Abstract
Inflammation is known to play a significant role in the process of atherogenesis and cardiovascular disease (CVD). Indeed, patients with chronic inflammatory diseases are at increased risk for cardiovascular events. However, the mechanisms linking chronic inflammation and CVD remain poorly understood. Psoriasis, a chronic inflammatory skin disease associated with a greater risk of early cardiovascular events, provides a suitable human model to study the pathophysiology of inflammatory atherogenesis in humans. Additionally, cytokines such as TNF-α, IL-17A, and other immune pathways are the common links between the pathogenesis of psoriasis and atherosclerosis, and hence the approved treatments for psoriasis, which include selective cytokine inhibition (e.g., anti-TNF, anti-IL-17A, and anti-IL-12/23) and immune modulation (e.g., methotrexate or cyclosporine), provide an opportunity to examine the effect of modulating these pathways on atherogenesis. We have been using this human model in a large, prospective cohort study, and this review summarizes our approach and results of using this human model to study inflammatory atherogenesis. Specifically, we review simultaneous multimodal imaging of several vascular beds using 18fludeoxyglucose positron emission tomography/computed tomography, 18fludeoxyglucose positron emission tomography/MRI, and coronary computed tomography angiography as well as cardiovascular biomarkers to better understand how modulation of inflammation may impact vascular diseases.
Keywords: inflammation, atherosclerosis, psoriasis, cardiovascular imaging, 18fludeoxyglucose-positron emission tomography/computed tomography
inflammation is well known to have a crucial role in the process of atherogenesis as well as cardiovascular disease (CVD) (36, 37). Chronic inflammatory diseases, such as psoriasis, are associated with a greater risk for myocardial infarction, stroke, coronary heart disease, peripheral vascular disease, and cardiomyopathy and, as such, provide a useful clinical human model to study inflammatory atherosclerosis and CVD (9, 20, 21, 40, 48, 50). Patients with severe psoriasis have a 50% increase in all-cause mortality (22). This mortality is frequently due to atherosclerosis and CVD (21, 40, 44) followed by deaths due to infection and cancer.
Psoriasis and CVD
Psoriasis is a chronic, immune-mediated systemic inflammatory disorder that affects the skin and joints and can manifest as various phenotypes, namely, plaque psoriasis, the most common form, as well as guttate psoriasis, nail psoriasis, and psoriatic arthritis (23, 51). It is a genetically driven autoimmune condition with innate and adaptive immunity activation both playing important roles in its pathogenesis (57). The factors vital in the causation of psoriasis are multiple and include genetic as well as environmental factors such as trauma, infection, and medications (57). Furthermore, multiple abnormalities involving immune cells such as T helper (Th)1 and Th17 cells and cytokines such as TNF-α, interferon-γ, IL-17A, IL-12, and IL-23 have been found to be critical in psoriasis pathophysiology (47). Finally, chronic systemic inflammation in psoriasis has been associated with the development of enthesitis, cardiovascular, and psychiatric comorbidities (31). Conversely, it has been noted that there is an increased prevalence of rheumatoid arthritis, celiac disease, and Crohn’s disease in patients with psoriasis (23).
There are roughly 125 million people worldwide with psoriasis, including 7 million in the United States, and an estimated >3 million people who are undiagnosed (3). The psoriatic plaque may be localized to the skin, but its effects are far reaching and systemic (1). During a psoriasis flare, there are >1 billion immune cells activated within the body (7). These cells are heavily activated in psoriasis and may be an important link between psoriasis and its comorbidities (8). The result of these activated immune cells is systemic inflammation that likely relates to the ensuing cardiometabolic dysfunction (1, 41).
Psoriasis severity has a dose-response relationship with an increase in the extent of obesity, magnitude of adipose inflammation, and severity of underlying insulin resistance (18, 25, 55). Furthermore, psoriasis patients have a high prevalence of lipid derangement that is evident in the directly measured clinical laboratory values (32). Additionally, emerging research shows that these effects go beyond the clinical parameters and may perhaps impact the functional status of these lipids and modulate their spectra while simultaneously causing atherosclerotic CVD (42, 63). Epidemiological studies have demonstrated a strong link between the incidence as well as prevalence of type 2 diabetes, metabolic syndrome, and psoriasis (2). These associations are not limited to subclinical CVD, as has been seen in large population-based studies (40). Furthermore, these studies have shown a relationship between psoriasis and an elevated burden of myocardial infarction (9, 21), cerebrovascular events (20), and overall cardiovascular morbidity and mortality (50).
