Abstract
Giant cell arteritis (GCA) is a granulomatous vasculitis of the aorta and its medium-sized branch vessels. CD4 T cells, macrophages, and dendritic cells (DCs) build granulomatous infiltrates that injure the vessel wall and elicit a maladaptive response to injury. Pathological consequences include fragmentation of elastic membranes, destruction of the medial layer, microvascular neoangiogenesis, massive outgrowth of myofibroblasts, and lumen-occlusive intimal hyperplasia. Antigens have been suspected to drive the local activation of vasculitogenic CD4 T cells, but recent data have suggested a more generalized defect in the threshold setting of such T cells, rendering them hyperreactive. Under physiological conditions, immune checkpoints provide negative signals to curb T cell activation and prevent inflammation-associated tissue destruction. This protective mechanism is disrupted in GCA. Vessel wall DCs fail to express the immunoinhibitory ligand programmed cell death ligand-1, leaving lesional T cells unchecked. Consequently, programmed cell death protein-1-positive CD4 T cells can enter the immunoprivileged vessel wall, where they produce a broad spectrum of inflammatory cytokines (interferon-γ, IL-17, and IL-21) and have a direct role in driving intimal hyperplasia and intramural neoangiogenesis. The deficiency of the programmed cell death protein-1 immune checkpoint in GCA, promoting unopposed T cell immunity, contrasts with checkpoint hyperactivity in cancer patients in whom excessive programmed cell death ligand-1 expression paralyzes the function of antitumor T cells. Excessive checkpoint activity is the principle underlying cancer-immune evasion and is therapeutically targeted by immunotherapy with checkpoint inhibitors. Such checkpoint inhibitors, which unleash anticancer T cells and induce immune-related toxicity, may lead to drug-induced vasculitis.
Keywords: dendritic cell, giant cell arteritis, immune checkpoint, programmed cell death protein-1, programmed cell death ligand-1
autoimmune inflammation, attacking the walls of the aorta and its side branches, results in aneurysm formation, rupture, and dissection and for medium-sized vessels in luminal occlusion. To protect the host from such life-threatening complications, the wall layers of vital arteries are immunoprivileged, rendering them resistant to localized immune and inflammatory responses (53). The role of the immunoprivilege in the aortic wall is broken in giant cell arteritis (GCA), an autoimmune and autoinflammatory disease causing aortitis and arteritis of the second to fifth aortic branches (3, 44, 52). Clinical consequences include wall damage to the aorta, aortic arch syndrome, and ischemic complications in the eye and the posterior brain. Almost always, the vascular inflammation in GCA patients is combined with a syndrome of systemic inflammation, manifesting as fever, failure to thrive, polymyalgia rheumatica, and exuberant production of acute-phase reactants (15). GCA is diagnosed by a biopsy of the temporal artery, typical findings on computer tomography, or magnetic resonance angiography. Therapeutically, induction therapy continues to rely on high doses of corticosteroids (52). Recently, newer immunosuppressants have been added to the therapeutic armamentarium, but they do not appear to induce durable disease remission (3, 50). Chronic smoldering vasculitis is now the most significant clinical challenge, complicated by the lack of data on the risk/benefit ratio of chronic immunosuppression and by incomplete understanding of the immunopathology of persistent vasculitis.
The Immune Lesion in GCA T Cells and Macrophages Rescinds the Artery’s Immune Privilege
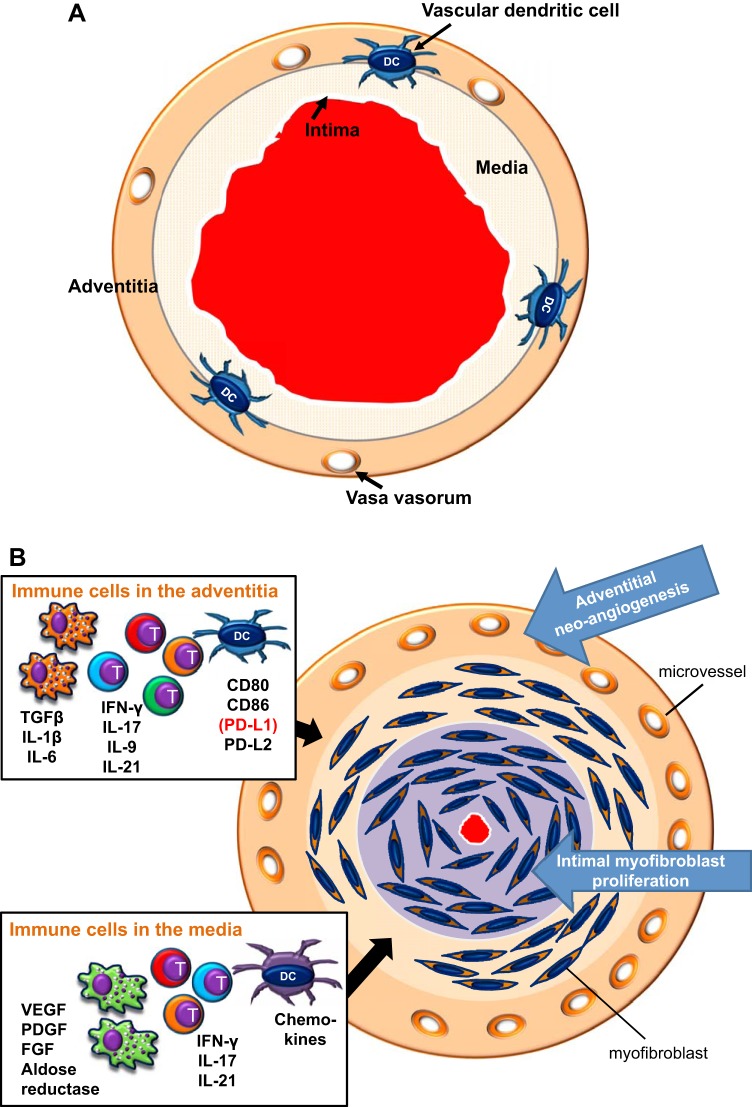
