Abstract
Cystic fibrosis-related diabetes is the most common comorbidity associated with cystic fibrosis (CF) and correlates with increased rates of lung function decline. Because glucose is a nutrient present in the airways of patients with bacterial airway infections and because insulin controls glucose metabolism, the effect of insulin on CF airway epithelia was investigated to determine the role of insulin receptors and glucose transport in regulating glucose availability in the airway. The response to insulin by human airway epithelial cells was characterized by quantitative PCR, immunoblot, immunofluorescence, and glucose uptake assays. Phosphatidylinositol 3-kinase/protein kinase B (Akt) signaling and cystic fibrosis transmembrane conductance regulator (CFTR) activity were analyzed by pharmacological and immunoblot assays. We found that normal human primary airway epithelial cells expressed glucose transporter 4 and that application of insulin stimulated cytochalasin B-inhibitable glucose uptake, consistent with a requirement for glucose transporter translocation. Application of insulin to normal primary human airway epithelial cells promoted airway barrier function as demonstrated by increased transepithelial electrical resistance and decreased paracellular flux of small molecules. This provides the first demonstration that airway cells express insulin-regulated glucose transporters that act in concert with tight junctions to form an airway glucose barrier. However, insulin failed to increase glucose uptake or decrease paracellular flux of small molecules in human airway epithelia expressing F508del-CFTR. Insulin stimulation of Akt1 and Akt2 signaling in CF airway cells was diminished compared with that observed in airway cells expressing wild-type CFTR. These results indicate that the airway glucose barrier is regulated by insulin and is dysfunctional in CF.
Keywords: airway epithelia, cystic fibrosis, glucose transporter 4, glucose metabolism, cystic fibrosis transmembrane conductance regulator, phosphatidylinositol 3-kinase, protein kinase B
cystic fibrosis (CF) is one of the most common inherited diseases, occurring at a rate of 1 in every 2,500 Caucasian births (32). Accelerated lung decline occurs in CF patients as they age and is further exacerbated in patients with CF-related diabetes (CFRD). CF and CFRD patients experience severe pulmonary exacerbations and recurring bacterial infections that collectively contribute to the precipitous decline in lung function (13, 66), a significant factor in patient morbidity and mortality.
Because bacterial infection is a driver of CF lung disease through inflammation, bacterial nutrient availability in the airway is suspected to be of importance for controlling airway infection (14, 23–25, 28, 57) and may contribute to the destructive effects of inflammation (1, 2, 27, 45, 59). In a model of bacterial pneumonia in a CF mouse, glucose was readily found in the bronchoalveolar lavage fluid (BALF) of both CF and CFRD mice (36). Similarly, hyperglycemia in mice correlated with higher airway glucose levels and increased bacterial load (28). Understanding how glucose accumulates in the airspace of CF patients (8, 70) will offer new explanations about the origin of airspace glucose and how it is regulated. Similarly, understanding how airspace glucose accumulation is associated with lung disease is important to controlling its impact on the progression of lung disease in CF and CFRD (11, 13, 17, 33, 62).
Metabolite regulation and homeostasis in human airway surface liquid (ASL) remains incompletely characterized. Therefore, we sought to understand the contributions of tight junctions and glucose transporters in controlling metabolite availability in ASL. The airway is lined by a pseudostratified epithelium that includes multiple cell types that work together to prevent infection by maintaining a physical barrier between the sterile serous compartment of the lung and the nonsterile hostile environment of the airway (30, 43, 63). The tight junctions of the airway barrier also act to regulate the composition of ASL by modulating paracellular fluxes (6, 30, 63). Paracellular barrier function previously was shown to be impaired in cells expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR) (54, 55) and by exposure to proinflammatory cytokines (16, 30). ASL composition is also regulated through the action of multiple channels and transporters in the plasma membranes of airway epithelial cells. Most of what is known about airway glucose homeostasis is based on studies of tumor-derived cell lines from club cells (H441) and alveolar epithelial cells (A549) that implicate passive uptake by the glucose transporters Glut2 and Glut10 (22, 39). How these data reflect glucose transport by nontransformed cells remains an open question.
In a previous study (18), Devaskar and deMello used whole lung homogenates of human lung tissue isolated 6–12 h postmortem to probe for glucose transporter proteins representing Glut-1, -2, -3, -4, and -5 and the sodium-glucose cotransporter SGLT1. All glucose transporters were examined by immunoblot and immunohistochemistry. Of these transporters, only Glut1 was found in human lung homogenates, presumed to be from the lower lung/alveolar region. By contrast, Glut2 was detected in rat lung tissue but not in rat tracheal tissue (18). Merigo et al. reported detectable expression of Glut2 and SGLT1 in specialized rat tracheal cells (51–53); however, they did not examine human tissue. Recently, a major difference in airway acidification between rodent and human airways was observed (64), suggesting that other regulatory mechanisms of lung physiology are likely to be fundamentally different in humans compared with rodents.
In Pezzulo et al. (57), the authors demonstrated that Glut1 and Glut10 protein was found in primary human airway epithelial cell cultures. Glucose transporter mRNA expression data were mined from a MIAME-compliant microarray GEO dataset, and all glucose transporters were screened for detection by immunocytochemistry of banked tissue samples. The dataset mRNA expression profile revealed broad gene expression of all 12 glucose transporters, notably including Glut4, and two of the three sodium-glucose cotransporters (SGLT-1 and -2). Pezzulo et al. reported no SGLT1 function detectable by Ussing chamber analysis. Also reported were the transepithelial flux rates of both l-glucose, which is not imported by transporters, and 2-deoxy-d-glucose, which is transported, but not metabolized. Surprisingly, both glucose analogs traversed wild-type ex vivo pig tracheal epithelia at similar rates, suggesting that transepithelial glucose transport can potentially occur by both the transcellular and paracellular routes. However, transcellular glucose transport is strongly influenced by rates of glycolysis that can be affected by several factors. In particular, the paracellular route of glucose transport may predominate when cells are highly glycolytic where imported glucose is degraded. To our knowledge, the effect of glycolytic stimulants and metabolite regulators, such as insulin, on glucose transport in nontransformed human airway epithelia has not been studied.
Here, we found that normal human airway cells have functional insulin receptors and insulin-dependent glucose transporters that assist in regulating intracellular glucose import. We also report that insulin tightens the paracellular barrier in non-CF airway epithelia, together creating an “airway metabolite barrier” where insulin-stimulated increases in glucose uptake and reduction of paracellular leak combine to decrease rates of glucose movement across the airway epithelium. In contrast, airway cells expressing F508del-CFTR are less responsive to insulin and have an impaired paracellular barrier, which enables the accumulation of glucose and other metabolites in the ASL.
MATERIALS AND METHODS
Cell lines and reagents.
Primary normal, non-CF human bronchial epithelial (NhBE) and primary CF human bronchial epithelial (CFhBE, F508del/F508del) cells from multiple donors were purchased from ChanTest (Cleveland, OH) and cultured according to the supplier’s instructions. Telomerase-immortalized normal (NuLi-1) and cystic fibrosis (CuFi-5) airway (CRL4011 and CRL4016, respectively; ATCC, Manassas, VA) cells were grown and matured at air-liquid interface as previously described (55), and passages 6–12 were used. All cells were shown to express CFTR protein (55), exhibit sodium and chloride currents, and acclimate to media containing 1.0 g/l (5.6 mM) glucose 24 h before and during experimentation. HeLa and T84 cells were grown according to standard protocols. All cells were negative for mycoplasma contamination as determined by the MycoAlert Mycoplasma Detection Kit (no. LT07-218; Lonza) used according to the manufacturer’s instructions.
Mouse studies.
All experimental procedures were carried out with approval from the Institutional Animal Care and Use Committee at Emory University (Atlanta, GA). The gut-corrected CFTR-null mouse [Cftrtm1UncTg(FABPCFTR)1Jaw/J] was used in this study to generate BALF and plasma for insulin, glucose, and urea analysis. Mice were purchased from the Emory+Children’s CF Animal Models Core. BALF was collected (2× 750 µl lavage) from each mouse using a collection solution containing glucose uptake and protease inhibitors and took an average of 15 s to perform. The collection solution consisted of 20 µM phlorizin (no. 4627; Tocris Bioscience), 500 µM phloretin (no. 191511; MP Biomedicals), 20 µM STF-31 (no. 4484; Tocris Bioscience), and one tablet of EDTA-free protease inhibitor (Roche) per 10 ml DPBS (with calcium and magnesium) supplemented with PMSF (0.1 mM) and NaVa2O4 (1 mM). The collected BALF was first centrifuged at 500 g for 5 min, and supernatant was transferred to a new tube and spun at 3,000 g for 10 min, followed by sterile filtration through a 0.22-μm syringe filter into a new tube before freezing. An ELISA for mouse insulin (no. 80-INSMSU-E01; Alpco Diagnostics) was used to quantify insulin in BALF and plasma from mice used in this study. A colorimetric glucose quantification kit (no. 10009582; Cayman Chemical) was used to quantify BALF and plasma glucose. A urea quantification kit (no. MAK006; Sigma) was used to quantify BALF and plasma urea from the mice according to the manufacturer’s instructions. The urea concentrations in plasma were used to correct insulin and glucose concentrations found in the BALF. The corrected values are reported as means ± SE.
Immunoblotting, immunofluorescence, immunohistochemistry, and antibodies.
Glucose transporter-positive control lysates were purchased as lyophilized whole cell lysates of HEK293 cells expressing the protein of interest (Glut1, no. LC416593; Glut10, no. LC410718; Origene). Protein kinase B (Akt) control lysates were purchased from Cell Signaling Technologies (CST) as Jurkat cells treated with either calyculin A or LY-294002 and provided as ready-to-load protein lysate solutions (no. 9273; CST). HeLa and T84 cell line lysates were made in-house. NuLi-1 and CuFi-5 cell lysates were prepared in 1× RIPA buffer (no. 9806; CST) and diluted in 4× Protein Sample Loading Buffer (no. 928–40004; Li-Cor) supplemented with fresh DTT (390 mM). Protein lysates were loaded on 4–20 or 10% TGX SDS-PAGE gels (Bio-Rad), transferred by a Trans-Blot Turbo Transfer System set for mixed molecular weights on nitrocellulose membranes, and processed for enhanced chemiluminescence (ECL) or infrared dye imaging (Li-Cor) using standard protocols. All immunoblots were blocked with TBS-based Odyssey Blocking Buffer (no. 927–50000; Li-Cor).
Antibodies used for immunoblotting include the following incubated overnight at room temperature, unless otherwise noted: rabbit monoclonal antibody (mAb) anti-human insulin receptor-β at 1:2,500 (no. 3025, 95 kDa; CST); mouse anti-actin at 1:20,000 (no. A5441, 47 kDa; Sigma) for 1 hour at room temperature (RT); rabbit anti-FLAG at 1:2,000 (no. F7425; Sigma); rabbit anti-human Glut4 at 1:2,500 (no. NBP1–49533, 54 kDa; Novus); rabbit anti-human SGLT1 at 1:1,000 (no. 07–1417, 72 kDa; Millipore); rabbit anti-human Glut1 at 1:1,000 (no. Ab15309, 54–60 kDa; Abcam); rabbit anti-human Glut10 at 1:1,000 (no. Ab33245, 52–60 kDa; Abcam); mouse anti-human panAKT at 1:1,000 (no. 2920, 60 kDa; CST); rabbit mAb anti-human Akt1 at 1:1,000 (no. 2938, 60 kDa; CST); rabbit mAb anti-human phospho-Akt1-S473 at 1:1,000 (no. 9018; CST); rabbit mAb anti-human Akt2 at 1:1,000 (no. 3063, 60 kDa; CST); rabbit mAb anti-human phospho-Akt2-S474 at 1:1,000 (no. 5899; CST); and mouse anti-human Akt3 at 1:1,000 (no. 8018, 60 kDa; CST).
