We report the first direct in vivo evidence that a smooth muscle cell (SMC)-produced collagen, collagen type XV (COL15A1), is critical for atherosclerotic lesion development. SMC Col15a1 knockout markedly attenuated advanced lesion formation, likely through reducing SMC proliferation and impairing multiple proatherogenic inflammatory processes.
Keywords: collagen, atherosclerosis, smooth muscle cells, lineage tracing, collagen type XV α1-gene
Abstract
Atherosclerotic plaque rupture with subsequent embolic events is a major cause of sudden death from myocardial infarction or stroke. Although smooth muscle cells (SMCs) produce and respond to collagens in vitro, there is no direct evidence in vivo that SMCs are a crucial source of collagens and that this impacts lesion development or fibrous cap formation. We sought to determine how conditional SMC-specific knockout of collagen type XV (COL15A1) in SMC lineage tracing mice affects advanced lesion formation given that 1) we have previously identified a Col15a1 sequence variant associated with age-related atherosclerosis, 2) COL15A1 is a matrix organizer enhancing tissue structural integrity, and 3) small interfering RNA-mediated Col15a1 knockdown increased migration and decreased proliferation of cultured human SMCs. We hypothesized that SMC-derived COL15A1 is critical in advanced lesions, specifically in fibrous cap formation. Surprisingly, we demonstrated that SMC-specific Col15a1 knockout mice fed a Western diet for 18 wk failed to form advanced lesions. SMC-specific Col15a1 knockout resulted in lesions reduced in size by 78%, with marked reductions in numbers and proliferating SMCs, and lacked a SMC and extracellular matrix-rich lesion or fibrous cap. In vivo RNA-seq analyses on SMC Col15a1 knockout and wild-type lesions suggested that a mechanism for these effects is through global repression of multiple proatherogenic inflammatory pathways involved in lesion development. These results provide the first direct evidence that a SMC-derived collagen, COL15A1, is critical during lesion pathogenesis, but, contrary to expectations, its loss resulted in marked attenuation rather than exacerbation of lesion pathogenesis.
NEW & NOTEWORTHY We report the first direct in vivo evidence that a smooth muscle cell (SMC)-produced collagen, collagen type XV (COL15A1), is critical for atherosclerotic lesion development. SMC Col15a1 knockout markedly attenuated advanced lesion formation, likely through reducing SMC proliferation and impairing multiple proatherogenic inflammatory processes.
atherosclerosis is a pervasive, progressive disease and the leading cause of death in the Western world (31, 40, 53, 69). Enhancement of atherosclerotic plaque stability, specifically the strength of the fibrous cap, is of great clinical relevance as a majority of sudden deaths from myocardial infarction result from plaque rupture or erosion causing subsequent embolic events (31, 40, 53, 69). Both the cell composition and collagen deposition and organization of collagen within atherosclerotic lesions affect plaque formation and stability. Specifically, assessment of human plaque pathology has suggested that an increase in the ratio of smooth muscle α-actin (ACTA2)+ to CD68+ cells within lesions and a collagen-rich fibrous cap characterize a stable plaque (26, 31, 69). An organized fibrillar collagen-rich matrix (i.e., collagen types I and III) within the fibrous cap provides mechanical tensile strength and resistance to rupture (14, 20, 39, 41, 61, 64). In addition, multiple nonfibrillar collagen subtypes have been identified within the fibrous cap (types IV and VI) and throughout atherosclerotic plaques (types VIII and XV−XIX) (10, 32, 46, 48, 54). Furthermore, conventional global knockout studies of specific nonfibrillar collagen subtypes VIII and XVIII in mouse models of atherosclerosis have provided evidence that collagens can contribute to plaque pathogenesis by impacting plaque size, cell composition, and indexes of stability (46, 48). However, a major limitation in the use of conventional global collagen knockout mouse models is that it precludes determination of the cellular source of collagens within atherosclerotic lesions. Consequently, while there is substantial evidence establishing collagen content and organization as important in plaque and fibrous cap formation and stability, the identity of the major collagen-producing cells within lesions remains unknown.
Nevertheless, it has been widely presumed in the field that smooth muscle cells (SMCs) are the primary source of collagen within the lesion and fibrous cap. This assumption has been supported by several in vitro observations including that cultured SMCs produce collagens in response to proatherogenic stimuli [e.g., transforming growth factor (TGF)-β1), IL-1β, and oxidized phospholipids] (1, 6, 22) and that collagens can influence SMC migration, proliferation, and adhesion (1, 72). However, definitive evidence for SMCs as the in vivo source of collagen within lesions is lacking as 1) collagen subtypes are secreted molecules, making it difficult to ascertain their cell source, and 2) recent rigorous lineage tracing studies have shown that cell marker genes traditionally used to identify cells within atherosclerotic lesions are nonspecific (33, 67). For example, cells other than SMCs, such as macrophages (7, 60) and endothelial cells (19), can activate traditional SMC marker genes within lesions (21, 30, 33, 34, 63). Furthermore, recent rigorous SMC lineage tracing mouse studies by our laboratory (21, 34, 63) have demonstrated that >80% of SMCs within advanced lesions cannot be identified using traditional SMC lineage markers such as ACTA2 or transgelin (TAGLN). Importantly, we also showed that nearly 40% of ACTA2− SMCs have activated multiple markers of macrophages and mesenchymal stem cells and therefore have likely been misidentified in previous studies in the field (63). Of most relevance to our understanding of the role of SMC-derived collagen production in human atherosclerosis, we presented compelling evidence that ~20% of cells previously identified as being macrophages within advanced human coronary artery lesions are actually of SMC rather than myeloid origin (63). The converse is also true; Caplice et al. (15) provided evidence based on analyses of advanced coronary artery lesions from subjects who had earlier undergone a cross-sex bone marrow transplant that a significant subset of ACTA2+ lesion cells are of myeloid origin. Finally, a comprehensive study by Allahverdian et al. (3) showed that nearly 50% of CD68+ cells within advanced human lesions are also ACTA2+, thus making it impossible to ascertain if these are SMCs that have activated macrophage markers, macrophages that activated SMC markers, or neither. Collectively, there are thus not only major ambiguities as to which cells within human and mouse lesions are SMCs versus non-SMCs but nearly complete uncertainties as to what cells are the major source of lesion collagens.
Of particular importance, we have previously identified a single-nucleotide polymorphism (rs4142986) within the collagen type XV α1-gene (COL15A1) that is significantly associated with atherosclerosis in aged (>55 yr) individuals (25). We also found that this polymorphism is associated with reduced COL15A1 expression levels and can be methylated and that its methylation is correlated with disease burden within human aortas (25). COL15A1 is a nonfibrillar multiplexin collagen that connects large fibrillar collagens and organizes cellular matrixes to enhance tissue structural integrity (5, 23, 29, 37, 43, 51, 56, 57). We discovered that COL15A1 is upregulated in human and mouse atherosclerosis and that small interfering (si)RNA-mediated knockdown of Col15a1 in cultured human aortic SMCs resulted in a decrease in SMC proliferation and an increase in SMC migration (25). Taken together, we thus hypothesized that SMC-derived COL15A1 would play a critical role in plaque stability by promoting collagen organization within the fibrous cap of advanced lesions and that its loss would be associated with increases in overall lesion size but reductions in indexes of plaque stability. However, completely contrary to expectations, we found that SMC-specific knockout of Col15a1 resulted in a marked attenuation of lesion development with lesions in SMC-specific Col15a1 knockout mice failing to advance beyond fatty streak formation despite 18 wk of Western diet feeding. That is, production of COL15A1 by SMCs had a major unanticipated novel atheropromoting role in mouse lesion development.
METHODS
Generation and Validation of Col15a1fl/fl Mice
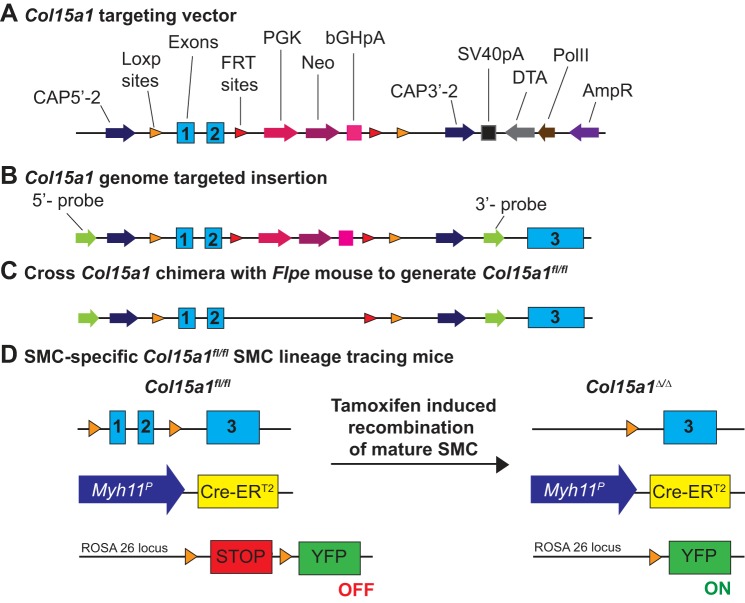
C57BL/6J/N-Col15a1tm1,2Jjc (Col15a1fl/fl) mice were generated by TransViragen. Excision of Col15a1 exons 1 and 2 has been previously shown to be sufficient for gene knockout (29). We therefore targeted these exons to generate Col15a1fl/fl mice, as shown in Fig. 1. In brief, the gene targeting cassette (Fig. 1A) was transfected into C57BL/6N embryonic stem cells, selected for neomycin resistance, and subsequently injected into albino C57BL/6J blastocysts (no. 000058, Jackson Laboratories) to generate chimeras on a C57BL/6 background (Fig. 1B). Chimeras were crossed to congenic C57BL/6J FLPe deleter mice [B6.129S4-Gt (ROSA) 26Sortm1(FLP1)Dym/RainJ; no. 009086, Jackson Laboratories] for FLP-FRT-mediated excision of the neomycin resistance component (Fig. 1C).
Fig. 1.
Generation of Col15a1fl/fl mice and smooth muscle cell (SMC)-specific Col15a1fl/fl SMC lineage tracing mice. A: schematic of collagen type XV α1-gene (Col15a1) targeting vector injected into embryonic stems cells. Vector insertion resulted in selective neomycin resistance of embryonic stem cells. B: schematic of Col15a1 genome targeted insertion contained within chimera pups. C: schematic showing cross of Col15a1 chimera with Flpe recombinase mice results in excision of neomycin selectivity. This represents the Col15a1 locus in the animals used in our studies. D: schematic representation of SMC-specific Col15a1fl/fl SMC lineage tracing mice before and after tamoxifen treatment. Myh11P is the Myh11 promoter. Cre-ERT2 is the estrogen receptor bound Cre recombinase.