The histomorphology of GCA-affected arteries describes intimal hyperplasia, fragmentation of the elastic lamina, and intramural neoangiogenesis, which are all understood as a maladaptive response to intramural granulomatous inflammation (Fig. 1). Typically, GCA-affected arteries are occupied by granulomatous infiltrates composed of CD4 T cells, highly activated macrophages, multinucleated giant cells, and dendritic cells (DCs) (53). CD4 T cell clones with identical T cell receptors have been isolated from independent tissue biopsies of GCA patients, nurturing the concept of a vasculitogenic antigen (13, 56). Functional analysis of tissue cytokines produced in vasculitic lesions has supported the notion of multifunctional T cells trapped in the lesions. Vascular wall resident T cells produce interferon (IFN)-γ, IL-17, IL-9, and IL-21, implicating T cell helper (Th)1, Th17, Th9, and follicular Th (TFH) cell lineages in the disease process (Fig. 1) (5, 6, 46, 51). Notably, tissue IL-17 production is highly sensitive to corticosteroid therapy, whereas IFN-γ-producing T cells seem unaffected by high doses of systemic steroids (6, 53), supporting the concept of functional heterogeneity among lesional T cell populations. The absence of CD4 and CD8 regulatory T cells in the vascular wall is in line with the observation that GCA immune lesions are driven by multiple effector T cell types.
Fig. 1.
Immune lesion in giant cell arteritis (GCA). A: healthy medium-sized artery with open lumen and three wall layers. Vascular dendritic cells (DCs) are placed at the media-adventitia border, where they may function to guard the immune privilege of the vessel wall. B: GCA is caused by granulomatous inflammation within the arterial wall layers. The major cellular players are CD4 T cells, highly activated macrophages, and DCs. Different inflammatory pathways dominate in different territories of the wall. Boxes indicate the cell types and their disease-relevant products that form the adventitial infiltrate and the medial infiltrate. The adventitia is an important site of antigen presentation and T cell activation. Multiple types of T effector cells are placed in the adventitia and media. Macrophage effector functions are critically involved in the tissue damage and wound-healing response centered in the media. The artery responds to inflammation with a maladaptive restructuring program. Neoangiogenesis in the adventitia opens the floodgates for infiltrating inflammatory cells. Myofibroblast mobilization and migration create rapidly progressive intimal hyperplasia, leading to luminal occlusion and the ischemic complications of GCA. TGF-β, transforming growth factor-β; T, T cell.
Many of the granuloma-residing cells are macrophages, often called histiocytes. Their functional commitment is tightly correlated to their location in the vessel wall (Fig. 1) (48), emphasizing that different microenvironments serve different functions in the disease process. Macrophages in the hyperplastic intima are proinflammatory, producing inducible nitric oxide in the vasculitic lesions (57). Medial macrophages have been implicated in vessel wall remodeling and oxidative stress protection, producing growth factors (platelet-derived growth factor, VEGF, and FGF), metalloproteinases, and aldose reductase (17, 18, 39, 40). Adventitial macrophages coproduce transforming growth factor-β1, IL-6, and IL-1β (57). Available data suggest that inhibition of vasculitic macrophages, effectively achieved by treating GCA patients with glucocorticoids, will not restore the vascular wall immune privilege (6). Studies of repeat biopsies in patients before and after glucocorticoid therapy have indicated that vasculitic infiltrates persist in most patients, even after 9–12 mo of therapy (27).
Evidence for antigen presentation within the granulomas derives from the prominent participation of DCs. As an indigenous population of the vessel wall (22, 26, 36), vascular DCs are believed to have a critical role in protecting the artery’s immune privilege. Equipped with costimulatory ligands and chemokines, they control cellular flux and amplify T cell responses (14, 22, 26, 32, 36, 55). DCs are also the source of immunoinhibitory ligands, providing negative signals to T cells, thus critically shaping the size and duration of immune responses (20, 59). Recent data demonstrate that the programmed cell death protein-1 (PD-1) immune checkpoint, through which programmed cell death ligand-1 (PD-L1)+ DCs provide a stop signal to PD-1+ T cells, is defective in GCA (62), emphasizing the regulatory importance of lesional DCs.
What Are Immune Checkpoints?