For ECL imaging, primary antibodies were diluted in DPBS supplemented with 0.1% (vol/vol) Tween 20 and 5% (wt/vol) BSA. Horseradish peroxidase (HRP)-conjugated goat anti-mouse or anti-rabbit secondary antibodies were incubated at 1:2,000 for 1 h at RT in DPBS supplemented with 0.1% (vol/vol) Tween 20 and 5% (wt/vol) BSA. Blots were exposed to Clarity Western ECL Substrate (no. 170–5060; Bio-Rad) for 3–10 min, depending on the antibody pair, before digital imaging with a Gel-Doc XR+ system (Bio-Rad).
For infrared immunoblot imaging, primary antibodies were diluted in a 1:1 mixture of DPBS with calcium/magnesium (DPBS++) and TBS-based Odyssey Blocking Buffer supplemented with 0.2% Tween 20. Fluorescent secondary antibodies were used as follows: goat anti-mouse 680RD, goat anti-mouse 800CW, goat anti-rabbit 680RD, and goat anti-rabbit 800CW, all diluted at 1:20,000 in a 1:1 mixture of DPBS++ and Odyssey Blocking Buffer with 0.2% Tween 20. All infrared blotting materials were purchased from Li-Cor Biosciences, and data were collected using an Odyssey Classic imager.
For immunofluorescence, cells grown on permeable supports were fixed and immunolabeled according to each cell type and labeling pair. For imaging of Glut4 (no. NBP1-49533, 1:100; Novus), β-catenin (no. 610153, 1:200; BD), and insulin receptor (no. ab5500; Abcam), cells were fixed in 3.7% paraformaldehyde in DPBS++ for 15 min at RT, washed one time, quenched with 1 M glycine for 15 min, blocked in DPBS++ with 0.5% Tween 20 (PBS-T) supplemented with 1% (wt/vol) BSA for 1 h at RT, and then incubated with primary antibodies overnight in 0.1% BSA in PBS-T. Fluorescent secondary antibodies conjugated to AlexaFluor 488 or 594 were used at 1:500 in PBS-T supplemented with 0.1% BSA for 1 h at RT. Wash steps were performed with PBS-T buffer, and after the final wash each filter was briefly rinsed in 70% ethanol before mounting in ProLong Gold Antifade Reagent with DAPI (no. P36931; Invitrogen). Immunofluorescence Z-stack images of Glut4 localization were collected at 0.25-μm steps on an inverted Olympus FV1000 Confocal Microscope and analyzed by Fiji Image Analysis Software version 1.49a (National Institutes of Health).
For immunohistochemistry, sections of human tissues were acquired through US Biomax (Derwood, MD) as FFPE samples on slides (catalog no. HuFPT). The immunohistochemistry procedure was performed by the Emory Winship Pathology Core Laboratory using a Biocare Decloaking Chamber and a DAKO Autostainer according to a protocol optimized as follows. Briefly, the sections were incubated for 1 h in a 60°C oven, deparaffinized in sequential xylene/alcohol/water solution changes, and then subjected to epitope retrieval with Target Retrieval Solution (DAKO) diluted 1:10. Endogenous peroxidase was blocked with H2O2 and washed, and sections were then incubated with Novus anti-Glut4 antibody (pAb no. NBP1-49533) at 1:200 for 40 min at room temperature, visualized with DAKO Envision+Dual Link System-HRP, developed with diaminobenzidine for 2 min, counterstained with hematoxylin, dehydrated and cleared with xylene, and then mounted and covered with a cover slip. Images were acquired on an Olympus BX41 microscope equipped with an Olympus XC10 camera controlled by cellSens Image Analysis Software.
mRNA analysis.
Quantitative RT-PCR was performed as previously described (55) using human specific and validated primer sets (PrimePCR system; Bio-Rad). PrimePCR reactions are optimized to produce single amplified cDNA products by using a unified PCR protocol for all primers used. The resulting amplified cDNAs were electrophoresed with a 2% agarose gel to resolve DNA products smaller than 500 bp and to confirm single cDNA products for each primer set tested.
Insulin stimulation and glucose uptake.
Methods used to determine insulin-mediated glucose uptake in airway cells grown at air-liquid interface (ALI) were based on methods described elsewhere for glucose uptake in differentiated L6 myotubes and 3T3-L1 adipocytes (72). Cells on Transwell permeable supports were washed three times on both apical and basolateral surfaces with Krebs-Ringer HEPES (KRH) buffer (1 g/l d-glucose, 50 mM HEPES, 137 mM NaCl, 4.7 mM KCl, 1.85 mM CaCl2, and 1.3 mM MgSO4 at pH 7.4) to remove residual growth medium. Transwells were then incubated in the cell culture incubator for 90 min in KRH to reduce residual effects of factors in the growth medium. Cells were briefly washed again with fresh prewarmed KRH to remove any mucus accumulation and then treated with 125 nM recombinant human insulin (human recombinant with zinc, no. 12585-014; Life Technologies) in KRH for the time indicated at 37°C. A d-glucose solution (1 g/l; Sigma) spiked with 2-deoxy-d-glucose (2-DG; Sigma) and 2-deoxy-d-[3H]glucose (NEN Radiochemicals, PerkinElmer), with or without insulin, was placed on the apical and/or basolateral surfaces at volumes of 250 and 500 µl, respectively. Each well contained 0.5 µCi of the 2-deoxy-d-[3H]glucose in a final total concentration of 5.6 mM (1 g/l) 2-DG in both the upper and lower chamber medium. After insulin and glucose incubation, the solutions were removed, and cells were washed multiple times with ice-cold KRH to remove excess 2-DG. Filters were then excised from the Transwell cups and carefully placed in a scintillation vial containing 200 µl of 0.1 M NaOH to lyse the cells. Next, 2 ml of scintillation fluid were added to each tube, which was then capped and vortexed. Vials were then measured for radioactivity using a Packard 2200CA scintillation counter.
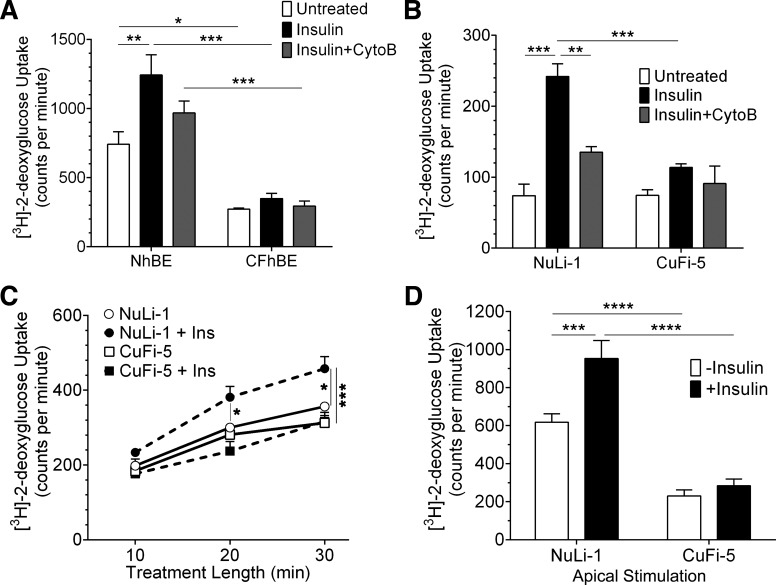
To differentiate insulin-specific stimulation of glucose transporter-mediated glucose uptake from nonspecific uptake, cells were treated with 125 nM bilateral insulin for 20 min before replacing the bilateral solutions with a solution containing labeled glucose and insulin (Fig. 6, A and B) for an additional 10 min (29). Some cells were treated with cytochalasin B (20 µM; Sigma), an inhibitor of Glut4-storage vesicle fusion, for 10 min before the addition of insulin. Data in Fig. 6, A and B, represent 30 min insulin stimulation including a 10-min glucose uptake period. Data in Fig. 6C represent glucose uptake when both 500 nM insulin and labeled glucose were added at time 0 min, and then cells were sampled every 10 min for a total of 30 min. In some cases, insulin was added only to one side of the epithelium (Fig. 6D).
Fig. 6.
Insulin stimulation fails to increase glucose uptake in human airway epithelia expressing F508del-CFTR. A: primary hBE cells were treated with bilateral insulin (black bars) for a total of 15 min in the presence of 2-deoxy-d-[3H]glucose to measure glucose uptake and retention within the cell layer. Cells were also treated with cytochalasin B (gray bars) to inhibit fusion of Glut4 storage vesicles (GSVs) to the plasma membrane. Unstimulated control cells are represented by white bars. NhBE cells responded to insulin by increasing their glucose uptake, whereas CFhBE cells did not respond to insulin. Cytochalasin B (CytoB) treatment prevented the full effect of insulin stimulation in NhBE cells but had no effect in CFhBE cells. B: comparably treated NuLi-1 and CuFi-5 cells had insulin stimulation responses similar to those seen in NhBE and CFhBE, respectively; n = 4 filters for each data point for each graph. C: temporal glucose uptake measurements indicated that CuFi-5 cells did not respond to treatment with bilateral insulin (■) and behaved similarly to unstimulated CuFi-5 cells (□) and unstimulated NuLi-1 cells (○) compared with stimulated NuLi-1 cells (●). D: NuLi-1 or CuFi-5 filters were pretreated with apical insulin solution for 15 min before measuring the uptake of 2-deoxy-d-[3H]glucose. The basolateral solution contained neither glucose nor insulin. Apical stimulation resulted in apical glucose uptake in NuLi-1 cells but not in CuFi-5 cells; n = 18 filters for each data bar. All data are shown as means ± SE where *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 by unprotected two-way ANOVA Fisher’s LSD test.
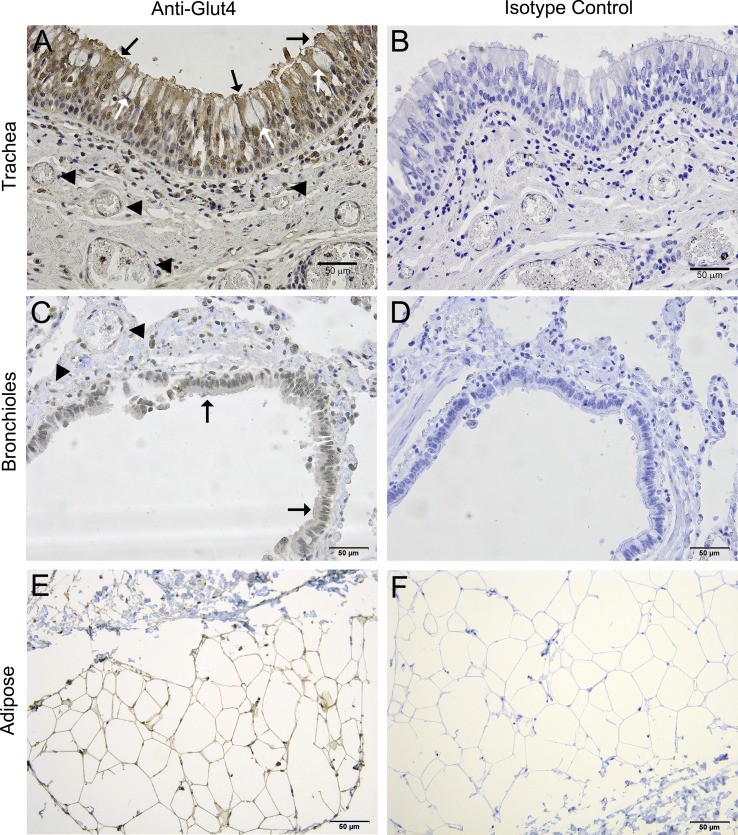
Fig. 4.
Glut4 protein is detectable in human tracheal and bronchial epithelial cells by immunohistochemistry. Glut4 appears to be expressed by ciliated cells (black arrows) and by nonciliated cells (white arrows) in the human trachea (A) and bronchioles (C). Blood vessels (arrowheads) are located within 50–100 μm from the tracheal and bronchial epithelial cells. B, D, and F: control sections processed with nonspecific isotype control antibody. E: adipose tissue was used as a positive control tissue where Glut4 is known to be expressed.
Phospho-Akt analysis.
