Abstract
B cells have emerged as important immune cells in cardiovascular disease. Initial studies have suggested that B cells protect against atherosclerosis development. However, subsequent studies demonstrating aggravation of atherosclerosis by B-2 cells have shed light on the subset-dependent effects of B cells. Here, we review the literature that has led to our current understanding of B cell regulation of atherosclerosis, touching on the importance of subsets, local regulation, human translation, and therapeutic potential.
Keywords: B cells, IgM, atherosclerosis, inflammation
atherosclerosis is a chronic inflammatory disease of major blood vessels and the primary underlying cause of cardiovascular disease (CVD). Circulating levels of LDL have been considered a major risk factor for atherosclerosis in humans. LDL accumulates in the artery wall, where it becomes oxidized (OxLDL), one of the major triggering events in the initiation of atherosclerotic lesion formation. OxLDL and other phospholipids (oxidative phospholipids) generate neoepitopes, termed oxidation-specific epitopes (10). These neoepitopes are recognized by both innate and adaptive immune cells, triggering a cascade of further events mediated by cytokines and chemokines that recruit more immune cells and further lipid accumulation. Together with immune cell recruitment, migration and proliferation of smooth muscle cells, apoptosis of foam cells, and the development of necrotic cores eventually lead to the formation of advanced lesions (36, 77, 101). Atherosclerotic lesions contain many immune cells, such as macrophages, mast cells, natural killer cells, T cells, and natural killer T cells, in the subendothelial space, i.e., the intima of the arterial wall (36, 102). In addition, immune cells, such as B cells, T cells, macrophages, and dendritic cells (DCs), occupy the vascular adventitia, perivascular adipose tissue (PVAT), and artery tertiary lymphoid organs (ATLOs) (30, 44, 69, 75, 91). The identification of how these cells regulate the response to oxidative phospholipids and other atherogenic stimuli is of key importance, and prior reviews have provided excellent overviews of this process (36, 37, 102). This review will focus on the role of B cells in atherosclerosis.
B Cells
B cells are lymphocytes that play important roles in both innate and adaptive immunity through both antibody (Ab) production and cytokine secretion. B cells are divided into two subtypes: B-1 and B-2 B cells.
B-1 B cells are innate-like B cells that produce natural Abs (NAbs). As B-1 cells show a rather restricted Ig variable region gene use, they undergo relatively limited or no affinity maturation, and their Abs show broad specificities with low-binding affinities. Interestingly, however, B-1 cells proliferate in response to self-antigens (Ags) and also form pools of short-lived, self-renewing B cells that produce most of the circulating NAbs of the IgM and IgA classes (5). Thus, B-1 cells are a subclass of B lymphocytes that are involved in innate humoral immune responses. B-1 cells arise predominantly from precursors in the fetal liver and constitute the earliest wave of mature peripheral B cells, undergoing self-renewal in the periphery. Recent studies have shown that B-1 cells can also arise from precursors in the bone marrow (BM) (Fig. 1) (23, 68). In adult mice, B-1 cells constitute a minor fraction (2–3%) of total B cells in secondary lymphoid organs (SLOs), such as the spleen, lymph nodes (LNs), and Peyer’s patches, but are abundant in the pleural and peritoneal cavities (50–70%) (38, 55). However, B-1 cells do not produce much Ab in the pleural and peritoneal cavities but migrate to the spleen for Ab production (Fig. 1) (15, 49, 104). Murine B-1 cells can be divided into B-1a (CD5+) and B-1b (CD5−) subtypes, based on CD5 expression (Fig. 1) (8). B-1a cells are important for the spontaneous production of IgM. Recent studies have shown that B-1b cells can confer T cell-independent, long-lasting, unmutated IgM memory to pathogens, such as Borrelia hermsii and Streptococcus pneumoniae (2, 33), and pathogen-associated polysaccharide Ag α(1,3)-dextran (26). The B-1b subtype shows IgA isotype switch capability and displays comparably high frequencies of somatic mutations in IgA-associated heavy-chain variable regions compared with B-1a cells (85, 86). These data suggest that B-1a and B-1b subtypes are not only phenotypically different but also have divergent functions.
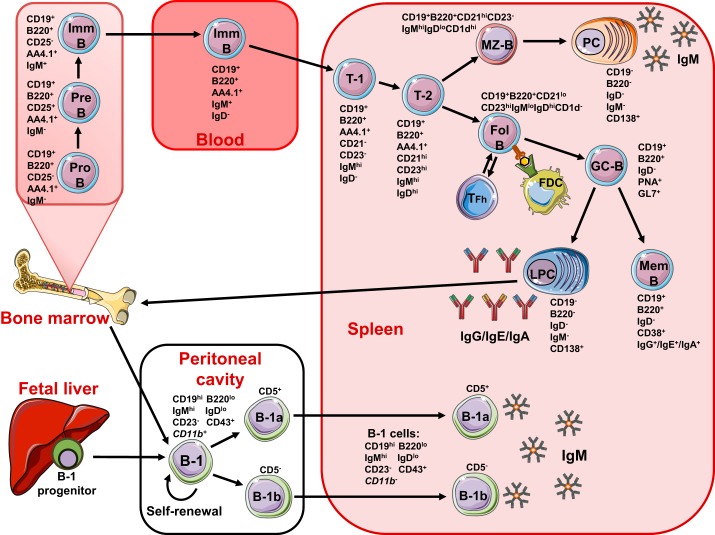
Fig. 1.
