The vascular effect of endothelium-derived extracellular vesicles (eEVs) is biphasic, with higher doses decreasing the magnitude of flow-induced dilation (FID) compared with lower doses that shift the mediator of FID from nitric oxide to H2O2. eEVs may cause vascular dysfunction via similar pathways despite being formed from different stimuli, although both require mitochondrial reactive oxygen species for their formation.
Keywords: extracellular vesicles, vasodilation, endothelium, mitochondria
Abstract
To examine the effect of endothelium-derived extracellular vesicles (eEVs) on the mediator of flow-induced dilation (FID), composition, formation, and functional effects on the mediator of FID were examined from two different eEV subtypes, one produced from ceramide, while the other was produced from plasminogen-activator inhibitor 1 (PAI-1). Using video microscopy, we measured internal-diameter changes in response to increases in flow in human adipose resistance arteries acutely exposed (30 min) to eEVs derived from cultured endothelial cells exposed to ceramide or PAI-1. FID was significantly impaired following exposure to 500K/ml (K = 1,000) of ceramide-induced eEVs (Cer-eEVs) but unaffected by 250K/ml. FID was reduced in the presence of PEG-catalase following administration of 250K/ml of Cer-eEVs and PAI-1 eEVs, whereas Nω-nitro-l-arginine methyl ester (l-NAME) had no effect. Pathway analysis following protein composition examination using liquid chromatography tandem mass spectrometry (LC-MS/MS) demonstrated that both subtypes were strongly linked to similar biological functions, primarily, mitochondrial dysfunction. Flow cytometry was used to quantify eEVs in the presence or absence of l-phenylalanine-4′-boronic acid (PBA) and mitochondria-targeted [93-boronophenyl)methyl]triphenyl-phosphonium (mito-PBA), cytosolic and mitochondrial-targeted antioxidants, respectively. eEV formation was significantly and dramatically reduced with mito-PBA treatment. In conclusion, eEVs have a biphasic effect, with higher doses impairing and lower doses shifting the mediator of FID from nitric oxide (NO) to hydrogen peroxide (H2O2). Despite differences in protein content, eEVs may alter vascular function in similar directions, regardless of the stimulus used for their formation. Furthermore, mitochondrial ROS production is required for the generation of these vesicles.
NEW & NOTEWORTHY The vascular effect of endothelium-derived extracellular vesicles (eEVs) is biphasic, with higher doses decreasing the magnitude of flow-induced dilation (FID) compared with lower doses that shift the mediator of FID from nitric oxide to H2O2. eEVs may cause vascular dysfunction via similar pathways despite being formed from different stimuli, although both require mitochondrial reactive oxygen species for their formation.
endothelium-derived extracellular vesicles (eEVs), small membrane-bound particles released from the cell under a variety of conditions, are emerging as important mediators of vascular homeostasis (8). Accumulating evidence indicates that these particles are capable of transporting proteins, lipids, and microRNA, suggesting a role in intercellular communication (9, 27). In addition, eEVs have been linked to pathophysiological processes, including inflammation, coagulation, and vascular dysfunction (29). Multiple studies have demonstrated that endothelium-dependent vasodilation is impaired by higher levels of circulating eEVs and, therefore, could be of use as a marker of endothelial dysfunction (1, 3). Despite their growing role in vascular pathophysiology, neither the events leading to the formation of, nor the composition of, eEVs has been sufficiently addressed.
We have recently discovered that ceramide, a sphingolipid known to be elevated in the plasma of patients with coronary artery disease (CAD), initiates a switch in the mediator of flow-induced dilation (FID) in human resistance arteries from healthy patients, from the anti-inflammatory molecule nitric oxide (NO) to the proinflammatory, prothrombotic, compound hydrogen peroxide (H2O2), derived from the mitochondria, as the mediator responsible for FID in microvessels from patients with CAD (13). In addition to being elevated in cardiovascular disease, previous studies suggest that ceramide is a critical signaling component for the initiation of cellular budding and expulsion of microvesicles (26). Although prior studies in animals have demonstrated that ACh-induced dilation is impaired in vessels treated with eEVs, the effect of eEVs on the mediator of FID in the human circulation has not been reported. We addressed this question by examining the mediator of FID in human resistance arteries following intraluminal exposure to eEVs generated from ceramide, as well as another known eEV-forming stimulus, plasminogen-activator inhibitor (PAI-1). Furthermore, we performed biological pathway analyses on proteins identified in both subtypes of eEVs. Finally, on the basis of data gathered from the pathway analyses, we investigated the role of the mitochondria in the formation of these specific shed endothelial particles.
MATERIALS AND METHODS
Tissue Acquisition
Fresh human adipose tissue (subcutaneous and peritoneal) from discarded surgical specimens was obtained from a total of 20 patients and placed in ice-cold HEPES buffer. Deidentified patient demographic data were collected using the Generic Clinical Research Database at the Medical College of Wisconsin. The average age of the subjects was 50.4 ± 14.6 (average ± SD) with an average BMI of 32.9 ± 9.4. Females represented 13 of the 20 patients. Of these patients, one had a history of hypertension, and one had a history of smoking. The others had no risk factors for CAD. All protocols were approved by the local Institutional Review Board (no. 10828) at the Medical College of Wisconsin.
Measurement of FID by Video Microscopy
As we have described before (20), isolated resistance arteries, size range 100–200 µm in diameter, were cannulated on glass micropipettes and secured in an organ chamber with warm circulating Krebs buffer. Internal diameters were measured at steady state before and after administration of endothelin-1 (ET-1) to produce 30–50% constriction, and then during intraluminal flow at pressure gradients of 5 to 100 cmH2O, which correspond to flow rates of 5–20 μl/min and shear stress between 5 and 90 dyn/cm2 (22). The following inhibitors were added to the bath 30 min before initiation of flow: nitric oxide synthase inhibitor Nω-nitro-l-arginine (l-NAME; 10−4 mol/l), or the H2O2 scavenger polyethylene glycol-catalase (PEG-catalase; 500 U/ml). Ceramide or PAI-1-generated eEVs were placed in the reservoirs connected to the micropipettes at varying concentrations. Resistance arteries were intraluminally injected with buffer containing the eEVs (250K/ml or 500K/ml) for 30 min before constriction with ET-1. Diameter changes to graded increases in flow were determined. To determine the vessel’s maximal diameter, papaverine, (10−4 mol/l) an endothelium-independent vasodilator, was added at the end of each experiment. To confirm matched impedance between pipettes, flow was reversed in the presence of maximal dilation. FID is expressed as a percentage of maximal relaxation from ET-1 constriction, with 100% representing full relaxation to the maximal diameter obtained by the addition of papaverine.