Female Col15a1fl/wt mice were initially crossed with congenic C57BL/6 Myh11-CreERT2 mice on an apolipoprotein E (Apoe)−/− background (71), resulting in the generation of SMC-specific Col15a1 floxed mice (Col15a1fl/fl Myh11-CreERT2, Apoe−/− or shorthand SMC Col15a1fl/fl, Apoe−/−). Female Col15a1fl/fl, Apoe−/− animals were then crossed to Myh11-CreERT2 ROSA26-STOP floxed eYFP Apoe−/− mice, as previously described (34, 63), to generate Col15a1fl/fl and Col15a1wt/wt Myh11-CreERT2 ROSA26-STOPflox eYFP Apoe−/− mice, which provide the added benefit of SMC-specific lineage tracing [yellow fluorescent protein (YFP)+; Fig. 1D]. Mice are designated as “SMC YFP+/+ Col15a1fl/fl, Apoe−/−” and “SMC YFP+/+ Col15a1wt/wt, Apoe−/−” for SMC Col15a1 knockout and wild type, respectively, throughout this report. Mice were genotyped as previously described (34, 70, 71). Col15a1 genotyping primers are shown in Table 1.
Table 1.
Genotyping and excision primers for Col15a1
| Col15a1 (TransViragen and BGD) | |
|---|---|
| DOC346 | GTGCCTCGGTCCCTTAAAAGCC |
| BGD15ex1R | ACCCGCGCTCTGAAACCCGACT |
| DOC349 | GGGATAGTTTGGCAGTGTGTTGTCAC |
| Doc346-BGD15ex1R-Doc349 | Col15a1wt/wt: 754 bp |
| Col15a1fl/fl: 872 bp | |
| Col15a1Δ/Δ: 339 bp | |
Col15a1, collagen type XV α1-gene.
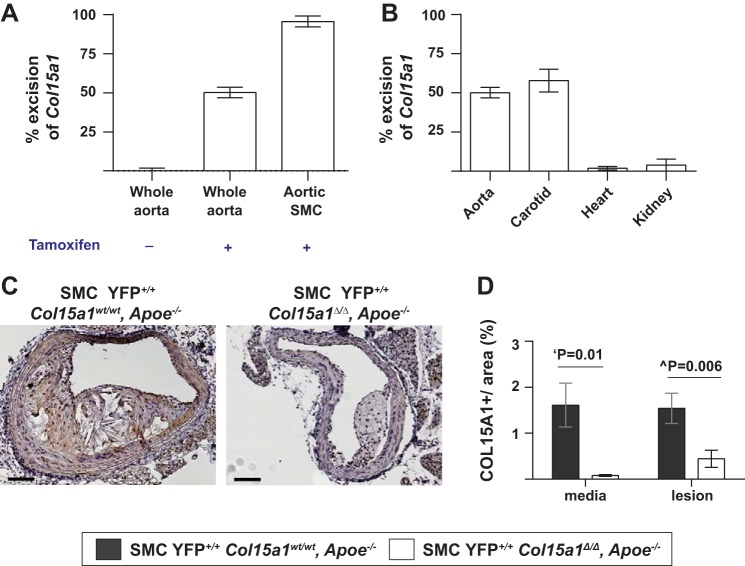
Myh11-CreERT2 resides on the Y chromosome; therefore, only male mice were used for our experiments. Cre-Lox-mediated knockout of Col15a1 was induced in 4-wk-old male mice after intraperitoneal injection of 1 mg tamoxifen (T-5648, Sigma) per mouse per day for 7 days (Fig. 1D). Col15a1 excision tissues (n = 9 for the aorta and n = 8 for the carotid, heart, and kidney) were harvested, and aortic SMCs (n = 10 pooled mouse thoracic aortas) were isolated at 8 wk of age. Col15a1 excision was assessed by Taqman Copy Number Variant Assay using a probe targeting exon 1 of Col15a1 (Mm00558775_cn, Life Technology; Fig. 2, A and B). No gross differences between SMC Col15a1 knockout and wild type mice were observed in any offspring. Information about the number of mice used for each analysis is shown in the figure legends and throughout the detailed methods. Animal usage for these experiments was approved by the University of Virginia Animal Care and Use Committee.
Fig. 2.
Validation of specificity of SMC Col15a1 knockout, SMC lineage tracing mice. A: excision of Col15a1 occurs only in presence of tamoxifen and shows nearly complete recombination in SMCs. B: SMC Col15a1 knockout is highly specific to SMC-rich vessels (aorta and carotid). C and D: staining for COL15A1 in advanced lesions from 18 wk Western diet-fed SMC lineage tracing [yellow fluorescent protein (YFP)]+/+ Col15a1wt/wt, apoliprotein E (Apoe)−/− (n = 11) and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− (n = 13) mice shows a significant reduction of COL15A1 in both the media and lesion of SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice. Values represent means ± SE. Scale bars = 100 µm. ‘P value was determined by an unpaired, two-tailed t-test with Welch’s correction; ^P value was determined by Mann-Whitney U-test.
Animal Diet and Tissue Processing
At 6 wk of age, mice were placed on a Western diet consisting of 21% milk fat and 0.15% cholesterol (TD.88137, Harlan Teklad) for 18 wk. All SMC YFP+/+ Col15a1wt/wt, Apoe−/− and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice were given the same tamoxifen regimen. In addition, all SMC YFP+/+ Col15a1wt/wt, Apoe−/− and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice were given the same Western diet regimen with the exception of mice used for the initial characterization of Col15a1 excision efficiency (i.e., Fig. 2, A and B). Mice were euthanized after a 4-h fast via CO2 asphyxiation. Blood was collected, and fasting plasma cholesterol and triglyceride level analyses were conducted by the University of Virginia Clinical Pathology Laboratory. After euthanasia by CO2 asphyxiation, mice were perfused with 10 ml PBS and 10 ml 4% paraformaldehyde via the left ventricle. Brachiocephalic arteries (BCAs) were dissected, postfixed overnight in 4% paraformaldehyde, and paraffin embedded. Paraffin-embedded BCAs were sectioned serially at 10 μm thickness from the aortic arch to right subclavian artery for analysis.
Immunohistochemical and Immunofluorescent Analysis
Murine tissues.
Modified Russell-Movat staining was conducted for vessel morphometry at three different locations on BCAs of SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 11) and SMC YFP+/+ Col15a1fl/fl, Apoe−/− (n = 13) mice. Immunohistochemical staining using an antibody specific for COL15A1 (0.48 μg/ml, HPA017913, Sigma Prestige) visualized with 3′,3-diaminobenzidine (DAB; Acros Organic) was performed at one location of the BCAs of SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 11) and SMC YFP+/+ Col15a1fl/fl, Apoe−/− (n = 13) mice. COL15A1 staining was used to confirm Col15a1 knockout in the SMC-rich media and assess lesion COL15A1 content. Picrosirius red staining was performed at one location of the BCA of SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 11) and SMC YFP+/+ Col15a1fl/fl, Apoe−/− (n = 13) mice to measure collagen content and maturation via fiber birefringence under polarized light. Immunofluorescent staining of BCAs from SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 11) and SMC YFP+/+ Col15a1fl/fl, Apoe−/− (n = 13) mice was done with primary antibodies specific for green fluorescent protein (GFP; 4 μg/ml, ab6673, Abcam), galectin 3 (LGALS3; 0.2 μg/ml, CL8942AP, Cedarlane), MKi67 (4 μg/ml, ab15580, Abcam), caspase-3 (CASP3; 0.84 µg/ml, no. 9661S, Cell Signaling), and DAPI (0.05 mg/ml, D3571, ThermoFisher Scientific) as well as with a conjugated ACTA2-Cy3 (1:500; C6198, Sigma-Aldrich) antibody. Secondary antibodies for immunofluorescence included the following: donkey anti-goat 488 (1:100, A11055, Invitrogen), donkey anti-goat 647 (1:100, A21447, Invitrogen), donkey anti-rat Dylight 650 (1:100, ab102263, Abcam), donkey anti-rat Dylight 550 (1:100, ab102261, Abcam), and donkey anti-rabbit 488 (1:100, A21206, Invitrogen). The number of locations assessed for each immunofluorescent primary antibody staining combination is indicated within the figure legends.
Human tissues.
Deidentified coronary artery specimens from coronary artery bypass graft (CABG) patients were paraformaldehyde fixed, paraffin embedded, and sectioned at 5 μm thickness. Arteries from six separate patients with advanced (>50% occluded) atherosclerotic lesions were selected. Two serial sections were stained. One section was stained using an antibody specific for COL15A1 (0.48 μg/ml, HPA017913, Sigma Prestige) and visualized with DAB (Acros Organic). The sequential section was stained with CD68 (2 μg/ml, sc20060 KP1 clone, Santa Cruz Technology) in conjunction with secondary goat anti-mouse 647 antibody (1:100, A21236, Invitrogen) and ACTA2-Cy3 (1:500, C6198, Sigma-Aldrich) for analysis via immunofluorescence. The Institutional Review Board of the University of Virginia approved the use of these specimens.
Species-matched immunoglobulins were used as a negative control for antibody staining in both human and murine tissues.
Sudan IV Analysis
The thoracic aorta through the iliac bifurcation was isolated from SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 9) and SMC YFP+/+ Col15a1fl/fl, Apoe−/− (n = 6) mice for en face Sudan IV staining analysis to assess aortic disease burden. The aorta was cleaned of periadventitial fat, dehydrated in 70% ethanol for 5 min, stained with Sudan IV [1 g S-4261 (Sigma) diluted in 100 ml of 70% ethanol and 100 ml of 100% acetone] for 6 min, differentiated in 80% ethanol for 3 min, and stored in PBS at 4°C.
Image Acquisition and Analysis
Movat, Sudan IV, and COL15A1 stained human and murine arteries were imaged using a Zeiss Axioskope2 microscope fitted with an AxioCamMR3 camera. Image acquisition was performed with AxioVision40 version 4.6.3.0 software (Carl Zeiss Imaging Solution). Digitized images of Movat, Sudan IV, and COL15A1 stained tissues were all analyzed directly using Image Pro Plus Software 7.0 (Media Cybernetics). Areas of interest were drawn within the software to delineate the external elastic lamina, inner elastic lamina, and lumen area with media and lesion areas extrapolated from these measurements. Positive staining color was selected at the pixel level and defined using a color cube-based method.
To determine fibrillar collagen content, images of BCA sections stained with picrosirius red were taken on an Olympus BX51 under a polarized lens. Digitized images were analyzed directly using Image Pro Plus Software 7.0 (Media Cybernetics) in the same manner as Movat, Sudan IV, and COL15A1 stained tissues.
To determine fibrillar collagen maturation, the picrosirius red-stained slides were assessed using quantitative polarization microscopy and an LC-PolScope (Perkin-Elmer). This system measures birefringence retardance of a sample in an orientation-independent manner and uses a polarization algorithm to compile images of the fibrillar organization of tissues. In the PolScope images, the color of each pixel is proportional to the amount of birefringent fibrillar collagen present in the tissue, as indicated on the spectrum key below the image, with blue representing less and red representing greater retardance. The sum of birefringence retardance was measured across the entire lesion and/or medial cross-sectional area and divided by the total area of lesion and/or media to obtain average retardance per pixel in each layer for each sample.
Immunofluorescent stained murine BCAs were imaged using a Zeiss LSM700 confocal microscope. Zen 2009 Light Edition Software (Zeiss) was used to acquire a series of eight z-stack images at 1-μm intervals. Careful analysis of each z-stack image was performed using Zen 2009 Light Edition Software (Zeiss) to assess colocalization of markers within a single cell (DAPI+ nucleus) throughout the entire lesion. Images are maximum intensity projections for the confocal z-stacks. For immunofluorescent imaging of human coronary arteries, an Olympus IX51 microscope fitted with an Olympus XM10 camera was used with image acquisition performed using Olympic standard cellSENS software.
