppresses glucose oxidation and potentiates hepatic glucose production. These metabolic patterns support flexibility in fetal metabolism in response to increased nutrient substrate supply while maintaining a relatively stable rate of oxidative metabolism.
Keywords: fetus, glucose, liver, amino acids, muscle
fetal oxidative metabolism is fueled by glucose, lactate, and amino acids (AA). Glucose and lactate are major carbohydrate fuels for the sheep fetus and their oxidation accounts for nearly 80% of fetal oxygen consumption (18). The remaining 20% is fueled by the oxidation of AAs, as the fetal uptake rate of AAs far exceeds the requirement for fetal tissue accretion rates (15, 24, 29) and previous studies have demonstrated AA oxidation in fetal sheep (3, 16, 37). The rate of fetal oxidative metabolism is relatively constant regardless of acute or chronic increases or decreases in substrate supply (12, 17, 30, 31). However, the oxidation rate of an individual substrate such as glucose or lactate increases when its concentration increases in the fetal blood (17, 18). This suggests that carbon substrates compete for mitochondrial oxidation, either on a simple supply or plasma concentration basis or by mechanisms that establish a preference for one substrate by upregulation of its oxidative pathway or downregulation of oxidative pathways of competing substrates.
Previous studies have investigated the effects of exogenously supplied AAs on fetal AA metabolism and protein synthesis rates in normally grown late gestation fetal sheep. These studies demonstrated that acute AA supplementation for 3 h, with somatostatin, to prevent a rise in insulin concentrations, produced a twofold increase in total AA delivered to the fetus from the placenta and experimental infusion combined. AA supplementation also increased net fetal leucine oxidation rates yet had no effect on fetal glucose uptake rates from the placenta or fetal oxygen utilization (7, 9). In skeletal muscle, the phosphorylation of mammalian target of rapamycin (mTOR) protein was increased supporting a direct AA effect in this tissue (9). In another study, a prolonged infusion (10 day) of AA alone in normal fetuses increased leucine oxidation rates, but in this case simultaneously decreased fetal glucose uptake rates from the placenta without an effect on fetal oxygen utilization (26). This combination of increased AA uptake and decreased glucose uptake suggests a reciprocal switch in substrate utilization for oxidation by the fetus. However, the effects of increased AA supply on glucose metabolism, specifically utilization and oxidation, during these AA infusions has not been directly measured.
Hepatic glucose production (HGP) is normally absent in the fetus until just before birth due to the transplacental supply of glucose from the mother. However, fetal sheep exposed to hypoglycemia or intrauterine growth restriction (IUGR) produced by placental insufficiency have an early activation of HGP and gluconeogenic gene expression, including phosphoenolpyruvate carboxykinase (PCK1, cytosolic form), glucose 6 phosphatase (G6PC), and the transcriptional coactivator PGC1A (13, 21, 25, 31–33). The mechanisms responsible for increased fetal HGP under these conditions are not fully understood. All AAs, except leucine and lysine, are considered glucogenic and can be used as carbon substrates for HGP in adults and animals postnatally (5, 23). Previously, we found that total net hepatic AA uptake was increased when hepatic glucose output was increased in response to hypoglycemia in fetal sheep (21), supporting the possibility that AA may contribute to HGP in the fetus under these conditions.
We hypothesized that an increased exogenous supply of AA to the fetus would suppress fetal glucose oxidation and activate HGP. Specifically, in skeletal muscle and liver, fetal glucose oxidation would be inhibited via increased pyruvate dehydrogenase (PDH) kinase 4 (PDK4), which inhibits PDH and thus the conversion of glycolytically derived pyruvate into acetyl-CoA for oxidation in the TCA cycle. Furthermore, genes supporting glucose uptake and utilization would be decreased and genes supporting AA catabolism would be increased. In the liver, we also hypothesized that increased AA supply would activate glucose production by increased expression of gluconeogenic genes supporting the flux of AA carbons into the gluconeogenic pathway. To test this, normal late gestation fetal sheep were infused intravenously for 3 h with saline or exogenous AA to double AA concentrations. Glucose metabolism studies were performed at the end of this 3-h period. Skeletal muscle and liver tissue samples also were taken and analyzed to determine the effect of the AA infusion on glucose and AA metabolic pathways. To further test the role of AA on activation of HGP, we performed studies in isolated primary fetal hepatocytes. Our results indicate that infusion of AAs into the late gestation fetal sheep suppresses fetal glucose oxidation rates and increases expression of PDK4 in fetal muscle and liver. AAs also potentiate gluconeogenic pathways in liver tissue and in isolated hepatocytes.
MATERIALS AND METHODS
Acute AA infusion in fetal sheep.
Experiments were conducted in pregnant Columbia-Rambouillet ewes carrying singletons or twins. At ~120–125 days gestational age, a surgery was performed to place fetal catheters. Infusion catheters were placed into the fetal femoral veins via hindlimb pedal veins. Sampling catheters were placed into the fetal abdominal aorta via a hindlimb pedal artery and in the umbilical vein. All catheters were tunneled subcutaneously through a flank incision on the ewe and kept within a plastic pouch attached to the ewe’s skin. The catheters were flushed daily with heparinized 0.9% sodium chloride. The ewe and fetus were allowed to recover for at least 5 days before the study. Ewes were kept in individual carts and given an ad libitum diet of alfalfa pellets, water, and mineral supplements. All animal procedures were in compliance with guidelines of the United States Department of Agriculture, the National Institutes of Health, and the American Association for the Accreditation of Laboratory Animal Care. The animal care and use protocols were approved by the University of Colorado Denver Institutional Animal Care and Use Committee.
Fetuses in the AA infusion group (n = 11; 8 singleton and 3 twin fetuses) first received a bolus of somatostatin (0.6 mg) followed by constant infusion (12 μg/min) to block insulin secretion in response to exogenously supplied AA (7, 9). After 20 min, an exogenous infusion of a mixed AA solution (TrophAmine) was given as a bolus (3 ml), followed by constant infusion (7.0 ml/h) to increase fetal AA concentrations by twofold (7, 9). After 2 h, a set of four steady-state basal blood samples were drawn from the fetal artery at 10- to 15-min intervals. Fetal blood was replaced isovolumetrically with heparinized maternal blood during the draw period (7, 9, 31). Another group of fetuses received a saline only infusion (n = 8; 6 singleton and 2 twin fetuses) and were studied similarly as the AA infusion group. In all twin pregnancies, both fetuses received treatments. The fetus in the right uterine horn received the AA infusion, while the fetus in the left horn received saline. In our previous work (8, 31), we have not detected any differences between singleton and twin fetuses for the related outcomes measured in this study, but we recognize that minor differences between singletons and twins may not be entirely appreciated in this smaller study. One twin fetus that received saline was excluded as it had a fetal weight of 1.2 kg, high arterial plasma lactate concentrations (5.4 mM), and low arterial oxygen concentrations (0.9 mM oxygen content) indicating fetal growth restriction.
Glucose tracer metabolic studies.