For the phospho-Akt (pAkt) analysis, cells on Transwells were washed in prewarmed KRH buffer and then incubated for 90 min in bilateral KRH in the cell culture incubator. Cells were then placed in their respective treatments of KRH with or without 125 nM insulin apically, basolaterally, or bilaterally for a total of 30 min. The chambers that did not contain insulin were washed and incubated in bilateral KRH. After insulin treatment, cells were washed one time in KRH buffer and lysed directly with 1× RIPA buffer supplemented with PMSF, NaF, Na2VO3, and protease inhibitors. SDS-PAGE gels were loaded with 20–25 µg of protein/lane for pAkt analysis using both the Odyssey Classic Imager (Li-Cor) and the ECL method (Bio-Rad).
Paracellular dye flux assays.
To reduce any effects from glucose stress in mature cells grown at ALI on Transwells, the cells were first acclimated to normal glucose conditions by replacing the basolateral growth media with normal glucose DMEM (no. 5546; Sigma) supplemented with 2% Ultroser G 24 h before initiating experiments. After acclimation, Transwells were placed in bilateral KRH buffer supplemented with 1 g/l glucose for 90 min to remove all growth factors and media supplements. After 90 min, the solutions were swapped with fresh solutions where the apical solution contained 4 µg/ml calcein (0.63 kDa) and 0.1 mg/ml Texas Red Dextran (10 kDa), in combination. These solutions contained insulin in either the apical, basolateral, or bilateral chambers along with apical dye solutions. Samples (100 µl) were taken from the basolateral chamber every 30 min, and fluorescence was measured with a BioTek Synergy plate reader at excitation/emission/gain settings for calcein at 485 nm/515 nm/50 and Texas Red at 585 nm/615 nm/150 in a 96-well White/Clear Flat Bottom Falcon wellplate. Rates were calculated by plotting each monolayer’s flux vs. time, generating a linear curve, and then taking the slope of the line as the rate of flux reported as change in fluorescence over minutes. Transepithelial resistance (TER) measurements were made as described previously (55) before and after each experiment.
For the calcium switch assay, mature cells were incubated in high-calcium media (HCM, no. 10-010-CV; Corning) and low-calcium media (LCM, no. M8167; Sigma-Aldrich) for 24 h before assay. Before the assay (90 min), cells were washed, and media were changed to KRH buffer either with or without calcium to match the culturing conditions. At time 0 min, dye was added to the apical chamber with either calcium-containing or calcium-free prewarmed KRH buffer. Measurements were taken every 30 min and analyzed as above.
Inhibitor studies.
The CFTR-specific inhibitor CFTRInh172 (20 µM, no. 219674; EMD/Millipore) and the phosphatidylinositol 3-kinase (PI3-kinase)-specific inhibitor LY-294002 (20 µM, no. 9901; CST) were used to pretreat ALI cultures bilaterally for 30 min before insulin stimulation and flux/TER measurements and were included with the insulin stimulation solutions during the experiments. DMSO was used as the vehicle control for untreated samples. Protein lysates were collected after 15 min of stimulation/inhibition for pAkt analysis as above. TER and flux measurements were taken at the described time points and analyzed as above.
Statistics and preparation of Figs. 1–9.
All data analysis and statistical calculations were performed using GraphPad Prism version 6 for Windows for at least triplicate experiments, except where noted. Figures 1–9 were compiled using Adobe Photoshop Elements version 12.1 for Windows 7.
RESULTS
Murine lung fluid contains insulin and glucose.
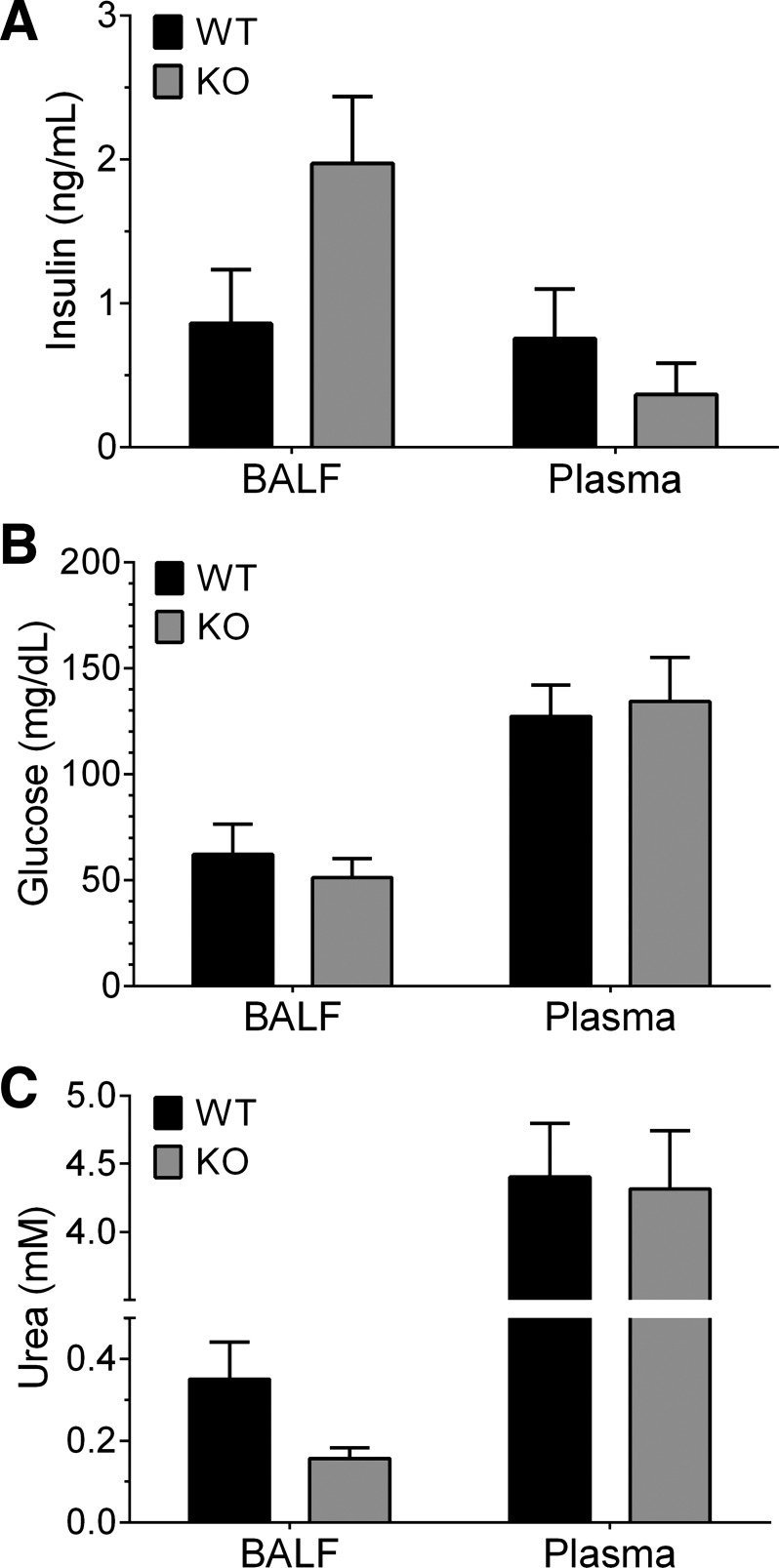
The detection and quantitation of metabolites in the lung lining fluid of humans, especially glucose, is currently controversial (8, 17, 32, 38, 56, 58, 70). To test if glucose and insulin could be detected in mouse BALF, we used an ELISA to measure insulin and an enzyme-coupled glucose oxidase assay to measure glucose concentrations in BALF from wild-type and CFTR-knockout (KO) mouse lungs (36). BALF from nondiabetic, nonfasted, wild-type mice contained an appreciable level of insulin (0.86 ± 0.37 ng/ml or 148 ± 64 pM) as shown in Fig. 1A, suggesting that airway insulin is present at baseline in vivo. CFTR-KO mice possessed an increased baseline insulin level (1.97 ± 0.46 ng/ml or 340 ± 80 pM), 2.3× higher than the wild-type amount, although not statistically significant (P = 0.059). In addition, there were no significant differences in plasma insulin levels between wild-type and CFTR-KO mice.
Fig. 1.
Insulin and glucose are detected in the bronchoalveolar lavage fluid (BALF) of wild-type littermates and gut-corrected cystic fibrosis transmembrane conductance regulator (CFTR)-knockout (KO) mice. BALF was collected from nonfasted nondiabetic CFTR-KO and wild-type (WT) littermate mice in two washes of 15 s each comprised of a PBS-based lavage fluid that contained inhibitors of glucose uptake and insulin degradation. A: mouse BALF was found to contain insulin. WT mouse BALF contained as much insulin as plasma, where KO mice showed more insulin in the BALF than in plasma. KO BALF contained nearly double the amount of insulin than WT BALF (P = 0.059). B: all mouse BALF was found to contain glucose at roughly one-half the amount found in plasma. C: urea was detected by a coupled enzymatic reaction and used to normalize the BALF insulin and glucose data. In A–C, data are reported from both BALF and blood plasma as means ± SE (n = 4 mice each) with statistics performed by unprotected two-way ANOVA Fisher’s least-significant difference (LSD) test.
Glucose also was readily detectable in urea-corrected BALF (Fig. 1B). There were no significant differences in BALF or plasma glucose in each of the groups; however, each group contained roughly 40% of the concentration of plasma glucose in the BALF. BALF glucose averaged 53.3 mg/dl (2.96 mM), and blood plasma glucose averaged 127 mg/dl (7.05 mM) across both groups. All BALF measurements were normalized to the concentration of urea, presumed to originate from the blood (Fig. 1C), where the reported normalized plasma levels of glucose and insulin were found to be in the normal range for mice (42, 61).
Human airway epithelial cells express apical insulin receptor and glucose transporters.
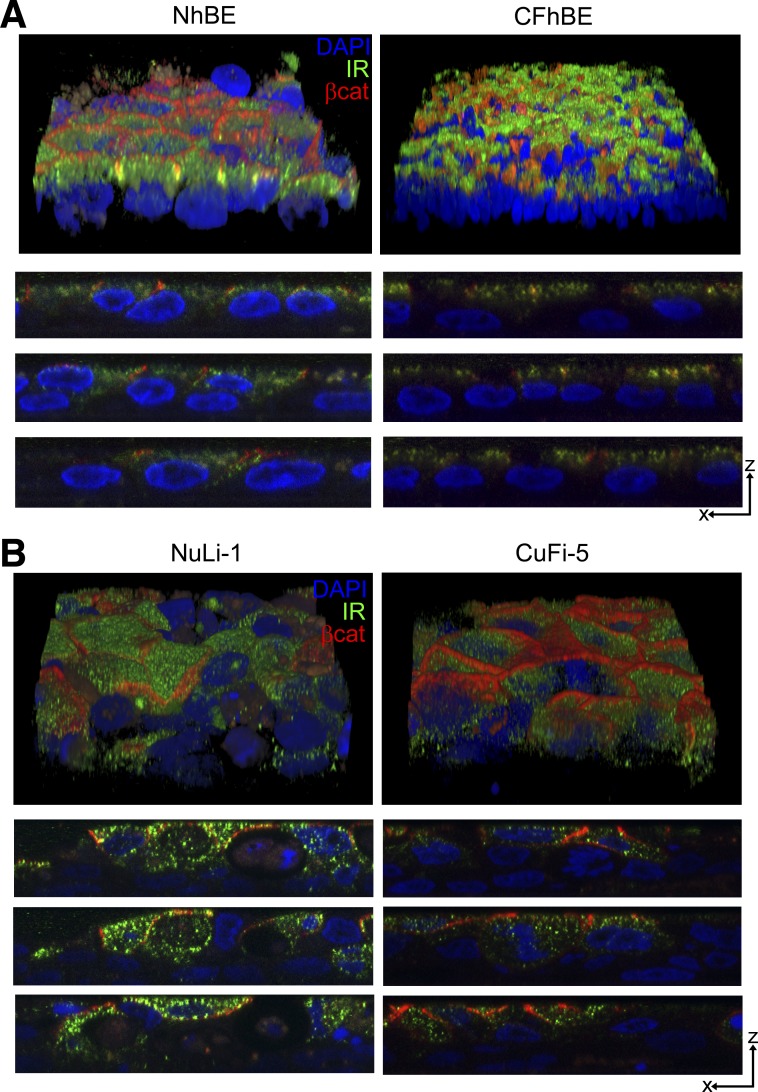
Because insulin is the key hormone disrupted in diabetes and compromised lung function is a significant pathology in CFRD, we examined whether human airway epithelial cells expressed insulin receptors and related proteins involved in insulin-stimulated signaling. As determined by Z-stacked immunofluorescence confocal microscopy (Fig. 2), normal, non-CF primary human airway cells (NhBE) and cystic fibrosis airway cells carrying the CFTRF508del/F508del mutation (CFhBE) expressed insulin receptors that were predominantly apically localized (Fig. 2A). Similarly, telomerase-immortalized non-CF NuLi-1 and CFTRF508del/F508del-expressing CuFi-5 human airway cells (55) expressed predominantly apically localized insulin receptors (Fig. 2B). Cells derived from non-CF and F508del-CFTR-expressing CF subjects had comparable insulin receptor expression and localization. This finding, that airway cells express apical insulin receptor, was surprising but is consistent with the data in Fig. 1 indicating that there may be conditions where the apical aspect of airway cells would respond to insulin present in ASL.