Schematic of murine B cell origin, development, and their surface markers expression. B cells are divided into two major subsets: B-1 and B-2 cells. B-2 cells arise from precursors in the bone marrow (BM). Common lymphoid progenitors in the BM differentiate into pre-B cell (Pre B), pro-B cell (Pro B), and immature B cells (Imm B). Immature B cells leave the BM, enter the bloodstream, and then travel to the spleen. Immature B cells undergo further transitional stages (T-1 and T-2) and then differentiate into mature marginal zone B cells (MZ-B) and follicular B cells (Fol B). After antigenic stimulation, MZ-B cells differentiate into IgM-secreting plasma cells (PCs). After antigen stimulation or presentation from follicular dendritic cells (FDCs) and with the help of follicular helper T cell (TFh), Fol-B cells enter into germinal center reactions (GC-B) followed by differentiation into memory B cells (Mem B) and antigen-specific, antibody-secreting long-lived PCs (LPCs). These LPCs migrate to the BM and stay for longer periods. B-1 B cells develop from B-1 precursors in the fetal liver and adult BM and migrate to and reside in the peritoneal and pleural cavity. B-1 cells are divided into CD5+ B-1a and CD5− B-1b cells. After activation, peritoneal B-1 cells lose surface CD11b expression and migrate to the spleen to secrete IgM.
B-2 cells are conventional B cells and participate in adaptive immune responses. B-2 cells arise from precursors in the BM. Common lymphoid progenitors in the BM differentiate into pre-B cell and pro-B cell stages by undergoing Ig heavy- and light-chain rearrangements and then develop into immature B cells. These immature B cells carry a mature B cell receptor (BCR) with unique specificity of IgM (6, 99). Immature B cells leave the BM, enter the bloodstream, and then travel to SLOs. Immature B cells undergo further transitional stages (T-1 and T-2) and then differentiate into mature B cells. These mature B cells differentiate into marginal zone (MZ)-B cells and follicular (Fol)-B cells (Fig. 1). MZ-B cells reside within the splenic marginal sinus. They mainly participate in the firstline defense against blood-borne pathogens and T cell-independent type II Ags, such as bacterial capsular polysaccharides, and differentiate into Ab-secreting plasma cells (PCs) (99). Due to participation in early immune responses, activation, and Ab production without T cell help, MZ-B cells are also considered innate immune cells. Fol-B cells are the major B-2 population in the periphery and differentiate into different B-2 cell subtypes during adaptive immune responses. Fol-B cells become activated by Ag stimulation and T cell help and then these activated B cells undergo germinal center (GC) reactions. B cells in GCs undergo class-switch recombination and somatic hypermutation steps and generate switched Ig and increased BCRs that are specific for Ag. These GC B cells undergo an affinity maturation and selection process for Ag with the help of Fol DCs and Fol helper T cells and then differentiate into Ag-specific, Ab-secreting, long-lived PCs or memory B cells (Fig. 1) (34). These PCs and memory B cells participate in long-lived, protective humoral immunity.
In addition to Ab-mediated immune responses, B cells regulate lymphoid tissue organization/development, modulate T cell and macrophage polarization, and regulate inflammatory reactions via secretion of discrete cytokines (57, 58, 89). Cytokine-producing B cells are referred to as “regulatory” B cells (Bregs) and “effector” B cells (25, 57, 58) and are not necessarily a unique subset but rather a functionally different phenotype derived from either B-1 or B-2 cells. IL-10-producing Bregs are called B-10 cells and have anti-inflammatory effects (103). IL-10, secreted by multiple cell types, including T cells, B cells, monocytes, macrophages, mast cells, eosinophils, and keratinocytes, is capable of suppressing both T cell helper types 1 and 2 polarized immune cells and inhibiting macrophage Ag presentation and proinflammatory cytokine production (4). Other studies have shown that Bregs can suppress inflammatory reactions by secreting other anti-inflammatory cytokines, such as transforming growth factor-β and IL-35 (74, 94, 100). Recently, Mauri and Menon (63) and Rosser and Mauri (83) reviewed Breg subtypes identified in both mice and humans and how these Breg subtypes can be induced in response to inflammation at different stages in development.
B Cells in Atherosclerosis
Evidence for a protective role.
B cells have emerged as important immune cells in the regulation of atherosclerosis. Caligiuri et al. (12) originally observed that after splenectomy, there was a reduction in B cells and aggravation of atherosclerosis in apolipoprotein E-deficient (ApoE−/−) mice. Immunomagnetic separation of spleen-derived T and B cells from diseased ApoE−/− mice and their adoptive transfer into splenectomized, young, nonatherosclerotic ApoE−/− mice, which were maintained on a high-fat diet (HFD) for 12 wk, have demonstrated that the atheroprotective effect was conferred by B cells. Consistent with these findings, LDL receptor-deficient (LDLR−/−) mice transplanted with BM from mice with a disrupted BCR gene [B cell deficient (µMT−/−)] exhibited a 30−40% increase in atherosclerotic lesions in the proximal and distal aortas compared with control mice [wild-type (WT) BM transplanted into LDLR−/−] (59). LDLR−/− mice reconstituted with BM cells from µMT−/− mice possess ≤1% of their normal B cell population, providing further evidence that B cells may protect from atherosclerosis development. Recent work from our group (18) demonstrated that adoptive transfer of splenic B cells reduced diet-induced atherosclerosis in µMT−/− (Table 1). Prior passive and active immunization studies (3, 9, 11, 24, 27, 28, 72, 73, 106) have supported an atheroprotective role for B cell-derived Abs, particularly IgM. Exogenous immunization of rabbits with malondialdehyde-modified LDL (MDA-LDL) (73) or other OxLDL (3) reduced atherosclerosis. Immunization studies in murine models have confirmed Ag-specific Ab responses to immunization and attenuation of atherosclerosis (28, 106).
Table 1.
