Abstract
We reported defective efferocytosis associated with cigarette smoking and/or airway inflammation in chronic lung diseases, including chronic obstructive pulmonary disease, severe asthma, and childhood bronchiectasis. We also showed defects in phagocytosis of nontypeable Haemophilus influenzae (NTHi), a common colonizer of the lower airway in these diseases. These defects could be substantially overcome with low-dose azithromycin; however, chronic use may induce bacterial resistance. The aim of the present study was therefore to investigate two novel macrolides—2′-desoxy-9-(S)-erythromycylamine (GS-459755) and azithromycin-based 2′-desoxy molecule (GS-560660)—with significantly diminished antibiotic activity against Staphylococcus aureus, Streptococcus pneumonia, Moraxella catarrhalis, and H. influenzae. We tested their effects on efferocytosis, phagocytosis of NTHi, cell viability, receptors involved in recognition of apoptotic cells and/or NTHi (flow cytometry), secreted and cleaved intracellular IL-1β (cytometric bead array, immunofluorescence/confocal microscopy), and nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) using primary alveolar macrophages and THP-1 macrophages ± 10% cigarette smoke extract. Dose-response experiments showed optimal prophagocytic effects of GS-459755 and GS-560660 at concentrations of 0.5–1 µg/ml compared with our findings with azithromycin. Both macrolides significantly improved phagocytosis of apoptotic cells and NTHi (e.g., increases in efferocytosis and phagocytosis of NTHi: GS-459755, 23 and 22.5%, P = 0.043; GS-560660, 23.5 and 22%, P = 0.043, respectively). Macrophage viability remained >85% following 24 h exposure to either macrolide at concentrations up to 20 µg/ml. Secreted and intracellular-cleaved IL-1β was decreased with both macrolides with no significant changes in recognition molecules c-mer proto-oncogene tyrosine kinase; scavenger receptor class A, member 1; Toll-like receptor 2/4; or CD36. Particulate cytoplasmic immunofluorescence of NLRP3 inflammasome was also reduced significantly. We conclude that GS-459755 and GS-560660 may be useful for reducing airway inflammation in chronic lung diseases without inducing bacterial resistance.
Keywords: nonantibiotic macrolides, azithromycin, chronic lung disease, chronic obstructive pulmonary disease, nontypeable H. influenzae
we have reported increased numbers of apoptotic bronchial epithelial cells and impaired clearance of these cells (efferocytosis) by alveolar macrophages in chronic lung diseases, including chronic obstructive pulmonary disease (COPD), severe asthma, childhood bronchiectasis, and chronic lung transplant rejection (6, 11, 13, 21).We showed that the uncleared material may undergo secondary necrosis with proinflammatory effects (9). These defects are thought to have various effects in the lung, potentially contributing to the development of COPD in smokers and perpetuating inflammation, infection, and tissue damage in established COPD and other chronic inflammatory lung diseases (26). We also showed defects in phagocytosis of nontypeable Haemophilus influenzae (NTHi), a common colonizer of the lower airway in these diseases (27). The improvement of the phagocytic ability of alveolar macrophages for bronchial epithelial cells may thus have clinical significance with regard to new or adjunct treatments for diseases, where there is inflammation associated with ineffective repair of the injured epithelium and loss of structural integrity.
There have been many reports of the anti-inflammatory properties of macrolide antibiotics. These agents are rapidly taken up by alveolar macrophages with high, prolonged levels in tissues (20). We have demonstrated both in vitro and in a human phase II study that in addition to its direct anti-inflammatory properties, the phagocytic defect in macrophages (for both apoptotic cells and bacteria) could be substantially overcome by administration of low doses of azithromycin (8, 10, 12). Azithromycin treatment significantly improved efferocytosis and decreased both proinflammatory cytokine production in response to LPS and airway inflammation (8). However, the use of long-term antibiotic therapy, even at a low dose, has obvious drawbacks, including the emergence of antibiotic-resistant strains, and its excess use might exacerbate the emergence of bacterial resistance, not just to azithromycin but also to many other existing antibiotics, with potentially serious public health consequences. To overcome this potential selection of resistant bacteria, two novel macrolides—2′-desoxy-9-(S)-erythromycylamine (GS-459755) and azithromycin-based 2′-desoxy molecule (GS-560660)—with negligible antibiotic activity were developed (23, 25). When assessed for minimum inhibitory concentrations by the broth microdilution method, recommended by the Clinical and Laboratory Standards Institute (2009), the compounds were reported to have greatly diminished efficacy against Staphylococcus aureus [e.g., minimum inhibitory concentration reached by 50% (MIC50) GS-560660: 256 μg/ml vs. azithromycin: 1 μg/ml], Streptococcus pneumonia (e.g., MIC50 GS-560660: 128 μg/ml vs. azithromycin: 0.12 μg/ml), Moraxella catarrhalis (e.g., MIC50 GS-560660: 64 μg/ml vs. azithromycin: 0.06 μg/ml), and H. influenzae (e.g., MIC50 GS-560660: 128 μg/ml vs. azithromycin: 2 μg/ml) (25).
This study further investigates the effects of these novel macrolides on efferocytosis, phagocytosis of NTHi, cell viability, receptors involved in recognition of apoptotic cells and/or NTHi, inflammation [nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome and released and cleaved IL-1β] using primary alveolar macrophages, and differentiated THP-1 macrophages exposed to cigarette smoke extract. Use of macrolides without antibiotic activity will allow better exploitation of other properties without compromising the effectiveness of first-line therapies.
METHODS
Synthesis and preparation of macrolide antibiotics and cigarette smoke extract.
Azithromycin (Max Pharma, Surrey Hills, VIC, Australia) was prepared as per the manufacturer’s instructions. Briefly, powder was dissolved at 100 mg/ml in sterile Milli-Q water and further diluted to 20 mg/ml in 0.9% sterile saline before being stored at −80°C. Macrolides GS-459755 and GS-560660 were synthesized by Gilead Sciences (Seattle, WA), as reported (23, 25). The MIC for the strain of NTHi used in this paper was performed as per the Clinical and Laboratory Standards Institute’s Guidelines for Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard (2009) using the recommendations for H. influenza. Briefly, NTHi was double subcultured on chocolate agar plates and a colony suspension produced in saline to 0.5 McFarland from a fresh-plate inoculum. The inhibition assay was inoculated at 5 × 105 colony-forming units/ml in the presence of azithromycin, GS-560660, or GS-459755 at 4.8 × 10−4 − 1 µg/ml for 16 h at 37°C in ambient air and growth assessed as any reading at an optical density of a sample measured at a wavelength of 600 nm above media-only control. The assay was performed in duplicate on three separate occasions. The MIC for the strain of NTHi used in this study was azithromycin ≥ 0.488 μg/ml, GS-560660 ≥ 1,000 μg/ml, and GS-459755 ≥ 500 μg/ml. GS-459755 and GS-560660 were suspended in DMSO at 20 mg/ml and stored at −80°C. All macrolide stocks were diluted in Roswell Park Memorial Institute (RPMI) 1640, supplemented with 10% FCS and 1% wt/vol penicillin/gentamicin (culture medium) and used at concentrations of 0.1, 0.5, 1.0, 10.0, and 20.0 µg/ml. Control cells were treated with culture medium. The effects of the macrolides, alone or in combination with cigarette smoke extract, were assessed after 24 h exposure. Cigarette smoke extract was prepared from 1R5F research cigarettes (University of Kentucky, Lexington, KY), as previously described (7, 8, 24) (10%; approximately equivalent to smoking 2 packs/day) (22).
