β-Adrenergic receptor (β-AR) stimulation can decrease the proliferation of embryonic ventricular cells in vitro and reduce the graft size after intracardiac cell transplantation. In contrast, β1-AR antagonists can abrogate the antiproliferative effects mediated by β-AR stimulation and increase graft size. These results highlight potential interactions between adrenergic drugs and cell transplantation.
Keywords: β-adrenergic receptor drugs, embryonic ventricular cells, proliferation and differentiation, intracardiac cell transplantation, donor cells and drug interactions
Abstract
β-Adrenergic receptors (β-ARs) and catecholamines are present in rodents as early as embryonic day (E)10.5. However, it is not known whether β-AR signaling plays any role in the proliferation and differentiation of ventricular cells in the embryonic heart. Here, we characterized expression profiles of β-AR subtypes and established dose-response curves for the nonselective β-AR agonist isoproterenol (ISO) in the developing mouse ventricular cells. Furthermore, we investigated the effects of ISO on cell cycle activity and differentiation of cultured E11.5 ventricular cells. ISO treatment significantly reduced tritiated thymidine incorporation and cell proliferation rates in both cardiac progenitor cell and cardiomyocyte populations. The ISO-mediated effects on DNA synthesis could be abolished by cotreatment of E11.5 cultures with either metoprolol (a β1-AR antagonist) or ICI-118,551 (a β2-AR antagonist). In contrast, ISO-mediated effects on cell proliferation could be abolished only by metoprolol. Furthermore, ISO treatment significantly increased the percentage of differentiated cardiomyocytes compared with that in control cultures. Additional experiments revealed that β-AR stimulation leads to downregulation of Erk and Akt phosphorylation followed by significant decreases in cyclin D1 and cyclin-dependent kinase 4 levels in E11.5 ventricular cells. Consistent with in vitro results, we found that chronic stimulation of recipient mice with ISO after intracardiac cell transplantation significantly decreased graft size, whereas metoprolol protected grafts from the inhibitory effects of systemic catecholamines. Collectively, these results underscore the effects of β-AR signaling in cardiac development as well as graft expansion after cell transplantation.
NEW & NOTEWORTHY β-Adrenergic receptor (β-AR) stimulation can decrease the proliferation of embryonic ventricular cells in vitro and reduce the graft size after intracardiac cell transplantation. In contrast, β1-AR antagonists can abrogate the antiproliferative effects mediated by β-AR stimulation and increase graft size. These results highlight potential interactions between adrenergic drugs and cell transplantation.
cardiac function is regulated by sympathetic and parasympathetic branches of the autonomic nervous system through adrenergic and cholinergic receptors. Recent evidence suggests that β-adrenergic receptor (β-AR) responses develop in the mouse heart at embryonic day (E)9.5 and cholinergic receptor responses develop 1 day later, by E10.5 (2). Although cardiac sympathetic neurons were first detected between E12.5 and E14.5 in rat or mouse hearts (2, 8), there is no information about the myocardial levels of naturally occurring catecholamines such as norepinephrine (NE) and epinephrine or the major β-AR subtypes (β1 and β2) during ontogeny. Analysis of whole embryo lysates from various developmental stages revealed the appearance of NE first at E10.5, whereas epinephrine was consistently detected in fetuses older than E13.5 (42). Despite these observations, there is no consensus in the literature regarding the onset of β-adrenergic regulation of the embryonic mouse heart rate (2, 3).
In addition to heart rate regulation, catecholamines also play a critical role in fetal growth and development. Targeted disruption of tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine biosynthesis, was shown to cause early embryonic lethality between E11.5 and E15.5, possibly due to cardiac structural defects (49). Another group of researchers (14) reported marked slowing of heart rate and perinatal lethality in an independent line of TH knockout mice. Similarly, mice lacking another enzyme involved in catecholamine biosynthesis [dopamine β-hydroxylase (DBH)] exhibited an embryonic lethality phenotype even before E11 (42). Furthermore, it is also known that most β1-AR knockout mice die prenatally between E10.5 and E18.5 (35). Although these studies have suggested a critical role for the adrenergic system in early stages of heart development, it is not clear whether catecholamines can directly influence proliferation and differentiation of cardiac progenitors and cardiomyocytes in the embryonic heart.
In previous studies, we showed that ~40% of ventricular cells at the E11.5 stage remain undifferentiated in two mouse strains (23, 48). Using novel lineage tracking reporter systems, it was confirmed that Nkx2.5+ undifferentiated cells represent a cardiac progenitor cell (CPC) population because they can differentiate into cardiomyocytes (CMs) in real time (23, 47, 48). Subsequent studies revealed that E11.5 ventricular cells can form larger grafts compared with E14.5 cells after direct intracardiac injection into recipient hearts (47). Given the midgestational and perinatal mortalities observed in TH, DBH, and β1-AR knockout mice, it is important to understand the role of β-AR signaling in the control of embryonic ventricular cell proliferation and differentiation. Moreover, there is scant information regarding the protective or detrimental effects of β-AR agonists or blockers on donor cell transplantation.
In the present study, we sought to characterize the largely unexplored role of β-AR signaling in cardiac cell proliferation and differentiation in the developing heart and the impact of various adrenergic drugs on cell-based interventions. To this end, we investigated the expression levels of β1- and β2-ARs as well as the associated second messenger responses in cardiac ventricular cell preparations from various developmental stages. Furthermore, we determined the effects of isoproterenol (ISO; a nonselective β-AR agonist) in the presence or absence of metoprolol (Meto; a β1-AR antagonist) and ICI-118,551 (ICI; a β2-AR antagonist) on cell cycle activity and differentiation of CPCs and CMs derived from E11.5 mouse ventricles. In addition, we evaluated the effects of these drugs on the graft size after intracardiac injection of E11.5 ventricular cells.
MATERIALS AND METHODS
Experimental animals.
All animal procedures were performed in accordance with Canadian Council on Animal Care guidelines and were approved by the Dalhousie University Committee on Laboratory Animal Care (protocols 14-013 and 16-048). CD1 and C57BL/6 (BL6) mice were obtained from Charles River Laboratories (Montreal, QC, Canada). Generation of mice with Cre recombinase inserted into the Nkx2.5 allele has been previously described (40). The R26R-lacZ reporter strain was obtained from Jackson Laboratories (Bar Harbor, ME). All knockin lines were maintained in a BL6 background, and genotyping was performed as described in our previous studies (47). Female mice were mated with male mice, and the E0.5 stage was designated as the noontime of the day when the copulation plug was found. Unless otherwise stated, CD1 mice were used for all experimental procedures.
Total RNA extractions and quantitative PCR analysis.
Total RNA was isolated from whole ventricles of embryonic (E11.5, E14.5, and E16.5), neonatal (1 day old), and adult (3 mo old) mice as well as from E11.5 ventricular cell cultures using TRIzol reagent (Invitrogen). Extracted RNA was then reverse transcribed into cDNA using a Superscript II reverse transcriptase kit (Invitrogen). Quantitative PCR (qPCR) was performed on all samples using EVOlution EvaGreen (Montreal Biotech, Quebec City, QC, Canada) according to the manufacturer’s instructions. Primer sequences are shown in Table 1. All qPCRs were performed for 40 cycles: 15 s at 95°C and 60 s at 60°C using an ECO thermocycler (Illumina, San Diego, CA) except for GAPDH, for which 62°C was used as the annealing temperature. qPCR efficiencies for all primer pairs were determined to be >90%. Gene expression was normalized to a control reference gene (GAPDH) using the ΔΔCt method as previously described (9, 10).
Table 1.
Primer sequences used for quantitative PCR analysis
| Target Gene | Primer Name | Sequence | Band Size |
|---|---|---|---|
| β1-AR | β1-AR-F | 5′-CGAGCTCTGGACTTCGGTAG-3′ | 119 |
| β1-AR-R | 5′-TCAGCAAACTCTGGTAGCGA-3′ | ||
| β2-AR | β2-AR-F | 5′-ATTTTGGCAACTTCTGGTGC-3′ | 97 |
| β2-AR-R | 5′-TAGCGATCCACTGCAATCAC-3′ | ||
| Cyclin D1 | Cyclin D1-F | 5′-CTCCTCTCCAAAATGCCAG-3′ | 112 |
| Cyclin D1-R | 5′-GGGTGGGTTGGAAATGAACT-3′ | ||
| CDK4 | CDK4-F | 5′-TGCCAGAGATGGAGGAGTCT-3′ | 109 |
| CDK4-R | 5′-TTGTGCAGGTAGGAGTGCTG-3′ | ||
| GAPDH | GAPDH-F | 5′-TCGTCCCGTAGACAAAATGG-3′ | 132 |
| GAPDH-R | 5′-TTGAGGTCAATGAAGGGGTC-3′ |
F, forward primer; R, reverse primer.
Preparation of dispersed ventricular cells.
Embryonic hearts from various stages of development were dissected using a stereomicroscope (MZ16F, Leica), and the atria and outflow tract were carefully removed from the ventricles. Tissue samples were digested in 0.2% collagenase type I (Worthington Biochemical, Lakewood, NJ), and cells were plated on fibronectin-coated chamber slides (Nalge Nunc, Rochester, NY), 35-mm dishes (Corning, New York, NY), or 384-well plates (Greiner Bio-One, Monroe, NC) in DMEM containing 10% FBS. Cells were maintained in a CO2 incubator at 37°C for primary cultures or freshly processed for FACS or second messenger studies.
FACS of embryonic ventricular cells.
For cell surface expression analysis of β1- and β2-ARs, dispersed E11.5 and E17.5 ventricular cells were obtained from 10−15 pooled ventricles for each experiment and passed through a 40-µm filter to ensure single cell preparations. Approximately 4–5 million unfixed cells were blocked with 4% BSA in PBS at room temperature for 45 min. For β-AR staining, cells were incubated for 1 h either with rabbit polyclonal β1-AR antibody conjugated to Cy5 (1:50, ABIN669355, Antibodies-Online, Atlanta, GA) or with rabbit polyclonal β2-AR antibody (1:50, ab36956, Abcam, Toronto, ON, Canada) in 4% BSA-PBS. For β2-AR staining, cells were further incubated for 1 h in secondary goat anti-rabbit antibodies conjugated to Alexa fluor 488 (1:200). Cells were subsequently washed in PBS and reconstituted in FACS buffer (0.1% BSA in PBS) and used for flow cytometry (FACSAria, BD Biosciences, Franklin Lakes, NJ). In some experiments, β1- and β2-AR-positive cells were sorted via FACS and cultured on chamber slides, fixed with methanol for 15 min at 4°C, and processed for immunofluorescence staining with antibodies for sarcomeric myosin (1:200, MF20, Developmental Studies Hybridoma Bank, Iowa City, IA) and nuclear staining with Hoechst dye as previously described (10, 23, 47).