Pressure Myography
The right carotid artery region just above the subclavian artery was harvested from 18-wk Western diet-fed SMC Col15a1wt/wt, Apoe−/− (n = 6), and SMC Col15a1Δ/Δ, Apoe−/− (n = 5) mice. Vessels were cannulated on glass micropipettes and secured with a 10-0 nylon monofilament suture to the arteriograph. Vessels were equilibrated at 90 mmHg for ~30 min in Krebs-HEPES containing 118.4 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 4 mM NaHCO3, 1.2 mM KH2PO4, 2 mM CaCl2, 10 mM HEPES, and 6 mM glucose and supplemented with 1% BSA before assessment of active tone (11). Pressure was subsequently dropped to 20 mmHg and increased in 5-min intervals (or until plateau was achieved) to 180 mmHg in 20-mmHg increments. Internal diameters were measured using digital calipers under a Danish Myotechnology microscope (DMT) using the DMT vessel acquisition suite. Carotids were then incubated in Ca2+-free Krebs-HEPES containing 2 mM EGTA and 10 µM sodium nitroprusside buffer for the assessment of passive tone. The pressure curve was then repeated for passive tone.
Isolation of Aortic SMCs
Mouse aortic SMCs were isolated as previously described (21, 63). Briefly, after tamoxifen injections at 4 wk of age, thoracic aortas from 10 individual SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice were harvested at 6 wk of age. Aortas were cleaned of periadventitial fat and enzymatically digested in a mix of 1 mg/ml collagenase II, 1 mg/ml soybean trypsin inhibitor, and 0.744 U/ml elastase (all from Worthington Biochemical) in HBSS for 10 min at 37°C in a 5% CO2 incubator. The adventitia was then carefully removed using autoclaved sterilized tools, and endothelial cells were gently scraped off the luminal surface. The 10 aortas were then pooled and cut into ~0.5-mm pieces, placed in enzyme, and incubated for an hour to dissociate SMCs. SMCs were then cultured in 20% serum containing media [DMEM-F-12 (GIBCO), FBS (Hyclone), 100 U/ml penicillin-streptomycin (GIBCO), and 1.6 mM/l l-glutamine (GIBCO)] and plated at a high density to ensure cell recovery. SMCs were changed to 10% serum-containing media after two passages. Flow cytometry analysis for endogenous YFP (data not shown) and Taqman Copy Number Assay for exon 1 of Col15a1 (Fig. 2A) were conducted to confirm isolation of Col15a1 knockout SMCs.
RNA-seq Analysis
The BCAs, aortic arch, and carotid arteries from 18-wk Western diet-fed SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− (n = 3) and SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 3) mice were flash frozen in liquid nitrogen for RNA-seq analysis. Total RNA was extracted from arteries using TRIzol RNA extraction per the manufacturer’s protocol (Invitrogen). RNA library and deep sequencing were performed by HudsonAlpha Institute for Biotechnology according to Illumina RNA Seq kit instructions with ribosomal reduction and strand specificity. The Agilent 2100 Bioanalyzer and Kapa Library Quantification Kit (Kapa Biosystems) were used for quality control and quantification of the RNA and RNA library according to the manufacturer’s protocol. Libraries were sequenced with the Illumina HiSeq 2000 (2 × 100 bp, 25 million reads).
One-hundred nucleotide paired-end reads were mapped to the mm9 reference genome using STAR software version 2.4 (27). A table of gene counts was generated using FeatureCounts in the Subread package (44). DESeq2 Bioconductor R package (47) was used to identify differentially expressed genes using Benjamini-Hochberg procedure to adjust P values. GENCODE/Ensembl gene ID mapping to known genes were used, whereas those mapping to predicted genes were excluded. We performed Ingenuity Pathway Analysis (35) on all differentially expressed genes to identify dysregulated pathways between SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− and SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions. We performed Upstream Regulator Analysis to predict the biological factors (e.g., gene, small molecule, transcription factor) most likely to be driving the differences in gene expression between SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− and SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions. Upstream Regulator Analysis (35) was done on the top ~3,000 differentially expressed genes with a log fold change of ±0.30 from the RNA-seq as this was the highest computational capacity of the program. The RNA-seq data are available at the NCBI Gene Ontology Omnibus (GEO) database under GEO Accession No. GSE94661.
Statistical Analysis
Statistics were performed using GraphPad Prism version 6 software. Normality of the data was determined via a Kolmogorov-Smirnoff normality test. In the case of Movat staining to ascertain overall morphometric parameters between SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− and SMC YFP+/+ Col15a1wt/wt, Apoe−/− animals, two-way ANOVA with repeated measures was performed to assess the consistency of the phenotype across the BCAs. In all other cases where multiple BCA locations were analyzed, the values across each location were averaged for each individual mouse in the analysis and an individual comparison analysis was subsequently conducted comparing SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− and SMC YFP+/+ Col15a1wt/wt, Apoe−/− mice. For individual comparisons of normally distributed data, unpaired two-tailed t-tests were performed with Welch’s correction applied only in cases of unequal variance between groups. Mann-Whitney U-tests were conducted if data were non-normally distributed. The statistical tests used for each data set are detailed in the figures.
RESULTS
SMC-Specific Col15a1 Deletion Resulted in Marked Reductions in the Size of BCA Lesions
To determine the effect of SMC-derived COL15A1 on plaque pathogenesis, we created a Col15a1fl/fl mouse and crossed it with a previously described tamoxifen-inducible SMC lineage tracing Apoe−/− mouse (Myh11-CreERT2 ROSA floxed STOP eYFP Apoe−/−) (21, 34, 63, 71) to generate a SMC-specific conditional Col15a1 knockout SMC-lineage tracing Apoe−/− mouse (SMC YFP+/+ Col15a1Δ/Δ, Apoe−/−; Fig. 1). After tamoxifen treatment, we placed 6-wk-old SMC YFP+/+ Col15a1wt/wt, Apoe–/− and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice on a Western diet for 18 wk and isolated BCAs and aortas to characterize advanced atherosclerotic lesion development. PCR genotyping of DNA from whole aortic and carotid samples from SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice showed 50% and 55% recombination efficiency, respectively, after tamoxifen treatment, which when corrected for the approximate >50–60% SMC composition (63) of these samples equates to >95% recombination efficiency within SMCs (Fig. 2, A and B). Consistent with these data, we observed nearly 100% recombination efficiency in cultured SMCs isolated from SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice (Fig. 2A). Similarly, results of COL15A1 immunostaining of BCA sections from SMC YFP+/+ Col15a1wt/wt, Apoe−/− and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice showed a 95% knockout efficiency within medial SMC (Fig. 2, C and D). In addition, these results showed a 71% reduction in COL15A1 within lesions, indicating that SMCs are a major, although not exclusive, source of COL15A1 in atherosclerotic lesions.
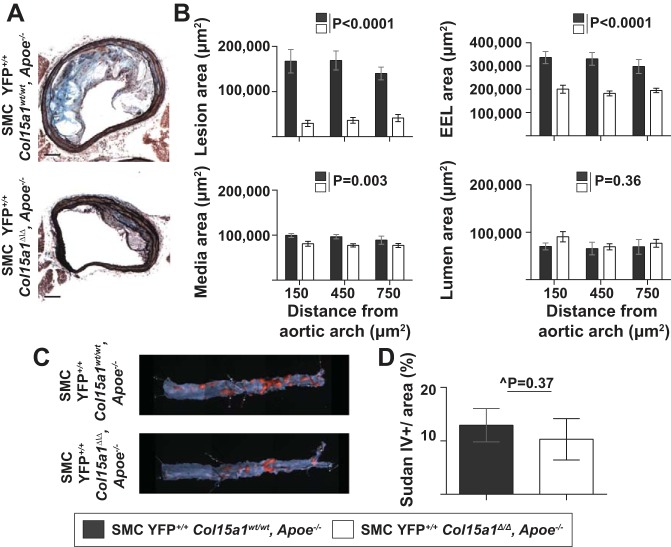
Given that COL15A1 is involved in fibrillar collagen organization and can influence mechanical stability as well as our prior observations of an association between a COL15A1 sequence variant and age-related human atherosclerosis (5, 25, 29, 56, 57), we predicted that SMC Col15a1 knockout would result in larger lesions with characteristics associated with less stable lesions. Surprisingly, however, we found that SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice exhibited a drastic, unexpected 78% decrease in BCA lesion size compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− mice (Fig. 3, A and B, left). Furthermore, SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− BCAs also had significantly smaller overall vessel size, as quantified by external elastic lamina area (Fig. 3B, top right) and media area (Fig. 3B, bottom left) but showed no difference in lumen area (Fig. 3B, bottom right). This suggests that SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− vessels are not undergoing outward remodeling likely due to reduced necessity as a result of small plaque burden. Interestingly, en face luminal Sudan IV staining for lipids within the thoracic and abdominal aorta showed no differences in overall atherosclerotic disease burden between SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− and SMC YFP+/+ Col15a1wt/wt, Apoe−/− mice (Fig. 3, C and D). This suggests that while both SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− and SMC YFP+/+ Col15a1wt/wt, Apoe−/− mice have equivalent atherosclerotic lesion burden, SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− develop drastically smaller lesions compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− mice within the BCAs indicative that SMC-derived COL15A1 is an essential component for lesion development and progression. Notably, no differences in body weight or fasting cholesterol and triglycerides were found between groups (Table 2). Taken together, these data provide the first direct in vivo evidence that a collagen, COL15A1, produced by a single cell type, SMCs, plays a critical role in atherosclerotic lesion pathogenesis. Contrary to predictions, however, loss of SMC-derived COL15A1 resulted in markedly impaired rather than exacerbated lesion development.
Fig. 3.
Loss of SMC-derived COL15A1 resulted in a marked decrease in lesion size. A: representative images of brachiocephalic artery (BCA) cross sections from SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 11) and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− (n = 13) mice fed a Western diet for 18 wk stained with Movat reagent for lesion morphometry. Scale bars = 100 µm. B: quantification of lesion area, external elastic lamina (EEL) area, media area, and lumen area measurements from Movat staining of BCAs. P values were determined by two-way ANOVA (repeated measures). C and D: representative images (C) and quantification (D) of Sudan IV-stained aortas from SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 9) and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− (n = 6) mice. Values represent means ± SE. ^P value was determined by Mann-Whitney U-test.
Table 2.
No differences in cholesterol, triglycerides, or body weight between SMC YFP+/+ Col15a1wt/wt, Apoe−/− and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice
| SMC YFP+/+ Col15a1wt/wt, Apoe−/− | SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− | P | |
|---|---|---|---|
| Cholesterol, mg/dl | 1,147 ± 79.49 | 973.6 ± 74.93 | 0.12† |
| Triglycerides, mg/dl | 152.9 ± 12.40 | 148.6 ± 15.83 | 0.34* |
| Body weight, g | 35.77 ± 0.86 | 34.04 ± 1.20 | 0.11* |
Values are means ± SE; n = 20 mice/group. SMC, smooth muscle cell; YFP, yellow fluorescent protein; Apoe, apolipoprotein E.