Formation and Quantification of eEVs
eEV formation.
Formation of eEVs for vascular and proteomic studies was accomplished using human cardiac microvascular endothelial cells (HMVECs) (Lonza) between passages 3 and 5, grown to 80% confluency in T-75 flasks using EBM-2 media (Lonza) with 10% FBS and was maintained at 37°C in 5% humidified CO2. Before eEV generation, cells were rinsed with HBSS three times and then incubated in serum-free media without antibiotics for 2 h. Cells were then treated with 15 µM of C-2 ceramide (Cayman Chemical) or 20 ng/ml PAI-1 (Millipore) for 3 h. After treatment, media were collected and pooled from 15 flasks and were placed into 50-ml conical tubes. Samples were centrifuged for 8 min at 140 g for debris removal. Supernatant was then transferred into 90-ml (Sorval) tubes and were centrifuged at 4°C for 1 h at 100,000 g. The remaining supernatant was discarded, and the pellet was resuspended in 100 µl of HBSS. Comparative quantification of eEVs was performed using HMVECs in 100-mm cell culture plates (~2 million cells) per treatment. Cells were rinsed with HBSS three times and then incubated in serum-free media without antibiotics in the presence or absence of 10 μM l-phenylalanine-4′-boronic acid (PBA) or 10 μM of the mitochondria-targeted [93-boronophenyl)methyl]triphenyl-phosphonium (mito-PBA), both synthesized in the Free Radical Research Center at the Medical College of Wisconsin. Cells were then treated with 15 μM ceramide, 20 ng/ml PAI-1, 10 μM antimycin A (Santa Cruz Biotechnology), or vehicle (DMSO) for 3 h in the presence or absence of PBA or mito-PBA. A total of 8 ml of media was combined and subjected to the centrifugation protocol as described above. eEVs were resuspended in 50 µl of HBSS before flow cytometry analysis.
Flow cytometric enumeration of eEVs.
Samples were transferred to 5-ml tubes containing: 100 µl of Annexin V buffer (BD Pharmingen), 7 µl of Cy5 Annexin V (BD Pharmingen), and 12 µl of phycoerythrin-coated mouse anti-human CD31 antibody (BD Pharmingen). Annexin buffer was used to stop the reaction after a 1-h incubation in the dark. For enumeration and size gating, 25,000 7-µm beads were added to each sample before analysis. Compensation beads (BD Pharmingen) were used to set gates for Annexin V+ and CD31+ vesicle populations. A size gate was used to quantify vesicles in the range of 0.2 to 1 µm. Flow cytometry for eEV batches used for vascular and proteomic studies was performed on a Becton Dickenson Flow Cytometer using allophycocyanin and phycoerythrin (PE) channels for fluorescence detection. Data were analyzed using FACSDiva software (BD Biosciences). An Accuri C6 cytometer (BD Biosciences) was utilized to quantitatively compare eEV formation from various treatments. Sample data were analyzed using Flow Jo software (Tree Star). Total eEV count was then normalized to total protein and presented as fold change from vehicle control.
LC-MS/MS Analysis of eEVs
eEV lysis and preparation for MS analysis.
Approximately 3.5 × 106 eEVs in HBSS were centrifuged at 100,000 g for 1 h at 4°C and then resuspended in 2 ml of cold hypotonic lysis buffer and set on ice for 10 min. eEVs were then transferred to a glass Dounce homogenizer and gently mechanically lysed on ice, followed by a 60-min water bath sonication step. The homogenate was then transferred to a 15-ml conical tube (Sorval), and an equal volume of prep buffer (280 mM sucrose, 50 mM MES pH 6.5, 450 mM NaCl, 10 mM MgCl2) was added. After 10 min on ice, the homogenate was centrifuged at 100,000 g at 4°C for 2 h (Surespin 630/17 rotor). The supernatant (soluble protein fraction) was then concentrated to a total volume of 300 μl in fresh ammonium bicarbonate (100 mM, pH 8.0) using Amicon Ultra 3K MWCO filters (5×, 15 min).
Protein digestion and LC-MS/MS analysis.
Protein digestion was performed in 300 μl of fresh 100 mM ammonium bicarbonate [pH 8.0. Stock 1% RapiGest (Waters)] solution was then added for a final concentration of 0.1%. Tris(2-carboxyethyl)phosphine hydrochloride (Sigma-Aldrich) was added to each digest to a final concentration of 10 mM. The sample was then placed on a thermomixer at 750 rpm for 20 min at 37°C for reduction. Alkylation was performed with iodoacetamide (Sigma-Aldrich) (20 mM) on a thermomixer at 750 rpm for 20 min at 37°C. Fifty nanograms per microliter of sequencing grade-modified trypsin (Promega) were added to each sample. Digestion continued overnight (18–20 h) at 37°C and was stopped using heat inactivation (95°C, 5 min) and acidification to pH 2–4. Samples were centrifuged at 14,000 g for 10 min for debris removal. All samples were desalted and concentrated using OMIX C18 zip-tips, followed by reconstitution in 8 μl of buffer A [98% H2O; 1.9% acetonitrile (ACN); 0.1% formic acid]. The peptide mixtures were injected via a NanoAccuity UPLC system (Waters) coupled to a C18 resin (Phenomenex) capillary column (15 cm long, 50 μm ID). A 240-min gradient of 98% HPLC H2O/1.9% ACN/0.1% formic acid to 98% ACN/1.9% HPLC H2O/0.1% formic acid was applied, and the eluted peptides were analyzed with a LTQ-Orbitrap Velos mass spectrometer (Thermo Scientific), as previously described (17). Dynamic exclusion was used, excluding any given mass observed more than once in a 30-s time frame for 180 s from selection for fragmentation.