Preparation of primary alveolar macrophages.
Flexible bronchoscopy with bronchoalveolar lavage (BAL) was performed on five non-COPD, never-smoker control subjects with normal spirometry, as previously described (10, 11). Cells from BAL fluid were centrifuged and resuspended in RPMI 1640, supplemented with 10% FCS and 1% wt/vol penicillin/gentamicin at a concentration of 4 × 105 macrophages/ml. Macrophages were purified by adhesion to plastic for 1 h, as previously described (10, 11). Written, informed consent was obtained and ethics approval granted by the Royal Adelaide Hospital Human Research Ethics Committee.
Culture and preparation of the THP-1 macrophage cell line.
As insufficient primary macrophages are isolated from BAL to enable extensive mechanistic investigations, a monocytic cell line, THP-1 (American Type Culture Collection, Manassas, VA), growing in continuous culture, was differentiated into macrophages following stimulation with 100 nM PMA (Sigma, St. Louis, MO) for 72 h, as previously reported (8). THP-1 cells were differentiated 3 days before use and maintained, as per American Type Culture Collection guidelines in RPMI-1640 media, supplemented with 2 mM l-glutamine, 10% FCS, and 1% wt/vol penicillin/gentamicin (all Thermo Fisher Scientific, Waltham, MA) and 0.05 mM β-mercaptoethanol (Sigma). Experiments were carried out within 25 passages.
Preparation of phagocytic targets.
NTHi, originally isolated from the sputum of an adult with pneumonia [confirmed by the Phadebact Hemophilus Test (Bactus, Huddinge, Sweden) and by PCR] and used in previous studies (13, 19, 27), was prepared as previously reported (27). Briefly, NTHi were permeabilized with 70% ethanol, washed and stained with Sytox Green (5 μM; Thermo Fisher Scientific), and adjusted to 4 × 107 colony-forming U/ml in RPMI + 10% FCS, ready for addition at a 100:1 ratio with macrophages for phagocytosis.
16HBE4o- normal bronchial epithelial cells were prepared as previously described (11, 27). Briefly, the cells were detached from culture flasks with trypsin, media were refreshed, and apoptosis induced by exposure to UV, as reported (11). Apoptotic cells were labeled with Sytox Orange (250 nM; Thermo Fisher Scientific), washed, and resuspended at 2 × 106 cells/ml (5:1 ratio of apoptotic cells:macrophage).
Investigation of macrophage phagocytic function.
A dual-target phagocytosis assay using both apoptotic epithelial cells and NTHi targets in a single well was performed as published (27). Briefly, patient macrophages or PMA-differentiated THP-1 macrophages were plated at 2 × 105 cells/well in 24-well tissue-culture plates and 500 µl Sytox Orange-labeled apoptotic 16HBEo- cells and 500 µl Sytox Green-labeled NTHi added for 90 min at 37°C, 5% CO2. The percentage of macrophages that had ingested NTHi or 16HBEo- cells was evaluated using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA), as published (27).
Flow cytometric analysis of receptors involved in recognition of apoptotic cells and/or NTHi.
Expression of c-mer proto-oncogene tyrosine kinase [MerTk; allophycocyanin (APC); R&D Systems, Minneapolis, MN], scavenger receptor class A, member 1 (SRA-1; CD204; APC; R&D Systems), Toll-like receptors 2 (TLR2; phycoerythrin) and 4 (TLR4; APC; both eBioscience, San Diego, CA), and CD36 (BD Biosciences) was assessed in THP-1 macrophages treated with 0.5 µg/ml azithromycin, GS-459755, or GS-560660, in combination with 10% cigarette smoke extract using flow cytometry, as previously described (7, 10, 24).
Assessment of macrophage viability.
THP-1 macrophages treated with varying concentrations (0.1–20 µg/ml) of azithromycin, GS-459755, or GS-560660 were stained with Annexin V-APC (BD Biosciences) to assess apoptosis and 1 µM Sytox Green nucleic acid stain (Thermo Fisher Scientific) in HEPES buffer containing 10 mmol HEPES NaOH (pH 7.4), 150 mmol NaCl, 5 mmol KCl, 1 mmol MgCl, 1.8 mmol CaCl, to assess cell death for 15 min at room temperature, as per product guidelines. Cells were washed with HEPES buffer and assessed using flow cytometry, as previously described (24).
CBA measurement of IL-1β.
IL-1β release in culture supernatant from azithromycin-, GS-459755-, or GS-560660-treated THP-1 macrophages was measured using a cytometric bead array (CBA) kit from BD Biosciences, as previously reported (5).
Immunofluorescence analysis of cleaved IL-1β and NLRP3.
Immunofluorescence and confocal microscopy were performed as published (24) using THP-1 macrophages treated with 0.5 µg/ml azithromycin, GS-459755, or GS-560660 in combination with 10% cigarette smoke extract as above. Primary antibodies were from Santa Cruz Biotechnology (Dallas, TX), including goat anti-cleaved IL-1β (h117, 10 µg/ml) and rabbit anti-NLRP3 (H-66, 5 µg/ml). Secondary antibodies were donkey IgG F(ab′)2 fragments obtained from Jackson ImmunoResearch (West Grove, PA), conjugated with Alexa Fluor 488 (anti-goat) or Alexa Fluor 594 (anti-rabbit). Specificity of the cleaved IL-1β antibody (h117; Santa Cruz Biotechnology) was confirmed using a competitive antigen (blocking peptide). NLRP3 antibody (H66; Santa Cruz Biotechnology) was used and validated in our previous study (24a). Images were taken using a laser confocal microscope (LSM 700; Carl Zeiss Australia, North Ryde, NSW, Australia). Quantitative analysis of immunofluorescence was carried out using ImageJ software (National Institutes of Health, Bethesda, MD). Briefly, the total number of cells in each image (8–20 per image under the ×40 objective) was counted in the 4′,6-diamidino-2-phenylindole channel using the “particle analysis” function of the software. In the AF594 (NLRP3) and AF488 (cleaved IL-1β) channels, uniform settings of fluorescence intensity lower threshold and particle size were then applied to all images, allowing the software to count the number of NLRP3 or cleaved IL-1β-positive cells (cells containing at least 1 fluorescence punctum of NLRP3 or cleaved IL-1β, respectively) in each of them.