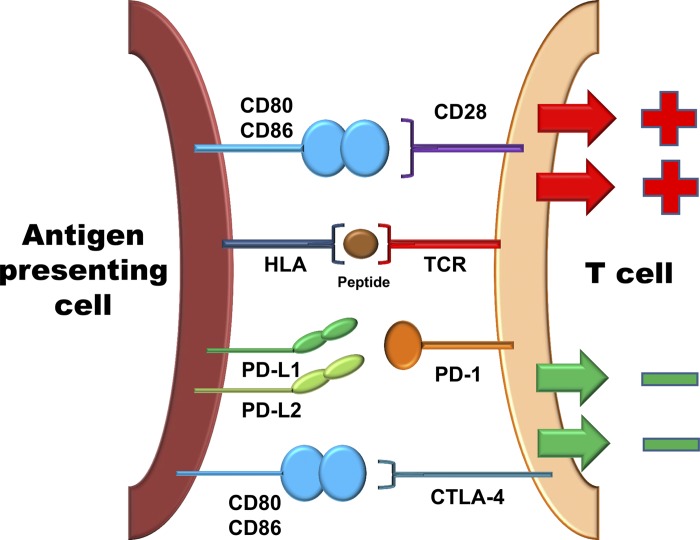
Protective and pathogenic T cell immunity is a multiple-step process centering on the clonal selection of antigen-specific cells. Upon encountering an antigen, T cells undergo activation and proliferate massively to expand the clonal size. Some cells will develop into memory T cells and enter storage sites in secondary lymphoid tissues. Others will traffic to peripheral tissues to reencounter the antigen, execute direct effector functions, or provide help (through cytokine release and membrane ligands) for a multitude of immune effector cells (45, 61). Each of these steps underlies regulation by counterbalancing stimulatory and inhibitory signals, ultimately to regulate the amplitude, durability, and quality of the immune response (Fig. 2) (12). Classical costimulatory signals derive from CD28-CD80 or CD28-CD86 interactions, which are an absolute requirement to drive the metabolic programs of expanding T cells (8). Incoming “go” signals are paired with “stop” signals, often called immune checkpoints. Well-recognized stop signals restraining the activation and clonal expansion of T cells are derived from cytotoxic T lymphocyte-associated antigen-4 (CTLA-4; CD152) and PD-1 (CD279). Both immune checkpoint receptors are critically involved in avoiding excessive immunity, protecting the host from collateral damage, and preventing autoimmunity (30, 38, 63). Both receptors are now recognized as targets for cancer immunotherapy (33, 35, 37), as tumors have learned how to usurp antitumor immunity by strengthening immune checkpoint signaling. Whereas CTLA-4 is believed to disrupt early steps in T cell activation, PD-1 is typically upregulated on T cells after activation, providing an opportunity to inhibit later stages of the clonal expansion program. PD-1 triggering, by its ligands PD-L1 and PD-L2, activates the phosphatase Src homology 2 domain-containing phosphatase-2 (1), thus inhibiting key kinases transmitting the T cell activation cascade. PD-L1 and PD-L2 are expressed on antigen-presenting cells, tumor cells, and tissue-residing cells, empowering them to determine the strength and longevity of immune responses (Fig. 2) (7, 9, 64).
Fig. 2.
Costimulatory and coinhibitory signals in T cell regulation. T cells interact with antigen-presenting cells to recognize their cognate antigen. The T cell receptor (TCR) binds to the human leukocyte antigen (HLA)-antigen complex to trigger the T cell activation cascade. Concomitant receptor-ligand interactions provide either positive signals (costimulation) or transmit negative signals (coinhibition), ultimately to adjust the quality, intensity, and duration of the T cell response. Shown is the major costimulatory molecule CD28, which binds to CD80 and CD86 to amplify the T cell activation cascade. Also shown are the two major inhibitory pathways. The inhibitory receptors cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1), expressed on T cells, are triggered by their ligands on the antigen-presenting cell and provide a “stop” signal to the T cell.
The PD-1 Immune Checkpoint in GCA
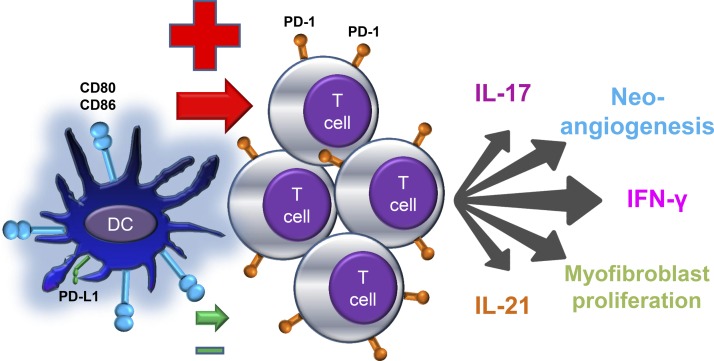
PD-L1 is expressed on antigen-presenting cells, some stromal cells, and tumor cells (7, 9, 64). Gene expression profiling has demonstrated high expression of PD-L1 transcripts in healthy, noninflamed human arteries (62), where this immunoprotective ligand may contribute to the immune privilege. Conversely, GCA-affected arteries had distinctly low PD-L1 transcript levels, and wall-embedded DCs had no demonstrable PD-L1 expression. Low PD-L1 protein expression held true for ex vivo-generated DCs from GCA patients, whereas DCs generated from patients with rheumatoid arthritis (RA) responded to activation signals with robust PD-L1 induction. Functional testing revealed that patient-derived PD-L1low DCs provided strong T cell-activating signals, and blockade of PD-L1, an intervention known to amplify T cell stimulation, failed to enhance further T cell responses. In essence, PD-L1 deficiency of GCA DCs leaves the patient with unopposed T cell-activating signals (Fig. 3).
Fig. 3.