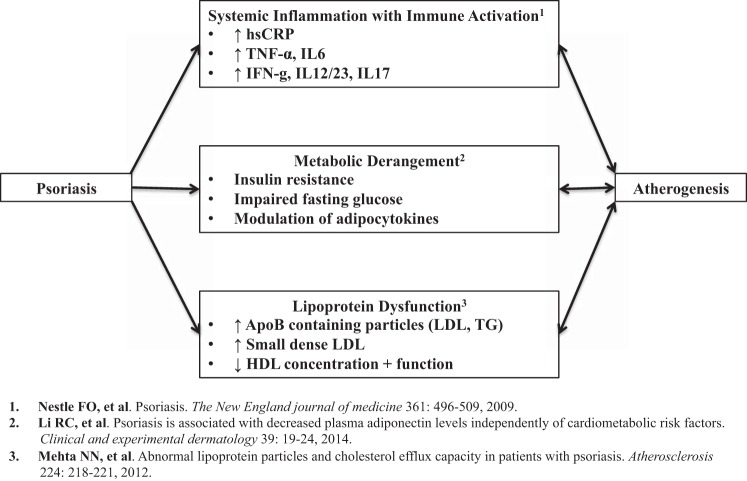
The current working hypothesis for the link between psoriasis and atherosclerosis revolves around three main factors: systemic inflammation (50) with immune activation (6, 7), metabolic derangement (12, 35), and lipoprotein dysfunction (42) (Fig. 1). Each of these factors has been well studied and known to be involved in the development of atherosclerosis and is beyond the scope of this review.
Fig. 1.
Potential pathways involved in accelerated atherogenesis observed in psoriasis. TG, triglycerides.
As the intricate details about psoriasis pathogenesis are being discovered, newer therapies targeting these pathways are also evolving. However, the lack of causal therapy remains a major problem with this disease and its high population burden (57). The available therapies at this point can be categorized as topical, phototherapy, and systemic/biological therapies. While topical and phototherapy have been in practice for the last few decades, systemic/biological therapies are novel and have shown potential in clearing the skin inflammation associated with psoriasis (23). The primary therapies currently in use are methotrexate, TNF inhibitors/blockers, anti-IL-17A agents, and anti-IL-12/23 agents. All of these therapies, especially biological therapy, have shown promise in clearing skin inflammation and improving the quality of life profoundly (61).
Multimodal and immunomodulatory drugs, such as statins, have been shown to reduce atherosclerotic plaque inflammation in patients at risk. In fact, a recent study (58) was the first to show that statin therapy leads to a reduction in the inflammation of the coronary arteries and that this anti-inflammatory impact of statins is substantially more in advanced coronary plaques. However, the anti-inflammatory effects of statins are yet to be studied in detail in psoriasis patients (62).
Inflammation and CVD
Inflammation measured by high-sensitivity C-reactive protein (CRP), a nonspecific marker of inflammation, is associated with future adverse events related to atherosclerosis (52, 54). Despite its utility in the general population, the clinical usefulness of CRP in CVD has been questioned during the last decade, specifically among patients with preexisting inflammatory conditions. Emerging literature suggests that CRP may not precisely capture the elevated risk of CVD in patients with preexisting inflammatory conditions such as systemic lupus erythematosus (29), psoriasis (59), rheumatoid arthritis (15), and human immunodeficiency virus (60). As an alternative to CRP, we recently showed that a newer biomarker of systemic inflammation, GlycA, predicted subclinical atherosclerosis in the form of vascular inflammation by 18fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) and coronary artery disease by coronary CT angiography (CCTA) in psoriasis (28).
Inflammation has come to the forefront of cardiovascular medicine such that there are two large, major ongoing trials aiming to assess the effect of anti-inflammatory treatment on prospective cardiovascular outcomes: 1) the Cardiovascular Inflammation Reduction Trial, which aims to evaluate if low-dose methotrexate will modulate major vascular events compared with placebo in postmyocardial infarction patients with metabolic syndrome or diabetes (16), and 2) the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study, which aims to understand if IL-1β inhibition by canakinumab compared with placebo reduces cardiovascular events among stable coronary artery disease patients who are at high cardiovascular risk as assessed as a persistent increase in CRP (≥2 mg/l) despite existent secondary prevention strategies (53).
Psoriasis as a Model to Study Inflammatory Atherogenesis
Lipid composition and function are modulated in psoriasis.
While the aforementioned trials regarding the association of inflammation with cardiometabolic dysfunction will be highly informative, they will take nearly a decade from initiation to conclusion and will not elucidate mechanisms of disease. Therefore, a human inflammatory model of disease such as psoriasis provides a model with several features that facilitate understanding of inflammatory cardiometabolic dysfunction and abnormal lipoprotein composition (42). Using nuclear magnetic resonance spectroscopy, it has been shown that patients with psoriasis had more intermediate-density lipoproteins as well as small, dense LDL particles compared with controls, suggesting a more atherogenic lipoprotein profile in psoriasis. In addition to composition, the function of lipids is also modulated in psoriasis. HDLs are beneficial as they perform reverse cholesterol transport to facilitate the excretion of cholesterol in the liver. Cholesterol efflux capacity, which is an estimate of HDL reverse cholesterol transport, can be estimated using an in vitro radiolabeled cholesterol assay (30). Compared with healthy control subjects, psoriasis patients have significantly lower levels of not only HDL concentration but also decreased HDL efflux capacity beyond CVD risk factors (42).
Psoriasis affects immune cells, which are known to be associated with atherosclerotic CVD.