Statistical analysis.
Data were analyzed using SPSS software (IBM, Armonk, NY). Results are reported as median (quartiles 1,3), unless otherwise indicated. Analysis was performed using the nonparametric Friedman test, with Wilcoxon signed-rank tests for post hoc pairwise comparisons. P < 0.05 was considered statistically significant.
RESULTS
Dose response experiments of macrophage phagocytic function in THP-1 macrophages.
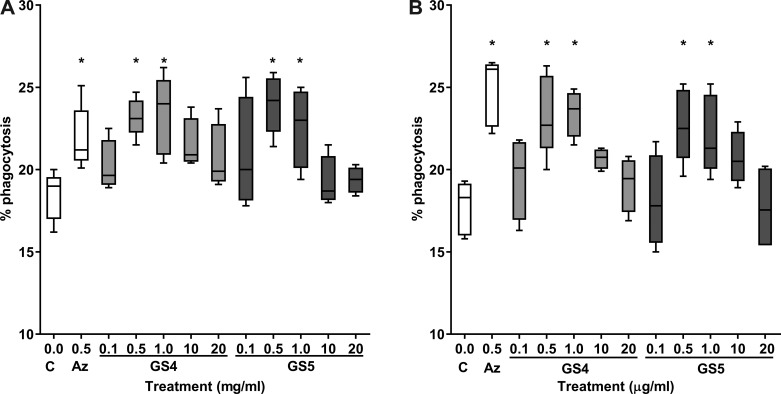
The optimal concentrations of GS-459755 and GS-560660 required to induce the maximum improvement in macrophage phagocytic function were shown to be 0.5–1 µg/ml for both agents, comparable with azithromycin (Fig. 1). These low-dose concentrations were therefore applied in subsequent experiments.
Fig. 1.
Dose-response experiments showing effects of various concentrations of GS-459755 (GS4) and GS-560660 (GS5) on phagocytic capacity of THP-1 macrophages. Phagocytosis of nontypeable Haemophilus influenzae (A) and efferocytosis of apoptotic bronchial epithelial cells (B). GS-459755 and GS-560660 were used at concentrations of 0.5–20 µg/ml. Azithromycin (Az; 0.5 µg/ml) was used as a positive control; n = 3 experiments from separate donors performed in triplicate. C, control. *P < 0.05 vs. control unless otherwise indicated. Box plots present the median and 25th and 75th percentiles (solid box), with the 10th and 90th percentiles shown by whiskers outside of the box. Data analyzed using Friedman and Wilcoxon signed-rank tests.
Investigation of macrophage phagocytic function in primary alveolar macrophages.
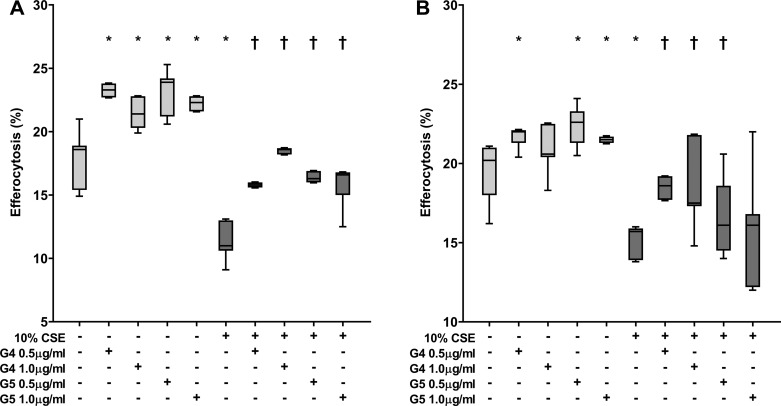
GS-459755 and GS-560660 (0.5–1 µg/ml) improved phagocytosis of apoptotic cells and NTHi by alveolar macrophages, with or without the presence of 10% cigarette smoke extract (e.g., increases in efferocytosis of apoptotic cells and phagocytosis of NTHi with 0.5 µg/ml macrolides and no cigarette smoke extract: GS-459755, P = 0.043 and P = 0.043; GS-560660, P = 0.043 and P = 0.042, respectively; Fig. 2).
Fig. 2.
Effect of macrolides ± cigarette smoke extract (CSE) on the phagocytic capacity of primary human alveolar macrophages. Phagocytosis of nontypeable Haemophilus influenzae (A) and efferocytosis of apoptotic bronchial epithelial cells (B). GS-459755 (G4) and GS-560660 (G5) were used at concentrations of 0.5 and 1 µg/ml; n = 5 experiments performed in triplicate. *P < 0.05 vs. control; †P < 0.05 vs. CSE, unless otherwise indicated. Data presented as box plots, as described in Fig. 1, and analyzed using Friedman and Wilcoxon signed-rank tests.
Macrophage phagocytic function.
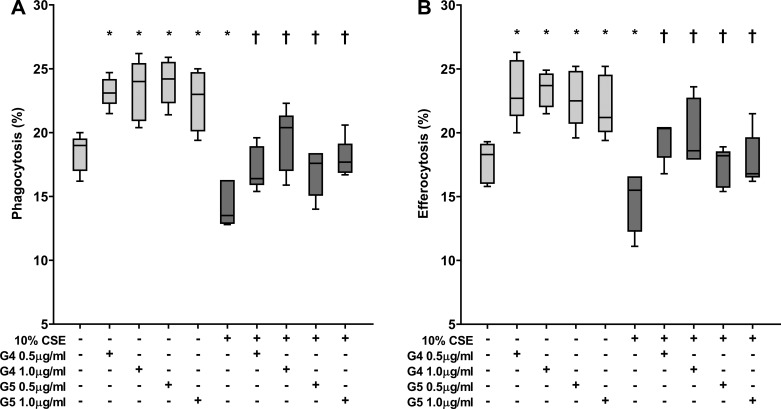
To confirm that GS-459755 and GS-560660 improved the phagocytic capacity of THP-1 macrophages to a similar extent as for alveolar macrophages, we investigated the effects of macrolides ± 10% cigarette smoke extract on phagocytic capacity of THP-1 macrophages. A significant increase in phagocytosis of apoptotic cells and NTHi was observed for both macrolides, consistent with findings with primary alveolar macrophages, e.g., increases in efferocytosis and phagocytosis of NTHi at concentrations of 0.5 µg/ml: GS-459755, 23 and 22.5%, P = 0.043; GS-560660, 23.5 and 22%, P = 0.043, respectively (Fig. 3). All subsequent experiments were therefore performed using THP-1 macrophages.
Fig. 3.