The defective PD-1 immune checkpoint in giant cell arteritis. DCs from patients with GCA express high levels of the costimulatory ligands CD80 and CD86 but low levels of inhibitory programmed cell death ligand-1 (PD-L1). As a consequence, they fail to inhibit interacting T cells. Uninhibited T cells proliferate and acquire multiple effector functions. In the vasculitic lesions of GCA, the majority of T cells carry the PD-1 receptor. Lesion-residing T cells are multifunctional, produce proinflammatory cytokines [e.g., interferon (IFN)-γ, IL-17, and IL-21] and promote vessel wall restructuring by enhancing microvascular neoangiogenesis and accelerating intimal hyperplasia.
How does weakness of PD-L1-induced stop signals affect the T cell response in GCA? In inflamed temporal arteries of GCA patients, essentially all T cells trapped in the granulomas are PD-1 positive. PD-1 is considered a delayed activation marker on T cells recognizing antigen. More importantly, the presence of this surface molecule has been associated with two outcomes: T cell exhaustion and Th activity in the germinal center, provided by so-called PD-1+ TFH cells (43, 58). Neither of these two scenarios applies to the granulomatous lesions in GCA. T cells occupying the inflamed vessel wall are certainly not exhausted. They are highly activated, and local production of several powerful T cell cytokines has been described. IFN-γ appears to be particularly important, and tissue IFN-γ has been associated with the intensity of the wall remodeling process (18). Lesional T cells also do not behave like TFH cells; they colocalize with macrophages but not with B cells. The granulomatous infiltrates are distinctly negative for B cells, and no autoantibody production has been identified for GCA patients (28).
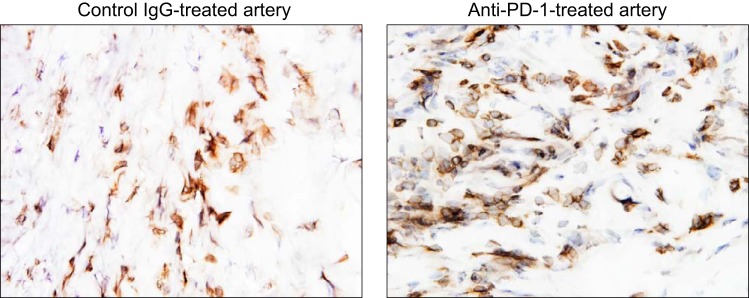
The functional activity of PD-1+ T cells in the inflamed arterial wall has been examined in a model system of GCA, created by engrafting normal human arteries into immunocompromised mice (34, 62). Such chimeric mice are then reconstituted with peripheral blood mononuclear cells, and vasculitis develops within 1–2 wk. Treatment of such human artery-mouse chimeras with anti-PD-1 antibody resulted in substantial aggravation of vessel wall inflammation and T cell infiltration (Fig. 4). Tissue gene expression analysis revealed marked enhancement of innate and adaptive immunity after PD-1/PD-L1 interactions had been dismantled. Artery grafts from anti-PD-1-treated chimeras were enriched for a broad array of cytokine transcripts: IL-1β, IL-6, TNF-α, IL-23 p19, IL-27 p28, IL-7, and IL-15. These data are most compatible with PD-1-dependent signaling, regulating tissue-residing T cells and macrophages. The impairment of the PD-1-derived stop signal ultimately aggravated a multitude of effector functions and overall enhanced the intensity of the vasculitogenic immune response.
Fig. 4.
Checkpoint inhibition with anti-PD-1 antibody treatment exacerbates vasculitis. Human arteries were engrafted into immunodeficient nonobese diabetic NSG-γ mice. To induce vasculitis in the engrafted vessels, chimeric mice were reconstituted with peripheral blood mononuclear cells from patients with GCA. Two weeks later, the human arteries were explanted, and the intensity of vasculitis was determined by immunostaining for human CD3+ T cells in tissue sections. Before harvesting of the human arteries, the chimeric mice were treated with anti-PD-1 antibodies (100 µg) or control IgG by alternative-day intraperitoneal injections. Anti-CD3-binding T cells (brown) in the tissue were visualized with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies. Compared with the IgG control (left), PD-1 blockade (right) markedly increased the density of the vascular T cell infiltrate. Original magnification: ×600.
Arguably, the most important observation made in the human artery-mouse chimera model (62) was the impact of disrupting the PD-1 signal on the remodeling of the inflamed arterial wall. The increase of the density of PD-1+ T cells in the vasculitic lesions led to aggravation of intramural neoangiogenesis and the induction of endothelial cell activation markers. Typically, GCA-affected arteries have dense networks of newly formed microvessels, which are believed to enable the fast recruitment of inflammatory cells and supply the growing inflammatory lesion with oxygen and nutrients. Higher density of PD-1+ T cells in the arterial wall was also associated with the size of the intimal layer, doubling intimal thickness. Intimal hyperplasia causes the ischemic complications of GCA, such as blindness and stroke (52, 54). These data place PD-1 signaling and the intensity of T cell activation at the pinnacle of the wall remodeling process. Further studies need to identify the cellular partners of PD-1+ T cells, e.g., endothelial cells, vascular smooth muscle cells, and others, that ultimately execute the pathological events.