For cell cycle analysis, propidum iodide (PI) staining was used as previously described (1). Initially, E11.5 ventricular cells were treated with β-AR agonists and antagonists for 18 h. Cells were subsequently trypsinized amd fixed by addition of ice-cold 70% ethanol (drop wise), and samples were incubated in PI solution (50 ng/ml PI and 100 U/ml ribonuclease A in PBS, both from Invitrogen) at room temperature for 30 min. Fluorescence intensities were determined using a Becton Dickinson FACSCalibur flow cytometer. The proportion of cells in various phases of the cell cycle was estimated using the ModFit program (ModFit LT, version 4, Verity Software House).
cAMP assay.
To determine the levels of cAMP in embryonic ventricular cells treated with or without various AR agonists and antagonists, competitive immunoassays were performed using a two-step protocol of the cAMP-Gs Dynamic 2 homogeneous time-resolved fluorescence (HTRF) assay kit (62AM4PEB, Cisbio) as previously described (9). The optimal cell density for cAMP assays was determined to be 4,000 cells/well. A standard curve was generated by plotting the change in fluoresence (ΔF) values from standards with known cAMP concentrations and covered an average range of 0.17−712 nM (final concentration of cAMP/well). Briefly, in step 1, a volume of 5 µl of cells in 10% FBS-DMEM was added to wells (4,000 cells/5 µl/well) of white 384-well low-volume plates (784075, Greiner Bio-One) followed by 5 µl of 10% DMEM containing the broad-spectrum phosphodiesterase inhibitor IBMX (500 µM, Sigma) and the drug (ISO, Meto, or ICI, all from Sigma) at the indicated concentrations. The plate was sealed and incubated at room temperature for 30 min. In step 2, 5 µl of the d2-cAMP analog and 5 µl of anti-cAMP-cryptate were added to each well, and the plate was sealed for 1 h at room temperature. The d2-cAMP fluorophore was excited at a wavelength of 337 nm, and emission was detected at 665 and 620 nm using a POLARstar Omega plate reader (BMG Labtech). Results were calculated using the 665-to-620-nm ratio and expressed as ΔF values using data reduction steps described in the manufacturer’s instructions. cAMP concentrations in samples were deduced from the standard curves and presented as molar concentrations.
Assessment of DNA synthesis, cell size, and apoptosis levels in E11.5 ventricular cells.
E11.5 ventricles were derived from crosses between Nkx2.5-Cre and Rosa-lacZ breeding pairs, and dispersed cells were plated in fibronectin-coated four-well chamber slides (Nalge Nunc) at a density of 1.2 × 105 cells/well. Cells were cultured in 10% FBS-DMEM in the presence or absence of various drugs (ISO, Meto, and ICI) at the indicated concentrations for 16 h. Subsequently, medium was aspirated and new medium supplemented with tritiated [3H]thymidine (GE Healthcare Life Sciences) was added to each well at a concentration of 1.0 μCi/1 ml medium for 6 h at 37°C. At the end of thymidine labeling, cells were fixed with methanol for 15 min at 4°C and processed for immunofluorescence staining and [3H]thymidine autoradiography as previously described (41, 47). Primary antibodies used to identify CPCs and CMs were β-galactosidase (β-Gal; 1:50, no. 55976, Cappel, ICN) and sarcomeric myosin (1:200, MF20, Developmental Studies Hybridoma Bank). Images were captured using a Leica DFC500 digital acquisition system. To quantitatively measure the impact of adrenergic drugs on cell size, surface area of CMs from each treatment group was determined using Color-Subtractive Computer-Assisted Image Analysis software as previously described (7, 23). TUNEL assays were performed for the detection of CM apoptosis using a TMR red in situ cell death detection kit (Roche Applied Science, Mannheim, Germany) as previously described (10).
CyQuant cell proliferation assay.
To examine the effects of β1- and β2-AR agonists and antagonists on the proliferation of E11.5 ventricular cells, CyQUANT assay (Invitrogen) was used according to the manufacturer’s instructions. Cells were seeded (1.6 × 104 cells/well) on fibronectin-coated (Sigma) 96-well plates (UV-Star microplate, no. 655809, Greiner Bio One), treated with or without ISO, Meto, and ICI for 18 h, and subsequently washed with PBS. Plates were frozen at −80°C to facilitate cell lysis. Later, plates were thawed, 200 µl of CyQUANT GR dye diluted in cell lysis buffer (Invitrogen) were added to each well, and plates were incubated in the dark at room temperature for 2 min. A plate reader was used to excite samples at 485 nm, and fluorescence readings were recorded at an emission of 530 nm using top optics. Cell numbers were determined by deducing the fluorescence readings from a standard curve generated with known numbers of cells using linear regression analysis and Prism software (version 5.01, GraphPad Software, San Diego, CA).
Protein extraction and Western blot analysis.
E11.5 ventricular cell cultures in 35-mm dishes (treated and untreated) were lysed using tumor lysis buffer [1% Nonidet P-40, 5 mM EDTA, 150 mM NaCl, 50 mM Tris·HCl (pH 8.0), 1.0 µl PMSF, and 1.0 µl aprotinin], left on ice for 15 min, and centrifuged at 13,300 revolutions/min for 15 min at 4°C to separate the soluble cytosolic and insoluble membrane fractions. Protein lysates (40 µg/lane) were separated on 12.5% SDS-polyacrylamide gels and transferred to a nitrocellulose membrane (GE Healthcare Life Sciences). Western blot analysis was performed using the following rabbit polyclonal antibodies (1:100 dilution) from Cell Signaling: p44/42 MAPK (no. 9102, Erk1/2), phospho-p44/42 MAPK (no. 9101, Erk1/2 Thr202/Tyr204), Akt (no. 4691), and phospho-Akt (no. 4060, Ser472). Subsequently, membranes were incubated with goat anti-rabbit antibodies conjugated to horseradish peroxidase (1:2,000, no. 172-1019, Bio-Rad), and signals were detected using an ECL Plus system (GE Healthcare Life Sciences) according to the manufacturer’s instructions. ECL films were scanned and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD) as described in our earlier study (6).
Cell transplantation, drug treatments, and graft analysis.
Adult C57BL/6 male mice (11–15 wk) were used as recipients, and E11.5 ventricular cells derived from crossing two knockin mouse models, Nkx2.5-Cre and Rosa-lacZ (NCRL), were used for cell transplantation. Recipient mice were anesthetized with 2.5% isoflurane, hearts were exposed via thoracotomy, and 3 × 105 NCRL E11.5 ventricular cells suspended in 5 µl of PBS were injected directly into the left ventricle of the heart as described in our previous studies (10, 47). Immediately after cell injection, miniosmotic pumps (model 2001, Alzet, Cupertino, CA) were implanted subcutaneously to administer ISO (0.025 g/ml) and Meto (0.0684 g/ml) as described in our earlier study (32). Each recipient heart was collected 3 days after cell transplantation, rinsed in PBS, and stored in a fixing solution (1% paraformaldehyde, 50 mM sodium cacodylate, and 114 mM sodium chloride, pH 7.4) while rocked at 4°C for 48 h. The entire heart was sectioned into 25-µm-thick tissue slices from the base to apex using a motorized advanced vibroslice (MA725, Campden Instruments, Lafayette, IN) in a PBS tissue bath. The tissue sections were subsequently rocked at 37°C overnight in X-Gal solution to visualize the blue chromogenic signal from the transplanted embryonic ventricular NCRL cells. Using this method of donor cell visualization, even a single donor cell can be tracked in vibroslice tissue sections (4, 47). All sections from each recipient heart were examined using a Leica MZ16 stereomicroscope, and sections positive for X-Gal staining were imaged and digitized. Graft size from each X-Gal-positive section was calculated as the area (in mm2) occupied by X-Gal staining using a previously described image analysis method (7) as reported in our previous studies (10, 47). Next, the graft volume (in mm3) for each section was calculated by multiplying the X-Gal positive area by 25 µm. The collective volume of all X-Gal-positive heart sections was then used to determine the total volume of the graft in each recipient heart as reported in our previous studies (10, 47).
Data analysis.
Data are presented as means ± SE. Multiple-group comparisons were analyzed by ANOVA and Tukey’s multiple-comparison post hoc test. A two-tailed, unpaired t-test was used to compare between two groups. Significance for all analyses was assigned at P < 0.05. Statistical analysis was performed using Prism version 5.01 (GraphPad). For each experiment, the number of experiments/replicates is represented in the corresponding figures.
RESULTS
β1- and β2-ARs are expressed at different levels during mouse ventricular development.
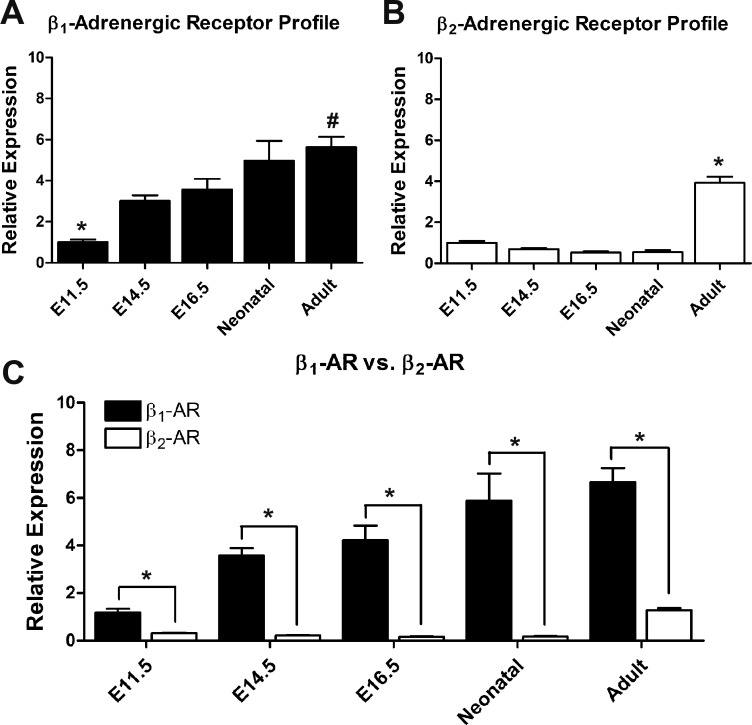
Using qPCR analysis, the relative mRNA abundance of β1- and β2-ARs was determined in ventricles harvested at various stages of development. Because GAPDH mRNA levels remain unchanged throughout ventricular development (10), β-AR expression data were normalized using GAPDH levels. Relative to E11.5 cardiac ventricles, β1-AR mRNA expression significantly increased by threefold at E14.5 and E16.5 and by fivefold at neonatal and adult stages (Fig. 1A). However, β1-AR gene expression levels did not differ significantly between E14.5, E16.5, and neonatal stages. In contrast, levels of β2-AR mRNA expression remained unchanged throughout embryonic development, whereas a fourfold increase in mRNA expression in the ventricles was evident in adult mice (Fig. 1B). Specific gene subsets are normally expressed in fetal ventricles during heart development and are repressed after birth (e.g., atrial natriuretic peptide, myosin heavy chain isoforms, and hyperpolarization-activated cyclic nucleotide-gated channels). However, these fetal genes are reinduced in the adult heart during disease processes as a compensatory response (17). Based on this notion, we examined whether there is any switch in the relative distribution of β-AR subtypes during ventricular development. Notably, β1-AR mRNA levels remained significantly higher compared with β2-AR mRNA levels at all stages of cardiac development (Fig. 1C). The fold difference in expression of β1- and β2-ARs ranged from 4-fold to 5-fold at E11.5 and adult stages to 17- to 34-fold at the remaining stages studied (Fig. 1C).