P value was determined by a Mann-Whitney U-test;
P value was determined by an unpaired two-tailed t-test.
SMC-Specific Deletion of Col15a1 Reduces BCA Collagen Content, Collagen Organization, and Carotid Artery Stiffness
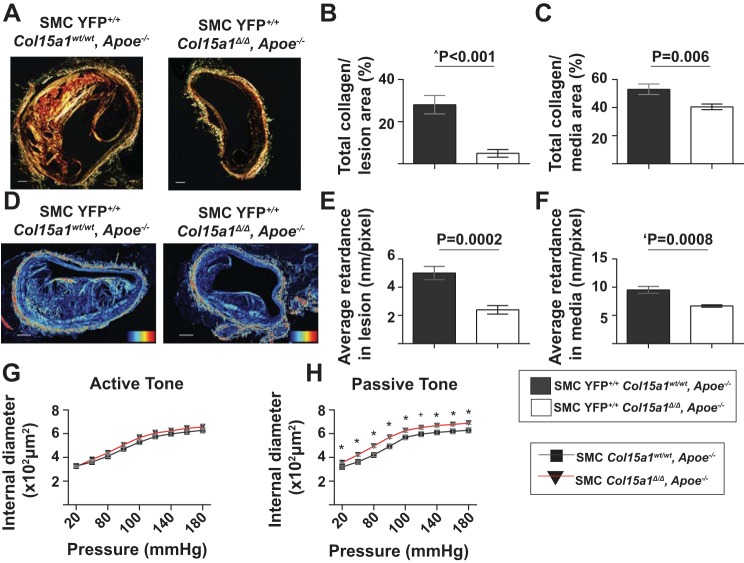
We next tested if collagen deposition is broadly affected in SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− lesions. Analysis of picrosirius red-stained BCAs demonstrated that SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice had an impressive 82% reduction in lesion and significant 24% reduction in media collagen content compared with SMC Col15a1wt/wt, Apoe−/− mice (Fig. 4, A–C). Moreover, SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice exhibited a reduced average birefringence retardance in both the media and lesion indicative of a less mature fibrillar collagen matrix in both regions compared with SMC Col15a1wt/wt, Apoe−/− mice (Fig. 4, D–F). Combined, these data provide evidence that SMCs produced COL15A1 contributes to fibrillar collagen deposition and organization within atherosclerotic lesions.
Fig. 4.
Genetic inactivation of Col15a1 in SMCs reduced collagen fiber content and maturation as well as vessel elasticity. A: representative images of picrosirius red-stained BCAs under polarized light of SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 11) and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− (n = 13) mice. Scale bars = 100 µm. Quantification of total collagen content per lesion (B) and media (C) area. D: representative images of collagen maturation by LC-Polscope analysis where pixel intensity is proportional to birefringent, organized fibrillar collagen. Average retardance measurements indicated a decrease in lesion (E) and media (F) collagen organization with SMC Col15a1 knockout. Heat map represents the amplitude of retardance (nm) from 0 (black) to 39.5 (red). Scale bars = 100 µM. P value was determined by an unpaired, two-tailed t-test; ‘P value was determined by an unpaired, two-tailed t-test with Welch’s correction; ^P value was determined by a Mann-Whitney U-test. G and H: quantification of pressure myography to assess active and passive tone of carotid arteries of 18-wk Western diet-fed SMC Col15a1wt/wt, Apoe−/− (n = 6) and SMC Col15a1Δ/Δ, Apoe−/− (n = 5) mice. G: no significant differences were detected at any point in active tone. H: passive tone was significantly increased in SMC Col15a1Δ/Δ, Apoe−/− compared with SMC Col15a1wt/wt, Apoe−/− animals. Values represent means ± SE. For passive tone, P values were determined by an unpaired, two-tailed t-test at each pressure. *P < 0.05; +P < 0.054.
To determine if these changes had consequences on vessel function, we analyzed active and passive vascular tone in the carotid arteries of 18-wk Western diet-fed SMC Col15a1wt/wt, Apoe−/− and SMC Col15a1Δ/Δ, Apoe−/− mice. Carotid arteries just above the right subclavian artery bifurcation were isolated and cannulated on glass micropipettes and then equilibrated in Krebs-HEPES-buffered physiological salt solution. By our observation, this region of the common right carotid, which is directly adjacent to the BCAs, in both SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− and SMC YFP+/+ Col15a1wt/wt, Apoe−/− was nearly devoid of lesions even after 18 wk of Western diet feeding. Vessels were then subjected to increasing transmural pressures, and internal lumen diameters were quantified. These experiments were done in the presence or absence of Ca2+ to assess spontaneous active and passive tone, respectively (Fig. 4, G and H). Carotid arteries from SMC Col15a1Δ/Δ, Apoe−/− mice showed no significant differences in active tone (Fig. 4G) but did show very modest but significant increases in passive tone (Fig. 4H) consistent with a minor decrease in overall vessel stiffness. Taken together, it therefore stands to reason that the decrease in media collagen content and maturation as a result of SMC Col15a1 knockout likely contributed to the observed functional decrease in stiffness.
Loss of COL15A1 in SMCs Alters the Number and Cell Type Composition of Lesions
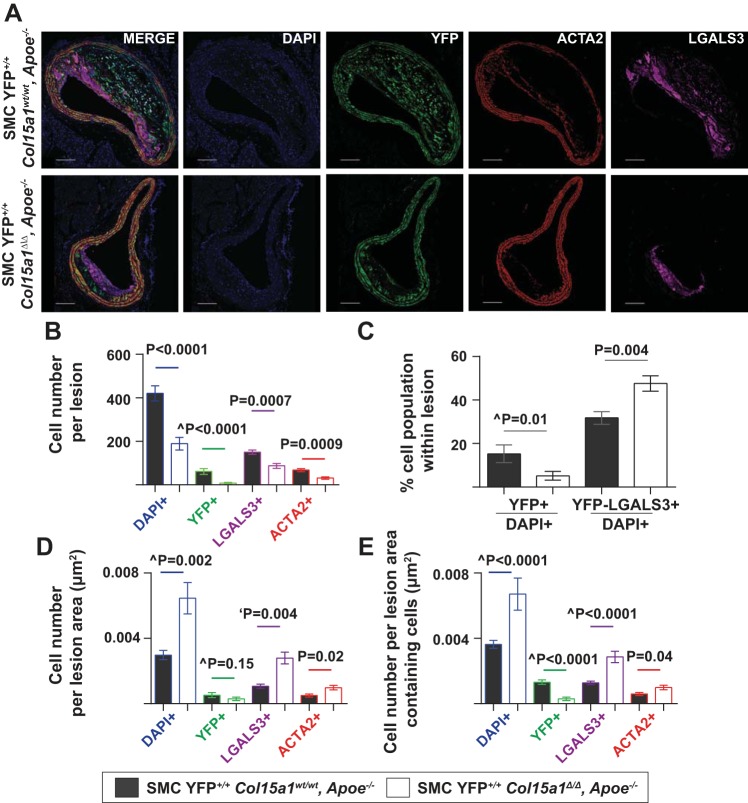
To understand how SMC-derived COL15A1 impacts the cellular composition of lesions, we performed high-resolution z-stack confocal microscopy for YFP (lineage tracing mark for SMCs) as well as for ACTA2 (a traditional SMC marker) and LGALS3 (MAC2 antigen, a macrophage marker; Fig. 5A). Remarkably, lesions from SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice exhibited a marked 55% decrease in the overall number of cells compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− mice (Fig. 5, A and B, DAPI). Notably, lesions from SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice had very few SMC-derived cells within lesions (Fig. 5C, YFP+/DAPI+) and virtually completely lacked an ACTA2+ fibrous cap (see the ACTA2 channel in Fig. 5A). In contrast, SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice had an increased proportion of macrophages (Fig. 5C, YFP−LGALS3+/DAPI+) compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions. Therefore, although SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− animals have a marked reduction in total number of cells within lesions, the cells that make up the lesions consist proportionately of more macrophages (YFP−LGALS3+) and fewer SMC (YFP+) compared with lesions from SMC YFP+/+ Col15a1wt/wt, Apoe−/− mice. Taken together, SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice develop less advanced, fatty streak-like lesions that are macrophage rich, SMC poor, and lack fibrillar collagens compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions.
Fig. 5.
Loss of SMC-specific COL15A1 resulted in overall reductions in the number of YFP+ SMC-derived cells within the lesions but an increase in YFP−galectin 3 (LGALS3)+ macrophage cells. A: representative immunofluorescence images of BCA sections of SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 11) and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− (n = 13) mice showing a marked decrease in overall cell number, a decrease in YFP+/DAPI+ cells, and an increase in YFP−LGALS3+/DAPI+ cells within lesions of SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice. Scale bars = 100 µm. B: quantification of cell number per lesion stained positive for DAPI, YFP, LGALS3, or smooth muscle α-actin (ACTA2) averaged across four locations of the BCA. C: quantification of percentage of SMCs (YFP) and non-SMC-derived macrophages (YFP−LGALS3+) over total number of cells (DAPI+) within lesions averaged across four separate locations of the BCA. D: quantification of cell density of the lesion by examining cell number normalized to lesion area (μm2). Given that SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− have more acellular, necrotic core regions in their lesions as compared with that of SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice, we also quantified cell density of the lesion (E) by examining cell number normalized to the lesion area (μm2) with the necrotic core regions excluded. Values represent means ± SE. P values were determined by an unpaired, two tailed t-test. ^P value was determined by a Mann-Whitney U-test.
Next, we analyzed whether cell density differed between SMC YFP+/+ Col15a1wt/wt, Apoe−/− and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice. We found that when normalized to lesion area, there was a significant increase in cell density of DAPI+, LGALS3+, and ACTA2+ cells but no change in YFP+ cell density within SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− lesions compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions (Fig. 5D). It is important to note that SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− animals contain much smaller, less complex lesions with a reduced frequency of acellular, necrotic core areas compared with SMC YFP+/+ Col15a1wt/wt Apoe−/− animals. Specifically, 8 of 11 SMC YFP+/+ Col15a1wt/wt, Apoe−/− animals in this analysis had large, established acellular necrotic cores, whereas only 2 of 13 SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− animals contained any small, acellular necrotic core-like regions (P = 0.01, Fisher’s exact test, data not shown). We also analyzed cell density as a function of the lesion area without inclusion of the acellular, necrotic core regions. Interestingly, we found that while the cell density of DAPI+, LGALS3+, and ACTA2+ cells remained increased in SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions, YFP+ cell density was found to be significantly reduced as a result of SMC Col15a1 knockout (Fig. 5E). This suggests that although SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− lesions overall appear to be more cell dense, there is a significant reduction in the proportion of SMCs present within SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions.