Fig. 2.
Insulin receptor is localized to the apical surface of human airway epithelial cells. A: pavement and XZ views of confocal immunofluorescence microscopy images of non-cystic fibrosis (CF) human bronchial epithelial (NhBE) and primary CF human bronchial epithelial (CFhBE) cells collected at 0.25-μm steps with a ×100 oil objective showing the localization of β-catenin (red) and insulin receptor (green) proteins. β-Catenin marks the apical junctional complex where insulin receptor is shown to be mostly lateral and apical to the nuclei (blue) in both NhBE and CFhBE cells. B: pavement and XZ views of telomerase-immortalized normal (NuLi-1) and cystic fibrosis (CuFi-5) airway cell images collected as in A.
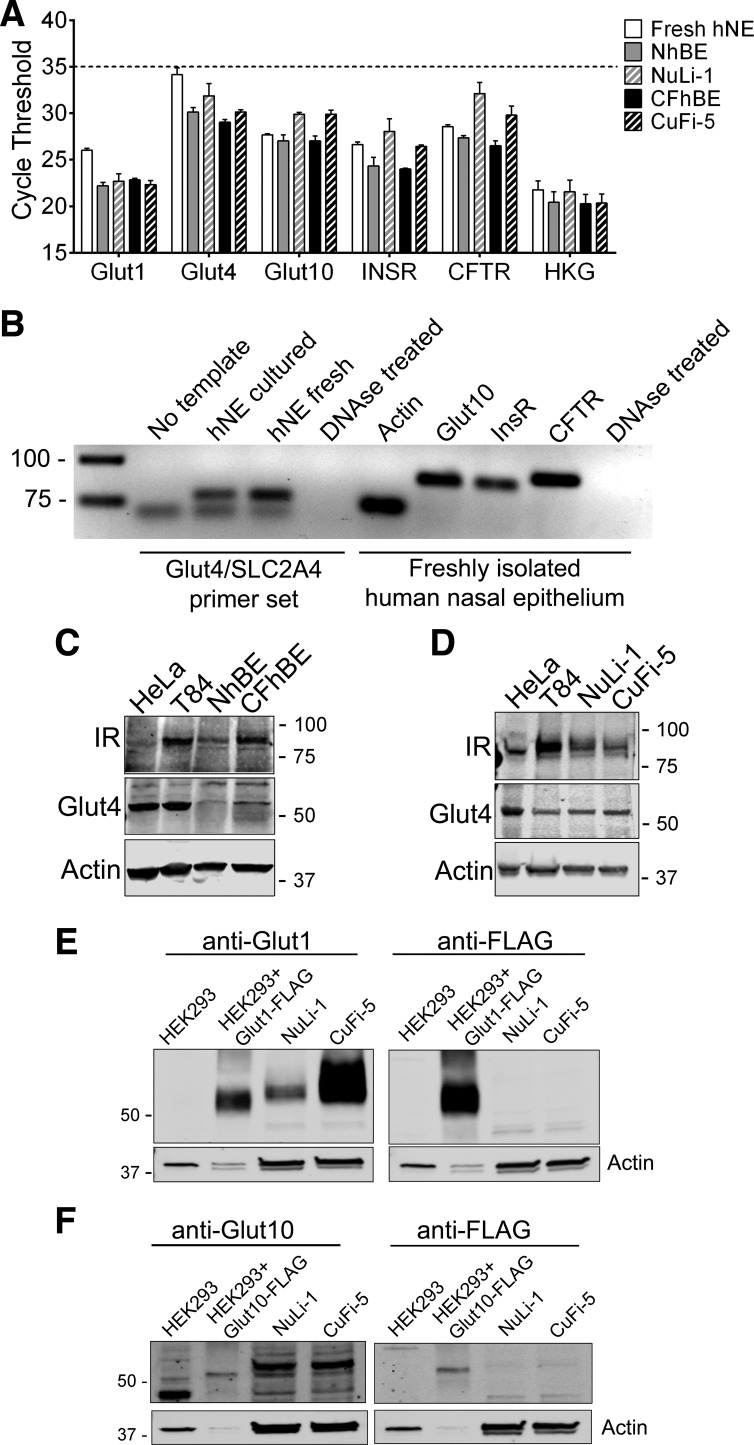
The proteins necessary for glucose transport were characterized by both qRT-PCR (Fig. 3A), mRNA expression (Fig. 3B), and immunoblot (Fig. 3, C–F) in normal and CFTRF508del/F508del-expressing airway cells. We isolated mRNA from freshly isolated human nasal epithelial cells obtained by nasal curettage, cultured human primary airway cells, and the NuLi-1 and CuFi-5 primary-like cell lines and analyzed them for cDNA products of Glut1 (SLC2A1), Glut4 (SLC2A4), Glut10 (SLC2A10), insulin receptor (INSR), cystic fibrosis transmembrane conductance regulator (CFTR), and an average of three housekeeping genes (GAPDH/ACTB/HPRT1). As shown in Fig. 3A, all airway cells tested expressed a broad complement of glucose transporters at appreciable levels similar to those seen in Pezzulo et al. (57) (Table 1). Analysis of the cDNA from the PrimePCR plates used in Fig. 3A by gel electrophoresis (Fig. 3B) shows that Glut4 cDNA is amplified with an apparent primer dimer since a nonspecific cDNA band is present in the no template control. This may account for the high Ct values observed in Fig. 3A. Most notably, freshly isolated nasal epithelial cells expressed INSR, SLC2A4 (Glut4), and the constitutive glucose transporter SLC2A10 (Glut10) (Fig. 3B). By immunoblot, insulin receptor (Fig. 3, C and D), Glut1 (Fig. 3E), and Glut10 (Fig. 3F) were all found to be expressed at the protein level. Notably, none of these cells, which are all noncancerous, expressed Glut2 (SLC2A2), despite previously published data suggesting expression (39) (Table 1). Differences in transcript abundance between normal and CF genotypes were generally small and did not reach statistical significance.
Fig. 3.
Human airway epithelial cells express proteins necessary for insulin-stimulated glucose transport. A: quantitative RT (qRT)-PCR analysis of a subset of glucose transporter and related gene transcripts in freshly obtained human nasal epithelial (hNE) cells and air-liquid interface-cultured primary human bronchial epithelial (hBE) cells. White, freshly isolated hNE; gray, NhBE; hatched gray, cultured NuLi-1 cells; black, CFhBE; hatched black, cultured CuFi-5 cells. HKG, average of three housekeeping genes (ACTB/GAPDH/HPRT1). The official gene symbols and common names of the full qRT-PCR screen are given in Table 1. A cycle threshold (Ct) result of 35 is considered not expressed (broken line). Data are shown as means ± SE; n = 4 freshly isolated nasal epithelial scrapes and n = 3 cultured filters from 3 different donors. B: gel electrophoresis results demonstrate a primer dimer in glucose transporter (Glut) 4 PrimePCR amplification products (left), which may explain the high Ct values observed; an additional band below the expected size was detected in reactions not containing template. The remaining genes detected produced single bands as PrimePCR amplified gene products in the freshly isolated nasal curretage samples (right). C and D: immunoblots demonstrating protein expression of the insulin receptor, β-subunit (IR-β), and insulin-stimulated Glut4 in primary hBE cells (C), T84 colon carcinoma cells, and HeLa cervical adenocarcinoma cells and NuLi-1 and CuFi-5 human airway cells (D). E: immunoblots demonstrating Glut1 expression in NuLi-1 and CuFi-5 human airway cells compared with HEK293 cell lysates containing overexpressed Glut1-FLAG protein. F: immunoblots demonstrating Glut10 expression in NuLi-1 and CuFi-5 human airway cells compared with HEK293 cell lysates containing overexpressed Glut10-FLAG protein.
Table 1.
Gene list and qRT-PCR results from freshly isolated nasal epithelia and cultured bronchial epithelia
| Gene | Fresh | Cultured | Protein |
|---|---|---|---|
| SLC2A1 | 26.0 ± 0.21 | 22.2 ± 0.38 | Solute carrier family 2 (facilitated glucose transporter), member 1; glucose transporter 1 |
| SLC2A2 | ND | ND | Solute carrier family 2 (facilitated glucose transporter), member 2; glucose transporter 2 |
| SLC2A3 | 28.5 ± 0.61 | 32.7 ± 1.95 | Solute carrier family 3 (facilitated glucose transporter), member 3; glucose transporter 3 |
| SLC2A4 | 34.2 ± 0.74 | 30.1 ± 0.48 | Solute carrier family 4 (facilitated glucose transporter), member 4; glucose transporter 4 |
| SLC2A5 | 31.5 ± 0.20 | 32.2 ± 1.93 | Solute carrier family 5 (facilitated glucose transporter), member 5; glucose transporter 5 |
| SLC2A6 | 33.6 ± 0.51 | 29.3 ± 0.51 | Solute carrier family 6 (facilitated glucose transporter), member 6; glucose transporter 6 |
| SLC2A7 | ND | ND | Solute carrier family 7 (facilitated glucose transporter), member 7; glucose transporter 7 |
| SLC2A8 | 30.6 ± 0.11 | 27.8 ± 0.70 | Solute carrier family 8 (facilitated glucose transporter), member 8; glucose transporter 8 |
| SLC2A9 | 27.4 ± 0.15 | 26.2 ± 0.35 | Solute carrier family 9 (facilitated glucose transporter), member 9; glucose transporter 9 |
| SLC2A10 | 27.7 ± 0.14 | 27.0 ± 0.64 | Solute carrier family 10 (facilitated glucose transporter), member 10; glucose transporter 10 |
| SLC2A11 | 27.8 ± 0.04 | 27.5 ± 0.09 | Solute carrier family 11 (facilitated glucose transporter), member 11; glucose transporter 11 |
| SLC2A12 | 26.7 ± 0.16 | 27.1 ± 0.50 | Solute carrier family 12 (facilitated glucose transporter), member 12; glucose transporter 12 |
| SLC2A13 | 26.7 ± 0.19 | 27.5 ± 0.50 | Solute carrier family 13 (facilitated glucose transporter), member 13; proton (H+) myoinositol symporter |
| SLC5A1 | 28.2 ± 0.46 | 28.3 ± 0.40 | Solute carrier family 5 (sodium/glucose cotransporter), member 1; sodium-glucose transporter 1 |
| SLC5A2 | ND | ND | Solute carrier family 5 (sodium/glucose cotransporter), member 2; sodium-glucose transporter 2 |
| SLC5A4 | ND | ND | Solute carrier family 5 (glucose-activated ion channel), member 4; sodium-glucose transporter 3 |
| CFTR | 28.6 ± 0.22 | 27.4 ± 0.23 | Cystic fibrosis transmembrane conductance regulator |
| INSR | 26.7 ± 0.25 | 24.3 ± 0.94 | Insulin receptor |
| ACTB | 21.0 ± 0.27 | 19.0 ± 0.72 | Actin, β |
| HPRT1 | 26.0 ± 0.19 | 24.8 ± 0.25 | Hypoxanthine guanine phosphoribosyltransferase 1 |
| GAPDH | 18.5 ± 0.17 | 17.5 ± 0.56 | Glyceraldehyde-3-phosphate dehydrogenase |
Cycle threshold (Ct) data are presented as means ± SE. The abbreviated dataset from qRT-PCR results is presented in Fig. 3. An autoadjusted cycle threshold value of ≥35 is considered not detected (ND). Fresh, freshly scraped nasal epithelial cells from non-cystic fibrosis (CF) donors; Cultured, non-CF human bronchial epithelial primary cells grown at the air-liquid interface for a minimum of 4 wk.
