We investigated epigenetic changes that occur in the hypertensive kidney and how H2S supplementation reverses adverse effects. Inflammation, aberrant methylation, and dysfunction were observed in the hypertensive kidney, and these effects were alleviated with H2S supplementation. We identify miR-129 as a potential regulator of blood pressure and H2S regulation.
Keywords: hypertension, hydrogen sulfide, microRNA, inflammation
Abstract
Hypertension is a major risk factor for chronic kidney disease (CKD), and renal inflammation is an integral part in this pathology. Hydrogen sulfide (H2S) has been shown to mitigate renal damage through reduction in blood pressure and ROS; however, the exact mechanisms are not clear. While several studies have underlined the role of epigenetics in renal inflammation and dysfunction, the mechanisms through which epigenetic regulators play a role in hypertension are not well defined. In this study, we sought to identify whether microRNAs are dysregulated in response to angiotensin II (ANG II)-induced hypertension in the kidney and whether a H2S donor, GYY4137, could reverse the microRNA alteration and kidney function. Wild-type (C57BL/6J) mice were treated without or with ANG II and GYY4137 for 4 wk. Blood pressure, renal blood flow, and resistive index (RI) were measured. MicroRNA microarrays were conducted and subsequent target prediction revealed genes associated with a proinflammatory response. ANG II treatment significantly increased blood pressure, decreased blood flow in the renal cortex, increased RI, and reduced renal function. These effects were ameliorated in mice treated with GYY4137. Microarray analysis revealed downregulation of miR-129 in ANG II-treated mice and upregulation after GYY4137 treatment. Quantitation of proteins involved in the inflammatory response and DNA methylation revealed upregulation of IL-17A and DNA methyltransferase 3a, whereas H2S production enzymes and anti-inflammatory IL-10 were reduced. Taken together, our data suggest that downregulation of miR-129 plays a significant role in ANG II-induced renal inflammation and functional outcomes and that GYY4137 improves renal function by reversing miR-129 expression.
NEW & NOTEWORTHY We investigated epigenetic changes that occur in the hypertensive kidney and how H2S supplementation reverses adverse effects. Inflammation, aberrant methylation, and dysfunction were observed in the hypertensive kidney, and these effects were alleviated with H2S supplementation. We identify miR-129 as a potential regulator of blood pressure and H2S regulation.
hypertension is recognized as a major risk factor contributing to the development of cardiovascular and renal disease, with an estimated one-third of the United States population being afflicted with hypertension (13, 25). Essential hypertension is the most common form, yet the initiation for this disease is not well understood because no single factor has been identified to be responsible (45). Chronic kidney disease (CKD) is linked with hypertension, and it is deemed that more than 20 million adults suffer from CKD, with an estimated 29% of these individuals having hypertension (9, 29). The renin-angiotensin system (RAS) is an important mechanism in regulating blood pressure in the kidney, and deregulation of this process, namely by increases in angiotensin II (ANG II) expression, can cause endothelial damage, fibrosis, inflammation, mesangial cell proliferation, vasoconstriction, and increased arteriolar resistance, leading to decreased blood flow and glomerular filtration rates (GFRs) (2, 23, 43, 51).
Among the different mechanisms shown to play a role in hypertension, inflammation has received considerable interest in recent years. Cytokines are one type of molecule that have been implicated in hypertension, including IL-6, IL-17, interferon (IFN)-γ, and TNF-α (19, 27, 38, 47). There are several isoforms of IL-17; however, IL-17A has been extensively studied compared with IL-17D-F and has been shown to have the most prominent role in hypertension, with IL-17 knockout mice displaying a reduction in blood pressure after ANG II treatment (1, 14, 27, 31). In addition, several studies have reported that IL-17A and TNF-α work in concert to stimulate and modulate inflammation through a variety of chemokines (27, 39, 41). Although inflammation in hypertension is one resultant pathology, anti-inflammatory therapy has not been rigorously investigated as a form of treatment in hypertension.
Hydrogen sulfide (H2S) is a ubiquitous gasotransmitter synthesized from cysteine primarily by the enzymes cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) through the transsulfuration pathway and is a potent vasodilator (48). H2S has been shown to be important in a variety of functions, including cardiovascular, renal, liver, respiratory, and neuronal. Reduced levels of H2S have been linked with increased damage due to ROS and an increase in blood pressure (7, 52). Supplementation with H2S has been shown to mitigate renal damage in hypertensive models by a reduction of blood pressure, proteinuria, and oxidative stress and inhibition of excessive collagen type I and collagen type III deposition (17, 18, 44). Whether H2S acts as a pro- or anti-inflammatory molecule in hypertension is not clear. In addition, although studies have examined knockout models or point mutations in regard to H2S regulation and function, to date, few studies have investigated the potential epigenetic roles of regulation in H2S production.
In recent years, a considerable number of studies have sought to investigate the epigenetic mechanisms of gene regulation in renal development and function and hypertension (15, 20, 37, 50, 53). Epigenetics is defined as the study of the molecular mechanisms that regulate gene expression often leading to reversible changes that can be stable throughout the lifespan of an organism and potentially be heritable (11, 40, 49). DNA hypermethylation of the RASAL1 gene and dysregulation of miR-132, miR-212, miR-181b, and miR-663 in the RAS are two epigenetic mechanisms that have been studied in the context of hypertension in the kidney (3, 4, 6, 10, 28). The latter, microRNAs, are short (~22 nucleotides in length), single-stranded RNA genes that regulate posttranscriptional gene expression. miRNAs target mRNAs by binding to complementary sequences in the 3′-untranslated region, repressing their translation, and are associated with a broad spectrum of cellular and physiological processes and disease (12, 22). Although a few studies have identified microRNAs in the kidney in the context of hypertension, the mechanism(s) and importance of these microRNAs have not been clearly defined.
In this study, we sought to examine whether dysregulation of epigenetic miRNAs in hypertension leads to chronic inflammation and CKD and to determine whether H2S has the potential to mitigate the effects. Previous studies have identified microRNAs that are altered in hypertensive models; however, the mechanism(s) of dysregulation and its effects in hypertensive kidney have not been studied. In addition, methylation of genes involved in the development of hypertension and H2S regulation have not been thoroughly investigated.
MATERIALS AND METHODS
Animals and protocol.
Wild-type (WT) C57BL/6J mice were obtained from The Jackson Laboratories (Bar Harbor, ME) and housed in the animal care facility on a 12:12-h light-dark cycle at the University of Louisville. The study was approved by University of Louisville Institutional Animal Care and Use Committee. All mice were fed standard chow (no. 5010, LabDiet, St. Louis, MO) with free access to food and water. Animals aged 10–12 wk were grouped based on treatment, with n = 5 animals/group: WT, WT + GYY4137, WT + ANG II, and WT + GYY4137 + ANG II. Administration of ANG II (Sigma, St. Louis, MO) was at a rate of 1,000 ng·kg−1·min−1 for 4 wk via Alzet miniosmotic pumps (Durect, Cupertino, CA) inserted subcutaneously on the back right of mice. GYY4137 (Sigma) treatments were given by injection (133 µM·kg−1·day−1 ip) (26).
Cell culture and transfections.
Mouse glomerular endothelial cells were maintained in endothelial cell medium + 10% FBS and antibiotics. Cells were transfected with mimics or inhibitor for miR-129-5p and controls obtained from Exiqon (Woburn, MA). Cells were seeded at 30% confluency in growth medium without antibiotics the day before transfections. A concentration of 50 nM for mimics, inhibitors, and controls was transfected using Lipofectamine RNAiMAX transfection reagent according to the manufacturer’s recommendations. Cells were transfected for a period of 48 h and subsequently collected in lysis buffer for Western blot analysis as described below.