There are many key immune factors that are important in psoriasis that overlap with atherosclerosis. It is well known that cell-mediated immunity has been shown to augment the atherosclerotic process, specifically via activated T cells, macrophages, and the response to injury. Immune cells, in response to various stimuli, produce cytokines (e.g., interferon-α, interferon-γ, IL-1β, IL-6, and TNF-α) that may be excessively secreted in autoimmune disease states such as psoriasis. Increased cytokine levels lead to activation of myeloid dendritic cells, which, in turn, present antigens and secrete additional cytokines leading to the differentiation of Th cells (Th1 and Th17) (38). T cells secrete intermediates (e.g., IL-17F, IL-17A, and IL-22), which, in turn, activate keratinocytes and stimulate more proinflammatory cytokines, antimicrobial peptides, chemokines, and S100 proteins (38). We also know that IL-17A, IL-1β, and TNF-α are proinflammatory cytokines that play a role in atherosclerosis by driving cell-mediated immunity. In addition to many other cytokines, these three inflammatory mediators are also known to be secreted by activated keratinocytes in psoriasis, which further supports the biological link between psoriasis and atherosclerosis (7).
Features of psoriasis that facilitate discovery.
Psoriasis is a common chronic inflammatory disease associated with an elevated burden of cardiovascular risk, CVD, and cardiovascular morbidity and mortality. Additionally, psoriasis pathogenesis has multiple shared pathways that link this chronic inflammatory condition with atherosclerosis, which is now known to be an immune-driven process with inflammation at its core. Furthermore, observational evidence has revealed that modulating these common pathways in psoriasis has a two-pronged benefit by alleviating skin inflammation while clearing skin disease and by simultaneously mitigating the subsequent cardiovascular risk. Finally, novel imaging modalities such as 18FDG-PET/CT allow for the quantification of directly visualized inflammation in the skin, joints, and blood vessels among psoriasis patients (5, 43, 45), thus providing a reliable surrogate to assess the future cardiovascular risk in this vulnerable population. Collectively, careful phenotyping of this population before and after therapy for psoriasis can be high yield for augmenting pathophysiological understanding of not only psoriasis but of atherosclerosis as well.
Psoriasis has proven to be a fruitful and reliable clinical human model to understand inflammatory cardiometabolic disease. As an inflammatory disease associated with early cardiovascular events, psoriasis is increasingly being recognized as a risk factor for developing inflammatory atherogenesis and can be used to study the relationship between inflammation and atherogenesis. Additionally, there are several other autoimmune conditions like systemic lupus erythematosus (26), chronic kidney disease (24), diabetes mellitus (27), rheumatoid arthritis (49), and inflammatory bowel disease (64) that also accelerate atherosclerosis through an increase in inflammatory signaling. These disease states require acute therapy for patient safety, thereby limiting the ability of clinical trials from having a placebo group. Moreover, the drugs used to treat these conditions are generally not targeted cytokine therapies as in the case of psoriasis, thereby limiting mechanistic understanding of treatment effects. Additionally, it is ethical to have a placebo controlled arm in psoriasis studies, which is not possible with disease states such as rheumatoid arthritis and inflammatory bowel disease. This is because treatment naïve rheumatoid arthritis is associated with destruction of the joint cavity (48) and patients with inflammatory bowel disease (64) have a poor quality of life without treatment. Psoriasis is also associated with vascular disease events a decade earlier than those without psoriasis. Furthermore, severe renal disease (24) and diabetic states (27) increase the calcification and production of insulin, which have been vasculopathic through immune-mediated mechanisms. The goal of our program is to study inflammatory atherogenesis from an immune-mediated standpoint to potentially discover targeted pathways to ameliorate immune-mediated atherosclerosis.
The Psoriasis Atherosclerosis Cardiometabolic Diseases Initiative.
To further study this relationship between inflammation and cardiometabolic diseases in psoriasis, we initiated a 4-yr longitudinal natural history cohort study recruiting patients with psoriasis [the Psoriasis Atherosclerosis Cardiometabolic Diseases Initiative (PACI)]. Furthermore, we compared them with healthy control subjects, diabetes patients, and patients with known coronary artery disease to provide context to the elevated cardiovascular risk in psoriasis patients. Our cohort of psoriasis patients is followed and deeply phenotyped during this 4-yr period, providing information at critical time points during the progression of their chronic inflammatory state. Additionally, we comprehensively evaluate psoriasis patients during a flare, thus providing significant findings regarding the immune system overdrive during these episodes and how they may modulate the patients’ cardiovascular risk. We evaluate the effect of this flare by quantifying vascular inflammation by 18FDG-PET/CT and comparing it with the preflare visit. We also evaluate the change in HDL function and inflammatory proteins during this visit. Careful evaluation at flare visits compared with earlier visits permit characterization of the impact of the immune system on the modulation of subclinical vascular disease in psoriasis to understand the possible mechanisms behind the elevated cardiovascular risk. Phenotyping of our cohort involves three main components: vascular imaging of multiple beds using 18FDG-PET/CT, 18FDG-PET/MRI, and CCTA, blood assays for HDL function, inflammatory mediators and isolated cells from whole blood, and skin and adipose tissue for characterization. These studies each provide a unique foundation of our scientific program to study pathophysiology and ask questions related to inflammatory atherogenesis.
