Abstract
Endothelial cell (EC) activation and vascular injury are hallmark features of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Caveolin-1 (Cav-1) is highly expressed in pulmonary microvascular ECs and plays a key role in maintaining vascular homeostasis. The aim of this study was to determine if the lung inflammatory response to Escherichia coli lipopolysaccharide (LPS) promotes priming of ECs via Cav-1 depletion and if this contributes to the onset of pulmonary vascular remodeling. To test the hypothesis that depletion of Cav-1 primes ECs to respond to profibrotic signals, C57BL6 wild-type (WT) mice (Tie2.Cre−;Cav1fl/fl) were exposed to nebulized LPS (10 mg; 1 h daily for 4 days) and compared with EC-specific Cav1−/− (Tie2.Cre+;Cav1fl/fl). After 96 h of LPS exposure, total lung Cav-1 and bone morphogenetic protein receptor type II (BMPRII) expression were reduced in WT mice. Moreover, plasma albumin leakage, infiltration of immune cells, and levels of IL-6/IL-6R and transforming growth factor-β (TGF-β) were elevated in both LPS-treated WT and EC-Cav1−/− mice. Finally, EC-Cav1−/− mice exhibited a modest increase in microvascular thickness basally and even more so on exposure to LPS (96 h). EC-Cav1−/− mice and LPS-treated WT mice exhibited reduced BMPRII expression and endothelial nitric oxide synthase uncoupling, which along with increased TGF-β promoted TGFβRI-dependent SMAD-2/3 phosphorylation. Finally, human lung sections from patients with ARDS displayed reduced EC Cav-1 expression, elevated TGF-β levels, and severe pulmonary vascular remodeling. Thus EC Cav-1 depletion, oxidative stress-mediated reduction in BMPRII expression, and enhanced TGF-β-driven SMAD-2/3 signaling promote pulmonary vascular remodeling in inflamed lungs.
Keywords: ARDS, vascular inflammation, endothelial dysfunction, caveolin-1, TGF-β signaling, vascular remodeling
acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) occur by several mechanisms, including shock, sepsis, pneumonia, and acid aspiration (35). The inflammatory response during ARDS promotes severe endothelial-alveolar barrier damage that progresses to edema, immune cell infiltration, increased pulmonary pressure, and mild pulmonary hypertension (PH) (21, 42). The short-term mortality in patients with ARDS is extremely high, and in survivors, morbidity can dramatically affect quality of life and lifespan (12, 15, 18, 19, 47). However, the critical determinants of the etiology of ALI/ARDS and their role in long-term survival of patients are poorly understood.
Quiescent endothelial cells (ECs) produce low levels of nitric oxide (NO) from endothelial NO synthase (eNOS), which is critical for maintaining vessel homeostasis (2). On the other hand, inflammatory diseases can elicit phenotypic changes in ECs, leading to reduced NO and increased superoxide and peroxynitrite production, which are thought to contribute to endothelial dysfunction (13). Physiologically, EC caveolin-1 (Cav-1) plays a key role as a negative-feedback regulator of eNOS (7, 14, 17). Recently, our group demonstrated that EC Cav-1 degradation initiates a sequence of events including eNOS uncoupling and oxidative stress that promotes EC dysfunction (5).
ECs exhibit different morphological and phenotypic characteristics that contribute to the control of vascular tone, permeability, chemotaxis, cell migration, and angiogenesis (2, 8, 10). In persistent inflammatory states, loss of EC markers such as vascular endothelial (VE)-cadherin and gain of mesenchymal markers (α-SMA, vimentin, and type I collagen) occur through a process known as endothelial-to-mesenchymal transition (EndoMT) (49), which may promote progressive vascular remodeling. Pulmonary ECs from global Cav1−/− mice are characterized by an immature phenotype and expression of mesenchymal markers, an effect mediated by TGF signaling (23, 28). In addition, Cav1−/− mice exhibit morphologic lung modifications such as thickening of the alveolar wall and EC hypercellularity (23, 28, 32). Hence, there is compelling evidence indicating that absence of Cav-1 and eNOS dysfunction are mechanistically linked to vascular damage and remodeling (51).
Here, we demonstrate that inflammatory cytokines promote EC Cav-1 depletion in vivo and in vitro, and that the restoration of Cav-1 expression is critical for the resolution of lipopolysaccharide (LPS)-induced ALI. Ablation of Cav-1 expression exacerbated LPS-induced ALI in mice. Assessment of lung sections from patients with ARDS revealed pulmonary vascular remodeling, indicating reduced expression of Cav-1 may enhance the sensitivity to a “second hit” that then elicits severe pulmonary vascular remodeling. We contend that the results presented here indicate that acute inflammatory conditions promote depletion of Cav-1, eNOS-driven oxidative stress, and resultant BMPRII depletion. Furthermore, inflammation-induced elevation in the level of TGF-β then drives the activation of SMAD-2/3 signaling via TGFβRI and reprogramming of ECs to a phenotype associated with chronic pulmonary vascular disease.
MATERIALS AND METHODS
Human Lung Tissue
De-identified human lung tissue (formalin-fixed, paraffin-embedded sections) from three male and one female control donors (tissue deemed nonsuitable for transplant), age 27–62 yr, and one male and three female ARDS donors, age 43–72 yr, were acquired from the Centre for Heart Lung Innovation lung registry (addendum to M16-00115, University of British Columbia, Vancouver, Canada).
Animals and ALI Model
Endothelial cell-specific Cav-1 null (EC-Cav1−/−) mice (generated by crossing Tie2.Cre+ mice with Cav1lox/lox mice) (41), strain-matched Tie2.Cre− mice and WT C57BL6 (8–12 wk) male (Jackson Laboratory, Bar Harbor, ME) mice were used in the experiments. ALI was induced by nebulization of Escherichia coli LPS (10 mg, diluted in 10 ml and nebulized over 1 h each day for up to 4 days). All mouse studies were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
Cell Culture
Murine lung endothelial cells (MLECs) from WT and Cav1−/− mice were isolated as previously described (34, 45). Human pulmonary artery ECs (HPAECs) were purchased from Lonza at passage 3 and used until passage 7.
Collection of Lung Tissue and Bronchoalveolar Lavage Fluid
Fully anesthetized mice (100/10 mg/kg ip ketamine/xylazine) were used to obtain lungs and bronchoalveolar lavage fluid (BALF). After cannulation of the trachea (by insertion of a polyethylene tube), mice were mechanically ventilated. Heparinized blood was fully cleared from lungs by perfusion of 5 ml of cold PBS via cannula placed in the right ventricle. BALF was collected by three washes with 1 ml of cold PBS; lungs were weighed and finally snap frozen in liquid nitrogen. For MLEC isolation and culture, cleared lungs were prepared as previously described (34, 45). For fresh MLEC isolation, a single cell suspension was obtained from digested lungs and incubated with CD31 antibody coupled to prewashed magnetic beads (dynabeads M-450 epoxy; Invitrogen, Carlsbad, CA) for 20 min (rotating at room temperature). After incubation, the CD31− population was separated from CD31+ cells using a magnetic tube rack, washed twice (PBS + 0.1% BSA), and centrifuged at 350 g (5 min, 4°C). The cell pellet was resuspended in RIPA buffer (containing 1% protease and phosphatase inhibitors) and incubated for 30 min at 4°C. After sonication and centrifugation, protein concentration in the cell lysates was determined by using a DC protein assay according to manufacturer instructions (Bio-Rad).
Immunohistochemistry and Immunocytochemistry
Formalin-fixed, paraffin-embedded lung sections were used to evaluate protein expression (primary antibodies: Cav-1, PECAM-1, α-SMA, eNOS, and TGF-β) and for histological analysis (after staining with hematoxylin and eosin or Masson’s trichrome) of vessel area, thickness, and collagen deposition. For immunocytochemistry, HPAECs grown on No. 1.5 glass coverslips were fixed in 4% paraformaldehyde, labeled with primary and secondary antibodies, and mounted on slides. Slides were analyzed with an LSM 880 confocal microscope (Carl Zeiss MicroImaging).