In a subset of four fetuses (1 singleton and 3 from twin pregnancies), fetal metabolic studies with a glucose tracer were performed under basal and AA infusion periods. Tracer infusions were initiated with a bolus of ethanol (300 mg) and d-[U-13C]glucose, 90 mg) to measure umbilical blood flow and glucose metabolism, respectively, followed by a constant infusion of ethanol (12.75 mg/min) and [13C]glucose (0.83 1.5 mg/min) (4, 11, 28). After 90 min of tracer equilibration, four steady-state basal blood samples were drawn simultaneously from the umbilical vein and fetal artery at 10- to 15-min intervals. The AA period was initiated as described above, and after 2 h, another set of four steady-state basal blood samples was drawn simultaneously from the umbilical vein and fetal artery at 10- to 15-min intervals.
Blood sample measurements.
Blood samples were analyzed for hematocrit, pH, Po2, and Pco2, oxygen saturation, and oxygen content using an ABL 825 Flex model blood gas analyzer (Radiometer America) and temperature was corrected to 39.1°C using manufacturer’s settings (9, 31). Plasma samples were analyzed for glucose and lactate using Yellow Springs Instrument 2700 (9, 31). AA concentrations were measured by HPLC as previously described (9). Branched chain amino acid (BCAA) concentrations represent the sum of Leu, Ile, and Val concentrations. Measurements were performed in all umbilical venous and arterial samples (4 per period) and mean values were calculated per period. Fetal plasma enrichment (MPE) of m + 6 glucose was measured by gas chromatography mass spectrometry (GC/MS) as described (21). Isotopic enrichments of 13CO2/12CO2 were measured using isotope ratio mass spectrometry (6). Plasma arterial insulin, cortisol, and glucagon concentrations were measured as previously described (9, 31).
Umbilical plasma and blood flow was determined by steady-state diffusion (ethanol) (4, 27, 36). Fetal (net) oxygen, lactate, and glucose uptake rates and fetal glucose utilization rate were calculated as described (19–21, 25). Fetal glucose production rate was calculated as the difference between glucose utilization rate and net glucose uptake rate (12, 25). Fetal glucose oxidation fractions and rates were calculated using 13CO2 enrichments and previously described calculations (18, 31). All results were normalized to fetal weight determined at necropsy.
Skeletal muscle and liver tissue samples.
After blood sampling was completed, under saline or AA study conditions, the ewe and fetus(es) were euthanized and muscle and liver tissue samples were collected for molecular analysis.
Gene expression.
RNA was extracted from muscle and liver, reverse transcribed, and used in real-time PCR (qPCR) as previously published (31, 32). To summarize, RNA was extracted from muscle and liver tissue with RNeasy Kits (Qiagen) or from hepatocytes with E.Z.N.A kits (Omega Biotek). RNA purity and concentration was measured with NanoDrop (Thermo Scientific). Reverse transcription was performed with 2 μg of total RNA using random primers with GoScript (Promega) following manufacturer’s guidelines. The cDNA was diluted 1:5 and used in 10-μl reaction with primers and 1× SYBR green master mix (Roche) for qPCR using the LightCycler 480 (Roche) and analyzed with LightCycler 480 software using the absolute quantification/2nd derivative maximum analysis method with relative standard curves produced from serial fourfold dilutions of a pooled liver cDNA sample (31, 32). Assays for PFK1, PK, PKLR, PC, PDK4, LDHA, LDHB, PCK1, G6PC, and PGC1A were used as previously reported (8, 32, 33). Primers were developed for real-time PCR assays for the following genes: BCAT2, BCKDK, ASNS, MCT1, GLUD1, GPT, GOT1, and GOT2 (Table 1). All primers are designed to span introns and avoid alternative spliced variants. Results were normalized to 18S rRNA expression for liver and muscle tissue and S15 for primary hepatocytes (31, 32). Each of these reference genes have similarly equal expression among all samples, as we have previously reported (8, 31–33).
Table 1.
Real-time PCR primer sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| BCAT2 | TGTCCTCCGTTTCCACAAGG | AGCTTTACACCGGGAGCATC |
| BCKDK | AAAGTGGGTGGACTTTGCCA | GCATCGGGATGAAGGGGAAA |
| ASNS | ACCCTCTCCAGACATTTGCG | TTCATGGTGTTCGCTCCCAA |
| MCT1 | GTGGCTTGATTGCTGCTTCC | GCCAATCATGGTCAAAGCCG |
| GLUD1 | AGCGCTCTGCCAGGCAAATCAT | GCGGCCGTTCTCAGGTCCAG |
| GPT | ACTGGAGCTGGAGCAGGAG | CAGGACCTGGGAGGAGTGAG |
| GOT1 | CATCTTTGTCCTCCACGCCT | GCCGAGTCAAAGAAGGGGAA |
| GOT2 | CACAACTGGCAGCACATCAC | GGAGAACTCCTTGGTCAGCC |
Protein expression analysis.
Whole cell protein lysates were prepared from liver tissue and Western immunoblotting was performed with antibodies against actin, PEPCK, and phosphorylated and total forms of AKT, AMPK, 4EBP1, and S6 as described (31–33). Antibodies against GLUT2 (no. 7580; Santa Cruz Biotechnology), LDHA (47010; Abcam), and glucocorticoid receptor (GR; no. 12041; Cell Signaling) were also used. Specificity of antibodies was verified based identification of a single band of the expected molecular weight. For phosphorylated proteins, protein specificity is further validated in separate experiments by differential expression in samples treated with or without an activator, such as insulin. Results were quantified on each blot and expressed relative to the reference sample on each blot. Equality of loading was verified by similar actin expression.
Tissue glycogen.
Tissue glycogen concentrations were determined in liver and skeletal muscle samples as described (25).
Primary fetal hepatocytes.
Primary fetal sheep hepatocytes were isolated as previously described (10, 31). Briefly, a piece of the medial right lobe was flushed with Hanks’ balanced salt solution (HBSS) containing 30 U/ml heparin, followed by perfusion with HBSS supplemented with 0.5 mM EGTA and 0.5% BSA, and then 500 ml of HBSS supplemented with 3 mM CaCl2, 1.2 mM MgSO4, 0.5% BSA, 0.05% collagenase, and 0.001% DNaseI. Digested tissue was filtered, cells were spun, washed three times, and resuspended in DMEM similar to fetal circulation (fetal DMEM: 1.1 mM glucose, 2 mM lactate, 2 mM glutamine, 1 mM pyruvate, 1× nonessential AAs, 1× penicillin-streptomycin) supplemented with 0.1 nM insulin, 10 mM dexamethasone, and 10% FBS and plated (3 × 105 cells/cm2, 6-well plates, and 5 μg/cm2 collagen). After a 3- to 4-h attachment period, cells were washed twice and media were replaced with fetal DMEM plus 0.2% BSA for overnight incubation.
Hepatocyte glucose production assay.
Hepatocytes were washed twice and incubated in phenol red free and glucose free DMEM with 10 mM HEPES and 0.369% NaHCO3. Cells were studied basally or treated with 2 mM sodium pyruvate and 20 mM sodium lactate, TrophAmine (20% vol/vol), or 500 nM dexamethasone and 100 μM cAMP to stimulate hormone activation for 24 h in duplicate wells. Glucose in the media (glucose oxidase assay) and protein content of cells (BCA assay) per well were measured (31).