Summary of different experimental studies that have demonstrated the role of B cell subtypes in murine atherosclerosis
| Experiment (Reference) | Atherosclerosis |
|---|---|
| B cell transfers | |
| Total splenic B cells (12) | ↓↓ |
| B-2 cells (5 × 106) (52) | ↑↑ |
| B-1 cells (5 × 106) (52) | ↓↓ |
| B-2 cells (30 and 60 × 106) (18) | ↓↓ |
| B-1a cells (1 × 105) (53) | ↓↓ |
| B-1b cells (1 × 105) (82) | ↓↓ |
| Bregs (CD21hiCD23hiCD24hi) (93) | Neointima formation ↓↓ |
| Bone marrow transplantation | |
| µMT−/− compared with WT → LDLR−/− (59) | ↑↑ |
| BAFFR−/− compared with WT → LDLR−/− (88) | ↓↓ |
| B cell depletion therapy | |
| Anti-CD20 treatment (1, 52) | ↓↓ |
| Anti-BAFFR treatment (50) | ↓↓ |
| BAFFR deletion (51) | ↓↓ |
Bregs, regulatory B cells; µMT−/−, B cell-deficient mice; WT, wild-type; LDLR−/−, LDL receptor knockout; BAFFR, B cell-activating factor receptor.
Evidence for an atherogenic role.
B cell activating factor (BAFF) is important for B cell survival, activation, and differentiation by binding to its receptors, such as BAFF receptor (BAFFR), B cell maturation Ag, and transmembrane activator and calcium-modulating ligand interactor (TACI). Studies that use approaches that deplete predominantly B-2 cells, such as anti-CD20 Ab, anti-BAFFR mAb, and BAFFR deficiency (BAFFR−/−), attenuated atherosclerosis development in ApoE−/− and LDLR−/− mice (1, 50–52, 88). Kyaw et al. (52) demonstrated that B cell depletion by anti-CD20 Ab attenuated atherosclerosis development in ApoE−/− mice maintained on an HFD and that adoptive transfer of 5 × 106 B-2, but not B-1, B cells into total lymphocyte-deficient ApoE−/− mice (ApoE−/−, recombination activating gene 2 deficient, common cytokine receptor γ-chain deficient) and ApoE−/−, µMT−/− mice aggravated atherosclerotic lesion size and macrophage accumulation in young mice maintained on a HFD. Similar results were reported at the same time by Ait-Oufella et al. (1), who observed that B cell depletion by anti-CD20 Ab reduced atherosclerosis in young ApoE−/− and LDLR−/− mice maintained on a HFD (Fig. 2 and Table 1).
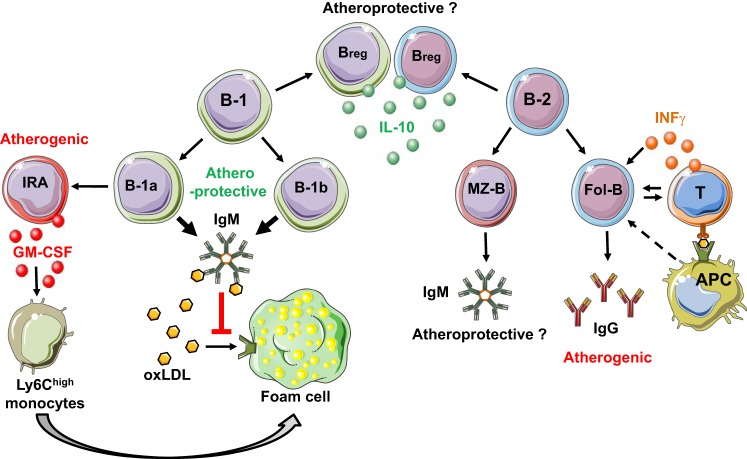
Fig. 2.
Simplified schematic of murine B cell subsets and their role in atherosclerosis. B cells are divided into two major subsets: B-1 and B-2 cells. B-1 cells (B-1a and B-1b) secrete natural IgM and attenuate diet-induced atherosclerosis. B-1a-derived innate response activator (IRA) B cells secrete granulocyte-macrophage colony-stimulating factor, which is important for Ly6Chi monocyte development, and promote atherosclerosis. B-2 cells are divided into MZ-B cells and Fol-B cells. Fol-B cells secrete IgG, stimulate inflammatory T cells (T), and aggravate atherosclerosis. The role of MZ-B cells and regulatory B cells (Bregs) in atherosclerosis is unclear. APC, antigen-presenting cells; INFγ, interferon-γ.
These two groups further demonstrated the impact of B-2 cells in promoting atherosclerosis by manipulating the BAFFR pathway. ApoE−/−/BAFFR−/− double-knockout mice showed reduced numbers of conventional B-2, but not B-1a, cells and attenuated atherosclerosis compared with control mice (ApoE−/−) maintained on a HFD (51). Treatment of ApoE−/− mice with anti-BAFFR mAb produced a similar effect (50). Sage et al. (88) observed reduced atherosclerosis in the aortic root in LDLR−/− mice reconstituted with BAFFR−/− BM compared with control mice (LDLR−/− mice reconstituted with WT BM) maintained on a HFD (Table 1).
B Cell Effects on Atherosclerosis Are Subset Dependent
Studies demonstrating that the reduction of B-2 cells attenuated atherosclerosis initially seemed at odds with prior immunization studies (3, 28, 73, 106) and the earlier studies of Caligiuri et al. (12), Doran et al. (18), and Major et al. (59), demonstrating that B cells attenuated atherosclerosis. Yet Ait-Oufella et al. (1), Kyaw et al. (50–52), and Sage et al. (88) demonstrated that anti-CD20 Ab and loss of BAFFR activity predominantly affected the B-2 and not B-1 cell population, shedding light on the important aspect of B cell subset-dependent effects on atherosclerosis. In contrast to B-2 cells, followup studies (42, 53, 82) revealed that B-1 cells are atheroprotective. B-1-derived natural IgM Abs can bind to the oxidation motifs in LDLs, the phosphocholine head group on cell wall polysaccharides of pathogens, such as S. pneumoniae and apoptotic cells (95). In vitro experiments have demonstrated that natural IgM can block the uptake of OxLDL by macrophages (Fig. 2) (41), a key pathogenic step in atherosclerosis formation. Lewis et al. (56) demonstrated that secreted IgM is important for atheroprotection. Increased atherosclerosis was observed in female secreted IgM-deficient LDLR−/− mice compared with control mice irrespective of type of diet (low fat or high fat). Kyaw et al. (53) demonstrated that adoptive transfer of peritoneal B-1a cells increased IgM in atherosclerotic lesions and attenuated disease in splenectomized ApoE−/− mice compared with control ApoE−/− mice. More recently, Kyaw and coworkers (42) demonstrated that Toll-like receptor 4 and myeloid differentiating factor 88 are essential for B-1a-derived, IgM-mediated atheroprotection in splenectomized ApoE−/− mice. Whereas B-1a cells had been thought to be the major source of IgM NAb, we (82) have recently shown that B-1b cells produce substantial IgM NAb in vivo, and adoptive transfer of B-1b cells attenuated diet-induced atherosclerosis in recombination activating gene 1-deficient ApoE−/− mice compared with PBS-injected control mice (Fig. 2 and Table 1).