The placement of the deficiency of the immunoinhibitory ligand PD-L1 to vascular DCs raises the question of how the presentation of endogenous and exogenous antigens is affected. Vasculitogenic antigens in GCA have remained unknown, and episodic reports on infectious antigens expressed in vasculitis-affected arteries have not been confirmed in independent studies. An appealing concept for endogenous antigens driving vascular disease has come from studying hypertension (16, 23, 29). In hypertension, increased oxidative stress in DCs has been associated with the formation of highly reactive γ-ketoaldehydes (21, 60). These highly reactive protein modifications have been implicated in altering protein structure and function and in the generation of neoantigens to which the immune system is not tolerant. Consequences include strong activation of DCs and inducing production of IL-6, IL-1β, and IL-23, which, in turn, enable interacting T cells to secrete IL-17, IFN-γ, and TNF-α. Whether similar mechanisms are at work in medium and large arteries susceptible to vasculitis is not understood. Obviously, the weakening of checkpoint function by PD-L1 loss could act as an amplification loop, supporting immune recognition of otherwise ignored self-antigens.
It is currently unknown whether insufficiency of the PD-1 checkpoint is a selective defect in GCA or is also of relevance in other autoimmune diseases. Since the defective PD-1 checkpoint appears to unleash immunity in the vessel wall, it would be equally important to explore its role in other cardiovascular diseases, such as atherosclerosis and hypertension. Available data in mouse models of atherosclerosis support the concept that an intact PD-1/PD-L1 pathway is necessary to suppress T cells that promote atherosclerotic lesion formation and plaque inflammation (2, 11). The risk of patients diagnosed with GCA to develop aortic aneurysm is doubled compared with age-matched controls (42), likely a direct manifestation of vasculitic involvement of the aorta. Furthermore, individuals affected by GCA are more likely to be diagnosed with cerebrovascular and cardiovascular disease, more so for older men from disadvantaged socioeconomic areas (41). Intriguingly, a recent study (47) has identified lower body mass index as a predictor of GCA risk, suggesting divergent risk scenarios for those susceptible to large vessel vasculitis versus those with classical atherosclerosis.
Conclusions, Open Questions, and Clinical Implications
Under physiological conditions, the wall of large arteries is protected from immune attack. In patients with large vessel vasculitis, such as GCA, this privilege breaks down, and immune cells (specifically T cells and macrophages) enter the wall layers to initiate wall remodeling and eventually luminal occlusion. Recent studies have implicated defects in immunoinhibitory signaling as upstream pathologies in GCA. Specifically, tissue-residing and ex vivo-generated DCs from patients with GCA have a defect in upregulating the immunoinhibitory ligand PD-L1, thus failing to counterbalance stimulatory signals with inhibitory signals. Thus, T cells in GCA patients are unleashed and able to infiltrate into the vessel wall, recruit macrophages, and form granulomatous assemblies. Molecular mechanisms underlying the defective induction of PD-L1 remain to be clarified, but a close correlation between inflammatory markers and DC PD-L1 expression levels in individual patients emphasizes the immediate impact that this molecular defect may have (62). Indeed, the accumulation of PD-1+ T cells within the vascular lesions appeared to have direct consequences for the quality and intensity of the vasculitic response. The more PD-1+ T cells were admitted to the vessel wall, the more T cell cytokines were produced, the more microvessels were generated, and the thicker the intimal layer grew (Table 1). These findings support a novel pathogenic model for GCA, assigning ultimate control over wall remodeling processes to T cell activity. Equally transformative is the recognition that antigen-nonspecific immune regulation determines the outcome of vascular inflammation.
Table 1.
Consequences of an impaired PD-1 checkpoint in vasculitis
| Biological Pathway | Clinical Consequence |
|---|---|
| T cell activation and polarization | Density of the T cell infiltrate |
| Interferon-γ, IL-17, and IL-21 production | |
| IL-7 and IL-15 production | |
| Macrophage activity | IL-1β, IL-6, IL-23, and TNF-α production |
| Intramural neoangiogenesis | Density of the microvascular lumina |
| Intimal hyperplasia | Thickness of the intimal layer |
As the implication of immune checkpoint regulation in the pathogenic events of GCA broadens the mechanistic understanding of the disease, it also raises several questions that will be instrumental to guide further studies. The PD-L1low phenotype of GCA DCs appears to be disease specific, not affecting DCs generated from patients with RA. Both disease states are considered autoimmune and inflammatory. These observations indicate that an inflammatory milieu, even when present over years, is insufficient to alter PD-L1 expression. Epigenetic studies are under way to assess the epigenetic landscape of the PD-L1 promoter in GCA, RA, and coronary artery disease. Most information about the regulation of PD-L1 expression is derived from investigations of tumor cells. So far, mechanisms driving PD-L1 expression are only superficially understood and appear to be strongly cell type specific (4, 19, 24, 25, 31, 49). Recent data collected in PD-L1-overexpressing tumors have suggested a role for novel structural variants in the 3′-untranslated region of the PD-L1 promoter to determine surface expression (19). Both IFN-γ and LPS are used in standard assay systems to upregulate the ligand, and DCs from GCA patients are particularly nonresponsive to IFN-γ (62). These data suggest that IFN-dependent signaling pathways may be primarily responsible for the defect.