Fig. 1.
Quantification of mRNA levels of β1- and β2-adrenergic receptors (ARs) during ontogeny of cardiac ventricles. A and B: relative expression levels of β1- and β2-ARs in different developmental stages of cardiac ventricles by quantitative PCR analysis. The relative expression of β1- and β2-ARs was determined in relation to the embryonic day (E)11.5 stage. A: *P < 0.05 compared with all stages, #P < 0.05 adult vs. E11.5, E14.5 and E16.5; B: *P < 0.05 compared with all stages (one-way ANOVA, Tukey's multiple-comparisons test). C: comparison of β1- and β2-AR expression levels at each developmental stage. Expression levels were normalized to GAPDH. *P < 0.05, β1-AR vs. β2-AR by unpaired Student’s t-test. Each bar represents means ± SE, n = 3 independent RNA extractions/developmental stage, analyzed in duplicate for each extraction.
Both β1- and β2-ARs are present on the cell surface in embryonic ventricular cells.
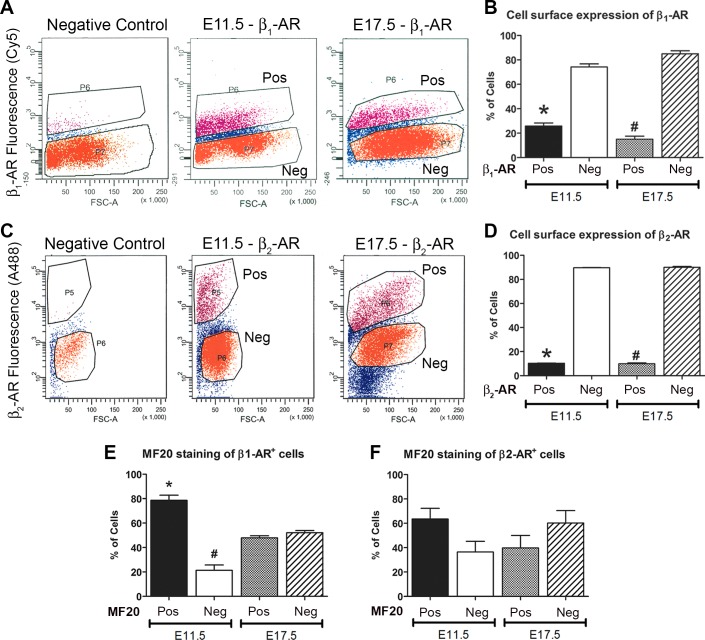
FACS was used to determine the percentage of cells positive for cell surface expression of β1- or β2-ARs in E11.5 and E17.5 embryonic ventricular cells. Unfixed and nonpermeabilized cells were immunostained using extracellular domain-specific β-AR antibodies and processed for FACS (Fig. 2, A–D). Quantification of the FACS results indicated that 25.8 ± 2.5% of E11.5 and 15.1 ± 2.5% of E17.5 ventricular cells express β1-AR on their cell surface, whereas the remaining cells are either β1-AR negative or have very little β1-AR on their cell surface (Fig. 2B). Multiple-group comparison using ANOVA revealed that there was no significant difference in β1-AR-positive cell numbers between E11.5 and E17.5 ventricular cells (Fig. 2B). Furthermore, it was found that 10.3 ± 1.7% of E11.5 and 9.9 ± 0.7% of E17.5 ventricular cells express β2-AR, whereas the remaining cells are either β2-AR negative or have very little β2-AR on their cell surface (Fig. 2D).
Fig. 2.
Characterization of cell surface expression of β1- and β2-ARs in embryonic ventricular cells. A and C: scatterplots of FACS-sorted E11.5 and E17.5 ventricular cells stained with extracellular domain-specific β1-AR (A) or β2-AR (C) antibodies. Negative control represents unstained E11.5 cells lacking the β1-AR antibody (A) or β2-AR antibody (C). B and D: relative distribution of β1-AR (B) or β2-AR (D) positive (Pos) and negative (Neg) cells in E11.5 and E17.5 ventricular cell preparations. B: *P < 0.005, E11.5 β1-AR Pos vs. E11.5 β1-AR Neg and E17.5 β1-AR Neg; #P < 0.005, E17.5 β2-AR Pos vs. E17.5 β2-AR Neg and E11.5 β2-AR Neg; D: *P < 0.005, E11.5 β2-AR Pos vs. E11.5 β2-AR Neg; #P < 0.005, E17.5 β2-AR Pos vs. E17.5 β2-AR Neg. E and F: relative distribution of MF20 Pos and Neg cells in β1-AR Pos (E; *P < 0.005, E11.5 β1-AR Pos/MF20 Pos vs. all other groups; #P < 0.05 E11.5 β1-AR Pos/MF20 Neg vs. all other groups) or β2-AR Pos (F) FACS-sorted cell fractions from E11.5 and E17.5 ventricular cells. In B and D–F, one-way ANOVA, Tukey’s multiple-comparisons test. Each bar represents means ± SE, n = 3 independent experiments.
To determine the cell type distribution, we performed MF20 staining on β1- and β2-AR-positive FACS fractions from E11.5 and E17.5 ventricular cells (Fig. 2, E and F). These analyses revealed that the majority of cells in E11.5 β1-AR-positive cell sorts were CMs (MF20 positive, 78.6 ± 4.3% vs. MF20 negative, 21.4 ± 4.3%; Fig. 2E). Whereas E17.5 β1-AR-positive cell sorts contained approximately equal numbers of myocyte and nonmyocyte (NMC) populations (MF20 positive, 47.9 ± 3.1% vs. MF20 negative, 52.1 ± 3.1%; Fig. 2E). Furthermore, there was a significant reduction in myocyte fraction (1.6-fold) with a concomitant increase of NMCs (2.4-fold) in β1-AR-positive cells from E11.5 to E17.5 stage (Fig. 2E). In contrast, there were no significant differences between myocyte and nonmyocyte numbers in β2-AR-positive cell sorts from both E11.5 and E17.5 ventricular cells (Fig. 2F). These data suggest that although β1- and β2-ARs are present on the cell surface of embryonic ventricular cells, neither of those markers can be used to fractionate pure populations of CMs and NMCs at this stage of heart development.
β-AR-associated second messenger responses progressively increase in developing ventricular cells.
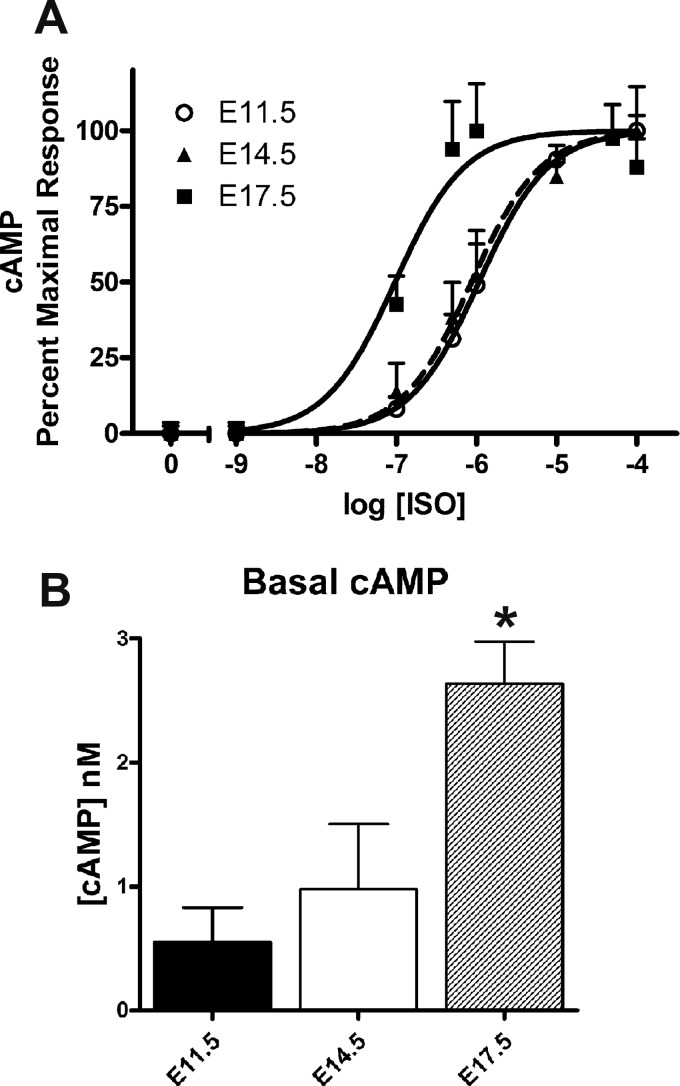
E11.5, E14.5, and E17.5 ventricular cells were treated with different concentrations of ISO to determine levels of cAMP production. Dose-response curves were generated after normalizing responses to the cell number (Fig. 3A). There was an apparent leftward shift of these dose-response curves with the advancement of ventricular development, which indicates that a lower dose of ISO is needed to elicit the same response in E17.5 cells compared with E11.5 cells. Based on these responses, the EC50 of each developmental stage was determined. EC50 values for E11.5, E14.5, and E17.5 ISO dose-response curves were found to be 1,083, 838.9, and 90.5 nM, respectively (Fig. 3A). Furthermore, it was evident that basal cAMP levels of E11.5 ventricular cells (0.55 ± 0.28 nM) were significantly lower compared with E17.5 ventricular cells (2.64 ± 0.34 nM; Fig. 3B). Notably, these results suggest that ISO acts more potently on E17.5 ventricular cells (∼9- to 12-fold) compared with E11.5 or E14.5 cells.
Fig. 3.
cAMP levels in embryonic ventricular cells treated with or without isoproterenol (ISO). A: dose-response curves of E11.5, E14.5, and E17.5 ventricular cells treated with ISO using a homogeneous time-resolved fluorescence (HTRF)-based cAMP assay. B: basal cAMP levels of E17.5 ventricular cells (untreated) were found to be significantly higher than those of E11.5 ventricular cells. *P < 0.05 vs. E11.5; one-way ANOVA, Tukey’s multiple-comparisons test. Each bar represents means ± SE; n = 3–5 independent experiments.