One of our study outcomes is vascular inflammation, which is quantified by vascular imaging of multiple beds using 18FDG-PET/CT. Vascular inflammation by 18FDG-PET/CT has been shown to be a reliable surrogate biomarker of cardiovascular risk (43) and is highly sensitive to modulation in risk factors with preventive strategies like statin therapy (62) and beneficial lifestyle changes (34), which are known to reduce cardiovascular risk. Another important outcome in our study is the burden of coronary artery disease analyzed by CCTA, which is validated against intravascular ultrasound (17) and provides a reliable framework to understand how emerging biomarkers and biological pathways may relate to in vivo coronary plaque (56).
Vascular imaging in PACI with a focus on 18FDG uptake, vascular inflammation, and PET MRI.
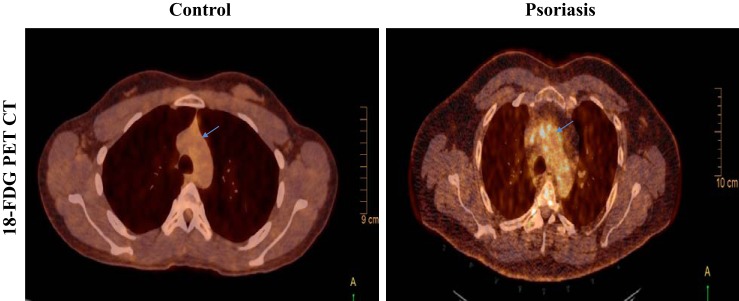
We have carefully selected a comprehensive multimodality imaging study panel to permit characterization of vascular disease at early (18FDG-PET/CT-derived vascular inflammation and CCTA-derived noncalcified burden of coronary artery disease), middle [18FDG-PET/MRI wall thickness and aortic distensibility (AD)], and late (coronary artery-dense Ca2+ burden) stages of atherosclerosis, which allow us to gain deeper insights into the underlying pathophysiology of atherosclerosis at various stages in the context of chronic inflammation in psoriasis patients. 18FDG-PET/CT and 18FDG-PET/MRI help assess the inflammatory burden in the aortic wall, whereas CCTA provides an estimate of the coronary artery disease burden and characterization of the subtypes of the coronary plaques, which serve as surrogate outcomes in our study since they have been shown to be associated with prospective cardiovascular events in large population-based studies (46). A higher amount of 18FDG is taken up by cells that are metabolically more active and therefore can be used as a novel biomarker to assess inflammation (10). Additionally, it can be used to quantify vascular inflammation in vivo as a target-to-background ratio that provides an idea of the burden of activated innate immune cells in the arterial wall causing inflammation, such as macrophages and neutrophils (13, 19). Using 18FDG-PET/CT, we have demonstrated that psoriasis patients have increased vascular inflammation of the entire aorta and that the strongest signal for increased vascular inflammation was in the aortic arch (Fig. 2) (43). 18FDG-PET/MRI offers an additive advantage of soft tissue localization with no additional radiation and the advantages of novel MRI sequences (14). Furthermore, 18FDG-PET/MRI is obtained at a delayed time point providing a longer duration for the tracer, 18FDG, to localize in the arterial wall and clear from the bloodstream and, therefore, may perhaps capture the arterial wall inflammation more precisely than 18FDG-PET/CT. Finally, MRI can also estimate AD, which correlates with aortic stiffness, a known predictor of cardiovascular events beyond risk factors (4). Cardiovascular disease also decreases AD compared with healthy control subjects, and it has been shown in the past that AD by MRI is associated with the severity of CVD (11).
Fig. 2.
Representative images from 18fluorodeoxyglucose-positron emission tomography computed tomography (18FDG-PET/CT) scans in a healthy volunteer control (left) and a patient with psoriasis (right) exhibiting increased uptake of 18FDG at the level of aortic arch (blue arrow) as seen in the transverse section images. The images shown here are from the fused PET scans.
While obtaining the images for 18FDG-PET/MRI, we also obtain high-frequency phase-contrast images for one complete cardiac cycle, which helps us derive the estimates of descending aortic wall thickness that provides an idea about the aortic stiffness in psoriasis patients. To measure wall thickness, both the outer and inner wall boundaries of the descending aorta are traced for each MRI slice during a cardiac cycle. The area between these boundaries is averaged over multiple transverse slices obtained during a cardiac cycle, which yields an index of wall thickness. We then calculate plaque index, another measure of wall thickness, by dividing wall volume by total vessel volume. Additionally, we measure AD by tracing the contours of the descending aorta throughout one cardiac cycle, which further help assess the maximum and minimum cross-sectional area of the aorta, and dividing this difference by the pulse pressure generates a value that provides an assessment of AD. Applying these measurements to our psoriasis patient sample, we are currently examining the relationship between vascular inflammation and anatomic as well as functional characteristics of the aorta.