However, many questions remain about the context-dependent nature of B cell subset functions. Rauch et al. (79) identified a unique B-1a cell-derived subtype, called innate response activator (IRA) B cells, in a sepsis model. They proposed that these IRA B cells are phenotypically and functionally distinct, develop in the spleen after LPS stimulation, and produce granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF is an atherogenic growth factor that promotes the differentiation of inflammatory Ly-6Chigh monocytes (Fig. 2) (79, 80). Mice deficient in IRA B cells were protected from atherosclerosis (39). These data provide the first evidence for an atherogenic role for B-1a-derived IRA B cells.
Interestingly, Jackson et al. (46) recently demonstrated that overexpression of BAFF attenuates atherosclerosis via TACI-mediated B cell activation in ApoE−/− mice. Transgenic BAFF ApoE−/− mice had higher levels of atheroprotective IgM, such as anti-PC IgM and anti-MDA-LDL IgM, and reduced cholesterol levels in serum compared with ApoE−/− and transgenic BAFF ApoE−/− TACI-deficient mice, suggesting that BAFF-dependent regulation of B cell survival and atheroprotection is linked to subset-dependent, specific BAFFR activity.
In addition to B-1 and B-2 cells, Bregs have been implicated in modulating atherosclerosis. Bregs produce immune-suppressive cytokines, such as IL-10 and transforming growth factor-β, and suppress other immune-mediated conditions, such as experimental autoimmune encephalomyelitis, collagen-induced arthritis, and colitis (25, 62, 66). Therefore, it is natural to speculate that they could be atheroprotective. Indeed, it is well established that depletion of IL-10 increased infiltration of inflammatory cells, production of inflammatory cytokines, and aggravated atherosclerosis in mice (13, 60). IL-10 levels within the aorta have been linked to Bregs accumulation and reduced overall aortic leukocyte content (29). Yet there are conflicting results as to the role of IL-10-producing Bregs in atherosclerosis. Strom et al. (93) found that the CD21hiCD23hiCD24hi Breg subset was increased in the draining LNs of ApoE−/− mice. Adoptive transfer of these cells into female ApoE−/− mice attenuated neointima formation in response to perivascular collar-induced carotid artery injury. Inhibition of IL-10 using a neutralizing Ab or adoptive transfer of B cells from IL-10-deficient mice prevented LN-derived B cell atheroprotection (Table 1). In contrast, Sage et al. (87) demonstrated that male LDLR−/− mice irradiated and reconstituted with 80% µMT−/− BM and 20% BM from IL-10-deficient mice had no difference in the size and cellular contents of the lesion compared with 20% WT BM, despite marked reductions of IL-10 in Bregs. As such, the role of IL-10-producing Bregs in atherosclerosis remains unclear (Fig. 2).
Local B Cell Immune Responses in Atherosclerosis
Ab production and disease-specific immune responses are thought to occur in SLOs. However, lymphocyte infiltration, followed by TLO formation in the adventitia adjacent to atherosclerotic plaque, has been observed in humans and aged mice (30, 43, 69). Moreover, Ig repertoire analysis of human arterial-wall lymphocytes demonstrated that resident B cells expressed switched isotypes of Ig heavy chains with hypermutated variable regions and inversion of the λ-to-κ ratio of L-chain use and activation-induced cytidine deaminase, suggesting that B cell recruitment and differentiation were taking place in the arterial wall (35). Recently, we have published (91) that ATLOs harbor both B-1 (B-1a and B-1b) and B-2 B cell subtypes. The B-2 B cell subtypes in ATLOs include transitional, Fol, GC, class-switched memory B cells, and PCs. In addition, B-1 cells and PCs in ATLOs can secrete IgM and IgG locally. These data, together with previous observations that B cells are important in Ag presentation in ATLOs, reveal atherosclerosis-specific B cell immunity, which includes effector B cells, PCs, and several immunosuppressive B cell subtypes (44, 91). These findings suggest that (auto) Ag-dependent hypermutation, proliferation, affinity maturation, Ig class switching, memory B cell generation, and differentiation into long-lived PCs may be carried out in the arterial adventitia. It has been suggested that ATLOs provide a new paradigm of atherosclerosis-specific B cell immunity and are the principal lymphoid tissue that orchestrates atherosclerosis-specific B cell immunity in the abdominal aorta of ApoE−/− mice during aging (67, 91, 105). All of these data suggest that disease-specific immune reactions may occur locally.
PVAT has also been implicated in the local regulation of atherosclerosis. Adipocytes in PVAT secrete both proinflammatory and anti-inflammatory cytokines (78), and PVAT-derived monocyte chemoattractant protein-1 increased carotid artery neointimal formation in response to wire injury in LDLR−/− mice (61). PVAT, adjacent to atherosclerotic plaque in humans, is more inflammatory than PVAT adjacent to nondiseased vessels, suggesting that PVAT may participate in local immune reactions that could modulate atherogenesis (14). Fat-associated lymphoid clusters (FALCs) have been found in adipose tissue, and these FALCs regulate local inflammation by secreting Ag-specific Abs (7, 47, 70). B-1 cells, B-2 cells, and FALCs are present in the PVAT of young and aged ApoE−/− mice (75, 105), suggesting that B cells and FALCs in PVAT may participate in local immune responses in atherosclerosis.