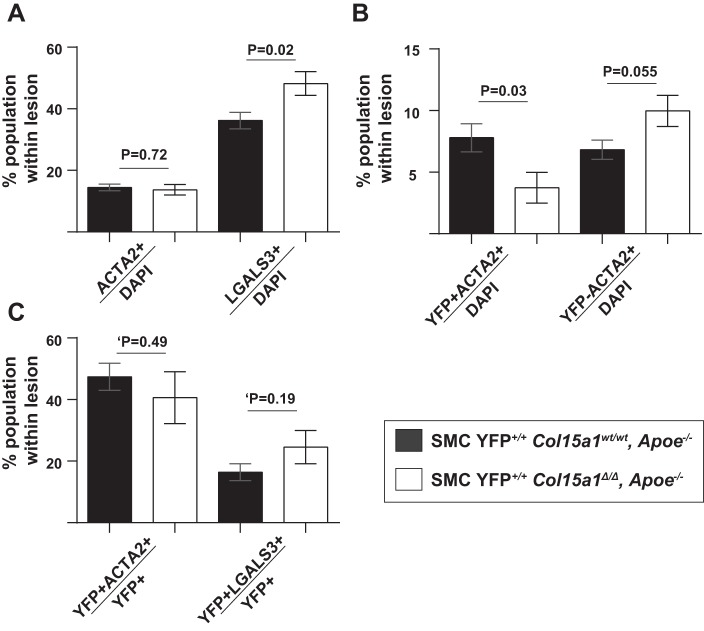
We then investigated the cells expressing the traditional SMC marker gene ACTA2. Of interest, we observed no difference in the proportion of ACTA2+ cells per total lesion cells (ACTA2+/DAPI+) between SMC YFP+/+ Col15a1wt/wt, Apoe−/− and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− lesions (Fig. 6A). This result and the observation of increased ACTA2+ cell density in SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− lesions differ from prior global collagen knockout studies where a decrease in ACTA2+ cells and ACTA2+ cell density within atherosclerotic lesions was consistently observed irrespective of the collagen type knockout (32, 46, 48). However, if we divided the ACTA2+ cells within the lesions in our studies into SMC and non-SMC derived, we found that there was a decrease in the proportion of ACTA2+ SMC-derived cells (YFP+ACTA2+/DAPI+) and a near significant increase in the proportion of non-SMC-derived ACTA2+ cells (YFP−ACTA2+/DAPI+) within SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− lesions compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions (Fig. 6B). Moreover, there was no difference in the proportion of SMC expressing ACTA2 (YFP+ACTA2+/YFP+) or LGALS3 (YFP+LGALS3+/YFP+), although a significant increase in the proportion of LGALS3+ cells (LGALS3+/DAPI+) was observed between SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− and SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions (Fig. 6, A and C). SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice therefore are still able to maintain ACTA2+ cell contribution to the lesion, but loss of SMC-produced COL15A1 reduces the proportion of ACTA2+ SMC contribution compared with wild-type lesions.
Fig. 6.
SMC-derived cell populations were reduced with SMC Col15a1 knockout. A: no changes were observed in the percentage of ACTA2+/DAPI+ cells but a significant increase was found in the percentage of LGALS3+/DAPI+ cells in SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− (n = 13) compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 11) lesions. B: a significant decrease in SMC-derived ACTA2+ (YFP+ACTA2+/DAPI+) cells and a near significant increase in non-SMC-derived ACTA2+ (YFP−ACTA2+/DAPI+) cell populations were also found in SMC Col15a1 knockout compared with wild type. C: no significant differences were seen in the proportion of SMCs able to express ACTA2 (YFP+ACTA2+/YFP+) or LGALS3 (YFP+LGALS3+/YFP+) as a consequence of SMC Col15a1 knockout. Values represent the average of three locations across the BCA for each genotype. Values represent means ± SE. ‘P value was determined by an unpaired two-tailed t-test analysis with Welch’s correction.
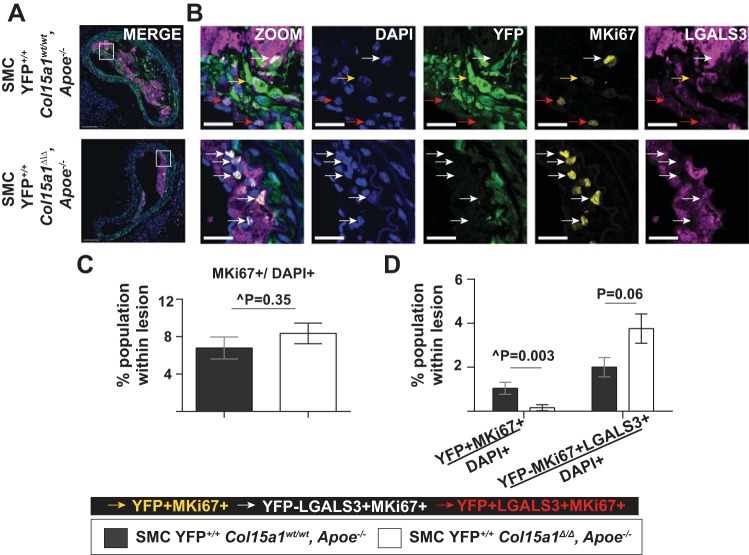
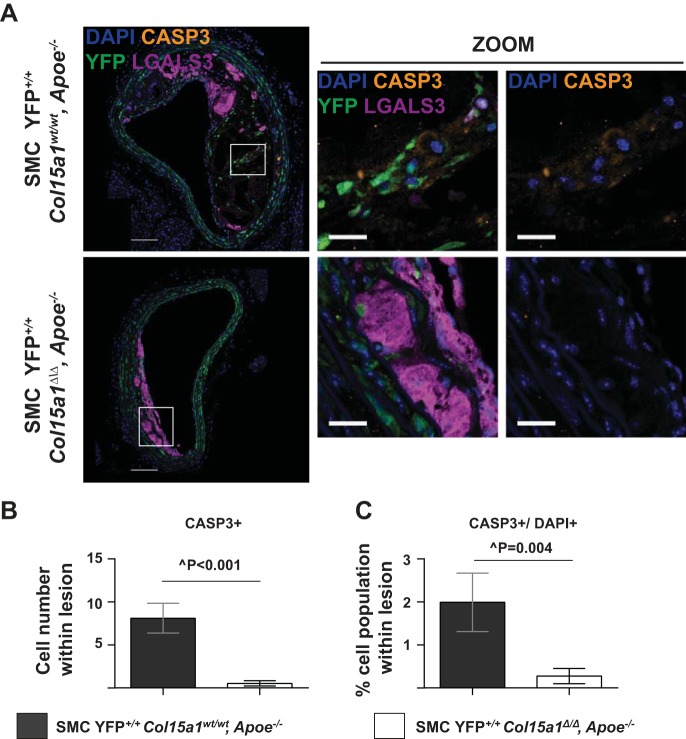
To determine the mechanisms by which loss of SMC-derived COL15A1 resulted in reduced SMC number within lesions, we performed fluorescence coimmunostaining for YFP, LGALS3, and MKi67 (a marker of proliferation) or YFP, LGALS3, and cleaved CASP3 (a marker of cell death). There was no difference in the overall amount of proliferating cells per total number of lesion cells (MKi67+/DAPI+) between groups (Fig. 7, A–C). However, we observed a significant 85% reduction in the proportion of SMC proliferating (YFP+MKi67+/DAPI+, Figs. 7B, yellow arrows, and D) within SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− lesions compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions. In contrast, there appeared to be a modest increase in the proliferation of macrophages (YFP−MKi67+LGALS3+/DAPI+, Figs. 7B, white arrows, and D) within SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− lesions compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions, although this did not reach statistical significance (P = 0.06). SMC Col15a1 knockout thus appears to differentially impact SMC versus non-SMC proliferation within lesions. SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− lesions also were nearly devoid of CASP3+ cells compared with their SMC YFP+/+ Col15a1wt/wt, Apoe−/− counterparts (Fig. 8, A–C), providing evidence that the reduction in overall cell number within SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− lesions is not due to increased cell death and likely reflects the reduced lesion complexity observed as a consequence of SMC Col15a1 knockout.
Fig. 7.
SMC-specific Col15a1 knockout was associated with a decrease in lesion SMC proliferation. A: representative immunofluorescence images of BCA sections stained for proliferating cells (MKi67+) within SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 11) and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− (n = 13) lesions. B: higher magnification and single marker immunostaining of the white box region in A. Arrows indicate subpopulations of proliferating cells including SMC (YFP+MKi67+, yellow arrows), non-SMC-derived macrophages (YFP−LGALS3+MKi67+, white arrows), and SMC-derived macrophage-like cells (YFP+LGALS3+MKi67+, red arrows). Scale bars = 100 µm (A) and 25 µm (B). B: quantification of the percentage of proliferating cells within the lesion (MKi67+/DAPI+) showed no difference between SMC YFP+/+ Col15a1wt/wt, Apoe−/− and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice. C: however, there was a significant decrease in the percentage of SMC-derived cells (YFP+MiKi67+/DAPI+) and an increase in the percentage of non-SMC-derived macrophages (YFP−MKi67+LGALS3+/DAPI+) proliferating within SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions. Values represent means ± SE of one location of the BCA. ^P value was determined by Mann-Whitney U-test analysis. P value was determined by an unpaired two-tailed t-test.
Fig. 8.
SMC Col15a1 knockout resulted in a decrease in lesion cell death. A: representative immunofluorescent images of SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 11) and SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− (n = 13) lesions stained for the cell death marker cleaved caspase-3 (CASP3+). B and C: quantification of CASP3+ cells counted per lesion (B) and percentages of CASP3+ cells within lesions (C; CASP3+/DAPI+) showed that SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice have significantly fewer dying cells compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− mice. Values represent means ± SE. Scale bars = 100 µm and on zoom in images 25 µm. ^P value from a Mann-Whitney U-test.
Loss of SMC-Derived COL15A1 Results in Dysregulation of Immune Cell Pathways Important in Driving Lesion Development
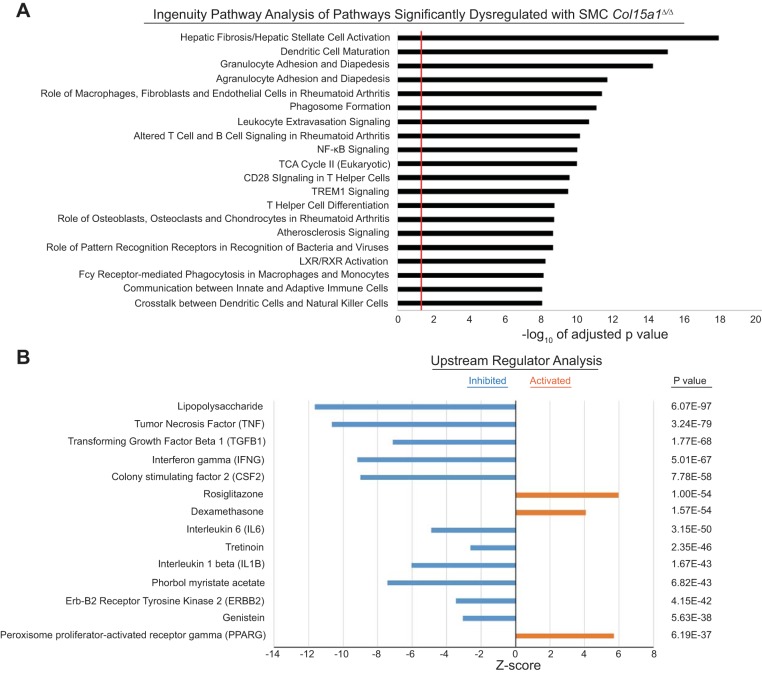
The preceding results indicate that the reduced lesion size and complexity observed in SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− lesions is due in part to an overall decrease in the number of cells within the lesions including both SMCs and macrophages. However, in aggregate, these two cell types only account for about half of the overall reduction in cell number within lesions of the SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− mice. To further understand the mechanism(s) by which loss of SMC-derived COL15A1 resulted in dramatic decreases in lesion size and complexity, we performed in vivo RNA-seq analysis on combined aortic arch, BCAs, and carotid artery tissue samples of 18-wk Western diet-fed SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− and SMC YFP+/+ Col15a1wt/wt, Apoe−/− mice. Analysis of complex tissue samples consisting of multiple cell types is usually highly problematic in that it is nearly impossible to rigorously ascertain what changes are contributed by one cell type versus another. In addition, many differences may be lost due to inadequate sensitivity in detecting changes in relatively low abundance cell types and/or offsetting changes in gene expression in different cell types. However, an extremely powerful aspect of our approach is that we know that changes were initiated by loss of one gene, Col15a1, in one cell type, SMCs. Of major interest, results of Ingenuity Pathway Analysis (35) of the RNA-seq showed marked dysregulation of multiple immune cell pathways including dendritic cell maturation, altered T and B cell signaling, NF-κB signaling, granulocyte adhesion and diapedesis, and leukocyte extravasation signaling in response to SMC Col15a1 knockout (Fig. 9A).