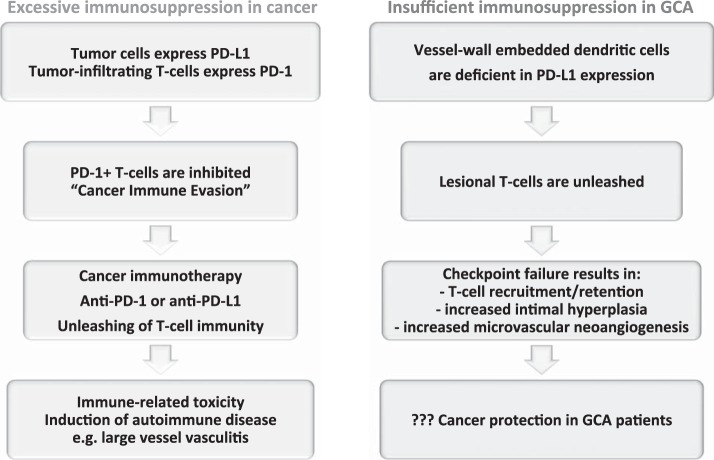
An intriguing aspect of identifying immune checkpoint abnormalities in the pathogenesis of large vessel vasculitis is the molecular interlinking of antitumor and antivessel wall immunity. CD4 T cells in vasculitis patients have a low threshold setting for activation and can survive in tissue niches from which they are otherwise excluded. In the absence of PD-L1 signaling, they are free to commit to a broad range of effector functions, including communications with vascular cells, such as endothelial cells and myofibroblasts. In tumor patients, cytotoxic T cells are banned from the tumor microenvironment but regain access once the PD-1 checkpoint is inhibited (Fig. 5) (35, 37, 64). Immune-mediated side effects of checkpoint inhibitor therapy have been implicated in inducing vasculitis (10). Physicians and patients need to be alerted to the possible risk of antivascular immunity as part of the growing spectrum of immune-related adverse events in cancer immunotherapy.
Fig. 5.
The PD-1 immune checkpoint in cancer and GCA interactions between PD-L1 and PD-1 provides negative signals to T cells, effectively suppressing T cell effector functions (see Fig. 2). Tumor cells escape from immune recognition by expressing the immunoinhibitory ligand PD-L1. T cells attempting to kill cancer cells are paralyzed by receiving negative signals through their PD-1 receptor. Recent breakthroughs in cancer immunotherapy capitalize on targeting PD-L1/PD-1 interactions. Checkpoint inhibitors prevent PD-L1/PD-1 interactions and have led to remarkable success in enhancing tumor immunity. The unleashing of T cell immunity in checkpoint-treated patients is associated with immune-related toxicity, e.g., the induction of autoimmune disease. Drug-induced vasculitis has been reported. In GCA, checkpoint signaling is impaired due to low PD-L1 expression on vessel wall DCs. The loss of this “immune break” enables PD-1+ T cells to infiltrate into the vessel wall and coordinate a wall-damaging inflammatory response. PD-1+ T cells have been implicated in regulating lesional cytokine production, driving microvascular neoangiogenesis, and enhancing lumen-occluding intimal hyperplasia. Given the deficiency of negative immune signaling implicated in cancer-immune evasion, GCA patients may have very effective antitumor immune responses.
GRANTS
Support for this work was provided by National Institutes of Health (NIH) Grants R01-AR-042527, R01-HL-117913, P01-HL-129941, and R01-AI-108906 and the Govenar Discovery Fund (to C. M. Weyand) as well as by NIH Grants R01-AI-108891, U19-AI-057266, and R01-AG-045779 and Biomedical Laboratory Research & Development Grant I01-BX-001669 (to J. J. Goronzy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.B., J.J.G., and C.M.W. conceived and designed research; R.W. and H.Z. performed experiments; R.W., H.Z., and J.J.G. analyzed data; R.W., H.Z., J.J.G., and C.M.W. interpreted results of experiments; R.W., H.Z., J.J.G., and C.M.W. prepared figures; J.J.G. and C.M.W. drafted manuscript; R.W., G.B., J.J.G., and C.M.W. edited and revised manuscript; J.J.G. and C.M.W. approved final version of manuscript.