Blood pressure measurements.
Blood pressure measurements were recorded by the noninvasive tail-cuff method in conscious animals using a CODA high-throughput acquisition system (Kent Scientific, Torrington, CT). Animals were placed on a warming platform and allowed to acclimatize for 10 min before readings were taken (34).
Plasma H2S and nitrite/nitrate measurements.
Levels of H2S in plasma were measured as previously described (24). Plasma nitrite/nitrate (NOx) as an index of nitric oxide was measured using a parameter assay kit according to the manufacturer’s recommendations (R&D Systems, Minneapolis, MN). Briefly, plasma samples were obtained using heparin to separate it from blood and subsequently underwent ultrafiltration using VIVASPIN 2 filters (Sartorius, Ireland). Samples were measured for total nitrite levels or converted to nitrite from nitrate using nitrate reductase and subsequently subjected to a Griess reaction and assayed at 540 nm.
Renal ultrasonography.
Renal ultrasound was performed with a Vevo 2100 system (Visual Sonics, Toronto, ON, Canada) as previously described (36). In brief, at the end of the experiment, animals were anesthetized with isoflurane inhalation with an induction rate of 4% and maintenance of 1–1.5% for a duration of 20–30 min and secured on a heated table. The transducer was immobilized during imaging using an acoustic gel (Other-Sonic, Pharmaceutical Innovations, Newark, NJ). Measurements were taken using the long and short axis on the left kidney. Renal arterial and cortical blood flow were measured (in mm/s) by pulsed-wave Doppler mode. The resistive index (RI) was calculated as peak systolic velocity (PSV) – end-diastolic velocity (EDV)/PSV.
Laser Doppler flowmetry.
Renal cortical blood flow was measured using a Speckle Contrast Imager (Moor FLPI, Wilmington, DE) as previously described (35). Animals were anesthetized with 2,2,2-tribromoethanol (TBE) at a rate of 125 mg/kg and placed in the right lateral position, and the left kidney was exposed through a paraspinal longitudinal incision. The camera was positioned 15 cm from the region of interest. A flux-units trace was recorded for 2 min and quantified.
RNA isolation and microarrays.
Total RNA was isolated from a portion of the kidney from all animals for each group and was used for microarrays. Tissue was homogenized in QIAzol reagent using a BenchMark BeadBlaster (Edison, NJ) using 45-s pulses six times and subsequently extracted and purified using the Qiagen miRNeasy Mini Kit (Valencia, CA) according to the manufacturer’s protocol. RNA concentration and quality were assessed using a NanoDrop Instrument (Thermo Scientific, Wilmington, DE), and integrity was assessed on an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). Affymetrix (Santa Clara, CA) miRNA 4.0 cartridges + FlashTag reagents were run according to the manufacturer’s protocol through the University of Louisville Genomics Core. Data were analyzed using a Partek Genomics Suite (Partek, St. Louis, MO) and underwent quantile normalization, and microRNA calls were made using a robust multichip average. ANOVA and a least-squared difference (LSD) test were used to determine statistical significance (P < 0.05), along with implementation of a fold change of 1.2 in either direction to be considered differentially expressed. TargetScan (http://www.targetscan.org/) and miRWalk 2.0 (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/) were used to identify potential targets of microRNAs. In both instances, the mouse database was searched using default settings and a list of hits was obtained and manually investigated to search for targets of interest. Probe data have been deposited into the National Center for Biotechnology Information database and have been assigned the corresponding GEO accession number GSE92857.
Quantitative PCR.
Quantitative PCR (qPCR) was carried out using the TaqMan system (Applied Biosystems, Carlsbad, CA) on RNA samples extracted and purified as described above. RNA (10 ng) was transcribed into cDNA using the TaqMan MicroRNA Reverse Transcription Kit using specific stem-loop primers for miR-132, miR-128, miR-369, miR-129-5p, and miR-129-3p according to the manufacturer’s protocol. Subsequent qPCRs were set up on a LightCycler 96 instrument (Roche, Indianapolis, IN) and run in triplicate using U6 snRNA as a reference.
Methylation-specific PCR.
Genomic DNA (gDNA) was extracted from kidney tissue using the Qiagen DNeasy Blood and Tissue Kit according to the manufacturer’s recommendations. Quality and quantity were assessed using a Nanodrop 1000 spectrophotometer. A methylated control was created by taking 1 µg control gDNA and artificially methylating the sample using Sss I methylase according to the manufacturer’s recommendations (M0226S, New England BioLabs, Ipswich, MA). Subsequently, 2 µg gDNA was converted using an EpiTect Bisulfite Kit (Qiagen) according to the manufacturer’s recommendations. Methylation-specific PCR was performed on 50 ng of the converted sample using GoTaq Hot Start Master Mix (Promega, Madison, WI) with primers designed through MethPrimer software. Products were run on a 1.0% agarose gel and visualized on a ChemiDoc XRS system (Bio-Rad, Hercules, CA). Primer sequences are shown in Table 1.
Table 1.
Primers used for methylation-specific PCR
| Gene | Forward | Reverse |
|---|---|---|
| CBS, methylated | 5′-AGTTTATTTAGATTCGGACGCG-3′ | 5′-GACCCAAAATTAACGAATATCGAC-3′ |
| CBS, unmethylated | 5′-GGAGTTTATTTAGATTTGGATGTGG-3′ | 5′-CAACCCAAAATTAACAAATATCAAC-3′ |
| CSE, methylated | 5′-AACGAAATGGGTAGTATTACGGTAC-3′ | 5′-ACCAACGATAATTAATTACGTCGAC-3′ |
| CSE, unmethylated | 5′-TGAAATGGGTAGTATTATGGTATGA-3′ | 5′-AACCAACAATAATTAATTACATCAAC-3′ |
CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase.
Immunohistochemistry.
Frozen kidney sections of 5 µm thickness were fixed in 4% paraformaldehyde. Immunofluorescence staining was performed for DNA methyltransferase (DNMT)3a, IL-17A, TNF-α, IL-10, and F4/80 with primary antibody incubation overnight and subsequently incubated with the appropriate secondary antibody and counterstained with DAPI. Images were captured using a confocal microscope (FluoView1000, Olympus) and analyzed by Image ProPlus (version 7.0, Media Cybernetics, Rockville, MD).
Western blot analysis.
Whole kidney tissues were homogenized with RIPA buffer (Boston BioProducts, Ashland, MA) containing protease inhibitor cocktail (Sigma-Aldrich) and phenylmethylsulfonyl fluoride (36). Protein (100 µg) from each sample was loaded and electrophoresed with SDS-PAGE and then transferred onto polyvinylidene membranes. Membranes were probed with appropriate primary antibody at 4°C overnight. The respective horseradish peroxidase-conjugated secondary antibody was incubated for 2 h at room temperature. Membranes were developed using chemiluminescence (Pierce ECL Western blotting substrate, Thermo Scientific). GAPDH was used as loading control, and band intensity was quantified using ImageJ software.
Plasma creatinine measurement.
The level of plasma creatinine was measured in mice using a Quantichrom Creatinine Assay Kit (DICT-500) as previously described (24). Briefly, 2 mg/dl standards were made and 30 µl of diluted samples were loaded onto a clear 96-well plate. Reagents (200 µl) were added to standards and samples and briefly mixed. The plate was immediately loaded into a SpectraMaxx spectrophotometer (Molecular Devices, Sunnyvale, CA) and read at 510-nm wavelength. A second measurement was taken 5 min after the addition of reagents. The creatinine concentration was calculated using the following formula: [(ODsample 5 − ODsample 0)/(ODstandard 5 − ODstandard 0)] × standard concentration (in mg/dl).