Fig. 9.
SMC-specific Col15a1 knockout resulted in downregulation of immune cell processes that impact atherosclerotic plaque formation. RNA-seq analysis of BCA, aortic arch, and carotid artery specimens from 18-wk Western diet-fed SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− (n = 3) compared with SMC YFP+/+ Col15a1wt/wt, Apoe−/− (n = 3) mice. A: Ingenuity Pathway Analysis showed dysregulation of inflammatory pathways. The red line marks the cut off for significantly enriched (adjusted P ≤ 0.05) pathways. Enrichment is shown as –log10 of the adjusted P value. B: Upstream Regulator Analysis was performed on the top ~3,000 differentially expressed genes containing a log fold change of ±0.30 from the RNA-seq to identify master regulators that are either inhibited or activated as a consequence of SMC Col15a1 knockout.
As there are myriad cell types present within atherosclerotic vessels, we are unable to make conclusions as to whether the pathway changes derived from this analysis are due to direct or indirect effects of SMC Col15a1 knockout. However, we used a recently developed bioinformatics analysis program referred to as “Upstream Regulator Analysis” (35) to attempt to identify the upstream drivers responsible for differences between SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− and SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions. That is, in our case, this analysis takes the RNA-seq differential gene expression data derived from the comparison of SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− and SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions and integrates it with cause-effect relationships reported in the literature to attempt to predict the master regulatory pathways (e.g., genes, transcription factors, small molecule-driven pathways) most responsible for the difference in phenotype observed between SMC Col15a1 knockout and wild-type lesions. Of major interest, these analyses identified significant repression of multiple factors as a result of SMC Col15a1 knockout, including lipopolysaccharide, TNF, TGF-β1, interferon (IFN)-γ, colony stimulating factor-2 (CSF2), IL-6, tretinoin (retinoic acid), IL-1β, PMA, Erb-B2 receptor tyrosine kinase 2 (ERBB2), and genistein (Fig. 9B). These results suggest that the mechanisms underlying the substantial decrease in lesion size and severity in our SMC Col15a1 knockout mice are extremely complex but likely to involve rather profound global repression of multiple proinflammatory processes and immune cell responses. In addition, results of these analyses also identified a number of pathways that are activated within SMC YFP+/+ Col15a1Δ/Δ, Apoe−/− versus SMC YFP+/+ Col15a1wt/wt, Apoe−/− lesions, including dexamethasone, peroxisome proliferator-activated receptor-γ (PPARG), and rosiglitazone (a PPARG agonist) pathways (Fig. 9B). While these pathways can impact a wide range of cellular functions, the role of dexamethasone in atherosclerosis is unclear although it is predominantly described as anti-inflammatory (8, 18). In addition, PPARG activation and the PPARG agonist rosiglitazone have been shown to be atheroprotective (9, 16, 17, 28, 42, 52). Taken together, our in vivo genomic analyses provide evidence that SMC-derived COL15A1 can positively impact a variety of proatherogenic processes, including immune cell activation, adhesion, migration, and cytokine expression, such that SMC-derived COL15A1 loss results in rather profound inhibition of lesion development and pathogenesis.
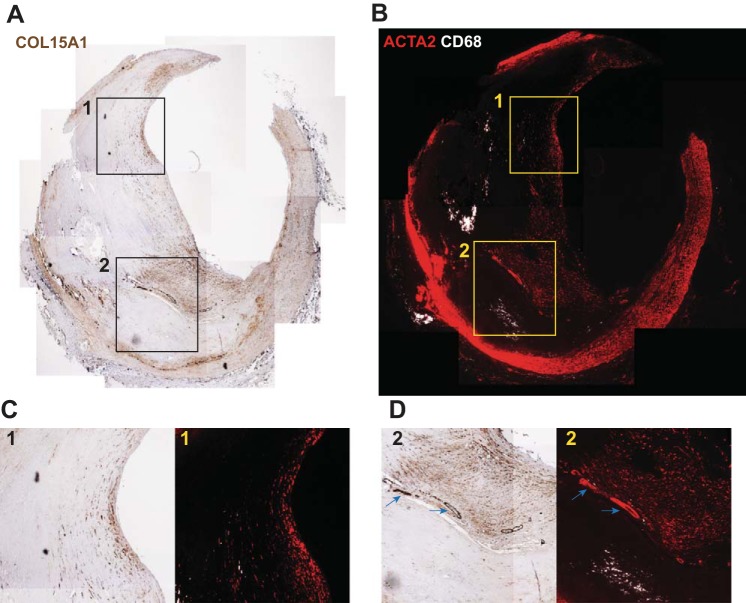
COL15A1 Is Prevalent in ACTA2+ Regions Within Advanced Human Atherosclerotic Plaques Including the Fibrous Cap
Given the importance of the fibrous cap in maintaining human plaque stability, we reasoned that identifying COL15A1 expression within human lesions would be important to our understanding of how COL15A1 may be contributing to plaque formation and stability. Coronary arteries with advanced lesions from six patients who underwent CABG were stained for COL15A1 (Fig. 10A). Serial slides were stained with the traditional macrophage marker CD68 and traditional SMC marker ACTA2 to provide a general characterization of the cell populations present within the lesion (Fig. 10B). We found that COL15A1 is highly expressed throughout ACTA2+-rich fibrous caps and within the plaque shoulder regions, suggesting a role for COL15A1 in the maintenance of overall plaque and fibrous cap stability (Fig. 10, C and D). Interestingly, COL15A1 also localized to intraplaque microvessels, which coincides with previous studies in mice showing COL15A1 expression within the microvasculature (29, 57) (Fig. 10D, blue arrows). These data therefore provide the first assessment of COL15A1 expression in advanced human lesions. In addition, the mouse and human data in this report provide the first detailed assessment of COL15A1 expression within lesions and COL15A1 function in atherosclerotic lesion development.
Fig. 10.
COL15A1 is pervasive within advanced human atherosclerotic plaques and localized to the fibrous cap and intraplaque microvessels. A representative image of a human coronary artery lesion from 1 of 6 patients analyzed who underwent coronary artery bypass surgery is shown. Serial sections were stained with COL15A1 (A) or traditional macrophage (CD68; B) and SMC marker (ACTA2) genes. C and D: boxes (1 and 2) represent regions of higher magnification to highlight COL15A1 presence in the ACTA2-rich fibrous cap (C and D) and intraplaque microvessels (D, blue arrows) with little to no COL15A1 staining in CD68-rich regions (D).
DISCUSSION
Fibrillar collagens are known to localize to the fibrous cap and provide mechanical stability to atherosclerotic plaques (31, 39, 45, 68a). COL15A1 serves as a linker of large fibrillar collagens providing structural integrity to collagen matrixes (5). We therefore predicted that SMC-derived COL15A1 would have an atheroprotective role in stabilizing advanced atherosclerotic lesions through organizing and fortifying the extracellular matrix (ECM) within the fibrous cap. Consistent with this hypothesis, we observed COL15A1 staining within the fibrous cap of advanced human lesions. Surprisingly, however, our data indicate that SMC Col15a1 knockout before the start of 18-wk Western diet-induced atherosclerosis led to the development of lesions 78% smaller than those of wild-type controls. In addition, SMC Col15a1 knockout lesions exhibit characteristics consistent with those of fatty streaks; they are macrophage rich, display a paucity of SMCs and collagen, and are devoid of a fibrous cap and necrotic core. In stark contrast, wild-type mice exhibited typical advanced BCA lesions, including a SMC-rich, collagen-rich fibrous cap. Taken together, these results provide the first direct evidence that a collagen produced by SMCs can impact plaque pathogenesis and that COL15A1 has a role in promoting lesion development.
The data presented here suggest that mechanisms of SMC COL15A1 action in impairing lesion development are both diverse and complex. One such mechanism we observed is that loss of SMC-derived COL15A1 results in reductions in the overall numbers of SMCs and macrophages within lesions. These findings are consistent with previous studies of conventional global Col15a1 knockout mice showing that global loss of COL15A1 from birth affects multiple cell types as these mice develop cardiovascular and skeletal myopathy (29, 57), impaired peripheral nerve maturation (56), and in a model of renal ischemia-reperfusion injury have reduced neutrophil infiltration but no change in macrophage (F4/80+) infiltration (73). Our work builds on and advances this knowledge; we are the first to provide evidence that the loss of COL15A1 production from a single cell type, SMCs, has a vast and varied effect on the function of multiple cell types during atherogenesis. Specifically, our RNA-seq analysis of BCA lesions indicates that the net effect of SMC Col15a1 knockout is the dysregulation of proinflammatory pathways associated with immune cells (i.e., T cells, B cells, macrophages, dendritic cells, granulocytes, and leukocytes), endothelial cells, and fibroblasts. SMC Col15a1 knockout also results in an overall increase in lesion cell density but a decrease in the proportion of SMCs and an increase in the proportion of macrophages populating the lesions compared with wild-type lesions. A second important role for COL15A1 is as a modulator of cell proliferation within lesions as loss of SMC-derived COL15A1 resulted in an 85% reduction in SMC proliferation and near significant increase in non-SMC-derived macrophage proliferation within lesions. SMC-derived COL15A1 thus has a major influence on the function of nearly all cell types involved in lesion development. We propose that COL15A1 is an integral component within the lesion environmental milieu crucial in driving atherosclerotic plaque formation. Finally, it is known that COL15A1 is an ECM-organizing molecule (5, 23, 29, 37, 43, 51, 56, 57). Collagen types I and III are, to our knowledge, the most well-studied collagens in regard to atherosclerosis (1, 39, 49, 58, 64). However, there are nearly 30 collagen types, all of which may be influenced by loss of COL15A1 production exclusively by SMCs. We therefore did not specifically measure collagen types I and III but instead used picrosirius red staining to evaluate the net changes in collagen content and maturation within the lesions. Notably, we observed that SMC Col15a1 knockout resulted in a 24% reduction in media and a drastic 82% reduction in lesion fibrillar collagen content, a reduced media and lesion collagen organization, as well as a functional increase in carotid artery elasticity. Likewise, we observed a 71% decrease in lesion COL15A1 expression with SMC Col15a1 knockout. Combined, this provides the first evidence that SMCs are a major, although not the sole, source of COL15A1 and likely collagens within lesions. We suspect that the effects on lesion development as a result of SMC Col15a1 knockout may therefore be due in part to impaired formation and organization of a three-dimensional matrix scaffold needed for SMCs and other lesion cells to attach, migrate, and proliferate into and within lesions. Importantly, together, these data represent a significant advance in the field by providing the first direct in vivo evidence that a SMC-derived collagen, COL15A1, directly influences plaque formation.