Hepatocyte gene expression.
Hepatocytes were washed twice and then incubated in fetal DMEM plus 0.2% BSA in the absence or presence of TrophAmine (20% vol/vol) for 24 h in duplicate wells. After treatment, media were aspirated, and cells were washed and lysed in buffer TRK (Omega) for RNA isolation. Real-time PCR was performed as described as above.
Statistical analysis.
Data were analyzed by t-test including adjustments for unequal variance when indicated by F-test ratio using SAS. For data obtained during the tracer study, results were analyzed by mixed model ANOVA with fixed effects of period (Basal, AA) and random effect of fetus to account for repeated measures between periods using SAS (PROC MIXED). Correlation analyses were performed using log-transformed data in SAS (PROC CORR). Glucose production rate during the basal and AA period was tested against a theoretical mean of zero by one-sample t-test. Statistical significance was declared at P < 0.05. Data are presented as means ± SE.
RESULTS
Fetal characteristics following AA infusion.
Fetal sheep (Table 2) were infused for 3 h with saline (CON) or TrophAmine (mixed AA solution, AA) to increase AA delivery as previously reported (9). Fetuses were of similar gestational age and weight (Table 2). Plasma BCAA concentrations were increased by nearly twofold in AA fetuses (Table 2). Fetal insulin concentrations were unchanged during AA infusion due to the concurrent somatostatin infusion. Plasma glucose concentrations were similar between groups, yet lactate concentrations increased by 80% in AA fetuses versus CON. Fetal arterial oxygen content was 35% lower in AA fetuses versus CON. Fetal cortisol concentrations increased by over twofold and glucagon concentrations increased 30% with AA infusion versus CON (Table 2).
Table 2.
Fetal characteristics following saline or amino acid infusion
| Saline | AA | P Value | |
|---|---|---|---|
| n | 8 | 11 | |
| Male/female/unkown, n | 2/5/1 | 7/4/0 | |
| Singletons/twins, n | 6/2 | 8/3 | |
| Age, day | 130.8 ± 0.8 | 132.4 ± 0.5 | 0.73 |
| Fetal weight, g | 3,133 ± 109 | 3,399 ± 153 | 0.21 |
| BCAA, µM | 643.7 ± 54.4 | 1,265.9 ± 48.7 | <0.001 |
| Insulin, ng/ml | 0.28 ± 0.06 | 0.25 ± 0.03 | 0.56 |
| Glucose, mM | 1.06 ± 0.11 | 1.12 ± 0.10 | 0.68 |
| Lactate, mM | 1.92 ± 0.10 | 3.49 ± 0.29 | <0.001 |
| Oxygen, mM | 3.11 ± 0.09 | 2.02 ± 0.19 | <0.001 |
| Cortisol, ng/ml | 13.4 ± 1.9 | 33.2 ± 8.4 | <0.05 |
| Glucagon, pg/ml | 31.5 ± 2.5 | 40.8 ± 2.6 | <0.05 |
| Muscle glycogen, mg/g | 35.2 ± 2.6 | 37.5 ± 2.7 | 0.56 |
| Liver glycogen, mg/g | 33.0 ± 3.4 | 28.8 ± 4.0 | 0.46 |
Data are means ± SE. AA, amino acid; BCAA, branched chain amino acids.
Effects of acute AA infusion on fetal glucose oxidation in vivo.
Metabolic studies were performed with a glucose tracer in a subset of AA fetuses to determine the effect of AA infusion on glucose metabolism (Table 3). In this group, plasma BCAA concentrations were increased nearly twofold in the AA period compared with basal period. Fetal arterial blood pH, hematocrit, hemoglobin, and oxygenation were significantly lower during the AA period (Table 3). Fetal plasma glucose concentrations decreased by <10% during the AA period (Table 3). Fetal lactate concentrations tended to increase during the AA period (P = 0.07, Table 3).
Table 3.
Fetal characteristics during basal and AA metabolic study periods
| Basal | AA | P Value | |
|---|---|---|---|
| Fetal artery | |||
| BCAA, µM | 636 ± 60 | 1,158 ± 61 | <0.001 |
| pH | 7.35 ± 0.01 | 7.33 ± 0.01 | <0.005 |
| Hematocrit, % | 33.9 ± 0.8 | 32.4 ± 0.9 | <0.05 |
| Hemoglobin, mM | 6.8 ± 0.2 | 6.5 ± 0.2 | <0.05 |
| %SO2 | 40.9 ± 4.3 | 28.4 ± 3.5 | <0.001 |
| Po2 | 20.7 ± 1.3 | 17.9 ± 0.8 | <0.01 |
| O2 content, mM | 2.8 ± 0.3 | 1.9 ± 0.3 | <0.001 |
| Glucose, mM | 1.08 ± 0.06 | 0.99 ± 0.07 | <0.005 |
| Lactate, mM | 2.8 ± 0.2 | 3.6 ± 0.5 | 0.07 |
| Umbilical blood flow, ml·min−1·kg−1 | 197 ± 23 | 176 ± 15 | 0.13 |
| Umbilical plasma flow, ml·min−1·kg−1 | 130 ± 17 | 119 ± 11 | 0.19 |
Data are means ± SE; n = 4 fetuses (1 singleton and 3 twin fetuses) each studied during basal and AA periods.
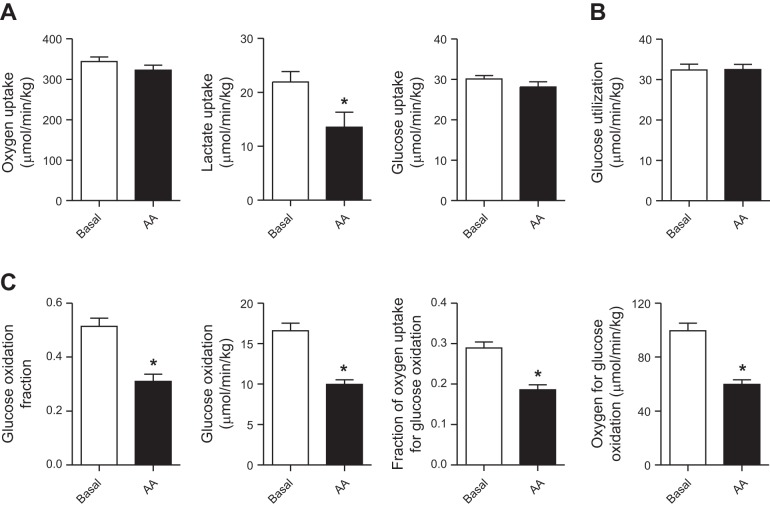
Umbilical blood and plasma flow rates, adjusted for fetal weight, were similar between periods (Table 3) and used to calculate rates of fetal nutrient uptake and glucose metabolism. The rate of net fetal oxygen uptake was similar between periods (Fig. 1A). The net uptake rate of lactate by the fetus from the placenta was decreased by 40% in AA compared with basal period (Fig. 1A). The rate of net fetal glucose uptake from the placenta was similar between periods (Fig. 1A), consistent with similar rates of glucose utilization measured with [U-13C]glucose tracer in each period (Fig. 1B). Despite similar glucose utilization rates between the basal and AA periods, the fraction of glucose oxidized and rate of glucose oxidation by the fetus was decreased by 40% in the AA period (Fig. 1C). The fractional rate of glucose oxidation and the fractional rate of oxygen used by the fetus for glucose oxidation also were reduced by 35–40% during the AA period (Fig. 1C).