Effect of macrolides ± cigarette smoke extract (CSE) on phagocytic capacity of THP-1 macrophages. Phagocytosis of nontypeable Haemophilus influenzae (A) and efferocytosis of apoptotic bronchial epithelial cells (B). GS-459755 (G4) and GS-560660 (G5) were used at concentrations of 0.5 and 1 µg/ml; n = 5 experiments performed in triplicate. *P < 0.05 vs. control; †P < 0.05 vs. CSE unless otherwise indicated. Data presented as box plots, as described in Fig. 1, and analyzed using Friedman and Wilcoxon signed-rank tests.
Flow cytometric analysis of receptors involved in recognition of apoptotic cells and/or NTHi.
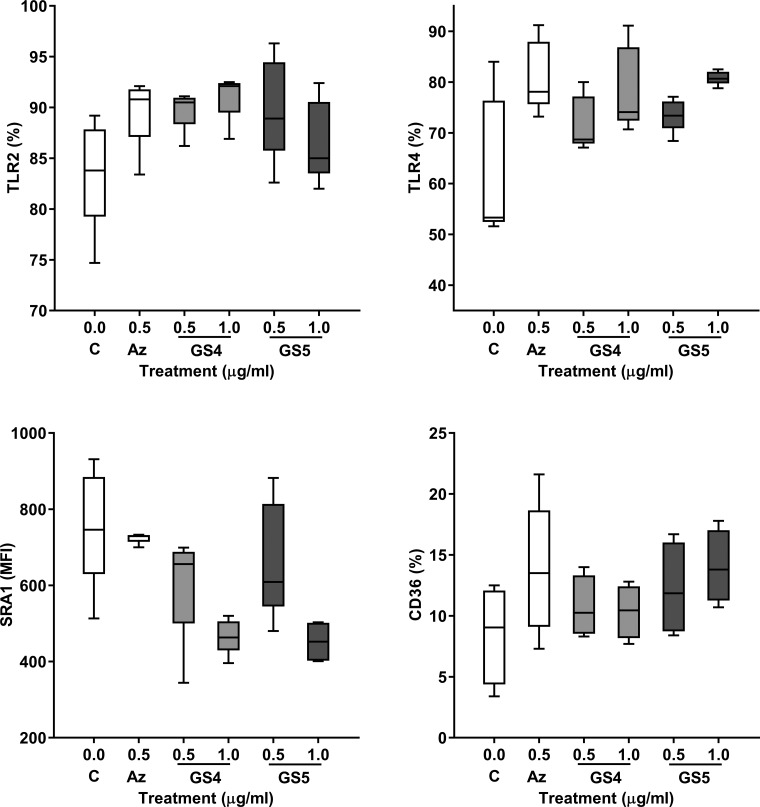
There were no significant changes in expression of phagocytic recognition molecules MerTk, TLR2/4, SRA-1, or CD36 on THP-1 macrophages following treatment with 10% cigarette smoke extract or azithromycin, GS-459755, or GS-560660, with or without 10% cigarette smoke extract (Fig. 4; MerTk and cigarette smoke extract experiments not shown).
Fig. 4.
Effect of macrolides on THP-1 expression of receptors involved in recognition of apoptotic cells and/or nontypeable Haemophilus influenzae. Azithromycin (Az), GS-459755 (GS4), and GS-560660 (GS5) were used at concentrations of 0.5 µg/ml; n = 5 experiments performed in triplicate. No significant changes were observed. Data presented as box plots, as described in Fig. 1, and analyzed using Friedman and Wilcoxon signed-rank tests. MFI, mean fluorescence intensity.
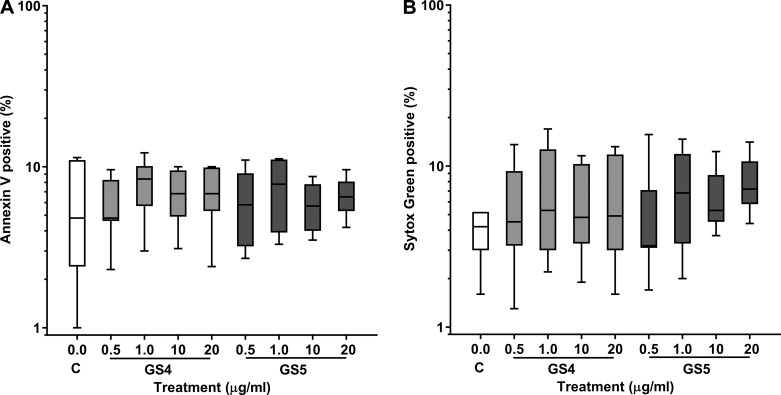
Assessment of macrophage viability.
As we have previously shown with alveolar macrophages (7, 8), there were no significant changes in the percentage of THP-1 macrophages staining with the apoptotic marker Annexin V or the necrotic marker Sytox Green following treatment with azithromycin or cigarette smoke extract. Macrophage viability also remained >85% following exposure to GS-459755 or GS-560660 at concentrations of 0.5–20 µg/ml (Fig. 5).
Fig. 5.
Effect of macrolides on THP-1 macrophage viability. Cells were treated with GS-459755 (GS4) and GS-560660 (GS5) and then stained with the apoptotic marker Annexin V (A) or the cell death marker Sytox Green (B) and analyzed using flow cytometry; n = 5 experiments performed in triplicate. Data presented as box plots, as described in Fig. 1, and analyzed using Friedman and Wilcoxon signed-rank tests.
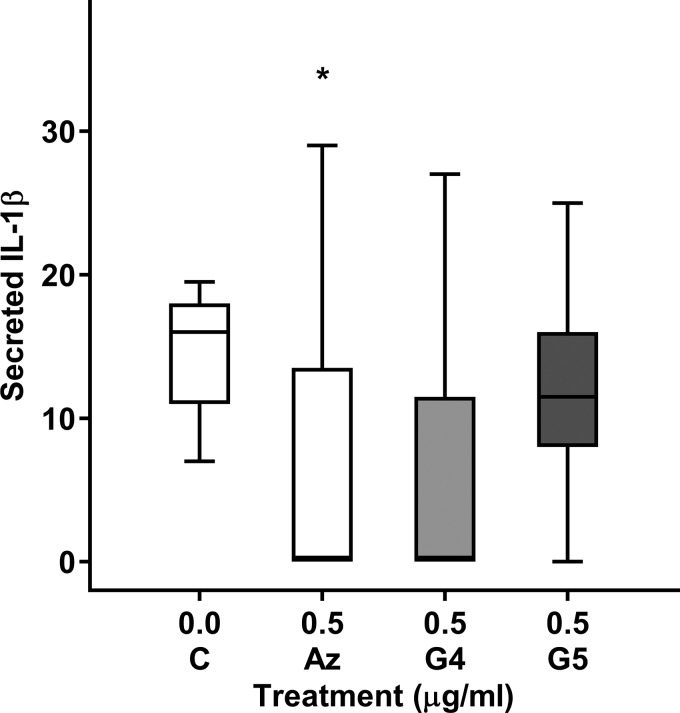
CBA measurement of IL-1β secretion by THP-1 macrophages.