Statistical analysis.
All results are expressed as means ± SD. Comparison between the groups was carried out using one-way ANOVA followed by a LSD post hoc test using Prism 5.0 (Graph Software, San Diego, CA). For percent change analysis, a Student’s t-test was used to compare the differences in percent change from treated animals against control animals. Controls were set to a value of 100% for the analysis. Significance was achieved with a value of P < 0.05.
RESULTS
H2S treatment reduces blood pressure and plasma creatinine levels and increases total NOx.
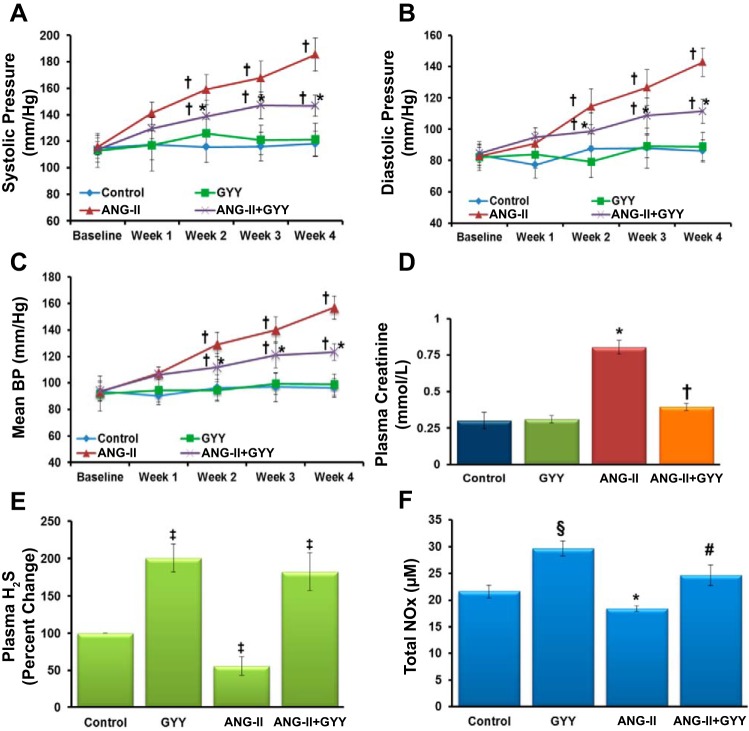
Baseline systolic, diastolic, and mean blood pressures were similar among the different treatment groups with no significant differences observed (Fig. 1, A–C). Over the course of the 4-wk treatment period, mean blood pressure rose steadily in mice that received ANG II treatment and was significantly higher than animals that received GYY4137 injections and controls (Fig. 1A). Systolic and diastolic blood pressures followed a similar trend (Fig. 1, B and C). Interestingly, mice treated with ANG II and GYY4137 showed a significant reduction in mean, systolic, and diastolic blood pressure compared with ANG II-treated animals (Fig. 1, A–C). Plasma creatinine levels were found to be significantly higher in ANG II-treated mice compared with controls and GYY4137-treated mice (Fig. 1D). Furthermore, the levels of creatinine in plasma were also significantly reduced in animals treated with ANG II and GYY4137 compared with ANG II alone, with levels similar to those of controls. H2S was measured in all animal groups from plasma collected at the end of the treatment period. Percent changes in H2S levels were significantly reduced in hypertensive mice compared with controls, whereas mice treated with the H2S donor GYY4137 had increased levels of plasma H2S (Fig. 1E). In addition, total NOx levels were altered among the different groups, with lower NOx found in mice treated with ANG II, whereas mice treated with GYY4137 showed an increase in NOx in plasma (Fig. 1F).
Fig. 1.
A–C: systolic, diastolic, and mean blood pressures were elevated in mice treated with ANG II. After 2 wk of GYY4137 (GYY) supplementation, all three pressures decreased significantly in mice cotreated with ANG II + GYY compared with mice treated with ANG II only. D: plasma creatinine levels were significantly elevated in hypertensive mice compared with control and GYY-treated animals. GYY supplementation significantly lowered levels of creatinine in hypertensive mice (n = 5). E: percent changes in plasma H2S levels were decreased in hypertensive mice; however, levels were increased in plasma of mice treated with GYY. F: nitrite/nitrate (NOx) levels in plasma followed a similar trend to that of H2S, with decreased levels observed in ANG II-treated mice and increased levels in plasma of mice treated with GYY. *P < 0.05 vs. control, GYY, and ANG II + GYY; †P < 0.05 vs. control and GYY; ‡P < 0.05 vs. control; §P < 0.05 vs. control, ANG II, and ANG II + GYY; #P < 0.05 vs. control, ANG II, and GYY.
Renal vascular resistance is reduced with GYY4137 treatment in animals treated with ANG II.
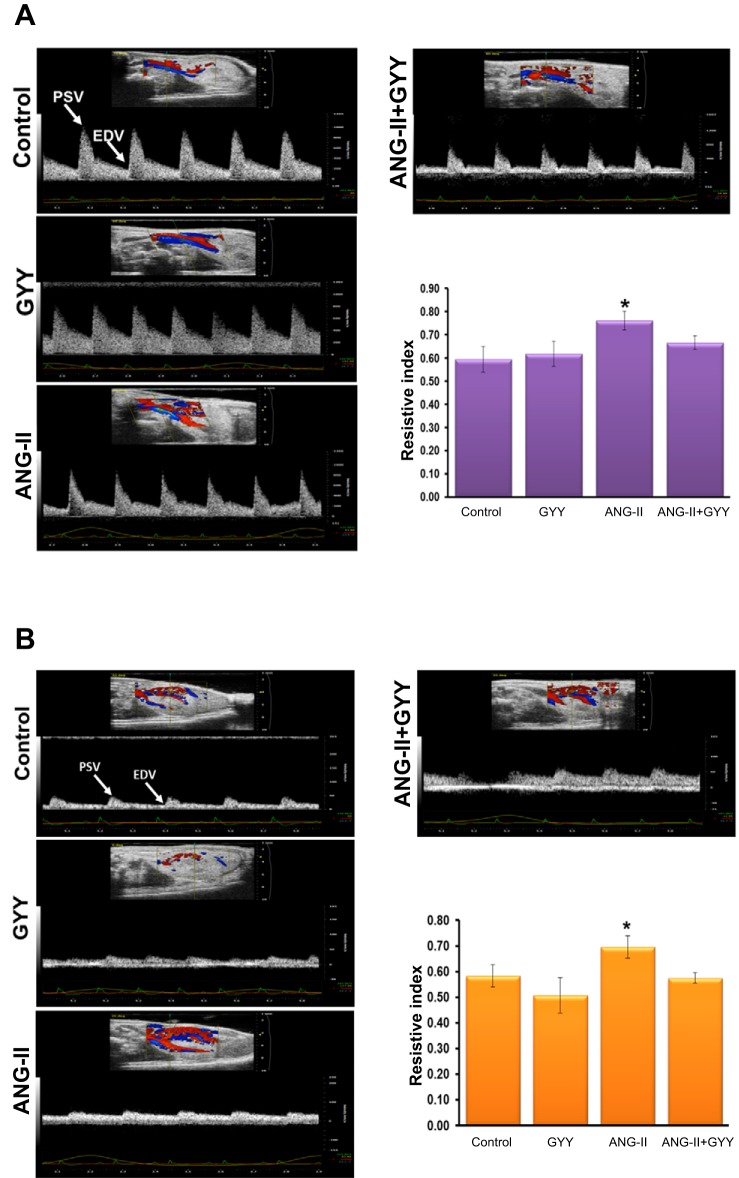
In an effort to assess the renal arterial and cortical vascular resistance of mice treated with ANG II and whether GYY4137 can reduce the constrictive effects of ANG II, we used ultrasonography to detect changes in blood flow. RI is a measure of vascular resistance and assessment for renal disease, and hypertension leads to an increase in resistance and overall RI. In ANG II-treated mice, RI increased significantly compared with mice treated with GYY4137 or control animals (Fig. 2A). Upon treatment with GYY4137, in animals cotreated with ANG II RI was significantly reduced and comparable to that of control animals, as displayed as a bar graph. A similar effect was observed in the renal cortex, with ANG II-treated animals showing a higher RI value compared with control and GYY4137-treated animals, with cotreatment of ANG II and GYY4137 having a RI value similar to that of control animals (Fig. 2B).