Fig. 1.
Effect of acute amino acid infusion on fetal glucose and oxidative metabolism. A: net fetal oxygen, lactate, and glucose uptake rates were measured in in late gestation control fetal sheep during basal and amino acid (AA) supplementation periods. B: fetal glucose utilization rate was measured with glucose tracer. C: the fetal glucose oxidation fraction, glucose oxidation rate, fraction of oxygen uptake for glucose oxidation, and rate of oxygen used for glucose oxidation were measured. Data are means ± SE (n = 4 fetuses studied in each period). *P < 0.05 in AA vs. basal period.
Skeletal muscle response to acute AA infusion.
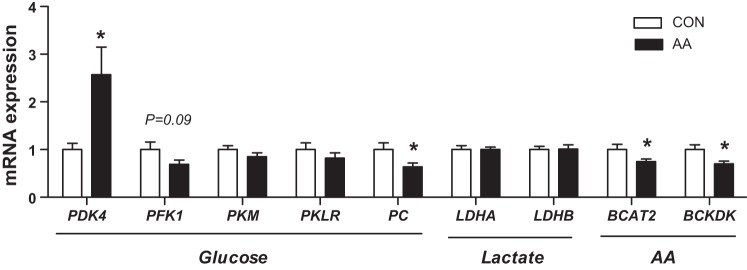
We next analyzed skeletal muscle tissue samples to determine the effect of acute AA infusion on pathways regulating glucose, lactate, and AA metabolism. Expression of PDK4 was increased over twofold versus controls with AA infusion, consistent with decreased glucose oxidation. Expression of pyruvate carboxylase (PC), but not phosphofructokinase (PFK1) or pyruvate kinase (PKM or PKLR), was decreased with AA infusion in fetal skeletal muscle versus CON. There was no change in the expression of the lactate dehydrogenase genes (LDHA or LDHB). Expression of the mitochondrial form of the branched chain aminotransferase BCAT2 was 30% lower in AA fetuses (Fig. 2A). Expression of BCKDK, the kinase that inhibits branched chain ketoacid dehydrogenase (BCKDH) activity, was decreased 30% in muscle in AA infused fetuses. Skeletal muscle glycogen content was not affected by AA infusion (Table 2).
Fig. 2.
Effect of acute amino acid supplementation on expression of metabolic genes in skeletal muscle. Expression of genes in glucose, lactate, and AA metabolism measured in fetal skeletal muscle collected under basal saline (CON) or AA supplementation conditions. Data are means ± SE for saline (white bars, n = 8) and AA (black bars, n = 11). *P < 0.05 in AA vs. saline group.
Liver response to acute AA infusion.
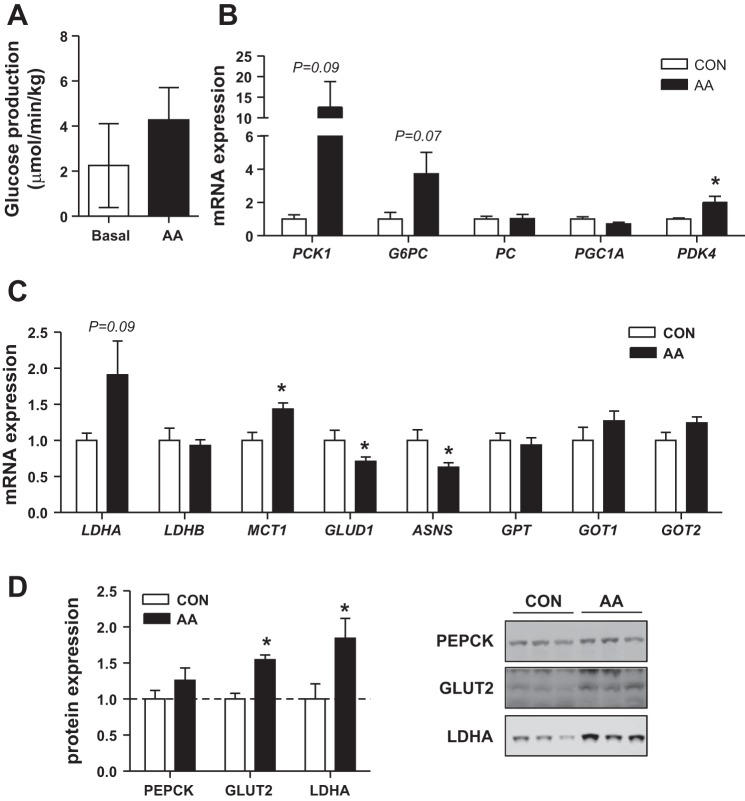
The difference between fetal glucose utilization rates and net fetal glucose uptake rates, presented in Fig. 1, represents the fetal glucose production rate (Fig. 3A). During the basal period, the rates of net fetal glucose uptake from the placenta (net umbilical glucose uptake) and fetal glucose utilization were similar and their difference was not greater than zero (P = 0.31). During the AA infusion period, however, the rate of fetal glucose utilization was significantly greater than the rate of umbilical glucose uptake (P = 0.05), indicating net fetal glucose production (Fig. 3A). We next measured the expression of genes in the gluconeogenic and glucose utilization pathways in liver samples from saline and AA infused fetus. AA infusion variably increased PCK1 (P = 0.09) and G6PC (P = 0.07) expression (Fig. 3B), with six out of nine AA fetuses having PCK1 expression greater than 2 SD above the mean in the CON group. There was no change in PC or PGC1A expression. PDK4 expression was increased twofold with AA infusion, consistent with expression in skeletal muscle and decreased fetal glucose oxidation rates. Hepatic glycogen content was not changed with AA infusion (Table 2).
Fig. 3.
Effect of acute amino acid supplementation on glucose production and related pathways in the liver. A: fetal rate of glucose production was measured during basal and AA periods. Expression of gluconeogenic genes (B) and genes coordinating glucose, lactate, and AA metabolism (C) measured in fetal liver collected under basal saline (CON, white bars, n = 6) or AA supplementation (black bars, n = 9) conditions. D: hepatic protein expression of PEPCK, GLUT2, and LDHA (n = 5 saline, n = 7 AA). Representative blots are shown. Data are means ± SE. *P < 0.05 in AA vs. saline group.