Azithromycin (0.5 µg/ml) significantly decreased IL-1β secretion by unstimulated THP-1 macrophages (P = 0.043; Fig. 6). There was a nonsignificant trend for an increase in IL-1β secretion after treatment with 0.5 µg/ml GS-459755 or GS-560660 (P = 0.066 and P = 0.144, respectively; Fig. 6). The considerable variation in expression of IL-1β using this technique necessitated our further investigations using immunofluorescence.
Fig. 6.
Effect of macrolides on IL-1β secretion by THP-1 macrophages. Azithromycin (Az), GS-459755 (G4), and GS-560660 (G5) were used at a concentration of 0.5 µg/ml and IL-1β secretion measured using a cytometric bead array assay; n = 5 experiments performed in triplicate. *P < 0.05 vs. control. Data presented as box plots, as described in Fig. 1, and analyzed using Friedman and Wilcoxon signed-rank tests.
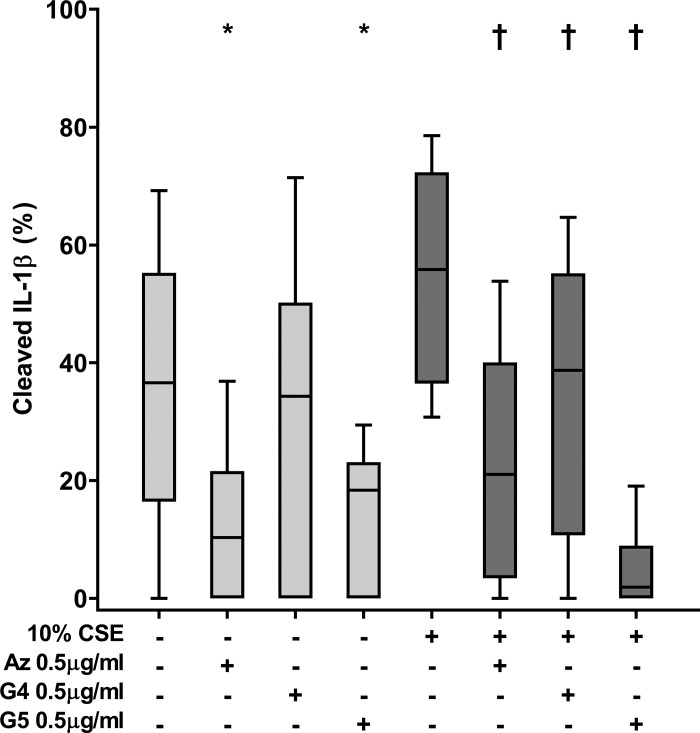
Immunofluorescence analysis of cleaved IL-1β.
Particulate staining of “spontaneously” cleaved IL-1β was detected in a subpopulation of PMA-differentiated THP-1 macrophages and increased after exposure of macrophages to cigarette smoke extract (Fig. 7). All macrolides, used at a concentration of 0.5 µg/ml, reduced immunofluorescence staining of cleaved intracellular IL-1β, both with or without cigarette smoke exposure (Fig. 7). Without cigarette smoke, the reduction was significant for azithromycin and GS-560660 (P = 0.021 and P = 0.038, respectively; GS-459755, nonsignificant), whereas all agents had a significant effect in the presence of cigarette smoke extract (P = 0.009, P = 0.017, and P = 0.005 for azithromycin, GS-459755, or GS-560660, respectively; Fig. 7).
Fig. 7.
Effect of macrolides ± cigarette smoke extract (CSE) on cleaved IL-1β in THP-1 macrophages. Azithromycin (Az), GS-459755 (G4), and GS-560660 (G5) were used at a concentration of 0.5 µg/ml and IL-1β measured using immunofluorescence/confocal microscopy. Serial images (n = 10 per well) were captured from 5 experiments. *P < 0.05 vs. control; †P < 0.05 vs. CSE unless otherwise indicated. Data analyzed using Friedman and Wilcoxon signed-rank tests.
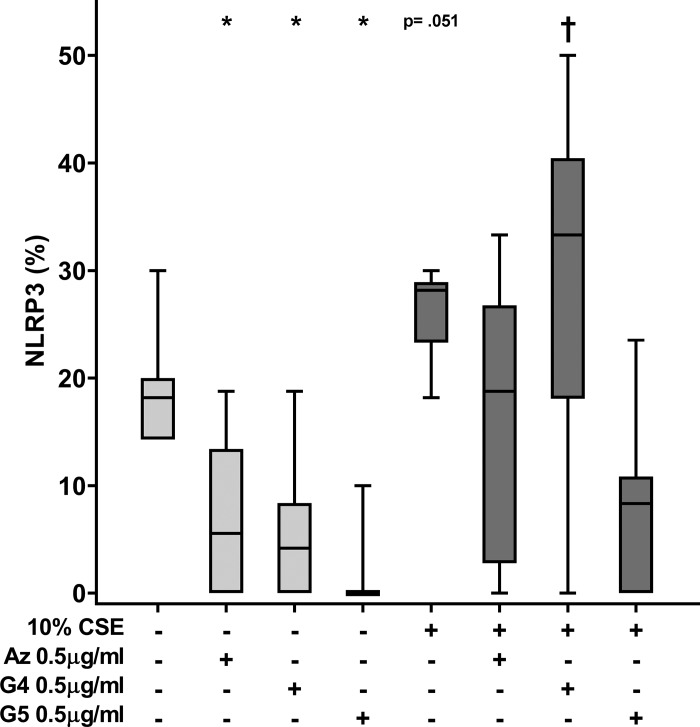
Immunofluorescence analysis of NLRP3.
Similarly to cleaved IL-1β, staining of NLRP3 revealed particulate immunofluorescence in both control and cigarette smoke-exposed cells. The number of particulate NLRP3-positive cells increased significantly in the cigarette smoke-exposed cells (Fig. 8). All macrolides, used at a concentration of 0.5 µg/ml, reduced immunofluorescence staining of NLRP3, both with or without cigarette smoke exposure. Without cigarette smoke, the reduction was significant for azithromycin and GS-560660 (P = 0.028 and P = 0.008, respectively), with a nonsignificant reduction with GS-459755 (P = 0.086). In the presence of cigarette smoke extract, GS-459755 significantly (P = 0.025) reduced NLRP3 expression, whereas for azithromycin, there was a nonsignificant trend for a decrease in NLRP3 (P = 0.051; Fig. 8).
Fig. 8.