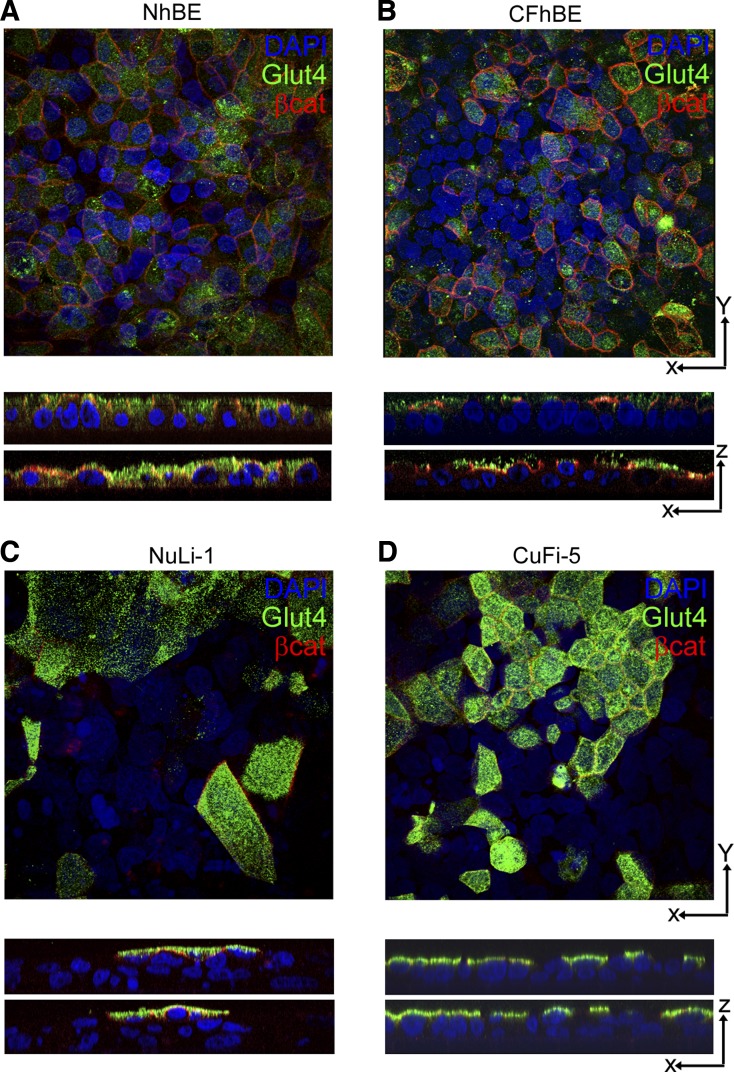
We then verified Glut4 protein expression in non-CF human donor trachea and bronchioles by immunohistochemistry (Fig. 4) and in cultured cells by immunofluorescence (Fig. 5). Glut4 was detected in the airway epithelial cells lining the trachea (Fig. 4A) and lower airway bronchioles (Fig. 4C) but was not detected in the surrounding connective tissues. Multiple capillaries were found within 50–100 μm of the epithelial layer that did not stain positive for Glut4 as expected; however, adipose tissue did show Glut4 labeling as anticipated (Fig. 4E). As observed by Z-stacked immunofluorescence confocal microscopy, Glut4 protein was detected in the apical spaces above the nuclei in both primary and immortalized human airway pseudostratified cell layers (Fig. 5). Taken together, mRNA, immunoblot, and localization data support the conclusion that the insulin receptor, Glut1, Glut4, and Glut10 are expressed in native human conducting airway epithelium and in air-liquid interface cultured cells at similar levels, and that these cells possess the proteins necessary to detect and respond to insulin in the ASL.
Fig. 5.
Primary and immortalized human airway epithelia exhibit apical organization of Glut4 storage vesicles. A and B: primary human airway epithelial cells without insulin stimulation show Glut4 (green) lateral to the nuclei (blue) in primary NhBE cells but show only apical organization relative to the nuclei in primary CFhBE cells. Apical β-catenin is pseudocolored in red to indicate cell borders. C and D: NuLi-1 and CuFi-5 cells showed prominent apical localization of Glut4 (green) relative to the nuclei (blue). Images were collected at ×60 magnification and generated with National Institutes of Health ImageJ software.
Airway cells exhibit insulin-stimulated glucose uptake that is impaired by F508del-CFTR.
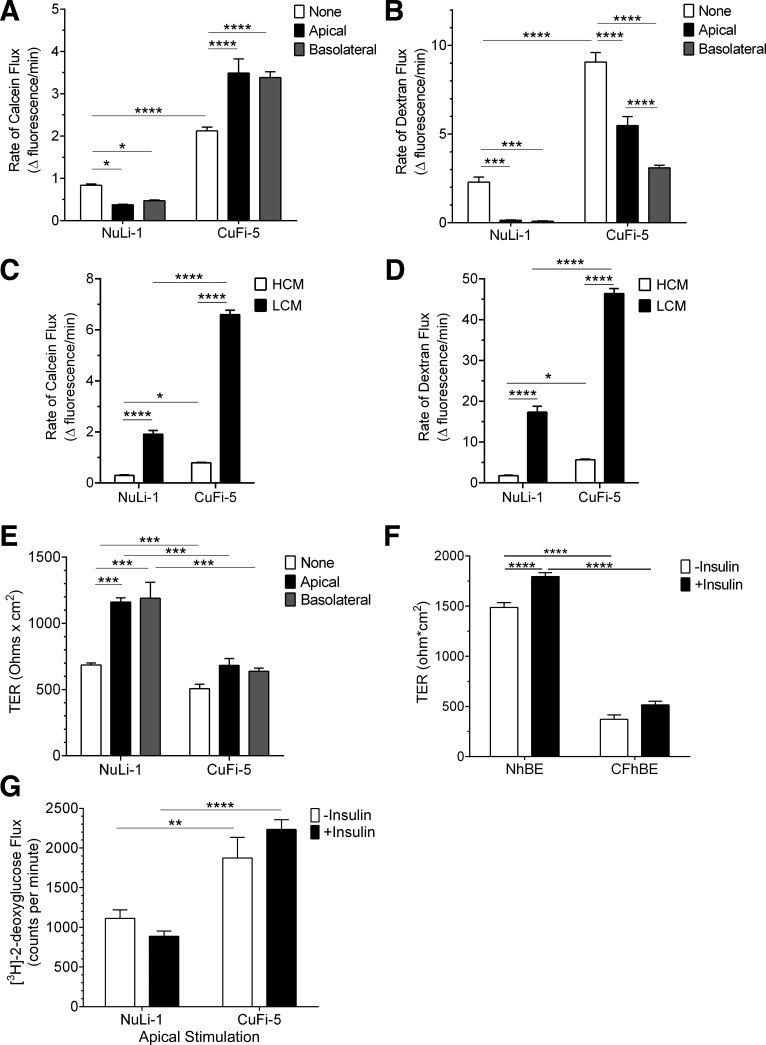
Airway cells were then further characterized to assess their response to insulin and whether this response stimulated glucose uptake. We used 2-deoxy-d-[3H]glucose as a nonmetabolizable proxy for transportable glucose, since uptake of this marker is a well-described insulin-stimulated function in other cell types (3, 4, 68). Application of bilateral insulin caused significant increases in 2-deoxy-d-[3H]glucose uptake by both NhBE cells (1.7-fold, Fig. 6A) and NuLi-1 cells (3.3-fold, Fig. 6B). In contrast to cells expressing wild-type CFTR, insulin did not stimulate 2-deoxy-d-[3H]glucose uptake by cells expressing F508del-CFTR, as shown in CFhBE cells (Fig. 6A) and CuFi-5 cells (Fig. 6B). Between 55 and 64% of insulin-stimulated 2-deoxy-d-[3H]glucose uptake in NhBE and NuLi-1 cells was inhibited by pretreatment with cytochalasin B, a mycotoxin used to inhibit insulin-stimulated glucose uptake by blocking fusion of Glut4 storage vesicles (GSVs) with the apical plasma membrane (10, 20). Cytochalasin had no significant effect on CFhBE or CuFi-5 cells. Stimulation of 2-deoxy-d-[3H]glucose uptake began as soon as 20 min after exposure to insulin, effectively increasing the rate of uptake in NuLi-1 cells but not in CuFi-5 cells (Fig. 6C). Both NhBE and NuLi-1 cells expressed Glut4 (Figs. 3 and 5), consistent with activation of this transporter as a mechanism for insulin-stimulated glucose uptake. Consistent with the predominantly apical localization of the insulin receptor (Fig. 2), NuLi-1 cells demonstrated a significant uptake of glucose from the apical side when stimulated with apically applied insulin (Fig. 6D). Conversely, CF airway cells did not respond to apically applied insulin (Fig. 6D) despite CFhBE and CuFi-5 cells expressing apical insulin receptors (Fig. 2) and apical GSVs (Fig. 5). Taken together, these data suggest that the critical insulin-responsive protein machinery is in place within airway epithelial cells regardless of whether they have wild-type or mutant CFTR. Thus a defective insulin signaling cascade in F508del CFTR-expressing cells is likely to contribute to the lack of insulin-stimulated glucose uptake.
Insulin-stimulated increases in airway barrier function are impaired by F508del-CFTR.
In addition to glucose transporters regulating the transcellular route, paracellular flux of glucose (referred to as glucose leak) can also alter glucose concentration in the vertebrate airway. In fact, paracellular flux of glucose metabolites (in the same size range of 0.18 kDa) has previously been demonstrated using human airway cells in coculture with live Pseudomonas aeruginosa bacteria where l-glucose flux was higher in CF airway cells at baseline and increased with P. aeruginosa treatment (25). Consistent with this idea, F508del-CFTR-expressing human airway epithelial cells have leakier barrier function than cells expressing wild-type CFTR (48, 55, 60, 71), suggesting the potential for increased penetration of small metabolites in the airways of CF lungs. However, whether glucose permeability (leak) across the airway epithelium is influenced by insulin is unknown (65).
We examined the effects of insulin on airway barrier function using two fluorescent tracer molecules, calcein (0.63 kDa) and Texas Red-labeled dextran (10 kDa) (Fig. 7, A and B), to simultaneously ascertain flux rates of differently sized molecules. In NuLi-1 cells, insulin treatment applied either apically or basolaterally significantly decreased the paracellular flux of both calcein and dextran. Moreover, insulin increased TER of NuLi-1 cells ~1.7-fold when applied either apically or basolaterally (Fig. 7E) and significantly increased the barrier function in NhBE cells (Fig. 7F), indicating another measure of barrier tightening. Taken together, these data provide the first demonstration that stimulating the insulin receptor has the capacity to improve airway tight junction barrier function in epithelial cells expressing wild-type CFTR.
Fig. 7.
Insulin enhances normal airway epithelial barrier function but diminishes barrier function of F508del-CFTR airway cells. A: paracellular permeability flux measurements indicated that insulin (white bars, untreated; black bars, apical insulin; gray bars, basolateral insulin) significantly decreased flux of calcein in NuLi-1 cells while significantly increasing paracellular calcein flux in CuFi-5 cells. B: paracellular flux measurements of dextran indicated that both NuLi-1 and CuFi-5 decreased flux of dextran in response to insulin treatment. For both calcein and dextran paracellular flux rate datasets, n = 20 filters/condition. C and D: a calcium-switch assay was employed to assess the contribution of the paracellular barrier mediated by claudins to the flux of calcein (C) or dextran (D) through NuLi-1 and CuFi-5 monolayers. High-calcium media (HCM) demonstrate that CuFi-5 monolayers maintain a higher flux at baseline compared with NuLi-1 monolayers. E: in NuLi-1 cells, either apical or basolateral insulin treatment induced an increase in transepithelial resistance (TER) compared with untreated cells; n = 4 filters for each bar. F: in primary NhBE cells (n = 9), bilateral insulin treatment results in a significant increase in TER, whereas in primary CFhBE cells (n = 4) this increase does not occur. G: an apical solution containing insulin and 2-deoxy-d-[3H]glucose was applied for 60 min to measure glucose flux through both NuLi-1 and CuFi-5 monolayers on filters by scintillation counting; n = 12 filters for each bar. All data are shown as means ± SE and where *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 by unprotected two-way ANOVA Fisher’s LSD test.