CCTA provides a platform to measure the coronary artery burden before luminal disease in psoriasis.
Using CCTA on a 320-row detector scanner (320 detector row Aquilion ONE VISION, Toshiba, Japan), we apply dedicated quantitative CTA software to quantify the coronary plaque burden in each of the three major coronary arteries in patients with coronary artery disease as well as psoriasis patients. We begin with the clinical reads of each of the CCTA scans that provide a measure of coronary artery Ca2+ as mean Agatston score. We then use dedicated software (Q Angio CT, Medis, Leiden, The Netherlands) to assesses plaque volume by performing a semiautomated analysis of outer wall characterization as well as luminal characterization on a series of multiplanar reconstructed images of each of the major coronary arteries, which facilitates the calculation of plaque volumes (volume under the outer wall – volume under the luminal border) and plaque index, which is calculated by dividing the sum of all plaque volumes in a given segment by the length of that segment [plaque index = (sum of all plaque volumes of segment)/(length of segment)]. We further assess plaque burden by stratifying it into calcified and noncalcified plaques based on previously defined standardized Hounsfield unit values (33, 56). CCTA is also a valuable tool as it provides an opportunity to further characterize coronary plaques as high-risk plaques based on standardized definitions (46). Characterization of plaque morphology in psoriasis patients and comparison of high-risk plaque prevalence across groups of psoriasis patients, patients with hyperlipidemia who are at high risk for myocardial infarction, and healthy control subjects has enabled us to gain deeper understanding of why psoriasis patients have a high risk of first myocardial infarction at a young age. The analysis of lipid-rich plaques, which are usually prone to rupture and cause myocardial infarction (39), has helped us understand the relationship between coronary artery disease and cholesterol efflux capacity (56). This exercise provided us with an important insights into the role of HDL in the pathogenesis of atherosclerosis and demonstrated that the function of this lipoprotein subtype is more critical than its concentration in the blood.
Summary and Future Directions
Psoriasis is a common, genetically driven immune-mediated condition with innate and adaptive immunity activation playing significant roles in its pathogenesis (57). It has a varied etiology including genetic as well as environmental factors such as infection, trauma, and medications (57). Psoriasis has been noted to have an increased prevalence of rheumatoid arthritis, celiac disease, and Crohn’s disease (23). Conversely, chronic systemic inflammation in psoriasis has been associated with the development of enthesitis and psychiatric and cardiovascular comorbidities (31).
Psoriasis is associated with immune cell dysfunction such as T cells and neutrophils, which are critical to atherosclerosis (23). More research into the pathophysiology of psoriasis has led to the development of novel systemic and biological therapies for psoriasis. Because of the lack of causal therapy, there has been an increasing search and need for newer biological agents and immune-response modifiers to tackle the current 2–4% (likely an underestimate) (23) prevalence of psoriasis. Also, our understanding of psoriasis as a risk factor of CVD needs further investigation, since it is unclear whether modulating immune activation with systemic and biological therapy can mitigate cardiovascular morbidity and mortality. In addition to this, the role of statins has also not been studied in detail in patients with psoriasis. Continued followup and evaluation of treatment effects on the disease will inform pathways important both in psoriasis and the understanding inflammatory atherogenesis.
As our natural history cohort study demonstrated increased utility in understanding pathophysiology, we also recognize the importance of analyzing how psoriasis treatments may have an impact on cardiovascular disease. Therefore, we have initiated multiple randomized controlled trials, namely, the Vascular Inflammation in Psoriasis (VIP) trials. The broad objective of these trials is to understand whether the treatment of severe psoriasis with novel biological therapies targeting the cytokine pathways involved in both the pathogenesis of psoriasis and atherosclerosis leads to an improvement in vascular inflammation and cardiovascular biomarkers. Specifically, the VIP NCT01866592 study is a double-blind, placebo-controlled trial of 96 patients enrolled under three arms studying adalimumab, a TNF inhibitor used to treat plaque psoriasis, versus phototherapy with ultraviolet light B versus placebo at 12 wk. Our main outcome is vascular inflammation by 18FDG-PET/CT, and we intend to analyze the effect of the psoriasis treatment on vascular inflammation at 12-wk followup. The VIP-E (NCT01866592) study is an open-label extension trial of VIP wherein all 96 patients enrolled under the VIP trial received TNF inhibitor therapy beyond the stipulated duration of 12 wk and underwent treatment with TNF inhibitor until the end of 52 wk from initial enrollment regardless of their primary randomization. The results of VIP-E will assist us to understand if there are durable effects of TNF inhibitor therapy on vascular inflammation over a longer duration.
GRANTS
The work described here was funded by National Heart, Lung, and Blood Institute Grant HL-006193-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.L.H. and N.N.M. conceived and designed research; C.L.H. and A.K.D. prepared figures; C.L.H., A.K.D., R.Y., A.A.J., and N.N.M. drafted manuscript; C.L.H., A.K.D., R.Y., A.A.J., and N.N.M. edited and revised manuscript; C.L.H., A.K.D., R.Y., A.A.J., and N.N.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ahmed Sadek for one figure included in the paper.