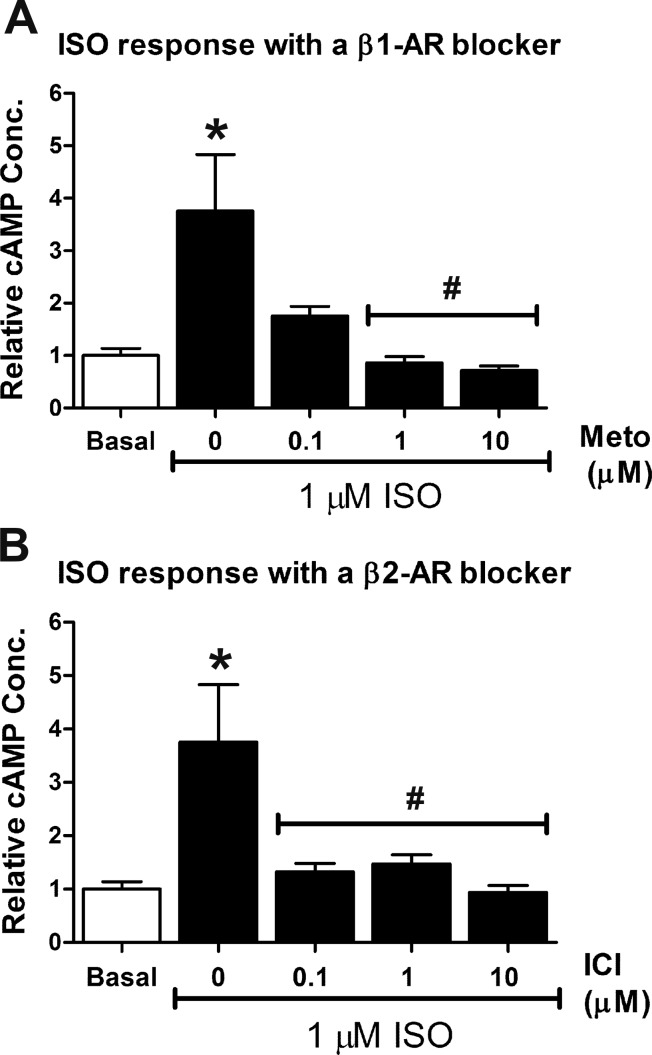
We next determined whether the ISO responses observed in E11.5 ventricular cells were due to β1- or β2-AR activation. For these experiments, cells were treated with 1 µM ISO (~EC50) in the presence or absence of varying concentrations (0.1, 1, and 10 μM) of Meto or ICI and the cAMP levels were measured (Fig. 4, A and B). As expected, a significant increase in cAMP concentration was evident in cells treated with 1 μM ISO compared with the basal untreated group (3.5- to 4-fold; Fig. 4, A and B), whereas cotreatment of cells with 1 μM ISO and 1 or 10 μM Meto significantly reduced cAMP levels back to basal levels (Fig. 4A). E11.5 ventricular cells cotreated with 1 μM ISO and 0.1, 1, and 10 μM ICI also showed a significant reduction in cAMP levels compared with the ISO-only group (Fig. 4B). These results suggest that both β1- and β2-ARs are involved in ISO-mediated cAMP generation in E11.5 ventricular cells.
Fig. 4.
Effects of β-AR stimulation and inhibition on cAMP production in E11.5 ventricular cells. Basal cAMP levels in untreated cells were set to a value of 1.0 and data represent fold changes in cAMP in response to ISO with or without β-AR antagonists. A: stimulation of E11.5 ventricular cells with 1 µM ISO was associated with an approximate fourfold increase in cAMP. Cotreatment of cells with ISO and metoprolol (Meto) abolished the increase in cAMP production observed with ISO alone. B: cotreatment of E11.5 cells with ISO and ICI-118,551 (ICI) also abolished the increase in cAMP production observed with ISO alone. *P < 0.05 vs. basal; #P < 0.05 vs. ISO alone, one-way ANOVA, Tukey’s multiple-comparisons test. Each bar represents means ± SE; n = 3–5 independent experiments.
β-AR stimulation can decrease DNA synthesis in both CPC and CM populations in midgestation ventricles.
To investigate the role of β1- and β2-AR signaling on cell cycle kinetics, E11.5 ventricular cells were treated with ISO alone or in combination with Meto or ICI and pulsed with [3H]thymidine. In this study, CPCs were distinguished from CMs using our previously reported lineage tracking approach, which relied on the generation of E11.5 embryos by crossing two knockin mouse strains [Nkx2.5-Cre and Rosa-lacZ (47)]. Ventricular cells positive for β-Gal but not MF20 were designated as CPCs (β-Gal+/MF20−) because Nkx2.5+/MF20− cells were shown to differentiate into CMs (23, 47). Additionally, cells positive for both β-Gal and MF20 were designated as CMs (β-Gal+/MF20+; Fig. 5, A and B).
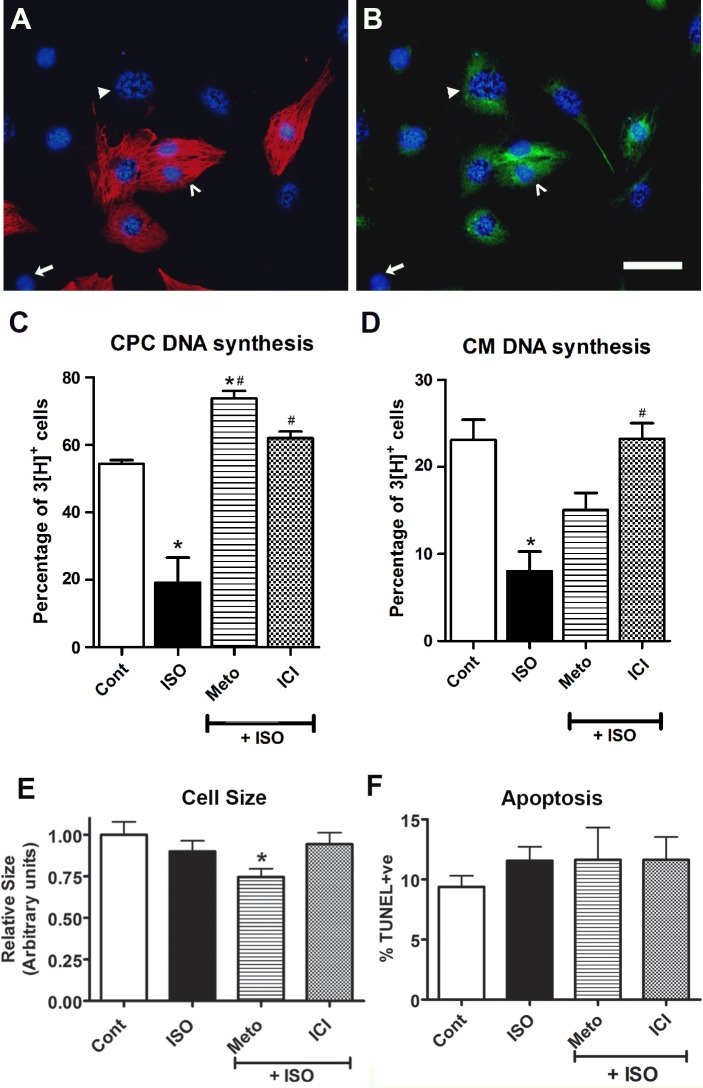
Fig. 5.
Assessment of DNA synthesis, cell size, and apoptosis levels in E11.5 ventricular cells in response to β-AR stimulation and inhibition. A and B: ventricular cells generated from double knockin embryos (Nkx2.5-Cre × Rosa-lacZ) were processed for [3H]thymidine incorporation assay. A: cells were labeled for sarcomeric myosin (MF20; red) and nuclei (Hoechst; blue). B: the same field of cells was also colabeled with β-galactosidase antibodies (β-Gal; green). Cells positive for MF20 and β-Gal (β-Gal+/MF20+) are mature cardiomyocytes (CMs; open arrowhead), whereas cells positive for β-Gal only (β-Gal+/MF20−) are cardiac progenitor cells (CPCs; solid arrowhead) and cells negative for both β-Gal and MF20 (β-Gal−/MF20−) are noncardiomyogenic cells (arrow). The CPC indicated by the solid arrowhead is also [3H]+ based on the presence of silver grains in the nucleus. Bar = 100 µm in A and B. C and D: quantification of cells undergoing DNA synthesis in CPC (C) and CM (D) populations after treatment with the indicated drugs: ISO (1 µM), Meto (1 µM), or ICI (1 µM). *P < 0.05 vs. control (Cont); #P < 0.05 vs. ISO alone, one-way ANOVA, Tukey’s multiple-comparisons test. Each bar represents means ± SE; n = 5 independent experiments. E and F: quantification of CM cell size (E) and apoptosis (F) levels after treatment with indicated drugs. *P < 0.05 vs. control, one-way ANOVA, Tukey’s multiple-comparisons test. Bars represent means ± SE; n = 3 independent experiments.
After drug treatment and [3H]thymidine labeling, cell cycle activity of ventricular cells was determined using an in situ autoradiography method. Using this technique, cells undergoing DNA synthesis (S phase) can be detected by the development of silver grains in the nucleus via autoradiography (Fig. 5, A and B). The thymidine labeling index (LI) was assessed as the percentage of cells positive for nuclear silver grains out of total cells counted in each experiment. In untreated (control) embryonic ventricular cells, thymidine LI values were found to be 54.4 ± 1.1% for CPCs and 22.4 ± 2.1% for CMs (Fig. 5, C and D). In response to 1 μM ISO treatment, there was a significant decrease (∼2.5-fold to 3-fold) in levels of DNA synthesis in both CPC and CM fractions (Fig. 5, C and D). Cotreatment of cells with ISO in the presence of Meto resulted in a significant increase in thymidine labeling of CPCs (73.8 ± 2.2%; Fig. 5C) compared with that of ISO-treated or untreated groups; however, a similar effect was not observed in the CM population (15.1 ± 2.0%; Fig. 5D). Cotreatment of cells with ICI and ISO fully rescued the inhibitory cell cycle effects mediated by ISO alone in both CPC (62.0 ± 2.0%; Fig. 5C) and CM (23.2 ± 1.8%; Fig. 5D) populations. These results indicate that both β1- and β2-ARs play an important role in the regulation of cell cycle activity in midgestation ventricles.