Western Blotting
Lung tissue, MLECs (freshly isolated or cultured), and HPAEC lysates (20–30 µg) were loaded on SDS-PAGE gels, transferred to nitrocellulose membranes, and, after blocking, incubated with primary antibody. Proteins were detected by using an ECL kit (Amersham, Piscataway, NJ) and scanned with a Li-Cor Odessy CLx (Lincoln, NE). Data were normalized to β-actin or GAPDH loading controls.
Inflammatory Profile
Albumin, protein, and cytokine concentrations were measured in BALF obtained from WT and EC-Cav1−/− mice with or without LPS pretreatment. Cell counts and myeloperoxidase (MPO) activity were evaluated in BALF and lung homogenates as described (37). Cytokine levels were measured using Multiplex kits and a Luminex 100 platform (R&D Systems, Minneapolis, MN). Albumin was measured with an Albumin blue fluorescent assay kit accordingly to manufacturer’s instructions (Active Motif, Carlsbad, CA).
Excess Lung Water
Excess lung water was determined as previously described (37). Briefly, the body weight of each mouse was measured and a blood sample was collected for hematocrit determination. The hemoglobin concentration was measured in the supernatant of homogenized total lung tissue and whole blood with an Hb 201 analyzer (Hemocue, Cypress, CA). Total lung homogenate, supernatant and a whole blood sample were weighed before and after drying (60°C) for 24 h.
Antibodies and Reagents
Primary antibodies.
Rabbit polyclonal antibodies against eNOS, TGFβRI, and GAPDH were acquired from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibody against CD31 (PECAM-1), rabbit polyclonal antibodies against P-SMAD1/5/9 (8), P-SMAD2, and BMPRII were purchased from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal antibody against α-SMA was purchased from Abcam (Cambridge, MA). Rabbit polyclonal antibody against TGF-β1 was obtained from Bioss. Mouse monoclonal antibodies against eNOS, phospho-Cav-1 (PY14), rabbit polyclonal antibodies against Cav-1, and rat anti-mouse CD31 (clone MEC 13.3) were purchased from BD PharMingen (San Jose, CA).
Secondary antibodies.
Alexa-Fluor 488, Alexa-Fluor 555, and Alexa-Fluor 546-conjugated goat anti-mouse and anti-rabbit IgG were purchased from Life Technologies (Grand Island, NY). Anti-mouse and anti-rabbit horseradish peroxidase-conjugated IgG were purchased from Cell Signaling Technology (Danvers, MA) or Kirkegaard & Perry Laboratories.
Reagents.
EBM-2/EGM-2 SingleQuots and fetal bovine serum were purchased from Lonza and Gemini Bio-Products (West Sacramento, CA), respectively. Recombinant IL-6, IL-6R, and TGF-β were obtained from Peprotech (Rocky Hill, NJ). 3-Morpholinosydnonimine hydrochloride (SIN-1) and Escherichia coli LPS were purchased from SIGMA Chemical (St. Louis, MO); mounting media (with DAPI) was obtained from Vector (Burlingame, CA).
Data Analysis
Data are presented as the arithmetic means ± SE of the indicated number of observations. Unpaired Student’s t-test was performed to determine the significance of differences between two groups. Alternatively, one-way analysis of variance (ANOVA), followed by post hoc Newman-Keuls analysis was performed, considering P < 0.05 as statistically significant.
RESULTS
LPS-Induced ALI/ARDS Promotes Depletion of Lung Cav-1 Expression Associated with Increased Vascular Permeability and Immune Cell Infiltration
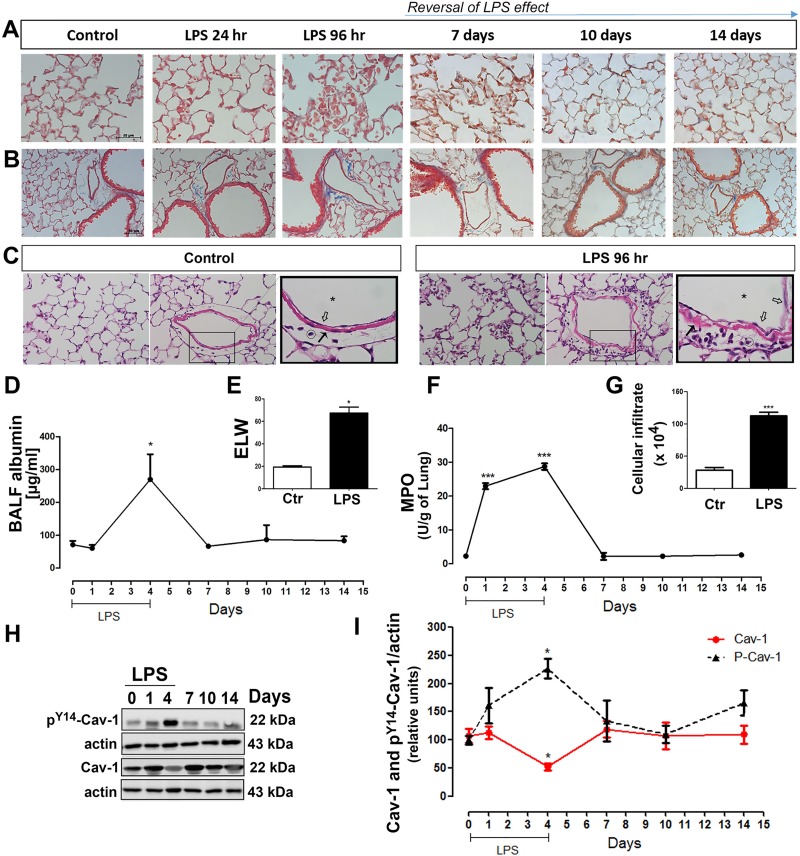
To better understand the mechanisms associated with the onset of endothelial dysfunction and pulmonary vascular pathology, we adapted the well-established LPS model of lung vascular injury by introducing intermittent daily exposures of WT mice to LPS for up to 4 days. Mice exposed to nebulized saline (vehicle control group) exhibited preserved lung structure with no cellular infiltration, whereas animals challenged with LPS exhibited an increase in perivascular collagen fibril deposition and infiltration of immune cells which returned to baseline by day 10 (Fig. 1, A–C). Normal parenchyma and vasculature morphology was observed in the WT control group, while 96 h after LPS exposure WT animals showed an increase in immune cell infiltration in the pulmonary tissue and EC swelling (Fig. 1C). Moreover, after 96 h of LPS exposure, mice showed an increase in BALF albumin accumulation (Fig. 1D) and excess lung water (Fig. 1E) indicative of alveolar-endothelial barrier damage and edema formation, both hallmarks of ALI/ARDS. Finally, MPO activity (Fig. 1F) and total BALF cell counts (Fig. 1G) were dramatically increased compared with the vehicle control, indicating daily inhalation of nebulized LPS induced EC activation and pulmonary inflammation.
Fig. 1.
Time course of lipopolysaccharide (LPS)-induced acute lung injury (ALI). Wild-type (WT) mice untreated or treated every 24 h with nebulized LPS for 1 h/day for up to 4 days were evaluated on day 1, 4, 7, 10, and 14. Masson’s trichrome staining of lung parenchyma (A) or vasculature (B) (red: cells; blue: collagen). C: hematoxylin and eosin stain of lung parenchyma (first panel) or vasculature (second panel) in vehicle or LPS-treated (96 h) WT mice. Inset: macrovasculature morphology; magnification equal to ×400. Endothelial cells (open arrows), smooth muscle layer (bold arrows), vascular lumen (*), and immune cells (○). Albumin concentration (µg/ml) of bronchoalveolar lavage fluid (BALF) (D), excess lung water (ELW) (E), myeloperoxidase (MPO) activity (F), total cells in BALF (G), phospho-Tyr14-Cav-1 (pY14-Cav-1), and total Cav-1 (I) expression. H: representative Western blot showing total Cav-1 and pY14-Cav-1 level relative to β-actin loading control (n = 3–4 animals/group). Ctl, control.