Protein Identification and Annotation
MS/MS spectra were searched against the human UniProtKB protein database using both Mascot (Matrix Science) and Sequest (Thermo Finnigan). Search criteria included variable modification of +57 Da for alkylation of cysteines and +16 Da oxidation of methionines. The best match for spectra from the Mascot and Sequest searches were used, and all redundancies were removed. Four technical replicates for each of the two biological replicates (eight runs total per group) were combined. Combined files for the ceramide and PAI-1 eEV data sets were then compared for label-free spectral counting analysis using Visualize analysis software (15). Within this analysis, each protein was normalized to the total scan count for a given data set to formulate a normalized log ratio and P value. Within Visualize analysis software, the statistical analysis for large proteomic data set comparison uses the G-test, a log-likelihood ratio test whose distribution can be approximated by a χ2 distribution with a single degree of freedom. Using this algorithm, we made assumptions that the expected proportion of scans for a given protein is directly related to the ratio of the total scans in each group. Stringent filters were then applied to the combined file, including removal of redundancies, P > 0.85 (FDR <5%), detection in ≥4 runs in either group, and a total scan count ≥8 in either group. This data set then underwent subsequent signaling protein composition and pathway analysis using a combination of UniProtKB, StringDB, Ingenuity Pathway Analysis, and Protein Center software platforms.
Statistics
All statistical analysis was completed using SigmaPlot 12.5, and normality was determined using a Shapiro-Wilk test. All data are presented as a means ± SE. To compare flow–response relationships, parametric statistical evaluation was performed using a two-way ANOVA or a nonparametric Friedman test with flow gradient and treatment as parameters. A one-way ANOVA was used to compare the number of eEVs generated per treatment. ANOVA analyses were followed by pairwise multiple-comparison testing (post hoc analysis) using the method of Holm-Sidak. Statistical significance was defined as P < 0.05. Differential expression of proteins between ceramide and PAI-1 eEVs was classified as those proteins having a normalized log ratio ≥ ±1.5 and a P value ≤ 0.05. All normalization and statistical comparisons of the protein differential expression were determined as noted above.
RESULTS
Ceramide-Generated eEVs Induce Endothelial Dysfunction
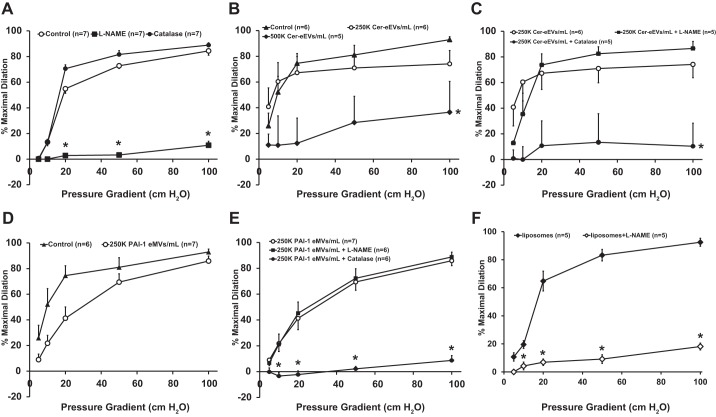
As illustrated in Fig. 1A, FID is significantly impaired in healthy resistance arteries following exposure to the nitric oxide synthase inhibitor l-NAME (100 μM), [%maximal dilation (MD) ± SE, 10.8% ± 3.1 of maximal dilator capacity; n = 7] compared with control (84.4 ± 3.3; n = 7), whereas PEG-Catalase (500 U) had no effect (88.9 ± 1.8; n = 7), suggesting that the primary mediator of FID in healthy controls is NO. The concentration effect of ceramide-induced eEVs (Cer-eEVs) on FID is shown in Fig. 1B. The vasodilatory response to flow was significantly decreased in vessels pretreated with 500K/ml of Cer-eEVs intraluminally for 30 min (36.5% ± 23.9, n = 5) compared with control (92.9 ± 2.8; n = 6). To assess whether FID is maintained following exposure to a decreased concentration of eEVs, resistance arteries were exposed to 250K/ml Cer-eEVs, which had no effect on maximal dilation (74.1 ± 10.3; n = 6). To determine the mediator of FID after exposure to 250K/ml of Cer-eEVs, vasodilation was assessed again in the presence of l-NAME or PEG-Catalase. As Fig. 1C indicates, incubation with l-NAME had no effect on FID in the presence of Cer-eEVs (86.6 ± 5.4, n = 5) compared with control (92.9 ± 2.8; n = 6); however, maximal dilation in response to flow was significantly attenuated in the presence of catalase (10.3 ± 17.9; n = 5). Maximal dilation to the endothelium-independent vasodilator papaverine did not differ between control and eEV-treated resistance arteries (data not shown).
Fig. 1.
Effect of plasminogen-activator inhibitor 1 endothelium-derived extracellular vesicles (PAI-1 eEVs) on flow-induced dilation (FID). A: overall magnitude of dilation is decreased in the presence of Nω-nitro-l-arginine methyl ester (l-NAME; 100 μM) compared with control, whereas PEG-catalase (500 U) has no effect (n = 7, all groups). B: dilation is not affected with intraluminal exposure to 250K/ml (K = 1,000) of ceramide-induced endothelium-derived extracellular vesicles (Cer-eEVs; n = 6), but it is significantly decreased with administration of 500K/ml Cer-eEVs (n = 5) compared with control (n = 6). C: FID is maintained in microvessels treated with 250K/ml Cer-eEVs in the presence of l-NAME compared with Cer-eEVs alone (n = 5 and n = 6, respectively); however, it is significantly decreased during exposure to PEG-catalase (n = 5). D: vasodilatory response to flow is maintained in resistance arteries exposed to 250K/ml PAI-1 eEVs compared with control (n = 7 and n = 6, respectively). E: dilation to flow is not affected in 250K/ml PAI-1 eEV-treated resistance arteries in the presence of l-NAME (100 μM) (n = 6) compared with 250K/ml PAI-1 eEV alone (n = 7), but it is significantly impaired following exposure to PEG-catalase (500 U) (n = 6). F: intraluminal administration of 250K/ml of liposomes does not affect the magnitude of dilation (n = 5) and FID is abolished in the presence of L-NAME (100 µM) (n = 5) compared with liposomes alone. *P < 0.05 vs. control at specific pressure gradients. n indicates number of patients.