We next looked at the expression of genes involved in the hepatic metabolism of lactate and AAs, which may provide carbon sources for glucose synthesis. The lactate dehydrogenase genes (LDHA and LDHB) that interconvert lactate and pyruvate were not significantly increased, yet the monocarboxylic acid transporter, MCT1 was increased 20% with AA infusion (Fig. 3C). Expression of GLUD1, which deaminates glutamate to α-ketoglutarate, was decreased 40% with AA infusion (Fig. 3C). The expression of asparagine synthetase (ASNS) was decreased 30% with AA infusion (Fig. 3C). There was no change in expression of glutamic pyruvic transaminase (GPT) or glutamic oxaloacetic transaminase (GOT1 or GOT2) (Fig. 3C) with AA infusion. At the protein level, PEPCK, encoded by PCK1, was not significantly increased in livers of AA fetuses (Fig. 3D). Expression of GLUT2, a bidirectional glucose transporter, was increased by 50% in the liver of AA fetuses (Fig. 3D). LDHA protein expression was increased 80% in the AA-infused fetuses (Fig. 3D).
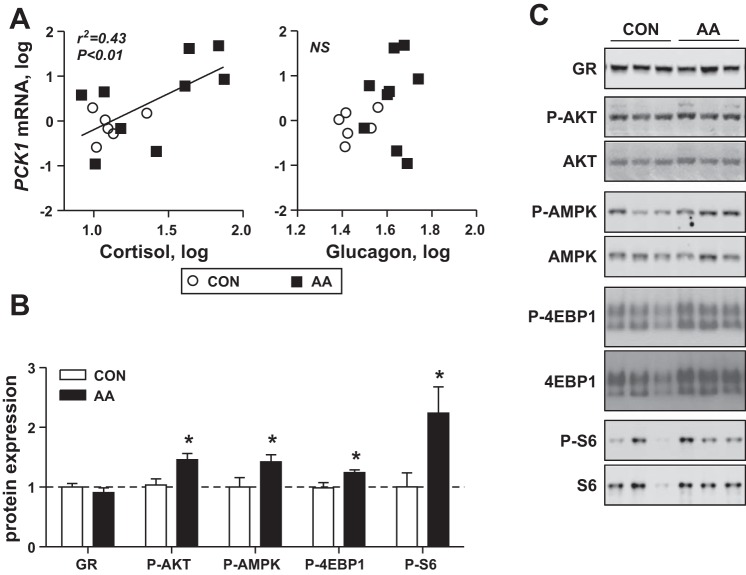
The expression of hepatic PCK1 mRNA correlated with fetal plasma cortisol but not glucagon concentrations (Fig. 4A). Expression of the GR was similar between CON and AA fetal livers (Fig. 4B). Despite similar insulin fetal plasma concentrations between CON and AA groups, hepatic AKT phosphorylation was increased 30% with AA infusion. Expression of phosphorylated AMPK also was increased 50% (Fig. 4B) in AA compared with CON fetal livers. Phosphorylation of the downstream mTOR targets 4EBP1 and S6 also were increased in AA compared with CON fetal livers (Fig. 4B).
Fig. 4.
Effect of AA supplementation on endocrine factors and signaling pathways in the fetal liver. A: correlation analysis of fetal plasma cortisol and glucagon concentrations with hepatic PCK1 expression in saline (CON) and AA fetuses. Significant correlation with cortisol is indicated. B: hepatic protein expression of GR and phosphorylation, of AKT, AMPK, 4EBP1, S6, and eIF2a, expressed as a ratio to total expression for (n = 5 saline, n = 7 AA). C: representative blots are shown (n = 3 per group). Lines indicate that samples shown are from different nonadjacent lanes on the same blot. Data are means ± SE. *P < 0.05 in AA vs. saline group.
Exogenous AA increase glucose production and PCK1 expression in isolated fetal hepatocytes.
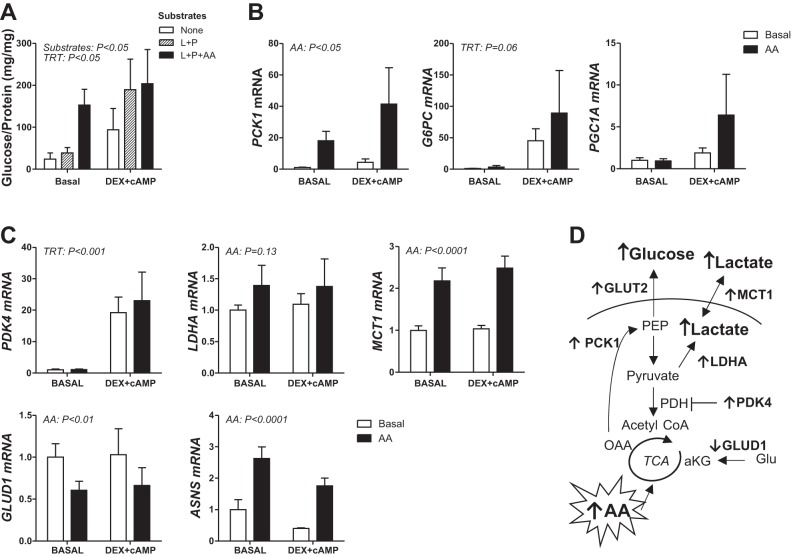
We next used primary hepatocytes that were isolated from normal late gestation fetal sheep to test the effect of supplementation of the incubation media with the same mixed AA solution used in vivo. Hepatocytes were also treated with the synthetic glucocorticoid agonist dexamethasone (DEX) and second messenger cAMP, both hormone-mediated activators of the gluconeogenic pathway. Basal glucose production increased fourfold in hepatocytes supplemented with AA in the presence of lactate and pyruvate compared with hepatocytes with no substrates or only lactate and pyruvate (Fig. 5A). Treatment with DEX + cAMP only increased glucose production in hepatocytes treated with lactate and pyruvate and had no further effect in combination with AA. AA supplementation also increased PCK1, LDHA, MCT1, and ASNS expression, decreased GLUD1 expression, and had no effect on G6PC, PGC1A, or PDK4 expression (Fig. 5B). Treatment with DEX + cAMP only increased G6PC and PDK4 expression.
Fig. 5.
Exogenous amino acids potentiate glucose production and supporting pathways in primary fetal hepatocytes. Primary hepatocytes were isolated from normal late gestation fetal livers. A: glucose production was measured after 24 h after treatment with substrates [lactate and pyruvate (L + P)] and 20% trophamine (AA) minus or plus hormone treatment (TRT) with 500 nM dexamethasone and 100 mM cAMP (DEX+cAMP) as indicated. Results are expressed as glucose produced per amount of protein per well (mg/mg). Expression of key gluconeogenic (B) and supporting metabolic (C) genes was measured in hepatocytes following AA treatment alone and in the presence of DEX + cAMP (TRT effect). Data are means ± SE (n = 3 for glucose production, n = 5 for gene expression studies). Significant effects from two-way ANOVA are indicated. D: summary of metabolic responses to AA treatment in hepatocytes and liver tissue.