As Cav-1 is thought to regulate aspects of the lung injury response (23), we measured Cav-1 expression and its phosphorylation in total lung homogenates following exposure to LPS. As shown in Fig. 1, H and I, Cav-1 expression was reduced after 96 h of LPS exposure while Cav-1 Y14 phosphorylation was maximal at the same time point, consistent with previous findings that persistent phosphorylation of Cav-1 leads to its ubiquitinylation and degradation (5). Finally, Cav-1 expression was restored to basal levels by day 7, indicating Cav-1 depletion is reversible and dependent on the presence of an ongoing inflammatory stimulus.
ALI/ARDS Reduces Pulmonary EC Cav-1 Expression Which Is Associated with eNOS Uncoupling and EC Dysfunction
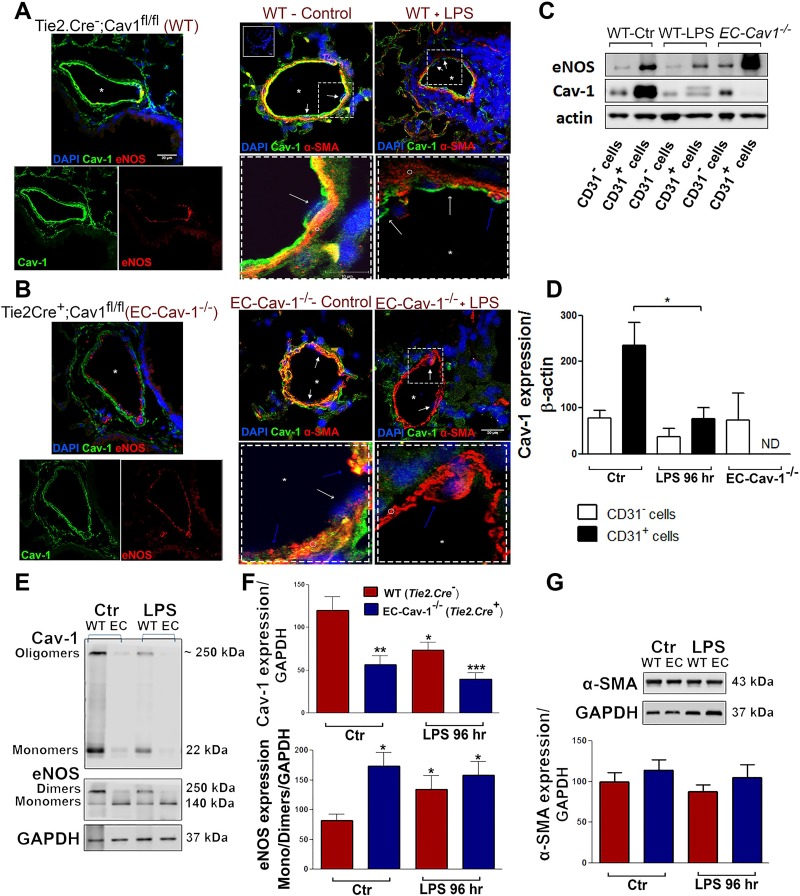
We next compared the phenotype of LPS-challenged mice with EC-specific Cav-1−/− (Tie2.Cre+;Cav1lox/lox) mice to determine the role of EC Cav-1 in the presence or absence of multiple hits of LPS on pulmonary vascular injury and remodeling. Immunohistochemical (IHC) analysis confirmed the presence of Cav-1 expression in ECs and smooth muscle cells (SMCs) of Tie.2.Cre− mice, whereas Cav-1 expression was absent in eNOS+ ECs but present in SMCs of EC-Cav1−/− (Fig. 2, A and B). CD31+ and eNOS+ cells from WT mice showed high levels of Cav-1 expression compared with other pulmonary cells (Fig 2, A and C). However, ALI reduced Cav-1 expression in CD31+ cells when compared with cells obtained from untreated WT mice; the extent of EC Cav-1 depletion in response to LPS was similar to that observed in the CD31+ cells obtained from EC-Cav1−/− mice (Fig. 2, B–D). Although Cav-1 expression was not observed in some α-SMA+ cells and slightly reduced in the CD31− cells after LPS exposure, statistically significant differences were not revealed between these groups. Moreover, EC Cav-1 depletion in WT and EC-Cav1−/− mouse lungs was associated with reduced eNOS dimerization, suggesting the inflammatory response promotes eNOS uncoupling, a key marker of EC dysfunction (Fig. 2, E and F).
Fig. 2.
LPS induced Cav-1 depletion and endothelial nitric oxide synthase (eNOS) uncoupling in pulmonary vasculature. Lung sections from WT (Tie2.Cre−;Cav1fl/fl mice) (A) and endothelial cell (EC) Cav-1−/− (Tie2.Cre+;Cav1fl/fl mice) (B) exposed to saline (control) or LPS for 96 h (LPS). The panels at left show Cav-1 (green) or eNOS (red) expression in the control groups (top = merged channels; bottom = split channels). The panels at right show Cav-1 (green) and α-SMA (red) expression in vehicle and LPS-treated WT (inset = negative control) and EC Cav-1−/− mice (top = 20 µm; dashed squares = 10 µm). Symbols indicate endothelial cells (white arrows), α-smooth muscle actin+ cells (blue arrows or ○), and vascular lumen (*). Nuclei were stained with DAPI. C: mouse lungs from vehicle-treated WT (control), 96-h LPS-treated WT mice, and EC Cav-1−/− were digested to obtain freshly isolated CD31 positive and negative cells (CD31+ or CD31−). EC lysates were used for Western blot analysis of Cav-1 and eNOS expression. D: densitometry of Cav-1 and eNOS expression (n = 3 animals/group). E: Western blots showing Cav-1 and eNOS expression in total lung lysates. F: densitometry of Cav-1 and eNOS expression in lung lysates. G: Western blot showing α-SMA expression in total lung lysates. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. WT control (CD31+ cells or total lungs) by one-way ANOVA followed by post hoc Newman-Keuls test (n = 4 animals/group).
Although IHC analysis of pulmonary sections revealed the presence of α-SMA+ cells in the vessel lumen (Fig. 2, A and B, dashed boxes, blue arrows) corroborating previous data showing selective EC Cav-1 deletion may promote expression of mesenchymal lineage-linked proteins (28), there was no difference in α-SMA expression in total lung lysates (Fig. 2G).