Fig. 2.
Renal arterial and cortical resistive indices are increased in hypertensive kidney. A and B: ultrasound analysis of the renal artery (A) and cortex (B) showing the resistive index in mice treated with ANG II and GYY. The bar graph represents the mean resistive index ± SD. n = 5 mice/group. *P < 0.05.
ANG II-induced hypertension reduces renal cortical blood flow and is restored with GYY4137 supplementation.
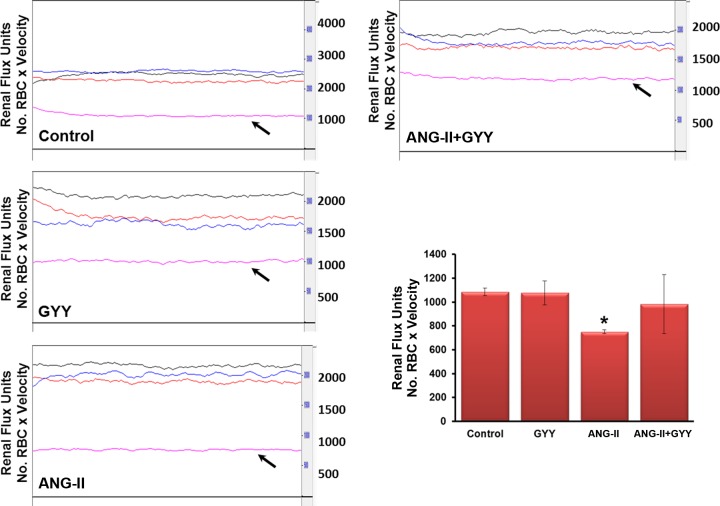
Because it is known that ANG II is a potent vasoconstrictor and reduces blood flow, we tested whether H2S supplementation would reverse the effects of ANG II and restore normal blood flow. There were no significant differences between control and GYY4137-treated animals; however, there was a significant reduction in blood flow in the renal cortex of mice treated with ANG II (Fig. 3). Moreover, GYY4137 treatment in animals also treated with ANG II showed an increase in blood flow, returning to levels near controls and significantly higher than hypertensive mice (Fig. 3).
Fig. 3.
Renal blood flow is decreased in the kidney of ANG II-treated mice and is restored with H2S treatment. Laser Doppler flowmetry showed reduced blood flow in the renal cortex (black arrow) of mice treated with ANG II compared with mice treated with GYY. The line trace represents blood flow in the aorta (black), renal artery (red), and renal vein (blue). The bar graph represents data as mean renal flux units ± SD. n = 5 mice/group. *P < 0.05.
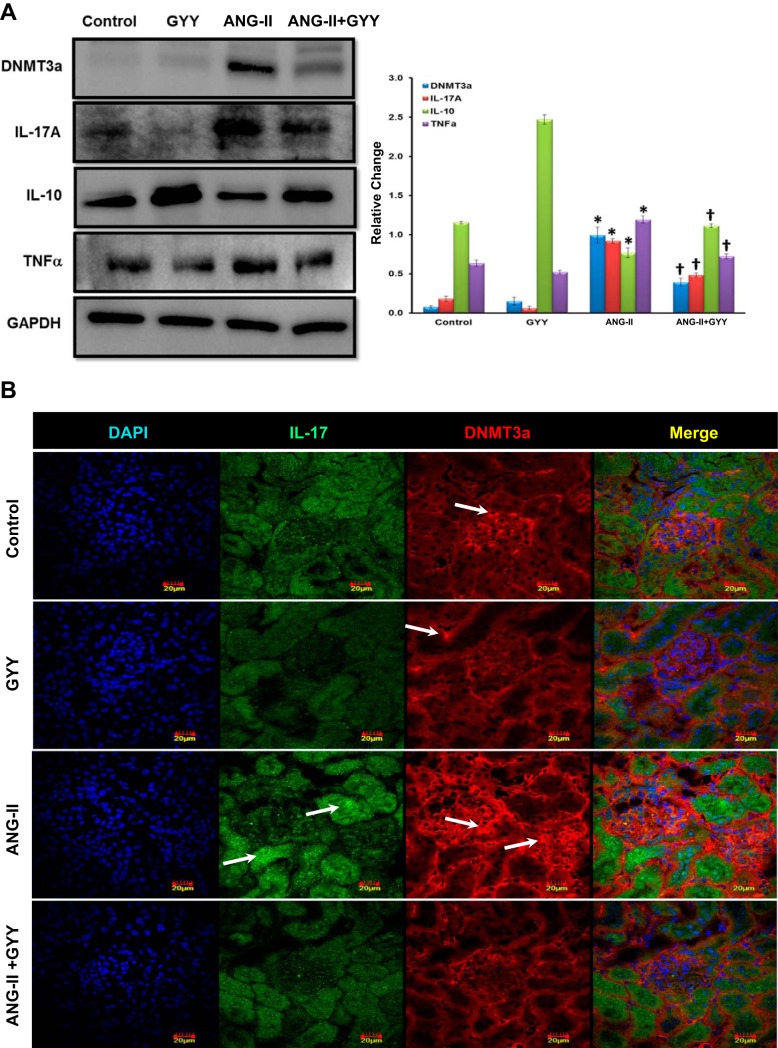
ANG II treatment significantly upregulates epigenetic modulator DNMT3a and proinflammatory cytokine IL-17A and downregulates the H2S production pathway enzymes CBS/CSE and anti-inflammatory cytokine IL-10.