In contrast with NuLi-1 cells, paracellular flux measurements of CuFi-5 cells revealed an insulin-sensitive selective paracellular leak pathway for small molecules such as 0.63 kDa calcein (Fig. 7A). Insulin added either apically or basolaterally significantly increased calcein flux by ~60% in CuFi-5 cell layers compared with an ~50% decrease in NuLi-1 cell layers. However, this was not the case for 10 kDa dextran, where paracellular flux for CuFi-5 cells was significantly decreased with insulin treatment, although dextran flux remained well above that in NuLi-1 cells (Fig. 7B).
Because we saw a differential effect of insulin on the flux of calcein and dextran in F508del-CFTR-expressing cells, we examined the effect of calcium depletion on transepithelial flux since this treatment causes tight junctions to disassemble, enabling an unimpeded path for paracellular flux (35, 67). Calcium-depleted NuLi-1 and CuFi-5 cells showed increased flux of both calcein (Fig. 7C) and dextran (Fig. 7D), indicating that the differential effects of insulin on flux of these two differently sized molecules was not an inherent property of the cells. Interestingly, the maximum flux of CuFi-5 cells was higher than that of NuLi-1 cells, in support of the notion that cells expressing F508del-CFTR are predisposed to be leakier than non-CF cells.
TER measurements of CuFi-5 cells (Fig. 7E) and primary human CFhBE (Fig. 7F) showed no significant change in response to insulin. Although the effects of insulin on CuFi-5 and CFhBE cell barrier function were complex, there is precedence for differential regulation of TER and paracellular flux, particularly when comparing differently sized tracers (5, 9, 44, 69).
We then investigated whether insulin had an effect on flux of 2-deoxy-d-[3H]glucose across the airway barrier, which has the capacity of moving through both the paracellular and transcellular routes (Fig. 7G). Glucose flux across CuFi-5 monolayers was consistently higher than across NuLi-1 monolayers. Although not significant, apical stimulation reduced the amount of glucose flux through NuLi-1 monolayers and increased the flux in CuFi-5 monolayers. This defect could allow metabolites to leak into and accumulate within the apical space of a CF airway. Taken together, these data support the hypothesis that insulin regulates the normal concentration of airway glucose by the combination of increased glucose uptake and increased tight junction barrier function, which we refer to collectively as the “airway glucose barrier.”
Claudins are important protein components responsible for paracellular tight junction sealing, so derangements in claudins could explain the differences in rates of paracellular flux. Expression of the major airway claudins comparing normal and F508del-CFTR-expressing cells demonstrated possible derangements of claudin expression and tight junction composition (55). Whether differences in claudin expression and tight junction organization that occur in CF airway cells affect the function of the airway glucose barrier remain to be determined and may explain the reduction of the airway glucose barrier in CF airway epithelial cells.
Activation of the Akt signaling pathway by insulin is impaired in F508del-CFTR expressing cells.
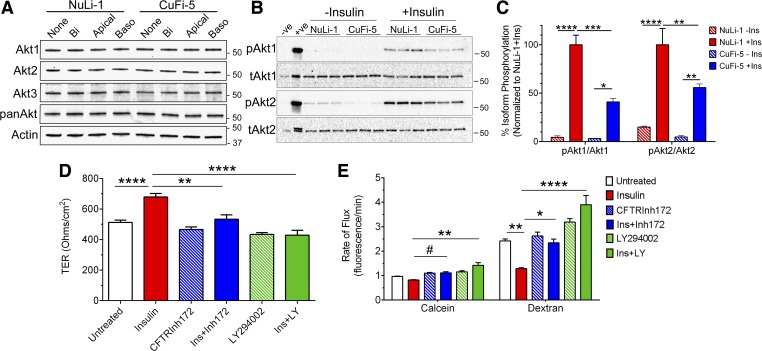
Because insulin can stimulate functional effects in both glucose uptake and paracellular transport in human airway epithelia and because insulin transduces signals through the PKB/Akt pathway (3), PKB/Akt activity was measured in response to insulin. Human airway epithelia express all three isoforms of Akt, which are Akt1, Akt2, and Akt3 (Fig. 8A). The phosphorylated forms of Akt represent the active kinase, but reliable phosphospecific antibodies for Akt3 are lacking, so we chose to focus on Akt1, pAkt1, Akt2, and pAkt2. Stimulation with insulin did not affect the observed total abundance of either isoform, and the addition of insulin stimulated phosphorylation of Akt1 and Akt2 in NuLi-1 and CuFi-5 cells (Fig. 8B), as expected. Addition of 125 nM bilateral insulin resulted in the phosphorylation of Akt1 to a much greater degree than Akt2 in both NuLi-1 cells and CuFi-5 cells (Fig. 8C); the response to insulin for both Akt1 and Akt2 was higher in NuLi-1 cells compared with CuFi-5 cells.
Fig. 8.
Functional phosphatidylinositol 3-kinase [PI3-kinase (PI3K)] and CFTR are required for protein kinase B (Akt) 2-stimulated paracellular barrier tightening in human airway epithelial cells. A: human airway epithelial cells express all three isoforms of PKB/Akt as shown by immunoblot. Treatment with bilateral (Bi), apical, or basolateral (Baso) insulin did not change total amounts of Akt protein. B: immunoblot of phospho-Akt1 and phospho-Akt2 after 30 min stimulation with bilateral 125 nM insulin. Calyculin A-treated phosphorylation-positive control Jurkat cells (+ve) were used as a control for phospho-Akt. C: fold-increase of phospho-Akt in n = 3 filters as in B. Akt stimulation was significantly higher for NuLi-1 cells than for CuFi-5 cells. D: insulin treatment significantly increased TER in NuLi-1 cells (red bar). Pretreatment with CFTRInh172 before and during insulin stimulation significantly diminished the insulin-stimulated increase in TER (blue bars). The insulin-stimulated increase in TER of NuLi-1 cells also was prevented by LY-294002 treatment (green bars). E: pretreatment with CFTRInh172 before insulin stimulation also prevented the decrease in the rates of paracellular flux of both calcein (blue bar, left) and dextran (blue bar, right) that is normally seen with insulin alone (red bars). NuLi-1 cells pretreated with LY-294002 responded to subsequent exposure to insulin with an increase of both calcein (green bar, left) and dextran (green bar, right) flux, whereas insulin alone (red bar) selectively decreased paracellular flux of dextran (right). All data are shown as means ± SE and n = 3–4 experiments for each data point for each graph where P ≤ 0.05 (*), 0.01 (**), 0.001 (***), 0.0001 (****), and 0.06 (#) by unprotected two-way ANOVA Fisher’s LSD test.
Insulin signaling is dysfunctional in diabetes where Akt2 plays a role in regulating glucose metabolism. We used two approaches to determine if insulin signaling is dysfunctional in airway epithelia with reduced CFTR function. The first approach was to inhibit CFTR channels in normal NuLi-1 cells (Fig. 8, D and E). In NuLi-1 cells, insulin stimulation increased TER (Fig. 8D) and simultaneously decreased paracellular permeability (Fig. 8E) compared with DMSO-treated vehicle control cells. Interestingly, inhibition of CFTR with CFTRInh172 before and during insulin stimulation prevented the insulin-stimulated increase in TER (Fig. 8D) and prevented the decrease in paracellular transport (Fig. 8E) that normally occurs in normal human airway cells in response to insulin. In the second approach, LY-294002 treatment to inhibit Akt signaling also prevented the insulin-mediated increase in TER (Fig. 8D) while simultaneously increasing paracellular permeability of tracer molecules (Fig. 8E). These results suggest that functional CFTR and Akt activation is a necessary component for the normal insulin stimulation of the glucose barrier in human airway epithelial cells.
Taken together, these results support the hypothesis that insulin-mediated regulation of paracellular permeability via the PI3-kinase/PKB/Akt pathway requires functional CFTR activity to achieve proper airway protection from metabolite accumulation. Coupled to the loss of insulin-mediated glucose clearance from CF monolayers, the observation that CF-affected airway epithelia do not respond appropriately to insulin suggests the potential for accumulation of metabolites and serum proteins in the CF airspace (Fig. 9). Thus the airway glucose barrier we defined here is regulated by insulin and acts to protect the airspace in the normal airway, but may be defective in the CF airway.
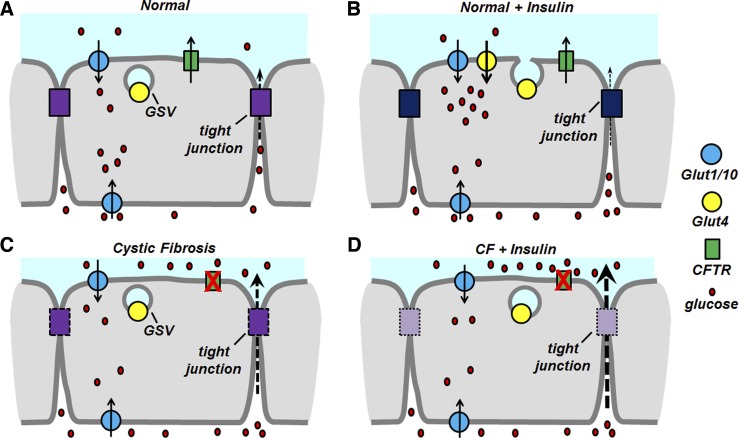
Fig. 9.
Model for CFTR-mediated regulation of the airway glucose barrier. A: in normal airway epithelia without insulin stimulation, nutrients like glucose (red) slowly traverse the tight junction-regulated paracellular pathway (purple) while constitutive glucose transporters (blue) mediate baseline glucose metabolism and clearance from the paracellular space. Activity of CFTR (green) is normal, whereas Glut4 transporter (yellow) is stored in GSVs near the apical plasma membrane. B: airway epithelia stimulated with insulin decrease the rate of paracellular flux while simultaneously reducing the amount of glucose in the apical space through the activity of translocated Glut4 in the apical plasma membrane. C: in cystic fibrosis airway epithelia, the paracellular route is less stringent (dashed purple box) compared with normal epithelia. This results in more glucose being transported through the tight junction-mediated barrier, where the constitutive glucose transporters act to reduce the amount of glucose in the apical space. CFTR activity is reduced, effectively changing the fluid and ion balance of CF airway epithelia. D: in CF epithelia, insulin fails to activate GSV translocation, effectively reducing insulin-stimulated Glut4 activity while simultaneously allowing small molecules <10 kDa through the paracellular barrier, effectively allowing nutrient accumulation in the apical space.
DISCUSSION
We found that normal human airway epithelia express functional insulin receptors and that at least two insulin-dependent pathways have the capacity to regulate airspace glucose: 1) uptake of glucose at the apical membrane and 2) regulation of paracellular leak. These insulin-dependent pathways are impaired in airway cells expressing F508del-CFTR. This finding has potentially important implications for CF, diabetic, and CFRD patients.
In the CF lung, bacterial infections dominate the airway where primary defects in CFTR enable acute bacterial infections to become chronic. Limiting a source of nutrients for airway bacteria presents a novel way to treat a contributing cause of CF and CFRD lung exacerbations that result in irreversible lung damage and decreased lung function. Bacterial infections, in turn, also induce proinflammatory mediators that further impair the paracellular barrier and recruit immune cells to airspaces, such as neutrophils, that can become hyperresponsive by using monosaccharides and amino acids as sources of energy (47).