eEVs Generated via PAI-1 Alter the Mediator of FID From NO to H2O2
To investigate whether eEVs generated by a different stimulus could produce a similar transition in the mediator, FID was assessed in microvessels exposed to eEVs formed from stimulation with PAI-1. The magnitude of dilation (maximal dilation observed at the highest flow rate achieved at a pressure gradient of 100 cmH2O) was maintained after exposure to 250K/ml PAI-1 eEVs compared with control (Fig. 1D; %MD 85.9 ± 3.8; n = 7 vs. 92.9 ± 2.8; n = 6, respectively). In resistance arteries treated with 250K/ml of PAI-1 eEVs, FID was decreased in the presence of catalase (Fig. 1E; %MD 8.6 ± 3.7; n = 6) but not after more exposure to l-NAME (%MD 96.5 ± 1.3; n = 6). Endothelium-independent vasodilation was not impaired in vessels exposed to PAI-eEVs, as assessed via response to papaverine (data not shown). Of note, healthy resistance arteries exposed to PAI-1 alone (20 ng/ml, 16–20 h) also transitions the mediator of FID from NO to H2O2 (data not shown). In addition, resistance arteries treated with commercially available liposomes displayed preserved FID that was inhibited by pretreatment with l-NAME, indicating that liposomal particles alone do not alter the mediator of FID (Fig. 1F).
Proteomic Comparison of Ceramide Versus PAI-1 eEVs
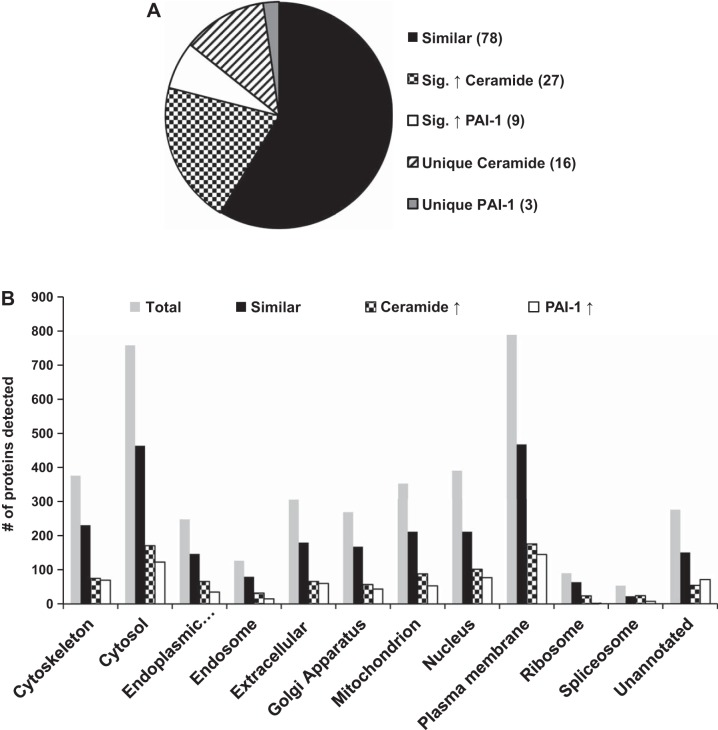
Since both ceramide and PAI-1 are elevated in the plasma of patients with cardiovascular disease, and both are capable of forming eEVs that cause endothelial dysfunction, we examined their contents for similarities using proteomic analyses. MS analysis detected 3,292 proteins in the ceramide and 2,941 proteins in PAI-1-generated eEVs. Altogether, the comparison of the total proteins identified in ceramide vs. PAI-1 data sets yielded 3,333 unique proteins (as shown in Fig. 2A), 1,930 proteins (57.9%) were not expressed differently between groups, 379 proteins (11.3%) were significantly increased in ceramide-induced eEVs, and 591 proteins (17.7%) were increased in PAI-1-generated eEVs; 392 proteins (11.7%) were unique to the ceramide eEVs, and 41 proteins (1.2%) were unique to eEVs generated from PAI-1. Using Protein Center software, we determined the cellular compartment derivation of the proteins. The categorization of data suggests the composition of both the ceramide and PAI-1 eEVs contain components from throughout the cell. Detected proteins were most prominently represented in descending order from the plasma membrane, cytosol, cytoskeleton, nucleus, and the mitochondria (Fig. 2B).
Fig. 2.
Protein composition of Cer-eEVs vs. PAI-1 eEVs, as detected by tandem MS analysis. A: total combined numbers of proteins identified in Cer-eEVs and PAI-1 eEVs are categorized on the basis of similarity, significantly elevated levels, or unique treatment. B: comparison of proteins identified in Cer-eEVs vs. PAI-1 eEVs based on cellular localization.