DISCUSSION
We have demonstrated that AA infusion for 3 h to normal fetal sheep in late gestation suppressed glucose oxidation and potentiated HGP. In liver and muscle, decreased glucose oxidation is supported by increased PDK4 expression. In the liver, increased AA supply potentiated HGP as evidenced by a modestly increased rate of glucose production in vivo, increased hepatic PCK1 expression, and increased in vitro PCK1 expression and glucose production in primary fetal hepatocytes. Overall, our studies in the fetus and analysis in muscle, liver, and primary hepatocytes provide novel results demonstrating that increased AA supply to tissues within the fetus can downregulate competing glucose substrate oxidation and upregulate AA oxidation, as shown in our previous studies (9), to coordinately maintain fetal metabolic rate (oxygen consumption rate). Increased AA supply also can activate HGP and may produce more glucogenic substrate (see summary Fig. 5D). Importantly, our results show that the supply of one nutrient substrate does more than passively compete with other substrates for oxidation, demonstrating how mechanisms have been established to defend the rate of fetal oxygen consumption.
We had hypothesized that the fetus would suppress glucose utilization and oxidation by downregulating the molecular pathways that stimulate glucose oxidation in liver and skeletal muscle when AA supply is increased. By study design, fetal plasma AA concentrations were increased by approximately twofold, similar to the increase achieved in our previous study where total AA uptake (infusion rate + umbilical uptake rate) increased twofold (9). As a result, total carbon uptake (sum of glucose, lactate, and AA carbons) by the fetus was increased (9). Importantly, despite increased AA and total carbon supply, the fetus maintained a relatively constant rate of oxygen uptake (Fig. 1) (9) and oxidative metabolism by decreasing glucose oxidation (shown herein) and increasing AA oxidation, as shown previously (9, 26). Gene expression profiles in skeletal muscle and liver tissue support decreased glucose oxidation via increased mRNA expression of the inhibitor PDK4 and increased BCAA catabolism in muscle via decreased expression of the inhibitor BCKDK. Together these results demonstrate mechanisms that would upregulate AA oxidation while simultaneously downregulating oxidation of other substrates.
Despite reduced fetal glucose oxidation rates, fetal total body glucose utilization rates were unchanged in response to AA. Maintained glucose utilization with decreased glucose oxidation could result in an accumulation of glucose and glycolytic metabolites in fetal tissues. There was no increase in liver and muscle glycogen content. However, we did find evidence in skeletal muscle for the early development of mechanisms to limit glucose utilization and oxidation, demonstrated by decreased expression of the rate-limiting glycolytic gene PFK1 and PC, which would decrease replenishment of oxaloacetate into the TCA cycle. We speculate that these changes would be sustained with a longer duration of AA infusion and would result in a decrease of glucose uptake, fetal glucose utilization, and oxidation rates as the fetus increases its utilization and oxidation of AA compared with glucose. Indeed, we have previously found that prolonged AA infusion for ~10 days decreased the rate of fetal glucose uptake; however, fetal glucose utilization and oxidation rates were not measured in that study (26). In addition, glucose-derived metabolites could be utilized in other pathways, including the pentose phosphate pathway.
Increased AA supply potentiated glucose production in the fetal liver. PCK1 is the rate-limiting enzyme for the gluconeogenic pathway and its expression is normally low in the fetal liver when glucose production is absent and activated just before birth. In our study, the activation of HGP and the increase in PCK1 expression in response to AA infusion in vivo was modest in comparison to other models with an early activation of fetal HGP, which include IUGR or hypoglycemia in the fetal sheep, which have over twofold increase in glucose production rate and 4- to 30-fold increase in PCK1 expression, in addition to reduced uptake rates of glucose from the placenta (21, 31, 33). The limited activation of HGP in comparison to models of chronic fetal hypoglycemia may in part be due to the presence of a normal fetal uptake rate of glucose from the placenta and absence of hypoglycemia. Alternatively, the 3-h time period for AA supplementation to the fetus may not have been long enough to activate regulatory signals and transcription of the gluconeogenic and other metabolic genes. In isolated fetal hepatocytes, AA infusion for 24 h robustly increased glucose production and PCK1 expression. We speculate that increased PCK1 may be sufficient for increased fetal glucose production in response to increased AA supply, as expression of G6PC, another rate-limiting enzyme in HGP, was not increased with AA treatment in vivo or in vitro. GLUT2 expression was also increased in liver of AA fetuses which allows for bidirectional transport of glucose. In contrast to these acute 3-h studies, we have found that a prolonged AA infusion into the fetus for ~10 days did not significantly increase PCK1 expression (26). Thus acute AA infusion results in activation of PCK1 and glucose production in vivo and in isolated hepatocytes, but additional studies with glucose tracers would be needed to determine whether activation of glucose production would be sustained with chronic 10-day AA supplementation.
PCK1 expression is activated in response to increased counter regulatory hormones and associated factors including the transcriptional coactivator PGC1α and the GR. Fetal plasma cortisol concentrations were increased in response to AA and correlated with PCK1, while glucagon concentrations also increased but did not correlate with PCK1. To further test the role of hormone activation of glucose production, we evaluated H3h.
AA.
Interestingly, neither PGC1A mRNA nor GR protein expression was increased in vivo in liver tissue with AA infusion or in isolated hepatocytes treated with AA or DEX + cAMP treatment, again supporting that PCK1 may be sufficient for the activation of glucose production. Previously, it has been shown that 24 h infusion of dexamethasone in fetal sheep was not sufficient to increase hepatic glucose output but did decrease hepatic glutamate release and hepatic uptake of glucogenic AA, suggesting a redirection of AA carbon flux (34). Additionally, fetal arterial oxygen content was decreased in AA fetuses and hypoxia has been linked to the activation of hepatic glucose release via glycogenolysis (1); hepatic glycogen content, however, was not decreased in our study. Thus it remains unclear from our study if the increase in PCK1 is caused by increased cortisol concentrations, increased AA supply, or decreased oxygen.
Increased carbon supply from AA metabolism or redirected AA carbon flux may drive increased HGP in the fetus, as AA, glucose, and oxidative metabolism in the liver are coordinately regulated (see Fig. 5D). Furthermore, the liver is a major site of AA metabolism, responsible for the disposal of ~90% of dietary AAs in the adult (5, 22). In our study, fetal lactate concentrations increased with AA infusion (9, 26). We speculate that this is due to increased hepatic lactate production as evidenced by increased hepatic tissue LDHA (mRNA and protein) and MCT1 expression and the absence of a change in LDHA in skeletal muscle. Furthermore, there was no increase in expression of LDHB or PC, which would convert lactate to oxaloacetate for use by PCK1. Thus, given that lactate is a key gluconeogenic carbon precursor, our data provide support for increased intrahepatic lactate production during AA supplementation and potentiation of in vitro glucose production in the presence of AA, lactate, and pyruvate.