REFERENCES
- 1.Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med 375: 1767–1778, 2016. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bu DX, Tarrio M, Maganto-Garcia E, Stavrakis G, Tajima G, Lederer J, Jarolim P, Freeman GJ, Sharpe AH, Lichtman AH. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol 31: 1100–1107, 2011. doi: 10.1161/ATVBAHA.111.224709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 315: 2442–2458, 2016. doi: 10.1001/jama.2016.5444. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol 27: 409–416, 2016. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 5.Ciccia F, Rizzo A, Guggino G, Cavazza A, Alessandro R, Maugeri R, Cannizzaro A, Boiardi L, Iacopino DG, Salvarani C, Triolo G. Difference in the expression of IL-9 and IL-17 correlates with different histological pattern of vascular wall injury in giant cell arteritis. Rheumatology (Oxford) 54: 1596–1604, 2015. doi: 10.1093/rheumatology/kev102. [DOI] [PubMed] [Google Scholar]
- 6.Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation 121: 906–915, 2010. doi: 10.1161/CIRCULATIONAHA.109.872903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8: 793–800, 2002. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 8.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity 16: 769–777, 2002. doi: 10.1016/S1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 9.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192: 1027–1034, 2000. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein BL, Gedmintas L, Todd DJ. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis Rheumatol 66: 768–769, 2014. doi: 10.1002/art.38282. [DOI] [PubMed] [Google Scholar]
- 11.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest 117: 2974–2982, 2007. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 23: 515–548, 2005. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 13.Grunewald J, Andersson R, Rydberg L, Gigliotti D, Schaufelberger C, Hansson GK, Wigzell H. CD4+ and CD8+ T cell expansions using selected TCR V and J gene segments at the onset of giant cell arteritis. Arthritis Rheum 37: 1221–1227, 1994. doi: 10.1002/art.1780370817. [DOI] [PubMed] [Google Scholar]
- 14.Han JW, Shimada K, Ma-Krupa W, Johnson TL, Nerem RM, Goronzy JJ, Weyand CM. Vessel wall-embedded dendritic cells induce T-cell autoreactivity and initiate vascular inflammation. Circ Res 102: 546–553, 2008. doi: 10.1161/CIRCRESAHA.107.161653. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman GS. Giant cell arteritis. Ann Intern Med 165: ITC65–ITC80, 2016. doi: 10.7326/AITC201611010. [DOI] [PubMed] [Google Scholar]
- 16.Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68: 123–132, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaiser M, Weyand CM, Björnsson J, Goronzy JJ. Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis Rheum 41: 623–633, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser M, Younge B, Björnsson J, Goronzy JJ, Weyand CM. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol 155: 765–774, 1999. doi: 10.1016/S0002-9440(10)65175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kataoka K, Shiraishi Y, Takeda Y, Sakata S, Matsumoto M, Nagano S, Maeda T, Nagata Y, Kitanaka A, Mizuno S, Tanaka H, Chiba K, Ito S, Watatani Y, Kakiuchi N, Suzuki H, Yoshizato T, Yoshida K, Sanada M, Itonaga H, Imaizumi Y, Totoki Y, Munakata W, Nakamura H, Hama N, Shide K, Kubuki Y, Hidaka T, Kameda T, Masuda K, Minato N, Kashiwase K, Izutsu K, Takaori-Kondo A, Miyazaki Y, Takahashi S, Shibata T, Kawamoto H, Akatsuka Y, Shimoda K, Takeuchi K, Seya T, Miyano S, Ogawa S. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature 534: 402–406, 2016. doi: 10.1038/nature18294. [DOI] [PubMed] [Google Scholar]
- 20.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol 19: 309–314, 2007. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J II, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 124: 4642–4656, 2014. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krupa WM, Dewan M, Jeon MS, Kurtin PJ, Younge BR, Goronzy JJ, Weyand CM. Trapping of misdirected dendritic cells in the granulomatous lesions of giant cell arteritis. Am J Pathol 161: 1815–1823, 2002. doi: 10.1016/S0002-9440(10)64458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loperena R, Harrison DG. Oxidative stress and hypertensive diseases. Med Clin North Am 101: 169–193, 2017. doi: 10.1016/j.mcna.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C, Paschall AV, Shi H, Savage N, Waller JL, Sabbatini ME, Oberlies NH, Pearce C, Liu K. The MLL1–H3K4me3 axis-mediated PD-L1 expression and pancreatic cancer immune evasion. J Natl Cancer Inst 109: djw283, 2017. doi: 10.1093/jnci/djw283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu C, Redd PS, Lee JR, Savage N, Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. OncoImmunology 5: e1247135, 2016. doi: 10.1080/2162402X.2016.1247135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma-Krupa W, Jeon MS, Spoerl S, Tedder TF, Goronzy JJ, Weyand CM. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med 199: 173–183, 2004. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maleszewski JJ, Younge BR, Fritzlen JT, Hunder GG, Goronzy JJ, Warrington KJ, Weyand CM. Clinical and pathological evolution of giant cell arteritis: a prospective study of follow-up temporal artery biopsies in 40 treated patients. Mod Pathol. In press. doi: 10.1038/modpathol.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Taboada V, Brack A, Hunder GG, Goronzy JJ, Weyand CM. The inflammatory infiltrate in giant cell arteritis selects against B lymphocytes. J Rheumatol 23: 1011–1014, 1996. [PubMed] [Google Scholar]
- 29.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033, 2015. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11: 141–151, 1999. doi: 10.1016/S1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 31.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211: 781–790, 2014. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill L, Molloy ES. The role of Toll like receptors in giant cell arteritis. Rheumatology (Oxford) 55: 1921–1931, 2016. doi: 10.1093/rheumatology/kew001. [DOI] [PubMed] [Google Scholar]