EC Cav-1−/− Mice Showed Elevated Levels of Proinflammatory and Regulatory Cytokines and Protein Accumulation in BALF After LPS Exposure
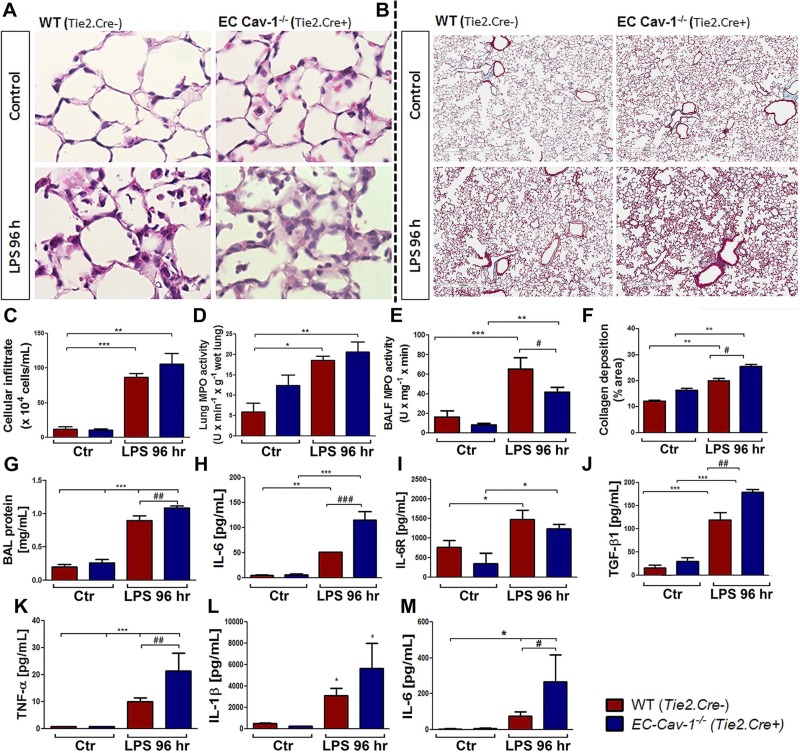
To evaluate vascular pathology induced by inflammatory mediators in the absence of EC Cav-1, we performed IHC to analyze the inflammatory profile. Untreated EC-Cav1−/− mice exhibited alveolar wall thickening, interstitial edema, intima swelling, and mild pulmonary remodeling compared with Cre− littermate controls (Fig. 3, A and B). Moreover, total BALF cell number (Fig. 3C) and the pathological profile of lung tissue was further increased after LPS challenge, as characterized by significant inflammatory injury and increase in MPO activity in both WT and EC-Cav1−/− lung tissue (Fig. 3D) and BALF (Fig. 3E). Finally, quantification of pulmonary collagen deposition revealed LPS-induced mild fibrosis and vascular damage (Fig. 3F).
Fig. 3.
Inflammatory profile analysis over time course of ALI. Lung histological evaluation of hematoxylin and eosin (A) and Masson’s trichrome (B) stainings of murine lung sections from vehicle- or LPS-treated (96 h) WT (Tie2.Cre−) and EC Cav-1−/− (Tie2.Cre+) mice. Magnification: ×400. Scale bar: 400 µm. C: BALF total cell counts. D: lung MPO activity (U·min−1·g−1 wet lung) in tissue and BALF (E). F: quantification of collagen content of lung sections revealed by Masson’s trichrome stain. Images were quantified by using ImageJ software (% area/field; 5 fields by slide). Protein concentration (G), IL-6 (H), IL-6R (I), TGF-β1 (J), and TNF-α (K) were measured in cell-free BALF or lungs (L: IL-1β; M: IL-6) after 96 h of LPS challenge in WT and EC Cav-1−/−; *P < 0.01, **P < 0.001, and ***P < 0.0001 indicate difference between vehicle and LPS-treated WT or EC Cav-1−/−; #P < 0.01, ##P < 0.001, and ###P < 0.0001 indicate difference between both LPS-treated WT and EC Cav-1−/− miceby one-way ANOVA followed by post hoc Newman-Keuls test (n = 3 animals/group).
In response to an infection, activated immune cells release inflammatory mediators to promote pathogen killing and restoration of tissue homeostasis. Thus, to assess the inflammatory profile and lung permeability, we measured protein and inflammatory cytokine levels in BALF from WT and EC-Cav1−/−, untreated and treated with LPS. LPS-induced ALI increased the protein concentration in BALF, indicative of increased endothelial-epithelial barrier permeability (Fig. 3G). Moreover, LPS exposure increased the levels of TNF-α, IL-1β, IL-6, IL-6R, and TGF-β levels in BALF and lungs from WT and EC-Cav1−/− (Fig. 3, H–M). However, in EC-Cav1−/− mice, we observed an even greater accumulation of proinflammatory cytokines IL-6, TNF-α, and IL-1β, and of the regulatory cytokine TGF-β1, when compared with WT mice (Fig. 3, H–M). Although no differences were observed in the number of cells in BALF or neutrophils present in lung homogenates from WT and EC-Cav1−/− mice following exposure to LPS, we did observe a reduction in the number of neutrophils in BALF and an increase in the number of perivascular macrophages in EC-Cav1−/− mice after LPS (data not shown), suggesting EC Cav-1 depletion may play a role in regulating immune cell recruitment and transmigration in ALI/ARDS.
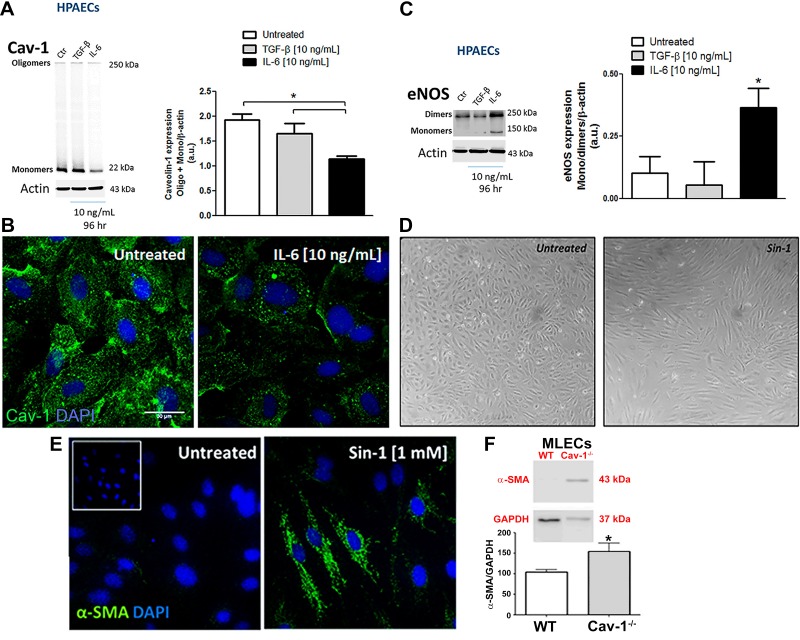
IL-6 Promotes Cav-1 Degradation, eNOS Uncoupling, Spindle-Shape Morphology, and α-SMA Expression in Human Pulmonary Artery ECs
IL-6/IL-6R and TGF-β are thought to induce tissue remodeling, EC dysfunction, dedifferentiation, and PH (20, 43), and both were increased after 96 h of LPS exposure. Thus we hypothesized that Cav-1 depletion and EC activation may be due in part to these inflammatory mediators. As shown in Fig. 4, A and B, treatment of HPAECs in vitro with IL-6/IL-6R, but not TGF-β, for 96 h led to depletion of Cav-1 protein. Moreover, IL-6/IL-6R-induced Cav-1 depletion was associated with eNOS monomerization (Fig. 4C), suggesting IL-6 may promote EC dysfunction through a mechanism dependent of Cav-1 depletion and eNOS uncoupling. In addition, we observed that treatment of HPAECs with peroxynitrite donor SIN-1, thus mimicking uncoupled eNOS, promoted the transition of EC monolayers from that with a cobblestone appearance to ECs with a spindle-shaped morphology that expressed α-SMA (Fig. 4, D and E) similar to that observed in MLECs isolated from Cav-1−/− mice (Fig. 4F). Thus loss of EC Cav-1 expression is associated with eNOS uncoupling, loss of typical cobblestone morphology, and EC dysfunction, which may be an initiating mechanism of EndoMT and vascular remodeling.
Fig. 4.