Before our studies, data from global knockout of nonfibrillar collagen types VIII and XVIII from birth and throughout lesion development provided evidence that collagens can have broad impacts on lesion development by observing changes in cellular and collagen content of lesions and in type XVIII knockouts, specifically changes in the size of lesions (46, 48). However, there are two inherent limitations in these prior global collagen knockout studies including 1) the inability to determine the cellular source of collagen and 2) global loss of the respective collagens from birth likely led to the activation of compensatory pathways that confound data interpretation. Notably, our studies and others in the field are also limited by the fact that collagens are extracellular proteins, making it nearly impossible to determine if the observations made in association with manipulating collagen levels in vivo are the consequence of direct or indirect effects. In the case of COL15A1, this is further complicated by several unique properties of the NH2-terminal and COOH-terminal domains of COL15A1. First, the noncollagenous NH2-terminal domain of COL15A1 contains chondroitin sulfate side chains similar to that of a proteoglycan (23, 43). There is evidence in the field that proteoglycans containing chondroitin sulfate side chains can bind apoB100 and LDL and are hypothesized to occur in the arterial wall contributing to early lesion development (13, 65, 68). However, there is currently no evidence in the field that COL15A1 itself can directly bind lipids. Second, the COOH-terminus of COL15A1 contains a cleavage product called restin (24). To our knowledge, the role of restin in atherosclerosis has not been previously investigated, and studies are conflicting over whether this molecule is involved in angiogenesis (23, 38, 50, 55, 62). Given these results and those of our in vivo RNA-seq analyses, it seems likely that COL15A1 production by SMCs is causing a number of direct and indirect changes that in aggregate result in profound inhibition of lesion development. Since in vitro cell culture approaches are unlikely to replicate the complex lesion environmental milieu, multicellularity, and extracellular architecture that exist within lesions in vivo, we took an alternative approach and performed an Upstream Regulator Analysis that focused on understanding the net effect of SMC-derived COL15A1 action on lesion development. These analyses identified SMC-derived COL15A1-dependent pathways that were highly significantly repressed, which included several proinflammatory and immune cell response factors previously implicated in lesion development, including lipopolysaccharides (66), TNF (12), TGF-β (59), IFN-γ (36), CSF2, and IL-1β (2), in lesions from SMC Col15a1 knockout versus wild-type mice. While these data fail to provide direct insights as to how loss of SMC-derived COL15A1 impacts the multiplicity of cells within developing lesions, the strength of the data lies in that they provide insight as to possible rate-limiting pathways essential for SMC COL15A1-dependent lesion development.
A critical question is to what extent our findings are relevant to human atherosclerosis. Of major interest, we provide evidence showing that COL15A1 is highly expressed within advanced human lesions, including plaque shoulders and caps, believed to be important in plaque stability. Moreover, our previous studies of COL15A1 in human atherosclerosis provided correlative evidence suggesting that a reduction in COL15A1 expression in patients carrying the risk allele may be associated with increased Sudan IV+ aortic lesion burden. However, these human studies were based on a very small patient cohort and did not include direct characterization of indexes of plaque stability. In addition, the results of the present studies showing that SMC-specific loss of Col15a1 before initiation of a Western diet greatly attenuates lesion development may not predict the role of this molecule in advanced lesions and/or if therapeutic targeting of COL15A1 is a viable means to promote plaque stabilization. To address these critical questions, future studies would need to include 1) a large-scale clinical study to assess if there is a correlation between COL15A1 levels within patient lesions and the incidence of S-T elevation-mediated myocardial infarctions or stroke and 2) experimental animal studies to test if a reduction of COL15A1 levels after establishment of advanced atherosclerotic lesions has beneficial effects on lesion composition and indexes of plaque stability.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-100257 and R00-HL-089412 (to J. J. Connelly); R01-HL-057353, R01-HL-087867, and R01-HL-098538 (to G. K. Owens); R01-HL-088554 (to B. E. Isakson); and T32-HL-007284 (to B. G. Durgin and J. T. Butcher); the American Heart Association (AHA) Grants 14PRE20380659 (to B. G. Durgin), 17IRG33370017 (to O. A. Cherepanova), 13POST17080043 and 15SDG25860021 (to D. Gomez), and 15PRE25730011 (to G. F. Alencar); Canadian Institutes of Health Research (CIHR) Grant MOP126042 (to M. P. Bendeck); and the Robert M. Berne Cardiovascular Research Center at the University of Virginia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.G.D., T.K., G.K.O., and J.J.C. conceived and designed research; B.G.D., D.G., J.T.B., and Y.-Q.Z. performed experiments; B.G.D., G.F.A., J.T.B., Y.-Q.Z., M.P.B., B.E.I., and J.J.C. analyzed data; B.G.D., O.A.C., D.G., G.F.A., J.T.B., Y.-Q.Z., M.P.B., B.E.I., G.K.O., and J.J.C. interpreted results of experiments; B.G.D., G.F.A., and J.T.B. prepared figures; B.G.D. drafted manuscript; B.G.D., M.P.B., G.K.O., and J.J.C. edited and revised manuscript; B.G.D., O.A.C., D.G., T.K., G.F.A., J.T.B., Y.-Q.Z., M.P.B., B.E.I., G.K.O., and J.J.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank TransViragen, specifically D. Crowley, for generating the Col15a1fl/fl mice and S. Offermanns from the Max Plank Institute for the Myh11-CreERT2 mice. We also thank M. Bevard and M. McCanna for histology expertise, R. Tripathi for assisting in isolating primary aortic SMCs, and S. Guillot at the Advanced Microscopy Facility for providing training for use of confocal microscopy and polarized light microscopy. We also recognize A. Newman, S. Sippl, M. Fawze, and A. Connelly for intellectual contributions and assistance at various points in the project.
Present address of T. Karaoli: University of Virginia, Charlottesville, VA.
Present address of J. T. Butcher: Vascular Biology Center, Augusta University, Augusta, GA.
REFERENCES
- 1.Adiguzel E, Ahmad PJ, Franco C, Bendeck MP. Collagens in the progression and complications of atherosclerosis. Vasc Med 14: 73–89, 2009. doi: 10.1177/1358863X08094801. [DOI] [PubMed] [Google Scholar]
- 2.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, Owens GK. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest 122: 70–79, 2012. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 129: 1551–1559, 2014. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 5.Amenta PS, Scivoletti NA, Newman MD, Sciancalepore JP, Li D, Myers JC. Proteoglycan-collagen XV in human tissues is seen linking banded collagen fibers subjacent to the basement membrane. J Histochem Cytochem 53: 165–176, 2005. doi: 10.1369/jhc.4A6376.2005. [DOI] [PubMed] [Google Scholar]
- 6.Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arter Thromb Vasc Biol 11: 1223–1230, 1991. 10.1161/01.ATV.11.5.1223. [DOI] [PubMed] [Google Scholar]
- 7.Andreeva ER, Pugach IM, Orekhov AN. Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis 135: 19–27, 1997. doi: 10.1016/S0021-9150(97)00136-6. [DOI] [PubMed] [Google Scholar]
- 8.Asai K, Funaki C, Hayashi T, Yamada K, Naito M, Kuzuya M, Yoshida F, Yoshimine N, Kuzuya F. Dexamethasone-induced suppression of aortic atherosclerosis in cholesterol-fed rabbits. Possible mechanisms. Arterioscler Thromb 13: 892–899, 1993. doi: 10.1161/01.ATV.13.6.892. [DOI] [PubMed] [Google Scholar]
- 9.Babaev VR, Yancey PG, Ryzhov SV, Kon V, Breyer MD, Magnuson MA, Fazio S, Linton MF. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 25: 1647–1653, 2005. doi: 10.1161/01.ATV.0000173413.31789.1a. [DOI] [PubMed] [Google Scholar]
- 10.Barnes MJ, Farndale RW. Collagens and atherosclerosis. Exp Gerontol 34: 513–525, 1999. doi: 10.1016/S0531-5565(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 11.Billaud M, Lohman AW, Straub AC, Parpaite T, Johnstone SR, Isakson BE. Characterization of the thoracodorsal artery: morphology and reactivity. Microcirculation 19: 360–372, 2012. doi: 10.1111/j.1549-8719.2012.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boesten LS, Zadelaar AS, van Nieuwkoop A, Gijbels MJ, de Winther MP, Havekes LM, van Vlijmen BJ. Tumor necrosis factor-alpha promotes atherosclerotic lesion progression in APOE*3-Leiden transgenic mice. Cardiovasc Res 66: 179–185, 2005. doi: 10.1016/j.cardiores.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Borén J, Olin K, Lee I, Chait A, Wight TN, Innerarity TL. Identification of the principal proteoglycan-binding site in LDL. A single-point mutation in apo-B100 severely affects proteoglycan interaction without affecting LDL receptor binding. J Clin Invest 101: 2658–2664, 1998. doi: 10.1172/JCI2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burleigh MC, Briggs AD, Lendon CL, Davies MJ, Born GV, Richardson PD. Collagen types I and III, collagen content, GAGs and mechanical strength of human atherosclerotic plaque caps: span-wise variations. Atherosclerosis 96: 71–81, 1992. doi: 10.1016/0021-9150(92)90039-J. [DOI] [PubMed] [Google Scholar]
- 15.Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci USA 100: 4754–4759, 2003. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castrillo A, Tontonoz P. PPARs in atherosclerosis: the clot thickens. J Clin Invest 114: 1538–1540, 2004. doi: 10.1172/JCI23705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR γ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell 7: 161–171, 2001. doi: 10.1016/S1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 18.Chen A, Geng Y, Ke H, Constant L, Yan Z, Pan Y, Lee P, Tan I, Williams K, George S, Munirathinam G, Reardon CA, Getz GS, Wang B, Zheng G. Cutting edge: Dexamethasone potentiates the responses of both regulatory T cells and B-1 cells to antigen immunization in the ApoE−/− mouse model of atherosclerosis. J Immunol 193: 35–39, 2014. doi: 10.4049/jimmunol.1302469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest 125: 4514–4528, 2015. doi: 10.1172/JCI82719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng GC, Loree HM, Kamm RD, Fishbein MC, Lee RT. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation 87: 1179–1187, 1993. doi: 10.1161/01.CIR.87.4.1179. [DOI] [PubMed] [Google Scholar]