Because it is important to account for any changes in cardiac cell growth and death after any cell cycle effects, we measured CM cell size in E11.5 ventricular cultures treated with various adrenergic agents. Based on these experiments, we did not observe any significant change in CM size in cultures treated with ISO or ISO + ICI compared with control cultures (Fig. 5E). In contrast, there was a significant decrease in CM size in cultures treated with ISO + Meto compared with control cultures (~26% reduction; Fig. 5E). We performed additional experiments and measured CM apoptosis in E11.5 ventricular cell cultures treated with ISO, Meto, and ICI. In those experiments, we did not observe any significant changes in the number of CMs undergoing apoptosis in any of the drug-treated groups (Fig. 5F).
β-AR stimulation decreases cell proliferation and decreases the number of cells in the G2-M phase in E11.5 ventricular cultures.
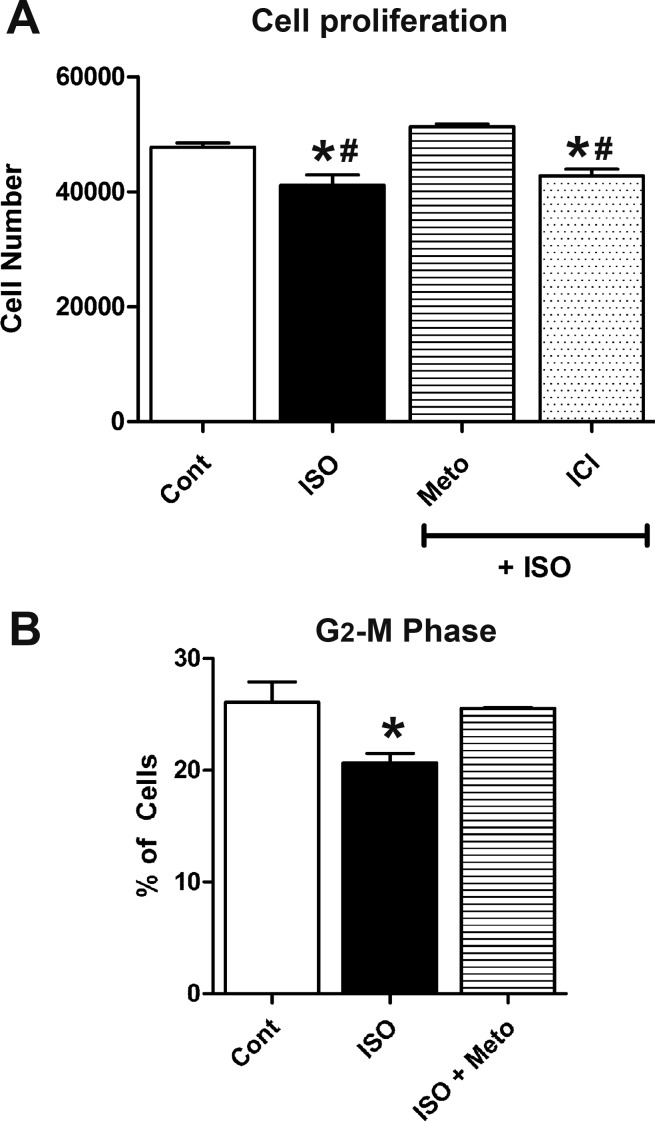
To further confirm the effects of β-AR stimulation on cell proliferation, E11.5 ventricular cells were treated with ISO alone or in combination with β-AR blockers for 18 h. Subsequently, these cultures were subjected to a CyQUANT cell proliferation assay to assess changes in cell number. Compared with the control group, the number of cells in cultures treated with ISO was significantly lower (control: 47,708 ± 763 cells vs. ISO: 41,104 ± 1,823 cells; Fig. 6A). Cotreatment of cells with Meto and ISO fully rescued the inhibitory effect on cell proliferation mediated by ISO alone, whereas cotreatment of cells with ISO and ICI failed to rescue the inhibitory effect on cell proliferation induced by ISO alone (Fig. 6A).
Fig. 6.
Stimulation of β1-ARs results in decreased proliferation of E11.5 ventricular cells. A: cells were treated with ISO (1 µM) in the presence and absence of either Meto (1 µM) or ICI (1 µM), and cell proliferation was assessed using a CyQUANT assay. *P < 0.05 vs. control; #P < 0.05 vs. Meto. B: percent distribution of cells in the G2/M phase in E11.5 cultures treated with ISO in the presence or absence of Meto. *P < 0.05 vs. ISO, one-way ANOVA with Tukey’s multiple-comparisons test. Each bar represents means ± SE; n = 5 independent experiments.
In another series of experiments, cells treated with or without ISO were stained with PI, and cellular DNA content frequency histograms were generated using FACS analysis. Because ICI did not rescue growth inhibitory effects of ISO in cell proliferation assays, FACS analyses did not include the ISO + ICI group. Further analysis of these histograms using deconvolution software revealed relative cell distributions in G0/G1, S, and G2/M stages. Interestingly, there were no significant differences in the percentage of G0/G1 or S cell populations among control, ISO, and ISO + Meto groups (data not shown). In contrast, the percentage of cells in the G2/M phase was significantly lower in ISO-treated cells compared with control cultures (ISO: 20.7 ± 0.8% vs. control: 26.1 ± 1.8%; Fig. 6B). Cotreatment of cells with Meto and ISO fully rescued the inhibitory cell cycle effects mediated by ISO alone, and the percentage distribution of cells in cotreated cultures was not significantly different from that observed with control cultures (Fig. 6B). From these results, it is apparent that stimulation of β-AR by catecholamines can decrease the proliferation rates of E11.5 ventricular cells by arresting them in the G1/S phase transition.
β-AR stimulation leads to decreased levels of phosphorylated Erk and phosphorylated Akt in E11.5 ventricular cultures.
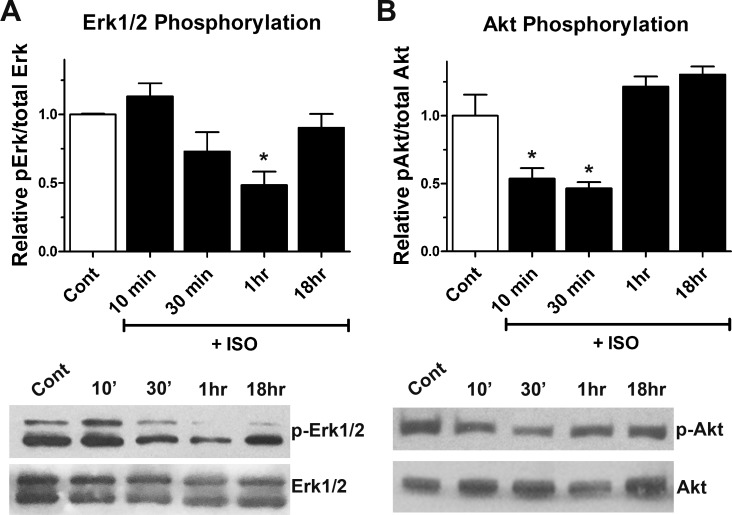
To determine the cellular mechanisms underlying cell cycle inhibition mediated by ISO, we examined whether there were any changes in levels of the mitogenic proteins Erk and Akt. These proteins are regulators of cell proliferation and cell survival, and both are activated via phosphorylation. E11.5 ventricular cells were treated with or without 1 μM ISO for various time periods (10 min, 30 min, 1 h, and 18 h), and protein lysates were processed for Western blot analysis with antibodies specific for total and phosphorylated forms of Erk and Akt (Fig. 7). Densitometric analysis revealed that there was a decreasing trend in phosphorylation levels of Erk, 30 min after ISO treatment (Fig. 7A). Normalization of phospho-Erk levels using densitometric values for total Erk revealed a significant decrease in phospho-Erk levels 1 h after ISO treatment (Fig. 7A). Phospho-Erk levels in ISO-treated cells returned to control levels 18 h after drug treatment. Similarly, a significant decrease in phospho-Akt levels was evident 10 and 30 min after treatment with 1 μM ISO (Fig. 7B). However, phospho-Akt levels returned to control levels at the 1- and 18-h time points (Fig. 7B).
Fig. 7.
Effect of β-adrenergic stimulation on phosphorylation of Akt and Erk. A and B: using Western blot analysis, levels of total and phosphorylated Erk and Akt were determined in E11.5 ventricular cells treated with ISO (1 μM) for various time periods. A: ratio of phosphorylated (p-)Erk1/2 to total Erk1/2 significantly decreased within 1 h of ISO treatment compared with untreated cultured cells. B: ratio of p-Akt to total Akt significantly decreased within 10–30 min of ISO treatment compared with the untreated E11.5 ventricular cells. *P < 0.05 vs. control (untreated), one-way ANOVA with Tukey’s multiple comparisons test. Results are means ± SE of three independent experiments/group.
ISO treatment of E11.5 ventricular cultures decreases the gene expression of G1/S cell cycle proteins and β1-ARs but not the expression of cardiomyogenic genes.