The effect of insulin signaling in normal airway epithelia is consistent with a protective response in that glucose is removed from the apical surface and the paracellular barrier becomes tighter. The data presented here demonstrate that insulin sensed by airway cells, either apically or basolaterally, acts through the PI3-kinase/PKB pathway to stimulate apical glucose uptake through the insulin-responsive glucose transporter Glut4 and to lower the rate of paracellular flux of molecules (Fig. 9B). At baseline, CF airway epithelial cells took up less glucose and possessed a higher rate of paracellular flux than normal airway epithelia (Fig. 9C). Upon stimulation with insulin, CF airway epithelia did not exhibit the protective response that normal airway cells did but rather enabled a selective leak of small molecules <10 kDa (such as 5.8 kDa insulin and 0.18 kDa glucose) through the paracellular route. Insulin stimulation also failed to trigger glucose uptake in CF airway epithelia (Fig. 9D). The combination of decreased glucose uptake and increased paracellular leak represents a potential mechanism to explain how bacteria are able to colonize the CF lung so easily, in combination with defects in innate and adaptive immune responses. Tight junction-mediated control of glucose flux is influenced by AMP-activated protein kinase (AMPK) signaling in non-CF airway epithelia where inhibition of AMPK signaling decreased TER and increased paracellular flux of l-glucose associated with Staphylococcus aureus coculture (23). Additionally, regulation of the tight junction barrier in the gut was shown to be controlled, in part, by glutamine where glutamine deprivation resulted in an Akt-dependent process where tight junctions were disassembled (49). Therefore, control of the tight junction barrier by nutrient-sensing signal transduction pathways could also be regulated by different nutrients present in the airway.
Insulin stimulates glucose metabolism, which is controlled in part by the downstream Akt2 kinase of the Akt/PKB pathway (15). We analyzed Akt activity as a possible mechanism controlling both paracellular flux and Glut4 activity; our results indicate that insulin-stimulated activation of both Akt1 and Akt2 is reduced in CF cells compared with non-CF cells (Fig. 8C). Akt1 and Akt2 represent the two most studied isoforms of the PKB pathway (19, 21). Akt knockout mice have defined the nonredundant activities of the three isoforms of Akt. Akt1 is known for its growth regulation and is needed for proper fetal development (21, 34, 41). By contrast, Akt2 has been described as the master regulator for glucose metabolism where Akt2 KO mice have resting hyperglycemia and weight gain (19, 37). Akt3 has roles in the brain, but this isoform also possesses functions redundant to Akt1 and Akt2 (19). Akt3 may be an important regulator of the pathways leading to fibrosis in lung fibroblasts (31), and, once reliable anti-pAkt3 antibodies become available, future studies will determine whether Akt3 has a unique function in airway epithelia.
Insulin stimulation resulted in strong Akt activation in normal airway epithelia that was diminished in CF airway cells (Fig. 8). The decreased protective function of insulin in CF cells was manifest as lower glucose uptake and a selective increase in paracellular flux of small molecules. This has the potential to lead to nutrient accumulation and availability in the airway lining fluid. Although we do not have an exact measure of glucose concentration in the ASL of insulin-stimulated airway epithelial cultures, we can predict that glucose levels will rise in the ASL of F508del-CFTR-expressing airway epithelia by analogy with previously published data showing increases in ASL glucose when basolateral glucose was increased (25, 26). Any failure of the insulin-stimulated glucose barrier as described here would likely exacerbate ASL glucose accumulation. Interestingly, our data support the idea that insulin and glucose are tightly regulated in the mouse airway (Fig. 1) and in human airway cell cultures (Fig. 6).
To our knowledge, we present the first demonstration that CFTR has an influence on insulin-stimulated PI3-kinase/Akt signaling. Membrane-localized CFTR is known to act as a hub for signal transduction of many other signal transducer proteins such as the kinases PKA, PKC, MAPK, ERK, and c-Src, the phosphatases PP2A and WNK1, and various phosphodiesterases affecting calcium and cAMP levels within the cell (7, 46). Whether or not PKB signaling in airway epithelium occurs solely through CFTR, or vice versa, is unknown at present. However, PKB (Akt) signaling has been implicated in controlling the hyperinflammatory status of CF macrophages (74), contributing to lipoxin A4-mediated alveolar fluid clearance (73), and mediating the inflammatory responses of platelets (50) and epithelial cells (12). Both PKB signaling and CFTR function are likely to be required for proper airway epithelial cell responses to insulin, where dysfunction in one or the other results in a diminished response as demonstrated here in normal NuLi-1 airway cells inhibited with either CFTRInh172 or LY-294002 (Fig. 8).
Taken together, our results are the first molecular demonstration of insulin-stimulated Glut4 activity in the airway through the insulin receptor and PKB/Akt signaling pathways. Our data also establish a link between insulin, PI3-kinase/Akt, and CFTR activities, which regulate the airway glucose barrier. The effects of systemic insulin replacement on metabolism in CFRD are profoundly positive for patients (13, 40); however, our data raise the possibility that insulin has a deleterious effect in the CF airway despite the overall benefit of controlling systemic glucose in CF patients.
GRANTS
This research project was supported in part by the Emory University Integrated Cellular Imaging Microscopy Core of the Emory+Children's Pediatric Research Center and the Winship Research Pathology Core of Emory University School of Medicine. This study was also supported in part by grants from Cystic Fibrosis Foundation (MCCART13I0, MCCART13P0, and MCCART14R0) and a CF@LANTA RDP Center Grant (MCCART15R0), from the National Heart, Lung, and Blood Institute (R01-HL-116958 to M. Koval and T32-HL-116271 to S. A. Molina), by the Emory+Children’s Center for Cystic Fibrosis and Airways Disease Research, and by the Emory+Children’s Marcus Professorship of Cystic Fibrosis research fund.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
SM, MK, JH and NM developed study concept and experimental design. Experiments were performed by SM, HM, BI, RV, AK, WH, JH, and DI. SM wrote the manuscript and compiled the figures. All authors edited and/or approved the final manuscript.
ACKNOWLEDGMENTS
We thank Assem G. Ziady for contributions of primary human airway epithelial cells for the study and Drs. Arlene Stecenko, Steven Goudy, and Roy Rajan for contributions to the nasal curettage. CF mice were provided by the Children's Healthcare of Atlanta and Emory University CF Animal Models Core. Cells were provided jointly by the Children's Healthcare of Atlanta and Emory University CF Discovery Core and the CF@LANTA RDP Experimental Support Models Core.
Present Address for J. M. Hansen: Brigham Young University, Dept. of Physiology and Developmental Biology, Provo, Utah.
REFERENCES
- 1.Adam D, Roux-Delrieu J, Luczka E, Bonnomet A, Lesage J, Mérol JC, Polette M, Abély M, Coraux C. Cystic fibrosis airway epithelium remodelling: involvement of inflammation. J Pathol 235: 408–419, 2015. doi: 10.1002/path.4471. [DOI] [PubMed] [Google Scholar]
- 2.Aldallal N, McNaughton EE, Manzel LJ, Richards AM, Zabner J, Ferkol TW, Look DC. Inflammatory response in airway epithelial cells isolated from patients with cystic fibrosis. Am J Respir Crit Care Med 166: 1248–1256, 2002. doi: 10.1164/rccm.200206-627OC. [DOI] [PubMed] [Google Scholar]
- 3.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev 8: 55–62, 1998. doi: 10.1016/S0959-437X(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 4.Alessi DR, Downes CP. The role of PI 3-kinase in insulin action. Biochim Biophys Acta 1436: 151–164, 1998. doi: 10.1016/S0005-2760(98)00133-7. [DOI] [PubMed] [Google Scholar]
- 5.Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115: 4969–4976, 2002. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1: a002584, 2009. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antigny F, Norez C, Becq F, Vandebrouck C. CFTR and Ca signaling in cystic fibrosis. Front Pharmacol 2: 67, 2011. doi: 10.3389/fphar.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker EH, Clark N, Brennan AL, Fisher DA, Gyi KM, Hodson ME, Philips BJ, Baines DL, Wood DM. Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J Appl Physiol (1985) 102: 1969–1975, 2007. doi: 10.1152/japplphysiol.01425.2006. [DOI] [PubMed] [Google Scholar]
- 9.Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 134: 1031–1049, 1996. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basketter DA, Widdas WF. Asymmetry of the hexose transfer system in human erythrocytes. Comparison of the effects of cytochalasin B, phloretin and maltose as competitive inhibitors. J Physiol 278: 389–401, 1978. doi: 10.1113/jphysiol.1978.sp012311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basset G, Saumon G, Bouchonnet F, Crone C. Apical sodium-sugar transport in pulmonary epithelium in situ. Biochim Biophys Acta 942: 11–18, 1988. doi: 10.1016/0005-2736(88)90269-6. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya S, Balakathiresan NS, Dalgard C, Gutti U, Armistead D, Jozwik C, Srivastava M, Pollard HB, Biswas R. Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J Biol Chem 286: 11604–11615, 2011. doi: 10.1074/jbc.M110.198390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan AL, Beynon J. Clinical updates in cystic fibrosis-related diabetes. Semin Respir Crit Care Med 36: 236–250, 2015. doi: 10.1055/s-0035-1547319. [DOI] [PubMed] [Google Scholar]
- 14.Brennan AL, Gyi KM, Wood DM, Johnson J, Holliman R, Baines DL, Philips BJ, Geddes DM, Hodson ME, Baker EH. Airway glucose concentrations and effect on growth of respiratory pathogens in cystic fibrosis. J Cyst Fibros 6: 101–109, 2007. doi: 10.1016/j.jcf.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB III, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728–1731, 2001. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 16.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell 13: 3218–3234, 2002. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Prost N, Saumon G. Glucose transport in the lung and its role in liquid movement. Respir Physiol Neurobiol 159: 331–337, 2007. doi: 10.1016/j.resp.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Devaskar SU, deMello DE. Cell-specific localization of glucose transporter proteins in mammalian lung. J Clin Endocrinol Metab 81: 4373–4378, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol 26: 8042–8051, 2006. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebstensen RD, Plagemann PG. Cytochalasin B: inhibition of glucose and glucosamine transport. Proc Natl Acad Sci USA 69: 1430–1434, 1972. doi: 10.1073/pnas.69.6.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke TF. PI3K/Akt: getting it right matters. Oncogene 27: 6473–6488, 2008. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 22.Garnett JP, Baker EH, Baines DL. Sweet talk: insights into the nature and importance of glucose transport in lung epithelium. Eur Respir J 40: 1269–1276, 2012. doi: 10.1183/09031936.00052612. [DOI] [PubMed] [Google Scholar]