Effect of macrolides ± cigarette smoke extract (CSE) on NLRP3 in THP-1 macrophages. Azithromycin (Az), GS-459755 (G4), and GS-560660 (G5) were used at a concentration of 0.5 µg/ml and NLRP3 measured using immunofluorescence/confocal microscopy. Serial images (n = 10 per well) were captured from 5 experiments. *P < 0.05 vs. control (no CSE); †P < 0.05 vs. CSE unless otherwise indicated. Data analyzed using Friedman and Wilcoxon signed-rank tests.
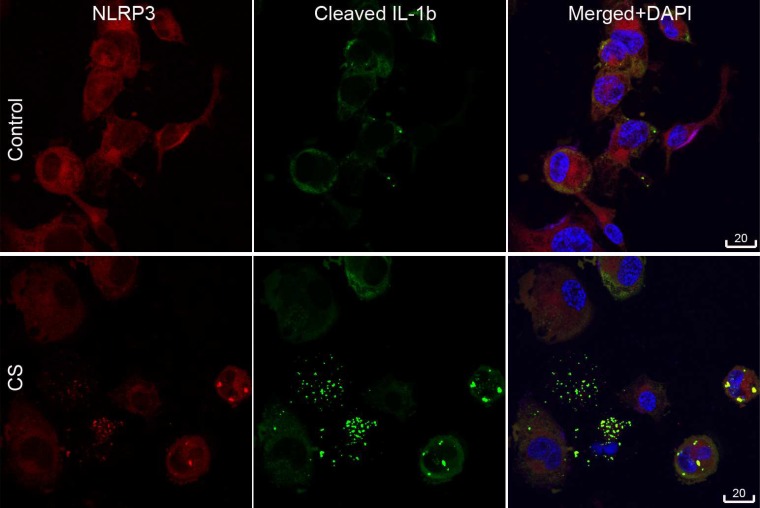
Colocalization of NLRP3 and IL-1β.
Immunofluorescence double-staining showed colocalization of particulate NLRP3 and IL-1β in the cytoplasm of PMA-induced THP-1 macrophages, both in control macrophages and those exposed to cigarette smoke extract for 24 h (Fig. 9).
Fig. 9.
Colocalization of NLRP3 and cleaved IL-1β (IL-1b) in cigarette smoke (CS) extract-exposed THP-1 macrophages. Immunofluorescence staining showing colocalization (yellow) of NLRP3 (red) and cleaved IL-1β (green) in cigarette smoke extract-exposed THP-1 macrophages. DAPI, 4′,6-diamidino-2-phenylindole.
DISCUSSION
Chronic inflammatory lung diseases cause considerable morbidity and health care costs. We reported defective efferocytosis associated with airway inflammation in several of these diseases, including COPD, severe asthma, and childhood bronchiectasis. We also showed associated defects in phagocytosis of NTHi, a common colonizer of the lower airway in these diseases. These defects could be substantially overcome with low-dose azithromycin; however, the use of antibacterial compounds in chronic indications raises concerns of potential resistance. Use of macrolides with their antibiotic activity removed would allow better exploitation of other properties without compromising the effectiveness of first-line therapies. We therefore investigated two novel macrolides: GS-459755 and its azithromycin-based analog, GS-560660. The macrolides were synthesized as reported (23, 25).
The major finding of the present study was the significant improvement in the capacity of primary human alveolar macrophages and cigarette smoke-exposed THP-1 macrophages to phagocytose both NTHi and apoptotic bronchial epithelial cells associated with a reduction in the inflammatory markers IL-1β and the NLRP3 inflammasome following exposure to GS-459755 and GS-560660. These effects were noted at low doses of macrolides (500–1000 ng/ml)—doses consistent with those previously reported by us for azithromycin (10, 12).
Azithromycin safety is established, and there have been many reports of its anti-inflammatory properties and it becoming increasingly used as an adjunct anti-inflammatory treatment for a range of chronic lung diseases, including COPD, cystic fibrosis, and severe asthma, and following lung transplantation, in addition to front-line antibiotics as required. We previously demonstrated both in vitro and in a human phase II study that the anti-inflammatory effects of low doses of azithromycin were accompanied by a substantial improvement in alveolar macrophage phagocytic function (for both apoptotic cells and bacteria) (10, 12). Nine COPD subjects were treated with 250 mg azithromycin, twice weekly for 12 wk. Bronchoscopy was performed pre- and posttreatment. Azithromycin treatment significantly improved phagocytosis associated with decreases in both proinflammatory cytokine production in response to LPS and airway inflammation (reduced C-reactive protein and total leukocyte count) (10, 12).
The macrolide-mediated improvement in the ability of alveolar macrophages to phagocytose bronchial epithelial cells may have clinical significance with regard to new or adjunct treatments for COPD and other chronic lung diseases, where there is inflammation associated with ineffective repair of the injured epithelium and loss of structural integrity. The resolution of inflammation is a key step for controlling chronic inflammatory lung diseases. In the airway, prompt phagocytosis of apoptotic epithelial cells by alveolar and lung tissue macrophages suppresses inflammation. However, as disease progresses, there is an increase in the number of noncleared apoptotic cells, suggesting that the efficiency of efferocytosis is impaired. Further evidence for the key role of macrophages in the resolution of inflammatory responses and a causative link between macrophage dysfunction and consequent accumulation of secondary necrotic material were presented by Knapp et al. (15). Mice selectively depleted of alveolar macrophages showed increased mortality and impaired resolution of inflammation directly related to an increased accumulation of apoptotic material and secondary necrosis (15). It has also been shown that many of these cellular abnormalities, including excess epithelial apoptosis and macrophage dysfunction, persist in COPD, despite smoking cessation (9), and thus provide relevant therapeutic targets for COPD.
Colonization of the airway with potentially pathogenic bacteria, including NTHi, and neutrophilic inflammation are hallmarks of chronic inflammatory lung diseases, including COPD, and it is widely accepted that the bacterial persistence is harmful to the airways. The colonization is likely to be exacerbated by the reduced capacity for alveolar macrophages to phagocytose NTHi and other bacteria. Our current findings of improved clearance of NTHi by macrophages in the presence of the nonantibiotic macrolides may thus have clinical significance.