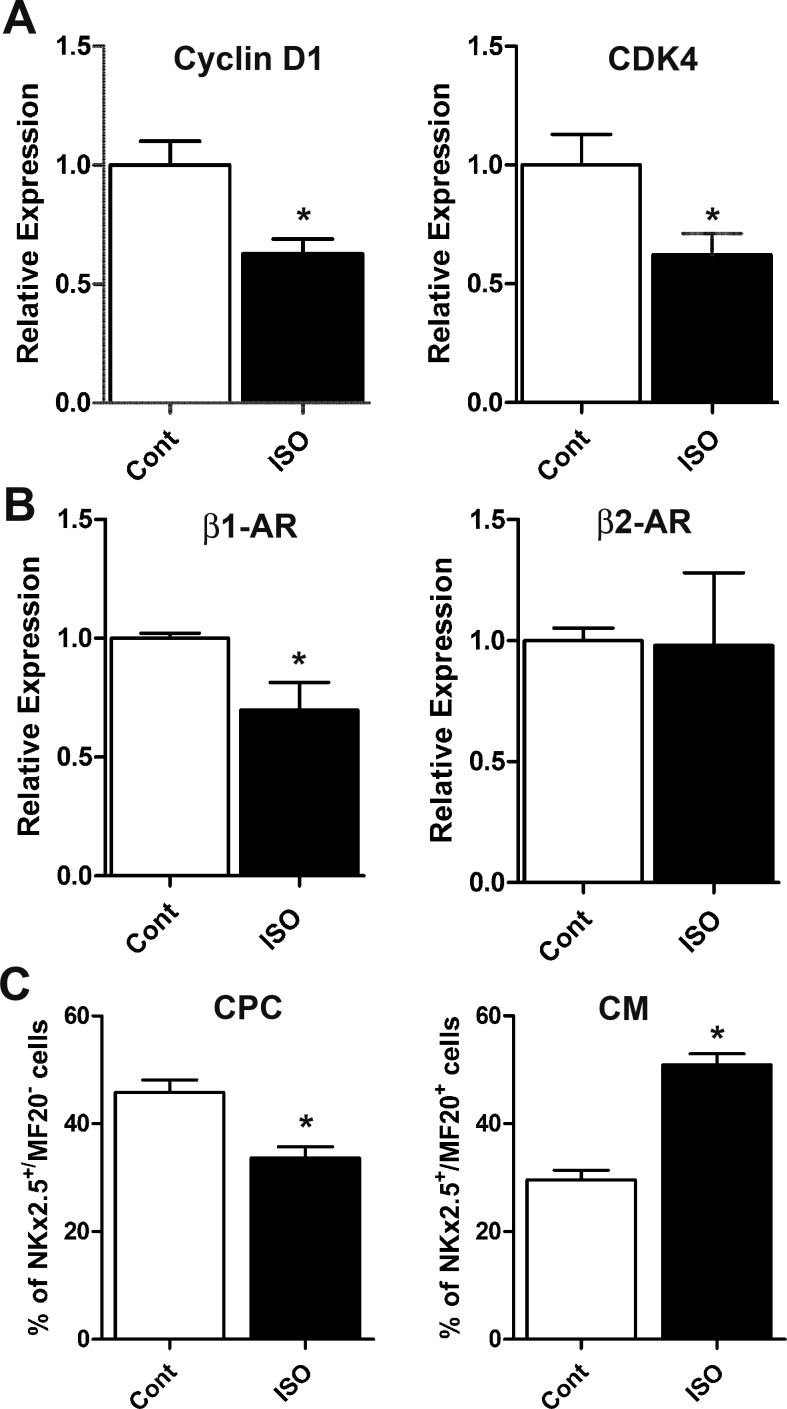
Because phospho-Erk and phospho-Akt play important roles in the regulation of gene expression, qPCR analysis was used to determine gene expression changes in several cell cycle regulatory genes, β-AR subtypes, and cardiomyogenic genes in E11.5 ventricular cultures after β-AR stimulation for 18 h (Fig. 8, A and B). Significant decreases (1.6-fold) in mRNA levels of cyclin D1 and cyclin-dependent kinase (CDK)4 were noted in cultures treated with ISO compared with controls (Fig. 8A). ISO treatment of E11.5 ventricular cells also led to a significant decrease in mRNA levels of β1-AR, whereas levels of β2-AR transcripts were unchanged (Fig. 8B). No significant changes were evident in mRNA levels of p27 and cyclin B1 (data not shown). Notably, there were no significant changes in mRNA levels of cardiomyogenic transcription factors Mef2C, Tbx5, and Hand2 in ISO-treated cultures compared with untreated cells (data not shown).
Fig. 8.
Effects of ISO on expression of cell cycle regulating genes and CM differentiation. A and B: relative expression of cell cycle genes cyclin D1 and cyclin-dependent kinase (CDK)4 (A) and β-AR subtypes (B) in E11.5 ventricular cultures treated with or without ISO (1 μM). *P < 0.05 vs. control, unpaired Student’s t-test. n = 3 independent experiments/treatment group, analyzed in duplicate for each experiment. C: percent distribution of CPCs and differentiated CMs in E11.5 cultures treated with ISO. *P < 0.05 vs. control, unpaired Student’s t-test. n = 5 independent experiments/treatment group.
ISO treatment increases cardiomyocyte differentiation in E11.5 ventricular cell cultures.
Given the heterogeneous nature of midgestation ventricular cells, cell differentiation status in E11.5 cell cultures after ISO treatment was assessed by immunofluorescence using various cell lineage-specific antibodies. Our results indicated that both control and ISO-treated cultures were negative for the endothelial cells marker von Willebrand factor, and >1% of the cells were positive for CD31 in both cultures (data not shown). Quantification of CPCs and CMs in untreated and ISO-treated ventricular cultures indicated that compared with control cultures, a significant reduction in the number of CPCs and a significant increase in the number of CMs were apparent in ISO-treated cultures (Fig. 8C). Collectively, these findings suggest that β-AR stimulation with ISO induces differentiation of CPCs into CMs.
Continuous infusion of ISO decreases graft size and cotreatment with Meto rescues the detrimental effect of ISO after intracardiac cell transplantation.
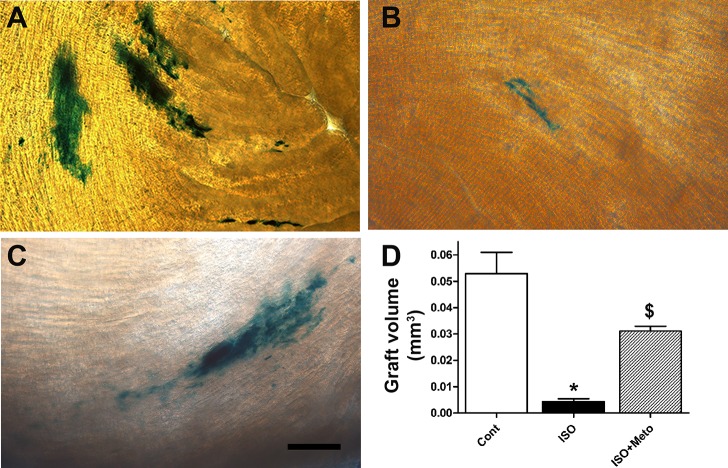
We next tested the effects of β-AR drugs on cell transplantation in vivo. To this end, ventricular cells were isolated from E11.5 NCRL embryos, ~3 × 105 ventricular cells were injected in the left ventricle of healthy C57Bl/6 mice, and animals were treated with ISO, Meto, or ISO + Meto via miniosmotic pumps for a period of 3 days. Animals were then euthanized, and the hearts were excised and processed for quantification of graft volume. Recipient hearts were sliced into 25-μm sections and placed in X-Gal solution to visualize the engrafted embryonic ventricular NCRL cells and to quantify the volume of blue intracardiac grafted cells as previously described (10, 47) (Fig. 9, A–D).
Fig. 9.
Quantification of graft volumes of intracardiac transplanted E11.5 ventricular cells in recipient mice treated with or without β-adrenergic agonist and antagonist drugs. A–C: representative micrographs of X-Gal-stained thick sections of recipient hearts transplanted with E11.5 ventricular cells derived from double knockin embryos (Nkx2.5-Cre × Rosa-lacZ). Bar = 100 µm. Recipient mice were either untreated (A), treated with ISO (B), or treated with ISO + Meto (C). D: quantification of graft volume of intracardiac transplanted embryonic ventricular cells. *P < 0.005 vs. control; $P < 0.05 vs. control and ISO, one-way ANOVA with Tukey’s multiple-comparisons test. Results are means ± SE of 3–4 experiments/group.
Quantification of graft volumes indicated that compared with the saline-treated group (Fig. 9, A and D), systemic infusion of ISO (Fig. 9, B and D) significantly reduced the graft volume (12-fold decrease in graft volume). On the other hand, cotreatment of recipient animals with ISO + Meto (Fig. 9C) resulted in a significant increase in graft volume compared with the ISO-treated group (7-fold increase in graft volume; Fig. 9D). Collectively, these results suggest that use of the β1-AR blocker Meto could remove deleterious effects of β-AR agonists on intracardiac graft formation.
DISCUSSION
The role of β-AR signaling in embryonic ventricular cell proliferation and differentiation is largely unexplored. In this context, we studied the gene expression and cell surface expression levels of β-AR subtypes as well as their second messenger responses in ventricular preparations from different stages of development (Figs. 1–3). A subsequent series of experiments was focused on the role of β-AR signaling in E11.5 ventricular cells (Figs. 4–9). E11.5 ventricular cells were selected for these studies mainly based on the notion that β-AR signaling may play a significant role in cardiac cell proliferation and differentiation around this stage. This notion is supported by previous reports on early embryonic lethality around stages E10.5−E11.5 in mice lacking enzymes involved in catecholamine biosynthesis or β1-ARs (35, 42, 49). Notably, improper cellular organization and differentiation in TH knockout embryonic ventricles underscores the importance of catecholamines in cardiac development (49). Moreover, catecholamines and their receptors were shown to be present in rodents as early as E10.5 (2, 42). In addition, it is well established that E11.5 ventricles harbor undifferentiated CPCs (23, 48) and exhibit higher cell cycle activity and engraftment efficiency (47) compared with ventricular cells from other developmental stages. Here, we sought to examine the effects of β-AR agonists and antagonists on E11.5 ventricular cell proliferation and differentiation in vitro and after intracardiac cell transplantation in vivo.
Left and right ventricles of the mammalian heart have been shown to exhibit different embryological origins and different structural, metabolic, and electrophysiological features (5). It is possible that β-AR subtype distribution may be different in each chamber. However, it is technically challenging to precisely separate left and right ventricular preparations during early stages of mouse development and hence the ventricular preparations used in the present study encompassed both left and right ventricles. Notably, β1- or β2-AR gene expression levels were significantly higher in adult ventricles compared with those in mice at stages E11.5, E14.5, and E16.5. Although previous studies did not document gene expression levels of β-AR subtypes, adult mouse or rat heart homogenates were shown to contain a significantly higher number of [3H]dihydroalprenolol (DHA; a nonselective β-AR blocker)-binding sites compared with embryonic heart homogenates (3, 16). Collectively, these findings suggest that an increase in β-AR mRNA levels in the adult heart may result in a higher number of β-AR-binding sites. Contrary to lack of differences in β1- or β2-AR gene expression levels between E14.5, E16.5, and neonatal stages in this study, an increasing trend in the overall β-AR-binding site densities was reported for both developing mouse and rat heart homogenates (3, 16). However, a clear downward trend in the β-AR-binding site densities has also been reported for developing chick hearts from stages E4−E18 (15).
Because it is difficult to determine the relative contribution of β-AR subtypes present on cell membrane versus cytosolic fractions from whole heart homogenate studies (3, 16), we specifically monitored cell surface staining of β1- and β2-ARs in unfixed E11.5 and E17.5 ventricular cell preparations by FACS analysis. Although there appears to be a declining trend in β1-AR-positive cell numbers, we did not find any significant difference in either β1- or β2-AR cell surface expression levels between E11.5 and E17.5 ventricular cells. It is important to note that extracellular domain-specific β1- and β2-AR antibodies do not recognize the receptor protein that is being trafficked in the intracellular compartments of unfixed cell preparations used for FACS analyses. Thus, β1- or β2-AR cell surface expression does not represent the total receptor binding sites and cannot be directly correlated with β1- or β2-AR mRNA levels within E11.5 and E17.5 cell preparations.