Proteins Identified in Ceramide and PAI-1 eEVs Annotate to Mitochondrial Dysfunction
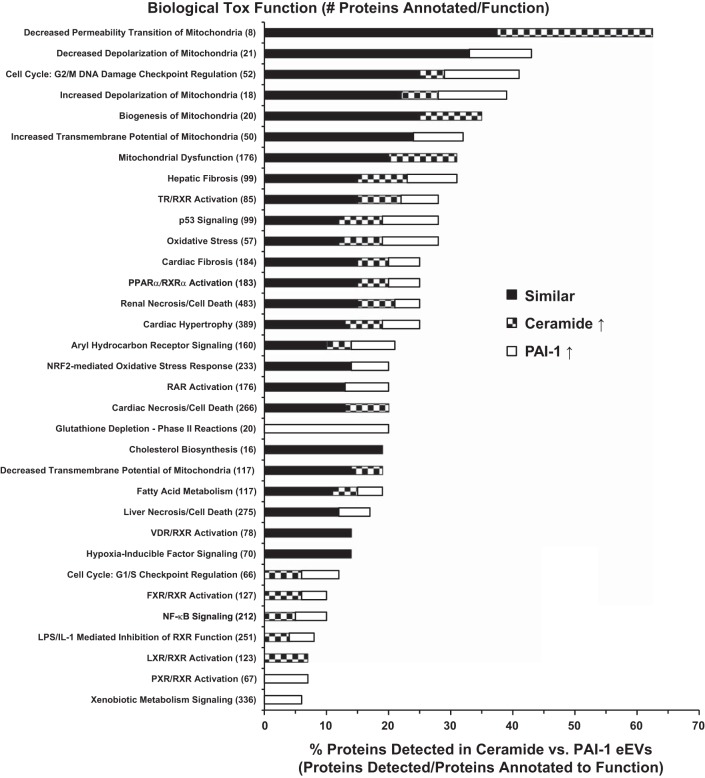
For further bioinformatic analysis, the proteins from the eEV comparison were divided into three distinct groupings: 1) proteins expressed in similar amounts between ceramide and PAI-1 eEVs (similar), 2) proteins expressed to a greater degree or only in ceramide eEVs (ceramide↑), and 3) proteins expressed more or only in PAI-1 eEVs (PAI-1 ↑). Ingenuity Pathway Analysis revealed that both Cer-eEVs and PAI-1 eEVs contained proteins that annotated to similar functions (Fig. 3). Proteins correlated strongly to mitochondrial dysfunction, membrane potential, and permeability. Oxidative stress, cell cycle regulation, and cardiovascular dysfunction pathways were among others. Only one biological toxicology function, glutathione depletion, was unique to PAI-1 eEVs.
Fig. 3.
Comparison of the top biological toxicology functions of proteins identified in ceramide vs. PAI-1 eEVs. Bottom axis indicates the percentage of proteins from the eEV data set attributed to that particular “Biological Tox Function” (detected proteins/total annotated to the particular function). Proteins not significantly different between ceramide and PAI-1 eEVs (black) and those significantly increased in ceramide (checkered) or significantly increased in PAI-1 (white) eEVs are indicated.
Mitochondrial ROS Contributes to the Formation of eEVs
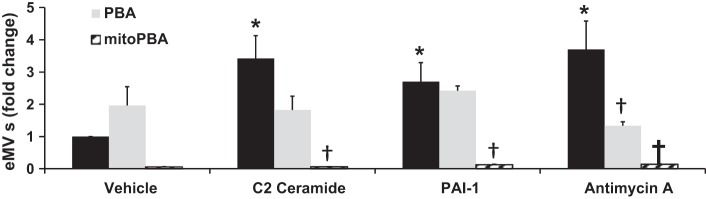
On the basis of the proteomic analysis, we suspected that changes in mitochondrial function play a role in the biological function of eEVs. To determine whether mitochondrial ROS is required for eEV formation, vesicles were quantified following treatment with ceramide, PAI-1, or antimycin A, which stimulates mitochondrial superoxide formation by inhibiting the mitochondrial ETC. As shown in Fig. 4, eEV formation compared with vehicle-treated control was significantly increased in all three treatment groups (3.4 ± 0.7, 2.7 ± 0.6, and 3.7 ± 0.9, presented as average fold change ± SE, in ceramide, PAI-1, and antimycin A, respectively; n ≥ 4 in all groups). The addition of PBA, an antioxidant that targets cytosolic peroxynitirite (ONOO−) and H2O2 (19), did not affect vesicle formation in the ceramide and PAI-1 group; however, it significantly decreased antimycin A-induced eEVs (1.3 ± 0.1; n = 3 vs. 3.7 ± 0.9; n = 4 in both groups, antimycin + PBA and antimycin alone, respectively). Interestingly, mito-PBA, a scavenger of mitochondrial-derived H2O2 (6), significantly impaired the generation of eEVs in all three treatment groups compared with their respective treatment alone (0.06 ± 0.01, 0.13 ± 0.02, and 0.14 ± 0.01, in ceramide, PAI-1, and antimycin A; n = 3, in all groups). Increases in endothelial cell mitochondrial H2O2 were not detected from administration of ceramide (15 μM), PAI-1 (20 ng/ml), or antimycin A (10 μM) in HMVECs during the same conditions in which eEVs are generated. This result is based on the use of three independent methods for detecting mitochondrial ROS, including visualization of mitochondrial peroxy yellow 1 (mitoPY1), HMVECs infected with a mitochondrial-targeted ROS-sensitive roGFP virus, as well as mass spectrometry analysis of mito-PBA oxidation products (data not shown).
Fig. 4.
Role of cellular and mitochondrial-derived ROS in the formation of ceramide and PAI-1 eEVs. The number of eEVs (fold change) is significantly increased following treatment with C2 ceramide (15 µM), PAI-1 (20 ng/ml), and antimycin A (10 µM) compared with vehicle-treated control (n = 4 for treatment groups, n = 6 for control). PBA (10 μM) decreased only antimycin A-generated eEV formation (n = 3) compared with antimycin A alone, whereas mito PBA (10 μM) significantly decreased eEV formation compared with all three respective treatments (n = 3 all groups). Black bars represent no antioxidant treatment. *P < 0.05 vs. vehicle; †P < 0.05 vs. treatment.
DISCUSSION
Vascular Effects and Formation of eEVs
The major novel findings of this study are threefold. First, intraluminal acute exposure to two different subtypes of eEVs induces a change in the mediator of FID from NO to H2O2. Second, pathway analysis performed on proteins identified via mass spectrometry indicates that despite differences in protein expression, the majority of proteins identified as having similar biological toxicology functions, primarily involve the mitochondria. Third, the use of a mitochondria-targeted antioxidant, mito-PBA, prevents the formation of eEVs produced from ceramide, PAI-1, and antimycin A. These findings suggest that the mitochondria may be critical to the formation of, and contribute to, the content of endothelium-derived vesicles that are capable of inducing endothelial dysfunction.