To further investigate the effect of increased AA supply on liver and muscle AA utilization, we measured the expression of genes involved in AA catabolism. GLUD1 expression in liver tissue and isolated hepatocytes was decreased, supporting a potential decrease in deamination of glutamate to α-ketoglutarate. Expression of ASNS, which produces asparagine from aspartate (2), also was decreased with AA supplementation. Decreases in both ASNS and GLUD1 may function to spare aspartate and glutamate from oxidation and rather these AAs, which are the major nitrogen acceptors, may be shuttled into the urea cycle. In contrast, we speculate that increased ASNS expression in isolated hepatocytes is an AA stress response (2) resulting from the higher AA concentrations used. In terms of BCAA catabolism, we found a decrease in muscle BCAT2 expression in response to increased AA supply, suggesting a paradoxical decrease in the first transamination step in BCAA catabolism in muscle (Fig. 2); while in liver we found no change in BCAT2 expression (data not shown). Studies in fetal rats have reported higher mitochondrial BCAT (BCATm, encoded by BCAT2 gene) activity in the fetal liver compared with both the fetal muscle and the postnatal liver (35). Thus, in our fetal data herein, we speculate that in response to increased AA supply, the fetal liver has sufficient capacity for the first transamination step via BCATm and that the subsequent keto-acids produced are then shuttled to muscle for oxidation via the BCDH enzyme. This interorgan shuttle would allow for the transamination step to occur in the liver where the nitrogen molecules could enter the urea cycle. Furthermore, carbons from isoleucine and valine can be glucogenic if further catabolized in the liver. Additional studies are needed to test whether increased AA supply will increase flux of AA through other pathways including the urea cycle, ornithine cycle, or polyamine production.
We also sought to determine whether additional AA flux through the liver would activate hepatic signaling pathways related to protein synthesis. Previously, we found that AA increased the phosphorylation and activation of mTOR in muscle (9). Here, we found that in liver, AA infusion increased phosphorylation of 4EBP1 and S6, both downstream mTOR targets. AA infusion also increased phosphorylation and activation of AMPK. Thus acute AA infusion activates key nutrient-sensing and protein synthesis pathways in the liver. Similarly, increased phosphorylation of S6 and p70S6K proteins was observed in the fetal liver following chronic AA infusion (14).
Perspectives and Significance
In conclusion, we found that in response to acute fetal AA infusion in normal pregnancies, AA oxidation is increased and glucose oxidation is decreased. Fetal HGP also increases. Coordinated changes in fetal glucose metabolism demonstrate tissue specific responses to increased AA supply to maintain energy balance and oxidative metabolism. These results have broader implications for understanding how increased nutrient supply to the fetus affects fetal metabolism. First, the metabolic patterns observed in this study demonstrate flexibility in normal fetal metabolism to meet variable nutrient substrate supply while maintaining a relatively stable rate of oxidative metabolism, an apparently necessary constant in fetal life. Second, our results set the stage for further investigation into how the fetus adapts to increases or decreases in other substrates, such as glucose, to determine whether extra glucose conversely diminishes AA oxidation and protein accretion by coordinated changes in these metabolic pathways, not just by changes in substrate concentrations via competition. Finally, our results are particularly important as we consider strategies to normalize fetal growth when nutrient (glucose, AA, and lipid) delivery may be restricted or in excess, such as IUGR or infants born large for gestational age as a result of maternal obesity and/or diabetes. Increasing the delivery of one or more nutrients to the fetus, especially in the chronic condition, might have profound effects on metabolic pathways for AA and glucose utilization, oxidative balance, and lactate production. Further investigation into these complex fetal responses, especially in the context of pathological growth conditions in the fetus, is warranted.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants K01-DK-090199 and R03-DK-102972 (to S. R. Wesolowski, Principal Investigator). LDB was supported by National Institute of Child Health and Human Development Grants K12-HD-057022 Building Interdisciplinary Careers in Women's Health Scholar Award and R01-HD-079404-01A1. P. J. Rozance was supported NIDDK Grant R01-DK-088139. W. W. Hay, Jr. was supported by National Institutes of Health (NIH) Grants T3-2007186-32 (Principal Investigator and Project Director), K12-HD-068372 (Project Director), and NIH NCATS UL1TR001082, TL1TR001081, and KL2TR001080 (Co-Director), and a Grand Challenges Exploration Grant from the Bill and Melinda Gates Foundation (OPP1061082).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.R.W., P.J.R, W.W.H, and L.D.B designed experiments; S.R.W., J.R.K., and L.D.B performed experiments; S.R.W. analyzed data; S.R.W., P.J.R, W.W.H, and L.D.B interpreted results of experiments; S.R.W., P.J.R, W.W.H, and L.D.B edited and revised manuscript; and S.R.W approved final version of manuscript.
ACKNOWLEDGMENTS
We thank David Caprio, Meagan O’Meara, Karen Trembler, Gates Roe, Dan LoTurco, David Goldstrohm, and Alex Cheung for technical assistance.
REFERENCES
- 1.Apatu RS, Barnes RJ. Release of glucose from the liver of fetal and postnatal sheep by portal vein infusion of catecholamines or glucagon. J Physiol 436: 449–468, 1991. doi: 10.1113/jphysiol.1991.sp018560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balasubramanian MN, Butterworth EA, Kilberg MS. Asparagine synthetase: regulation by cell stress and involvement in tumor biology. Am J Physiol Endocrinol Metab 304: E789–E799, 2013. doi: 10.1152/ajpendo.00015.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battaglia FC. Amino acid oxidation and urea production rates in fetal life. Biol Neonate 67: 149–153, 1995. doi: 10.1159/000244156. [DOI] [PubMed] [Google Scholar]
- 4.Bonds DR, Anderson S, Meschia G. Transplacental diffusion of ethanol under steady state conditions. J Dev Physiol 2: 409–416, 1980. [PubMed] [Google Scholar]
- 5.Brosnan JT. Glutamate, at the interface between amino acid and carbohydrate metabolism. J Nutr 130, Suppl: 988S–990S, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Brown LD, Hay WW Jr. Effect of hyperinsulinemia on amino acid utilization and oxidation independent of glucose metabolism in the ovine fetus. Am J Physiol Endocrinol Metab 291: E1333–E1340, 2006. doi: 10.1152/ajpendo.00028.2006. [DOI] [PubMed] [Google Scholar]