IL-6-induced Cav-1 depletion and eNOS uncoupling associated with endothelial cell reprogramming. Cav-1 expression by Western blotting (A) or immunocytochemistry (green; B) and eNOS dimer/monomer expression (C) were measured in human pulmonary artery endothelial cells (HPAECs) after treatment with TGF-β and IL-6/IL-6R for 96 h. D: phase contrast micrograph showing HPAEC morphology after SIN-1 (peroxinitrite donor) treatment for 96 h. E: α-SMA expression in murine lung endothelial cells (MLECs) from WT and Cav-1−/−. *P < 0.01 by one-way ANOVA followed by post hoc Newman-Keuls test (n = 4 cultures; passage 4–7).
EC Cav-1 Depletion and Uncoupled eNOS in Response to Lung Inflammation Promotes the Switch from BMPRII to TGFβRI Signaling
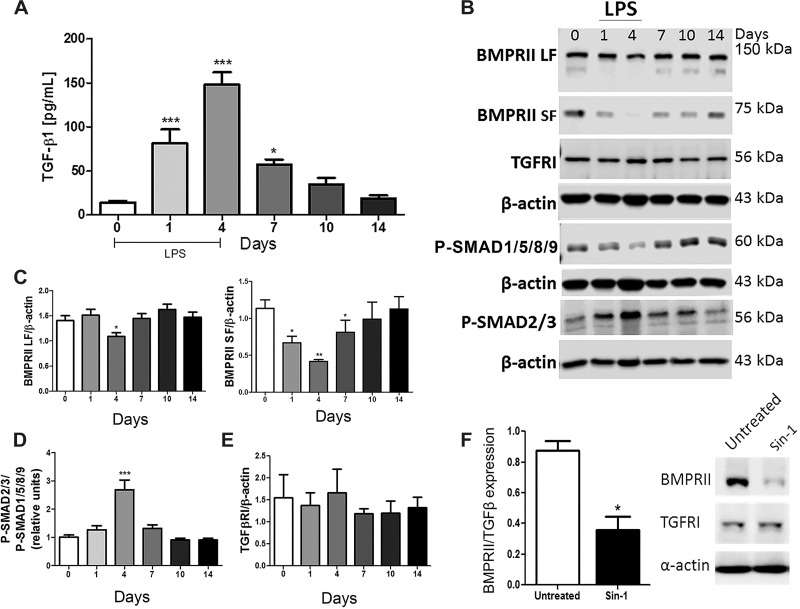
MLECs isolated from Cav-1−/− mice exhibit a mesenchymal phenotype associated with TGF-β signaling (28). Although TGF-β did not alter Cav-1 expression level when applied to HPAECs in vitro, we hypothesized that EC Cav-1 depletion induced by proinflammatory mediators such as IL-6 in association with TGF-β signaling (as a second hit) may be an initiating mechanism of inflammation-induced pulmonary vascular remodeling. To test this hypothesis, we evaluated TGF-β signaling pathways during the course of LPS-induced ALI. As shown in Fig. 5A, daily intermittent LPS exposure increased the level of TGF-β in WT mouse BALF which peaked after 96 h and then gradually, on termination of LPS, returned to basal level by 14 days. Similar to the time course observed for the reduction in Cav-1 expression, at 96 h of LPS exposure, the long form (LF) and short form (SF) of BMPRII as well as phospho-SMAD1/5/8 downstream of BMPRII activation were reduced, whereas activation of the TGFβRI pathway, based on elevated level of phospho-SMAD2/3, was enhanced despite no change in TGFβRI expression (Fig. 5, B–E). In vitro, treatment of HPAECs with peroxinitrite donor SIN-1 reduced BMPRII expression without altering TGFβRI expression (Fig. 5F). Thus EC Cav-1 depletion and eNOS uncoupling may in part promote the molecular switch from BMPRII to TGFβRI signaling and thereby promote the transition of ECs to a mesenchymal phenotype associated with pulmonary vascular remodeling.
Fig. 5.
Bone morphogenetic protein receptor type II (BMPRII) vs. transforming growth factor-β (TGF-β1) receptor I (TGFR1) signaling during ALI. A: TGF-β1 was measured in cell-free BALF after 1 or 4 days of LPS challenge in WT mice. B: representative Western blot showing BMPRII, TGFRI, P-SMAD1/5/8/9, P-SMAD2/3, and β-actin expression levels in lung lysates from WT mice exposed to saline or LPS (up to 4 days). C, D, and E: densitometry analysis of BMPRII short (SF) and long form (LF), P-SMADs, and TGFRI expression. F: BMPRII and TGFRI expression in HPAECs treated with IL-6/IL-6R or 3-morpholinosydnonimine (SIN-1) (96 h) compared with control. *P < 0.01, **P < 0.001, and ***P < 0.0001 by one-way ANOVA followed by post hoc Newman-Keuls test (n = 3–4 mice per experimental group).
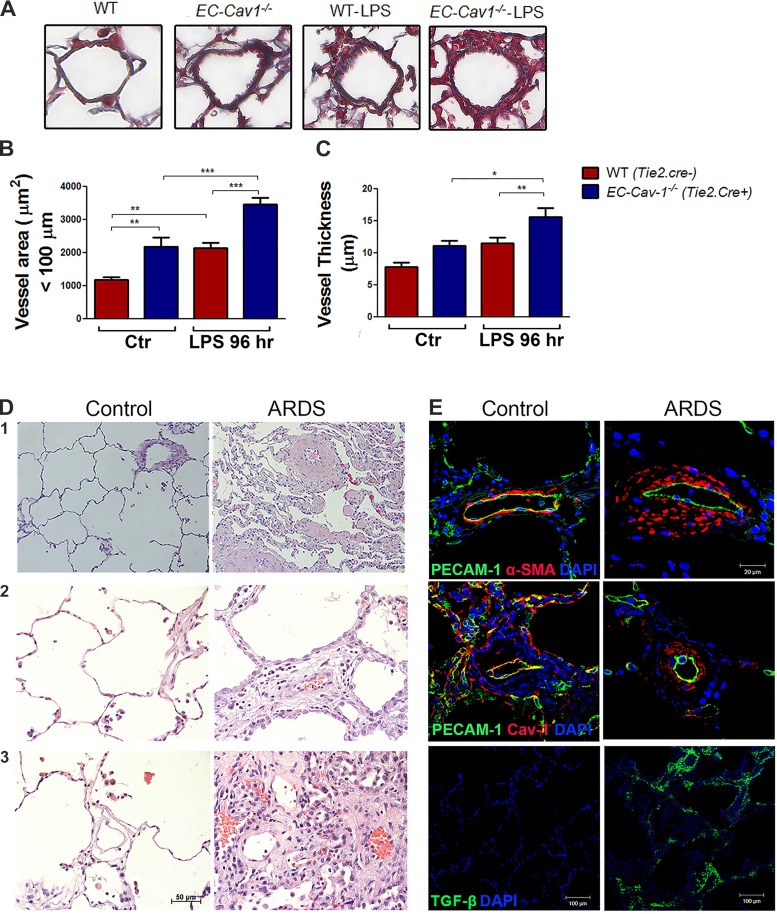
EC Cav-1 Depletion During ALI/ARDS Is Associated with Pulmonary Vascular Remodeling in Mice and Humans
In ALI/ARDS, EC activation contributes to vascular injury. We next tested the hypothesis that LPS-induced depletion of Cav-1 is associated with the initiation of pulmonary vascular remodeling. We evaluated the microvasculature in Masson’s trichrome-stained lungs obtained from mice and observed increased vessel area and thickness in untreated EC-Cav-1−/− mice and LPS-treated WT and EC-Cav-1−/− mice indicative of pulmonary vascular remodeling (Fig. 6, A–C). Although the degree of vascular remodeling was modest in mice, IHC analysis of human lung sections from ARDS patients showed dramatically remodeled pulmonary vessels, severe inflammation, and alveolar thickening as compared with “healthy” donor lungs (Fig. 6D). Moreover, remodeled vessels in ARDS lungs, identified by increased wall thickness composed of α-SMA+ cells, was associated with reduced expression of Cav-1 in ECs compared with control lung sections (Fig. 6E). Thus a severe inflammatory response in human lung is also associated with EC Cav-1 depletion and vascular remodeling. Finally, human lungs from ARDS patients showed increased TGF-β staining when compared with control lungs (Fig. 6E), suggesting EC dysfunction mediated by inflammatory signals may play a role in initiating pulmonary vascular remodeling.