- 33.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252–264, 2012. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piggott K, Deng J, Warrington K, Younge B, Kubo JT, Desai M, Goronzy JJ, Weyand CM. Blocking the NOTCH pathway inhibits vascular inflammation in large-vessel vasculitis. Circulation 123: 309–318, 2011. doi: 10.1161/CIRCULATIONAHA.110.936203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 33: 1974–1982, 2015. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation 118: 1276–1284, 2008. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramsay AG. Immune checkpoint blockade immunotherapy to activate anti-tumour T-cell immunity. Br J Haematol 162: 313–325, 2013. doi: 10.1111/bjh.12380. [DOI] [PubMed] [Google Scholar]
- 38.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science 290: 84–89, 2000. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 39.Rittner HL, Hafner V, Klimiuk PA, Szweda LI, Goronzy JJ, Weyand CM. Aldose reductase functions as a detoxification system for lipid peroxidation products in vasculitis. J Clin Invest 103: 1007–1013, 1999. doi: 10.1172/JCI4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rittner HL, Kaiser M, Brack A, Szweda LI, Goronzy JJ, Weyand CM. Tissue-destructive macrophages in giant cell arteritis. Circ Res 84: 1050–1058, 1999. doi: 10.1161/01.RES.84.9.1050. [DOI] [PubMed] [Google Scholar]
- 41.Robson JC, Kiran A, Maskell J, Hutchings A, Arden N, Dasgupta B, Hamilton W, Emin A, Culliford D, Luqmani R. Which patients with giant cell arteritis will develop cardiovascular or cerebrovascular disease? A clinical practice research datalink study. J Rheumatol 43: 1085–1092, 2016. doi: 10.3899/jrheum.151024. [DOI] [PubMed] [Google Scholar]
- 42.Robson JC, Kiran A, Maskell J, Hutchings A, Arden N, Dasgupta B, Hamilton W, Emin A, Culliford D, Luqmani RA. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Ann Rheum Dis 74: 129–135, 2015. doi: 10.1136/annrheumdis-2013-204113. [DOI] [PubMed] [Google Scholar]
- 43.Sage PT, Ron-Harel N, Juneja VR, Sen DR, Maleri S, Sungnak W, Kuchroo VK, Haining WN, Chevrier N, Haigis M, Sharpe AH. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol 17: 1436–1446, 2016. doi: 10.1038/ni.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet 372: 234–245, 2008. doi: 10.1016/S0140-6736(08)61077-6. [DOI] [PubMed] [Google Scholar]
- 45.Taylor JJ, Jenkins MK. CD4+ memory T cell survival. Curr Opin Immunol 23: 319–323, 2011. doi: 10.1016/j.coi.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Terrier B, Geri G, Chaara W, Allenbach Y, Rosenzwajg M, Costedoat-Chalumeau N, Fouret P, Musset L, Benveniste O, Six A, Klatzmann D, Saadoun D, Cacoub P. Interleukin-21 modulates Th1 and Th17 responses in giant cell arteritis. Arthritis Rheum 64: 2001–2011, 2012. doi: 10.1002/art.34327. [DOI] [PubMed] [Google Scholar]
- 47.Ungprasert P, Thongprayoon C, Warrington KJ. Lower body mass index is associated with a higher risk of giant cell arteritis: a systematic review and meta-analysis. Ann Transl Med 3: 232, 2015. doi: 10.3978/j.issn.2305-5839.2015.09.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner AD, Goronzy JJ, Weyand CM. Functional profile of tissue-infiltrating and circulating CD68+ cells in giant cell arteritis. Evidence for two components of the disease. J Clin Invest 94: 1134–1140, 1994. doi: 10.1172/JCI117428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang WB, Yen ML, Liu KJ, Hsu PJ, Lin MH, Chen PM, Sudhir PR, Chen CH, Chen CH, Sytwu HK, Yen BL. Interleukin-25 mediates transcriptional control of PD-L1 via STAT3 in multipotent human mesenchymal stromal cells (hMSCs) to suppress Th17 responses. Stem Cell Reports 5: 392–404, 2015. doi: 10.1016/j.stemcr.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe R, Goronzy JJ, Berry G, Liao YJ, Weyand CM. Giant cell arteritis: from pathogenesis to therapeutic management. Curr Treatm Opt Rheumatol 2: 126–137, 2016. doi: 10.1007/s40674-016-0043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watanabe R, Hosgur E, Zhang H, Wen Z, Berry G, Goronzy JJ, Weyand CM. Pro-inflammatory and anti-inflammatory T cells in giant cell arteritis. Joint Bone Spine. In press. doi: 10.1016/j.jbspin.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weyand CM, Goronzy JJ. Clinical practice. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 371: 50–57, 2014. doi: 10.1056/NEJMcp1214825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol 9: 731–740, 2013. doi: 10.1038/nrrheum.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med 349: 160–169, 2003. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 55.Weyand CM, Ma-Krupa W, Pryshchep O, Gröschel S, Bernardino R, Goronzy JJ. Vascular dendritic cells in giant cell arteritis. Ann NY Acad Sci 1062: 195–208, 2005. doi: 10.1196/annals.1358.023. [DOI] [PubMed] [Google Scholar]
- 56.Weyand CM, Schönberger J, Oppitz U, Hunder NN, Hicok KC, Goronzy JJ. Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes. J Exp Med 179: 951–960, 1994. doi: 10.1084/jem.179.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weyand CM, Wagner AD, Björnsson J, Goronzy JJ. Correlation of the topographical arrangement and the functional pattern of tissue-infiltrating macrophages in giant cell arteritis. J Clin Invest 98: 1642–1649, 1996. doi: 10.1172/JCI118959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15: 486–499, 2015. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol 33: 445–474, 2015. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 60.Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, Chen W, Mernaugh RL, Cai H, Bernstein KE, Goronzy JJ, Weyand CM, Curci JA, Barbaro NR, Moreno H, Davies SS, Roberts LJ II, Madhur MS, Harrison DG. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest 126: 50–67, 2016. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Youngblood B, Hale JS, Ahmed R. T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology 139: 277–284, 2013. doi: 10.1111/imm.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H, Watanabe R, Berry GJ, Vaglio A, Liao YJ, Warrington KJ, Goronzy JJ, Weyand CM. Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc Natl Acad Sci USA 114: E970–E979, 2017. doi: 10.1073/pnas.1616848114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Q, Vignali DA. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity 44: 1034–1051, 2016. doi: 10.1016/j.immuni.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 8: 328rv4, 2016. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]