The endothelium is responsible for a wide range of homeostatic functions, including hemostasis, angiogenesis, and the regulation of vascular tone (11). To facilitate regulation of tone and ensure proper tissue perfusion, endothelial cells rely on various types of intercellular communication (24). Vasoactive substances released from the endothelium in response to an increase in blood flow rapidly act in a paracrine manner to cause smooth muscle relaxation and vasodilation (2). The inability of a vessel to dilate in response to increases in shear, or an alteration in the environment that favors inflammation and thrombosis, are both considered forms of endothelial dysfunction, and have been linked with most forms of cardiovascular disease (5, 28). Prior studies from our laboratory, as well as others, have shown that resistance arteries from healthy adults primarily vasodilate to NO in response to flow, promoting an anti-inflammatory, antithrombotic environment, in contrast to disease states, such as coronary artery disease, where the proinflammatory mediator H2O2 predominates (22). Therefore, the vasculature is capable of maintaining vasodilation in a disease state via a compensatory pathway, however, one that supports a proatherosclerotic environment as opposed to the antiatherosclerotic actions of NO. Although elevated levels of extracellular vesicles in patients with chronic disease have been correlated with reduced endothelium-dependent dilation (1), this study is the first to demonstrate that eEVs are capable of shifting the mediator of dilation from NO to H2O2 in response to flow.
In addition to paracrine mediators, extracellular vesicles, small membrane-bound particles released from cells, are capable of acting both locally and remotely by delivering their cargo via the circulation to distal target cells (25). Collected data suggest that the presence of eEVs in serum is associated with endothelial dysfunction (3). The eEV stimuli used in the current study, ceramide and PAI-1, are known to be elevated in the plasma of patients with cardiovascular disease (16). Although both of these compounds have been shown to initiate the transition from NO to H2O2 in isolated human resistance arterioles, the current study supports that both are also capable of producing eEVs from the endothelium, which may circulate throughout the vasculature and augment endothelial dysfunction. Therefore, the production of these vesicles may serve as a feed-forward mechanism by which the compensatory H2O2 pathway predominates during disease.
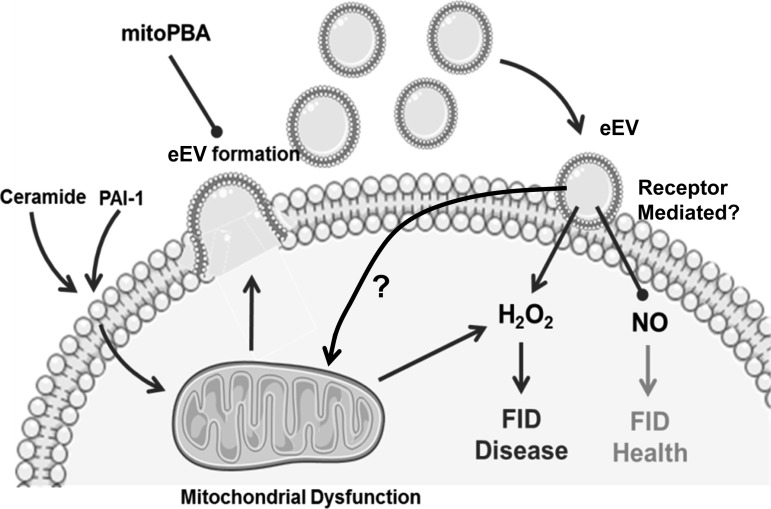
The exact events that cause the conversion from NO to H2O2 as the primary mediator of dilation are unknown and are continually being investigated. Our laboratory has shown that the predominant source of H2O2 in vessels from patients with CAD responsible for dilation is via the mitochondria, as evidenced by the decreased dilation observed with the use of the mitochondrial respiratory chain complex I inhibitor rotenone (20). More recent work from our laboratory has indicated that chronic exposure to exogenous ceramide in healthy microvessels mirrors what is observed in vessels from diseased patients, that ceramide is capable of increasing mitochondrial ROS, which replaces NO as the primary mediator of FID (13). In the current study, eEVs produced from ceramide and PAI-1 initiated this similar switch in the dilator mechanism when exposed to the lumen of human resistance arteries. This observed phenomenon may be triggered via multiple pathways, including activation of an extracellular receptor, alteration of the cellular environment following incorporation of the eEV into the endothelium, or by merely transporting ceramide and PAI-1 into the cell (Fig. 5). Of these possible mechanisms, it is unlikely that the particles act as a transporter for ceramide and PAI-1 as 1) they were rinsed thoroughly before being introduced into the vessel lumen, and 2) previous data have concluded that chronic exposure to ceramide (16–20 h) is necessary for the transition from NO to H2O2, whereas 30 min of ceramide exposure did not produce this effect (data not shown). In the current study, acute exposure (30 min) of the vessels to the eEVs was sufficient to induce the change in the mediator, suggesting an alternate mechanism. Although studies are starting to emerge that shed light on the interaction between microvesicles and the endothelium, we still have an incomplete understanding of how these particles interact extracellularly with the endothelial cell or how they get incorporated into the cell itself. Determining how eEVs interact with the endothelium and initiate this switch in the mediator is a future area of investigation.
Fig. 5.
Schematic diagram illustrating proposed mechanism of eEV-induced endothelial dysfunction. Exogenous ceramide and PAI-1 trigger formation of eEVs that is dependent on the generation of mitochondrial reactive oxygen species (ROS). Once formed eEVs can circulate throughout the vasculature and interact with downstream endothelium to initiate or contribute to the transition from NO to H2O2 as the primary mediator of FID. eEVs may be directly incorporated into the cell or may act through a cell membrane receptor. After endothelial cell interaction the eEV may increase mitochondrially derived H2O2 and replace NO as the mediator of FID. mitoPBA, mitochondria-targeted [93-boronophenyl)methyl]triphenyl-phosphonium.