- 7.Brown LD, Rozance PJ, Barry JS, Friedman JE, Hay WW Jr. Insulin is required for amino acid stimulation of dual pathways for translational control in skeletal muscle in the late-gestation ovine fetus. Am J Physiol Endocrinol Metab 296: E56–E63, 2009. doi: 10.1152/ajpendo.90310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown LD, Rozance PJ, Bruce JL, Friedman JE, Hay WW Jr, Wesolowski SR. Limited capacity for glucose oxidation in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 309: R920–R928, 2015. doi: 10.1152/ajpregu.00197.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW Jr. Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab 303: E352–E364, 2012. doi: 10.1152/ajpendo.00059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culpepper C, Wesolowski SR, Benjamin J, Bruce JL, Brown LD, Jonker SS, Wilkening RB, Hay WW Jr, Rozance PJ. Chronic anemic hypoxemia increases plasma glucagon and hepatic PCK1 mRNA in late-gestation fetal sheep. Am J Physiol Regul Integr Comp Physiol 311: R200–R208, 2016. doi: 10.1152/ajpregu.00037.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vrijer B, Regnault TR, Wilkening RB, Meschia G, Battaglia FC. Placental uptake and transport of ACP, a neutral nonmetabolizable amino acid, in an ovine model of fetal growth restriction. Am J Physiol Endocrinol Metab 287: E1114–E1124, 2004. doi: 10.1152/ajpendo.00259.2004. [DOI] [PubMed] [Google Scholar]
- 12.DiGiacomo JE, Hay WW Jr. Fetal glucose metabolism and oxygen consumption during sustained hypoglycemia. Metabolism 39: 193–202, 1990. doi: 10.1016/0026-0495(90)90075-N. [DOI] [PubMed] [Google Scholar]
- 13.DiGiacomo JE, Hay WW Jr. Regulation of placental glucose transfer and consumption by fetal glucose production. Pediatr Res 25: 429–434, 1989. doi: 10.1203/00006450-198905000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Gadhia MM, Maliszewski AM, O’Meara MC, Thorn SR, Lavezzi JR, Limesand SW, Hay WW Jr, Brown LD, Rozance PJ. Increased amino acid supply potentiates glucose-stimulated insulin secretion but does not increase β-cell mass in fetal sheep. Am J Physiol Endocrinol Metab 304: E352–E362, 2013. doi: 10.1152/ajpendo.00377.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gresham EL, James EJ, Raye JR, Battaglia FC, Makowski EL, Meschia G. Production and excretion of urea by the fetal lamb. Pediatrics 50: 372–379, 1972. [PubMed] [Google Scholar]
- 16.Guyton TS, De Wilt H, Fennessey PV, Meschia G, Wilkening RB, Battaglia FC. Alanine umbilical uptake, disposal rate, and decarboxylation rate in the fetal lamb. Am J Physiol Endocrinol Metab 265: E497–E503, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Hay WW Jr, DiGiacomo JE, Meznarich HK, Hirst K, Zerbe G. Effects of glucose and insulin on fetal glucose oxidation and oxygen consumption. Am J Physiol Endocrinol Metab 256: E704–E713, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Hay WW Jr, Myers SA, Sparks JW, Wilkening RB, Meschia G, Battaglia FC. Glucose and lactate oxidation rates in the fetal lamb. Proc Soc Exp Biol Med 173: 553–563, 1983. doi: 10.3181/00379727-173-41686. [DOI] [PubMed] [Google Scholar]
- 19.Hay WW Jr, Sparks JW, Battaglia FC, Meschia G. Maternal-fetal glucose exchange: necessity of a three-pool model. Am J Physiol Endocrinol Metab 246: E528–E534, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Hay WW Jr, Sparks JW, Quissell BJ, Battaglia FC, Meschia G. Simultaneous measurements of umbilical uptake, fetal utilization rate, and fetal turnover rate of glucose. Am J Physiol Endocrinol Metab 240: E662–E668, 1981. [DOI] [PubMed] [Google Scholar]
- 21.Houin SS, Rozance PJ, Brown LD, Hay WW Jr, Wilkening RB, Thorn SR. Coordinated changes in hepatic amino acid metabolism and endocrine signals support hepatic glucose production during fetal hypoglycemia. Am J Physiol Endocrinol Metab 308: E306–E314, 2015. doi: 10.1152/ajpendo.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jungas RL, Halperin ML, Brosnan JT. Quantitative analysis of amino acid oxidation and related gluconeogenesis in humans. Physiol Rev 72: 419–448, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Krebs M, Brehm A, Krssak M, Anderwald C, Bernroider E, Nowotny P, Roth E, Chandramouli V, Landau BR, Waldhäusl W, Roden M. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia 46: 917–925, 2003. doi: 10.1007/s00125-003-1129-1. [DOI] [PubMed] [Google Scholar]
- 24.Lemons JA, Adcock EW III, Jones MD Jr, Naughton MA, Meschia G, Battaglia FC. Umbilical uptake of amino acids in the unstressed fetal lamb. J Clin Invest 58: 1428–1434, 1976. doi: 10.1172/JCI108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limesand SW, Rozance PJ, Smith D, Hay WW Jr. Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 293: E1716–E1725, 2007. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- 26.Maliszewski AM, Gadhia MM, O’Meara MC, Thorn SR, Rozance PJ, Brown LD. Prolonged infusion of amino acids increases leucine oxidation in fetal sheep. Am J Physiol Endocrinol Metab 302: E1483–E1492, 2012. doi: 10.1152/ajpendo.00026.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meschia G, Cotter JR, Breathnach CS, Barron DH. Simultaneous measurement of uterine and umbilical blood flows and oxygen uptake. Q J Exp Physiol 52: 1–8, 1966. doi: 10.1113/expphysiol.1967.sp001877. [DOI] [Google Scholar]
- 28.Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Development and mechanisms of fetal hypoxia in severe fetal growth restriction. Placenta 28: 714–723, 2007. doi: 10.1016/j.placenta.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Umbilical uptakes and transplacental concentration ratios of amino acids in severe fetal growth restriction. Pediatr Res 73: 602–611, 2013. doi: 10.1038/pr.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozance PJ, Crispo MM, Barry JS, O’Meara MC, Frost MS, Hansen KC, Hay WW Jr, Brown LD. Prolonged maternal amino acid infusion in late-gestation pregnant sheep increases fetal amino acid oxidation. Am J Physiol Endocrinol Metab 297: E638–E646, 2009. doi: 10.1152/ajpendo.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorn SR, Brown LD, Rozance PJ, Hay WW Jr, Friedman JE. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes 62: 65–73, 2013. doi: 10.2337/db11-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorn SR, Regnault TR, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 150: 3021–3030, 2009. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorn SR, Sekar SM, Lavezzi JR, O’Meara MC, Brown LD, Hay WW Jr, Rozance PJ. A physiological increase in insulin suppresses gluconeogenic gene activation in fetal sheep with sustained hypoglycemia. Am J Physiol Regul Integr Comp Physiol 303: R861–R869, 2012. doi: 10.1152/ajpregu.00331.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timmerman M, Teng C, Wilkening RB, Fennessey P, Battaglia FC, Meschia G. Effect of dexamethasone on fetal hepatic glutamine-glutamate exchange. Am J Physiol Endocrinol Metab 278: E839–E845, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Torres N, Vargas C, Hernández-Pando R, Orozco H, Hutson SM, Tovar AR. Ontogeny and subcellular localization of rat liver mitochondrial branched chain amino-acid aminotransferase. Eur J Biochem 268: 6132–6139, 2001. doi: 10.1046/j.0014-2956.2001.02563.x. [DOI] [PubMed] [Google Scholar]
- 36.Van Veen LC, Hay WW Jr, Battaglia FC, Meschia G. Fetal CO2 kinetics. J Dev Physiol 6: 359–365, 1984. [PubMed] [Google Scholar]
- 37.van Veen LC, Teng C, Hay WW Jr, Meschia G, Battaglia FC. Leucine disposal and oxidation rates in the fetal lamb. Metabolism 36: 48–53, 1987. doi: 10.1016/0026-0495(87)90062-X. [DOI] [PubMed] [Google Scholar]