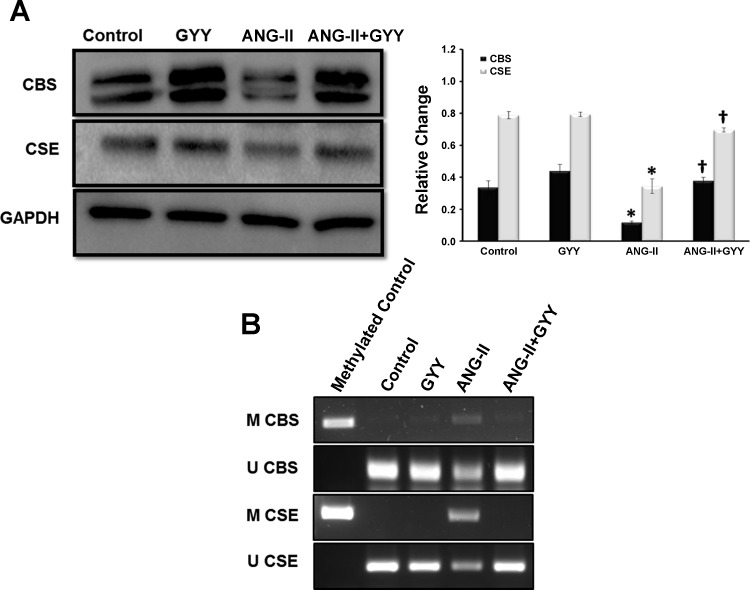
Methylation has been shown to play a role in hypertension and renal pathologies such as fibrosis (33). In addition, although H2S levels have been shown to be low in hypertension, the mechanism(s) involved remains unanswered. Moreover, animal models have supported a role for inflammation in renal dysfunction. We investigated the protein levels of H2S production enzymes CBS and CSE, the de novo methyltransferase DNMT3a, and the proinflammatory and anti-inflammatory markers IL-17A, TNF-α, and IL-10, respectively. The H2S production pathway enzymes CBS and CSE were significantly downregulated in the kidneys of mice treated with ANG II; these enzymes were restored to baseline levels in animals cotreated with ANG II and GYY4137 (Fig. 4A). Methylation-specific PCR showed an increase in promoter methylation of both CBS and CSE in the kidneys of hypertensive mice, whereas GYY4137 supplementation reduces the methylation status of these enzymes (Fig. 4B). The expression level of DNMT3a was significantly increased in the kidneys of mice treated with ANG II compared with control and GYY4137-treated animals. Protein levels of DNMT3a were significantly reduced when GYY4137 was supplemented in hypertensive mice, bringing expression levels closer to those of controls (Fig. 5A). The proinflammatory marker IL-17A was found to be upregulated in the ANG II-treated group and was significantly reduced with GYY4137 supplementation in hypertensive mice (Fig. 5A). IL-10, an anti-inflammatory marker, was significantly reduced in ANG II-treated mice, with levels being restored when GYY4137 was administered in mice treated with ANG II (Fig. 5A).
Fig. 4.
The hydrogen sulfide production enzymes cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) are downregulated in the hypertensive kidney. A: protein (100 µg) was resolved on SDS-PAGE gels and probed with the appropriate antibodies overnight. Band intensities were quantified using ImageJ software. Bar graphs indicate mean intensities ± SD. B: methylation-specific PCR showed an increase in promoter methylation of CBS and CSE of hypertensive mice compared with control and GYY-supplemented mice. n = 5 mice/group. *P < 0.05, control vs. ANG II; †P < 0.05 ANG II vs. ANG II + GYY.
Fig. 5.
Epigenetic regulator DNA methyltransferase (DNMT)3a and proinflammatory IL-17A are increased and anti-inflammatory IL-10 is downregulated in the hypertensive kidney. A: protein (100 µg) was resolved on SDS-PAGE gels and probed with the appropriate antibodies overnight. Band intensities were quantified using ImageJ software. The bar graphs indicate mean intensities ± SD. n = 5 mice/group. *P < 0.05, control vs. ANG II; †P < 0.05, ANG II vs. ANG II + GYY. B: DNMT3a and IL-17 expression were increased in glomeruli and tubular areas of the kidney. Immunostaining for DNMT3a and IL-17A showed increased expression in glomeruli and proximal tubules, supporting Western blot data (white arrows). Magnification: ×60. Scale bars = 20 µm.
The protein expression of DNMT3a and IL-17A was further confirmed by immunostaining. No changes were found in groups with or without GYY4137 treatment for both DNMT3a and IL-17A (Fig. 5B). In contrast, both DNMT3a and IL-17A were upregulated in ANG II-treated animals, with DNMT3a showing localized expression in the glomeruli and IL-17A predominately in proximal tubules. Treatment with GYY4137 in these animals mitigated their expression (Fig. 5B).
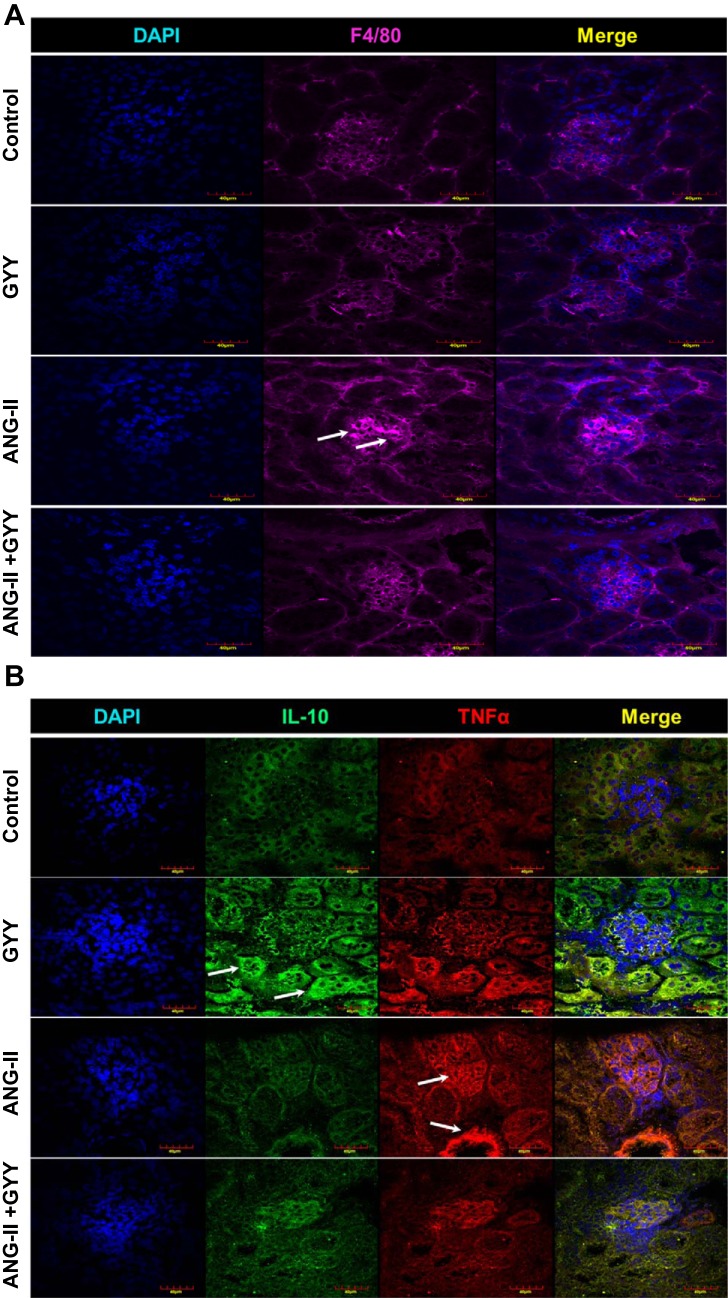
Macrophage-induced inflammation in kidneys of hypertensive mice is alleviated with H2S supplementation.
Kidneys from hypertensive mice showed an increased signal in the glomeruli for F4/80, a marker for macrophage infiltration, whereas mice given H2S showed minimal expression (Fig. 6A). Macrophage-mediated inflammation is supported by an increased signal of TNF-α in the glomeruli and blood vessels of the kidney in mice treated with ANG II, whereas anti-inflammatory cytokine IL-10 expression is increased in mice supplemented with H2S (Fig. 6B).
Fig. 6.