Despite no significant differences in cell surface expression levels of β1- and β2-ARs in E11.5 and E17.5 ventricles, ISO-induced cAMP production is significantly higher in E17.5 ventricular cells compared with E11.5 or E14.5 ventricular cells. Such developmental differences in cAMP production could be attributed to either an increased adenylyl cyclase (AC) activity or possibly to changes in the expression pattern of AC isoforms in late-gestation ventricular cells. Ablation of ISO-induced cAMP generation in response to either β1- or β2-AR antagonists in our study can be explained by the ability of β-ARs to dimerize (18). It has been suggested that β1- and β2-ARs require support from each other to maintain optimal sympathetic control over their functionality (50) and, thus, blockade of one of the two receptors may be sufficient to block activation of the other receptor.
It is well established that expression levels of various positive cell cycle regulatory proteins are decreased during heart development. However, the mechanisms regulating these changes in embryonic heart cells remain poorly characterized (11, 31, 34). Fetal catecholamine levels were found to be significantly lower at the E11.5 stage compared with late-gestation or neonatal stages (42). Previous studies have demonstrated that a drastic reduction in cell cycle activity occurs during late gestation and early postnatal stages in mouse heart development (39, 47). Although these studies clearly point to an inverse relationship between levels of catecholamines and cardiac cell cycle activity, such a notion is further corroborated by our current finding that β-AR stimulation can induce a significant decrease in cell cycle activity of E11.5 ventricular cell cultures. It is likely that β-AR signaling via endogenous catecholamines along with other known cardiac growth agonists such as ANG II or endothelin (ET)-1 would promote cell cycle exit and cardiomyocyte differentiation in the postnatal heart. Although ANG II treatment has been shown to increase DNA synthesis in both fetal ovine and neonatal rat CMs (25, 43), it is known to trigger mainly hypertrophic growth but not proliferation of neonatal CMs (25, 36). Similarly, treatment of fetal rat CMs with ET-1 led to decreased DNA synthesis and an increase in binucleation index (28). In addition, treatment of fetal cultures with a nonselective ET receptor antagonist blocked the effects of ET-1 on CM proliferation and binucleation (28). Administration of an ETA receptor-specific antagonist to newborn rats increased CM proliferation and cell size (29). Collectively, these studies suggest that combined actions of blockers specific for β-ARs, ANG II, and ET receptors may provide new means for increasing CM proliferation in postnatal hearts.
In this study, we found that ISO-induced second messenger responses were followed by significant decreases in cell cycle activity of CPCs and CMs in E11.5 ventricular cell cultures. Previous studies have demonstrated that the addition of cAMP, analogs of cAMP, or agents that raise intracellular cAMP can either inhibit or promote proliferation of different noncardiac cell types in a concentration-dependent manner (30). Further comparison of different cell cycle analyses indicated that ISO-induced reduction in DNA synthesis levels (~50% vs. control) did not closely mirror a proportionate reduction in cell numbers using a CyQUANT assay (~15% vs. control). This discrepancy could be attributed to the fact that [3H]thymidine assays allowed for quantification of DNA synthesis specifically in CPCs and CMs, whereas the CyQUANT assay measured the overall cell number and did not distinguish between CPC, CM, and NMC populations. It is possible that stimulation of cultures with ISO may have potentially increased the proliferation of NMCs in E11.5 cultures, which may account for a smaller change in cell number using CyQUANT assay. Although ISO-mediated cell cycle inhibition is not immediately accompanied by a hypertrophic growth response in E11.5 ventricular myocytes, the cell size reduction in the Meto-treated group may be correlated with previous clinical reports that found that use of β-AR blockers in early pregnancy can lead to fetal growth retardation and reductions in birth weight (24).
Currently, it is not clear how β-AR activation leads to decreases in Erk and Akt phosphorylation levels in E11.5 ventricular cells. Several studies conducted on other cell types have suggested potential mechanisms that may explain a connection between the cAMP pathway and Erk1/2 and Akt phosphorylation. For instance, inactivation of Ras via PKA signaling (13), hyperphosphorylation of MEK (44), or activation of Rap-1 and inactivation of the Ras/MEK pathway (37) may be involved in ISO-mediated effects on Erk 1/2 and Akt phosphorylation. Additional experiments are required to precisely define the signaling events linking β-AR activation to changes in Erk 1/2 and Akt phosphorylation in midgestation ventricular cells. In addition to decreased phosphorylation of Erk1/2 and Akt, ISO treatment of E11.5 ventricular cells also resulted in significant decreases in gene expression of the G1/S regulatory proteins cyclin D1 and CDK4 (33). The activated Akt (26, 27) and Erk1/2 (19, 45) pathways are known to be involved in the induction of cyclin D1 expression in several noncardiac cell types via both transcriptional and posttranscriptional mechanisms. Similarly, activated Erk1/2 and/or Akt pathways may be necessary for the regulation of CDK4 gene expression.
ISO treatment significantly reduced the number of CPCs and increased the number of CMs in E11.5 ventricular cultures. This observation suggests a direct role for β-AR signaling in CM differentiation of midgestation ventricles, and it is also in agreement with data from murine knockout models (14, 42, 49) and other in vitro studies that demonstrated the importance of catecholamines and the β-ARs in cardiomyocyte lineage commitment. For instance, Lehmann and colleagues (20) demonstrated that depletion of catecholamines using reserpine or pharmacological blockade of α- and β-ARs in embryonic stem cell cultures can significantly delay the induction of cardiac lineage and delay differentiation of embryonic stem cell cells toward CMs. However, Lehmann et al. did not formally examine the effects of pharmacological agents on the proliferation and differentiation of CPCs and CMs.
In this study, phosphorylation of the prosurvival protein Akt was restored to control levels after treatment of E11.5 ventricular cells with 1 µM ISO for 18 h, yet the graft volume was decreased in mice subjected to continuous infusion of ISO over a 3-day period in in vivo experiments. It is known that β-ARs are subjected to desensitization and/or downregulation when exposed to prolonged stimulation with the ligands. Therefore, we examined the effects of ISO on feedback regulation of β1- and β2-AR expression and found a significant decrease in the expression of β1- but not β2-AR levels. Although we have not studied the effects of ISO on β-AR desensitization, it was shown that short-term treatment of adult rat ventricular myocyte cultures with 1 µM ISO for 24 h was sufficient to significantly increase the levels of β-adrenergic receptor kinase 1, an enzyme involved in the desensitization of β-ARs (12). Taken together, it is likely that one-time treatment of 1 µM ISO for 18 h in the present study would have led to the dampening of adrenergic responses and facilitated the restoration of Akt phosphorylation in our primary culture studies. In contrast to these in vitro observations, we have not studied the effects of continuous delivery of ISO via miniosmotic pumps on the desensitization and/or downregulation of β-ARs in cell transplantation experiments. Considering a significant reduction in the graft volume with ISO group, we speculate that a controlled delivery of ISO via miniosmotic pumps may have maintained a steady state of adrenergic responses in vivo over a 3-day period and thus preserving the growth inhibitory actions of ISO on transplanted cells.
Our results pertaining to protective effects of the β1-AR blocker Meto on graft formation are distinct from previously documented drug interactions with progenitor or stem cell-based interventions (22). For instance, use of heparin in the BOOST (46) and CADUCEUS (21) trials has come into question because recent findings have suggested that heparin interferes with the migration and homing potential of bone marrow-derived mononuclear cells (38). Additionally, the graft volume of intracardiac transplanted midgestation embryonic ventricular cells was significantly reduced in mice treated with the L-type Ca2+ blocker nifedipine (10). Collectively, these findings provide strong evidence for a potential interaction between transplanted cells and systemically administered drugs and raise a cautionary note for the ongoing clinical trials as well as future interventions involving cell-based therapies.
In summary, we showed that stimulation of the β-AR signaling pathway with a nonselective β-AR agonist, ISO, can significantly decrease the proliferation of midgestation ventricular cells by decreasing the levels of phospho-Erk 1/2, phospho-Akt, cyclin D1, and CDK4. ISO-induced changes in cell cycle activity were also accompanied by a significant increase in CM differentiation. In addition, we found that β-AR stimulation can significantly reduce the grafting efficiency of donor cells, whereas β1-AR antagonists can protect transplanted cells from the deleterious effects caused by β-AR stimulation in vivo.
GRANTS
This work was supported by Heart and Stroke Foundation of Canada (HSFC) Grant-In-Aid G-15-0009233 and Faculty of Medicine Bridge Fund 48597. T. Feridooni and A. Hotchkiss received graduate studentships from the Nova Scotia Health Research Foundation. B. Allen received a BrightRed studentship from HSFC.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.F. and K.B.P. conceived and designed research; T.F., A.H., M.B.-N., F.Z., B.A., and S.C. performed experiments; T.F., A.H., M.B.-N., F.Z., B.A., S.C., and K.B.P. analyzed data; T.F., A.H., M.B.-N., F.Z., B.A., S.C., and K.B.P. interpreted results of experiments; T.F., B.A., and K.B.P. prepared figures; T.F. and K.B.P. drafted manuscript; T.F., A.H., M.B.-N., and K.B.P. edited and revised manuscript; T.F., A.H., M.B.-N., F.Z., B.A., S.C., and K.B.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Derek Rowter of Flow Cytometry CORES for help with FACS experiments.