B Cells in Human Atherosclerosis
How these B cell findings in animal models will apply to understand the pathogenesis of human atherosclerosis or how this may impact on therapy remain unclear. An unbiased systems biology approach using the Framingham Heart Study participants identified that genes associated with B cell activation were most strongly enriched in control subjects but not in coronary heart disease patients, suggesting that B cells may play a protective role in human atherosclerosis (45). Notably, plasma levels of IgM to MDA-LDL in humans are associated with less coronary artery disease and fewer cardiovascular events (97, 98) and can predict 15-yr CVD outcomes (98), suggesting that IgM to MDA-LDL-producing B cells in humans may also be atheroprotective. However, which B cell fraction was responsible for this IgM production was unknown. Recently, Griffin et al. (32) identified a circulating human B cell subset with functional properties similar to those associated with murine B-1 cells. This human B-1 cell subset (CD19+ CD20+ CD27+ CD43+) secreted IgM spontaneously, stimulated T cells to proliferate, and demonstrated tonic intracellular signaling. Engelbertsen et al. (22) found that these human B-1 cells produce IgM to a modified epitope on LDL ApoB, a marker inversely associated with CVD events. Further studies are needed to characterize fully human B-1 cells producing IgM specific to an oxidation-specific epitope.
Biologicals that target B cells have entered the clinical arena for the treatment of autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus (16, 17, 19–21, 31, 48, 54, 64, 65, 76, 84, 90, 92). Interestingly, patients with rheumatoid arthritis and systemic lupus erythematosus are at increased risk of CVD (40, 81), yet the impact of these therapies on CVD risk in humans remains unclear (71, 96). We have recently reviewed this literature and performed a meta-analysis of existing studies with rituximab that included CVD end points; however, larger and longer studies are needed to draw any conclusions (71).
Conclusions
Significant advances have been made in our understanding of the role of B cells in murine atherosclerosis over the last two decades. Yet many unanswered questions remain, such as the impact of local versus systemic effects, identification of all of the molecular and cellular regulators of B cell functions linked to atherosclerosis, and the impact of biological agents that deplete B cells on human CVD. The answers to these questions have promising potential to provide for novel strategies for CVD prevention.
GRANTS
Funding for this work was provided by National Heart, Lung, and Blood Institute Grants R01-HL-136098, P01-HL-055798, and R01-HL-107490 (to C. A. McNamara).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.S. and C.A.M. conceived and designed the research; P.S. prepared figures; P.S. drafted manuscript; P.S. and C.A.M. edited and revised manuscript; P.S. and C.A.M. approved final version of manuscript.
REFERENCES
- 1.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vré E, Esposito B, Vilar J, Sirvent J, Van Snick J, Tedgui A, Tedder TF, Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med 207: 1579–1587, 2010. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21: 379–390, 2004. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Ameli S, Hultgårdh-Nilsson A, Regnström J, Calara F, Yano J, Cercek B, Shah PK, Nilsson J. Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol 16: 1074–1079, 1996. doi: 10.1161/01.ATV.16.8.1074. [DOI] [PubMed] [Google Scholar]
- 4.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy−review of a new approach. Pharmacol Rev 55: 241–269, 2003. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 5.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 11: 34–46, 2011. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 6.Baumgarth N. B-cell immunophenotyping. Methods Cell Biol 75: 643–662, 2004. doi: 10.1016/S0091-679X(04)75027-X. [DOI] [PubMed] [Google Scholar]
- 7.Bénézech C, Luu NT, Walker JA, Kruglov AA, Loo Y, Nakamura K, Zhang Y, Nayar S, Jones LH, Flores-Langarica A, McIntosh A, Marshall J, Barone F, Besra G, Miles K, Allen JE, Gray M, Kollias G, Cunningham AF, Withers DR, Toellner KM, Jones ND, Veldhoen M, Nedospasov SA, McKenzie AN, Caamaño JH. Inflammation-induced formation of fat-associated lymphoid clusters. Nat Immunol 16: 819–828, 2015. doi: 10.1038/ni.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol 20: 253–300, 2002. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 9.Binder CJ, Hörkkö S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med 9: 736–743, 2003. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 10.Binder CJ, Papac-Milicevic N, Witztum JL. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol 16: 485–497, 2016. doi: 10.1038/nri.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caligiuri G, Khallou-Laschet J, Vandaele M, Gaston AT, Delignat S, Mandet C, Kohler HV, Kaveri SV, Nicoletti A. Phosphorylcholine-targeting immunization reduces atherosclerosis. J Am Coll Cardiol 50: 540–546, 2007. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 12.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest 109: 745–753, 2002. doi: 10.1172/JCI7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med 9: 10–17, 2003. [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee TK, Aronow BJ, Tong WS, Manka D, Tang Y, Bogdanov VY, Unruh D, Blomkalns AL, Piegore MG Jr, Weintraub DS, Rudich SM, Kuhel DG, Hui DY, Weintraub NL. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics 45: 697–709, 2013. doi: 10.1152/physiolgenomics.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol 42: 120–129, 2012. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, Keystone EC, Loveless JE, Burmester GR, Cravets MW, Hessey EW, Shaw T, Totoritis MC; REFLEX Trial Group . Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 54: 2793–2806, 2006. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 17.Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Sirinian C, Karampetsou M, Yiannopoulos G, Andonopoulos AP. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford) 49: 271–280, 2010. doi: 10.1093/rheumatology/kep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doran AC, Lipinski MJ, Oldham SN, Garmey JC, Campbell KA, Skaflen MD, Cutchins A, Lee DJ, Glover DK, Kelly KA, Galkina EV, Ley K, Witztum JL, Tsimikas S, Bender TP, McNamara CA. B-cell aortic homing and atheroprotection depend on Id3. Circ Res 110: e1–e12, 2012. doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 350: 2572–2581, 2004. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 20.Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, Latinis K, Abud-Mendoza C, Szczepanski LJ, Roschmann RA, Chen A, Armstrong GK, Douglass W, Tyrrell H. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)). Ann Rheum Dis 69: 1629–1635, 2010. doi: 10.1136/ard.2009.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, Racewicz AJ, van Vollenhoven RF, Li NF, Agarwal S, Hessey EW, Shaw TM; DANCER Study Group . The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 54: 1390–1400, 2006. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 22.Engelbertsen D, Vallejo J, Quách TD, Fredrikson GN, Alm R, Hedblad B, Björkbacka H, Rothstein TL, Nilsson J, Bengtsson E. Low levels of IgM antibodies against an advanced glycation endproduct-modified apolipoprotein B100 peptide predict cardiovascular events in nondiabetic subjects. J Immunol 195: 3020–3025, 2015. doi: 10.4049/jimmunol.1402869. [DOI] [PubMed] [Google Scholar]
- 23.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci USA 106: 5773–5778, 2009. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faria-Neto JR, Chyu KY, Li X, Dimayuga PC, Ferreira C, Yano J, Cercek B, Shah PK. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis 189: 83–90, 2006. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3: 944–950, 2002. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 26.Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J Immunol 183: 6359–6368, 2009. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fredrikson GN, Söderberg I, Lindholm M, Dimayuga P, Chyu KY, Shah PK, Nilsson J. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler Thromb Vasc Biol 23: 879–884, 2003. doi: 10.1161/01.ATV.0000067937.93716.DB. [DOI] [PubMed] [Google Scholar]
- 28.George J, Afek A, Gilburd B, Levkovitz H, Shaish A, Goldberg I, Kopolovic Y, Wick G, Shoenfeld Y, Harats D. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis 138: 147–152, 1998. doi: 10.1016/S0021-9150(98)00015-X. [DOI] [PubMed] [Google Scholar]
- 29.Gjurich BN, Taghavie-Moghadam PL, Ley K, Galkina EV. L-selectin deficiency decreases aortic B1a and Breg subsets and promotes atherosclerosis. Thromb Haemost 112: 803–811, 2014. doi: 10.1160/TH13-10-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gräbner R, Lötzer K, Döpping S, Hildner M, Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, Fu YX, Hehlgans T, Mebius RE, van der Wall M, Kruspe D, Englert C, Lovas A, Hu D, Randolph GJ, Weih F, Habenicht AJ. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med 206: 233–248, 2009. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenwald MW, Shergy WJ, Kaine JL, Sweetser MT, Gilder K, Linnik MD. Evaluation of the safety of rituximab in combination with a tumor necrosis factor inhibitor and methotrexate in patients with active rheumatoid arthritis: results from a randomized controlled trial. Arthritis Rheum 63: 622–632, 2011. doi: 10.1002/art.30194. [DOI] [PubMed] [Google Scholar]
- 32.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70−. J Exp Med 208: 67–80, 2011. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23: 7–18, 2005. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Hamel KM, Liarski VM, Clark MR. Germinal center B-cells. Autoimmunity 45: 333–347, 2012. doi: 10.3109/08916934.2012.665524. [DOI] [PubMed] [Google Scholar]
- 35.Hamze M, Desmetz C, Berthe ML, Roger P, Boulle N, Brancherau P, Picard E, Guzman C, Tolza C, Guglielmi P. Characterization of resident B cells of vascular walls in human atherosclerotic patients. J Immunol 191: 3006–3016, 2013. doi: 10.4049/jimmunol.1202870. [DOI] [PubMed] [Google Scholar]
- 36.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 12: 204–212, 2011. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 37.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6: 508–519, 2006. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 38.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med 161: 1554–1568, 1985. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilgendorf I, Theurl I, Gerhardt LM, Robbins CS, Weber GF, Gonen A, Iwamoto Y, Degousee N, Holderried TA, Winter C, Zirlik A, Lin HY, Sukhova GK, Butany J, Rubin BB, Witztum JL, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation 129: 1677–1687, 2014. doi: 10.1161/CIRCULATIONAHA.113.006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollan I, Meroni PL, Ahearn JM, Cohen Tervaert JW, Curran S, Goodyear CS, Hestad KA, Kahaleh B, Riggio M, Shields K, Wasko MC. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev 12: 1004–1015, 2013. doi: 10.1016/j.autrev.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Hörkkö S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest 103: 117–128, 1999. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosseini H, Li Y, Kanellakis P, Tay C, Cao A, Liu E, Peter K, Tipping P, Toh BH, Bobik A, Kyaw T. Toll-like receptor (TLR)4 and MyD88 are essential for atheroprotection by peritoneal B1a B cells. J Am Heart Assoc 5: e002947, 2016. doi: 10.1161/JAHA.115.002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houtkamp MA, de Boer OJ, van der Loos CM, van der Wal AC, Becker AE. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J Pathol 193: 263–269, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 44.Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, Grassia G, MacRitchie N, Dever G, Gordon P, Burton FL, Ialenti A, Sabir SR, McInnes IB, Brewer JM, Garside P, Weber C, Lehmann T, Teupser D, Habenicht L, Beer M, Grabner R, Maffia P, Weih F, Habenicht AJ. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin β receptors. Immunity 42: 1100–1115, 2015. doi: 10.1016/j.immuni.