REFERENCES
- 1.Alexandroff AB, Pauriah M, Camp RD, Lang CC, Struthers AD, Armstrong DJ. More than skin deep: atherosclerosis as a systemic manifestation of psoriasis. Br J Dermatol 161: 1–7, 2009. doi: 10.1111/j.1365-2133.2009.09281.x. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol 149: 84–91, 2013. doi: 10.1001/2013.jamadermatol.406. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003−2011. JAMA Dermatol 149: 1180–1185, 2013. doi: 10.1001/jamadermatol.2013.5264. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646, 2014. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bissonnette R, Tardif JC, Harel F, Pressacco J, Bolduc C, Guertin MC. Effects of the tumor necrosis factor-α antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging 6: 83–90, 2013. doi: 10.1161/CIRCIMAGING.112.975730. [DOI] [PubMed] [Google Scholar]
- 6.Boehncke S, Salgo R, Garbaraviciene J, Beschmann H, Hardt K, Diehl S, Fichtlscherer S, Thaçi D, Boehncke WH. Effective continuous systemic therapy of severe plaque-type psoriasis is accompanied by amelioration of biomarkers of cardiovascular risk: results of a prospective longitudinal observational study. J Eur Acad Dermatol Venereol 25: 1187–1193, 2011. doi: 10.1111/j.1468-3083.2010.03947.x. [DOI] [PubMed] [Google Scholar]
- 7.Boehncke WH, Boehncke S, Tobin AM, Kirby B. The ‘psoriatic march’: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol 20: 303–307, 2011. doi: 10.1111/j.1600-0625.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 8.Boehncke WH, Gladman DD, Chandran V. Cardiovascular comorbidities in psoriasis and psoriatic arthritis: pathogenesis, consequences for patient management, and future research agenda: a report from the GRAPPA 2009 annual meeting. J Rheumatol 38: 567–571, 2011. doi: 10.3899/jrheum.101124. [DOI] [PubMed] [Google Scholar]
- 9.Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case-control analysis. Br J Dermatol 160: 1048–1056, 2009. doi: 10.1111/j.1365-2133.2008.09020.x. [DOI] [PubMed] [Google Scholar]
- 10.Bural GG, Torigian DA, Chamroonrat W, Alkhawaldeh K, Houseni M, El-Haddad G, Alavi A. Quantitative assessment of the atherosclerotic burden of the aorta by combined FDG-PET and CT image analysis: a new concept. Nucl Med Biol 33: 1037–1043, 2006. doi: 10.1016/j.nucmedbio.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Choudhury RP, Birks JS, Mani V, Biasiolli L, Robson MD, L’Allier PL, Gingras MA, Alie N, McLaughlin MA, Basson CT, Schecter AD, Svensson EC, Zhang Y, Yates D, Tardif JC, Fayad ZA. Arterial effects of canakinumab in patients with atherosclerosis and type 2 diabetes or glucose intolerance. J Am Coll Cardiol 68: 1769–1780, 2016. doi: 10.1016/j.jacc.2016.07.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology 216: 152–155, 2008. doi: 10.1159/000111512. [DOI] [PubMed] [Google Scholar]
- 13.Courtois A, Nusgens BV, Hustinx R, Namur G, Gomez P, Somja J, Defraigne JO, Delvenne P, Michel JB, Colige AC, Sakalihasan N. 18F-FDG uptake assessed by PET/CT in abdominal aortic aneurysms is associated with cellular and molecular alterations prefacing wall deterioration and rupture. J Nucl Med 54: 1740–1747, 2013. doi: 10.2967/jnumed.112.115873. [DOI] [PubMed] [Google Scholar]
- 14.Einspieler I, Thürmel K, Pyka T, Eiber M, Wolfram S, Moog P, Reeps C, Essler M. Imaging large vessel vasculitis with fully integrated PET/MRI: a pilot study. Eur J Nucl Med Mol Imaging 42: 1012–1024, 2015. doi: 10.1007/s00259-015-3007-8. [DOI] [PubMed] [Google Scholar]
- 15.Emami H, Vijayakumar J, Subramanian S, Vucic E, Singh P, MacNabb MH, Corsini E, Hoffmann U, Bathon JM, Solomon DH, Tawakol A. Arterial 18F-FDG uptake in rheumatoid arthritis correlates with synovial activity. JACC Cardiovasc Imaging 7: 959–960, 2014. doi: 10.1016/j.jcmg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 166: 199–207.e115, 2013. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer C, Hulten E, Belur P, Smith R, Voros S, Villines TC. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: a meta-analysis. J Cardiovasc Comput Tomogr 7: 256–266, 2013. doi: 10.1016/j.jcct.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Fleming P, Kraft J, Gulliver WP, Lynde C. The relationship of obesity with the severity of psoriasis: a systematic review. J Cutan Med Surg 19: 450–456, 2015. doi: 10.1177/1203475415586332. [DOI] [PubMed] [Google Scholar]
- 19.Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, Di Carli MF, Libby P. Hypoxia but not inflammation augments glucose uptake in human macrophages: implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-d-glucose positron emission tomography. J Am Coll Cardiol 58: 603–614, 2011. doi: 10.1016/j.jacc.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 20.Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, Troxel AB. The risk of stroke in patients with psoriasis. J Invest Dermatol 129: 2411–2418, 2009. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 296: 1735–1741, 2006. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 22.Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, Margolis DJ, Strom BL. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol 143: 1493–1499, 2007. doi: 10.1001/archderm.143.12.1493. [DOI] [PubMed] [Google Scholar]