Macrophage infiltration in the kidney promotes inflammation. A: F4/80 expression was increased in glomeruli of the kidney. Immunostaining for F4/80 showed increased expression in glomeruli of the kidney, supporting macrophage-mediated infiltration and promotion of inflammation in hypertensive mice (white arrows). B: TNF-α expression was increased in glomeruli and tubular areas of the kidney. IL-10 was low in hypertensive mice but elevated in kidneys of mice treated with GYY, supporting Western blot data (white arrows). Magnification: ×60. Scale bars = 40 µm.
Dysregulation of the miRNome by ANG II-induced hypertension in the kidney.
Microarray analysis of all known microRNAs and stem-loop RNAs in the mouse genome were used to identify microRNAs that were altered in the kidneys of the various groups of treated mice. A total of 187 microRNAs were found to be significantly altered in the kidneys of hypertensive mice, whereas 150 microRNAs were altered in hypertensive mice treated with GYY4137 (see Supplemental Tables S1 and S2 in the Supplemental Material; Supplemental Material is available at the American Journal of Physiology-Heart and Circulatory Physiology website). Mice that received H2S supplementation displayed the fewest number of changed microRNAs, with 116 microRNAs being disrupted (Supplemental Table S3). Table 2 shows qPCR analysis of several microRNAs identified to be changed in one or more treated animals, with high agreement between microarrays and qPCR. miR-129 family members were found to be altered in all three treatment groups, showing suppression in hypertensive mice but an induction in mice treated with H2S alone or with both.
Table 2.
MicroRNAs found to be significantly altered by arrays were further confirmed by quantitative PCR
| MicroRNA | ANG II |
GYY |
ANG II + GYY |
|||
|---|---|---|---|---|---|---|
| Array | Quantitative PCR | Array | Quantitative PCR | Array | Quantitative PCR | |
| miR-132 | 2.06* | 3.74† | −1.01 | −1.30† | 1.618 | 2.39† |
| miR-128 | −1.28* | −1.47† | −1.05 | −1.03 | −1.26* | 1.14 |
| miR-369 | 1.37* | 1.94† | 1.08 | 1.13 | −1.23* | −1.11 |
| miR-129-5p | 1.05 | −1.14† | 1.90* | 2.07† | 2.04* | 1.91† |
| miR-129-3p | −1.58* | −1.04 | −1.31 | −1.23† | −1.04 | −1.10 |
GYY, GYY4137.
MicroRNA found to be significantly altered by array.
MicroRNA found to be significantly altered by quantitative PCR in the respective treatment (n = 5).
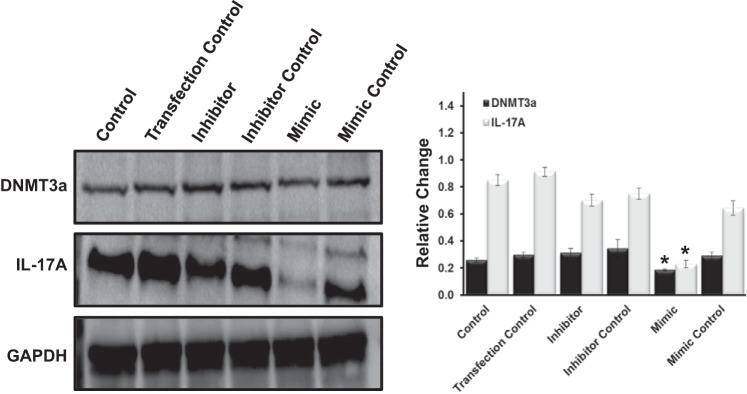
miR-129 targets IL-17A and DNMT3a in mouse glomerular endothelial cells.
In an effort to support a role for miR-129 in mediating hypertensive effects in the kidney, mouse glomerular endothelial cells were transfected with mimics, inhibitors, and controls against miR-129. Increased expression of miR-129 by the mimic form transfected into cells showed a significant decrease in levels of IL-17A and DNMT3a compared with controls and inhibitor (Fig. 7). Controls and an inhibitor against miR-129 showed similar levels of IL-17A and DNMT3a among these groups.
Fig. 7.
miR-129 overexpression suppresses IL-17A and DNMT3a expression in endothelial cells. Mouse glomerular endothelial cells were transfected with mimics, inhibitors, and controls against miR-129. Protein (100 µg) was resolved on SDS-PAGE gels and probed with the appropriate antibodies overnight. Band intensities were quantified using ImageJ software. Bar graphs indicate mean intensities ± SD. n = 5 mice/group. *P < 0.05.
DISCUSSION
The principal findings of this study offer new insights into the epigenetic regulators that are disrupted in the hypertensive kidney, relating miR-129 and DNMT3a to inflammation, H2S deficiency, and how supplementation with GYY4137 can regulate blood pressure by acting as an anti-inflammatory agent through epigenetic mechanisms. We showed that H2S supplementation in ANG II-induced hypertension decreases blood pressure and plasma creatinine levels, increases blood flow, and decreases arterial stiffening. In addition, NOx bioavailability was increased in mice with GYY4137 supplementation, as evidenced by higher plasma levels of NOx. This increase may aide in the increased function of the kidney; however, more studies are needed to understand the interaction between H2S and NOx because these are limited to date. This is of particular interest because the assays used in this study have limitations, notably the sensitivity, limits of detection, and collection and storage of samples for H2S, nitrites, and nitrates in plasma (5, 42). The proinflammatory cytokine IL-17A was found to be significantly reduced in the kidneys of hypertensive mice supplemented with H2S, whereas anti-inflammatory response molecule IL-10 protein expression was increased in these same animals. F4/80 and TNF-α expression were also higher in the kidneys of hypertensive mice, supporting a role for macrophage-induced inflammation in the kidney, and was alleviated by H2S supplementation. The two principle enzymes responsible in H2S production, CBS and CSE, were found to be decreased in hypertensive mice and were restored with H2S supplementation. In addition, the promoter region of these enzymes was found to be hypermethylated in the hypertensive kidney, whereas GYY4137 supplementation reduced methylation at these sites. Interestingly, protein expression of DNMT3a was found to be increased in mice treated with ANG II compared with mice treated with H2S and controls. These findings were further supported by DNMT3a expression being found higher in the glomerulus and IL-17A in the proximal tubules of the kidney in hypertensive mice, with expression being reduced with H2S supplementation. Global profiling of the miRNome in the kidney of these animals revealed several microRNAs altered in the hypertensive kidney and with H2S treatment. Among these, miR-129 was found to be downregulated in ANG II-treated animals; however, miR-129 was upregulated when GYY4137 was used as a treatment either alone or along with ANG II. Transfection experiments with a mimic of miR-129 showed decreased expression of IL-17A and DNMT3a, supporting a direct regulatory role for miR-129 and these two molecules in hypertension.
A limited number of studies have investigated changes in the miRNome in the context of the kidney in hypertension (10, 28). Moreover, these studies offer a snapshot of changes occurring in microRNAs in the kidney and do not tie these altered microRNAs to molecular or physiological changes. To identify new microRNAs that are disrupted in hypertension and how these changes affect the inflammatory response, we performed microRNA arrays on the kidney from animals treated with ANG II, GYY4137, or both. We found close to 200 microRNAs altered in the hypertensive kidney. Previously identified miR-132, found to be upregulated after ANG II treatment (10), was also identified in our data set, along with miR-212 in the cotreatment group. miR-483-3p was found to be suppressed in response to ANG II treatment and targets several members of the RAS in a previous study of vascular smooth muscle cells (21), whereas here we identified miR-483-5p as also being downregulated in the kidneys of ANG II-treated mice. One microRNA that was of interest to us was miR-129, which has not been previously identified in any hypertension system and has been investigated only in terms of renal cell carcinoma (8). We found miR-129-3p to be suppressed in the kidneys of mice treated with ANG II; however, miR-129-5p was upregulated by H2S supplementation and was still increased in mice cotreated with ANG II and GYY4137.