Fig. 6.
Acute respiratory distress syndrome (ARDS) is associated with reduced Cav-1 expression and pulmonary vascular remodeling. Mouse lung sections from WT (Tie2.Cre−;Cav1fl/fl mice) and EC Cav-1−/− (Tie2.Cre+;Cav1fl/fl mice) were stained with Masson’s trichrome (A), and the microvasculature area (B; µm2) and thickness (C; µm2) were quantified in scanned micrographs. Human lung sections from control (left panels) and ARDS donors (right panels) were stained with hematoxylin and eosin (D) and anti-PECAM-1 (green), anti-α-SMA (red), anti-PECAM-1 (green), and Cav-1 (red) or anti-TGF-β antibody (green) (E). Nuclei were stained with DAPI (blue). Human lung stainings and histology are representative sections from n = 3 control and n = 3 ARDS lungs. *P < 0.01, **P < 0.001, and ***P < 0.0001 by one-way ANOVA followed by post hoc Newman-Keuls test (n = 3 mice per experimental group).
DISCUSSION
Severe bacterial infection is recognized as a primary cause of ARDS. The initial phase of ALI/ARDS is characterized by pulmonary endothelial and epithelial barrier dysfunction, leukocyte infiltration, production of inflammatory mediators, and edema (35). Persistent inflammatory stimuli promotes disease progression into a proliferative/fibrotic phase (46). The late phase of ARDS is associated with growth factor signaling, increased cellular proliferation, production of anti-inflammatory cytokines, tissue remodeling, and increased pulmonary vascular resistance (27). The present work indicates LPS-induced ALI in mice and patients with ARDS exhibit vascular wall thickening associated with EC Cav-1 depletion, suggesting loss of Cav-1 may play a role in inflammation-induced pulmonary vascular remodeling.
We and others have shown that Cav-1 is highly expressed in pulmonary ECs (30), which is thought to regulate inflammatory cell recruitment and vascular hyperpermeability during ALI (23). In this study, we show that the lung inflammatory response to intermittent LPS exposure promotes a transient depletion of Cav-1 in the mouse lung, and that restoration of its expression correlates with recovery. Specific knockdown of EC Cav-1 promoted abnormal lung histology, and intermittent airway exposure of EC-Cav-1−/− mice to LPS reproduced several of the characteristics of human disease, indicating Cav-1 depletion may contribute to the short and long-term morbidity of ARDS.
Whereas the initial inflammatory response in ARDS involves increased proinflammatory mediators, during the proliferative/fibrotic stage, anti-inflammatory and regulatory cytokines accumulate in the lung from macrophages, which are thought to be required for both the initiating and repairative phases of ARDS (1, 6). In Pseudomonas aeruginosa-induced ALI, macrophage influx into the alveolar space peaks at day 4, coinciding with an increase in M2 macrophage markers and initial resolution of ALI (24). Similarly, we observed increased numbers of perivascular macrophages after 4 days of LPS exposure in EC-Cav1−/− mice as compared with WT control mice, suggesting that dysfunctional ECs marked by Cav-1 depletion may be linked functionally to specific immune cell recruitment (26). In addition, EC-Cav1−/− mice also exhibited elevated levels of IL-6 and TGF-β when compared with WT mice, implicating Cav-1 expression as an important regulator of EC immunobiology and the inflammatory response.
IL-6/IL-6R secreted by immune cells and ECs promote the activation and recruitment of neutrophils and then monocytes to the site of infection (25). Moreover, it was described that overexpression of IL-6 increases TGF-β signaling by reducing TGFβRI association with Cav-1 (50), thereby promoting vascular remodeling and PH in mice (43). Thus absence of Cav-1 may play a critical role during the inflammatory response by promoting TGF-β-mediated vascular remodeling. On the other hand, global Cav-1−/− mice are protected from acute ventilator-induced lung injury and exhibit reduced permeability and neutrophil migration (31), thus indicating the lung inflammatory response may be differentially regulated by Cav-1 expression in ECs and other cell types.
Reduced or absent expression of EC Cav-1 was associated with eNOS uncoupling and presence of α-SMA+ “endothelial” cells, indicating Cav-1 depletion during ARDS may be the initiating mechanism of EndoMT and pulmonary vascular remodeling. Peroxynitrite generation from uncoupled eNOS contributes to EC dysfunction during the inflammatory response (11). Previously, our group showed that MLECs isolated from Cav1−/− mice basally produce more peroxynitrite, as compared with WT ECs, which can be abolished in Cav1/eNOS double knockout MLECs (33). These data indicate that in the absence of Cav-1, dysfunctional eNOS is a source of peroxynitrite. In addition, nitration of critical tyrosine residues contribute to chronic lung diseases such as chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis (22, 40) via enhanced cytokine and TGF-β production by fibroblasts (44). Accordingly, previous data showed that after 6 days of proinflammatory cytokine treatment, TGF-β was able to induce EndoMT in HPAECs (16) and that in the absence of Cav-1, EndoMT occurs in MLECs via TGF-β-driven signaling (28). Taken together, these data support the observation that proinflammatory signals in vivo, including TGF-β signaling in EC-Cav-1−/− mice and LPS-induced Cav-1-depleted mice, may be sufficient to promote EndoMT and pulmonary vascular remodeling.
Increased vascular remodeling was observed in a mouse model of ALI and in the late phase of ARDS in humans. To the best of our knowledge, the link between Cav-1 depletion as a trigger of EC dysfunction and vascular remodeling in severe ARDS has not been shown previously. Thus these data indicate that absence of pulmonary EC Cav-1 associated with inflammatory cytokine signaling may be an initiating mechanism of pulmonary vascular remodeling.
In addition to Cav-1 depletion, reduced signaling and expression of BMPRII is considered to be a key factor in the development of familial and primary PH, a vascular pathology associated with significant vascular remodeling (4, 20, 29). BMPRII is localized in lipid rafts/caveolae in ECs (38) and vascular SMCs (48), and evidence in the literature indicates Cav-1 is required for BMPRII dimerization and signaling (36). Different molecular weights have been reported for BMPRII. The differences in the size of BMPRII protein observed by SDS-PAGE may be due both to different mRNA products found in several organs (39) as well as posttranscriptional mechanisms, which include secretory and endocytic trafficking, localization in membrane microdomains, degradation, and cleavage (3, 38). In addition, an imbalance between the two alternatively spliced isoforms (known as LF and SF) has been implicated in the development of PH (9). Interestingly, we observed BMPRII in the mouse lung at 150- and 75-kDa molecular weight, corresponding to the LF and SF of the receptor, and both were reduced after 4 days of LPS exposure, although more dramatically for the SF. Consistently, the 75-kDa isoform has been identified in caveolar and dense lipid fractions derived from rat pulmonary vascular endothelium (38), suggesting the SF may interact with Cav-1 in caveolae.
Antagonism between BMPRII and TGF-β signaling indicates reduced BMPRII signaling may increase TGFβRI-mediated SMAD-2/3 signaling and thereby promote pulmonary vascular remodeling. In LPS-treated mice and EC-Cav-1−/− mice, the initiating mechanism of pulmonary vascular remodeling may also be associated with EC dedifferentiation via EndoMT and expansion of apoptotic-resistant EC clones (29). In absence of Cav-1 and BMPRII signaling, not only ECs but also pulmonary artery SMC proliferation is known to contribute to the increase in vessel thickness, vascular occlusion, and pulmonary vascular resistance.