REFERENCES
- 1.Baguma-Nibasheka M, Li AW, Murphy PR. The fibroblast growth factor-2 antisense gene inhibits nuclear accumulation of FGF-2 and delays cell cycle progression in C6 glioma cells. Mol Cell Endocrinol 267: 127–136, 2007. doi: 10.1016/j.mce.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Chen F, Klitzner TS, Weiss JN. Autonomic regulation of calcium cycling in developing embryonic mouse hearts. Cell Calcium 39: 375–385, 2006. doi: 10.1016/j.ceca.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Chen FM, Yamamura HI, Roeske WR. Ontogeny of mammalian myocardial beta-adrenergic receptors. Eur J Pharmacol 58: 255–264, 1979. doi: 10.1016/0014-2999(79)90474-6. [DOI] [PubMed] [Google Scholar]
- 4.Dowell JD, Rubart M, Pasumarthi KB, Soonpaa MH, Field LJ. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res 58: 336–350, 2003. doi: 10.1016/S0008-6363(03)00254-2. [DOI] [PubMed] [Google Scholar]
- 5.Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. Circ Res 107: 1428–1444, 2010. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feridooni T, Hotchkiss A, Remley-Carr S, Saga Y, Pasumarthi KB. Cardiomyocyte specific ablation of p53 is not sufficient to block doxorubicin induced cardiac fibrosis and associated cytoskeletal changes. PLoS One 6: e22801, 2011. doi: 10.1371/journal.pone.0022801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaspard GJ, Pasumarthi KB. Quantification of cardiac fibrosis by colour-subtractive computer-assisted image analysis. Clin Exp Pharmacol Physiol 35: 679–686, 2008. doi: 10.1111/j.1440-1681.2008.04930.x. [DOI] [PubMed] [Google Scholar]
- 8.Gomez H. The development of the innervation of the heart in the rat embryo. Anat Rec 130: 53–71, 1958. doi: 10.1002/ar.1091300106. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss A, Feridooni T, Baguma-Nibasheka M, McNeil K, Chinni S, Pasumarthi KB. Atrial natriuretic peptide inhibits cell cycle activity of embryonic cardiac progenitor cells via its NPRA receptor signaling axis. Am J Physiol Cell Physiol 308: C557–C569, 2015. doi: 10.1152/ajpcell.00323.2014. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss A, Feridooni T, Zhang F, Pasumarthi KB. The effects of calcium channel blockade on proliferation and differentiation of cardiac progenitor cells. Cell Calcium 55: 238–251, 2014. doi: 10.1016/j.ceca.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss A, Robinson J, MacLean J, Feridooni T, Wafa K, Pasumarthi KB. Role of D-type cyclins in heart development and disease. Can J Physiol Pharmacol 90: 1197–1207, 2012. doi: 10.1139/y2012-037. [DOI] [PubMed] [Google Scholar]
- 12.Iaccarino G, Dolber PC, Lefkowitz RJ, Koch WJ. β-Adrenergic receptor kinase-1 levels in catecholamine-induced myocardial hypertrophy: regulation by β- but not α1-adrenergic stimulation. Hypertension 33: 396–401, 1999. doi: 10.1161/01.HYP.33.1.396. [DOI] [PubMed] [Google Scholar]
- 13.Indolfi C, Avvedimento EV, Di Lorenzo E, Esposito G, Rapacciuolo A, Giuliano P, Grieco D, Cavuto L, Stingone AM, Ciullo I, Condorelli G, Chiariello M. Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat Med 3: 775–779, 1997. doi: 10.1038/nm0797-775. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi K, Morita S, Sawada H, Mizuguchi T, Yamada K, Nagatsu I, Hata T, Watanabe Y, Fujita K, Nagatsu T. Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J Biol Chem 270: 27235–27243, 1995. doi: 10.1074/jbc.270.45.27235. [DOI] [PubMed] [Google Scholar]
- 15.Kockova R, Svatunkova J, Novotny J, Hejnova L, Ostadal B, Sedmera D. Heart rate changes mediate the embryotoxic effect of antiarrhythmic drugs in the chick embryo. Am J Physiol Heart Circ Physiol 304: H895–H902, 2013. doi: 10.1152/ajpheart.00679.2012. [DOI] [PubMed] [Google Scholar]
- 16.Kojima M, Ishima T, Taniguchi N, Kimura K, Sada H, Sperelakis N. Developmental changes in β-adrenoceptors, muscarinic cholinoceptors and Ca2+ channels in rat ventricular muscles. Br J Pharmacol 99: 334–339, 1990. doi: 10.1111/j.1476-5381.1990.tb14704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwahara K, Nishikimi T, Nakao K. Transcriptional regulation of the fetal cardiac gene program. J Pharmacol Sci 119: 198–203, 2012. doi: 10.1254/jphs.12R04CP. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie C, Mercier JF, Salahpour A, Umapathy D, Breit A, Villeneuve LR, Zhu WZ, Xiao RP, Lakatta EG, Bouvier M, Hébert TE. β1/β2-Adrenergic receptor heterodimerization regulates β2-adrenergic receptor internalization and ERK signaling efficacy. J Biol Chem 277: 35402–35410, 2002. doi: 10.1074/jbc.M204163200. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie JN, L’Allemain G, Brunet A, Müller R, Pouysségur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem 271: 20608–20616, 1996. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann M, Nguemo F, Wagh V, Pfannkuche K, Hescheler J, Reppel M. Evidence for a critical role of catecholamines for cardiomyocyte lineage commitment in murine embryonic stem cells. PLoS One 8: e70913, 2013. doi: 10.1371/journal.pone.0070913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379: 895–904, 2012. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMullen NM, Pasumarthi KB. Donor cell transplantation for myocardial disease: does it complement current pharmacological therapies? Can J Physiol Pharmacol 85: 1–15, 2007. doi: 10.1139/Y06-105. [DOI] [PubMed] [Google Scholar]
- 23.McMullen NM, Zhang F, Hotchkiss A, Bretzner F, Wilson JM, Ma H, Wafa K, Brownstone RM, Pasumarthi KB. Functional characterization of cardiac progenitor cells and their derivatives in the embryonic heart post-chamber formation. Dev Dyn 238: 2787–2799, 2009. doi: 10.1002/dvdy.22112. [DOI] [PubMed] [Google Scholar]
- 24.Meidahl Petersen K, Jimenez-Solem E, Andersen JT, Petersen M, Brødbæk K, Køber L, Torp-Pedersen C, Poulsen HE. β-Blocker treatment during pregnancy and adverse pregnancy outcomes: a nationwide population-based cohort study. BMJ Open 2: e001185, 2012. doi: 10.1136/bmjopen-2012-001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mel’nikova NP, Timoshin SS, Jivotova EY, Pelliniemi LJ, Jokinen E, Abdelwahid E. Angiotensin-II activates apoptosis, proliferation and protein synthesis in the left heart ventricle of newborn albino rats. Int J Cardiol 112: 219–222, 2006. doi: 10.1016/j.ijcard.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Muise-Helmericks RC, Grimes HL, Bellacosa A, Malstrom SE, Tsichlis PN, Rosen N. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem 273: 29864–29872, 1998. doi: 10.1074/jbc.273.45.29864. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang W, Li J, Ma Q, Huang C. Essential roles of PI-3K/Akt/IKKβ/NFκB pathway in cyclin D1 induction by arsenite in JB6 Cl41 cells. Carcinogenesis 27: 864–873, 2006. doi: 10.1093/carcin/bgi321. [DOI] [PubMed] [Google Scholar]
- 28.Paradis A, Xiao D, Zhou J, Zhang L. Endothelin-1 promotes cardiomyocyte terminal differentiation in the developing heart via heightened DNA methylation. Int J Med Sci 11: 373–380, 2014. doi: 10.7150/ijms.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paradis AN, Gay MS, Wilson CG, Zhang L. Newborn hypoxia/anoxia inhibits cardiomyocyte proliferation and decreases cardiomyocyte endowment in the developing heart: role of endothelin-1. PLoS One 10: e0116600, 2015. doi: 10.1371/journal.pone.0116600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastan IH, Johnson GS, Anderson WB. Role of cyclic nucleotides in growth control. Annu Rev Biochem 44: 491–522, 1975. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- 31.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res 90: 1044–1054, 2002. doi: 10.1161/01.RES.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 32.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res 96: 110–118, 2005. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- 33.Reed SI. Control of the G1/S transition. Cancer Surv 29: 7–23, 1997. [PubMed] [Google Scholar]
- 34.Regula KM, Rzeszutek MJ, Baetz D, Seneviratne C, Kirshenbaum LA. Therapeutic opportunities for cell cycle re-entry and cardiac regeneration. Cardiovasc Res 64: 395–401, 2004. doi: 10.1016/j.cardiores.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP Jr, Barsh GS, Bernstein D, Kobilka BK. Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci USA 93: 7375–7380, 1996. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadoshima J, Aoki H, Izumo S. Angiotensin II and serum differentially regulate expression of cyclins, activity of cyclin-dependent kinases, and phosphorylation of retinoblastoma gene product in neonatal cardiac myocytes. Circ Res 80: 228–241, 1997. doi: 10.1161/01.RES.80.2.228. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt JM, Stork PJ. PKA phosphorylation of Src mediates cAMP’s inhibition of cell growth via Rap1. Mol Cell 9: 85–94, 2002. doi: 10.1016/S1097-2765(01)00432-4. [DOI] [PubMed] [Google Scholar]
- 38.Seeger FH, Rasper T, Fischer A, Muhly-Reinholz M, Hergenreider E, Leistner DM, Sommer K, Manavski Y, Henschler R, Chavakis E, Assmus B, Zeiher AM, Dimmeler S. Heparin disrupts the CXCR4/SDF-1 axis and impairs the functional capacity of bone marrow-derived mononuclear cells used for cardiovascular repair. Circ Res 111: 854–862, 2012. doi: 10.1161/CIRCRESAHA.112.265678. [DOI] [PubMed] [Google Scholar]
- 39.Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol Heart Circ Physiol 271: H2183–H2189, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Stanley EG, Biben C, Elefanty A, Barnett L, Koentgen F, Robb L, Harvey RP. Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3′UTR-ires-Cre allele of the homeobox gene Nkx2-5. Int J Dev Biol 46: 431–439, 2002. [PubMed] [Google Scholar]
- 41.Sun Q, Zhang F, Wafa K, Baptist T, Pasumarthi KB. A splice variant of cyclin D2 regulates cardiomyocyte cell cycle through a novel protein aggregation pathway. J Cell Sci 122: 1563–1573, 2009. doi: 10.1242/jcs.047738. [DOI] [PubMed] [Google Scholar]
- 42.Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature 374: 643–646, 1995. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 43.Thornburg K, Jonker S, O’Tierney P, Chattergoon N, Louey S, Faber J, Giraud G. Regulation of the cardiomyocyte population in the developing heart. Prog Biophys Mol Biol 106: 289–299, 2011. doi: 10.1016/j.pbiomolbio.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogel S, Kubin T, von der Ahe D, Deindl E, Schaper W, Zimmermann R. MEK hyperphosphorylation coincides with cell cycle shut down of cultured smooth muscle cells. J Cell Physiol 206: 25–34, 2006. doi: 10.1002/jcp.20437. [DOI] [PubMed] [Google Scholar]
- 45.Weber JD, Raben DM, Phillips PJ, Baldassare JJ. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J 326: 61–68, 1997. doi: 10.1042/bj3260061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364: 141–148, 2004. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 47.Zhang F, Feridooni T, Hotchkiss A, Pasumarthi KB. Divergent cell cycle kinetics of midgestation ventricular cells entail a higher engraftment efficiency after cell transplantation. Am J Physiol Cell Physiol 308: C220–C228, 2015. doi: 10.1152/ajpcell.00319.2014. [DOI] [PubMed] [Google Scholar]
- 48.Zhang F, Pasumarthi KB. Ultrastructural and immunocharacterization of undifferentiated myocardial cells in the developing mouse heart. J Cell Mol Med 11: 552–560, 2007. doi: 10.1111/j.1582-4934.2007.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature 374: 640–643, 1995. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
- 50.Zhu WZ, Chakir K, Zhang S, Yang D, Lavoie C, Bouvier M, Hébert TE, Lakatta EG, Cheng H, Xiao RP. Heterodimerization of β1- and β2-adrenergic receptor subtypes optimizes β-adrenergic modulation of cardiac contractility. Circ Res 97: 244–251, 2005. doi: 10.1161/01.RES.0000176764.38934.86. [DOI] [PubMed] [Google Scholar]