Efferocytosis is mediated by a range of macrophage receptors that recognizes specific molecules on, or secreted by, the apoptotic cell. We previously showed that azithromycin did not significantly improve the cigarette smoke-associated reduction in expression of phagocytic recognition molecules CD31 (promotes tethering of apoptotic cells to macrophages) (2), CD44 (involved in binding and metabolism of hyaluronan), and CD36 (recognizes thrombospondin 1). In the present study, we further investigated the effects of azithromycin, GS-459755, and GS-560660 on receptors that may mediate binding and internalization of either bacteria or apoptotic cells: MerTK (involved in engulfment of the tethered apoptotic cell; increased expression has been reported on airway macrophages from cigarette smokers) (14), SRA-1 (significantly underexpressed in COPD airways) (24, 29), and TLR2 and -4 (lipid-recognizing molecules). Despite the potential importance of these receptors and a report that clarithromycin downregulated TLR4 expression on monocytes (30), no significant changes were noted in the presence of azithromycin, GS-459755, or GS-560660, suggesting that the mechanisms of action of macrolide antibiotics do not involve significant changes to these molecules involved in the recognition of apoptotic cells or NTHi. It was previously reported that azithromycin shifted the polarization of a mouse macrophage cell line toward an alternatively activated “M2” phenotype (17). Consistent with this finding, we reported that azithromycin significantly increased macrophage expression of the M2 marker mannose receptor by 50% and established a functional link between the reduced expression of the mannose receptor and phagocytosis in COPD by blocking the expression of the mannose receptor on alveolar macrophages using a specific blocking antibody (10). As the shift to an M2 phenotype may decrease inflammation and encourage tissue repair, further investigation of GS-459755 or GS-560660 in this regard may be warranted.
We previously showed that anti-inflammatory effects of low-dose azithromycin in COPD subjects included a reduction in IL-1β levels in BAL (10), consistent with data from Bosnar et al. (1), who reported that azithromycin inhibited LPS-induced IL-1β concentrations in lung tissue macrophages in mice. We now report that anti-inflammatory properties are retained in GS-459755 and GS-560660, despite the loss of antibiotic properties. In a previous study, using a mucin 5AC inhibition assay, GS-560660 demonstrated potency equivalent to azithromycin (mucin 5AC MIC50, azithromycin 38 µM, GS-560660 30 µM) (25), and GS-459755 was shown to prevent human neutrophil elastase-induced mucus stasis and airway surface liquid volume depletion (23). In the present study, both GS-459755 and GS-560660 reduced intracellular levels of the proinflammatory-cleaved IL-1β with a nonsignificant trend for a decrease in IL-1β secretion by unstimulated or cigarette smoke-exposed macrophages.
Although an effect of azithromycin on reduction of IL-1β production has been reported in LPS-stimulated mice, in human COPD patients and in patients with bronchiolitis obliterans organizing pneumonia, the mechanisms of the effect are relatively unknown (1, 3, 16). Data from a recent study suggest that azithromycin may inhibit the noncanonical inflammasome pathway via impaired induction of the LPS-sensing caspase-4 (4). In the current study, the colocalization of active IL-1β with particulate NLRP3 in PMA-induced THP-1 macrophages and reduction of these complexes in the presence of azithromycin, GS-560660, and GS-459755 suggest that anti-inflammatory effects of azithromycin at low dose may be mediated further via inhibition of NLRP3 inflammasome activation.
Our previous studies suggest that smoking per se does not hinder the effects of macrolides on macrophage function or inflammatory mediators. Azithromycin improved the phagocytic capacity of alveolar macrophages from control and COPD subjects to a comparable extent in vitro and induced a similar effect in vivo in COPD patients, whether they were currently smoking or not. In the present study, the nonantibiotic compounds showed effects on primary alveolar macrophages from control subjects that were consistent with our previous findings with azithromycin. We confirmed these findings in smoke-exposed THP-1 macrophages, although further confirmation using macrophages from COPD subjects would strengthen the data. It would also be of interest to investigate the potential effects of pre-exposing macrophages to cigarette smoke before addition of the macrolides.
Whereas azithromycin is well absorbed following oral administration, the oral bioavailability of GS-459755 is low (<1% in the rat; Sibylle Wilbert, personal communication). GS-560660, on the other hand, may have the potential to be orally bioavailable, due to its structural similarity with azithromycin. Alternatively, macrolides could be administered to the lung by inhalation. As shown in a sheep model for neutrophil elastase-induced mucociliary dysfunction, the protective efficacy of inhaled GS-459755 was comparable with azithromycin (29), and GS-459755 prevented neutrophil elastase-induced mucus dehydration to a similar extent as azithromycin (23).
We conclude that the use of nonantibiotic macrolides GS-459755 and GS-560660 for reducing airway inflammation in chronic lung diseases without inducing bacterial resistance opens the door to a new generation of adjunct treatments.
GRANTS
This study was supported by National Health and Medical Research Council Project Grant 1106377 and Career Development Award 104551.
DISCLOSURES
A.Y., J.T., and S.W. are employees of Gilead Sciences and own shares in Gilead Sciences Inc. The other authors have no financial disclosures.
AUTHOR CONTRIBUTIONS
S.H., H.B.T., and S.W. conceived and designed research; H.B.T., R.H., E.R., and M.W. performed experiments; S.H., H.B.T., R.H., E.R., G.H., and M.W. analyzed data; S.H., H.B.T., E.R., G.H., H.J., P.N.R., A.Y., J.T., and S.W. interpreted results of experiments; S.H. drafted manuscript; S.H., H.B.T., R.H., E.R., G.H., H.J., M.W., P.N.R., A.Y., J.T., and S.W. edited and revised manuscript; S.H., H.B.T., R.H., E.R., G.H., H.J., M.W., P.N.R., A.Y., J.T., and S.W. approved final version of manuscript.