To identify potential mechanisms of action, in silico analysis of miR-129-5p revealed several members of the inflammatory response pathway to be potential targets of miR-129-5p, including IL-17A, and the de novo methyltransferase DNMT3a. IL-17A has been implicated in a variety of inflammation-associated diseases, including heart, rheumatoid arthritis, psoriasis, and periodontal disease (16, 30, 32). The role of IL-17A in regulating blood pressure has received attention as of late, and knockout of this cytokine has been shown to lower blood pressure, albeit not to baseline levels (27). Although DNMTs have been implicated in numerous biological processes and diseases, very little to no evidence has examined this epigenetic regulator in the hypertensive kidney. Here, we report DNMT3a to be upregulated in the kidneys of hypertensive mice, suggesting aberrant methylation may be occurring in these animals. We found the CBS and CSE protein levels, which are two of the main enzymes involved in H2S production pathway, to be downregulated in hypertensive mice. In addition, hypermethylation of CBS and CSE supports the downregulation of these enzymes. This inverse relationship points to an epigenetic mechanism of silencing of these two genes by DNMT3a in hypertension, because a link between lower H2S levels and hypertension has been previously shown (46, 52).
Because IL-17A and DNMT3a are both predicted to be targets of miR-129-5p, it is interesting to hypothesize that this microRNA may be an upstream regulator of both these genes and, therefore, a critical modulator of inflammation and H2S in the kidney. During hypertension, as miR-129 expression is suppressed, aberrant methylation of CBS and CSE genes by DNMT3a leads to a decrease in H2S production, and increased IL-17A production leads to a prolonged inflammatory response, both events leading to kidney damage and reduced function. Supplementation with the H2S donor GYY4137 alleviates these effects by reversing the expression of miR-129, and therefore can target and suppress DNMT3a and IL-17A and decrease inflammation. How exactly H2S supplementation acts on miR-129 is a question for future studies.
In summary, we demonstrated that ANG II downregulates miR-129, CBS, CSE, and IL-10 while increasing IL-17A and DNMT3a in the hypertensive kidney, leading to increased inflammation and kidney dysfunction. These effects were alleviated with H2S supplementation. Furthermore, H2S restored blood flow and lowered RI of the renal artery and cortex. miR-129 is predicted to target both IL-17A and DNMT3a, identifying a potential mechanism of blood pressure and H2S regulation, and predicts a novel therapeutic target in hypertension. Future studies are needed to confirm whether IL-17A and DNMT3a are direct targets of miR-129.
GRANTS
Support for this work was provided in part by National Institutes of Health Grants HL-104103 and DK-104653 (to U. Sen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.W. and S.P. performed experiments; G.W. and S.P. analyzed data; G.W. and S.P. interpreted results of experiments; G.W. prepared figures; G.W. drafted manuscript; G.W., S.P., and U.S. edited and revised manuscript; G.W., S.P., and U.S. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sabine Waigel, Vennila Arumugam, and staff in the University of Louisville Genomics Core for their assistance with performing the miRNA arrays and data analysis. We also thank Geetansh Tyagi for help with technical assistance.
REFERENCES
- 1.Amador CA, Barrientos V, Peña J, Herrada AA, González M, Valdés S, Carrasco L, Alzamora R, Figueroa F, Kalergis AM, Michea L. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 63: 797–803, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02883. [DOI] [PubMed] [Google Scholar]
- 2.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm 13, Suppl B: 9–20, 2007. doi: 10.18553/jmcp.2007.13.s8-b.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bechtel W, McGoohan S, Zeisberg EM, Müller GA, Kalbacher H, Salant DJ, Müller CA, Kalluri R, Zeisberg M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med 16: 544–550, 2010. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckerman P, Ko Y-A, Susztak K. Epigenetics: a new way to look at kidney diseases. Nephrol Dial Transplant 29: 1821–1827, 2014. doi: 10.1093/ndt/gfu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellavia L, Kim-Shapiro DB, King SB. Detecting and monitoring NO, SNO and nitrite in vivo. Future Sci OA 1: FSO36, 2015. doi: 10.4155/fso.15.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bomotti SM, Smith JA, Zagel AL, Taylor JY, Turner ST, Kardia SL. Epigenetic markers of renal function in African Americans. Nurs Res Pract 2013: 687519, 2013. doi: 10.1155/2013/687519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos EM, Wang R, Snijder PM, Boersema M, Damman J, Fu M, Moser J, Hillebrands JL, Ploeg RJ, Yang G, Leuvenink HG, van Goor H. Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol 24: 759–770, 2013. doi: 10.1681/ASN.2012030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Ruan A, Wang X, Han W, Wang R, Lou N, Ruan H, Qiu B, Yang H, Zhang X. miR-129-3p, as a diagnostic and prognostic biomarker for renal cell carcinoma, attenuates cell migration and invasion via downregulating multiple metastasis-related genes. J Cancer Res Clin Oncol 140: 1295–1304, 2014. doi: 10.1007/s00432-014-1690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension 52: 818–827, 2008. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 10.Eskildsen TV, Jeppesen PL, Schneider M, Nossent AY, Sandberg MB, Hansen PB, Jensen CH, Hansen ML, Marcussen N, Rasmussen LM, Bie P, Andersen DC, Sheikh SP. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int J Mol Sci 14: 11190–11207, 2013. doi: 10.3390/ijms140611190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friso S, Carvajal CA, Fardella CE, Olivieri O. Epigenetics and arterial hypertension: the challenge of emerging evidence. Transl Res 165: 154–165, 2015. doi: 10.1016/j.trsl.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Gebeshuber CA, Kornauth C, Dong L, Sierig R, Seibler J, Reiss M, Tauber S, Bilban M, Wang S, Kain R, Böhmig GA, Moeller MJ, Gröne HJ, Englert C, Martinez J, Kerjaschki D. Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat Med 19: 481–487, 2013. doi: 10.1038/nm.3142. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129: e28–e292, 2014. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine 64: 477–485, 2013. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilliard SA, El-Dahr SS. Epigenetics mechanisms in renal development. Pediatr Nephrol 31: 1055−1060, 2016. 10.1007/s00467-015-3228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmlund A, Holm G, Lind L. Severity of periodontal disease and number of remaining teeth are related to the prevalence of myocardial infarction and hypertension in a study based on 4,254 subjects. J Periodontol 77: 1173–1178, 2006. doi: 10.1902/jop.2006.050233. [DOI] [PubMed] [Google Scholar]
- 17.Holwerda KM, Burke SD, Faas MM, Zsengeller Z, Stillman IE, Kang PM, van Goor H, McCurley A, Jaffe IZ, Karumanchi SA, Lely AT. Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J Am Soc Nephrol 25: 717–725, 2014. doi: 10.1681/ASN.2013030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang P, Shen Z, Liu J, Huang Y, Chen S, Yu W, Wang S, Ren Y, Li X, Tang C, Du J, Jin H. Hydrogen sulfide inhibits high-salt diet-induced renal oxidative stress and kidney injury in Dahl rats. Oxid Med Cell Longev 2016: 2807490, 2016. doi: 10.1155/2016/2807490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishimitsu T, Uehara Y, Numabe A, Tsukada H, Ogawa Y, Iwai J, Ikeda T, Matsuoka H, Sugimoto T, Yagi S. Interferon gamma attenuates hypertensive renal injury in salt-sensitive Dahl rats. Hypertension 19: 804–808, 1992. doi: 10.1161/01.HYP.19.6.804. [DOI] [PubMed] [Google Scholar]