- 23.Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, Mehta NN, Finlay AY, Gottlieb AB. Psoriasis. Nat Rev Dis Primers 2: 16082, 2016. doi: 10.1038/nrdp.2016.82. [DOI] [PubMed] [Google Scholar]
- 24.Grunwald JE, Pistilli M, Ying G-S, Maguire M, Daniel E, Whittock-Martin R, Parker-Ostroff C, Mohler E, Lo JC, Townsend RR, Gadegbeku CA, Lash JP, Fink JC, Rahman M, Feldman H, Kusek JW, Xie D; CRIC Study Investigators . Retinopathy and the risk of cardiovascular disease in patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort study). Am J Cardiol 116: 1527–1533, 2015. doi: 10.1016/j.amjcard.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gyldenløve M, Storgaard H, Holst JJ, Vilsbøll T, Knop FK, Skov L. Patients with psoriasis are insulin resistant. J Am Acad Dermatol 72: 599–605, 2015. doi: 10.1016/j.jaad.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses’ health study. Arthritis Rheum 61: 1396–1402, 2009. doi: 10.1002/art.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanley AJG, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care 25: 1177–1184, 2002. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- 28.Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, Krishnamoorthy P, Ng Q, Aridi TZ, Salahuddin T, Natarajan B, Lockshin BN, Ahlman MA, Chen MY, Rader DJ, Reilly MP, Remaley AT, Bluemke DA, Playford MP, Gelfand JM, Mehta NN. GlycA is a novel biomarker of inflammation and subclinical cardiovascular disease in psoriasis. Circ Res 119: 1242–1253, 2016. doi: 10.1161/CIRCRESAHA.116.309637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kay SD, Poulsen MK, Diederichsen AC, Voss A. Coronary, carotid, and lower-extremity atherosclerosis and their interrelationship in Danish patients with systemic lupus erythematosus. J Rheumatol 43: 315–322, 2016. doi: 10.3899/jrheum.150488. [DOI] [PubMed] [Google Scholar]
- 30.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364: 127–135, 2011. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim N, Thrash B, Menter A. Comorbidities in psoriasis patients. Semin Cutan Med Surg 29: 10–15, 2010. doi: 10.1016/j.sder.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Kimball AB, Szapary P, Mrowietz U, Reich K, Langley RG, You Y, Hsu MC, Yeilding N, Rader DJ, Mehta NN. Underdiagnosis and undertreatment of cardiovascular risk factors in patients with moderate to severe psoriasis. J Am Acad Dermatol 67: 76–85, 2012. doi: 10.1016/j.jaad.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 33.Kwan AC, May HT, Cater G, Sibley CT, Rosen BD, Lima JAC, Rodriguez K, Lappe DL, Muhlestein JB, Anderson JL, Bluemke DA. Coronary artery plaque volume and obesity in patients with diabetes: the factor-64 study. Radiology 272: 690–699, 2014. doi: 10.1148/radiol.14140611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med 49: 1277–1282, 2008. doi: 10.2967/jnumed.108.052233. [DOI] [PubMed] [Google Scholar]
- 35.Li RC, Krishnamoorthy P, DerOhannessian S, Doveikis J, Wilcox M, Thomas P, Rader DJ, Reilly MP, Van Voorhees A, Gelfand JM, Mehta NN. Psoriasis is associated with decreased plasma adiponectin levels independently of cardiometabolic risk factors. Clin Exp Dermatol 39: 19–24, 2014. doi: 10.1111/ced.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 368: 2004–2013, 2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 37.Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis . Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 54: 2129–2138, 2009. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 105: 1135–1143, 2002. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 39.Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 11: 390–402, 2014. doi: 10.1038/nrcardio.2014.60. [DOI] [PubMed] [Google Scholar]
- 40.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J 31: 1000–1006, 2010. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta NN, Li K, Szapary P, Krueger J, Brodmerkel C. Modulation of cardiometabolic pathways in skin and serum from patients with psoriasis. J Transl Med 11: 194, 2013. doi: 10.1186/1479-5876-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehta NN, Li R, Krishnamoorthy P, Yu Y, Farver W, Rodrigues A, Raper A, Wilcox M, Baer A, DerOhannesian S, Wolfe M, Reilly MP, Rader DJ, VanVoorhees A, Gelfand JM. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis 224: 218–221, 2012. doi: 10.1016/j.atherosclerosis.2012.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehta NN, Torigian DA, Gelfand JM, Saboury B, Alavi A. Quantification of atherosclerotic plaque activity and vascular inflammation using [18-F] fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT). J Vis Exp 2012: e3777, 2012. doi: 10.3791/3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta NN, Yu Y, Pinnelas R, Krishnamoorthy P, Shin DB, Troxel AB, Gelfand JM. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med 124: 775.e1–775.e6, 2011. doi: 10.1016/j.amjmed.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehta NN, Yu Y, Saboury B, Foroughi N, Krishnamoorthy P, Raper A, Baer A, Antigua J, Van Voorhees AS, Torigian DA, Alavi A, Gelfand JM. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol 147: 1031–1039, 2011. doi: 10.1001/archdermatol.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H, Naruse H, Ishii J, Hecht H, Shaw LJ, Ozaki Y, Narula J. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 66: 337–346, 2015. doi: 10.1016/j.jacc.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 47.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 361: 496–509, 2009. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 48.Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, Troxel AB, Hennessy S, Kimmel SE, Margolis DJ, Choi H, Mehta NN, Gelfand JM. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis 74: 326–332, 2015. doi: 10.1136/annrheumdis-2014-205675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, Troxel AB, Hennessy S, Kimmel SE, Margolis DJ, Choi H, Mehta NN, Gelfand JM. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis 74: 326–332, 2015. doi: 10.1136/annrheumdis-2014-205675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol 145: 700–703, 2009. doi: 10.1001/archdermatol.2009.94. [DOI] [PubMed] [Google Scholar]
- 51.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 70: 512–516, 2014. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 49: 2129–2138, 2007. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 53.Ridker PM. Closing the loop on inflammation and atherothrombosis: why perform the CIRT and CANTOS trials? Trans Am Clin Climatol Assoc 124: 174–190, 2013. [PMC free article] [PubMed] [Google Scholar]
- 54.Ridker PM. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. Am Heart J Suppl 148: S19–S26, 2004. doi: 10.1016/j.ahj.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 55.Rose S, Stansky E, Dagur PK, Samsel L, Weiner E, Jahanshad A, Doveikis J, Naik HB, Playford MP, McCoy JP, Mehta NN. Characterization of immune cells in psoriatic adipose tissue. J Transl Med 12: 258, 2014. doi: 10.1186/s12967-014-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salahuddin T, Natarajan B, Playford MP, Joshi AA, Teague H, Masmoudi Y, Selwaness M, Chen MY, Bluemke DA, Mehta NN. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. Eur Heart J 36: 2662–2665, 2015. doi: 10.1093/eurheartj/ehv339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schön MP, Boehncke W-H. Psoriasis. N Engl J Med 352: 1899–1912, 2005. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 58.Singh P, Emami H, Subramanian S, Maurovich-Horvat P, Marincheva-Savcheva G, Medina HM, Abdelbaky A, Alon A, Shankar SS, Rudd JHF, Fayad ZA, Hoffmann U, Tawakol A. Coronary plaque morphology and the anti-inflammatory impact of atorvastatin: a multicenter 18F-fluorodeoxyglucose positron emission tomographic/computed tomographic study. Circ Cardiovasc Imaging 9: e004195–e004195, 2016. doi: 10.1161/CIRCIMAGING.115.004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Staniak HL, Bittencourt MS, de Souza Santos I, Sharovsky R, Sabbag C, Goulart AC, Lotufo PA, Benseñor IM. Association between psoriasis and coronary calcium score. Atherosclerosis 237: 847–852, 2014. doi: 10.1016/j.atherosclerosis.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, Williams KC, Lo J, Grinspoon SK. Arterial inflammation in patients with HIV. JAMA 308: 379–386, 2012. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi H, Iinuma S, Tsuji H, Honma M, Iizuka H. Biologics are more potent than other treatment modalities for improvement of quality of life in psoriasis patients. J Dermatol 41: 686–689, 2014. doi: 10.1111/1346-8138.12544. [DOI] [PubMed] [Google Scholar]
- 62.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, Nunes IO, Beals CR, Shankar SS. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol 62: 909–917, 2013. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 63.Yu Y, Sheth N, Krishnamoorthy P, Saboury B, Raper A, Baer A, Ochotony R, Doveikis J, Derohannessian S, Voorhees AS, Torigian DA, Alavi A, Gelfand JM, Mehta NN. Aortic vascular inflammation in psoriasis is associated with HDL particle size and concentration: a pilot study. Am J Cardiovasc Dis 2: 285–292, 2012. [PMC free article] [PubMed] [Google Scholar]
- 64.Zuin M, Rigatelli G, Del Favero G, Andreotti AN, Picariello C, Zuliani G, Carraro M, Galasso MP, Roncon L. Cardiovascular disease in patients with inflammatory bowel disease: An issue in no guidelines land. Int J Cardiol 222: 984–985, 2016. doi: 10.1016/j.ijcard.2016.08.101. [DOI] [PubMed] [Google Scholar]