- 21.Cherepanova OA, Gomez D, Shankman LS, Swiatlowska P, Williams J, Sarmento OF, Alencar GF, Hess DL, Bevard MH, Greene ES, Murgai M, Turner SD, Geng YJ, Bekiranov S, Connelly JJ, Tomilin A, Owens GK, Sox S. Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective. Nat Med 22: 657–665, 2016. doi: 10.1038/nm.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherepanova OA, Pidkovka NA, Sarmento OF, Yoshida T, Gan Q, Adiguzel E, Bendeck MP, Berliner J, Leitinger N, Owens GK. Oxidized phospholipids induce type VIII collagen expression and vascular smooth muscle cell migration. Circ Res 104: 609–618, 2009. doi: 10.1161/CIRCRESAHA.108.186064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clementz AG, Harris A. Collagen XV: exploring its structure and role within the tumor microenvironment. Mol Cancer Res 11: 1481–1486, 2013. doi: 10.1158/1541-7786.MCR-12-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clementz AG, Mutolo MJ, Leir SH, Morris KJ, Kucybala K, Harris H, Harris A. Collagen XV inhibits epithelial to mesenchymal transition in pancreatic adenocarcinoma cells. PLoS One 8: e72250, 2013. doi: 10.1371/journal.pone.0072250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connelly JJ, Cherepanova OA, Doss JF, Karaoli T, Lillard TS, Markunas CA, Nelson S, Wang T, Ellis PD, Langford CF, Haynes C, Seo DM, Goldschmidt-Clermont PJ, Shah SH, Kraus WE, Hauser ER, Gregory SG. Epigenetic regulation of COL15A1 in smooth muscle cell replicative aging and atherosclerosis. Hum Mol Genet 22: 5107–5120, 2013. doi: 10.1093/hmg/ddt365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J 69: 377–381, 1993. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duval C, Chinetti G, Trottein F, Fruchart JC, Staels B. The role of PPARs in atherosclerosis. Trends Mol Med 8: 422–430, 2002. doi: 10.1016/S1471-4914(02)02385-7. [DOI] [PubMed] [Google Scholar]
- 29.Eklund L, Piuhola J, Komulainen J, Sormunen R, Ongvarrasopone C, Fássler R, Muona A, Ilves M, Ruskoaho H, Takala TE, Pihlajaniemi T. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc Natl Acad Sci USA 98: 1194–1199, 2001. doi: 10.1073/pnas.98.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res 115: 662–667, 2014. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 31.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol 30: 1282–1292, 2010. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 32.Fukumoto Y, Deguchi JO, Libby P, Rabkin-Aikawa E, Sakata Y, Chin MT, Hill CC, Lawler PR, Varo N, Schoen FJ, Krane SM, Aikawa M. Genetically determined resistance to collagenase action augments interstitial collagen accumulation in atherosclerotic plaques. Circulation 110: 1953–1959, 2004. doi: 10.1161/01.CIR.0000143174.41810.10. [DOI] [PubMed] [Google Scholar]
- 33.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res 95: 156–164, 2012. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez D, Shankman LS, Nguyen AT, Owens GK. Detection of histone modifications at specific gene loci in single cells in histological sections. Nat Methods 10: 171–177, 2013. doi: 10.1038/nmeth.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30: 523–530, 2014. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-γ potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest 99: 2752–2761, 1997. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris A, Harris H, Hollingsworth MA. Complete suppression of tumor formation by high levels of basement membrane collagen. Mol Cancer Res 5: 1241–1245, 2007. doi: 10.1158/1541-7786.MCR-07-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.John H, Radtke K, Ständker L, Forssmann WG. Identification and characterization of novel endogenous proteolytic forms of the human angiogenesis inhibitors restin and endostatin. Biochim Biophys Acta 1747: 161–170, 2005. doi: 10.1016/j.bbapap.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Katsuda S, Okada Y, Minamoto T, Oda Y, Matsui Y, Nakanishi I. Collagens in human atherosclerosis. Immunohistochemical analysis using collagen type-specific antibodies. Arterioscler Thromb 12: 494–502, 1992. doi: 10.1161/01.ATV.12.4.494. [DOI] [PubMed] [Google Scholar]
- 40.Klein LW. Clinical implications and mechanisms of plaque rupture in the acute coronary syndromes. Am Heart Hosp J 3: 249–255, 2005. doi: 10.1111/j.1541-9215.2005.03221.x. [DOI] [PubMed] [Google Scholar]
- 41.Lendon CL, Davies MJ, Born GV, Richardson PD. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis 87: 87–90, 1991. doi: 10.1016/0021-9150(91)90235-U. [DOI] [PubMed] [Google Scholar]
- 42.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor γ ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest 106: 523–531, 2000. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D, Clark CC, Myers JC. Basement membrane zone type XV collagen is a disulfide-bonded chondroitin sulfate proteoglycan in human tissues and cultured cells. J Biol Chem 275: 22339–22347, 2000. doi: 10.1074/jbc.M000519200. [DOI] [PubMed] [Google Scholar]
- 44.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930, 2014. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 45.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 473: 317–325, 2011. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 46.Lopes J, Adiguzel E, Gu S, Liu SL, Hou G, Heximer S, Assoian RK, Bendeck MP. Type VIII collagen mediates vessel wall remodeling after arterial injury and fibrous cap formation in atherosclerosis. Am J Pathol 182: 2241–2253, 2013. doi: 10.1016/j.ajpath.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moulton KS, Olsen BR, Sonn S, Fukai N, Zurakowski D, Zeng X. Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation 110: 1330–1336, 2004. doi: 10.1161/01.CIR.0000140720.79015.3C. [DOI] [PubMed] [Google Scholar]
- 49.Murata K, Motayama T, Kotake C. Collagen types in various layers of the human aorta and their changes with the atherosclerotic process. Atherosclerosis 60: 251–262, 1986. doi: 10.1016/0021-9150(86)90172-3. [DOI] [PubMed] [Google Scholar]
- 50.Mutolo MJ, Morris KJ, Leir SH, Caffrey TC, Lewandowska MA, Hollingsworth MA, Harris A. Tumor suppression by collagen XV is independent of the restin domain. Matrix Biol 31: 285–289, 2012. doi: 10.1016/j.matbio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myers JC, Dion AS, Abraham V, Amenta PS. Type XV collagen exhibits a widespread distribution in human tissues but a distinct localization in basement membrane zones. Cell Tissue Res 286: 493–505, 1996. doi: 10.1007/s004410050719. [DOI] [PubMed] [Google Scholar]
- 52.Ohshima K, Mogi M, Horiuchi M. Role of peroxisome proliferator-activated receptor- γ in vascular inflammation. Int J Vasc Med 2012: 508416, 2012. doi: 10.1155/2012/508416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pelisek J, Eckstein HH, Zernecke A. Pathophysiological mechanisms of carotid plaque vulnerability: impact on ischemic stroke. Arch Immunol Ther Exp (Warsz) 60: 431–442, 2012. doi: 10.1007/s00005-012-0192-z. [DOI] [PubMed] [Google Scholar]
- 54.Plenz GA, Deng MC, Robenek H, Völker W. Vascular collagens: spotlight on the role of type VIII collagen in atherogenesis. Atherosclerosis 166: 1–11, 2003. doi: 10.1016/S0021-9150(01)00766-3. [DOI] [PubMed] [Google Scholar]
- 55.Ramchandran R, Dhanabal M, Volk R, Waterman MJF, Segal M, Lu H, Knebelmann B, Sukhatme VP. Antiangiogenic activity of restin, NC10 domain of human collagen XV: comparison to endostatin. Biochem Biophys Res Commun 255: 735–739, 1999. doi: 10.1006/bbrc.1999.0248. [DOI] [PubMed] [Google Scholar]
- 56.Rasi K, Hurskainen M, Kallio M, Stavén S, Sormunen R, Heape AM, Avila RL, Kirschner D, Muona A, Tolonen U, Tanila H, Huhtala P, Soininen R, Pihlajaniemi T. Lack of collagen XV impairs peripheral nerve maturation and, when combined with laminin-411 deficiency, leads to basement membrane abnormalities and sensorimotor dysfunction. J Neurosci 30: 14490–14501, 2010. doi: 10.1523/JNEUROSCI.2644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rasi K, Piuhola J, Czabanka M, Sormunen R, Ilves M, Leskinen H, Rysä J, Kerkelä R, Janmey P, Heljasvaara R, Peuhkurinen K, Vuolteenaho O, Ruskoaho H, Vajkoczy P, Pihlajaniemi T, Eklund L. Collagen XV is necessary for modeling of the extracellular matrix and its deficiency predisposes to cardiomyopathy. Circ Res 107: 1241–1252, 2010. doi: 10.1161/CIRCRESAHA.110.222133. [DOI] [PubMed] [Google Scholar]
- 58.Rekhter MD. Collagen synthesis in atherosclerosis: too much and not enough. Cardiovasc Res 41: 376–384, 1999. doi: 10.1016/S0008-6363(98)00321-6. [DOI] [PubMed] [Google Scholar]
- 59.Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J Clin Invest 112: 1342–1350, 2003. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci USA 100: 13531–13536, 2003. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ross R, Wight TN, Strandness E, Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 114: 79–93, 1984. [PMC free article] [PubMed] [Google Scholar]
- 62.Sasaki T, Larsson H, Tisi D, Claesson-Welsh L, Hohenester E, Timpl R. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J Mol Biol 301: 1179–1190, 2000. doi: 10.1006/jmbi.2000.3996. [DOI] [PubMed] [Google Scholar]
- 63.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AAC, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21: 628–637, 2015. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shekhonin BV, Domogatsky SP, Muzykantov VR, Idelson GL, Rukosuev VS. Distribution of type I, III, IV and V collagen in normal and atherosclerotic human arterial wall: immunomorphological characteristics. Coll Relat Res 5: 355–368, 1985. doi: 10.1016/S0174-173X(85)80024-8. [DOI] [PubMed] [Google Scholar]
- 65.Skålén K, Gustafsson M, Rydberg EK, Hultén LM, Wiklund O, Innerarity TL, Borén J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417: 750–754, 2002. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 66.Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol 24: 2227–2236, 2004. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 67.Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol 209: 13–22, 2015. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116: 1832–1844, 2007. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 68a.van der Wal AC, Becker AE. Atherosclerotic plaque rupture–pathologic basis of plaque stability and instability. Cardiovasc Res 41: 334–344, 1999. doi: 10.1016/S0008-6363(98)00276-4. [DOI] [PubMed] [Google Scholar]
- 69.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 20: 1262–1275, 2000. doi: 10.1161/01.ATV.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 70.Vooijs M, Jonkers J, Berns A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep 2: 292–297, 2001. doi: 10.1093/embo-reports/kve064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horváth B, Maser-Gluth C, Greiner E, Lemmer B, Schütz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14: 64–68, 2008. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 72.Yurdagul A Jr, Finney AC, Woolard MD, Orr AW. The arterial microenvironment: the where and why of atherosclerosis. Biochem J 473: 1281–1295, 2016. doi: 10.1042/BJ20150844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaferani A, Talsma DT, Yazdani S, Celie JW, Aikio M, Heljasvaara R, Navis GJ, Pihlajaniemi T, van den Born J. Basement membrane zone collagens XV and XVIII/proteoglycans mediate leukocyte influx in renal ischemia/reperfusion. PLoS One 9: e106732, 2014. doi: 10.1371/journal.pone.0106732. [DOI] [PMC free article] [PubMed] [Google Scholar]