- 20.Julian CG, Pedersen BS, Salmon CS, Yang IV, Gonzales M, Vargas E, Moore LG, Schwartz DA. Unique DNA methylation patterns in offspring of hypertensive pregnancy. Clin Transl Sci 8: 740–745, 2015. doi: 10.1111/cts.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemp JR, Unal H, Desnoyer R, Yue H, Bhatnagar A, Karnik SS. Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin-angiotensin system. J Mol Cell Cardiol 75: 25–39, 2014. doi: 10.1016/j.yjmcc.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khella HW, Bakhet M, Lichner Z, Romaschin AD, Jewett MA, Yousef GM. MicroRNAs in kidney disease: an emerging understanding. Am J Kidney Dis 61: 798–808, 2013. doi: 10.1053/j.ajkd.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 24.Kundu S, Pushpakumar S, Sen U. MMP-9- and NMDA receptor-mediated mechanism of diabetic renovascular remodeling and kidney dysfunction: hydrogen sulfide is a key modulator. Nitric Oxide 46: 172–185, 2015. doi: 10.1016/j.niox.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260, 2012. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Han Y, Li L, Lu H, Meng G, Li X, Shirhan M, Peh MT, Xie L, Zhou S, Wang X, Chen Q, Dai W, Tan CH, Pan S, Moore PK, Ji Y. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E−/− mice. Br J Pharmacol 169: 1795–1809, 2013. doi: 10.1111/bph.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YH, Charchar FJ, Morris BJ. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension 58: 1093–1098, 2011. doi: 10.1161/HYPERTENSIONAHA.111.180729. [DOI] [PubMed] [Google Scholar]
- 29.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O’Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT Jr; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators . Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 55: 441–451, 2010. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 55: 829–835, 2006. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res 97: 696–704, 2013. doi: 10.1093/cvr/cvs422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panoulas VF, Douglas KM, Milionis HJ, Stavropoulos-Kalinglou A, Nightingale P, Kita MD, Tselios AL, Metsios GS, Elisaf MS, Kitas GD. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 46: 1477–1482, 2007. doi: 10.1093/rheumatology/kem169. [DOI] [PubMed] [Google Scholar]
- 33.Pushpakumar S, Kundu S, Narayanan N, Sen U. DNA hypermethylation in hyperhomocysteinemia contributes to abnormal extracellular matrix metabolism in the kidney. FASEB J 29: 4713–4725, 2015. doi: 10.1096/fj.15-272443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pushpakumar S, Kundu S, Pryor T, Givvimani S, Lederer E, Tyagi SC, Sen U. Angiotensin-II induced hypertension and renovascular remodelling in tissue inhibitor of metalloproteinase 2 knockout mice. J Hypertens 31: 2270–2281, 2013. doi: 10.1097/HJH.0b013e3283649b33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pushpakumar SB, Kundu S, Metreveli N, Sen U. Folic acid mitigates angiotensin-II-induced blood pressure and renal remodeling. PLoS One 8: e83813, 2013. doi: 10.1371/journal.pone.0083813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pushpakumar SB, Kundu S, Metreveli N, Tyagi SC, Sen U. Matrix metalloproteinase inhibition mitigates renovascular remodeling in salt-sensitive hypertension. Physiol Rep 1: e00063, 2013. doi: 10.1002/phy2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raftopoulos L, Katsi V, Makris T, Tousoulis D, Stefanadis C, Kallikazaros I. Epigenetics, the missing link in hypertension. Life Sci 129: 22–26, 2015. doi: 10.1016/j.lfs.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Ramseyer VD, Hong NJ, Garvin JL. Tumor necrosis factor α decreases nitric oxide synthase type 3 expression primarily via Rho/Rho kinase in the thick ascending limb. Hypertension 59: 1145–1150, 2012. doi: 10.1161/HYPERTENSIONAHA.111.189761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-α is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem 279: 2559–2567, 2004. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 40.Scherrer U, Rimoldi SF, Sartori C, Messerli FH, Rexhaj E. Fetal programming and epigenetic mechanisms in arterial hypertension. Curr Opin Cardiol 30: 393–397, 2015. doi: 10.1097/HCO.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 41.Sharma AK, Mulloy DP, Le LT, Laubach VE. NADPH oxidase mediates synergistic effects of IL-17 and TNF-α on CXCL1 expression by epithelial cells after lung ischemia-reperfusion. Am J Physiol Lung Cell Mol Physiol 306: L69–L79, 2014. doi: 10.1152/ajplung.00205.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med 50: 1021–1031, 2011. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siragy HM, Carey RM. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol 31: 541–550, 2010. doi: 10.1159/000313363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snijder PM, Frenay AR, Koning AM, Bachtler M, Pasch A, Kwakernaak AJ, van den Berg E, Bos EM, Hillebrands JL, Navis G, Leuvenink HG, van Goor H. Sodium thiosulfate attenuates angiotensin II-induced hypertension, proteinuria and renal damage. Nitric Oxide 42: 87–98, 2014. doi: 10.1016/j.niox.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, Kanbay M. Hypertension as an autoimmune and inflammatory disease. Hypertens Res 39: 567–573, 2016. doi: 10.1038/hr.2016.35. [DOI] [PubMed] [Google Scholar]
- 46.Sun L, Sun S, Li Y, Pan W, Xie Y, Wang S, Zhang Z. Potential biomarkers predicting risk of pulmonary hypertension in congenital heart disease: the role of homocysteine and hydrogen sulfide. Chin Med J (Engl) 127: 893–899, 2014. [PubMed] [Google Scholar]
- 47.Vázquez-Oliva G, Fernández-Real JM, Zamora A, Vilaseca M, Badimón L. Lowering of blood pressure leads to decreased circulating interleukin-6 in hypertensive subjects. J Hum Hypertens 19: 457–462, 2005. doi: 10.1038/sj.jhh.1001845. [DOI] [PubMed] [Google Scholar]
- 48.Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov 14: 329–345, 2015. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Gong L, Tan Y, Hui R, Wang Y. Hypertensive epigenetics: from DNA methylation to microRNAs. J Hum Hypertens 29: 575–582, 2015. doi: 10.1038/jhh.2014.132. [DOI] [PubMed] [Google Scholar]
- 50.Watson CJ, Horgan S, Neary R, Glezeva N, Tea I, Corrigan N, McDonald K, Ledwidge M, Baugh J. Epigenetic therapy for the treatment of hypertension-induced cardiac hypertrophy and fibrosis. J Cardiovasc Pharmacol Ther 21: 127–137, 2016. doi: 10.1177/1074248415591698. [DOI] [PubMed] [Google Scholar]
- 51.Wolf G, Butzmann U, Wenzel UO. The renin-angiotensin system and progression of renal disease: from hemodynamics to cell biology. Nephron Physiol 93: 3–13, 2003. doi: 10.1159/000066656. [DOI] [PubMed] [Google Scholar]
- 52.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322: 587–590, 2008. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yara S, Lavoie JC, Levy E. Oxidative stress and DNA methylation regulation in the metabolic syndrome. Epigenomics 7: 283–300, 2015. doi: 10.2217/epi.14.84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.