- 23.Garnett JP, Baker EH, Naik S, Lindsay JA, Knight GM, Gill S, Tregoning JS, Baines DL. Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose. Thorax 68: 835–845, 2013. doi: 10.1136/thoraxjnl-2012-203178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garnett JP, Braun D, McCarthy AJ, Farrant MR, Baker EH, Lindsay JA, Baines DL. Fructose transport-deficient Staphylococcus aureus reveals important role of epithelial glucose transporters in limiting sugar-driven bacterial growth in airway surface liquid. Cell Mol Life Sci 71: 4665–4673, 2014. doi: 10.1007/s00018-014-1635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garnett JP, Gray MA, Tarran R, Brodlie M, Ward C, Baker EH, Baines DL. Elevated paracellular glucose flux across cystic fibrosis airway epithelial monolayers is an important factor for Pseudomonas aeruginosa growth. PLoS One 8: e76283, 2013. doi: 10.1371/journal.pone.0076283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garnett JP, Kalsi KK, Sobotta M, Bearham J, Carr G, Powell J, Brodlie M, Ward C, Tarran R, Baines DL. Hyperglycaemia and Pseudomonas aeruginosa acidify cystic fibrosis airway surface liquid by elevating epithelial monocarboxylate transporter 2 dependent lactate-H(+) secretion. Sci Rep 6: 37955, 2016. doi: 10.1038/srep37955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garnett JP, Nguyen TT, Moffatt JD, Pelham ER, Kalsi KK, Baker EH, Baines DL. Proinflammatory mediators disrupt glucose homeostasis in airway surface liquid. J Immunol 189: 373–380, 2012. doi: 10.4049/jimmunol.1200718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill SK, Hui K, Farne H, Garnett JP, Baines DL, Moore LS, Holmes AH, Filloux A, Tregoning JS. Increased airway glucose increases airway bacterial load in hyperglycaemia. Sci Rep 6: 27636, 2016. doi: 10.1038/srep27636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould GW, Holman GD. The glucose transporter family: structure, function and tissue-specific expression. Biochem J 295: 329–341, 1993. doi: 10.1042/bj2950329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525–569, 2013. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo J, Yao H, Lin X, Xu H, Dean D, Zhu Z, Liu G, Sime P. IL-13 induces YY1 through the AKT pathway in lung fibroblasts. PLoS One 10: e0119039, 2015. doi: 10.1371/journal.pone.0119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hameed S, Jaffé A, Verge CF. Advances in the detection and management of cystic fibrosis related diabetes. Curr Opin Pediatr 27: 525–533, 2015. doi: 10.1097/MOP.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 33.Hardin DS, Ahn C, Rice J, Rice M, Rosenblatt R. Elevated gluconeogenesis and lack of suppression by insulin contribute to cystic fibrosis-related diabetes. J Investig Med 56: 567–573, 2008. doi: 10.2310/JIM.0b013e3181671788. [DOI] [PubMed] [Google Scholar]
- 34.Héron-Milhavet L, Franckhauser C, Rana V, Berthenet C, Fisher D, Hemmings BA, Fernandez A, Lamb NJ. Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol Cell Biol 26: 8267–8280, 2006. doi: 10.1128/MCB.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci 116: 725–742, 2003. doi: 10.1242/jcs.00300. [DOI] [PubMed] [Google Scholar]
- 36.Hunt WR, Zughaier SM, Guentert DE, Shenep MA, Koval M, McCarty NA, Hansen JM. Hyperglycemia impedes lung bacterial clearance in a murine model of cystic fibrosis-related diabetes. Am J Physiol Lung Cell Mol Physiol 306: L43–L49, 2014. doi: 10.1152/ajplung.00224.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen PJ, Gunter LB, Carayannopoulos MO. Akt2 modulates glucose availability and downstream apoptotic pathways during development. J Biol Chem 285: 17673–17680, 2010. doi: 10.1074/jbc.M109.079343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalsi KK, Baker EH, Fraser O, Chung YL, Mace OJ, Tarelli E, Philips BJ, Baines DL. Glucose homeostasis across human airway epithelial cell monolayers: role of diffusion, transport and metabolism. Pflugers Arch 457: 1061–1070, 2009. doi: 10.1007/s00424-008-0576-4. [DOI] [PubMed] [Google Scholar]
- 39.Kalsi KK, Baker EH, Medina RA, Rice S, Wood DM, Ratoff JC, Philips BJ, Baines DL. Apical and basolateral localisation of GLUT2 transporters in human lung epithelial cells. Pflugers Arch 456: 991–1003, 2008. doi: 10.1007/s00424-008-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly A, Moran A. Update on cystic fibrosis-related diabetes. J Cyst Fibros 12: 318–331, 2013. doi: 10.1016/j.jcf.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Kim YB, Peroni OD, Franke TF, Kahn BB. Divergent regulation of Akt1 and Akt2 isoforms in insulin target tissues of obese Zucker rats. Diabetes 49: 847–856, 2000. doi: 10.2337/diabetes.49.5.847. [DOI] [PubMed] [Google Scholar]
- 42.Klueh U, Liu Z, Cho B, Ouyang T, Feldman B, Henning TP, Kaur M, Kreutzer D. Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technol Ther 8: 402–412, 2006. doi: 10.1089/dia.2006.8.402. [DOI] [PubMed] [Google Scholar]
- 43.Koval M. Claudin heterogeneity and control of lung tight junctions. Annu Rev Physiol 75: 551–567, 2013. doi: 10.1146/annurev-physiol-030212-183809. [DOI] [PubMed] [Google Scholar]
- 44.Krug SM, Amasheh M, Dittmann I, Christoffel I, Fromm M, Amasheh S. Sodium caprate as an enhancer of macromolecule permeation across tricellular tight junctions of intestinal cells. Biomaterials 34: 275–282, 2013. doi: 10.1016/j.biomaterials.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 45.Kube D, Sontich U, Fletcher D, Davis PB. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am J Physiol Lung Cell Mol Physiol 280: L493–L502, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Kunzelmann K, Mehta A. CFTR: a hub for kinases and crosstalk of cAMP and Ca2+. FEBS J 280: 4417–4429, 2013. doi: 10.1111/febs.12457. [DOI] [PubMed] [Google Scholar]
- 47.Laval J, Touhami J, Herzenberg LA, Conrad C, Taylor N, Battini JL, Sitbon M, Tirouvanziam R. Metabolic adaptation of neutrophils in cystic fibrosis airways involves distinct shifts in nutrient transporter expression. J Immunol 190: 6043–6050, 2013. doi: 10.4049/jimmunol.1201755. [DOI] [PubMed] [Google Scholar]
- 48.LeSimple P, Liao J, Robert R, Gruenert DC, Hanrahan JW. Cystic fibrosis transmembrane conductance regulator trafficking modulates the barrier function of airway epithelial cell monolayers. J Physiol 588: 1195–1209, 2010. doi: 10.1113/jphysiol.2009.182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li N, Neu J. Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J Nutr 139: 710–714, 2009. doi: 10.3945/jn.108.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattoscio D, Evangelista V, De Cristofaro R, Recchiuti A, Pandolfi A, Di Silvestre S, Manarini S, Martelli N, Rocca B, Petrucci G, Angelini DF, Battistini L, Robuffo I, Pensabene T, Pieroni L, Furnari ML, Pardo F, Quattrucci S, Lancellotti S, Davì G, Romano M. Cystic fibrosis transmembrane conductance regulator (CFTR) expression in human platelets: impact on mediators and mechanisms of the inflammatory response. FASEB J 24: 3970–3980, 2010. doi: 10.1096/fj.10-159921. [DOI] [PubMed] [Google Scholar]
- 51.Merigo F, Benati D, Cristofoletti M, Amarù F, Osculati F, Sbarbati A. Glucose transporter/T1R3-expressing cells in rat tracheal epithelium. J Anat 221: 138–150, 2012. doi: 10.1111/j.1469-7580.2012.01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merigo F, Benati D, Cristofoletti M, Osculati F, Sbarbati A. Glucose transporters are expressed in taste receptor cells. J Anat 219: 243–252, 2011. doi: 10.1111/j.1469-7580.2011.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merigo F, Boschi F, Lasconi C, Benati D, Sbarbati A. Molecules implicated in glucose homeostasis are differentially expressed in the trachea of lean and obese Zucker rats. Eur J Histochem 60: 2557, 2016. doi: 10.4081/ejh.2016.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molenda N, Urbanova K, Weiser N, Kusche-Vihrog K, Günzel D, Schillers H. Paracellular transport through healthy and cystic fibrosis bronchial epithelial cell lines–do we have a proper model? PLoS One 9: e100621, 2014. doi: 10.1371/journal.pone.0100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molina SA, Stauffer B, Moriarty HK, Kim AH, McCarty NA, Koval M. Junctional abnormalities in human airway epithelial cells expressing F508del CFTR. Am J Physiol Lung Cell Mol Physiol 309: L475–L487, 2015. doi: 10.1152/ajplung.00060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monge ME, Pérez JJ, Dwivedi P, Zhou M, McCarty NA, Stecenko AA, Fernández FM. Ion mobility and liquid chromatography/mass spectrometry strategies for exhaled breath condensate glucose quantitation in cystic fibrosis studies. Rapid Commun Mass Spectrom 27: 2263–2271, 2013. doi: 10.1002/rcm.6683. [DOI] [PubMed] [Google Scholar]
- 57.Pezzulo AA, Gutiérrez J, Duschner KS, McConnell KS, Taft PJ, Ernst SE, Yahr TL, Rahmouni K, Klesney-Tait J, Stoltz DA, Zabner J. Glucose depletion in the airway surface liquid is essential for sterility of the airways. PLoS One 6: e16166, 2011. doi: 10.1371/journal.pone.0016166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts K, Jaffe A, Verge C, Thomas PS. Noninvasive monitoring of glucose levels: is exhaled breath the answer? J Diabetes Sci Technol 6: 659–664, 2012. doi: 10.1177/193229681200600322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rottner M, Freyssinet JM, Martínez MC. Mechanisms of the noxious inflammatory cycle in cystic fibrosis. Respir Res 10: 23, 2009. doi: 10.1186/1465-9921-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruan YC, Wang Y, Da Silva N, Kim B, Diao RY, Hill E, Brown D, Chan HC, Breton S. CFTR interacts with ZO-1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J Cell Sci 127: 4396–4408, 2014. doi: 10.1242/jcs.148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes 54: 2314–2319, 2005. doi: 10.2337/diabetes.54.8.2314. [DOI] [PubMed] [Google Scholar]
- 62.Sc NN, Shoseyov D, Kerem E, Zangen DH. Patients with cystic fibrosis and normoglycemia exhibit diabetic glucose tolerance during pulmonary exacerbation. J Cyst Fibros 9: 199–204, 2010. doi: 10.1016/j.jcf.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Schlingmann B, Molina SA, Koval M. Claudins: Gatekeepers of lung epithelial function. Semin Cell Dev Biol 42: 47–57, 2015. doi: 10.1016/j.semcdb.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, Karp PH, Wohlford-Lenane CL, Heilmann KP, Leidinger MR, Allen PD, Zabner J, McCray PB Jr, Ostedgaard LS, Stoltz DA, Randak CO, Welsh MJ. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351: 503–507, 2016. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh S, Prakash YS, Linneberg A, Agrawal A. Insulin and the lung: connecting asthma and metabolic syndrome. doi: 10.1155/2013/627384. J Allergy (Cairo) 2013: 627384, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stecenko AA, Moran A. Update on cystic fibrosis-related diabetes. Curr Opin Pulm Med 16: 611–615, 2010. doi: 10.1097/MCP.0b013e32833e8700. [DOI] [PubMed] [Google Scholar]
- 67.Stuart RO, Sun A, Panichas M, Hebert SC, Brenner BM, Nigam SK. Critical role for intracellular calcium in tight junction biogenesis. J Cell Physiol 159: 423–433, 1994. doi: 10.1002/jcp.1041590306. [DOI] [PubMed] [Google Scholar]
- 68.Tsuchiya A, Kanno T, Nishizaki T. PI3 kinase directly phosphorylates Akt1/2 at Ser473/474 in the insulin signal transduction pathway. J Endocrinol 220: 49–59, 2013. doi: 10.1530/JOE-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107: 1319–1327, 2001. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Sambeek L, Cowley ES, Newman DK, Kato R. Sputum glucose and glycemic control in cystic fibrosis-related diabetes: a cross-sectional study. PLoS One 10: e0119938, 2015. doi: 10.1371/journal.pone.0119938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weiser N, Molenda N, Urbanova K, Bähler M, Pieper U, Oberleithner H, Schillers H. Paracellular permeability of bronchial epithelium is controlled by CFTR. Cell Physiol Biochem 28: 289–296, 2011. doi: 10.1159/000331742. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto N, Ueda M, Sato T, Kawasaki K, Sawada K, Kawabata K, Ashida H.. Measurement of glucose uptake in cultured cells. Curr Protoc Pharmacol 71: 1–26, 2015. doi: 10.1002/0471141755.ph1214s71. [DOI] [PubMed] [Google Scholar]
- 73.Yang Y, Cheng Y, Lian QQ, Yang L, Qi W, Wu DR, Zheng X, Liu YJ, Li WJ, Jin SW, Smith FG. Contribution of CFTR to alveolar fluid clearance by lipoxin A4 via PI3K/Akt pathway in LPS-induced acute lung injury. Mediators Inflamm 2013: 862628, 2013. doi: 10.1155/2013/862628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang PX, Cheng J, Zou S, D’Souza AD, Koff JL, Lu J, Lee PJ, Krause DS, Egan ME, Bruscia EM. Pharmacological modulation of the AKT/microRNA-199a-5p/CAV1 pathway ameliorates cystic fibrosis lung hyper-inflammation. Nat Commun 6: 6221, 2015. doi: 10.1038/ncomms7221. [DOI] [PMC free article] [PubMed] [Google Scholar]