REFERENCES
- 1.Bosnar M, Čužić S, Bošnjak B, Nujić K, Ergović G, Marjanović N, Pašalić I, Hrvačić B, Polančec D, Glojnarić I, Eraković Haber V. Azithromycin inhibits macrophage interleukin-1β production through inhibition of activator protein-1 in lipopolysaccharide-induced murine pulmonary neutrophilia. Int Immunopharmacol 11: 424–434, 2011. doi: 10.1016/j.intimp.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 418: 200–203, 2002. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 3.Cai M, Bonella F, Dai H, Sarria R, Guzman J, Costabel U. Macrolides inhibit cytokine production by alveolar macrophages in bronchiolitis obliterans organizing pneumonia. Immunobiology 218: 930–937, 2013. doi: 10.1016/j.imbio.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Gualdoni GA, Lingscheid T, Schmetterer KG, Hennig A, Steinberger P, Zlabinger GJ. Azithromycin inhibits IL-1 secretion and non-canonical inflammasome activation. Sci Rep 5: 12016, 2015. doi: 10.1038/srep12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodge G, Osborn M, Hodge S, Nairn J, Tapp H, Kirby M, Sepulveda H, Morgan E, Revesz T, Zola H. Rapid simultaneous measurement of multiple cytokines in childhood oncology patients with febrile neutropenia: increased interleukin (IL)-8 or IL-5 correlates with culture-positive infection. Br J Haematol 132: 247–248, 2006. doi: 10.1111/j.1365-2141.2005.05870.x. [DOI] [PubMed] [Google Scholar]
- 6.Hodge S, Dean M, Hodge G, Holmes M, Reynolds PN. Decreased efferocytosis and MBL in the airway in bronchiolitis obliterans syndrome. J Heart Lung Transplant 30: 589–595, 2011. doi: 10.1016/j.healun.2011.01.710. [DOI] [PubMed] [Google Scholar]
- 7.Hodge S, Hodge G, Ahern J, Jersmann H, Holmes M, Reynolds PN. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in COPD. Am J Respir Cell Mol Biol 37: 748–755, 2007. doi: 10.1165/rcmb.2007-0025OC. [DOI] [PubMed] [Google Scholar]
- 8.Hodge S, Hodge G, Brozyna S, Jersmann H, Holmes M, Reynolds PN. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur Respir J 28: 486–495, 2006. doi: 10.1183/09031936.06.00001506. [DOI] [PubMed] [Google Scholar]
- 9.Hodge S, Hodge G, Holmes M, Reynolds PN. Increased apoptosis in the airways in COPD persists after smoking cessation. Eur Respir J 25: 447–454, 2005. doi: 10.1183/09031936.05.00077604. [DOI] [PubMed] [Google Scholar]
- 10.Hodge S, Hodge G, Jersmann H, Matthews G, Ahern J, Holmes M, Reynolds PN. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in COPD. Am J Respir Crit Care Med 178: 139–148, 2008. doi: 10.1164/rccm.200711-1666OC. [DOI] [PubMed] [Google Scholar]
- 11.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with COPD are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol 81: 289–296, 2003. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- 12.Hodge S, Reynolds PN. Low-dose azithromycin improves phagocytosis of bacteria by both alveolar and monocyte-derived macrophages in chronic obstructive pulmonary disease subjects. Respirology 17: 802–807, 2012. doi: 10.1111/j.1440-1843.2012.02135.x. [DOI] [PubMed] [Google Scholar]
- 13.Hodge S, Upham JW, Pizzutto S, Petsky HL, Yerkovich S, Baines KJ, Gibson P, Simpson JL, Buntain H, Chen ACH, Hodge G, Chang AB. Is alveolar macrophage phagocytic dysfunction in children with protracted bacterial bronchitis a forerunner to bronchiectasis? Chest 149: 508–515, 2016. doi: 10.1016/j.chest.2015.10.066. [DOI] [PubMed] [Google Scholar]
- 14.Kazeros A, Harvey BG, Carolan BJ, Vanni H, Krause A, Crystal RG. Overexpression of apoptotic cell removal receptor MERTK in alveolar macrophages of cigarette smokers. Am J Respir Cell Mol Biol 39: 747–757, 2008. doi: 10.1165/rcmb.2007-0306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med 167: 171–179, 2003. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- 16.Marjanović N, Bosnar M, Michielin F, Willé DR, Anić-Milić T, Culić O, Popović-Grle S, Bogdan M, Parnham MJ, Eraković Haber V. Macrolide antibiotics broadly and distinctively inhibit cytokine and chemokine production by COPD sputum cells in vitro. Pharmacol Res 63: 389–397, 2011. doi: 10.1016/j.phrs.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Murphy BS, Sundareshan V, Cory TJ, Hayes D Jr, Anstead MI, Feola DJ. Azithromycin alters macrophage phenotype. J Antimicrob Chemother 61: 554–560, 2008. doi: 10.1093/jac/dkn007. [DOI] [PubMed] [Google Scholar]
- 19.Pizzutto SJ, Yerkovich ST, Upham JW, Hales BJ, Thomas WR, Chang AB. Children with chronic suppurative lung disease have a reduced capacity to synthesize interferon-gamma in vitro in response to non-typeable Haemophilus influenzae. PLoS One 9: e104236, 2014. doi: 10.1371/journal.pone.0104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodvold KA, Danziger LH, Gotfried MH. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob Agents Chemother 47: 2450–2457, 2003. doi: 10.1128/AAC.47.8.2450-2457.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson JL, Gibson PG, Yang IA, Upham J, James A, Reynolds PN, Hodge S; AMAZES Study Research Group . Impaired macrophage phagocytosis in non-eosinophilic asthma. Clin Exp Allergy 43: 29–35, 2013. doi: 10.1111/j.1365-2222.2012.04075.x. [DOI] [PubMed] [Google Scholar]
- 22.Su Y, Han W, Giraldo C, De Li Y, Block ER. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. Am J Respir Cell Mol Biol 19: 819–825, 1998. doi: 10.1165/ajrcmb.19.5.3091. [DOI] [PubMed] [Google Scholar]
- 23.Tarran R, Sabater JR, Clarke TC, Tan CD, Davies CM, Liu J, Yeung A, Garland AL, Stutts MJ, Abraham WM, Phillips G, Baker WR, Wright CD, Wilbert S. Nonantibiotic macrolides prevent human neutrophil elastase-induced mucus stasis and airway surface liquid volume depletion. Am J Physiol Lung Cell Mol Physiol 304: L746–L756, 2013. doi: 10.1152/ajplung.00292.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran HB, Ahern J, Hodge G, Holt P, Dean MM, Reynolds PN, Hodge S. Oxidative stress decreases functional airway mannose binding lectin in COPD. PLoS One 9: e98571, 2014. doi: 10.1371/journal.pone.0098571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Tran HB, Lewis MD, Tan LW, Lester SE, Baker LM, Ng J, Hamilton-Bruce MA, Hill CL, Koblar SA, Rischmueller M, Ruffin RE, Wormald PJ, Zalewski PD, Lang CJ. Immunolocalization of NLRP3 inflammasome in normal murine airway epithelium and changes following induction of ovalbumin-induced airway inflammation. J Allergy (Cairo) 2012: 819176, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treiberg J, Yeung A, Baker B, Liu J, Kenney T, MacLeod D, Therrien J, Wilbert S, Wright C, Phillips G. Non-antibacterial azithromycin derivatives for COPD. 248th American Chemical Society National Meeting and Exposition, San Francisco, CA, August 10–14, 2014, Pub #82. [Google Scholar]
- 26.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 129: 1673–1682, 2006. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 27.Ween M, Carroll A, Ahern J, Hodge G, Pizzutto S, Jersmann H, Reynolds P, Hodge S. A small volume technique to examine and compare alveolar macrophage phagocytosis of apoptotic cells and NTHi. J Immunol Methods 12: 7–14, 2016. doi: 10.1016/j.jim.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Wilbert S, Sabater JR, Clarke TC, Liu J, Yeung A, Wright CD, Abraham WM. Azithromycin and the non-antibacterial macrolide GS-459755 prevent neutrophil elastase-induced mucociliary dysfunction in sheep. American Thoracic Society 2012 International Conference, San Francisco, CA, May 18–23, 2012. [Google Scholar]
- 30.Williams JC, Craven RR, Earp HS, Kawula TH, Matsushima GK. TAM receptors are dispensable in the phagocytosis and killing of bacteria. Cell Immunol 259: 128–134, 2009. doi: 10.1016/j.cellimm.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]