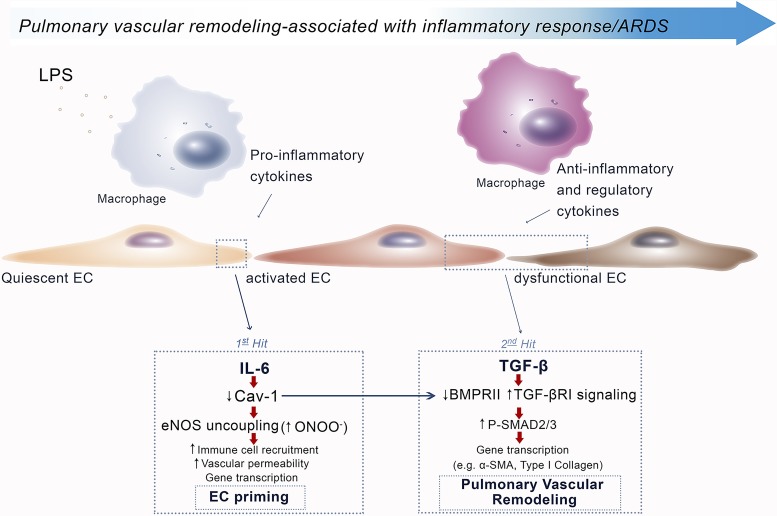
The model of multiple-hit LPS exposure over 4 days increased microvessel area in WT mice, similar to that observed in EC-Cav1−/− mice, suggesting EC Cav-1 is a negative regulator of pulmonary vascular remodeling. Taking this into consideration, we hypothesized that vascular remodeling in severe inflammatory disease may occur in a two-step manner (Fig. 7). First, EC injury caused by proinflammatory mediators leads to Cav-1 depletion and eNOS uncoupling that results in priming of ECs into a proinflammatory phenotype. The mechanism of “priming” may be linked to peroxynitrite-mediated BMPRII depletion. Subsequently, under persistent stimulation via elevated TGF-β levels, ECs then transition to a mesenchymal-like phenotype (EndoMT) associated with collagen deposition and progressive vascular remodeling. Taken together, our study indicates that lung inflammatory mediators in ALI/ARDS may promote EC dysfunction by reducing EC Cav-1 expression and thereby promote eNOS uncoupling and peroxynitrite-mediated depletion of BMPRII. Inflammatory cytokine-mediated EC Cav-1 depletion may therefore play a significant role in the onset of inflammatory pulmonary vascular remodeling.
Fig. 7.
Schematic indicating sequence of signaling events associated with Cav-1 depletion, eNOS uncoupling, and vascular injury/remodeling. During LPS-induced ALI, EC injury induced by proinflammatory mediators, such as IL-6, leads to Cav-1 depletion and eNOS uncoupling, resulting in priming of ECs to a proinflammatory phenotype. Uncoupled eNOS induces junctional destabilization, increase in vascular permeability, and inflammatory cell migration. Under persistent stimulation, regulatory cytokines such as TGF-β activate primed ECs to promote EndoMT, proliferation, and pulmonary vascular remodeling.
GRANTS
This work was supported in part by National Institutes of Health Grants P01-HL-60678, R01-HL-71626, and R01-HL-125356 (to R. Minshall and M. Bonini) and R01-HL-127342, R01-HL-111656, 1R01-HL-133951 (to R. Machado), and R01-EY-019494 (to M. Elliott); American Heart Association SDG 09SDG2250933 and DOD 67263-RT-REP (to M. Bonini and R. Minshall); and a Postdoctoral Fellowship from CNPq/Science without Borders-Brazil (to S. Oliveira).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.D.S.O. and R.D.M. conceived and designed research; S.D.S.O., M.C., and J.C. performed experiments; S.D.S.O., J.C., M.G.B., and R.F.M. analyzed data; S.D.S.O., R.F.M., and R.D.M. interpreted results of experiments; S.D.S.O. prepared figures; S.D.S.O. drafted manuscript; S.D.S.O., J.C., M.G.B., X.G., M.H.E., R.F.M., and R.D.M. edited and revised manuscript; R.F.M. and R.D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the James Hogg Research Centre Lung Registry at the University of British Columbia, Vancouver, BC, for providing human lung tissue sections for this study; Dr. Timothy Thompson, University of Texas at Houston, for providing Cav1lox/lox mice; and the Research Histology and Tissue Imaging Core in the UIC Research Resources Center for technical assistance. This work was presented in part at the American Thoracic Society annual meeting in San Francisco (2016).
REFERENCES
- 1.Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol 306: L709–L725, 2014. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 3.Amsalem AR, Marom B, Shapira KE, Hirschhorn T, Preisler L, Paarmann P, Knaus P, Henis YI, Ehrlich M. Differential regulation of translation and endocytosis of alternatively spliced forms of the type II bone morphogenetic protein (BMP) receptor. Mol Biol Cell 27: 716–730, 2016. doi: 10.1091/mbc.E15-08-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105: 1672–1678, 2002. doi: 10.1161/01.CIR.0000012754.72951.3D. [DOI] [PubMed] [Google Scholar]
- 5.Bakhshi FR, Mao M, Shajahan AN, Piegeler T, Chen Z, Chernaya O, Sharma T, Elliott WM, Szulcek R, Bogaard HJ, Comhair S, Erzurum S, van Nieuw Amerongen GP, Bonini MG, Minshall RD. Nitrosation-dependent caveolin 1 phosphorylation, ubiquitination, and degradation and its association with idiopathic pulmonary arterial hypertension. Pulm Circ 3: 816–830, 2013. doi: 10.1086/674753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol 75: 593–615, 2013. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 7.Bonini MG, Dull RO, Minshall RD. Systems Biology of Free Radicals and Antioxidants, Laher I, editor. Berlin: Springer, 2014, p. 1343–1363. doi: 10.1007/978-3-642-30018-9_183. [DOI] [Google Scholar]
- 8.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood 84: 2068–2101, 1994. [PubMed] [Google Scholar]
- 9.Cogan J, Austin E, Hedges L, Womack B, West J, Loyd J, Hamid R. Role of BMPR2 alternative splicing in heritable pulmonary arterial hypertension penetrance. Circulation 126: 1907–1916, 2012. doi: 10.1161/CIRCULATIONAHA.112.106245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol 178: 6017–6022, 2007. doi: 10.4049/jimmunol.178.10.6017. [DOI] [PubMed] [Google Scholar]
- 11.Eligini S, Habib A, Lebret M, Créminon C, Lévy-Toledano S, Maclouf J. Induction of cyclo-oxygenase-2 in human endothelial cells by SIN-1 in the absence of prostaglandin production. Br J Pharmacol 133: 1163–1171, 2001. doi: 10.1038/sj.bjp.0704163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott CG, Rasmusson BY, Crapo RO, Morris AH, Jensen RL. Prediction of pulmonary function abnormalities after adult respiratory distress syndrome (ARDS). Am Rev Respir Dis 135: 634–638, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol 15: 1983–1992, 2004. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Cardeña G, Comander J, Anderson KR, Blackman BR, Gimbrone MA Jr. Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA 98: 4478–4485, 2001. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghio AJ, Elliott CG, Crapo RO, Berlin SL, Jensen RL. Impairment after adult respiratory distress syndrome. An evaluation based on American Thoracic Society recommendations. Am Rev Respir Dis 139: 1158–1162, 1989. doi: 10.1164/ajrccm/139.5.1158. [DOI] [PubMed] [Google Scholar]
- 16.Good RB, Gilbane AJ, Trinder SL, Denton CP, Coghlan G, Abraham DJ, Holmes AM. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am J Pathol 185: 1850–1858, 2015. doi: 10.1016/j.ajpath.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Gratton JP, Fontana J, O’Connor DS, García-Cardeña G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem 275: 22268–22272, 2000. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 18.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS; Canadian Critical Care Trials Group . One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348: 683–693, 2003. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 19.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM; Canadian Critical Care Trials Group . Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 364: 1293–1304, 2011. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 20.Hong K-H, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, Oh SP. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 118: 722–730, 2008. doi: 10.1161/CIRCULATIONAHA.107.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui-li G. The management of acute pulmonary arterial hypertension. Cardiovasc Ther 29: 153–175, 2011. doi: 10.1111/j.1755-5922.2009.00095.x. [DOI] [PubMed] [Google Scholar]