Previous data have also suggested that the effect of eEVs on endothelial function is both concentration- and subtype-dependent. Extracellular vesicles from patients with acute MI caused impairment of endothelium-dependent relaxation at a low concentration (0.7-fold higher plasma levels), with an even greater reduction using a higher dose of particles (twofold higher plasma levels). Interestingly, particles collected from nonischemic patients had no effect on vascular function (3). Indeed, with high concentrations of eEVs, we observed an overall reduction in endothelium-dependent (but not endothelium-independent) dilatory capacity. The mechanisms by which a decrease in overall magnitude of dilation occurs in response to flow as opposed to the conversion to a compensatory dilator pathway remain unclear. However, to our knowledge, this study is the first to show that acute (30 min) intraluminal exposure to a lower dose of eEVs results in a shift in endothelial dilator function. The question of whether eEVs collected from healthy patients can recapitulate the NO-mediated mechanism of FID in those with chronic disease requires further investigation.
While the exact mechanism of eEV formation remains unknown, the list of stimuli known to produce extracellular vesicles is continually expanding (8). Ceramide has been shown to play a critical role in the development of cellular particles, as evidenced by the inability of mouse oligodendroglial cells to form vesicles during inhibition of neutral sphingomyelinase (NSmase), an enzyme responsible for the generation of ceramide (26). Prior evidence suggests that ROS levels correlate with extracellular vesicle formation. ANG II, another known stimulus for eEV formation, relies on the activation of NADPH oxidase and, thus, superoxide for vesicle generation. Furthermore, administration of ANG II-induced eEVs to cultured cells stimulates ROS production (4). Our present data suggest that mitochondrial ROS are not only involved but required for the formation of eEVs, based on the fact that mito-PBA, a mitochondria-targeted antioxidant, dramatically impaired generation of eEVs in response to ceramide, PAI-1, and to the ROS-generating complex III inhibitor antimycin A, while nonmitochondrially targeted PBA in the same concentration was found to have no effect. Interestingly, we were unable to detect changes in mitochondrial H2O2 using the same dose of each stimulant that produced eEV formation. This is surprising, since prior work supports an effect of ceramide on mitochondrial function (14) via a similar mechanism by which antimycin A elevates mitochondrial ROS (7, 14). One explanation for the lack of mitochondrial ROS detection could be that the amount of ROS produced is sufficient for stimulating eEV production but is subthreshold for fluorescence detection. Nearly 50 µM of C2 ceramide has been required to detect mitochondrial ROS production in bovine aortic endothelial cells using 2′,7′-dichlorofluorescein (DCF) (21), as compared with 15 µM used in the present study. Alternatively, it may require only a transient “burst” of oxidative stress to trigger vesicle generation, something not detectable with our protocol.
The present study reveals that although differences in protein composition exist between eEVs generated by different stimuli, the majority of proteins correlate to similar biological pathways, primarily involving the mitochondria. Interestingly, both Parkin and mitochondrial fission regulator 1, two key proteins involved in mitophagy and mitochondrial fission, respectively, were equally identified in both eEV subtypes (31). While ROS has been implicated in the process of mitophagy and fission, compelling evidence suggests that only mild or transient increases in oxidative stress are required for the induction of mitophagy, as well as fission (12). These data prompt speculation as to the interlinked roles of mitochondria-derived ROS, mitophagy, and mitochondrial fission, in vesicle formation from ceramide, PAI-1, and antimycin A. In addition to being antioxidants, mitochondria-targeted compounds have been shown to affect mitochondrial function (10); thus, the effect of mito-PBA observed in the current study may be altering the mitochondria via an unknown mechanism. Future investigations will aim to shed light on these important questions.
Potential Study Limitations
The present study has focused on the effect of shed extracellular vesicles from endothelial cells, as opposed to specific subtypes of vesicles. The vesicles used in this study were derived by centrifugation and likely consisted of a range of sizes all under 1 μm, including both microparticles (size range 100 nm to 1 μm), as well as exosomes (size range <100 nm) (9). The reported concentration of eEVs administered intraluminally to the resistance vessels was based on the number of the larger microparticles, since flow cytometers cannot detect particles less than 200 nm in diameter. Therefore, the actual concentration of delivered vesicles might be higher due to exosomes present in the infusate.
Although there is great translational relevance to studying human resistance arteries, variability among subjects poses some limitations. The resistance arteries used in the current study were collected from patients classified as healthy, meaning they have not been previously diagnosed with CAD. However, these patients do have other comorbidities and may have been taking one or more medications at the time of collection. To reduce variability and mitigate the effect of medications, we studied microvessels from patients with no more than one risk factor for CAD and washed resistance arteries with 60 ml of buffer before examining vascular function. Another source of variability is that resistance arteries were dissected from surgically discarded adipose tissue that was collected from various anatomical locations (e.g., subcutaneous and visceral); however, differences in the FID mediator have not been observed between these tissues (23).
Shear stress, the tangential force exerted on the endothelium due to blood flow, also affects the formation and release of extracellular vesicles. Vion et al. (30) demonstrated that low shear stress (2 dyn/cm2) activates the ERK 1/2 pathway, resulting in increased levels of eEVs, whereas higher shear forces (20 dyn/cm2) prevented vesicle release. Likewise, prolonged laminar shear stress (20 dyn/cm2 for 36 h) was recently shown to reduce the release of eEVs in cultured cells (18). It is possible that the number of eEVs produced in the current study would differ if the cultured cells had been preconditioned with physiological levels of shear stress.
Conclusions and Clinical Significance
eEVs are being recognized as intercellular transporters, biomarkers, and regulators of vascular function. This study for the first time demonstrates that eEVs formed from two different stimuli, ceramide and PAI-1, are capable of altering the mediator of FID from NO, which is vasculo-protective, to H2O2, thus promoting a proinflammatory, prothrombotic environment. Further, comparison of these two subtypes indicates that despite differences in protein composition, the biological functions mapped to these proteins are similar and suggest that the mitochondria play a role in vesicle formation. To our knowledge, this is the first study to demonstrate that eEV formation can be dramatically reduced following use of a mitochondria-targeted antioxidant. The current study provides mechanistic insight into the formation, composition, and functional effects of eEVs, contributing to our understanding of the role of these vesicles in cardiovascular diseas, as well as other acute and chronic pathologies.