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huan T, Zhang B, Wang Z, Joehanes R, Zhu J, Johnson AD, Ying S, Munson PJ, Raghavachari N, Wang R, Liu P, Courchesne P, Hwang SJ, Assimes TL, McPherson R, Samani NJ, Schunkert H, Meng Q, Suver C, O’Donnell CJ, Derry J, Yang X, Levy D; Coronary Artery Disease Genome wide Replication and Meta-analysis (CARDIoGRAM) Consortium, International Consortium for Blood Pressure GWAS (ICBP), Meng Q, Suver C, O'Donnell CJ, Derry J, Yang X, Levy D. A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler Thromb Vasc Biol 33: 1427–1434, 2013. doi: 10.1161/ATVBAHA.112.300112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson SW, Scharping NE, Jacobs HM, Wang S, Chait A, Rawlings DJ. Cutting edge: BAFF overexpression reduces atherosclerosis via TACI-dependent B cell activation. J Immunol 197: 4529–4534, 2016. doi: 10.4049/jimmunol.1601198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson-Jones LH, Duncan SM, Magalhaes MS, Campbell SM, Maizels RM, McSorley HJ, Allen JE, Bénézech C. Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat Commun 7: 12651, 2016. doi: 10.1038/ncomms12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V, van Paassen P, Walsh D, Walsh M, Westman K, Jayne DR; European Vasculitis Study Group . Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 363: 211–220, 2010. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 49.Kawahara T, Ohdan H, Zhao G, Yang YG, Sykes M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol 171: 5406–5414, 2003. doi: 10.4049/jimmunol.171.10.5406. [DOI] [PubMed] [Google Scholar]
- 50.Kyaw T, Cui P, Tay C, Kanellakis P, Hosseini H, Liu E, Rolink AG, Tipping P, Bobik A, Toh BH. BAFF receptor mAb treatment ameliorates development and progression of atherosclerosis in hyperlipidemic ApoE−/− mice. PLoS One 8: e60430, 2013. doi: 10.1371/journal.pone.0060430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kyaw T, Tay C, Hosseini H, Kanellakis P, Gadowski T, MacKay F, Tipping P, Bobik A, Toh BH. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS One 7: e29371, 2012. doi: 10.1371/journal.pone.0029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, Kehry M, Dunn R, Agrotis A, Tipping P, Bobik A, Toh BH. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol 185: 4410–4419, 2010. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 53.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, Bobik A, Toh BH. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res 109: 830–840, 2011. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 54.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol 8: 348–358, 2011. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
- 55.Lalor PA, Stall AM, Adams S, Herzenberg LA. Permanent alteration of the murine Ly-1 B repertoire due to selective depletion of Ly-1 B cells in neonatal animals. Eur J Immunol 19: 501–506, 1989. doi: 10.1002/eji.1830190314. [DOI] [PubMed] [Google Scholar]
- 56.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 120: 417–426, 2009. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lund FE. Cytokine-producing B lymphocytes−key regulators of immunity. Curr Opin Immunol 20: 332–338, 2008. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun 8: 25–54, 2005. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- 59.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol 22: 1892–1898, 2002. doi: 10.1161/01.ATV.0000039169.47943.EE. [DOI] [PubMed] [Google Scholar]
- 60.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res 85: e17–e24, 1999. doi: 10.1161/01.RES.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 61.Manka D, Chatterjee TK, Stoll LL, Basford JE, Konaniah ES, Srinivasan R, Bogdanov VY, Tang Y, Blomkalns AL, Hui DY, Weintraub NL. Transplanted perivascular adipose tissue accelerates injury-induced neointimal hyperplasia: role of monocyte chemoattractant protein-1. Arterioscler Thromb Vasc Biol 34: 1723–1730, 2014. doi: 10.1161/ATVBAHA.114.303983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 197: 489–501, 2003. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mauri C, Menon M. The expanding family of regulatory B cells. Int Immunol 27: 479–486, 2015. doi: 10.1093/intimm/dxv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meijer JM, Meiners PM, Vissink A, Spijkervet FK, Abdulahad W, Kamminga N, Brouwer E, Kallenberg CG, Bootsma H. Effectiveness of rituximab treatment in primary Sjögren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 62: 960–968, 2010. doi: 10.1002/art.27314. [DOI] [PubMed] [Google Scholar]
- 65.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh HJ, Zhang D, Brunetta PG. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 62: 222–233, 2010. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16: 219–230, 2002. doi: 10.1016/S1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 67.Mohanta SK, Yin C, Peng L, Srikakulapu P, Bontha V, Hu D, Weih F, Weber C, Gerdes N, Habenicht AJ. Artery tertiary lymphoid organs contribute to innate and adaptive immune responses in advanced mouse atherosclerosis. Circ Res 114: 1772–1787, 2014. doi: 10.1161/CIRCRESAHA.114.301137. [DOI] [PubMed] [Google Scholar]
- 68.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol 7: 293–301, 2006. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 69.Moos MP, John N, Gräbner R, Nossmann S, Günther B, Vollandt R, Funk CD, Kaiser B, Habenicht AJ. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 25: 2386–2391, 2005. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 70.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463: 540–544, 2010. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 71.Morris-Rosenfeld S, Lipinski MJ, McNamara CA. Understanding the role of B cells in atherosclerosis: potential clinical implications. Expert Rev Clin Immunol 10: 77–89, 2014. doi: 10.1586/1744666X.2014.857602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nicoletti A, Kaveri S, Caligiuri G, Bariéty J, Hansson GK. Immunoglobulin treatment reduces atherosclerosis in apo E knockout mice. J Clin Invest 102: 910–918, 1998. doi: 10.1172/JCI119892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci USA 92: 821–825, 1995. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J Immunol 170: 5897–5911, 2003. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- 75.Perry HM, Oldham SN, Fahl SP, Que X, Gonen A, Harmon DB, Tsimikas S, Witztum JL, Bender TP, McNamara CA. Helix-loop-helix factor inhibitor of differentiation 3 regulates interleukin-5 expression and B-1a B cell proliferation. Arterioscler Thromb Vasc Biol 33: 2771–2779, 2013. doi: 10.1161/ATVBAHA.113.302571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS; Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 361: 2143–2152, 2009. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ponnuswamy P, Van Vre EA, Mallat Z, Tedgui A. Humoral and cellular immune responses in atherosclerosis: spotlight on B- and T-cells. Vascul Pharmacol 56: 193–203, 2012. doi: 10.1016/j.vph.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol 10: 191–196, 2010. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, Gorbatov R, Waring MT, Chicoine AT, Mouded M, Pittet MJ, Nahrendorf M, Weissleder R, Swirski FK. Innate response activator B cells protect against microbial sepsis. Science 335: 597–601, 2012. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 125: 364–374, 2012. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roman MJ, Salmon JE. Cardiovascular manifestations of rheumatologic diseases. Circulation 116: 2346–2355, 2007. doi: 10.1161/CIRCULATIONAHA.106.678334. [DOI] [PubMed] [Google Scholar]
- 82.Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, Das D, McSkimming C, Taylor AM, Tsimikas S, Bender TP, Witztum JL, McNamara CA. B-1b cells secrete atheroprotective IgM and attenuate atherosclerosis. Circ Res 117: e28–e39, 2015. doi: 10.1161/CIRCRESAHA.117.306044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity 42: 607–612, 2015. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 84.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg JP, Brunetta P, Appel G; LUNAR Investigator Group . Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 64: 1215–1226, 2012. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 85.Roy B, Brennecke AM, Agarwal S, Krey M, Duber S, Weiss S. An intrinsic propensity of murine peritoneal B1b cells to switch to IgA in presence of TGF-beta and retinoic acid. PLoS One 8: e82121, 2013. doi: 10.1371/journal.pone.0082121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roy B, Shukla S, Lyszkiewicz M, Krey M, Viegas N, Duber S, Weiss S. Somatic hypermutation in peritoneal B1b cells. Mol Immunol 46: 1613–1619, 2009. doi: 10.1016/j.molimm.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 87.Sage AP, Nus M, Baker LL, Finigan AJ, Masters LM, Mallat Z. Regulatory B cell-specific interleukin-10 is dispensable for atherosclerosis development in mice. Arterioscler Thromb Vasc Biol 35: 1770–1773, 2015. doi: 10.1161/ATVBAHA.115.305568. [DOI] [PubMed] [Google Scholar]
- 88.Sage AP, Tsiantoulas D, Baker L, Harrison J, Masters L, Murphy D, Loinard C, Binder CJ, Mallat Z. BAFF receptor deficiency reduces the development of atherosclerosis in mice—brief report. Arterioscler Thromb Vasc Biol 32: 1573–1576, 2012. doi: 10.1161/ATVBAHA.111.244731. [DOI] [PubMed] [Google Scholar]
- 89.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol 15: 441–451, 2015. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 90.Sneller MC, Hu Z, Langford CA. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum 64: 835–842, 2012. doi: 10.1002/art.34322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Srikakulapu P, Hu D, Yin C, Mohanta SK, Bontha SV, Peng L, Beer M, Weber C, McNamara CA, Grassia G, Maffia P, Manz RA, Habenicht AJ. Artery tertiary lymphoid organs control multilayered territorialized atherosclerosis B-cell responses in aged ApoE−/− mice. Arterioscler Thromb Vasc Biol 36: 1174–1185, 2016. doi: 10.1161/ATVBAHA.115.306983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U; RAVE-ITN Research Group . Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221–232, 2010. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strom AC, Cross AJ, Cole JE, Blair PA, Leib C, Goddard ME, Rosser EC, Park I, Hultgårdh Nilsson A, Nilsson J, Mauri C, Monaco C. B regulatory cells are increased in hypercholesterolaemic mice and protect from lesion development via IL-10. Thromb Haemost 114: 835–847, 2015. doi: 10.1160/TH14-12-1084. [DOI] [PubMed] [Google Scholar]
- 94.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol 167: 1081–1089, 2001. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- 95.Tsiantoulas D, Gruber S, Binder CJ. B-1 cell immunoglobulin directed against oxidation-specific epitopes. Front Immunol 3: 415, 2013. doi: 10.3389/fimmu.2012.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsiantoulas D, Sage AP, Mallat Z, Binder CJ. Targeting B cells in atherosclerosis: closing the gap from bench to bedside. Arterioscler Thromb Vasc Biol 35: 296–302, 2015. doi: 10.1161/ATVBAHA.114.303569. [DOI] [PubMed] [Google Scholar]
- 97.Tsimikas S, Brilakis ES, Lennon RJ, Miller ER, Witztum JL, McConnell JP, Kornman KS, Berger PB. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J Lipid Res 48: 425–433, 2007. doi: 10.1194/jlr.M600361-JLR200. [DOI] [PubMed] [Google Scholar]
- 98.Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, Xu Q, Mayr A, Witztum JL, Kiechl S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol 60: 2218–2229, 2012. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 99.Vaughan AT, Roghanian A, Cragg MS. B cells—masters of the immunoverse. Int J Biochem Cell Biol 43: 280–285, 2011. doi: 10.1016/j.biocel.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 100.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med 20: 633–641, 2014. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 17: 1410–1422, 2011. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 102.Wigren M, Nilsson J, Kolbus D. Lymphocytes in atherosclerosis. Clin Chim Acta 413: 1562–1568, 2012. doi: 10.1016/j.cca.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 103.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28: 639–650, 2008. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 104.Yang Y, Tung JW, Ghosn EE, Herzenberg LA, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci USA 104: 4542–4546, 2007. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yin C, Mohanta SK, Srikakulapu P, Weber C, Habenicht AJ. Artery tertiary lymphoid organs: powerhouses of atherosclerosis immunity. Front Immunol 7: 387, 2016. doi: 10.3389/fimmu.2016.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol 21: 108–114, 2001. doi: 10.1161/01.ATV.21.1.108. [DOI] [PubMed] [Google Scholar]