- 22.Ichinose M, Sugiura H, Yamagata S, Koarai A, Shirato K. Increase in reactive nitrogen species production in chronic obstructive pulmonary disease airways. Am J Respir Crit Care Med 162: 701–706, 2000. doi: 10.1164/ajrccm.162.2.9908132. [DOI] [PubMed] [Google Scholar]
- 23.Jin Y, Lee S-J, Minshall RD, Choi AMK. Caveolin-1: a critical regulator of lung injury. Am J Physiol Lung Cell Mol Physiol 300: L151–L160, 2011. doi: 10.1152/ajplung.00170.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol 47: 417–426, 2012. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol 24: 25–29, 2003. doi: 10.1016/S1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 26.Karmpaliotis D, Kosmidou I, Ingenito EP, Hong K, Malhotra A, Sunday ME, Haley KJ. Angiogenic growth factors in the pathophysiology of a murine model of acute lung injury. Am J Physiol Lung Cell Mol Physiol 283: L585–L595, 2002. doi: 10.1152/ajplung.00048.2002. [DOI] [PubMed] [Google Scholar]
- 27.Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells 6: 552–570, 2014. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Wermuth PJ, Benn BS, Lisanti MP, Jimenez SA. Caveolin-1 deficiency induces spontaneous endothelial-to-mesenchymal transition in murine pulmonary endothelial cells in vitro. Am J Pathol 182: 325–331, 2013. doi: 10.1016/j.ajpath.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, Moore SD, Drake KM, Aldred MA, Yu PB, Upton PD, Morrell NW. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 21: 777–785, 2015. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maniatis NA, Chernaya O, Shinin V, Minshall RD. Caveolins and lung function. Adv Exp Med Biol 729: 157–179, 2012. doi: 10.1007/978-1-4614-1222-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maniatis NA, Kardara M, Hecimovich D, Letsiou E, Castellon M, Roussos C, Shinin V, Votta-Vellis EG, Schwartz DE, Minshall RD. Role of caveolin-1 expression in the pathogenesis of pulmonary edema in ventilator-induced lung injury. Pulm Circ 2: 452–460, 2012. doi: 10.4103/2045-8932.105033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maniatis NA, Shinin V, Schraufnagel DE, Okada S, Vogel SM, Malik AB, Minshall RD. Increased pulmonary vascular resistance and defective pulmonary artery filling in caveolin-1−/− mice. Am J Physiol Lung Cell Mol Physiol 294: L865–L873, 2008. doi: 10.1152/ajplung.00079.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao M, Varadarajan S, Fukai T, Bakhshi FR, Chernaya O, Dudley SC Jr, Minshall RD, Bonini MG. Nitroglycerin tolerance in caveolin-1 deficient mice. PLoS One 9: e104101, 2014. doi: 10.1371/journal.pone.0104101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marelli-Berg FM, Peek E, Lidington EA, Stauss HJ, Lechler RI. Isolation of endothelial cells from murine tissue. J Immunol Methods 244: 205–215, 2000. doi: 10.1016/S0022-1759(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 35.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 33: 319–327, 2005. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nohe A, Keating E, Underhill TM, Knaus P, Petersen NO. Dynamics and interaction of caveolin-1 isoforms with BMP-receptors. J Cell Sci 118: 643–650, 2005. doi: 10.1242/jcs.01402. [DOI] [PubMed] [Google Scholar]
- 37.Piegeler T, Dull RO, Hu G, Castellon M, Chignalia AZ, Koshy RG, Votta-Velis EG, Borgeat A, Schwartz DE, Beck-Schimmer B, Minshall RD. Ropivacaine attenuates endotoxin plus hyperinflation-mediated acute lung injury via inhibition of early-onset Src-dependent signaling. BMC Anesthesiol 14: 57, 2014. doi: 10.1186/1471-2253-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramos M, Lamé MW, Segall HJ, Wilson DW. The BMP type II receptor is located in lipid rafts, including caveolae, of pulmonary endothelium in vivo and in vitro. Vascul Pharmacol 44: 50–59, 2006. doi: 10.1016/j.vph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA 92: 7632–7636, 1995. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saleh D, Barnes PJ, Giaid A. Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 155: 1763–1769, 1997. doi: 10.1164/ajrccm.155.5.9154889. [DOI] [PubMed] [Google Scholar]
- 41.Sethna S, Chamakkala T, Gu X, Thompson TC, Cao G, Elliott MH, Finnemann SC. Regulation of phagolysosomal digestion by caveolin-1 of the retinal pigment epithelium is essential for vision. J Biol Chem 291: 6494–6506, 2016. doi: 10.1074/jbc.M115.687004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Souza R, Amato MBP, Demarzo SE, Deheinzelin D, Barbas CSV, Schettino GPP, Carvalho CRR. Pulmonary capillary pressure in pulmonary hypertension. Crit Care 9: R132–R138, 2005. doi: 10.1186/cc3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 104: 236–244, 2009. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugiura H, Liu X, Kobayashi T, Togo S, Ertl RF, Kawasaki S, Kamio K, Wang XQ, Mao L, Shen L, Hogaboam CM, Rennard SI. Reactive nitrogen species augment fibroblast-mediated collagen gel contraction, mediator production, and chemotaxis. Am J Respir Cell Mol Biol 34: 592–599, 2006. doi: 10.1165/rcmb.2005-0339OC. [DOI] [PubMed] [Google Scholar]
- 45.Sverdlov M, Shinin V, Place AT, Castellon M, Minshall RD. Filamin A regulates caveolae internalization and trafficking in endothelial cells. Mol Biol Cell 20: 4531–4540, 2009. doi: 10.1091/mbc.E08-10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walkey AJ, Summer R, Ho V, Alkana P. Acute respiratory distress syndrome: epidemiology and management approaches. Clin Epidemiol 4: 159–169, 2012. doi: 10.2147/CLEP.S28800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang CY, Calfee CS, Paul DW, Janz DR, May AK, Zhuo H, Bernard GR, Matthay MA, Ware LB, Kangelaris KN. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med 40: 388–396, 2014. doi: 10.1007/s00134-013-3186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wertz JW, Bauer PM. Caveolin-1 regulates BMPRII localization and signaling in vascular smooth muscle cells. Biochem Biophys Res Commun 375: 557–561, 2008. doi: 10.1016/j.bbrc.2008.08.066. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimatsu Y, Watabe T. Roles of TGF-β signals in endothelial-mesenchymal transition during cardiac fibrosis. Int J Inflamm 2011: 724080, 2011. doi: 10.4061/2011/724080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang XL, Topley N, Ito T, Phillips A. Interleukin-6 regulation of transforming growth factor (TGF)-β receptor compartmentalization and turnover enhances TGF-β1 signaling. J Biol Chem 280: 12239–12245, 2005. doi: 10.1074/jbc.M413284200. [DOI] [PubMed] [Google Scholar]
- 51.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HHSK, Vogel SM, Brovkovych V, Yuan JXJ, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest 119: 2009–2018, 2009. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]