GRANTS
This work was supported by the National Institutes of Health through Grants RO1-HL-113612-02 to D. Gutterman, PO1-HL-082798 to A. Greene, and GM-089586 to J. K. Freed.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K.F., M.J.D., and B.R.H. performed experiments; J.K.F., M.J.D., and B.R.H. analyzed data; J.K.F., M.J.D., B.R.H., A.S.G., and D.D.G. interpreted results of experiments; J.K.F. and B.R.H. prepared figures; J.K.F. and B.R.H. drafted manuscript; J.K.F., M.J.D., B.R.H., J.C.D., A.S.G., and D.D.G. edited and revised manuscript; J.K.F., M.J.D., B.R.H., J.C.D., A.S.G., and D.D.G. approved final version of manuscript.
ACKNOWLEDGMENTS
All mass spectrometry work was performed in the Medical College of Wisconsin Biotechnology and Bioengineering Center. The authors thank the surgeons and nurses at Froedtert Memorial Lutheran Hospital, the Division of Cardiothoracic Surgery at the Medical College of Wisconsin, the Aurora Medical Group Cardiovascular and Thoracic Surgery, the Cardiothoracic Surgery Group of Milwaukee, and the Wheaton Franciscan Healthcare Group.
REFERENCES
- 1.Amabile N, Guérin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol 16: 3381–3388, 2005. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 2.Beyer AM, Gutterman DD. Regulation of the human coronary microcirculation. J Mol Cell Cardiol 52: 814–821, 2012. doi: 10.1016/j.yjmcc.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulanger CM, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A, Mallat Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 104: 2649–2652, 2001. doi: 10.1161/hc4701.100516. [DOI] [PubMed] [Google Scholar]
- 4.Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol 31: 1898–1907, 2011. doi: 10.1161/ATVBAHA.110.222703. [DOI] [PubMed] [Google Scholar]
- 5.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000. doi: 10.1161/01.RES.87.10.840. [DOI] [PubMed] [Google Scholar]
- 6.Cairns AG, McQuaker SJ, Murphy MP, Hartley RC. Targeting mitochondria with small molecules: the preparation of MitoB and MitoP as exomarkers of mitochondrial hydrogen peroxide. Methods Mol Biol 1265: 25–50, 2015. doi: 10.1007/978-1-4939-2288-8_3. [DOI] [PubMed] [Google Scholar]
- 7.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J Biol Chem 275: 25130–25138, 2000. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 8.Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res 335: 143–151, 2009. doi: 10.1007/s00441-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 9.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 31: 27–33, 2011. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 10.Dranka BP, Zielonka J, Kanthasamy AG, Kalyanaraman B. Alterations in bioenergetic function induced by Parkinson’s disease mimetic compounds: lack of correlation with superoxide generation. J Neurochem 122: 941–951, 2012. doi: 10.1111/j.1471-4159.2012.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durand MJ, Gutterman DD. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation 20: 239–247, 2013. doi: 10.1111/micc.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission-dependent manner. Biochim Biophys Acta 1823: 2297–2310, 2012. doi: 10.1016/j.bbamcr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res 115: 525–532, 2014. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Ruiz C, Colell A, Marí M, Morales A, Fernández-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem 272: 11369–11377, 1997. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 15.Halligan BD, Greene AS. Visualize: a free and open source multifunction tool for proteomics data analysis. Proteomics 11: 1058–1063, 2011. doi: 10.1002/pmic.201000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havulinna AS, Sysi-Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, Salomaa V, Laaksonen R. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol 36: 2424–2430, 2016. doi: 10.1161/ATVBAHA.116.307497. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann BR, Wagner JR, Prisco AR, Janiak A, Greene AS. Vascular endothelial growth factor-A signaling in bone marrow-derived endothelial progenitor cells exposed to hypoxic stress. Physiol Genomics 45: 1021–1034, 2013. doi: 10.1152/physiolgenomics.00070.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JS, Kim B, Lee H, Thakkar S, Babbitt DM, Eguchi S, Brown MD, Park JY. Shear stress-induced mitochondrial biogenesis decreases the release of microparticles from endothelial cells. Am J Physiol Heart Circ Physiol 309: H425–H433, 2015. doi: 10.1152/ajpheart.00438.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippert AR, Van de Bittner GC, Chang CJ. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc Chem Res 44: 793–804, 2011. doi: 10.1021/ar200126t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 21.Matsunaga T, Kotamraju S, Kalivendi SV, Dhanasekaran A, Joseph J, Kalyanaraman B. Ceramide-induced intracellular oxidant formation, iron signaling, and apoptosis in endothelial cells: protective role of endogenous nitric oxide. J Biol Chem 279: 28614–28624, 2004. doi: 10.1074/jbc.M400977200. [DOI] [PubMed] [Google Scholar]
- 22.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: e31–e40, 2003. doi: 10.1161/01.RES.0000054200.44505.AB. [DOI] [PubMed] [Google Scholar]
- 23.Sato A, Sakuma I, Gutterman DD. Mechanism of dilation to reactive oxygen species in human coronary arterioles. Am J Physiol Heart Circ Physiol 285: H2345–H2354, 2003. doi: 10.1152/ajpheart.00458.2003. [DOI] [PubMed] [Google Scholar]
- 24.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation 12: 33–45, 2005. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 25.Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA. Microvesicles and exosomes for intracardiac communication. Cardiovasc Res 102: 302–311, 2014. doi: 10.1093/cvr/cvu022. [DOI] [PubMed] [Google Scholar]
- 26.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247, 2008. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 27.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 28.Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease—a thirthieth anniversary update. Acta Physiol (Oxf) 219: 22−96, 2017. doi: 10.1111/apha.12646. [DOI] [PubMed] [Google Scholar]
- 29.VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res 59: 277–287, 2003. doi: 10.1016/S0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- 30.Vion AC, Ramkhelawon B, Loyer X, Chironi G, Devue C, Loirand G, Tedgui A, Lehoux S, Boulanger CM. Shear stress regulates endothelial microparticle release. Circ Res 112: 1323–1333, 2013. doi: 10.1161/CIRCRESAHA.112.300818. [DOI] [PubMed] [Google Scholar]
- 31.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12: 9–14, 2011. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]