Abstract
Right ventricular (RV) dysfunction is associated with numerous smoking-related illnesses, including chronic obstructive pulmonary disease (COPD), in which it is present even in the absence of pulmonary hypertension. It is unknown whether exposure to cigarette smoke (CS) has direct effects on RV function and cardiac fibroblast (CF) proliferation or collagen synthesis. In this study, we evaluated cardiac function and fibrosis in mice exposed to CS and determined mechanisms of smoke-induced changes in CF signaling and fibrosis. AKR mice were exposed to CS for 6 wk followed by echocardiography and evaluation of cardiac hypertrophy, collagen content, and pulmonary muscularization. Proliferation and collagen content were evaluated in primary isolated rat CFs exposed to CS extract (CSE) or nicotine. Markers of cell proliferation, fibrosis, and proliferative signaling were determined by immunoblot or Sircol collagen assay. Mice exposed to CS had significantly decreased RV function, as determined by tricuspid annular plane systolic excursion. There were no changes in left ventricular parameters. RV collagen content was significantly elevated, but there was no change in RV hypertrophy or pulmonary vascular muscularization. CSE directly increased CF proliferation and collagen content in CF. Nicotine alone reproduced these effects. CSE and nicotine-induced fibroblast proliferation and collagen content were mediated through α7 nicotinic acetylcholine receptors and were dependent on PKC-α, PKC-δ, and reduced p38-MAPK phosphorylation. CS and nicotine have direct effects on CFs to induce proliferation and fibrosis, which may negatively affect right heart function.
Keywords: chronic obstructive pulmonary disease, fibrosis, nicotine, nicotinic acetylcholine receptor, right ventricle
right ventricular (RV) dysfunction is a poor prognostic marker in a number of cardiopulmonary diseases, such as heart failure, pulmonary hypertension, and chronic obstructive pulmonary disease (COPD). Cigarette smoking is a major cause of COPD. RV dysfunction in smokers is thought to be precipitated by vascular remodeling associated with COPD resulting in pulmonary hypertension and subsequently increased RV afterload (12, 22). However, recent evidence has shown that RV dysfunction can be present in patients with COPD without pulmonary hypertension (12). RV dysfunction and failure are associated with increased RV fibrosis (2), demonstrated in autopsy specimens and in animal models of RV failure (2, 11). Myocardial fibrosis is mediated by proliferation and activation of cardiac fibroblasts (CFs). However, it is unknown whether exposure to cigarette smoke (CS) has direct effects on RV function, CF proliferation, or collagen synthesis.
CS is a complicated chemical mixture. The effects of CS on cellular function have been attributed to various components, such as nicotine, reactive oxygen species (ROS), and reactive aldehydes, among others. In endothelial cells, exposure to CS extract (CSE) induces apoptosis via ROS and reactive aldehydes (6, 24). CSE can also promote increased apoptosis of cardiomyocytes (7, 34). In contrast, in cancer cells, nicotine may promote tumor cell proliferation and survival (25). Furthermore, nicotine is fibrogenic in multiple organs, including the lungs and kidneys (13). However, the effect of exposure to CS constituents on ventricular fibroblasts and the underlying mechanism(s) remains unclear.
In this study, we sought to evaluate the effect of CS exposure on RV function. Furthermore, we sought to determine the effects of CS on primary CF proliferation and elucidate the underlying mediators and the mechanism. We demonstrate that CS exposure in mice increases RV collagen content and significantly reduces RV function in the absence of RV hypertrophy and vascular remodeling. In addition, nicotine exposure recapitulates the effect of CS on CFs, including increased fibroblast proliferation and collagen content mediated via α7 nicotinic acetylcholine receptor (nAChR) activation.
MATERIALS AND METHODS
Materials.
All materials were obtained from Sigma (St. Louis, MO) unless otherwise noted. Collagenase II and DNAse were purchased from Worthington Biochemical (Lakewood, NJ). α-Smooth muscle actin (α-SMA, ab7817) antibody and α-bungarotoxin (α-BTX) were purchased from Abcam (Cambridge, MA). Aldehyde dehydrogenase (Alda-1) was purchased from EMD Millipore (Billerica, MA). The vectors encoding dominant-negative cDNA for PKC-δ (pHACE-PKCδK376R) and PKC-α (pHACE-PKCαK368R) were gifts from Bernard Weinstein [Addgene plasmids no. 16389 and 21235, respectively; Cambridge, MA (27)]. cDNA for green fluorescent protein (pGFP-C1) was obtained from Clontech (Mountain View, CA). Sircol collagen assay was purchased through Accurate (Westbury, NY). Antibodies against phosphorylated Erk (T202/Y204, no. 9101), phosphorylated p38 (T180/Y182, no. 9125), phosphorylated Akt (T308, no. 9272), total Erk (no. 9102), total p38 (no. 9212), total Akt (no. 9272), and caspase-3 (no. 9662) were purchased through Cell Signaling (Beverly, MA). α7 nAChR siRNA (sc-270402), control siRNA-FITC (sc-36869), antibodies for hemagglutinin (HA) (sc-805), PKC-α (sc-208), PKC-δ (sc-8402), PKC-βII (sc-210), procollagen (sc-8787), α7 nAChR (sc-554), and proliferating cell nuclear antigen (PCNA) (sc-25280) were purchased from Santa Cruz Biotechnology (Dallas, TX). von Willebrand factor (vWF) antibody (no. 2022-04) was purchased from Dako (Carpinteria, CA). Lipofectamine3000 was purchased through Life Technologies (Grand Island, NY).
Animals.
All animal protocols were approved by the Institutional Animal Care and Use Committee of the Providence VA Medical Center (protocols 2010-026 and 2013-010). Male AKR mice ages 6–8 wk purchased from Jackson Laboratory were either exposed to room air (RA) or CS for 6 weeks at 6 h per day, 4 days a week using a TE-10 mouse smoking machine (Teague Enterprises, Woodland, CA) with 3R4F reference cigarettes (University of Kentucky, Tobacco Research Inst., Lexington, KY) as previously described (24). The smoking machine produced a mixture of 89% sidestream and 11% mainsteam smoke, and the chamber was monitored for 120 mg/m3 of total suspended particulate matter. After 6 wk, mice were weighed and were subjected to either transthoracic echocardiography or in vivo hemodynamic measurements and then euthanized. These studies were performed in two cohorts with all animals in the first groups receiving echocardiography and all animals in the second group subjected to invasive hemodynamics. Lung and heart tissue were collected. Lungs were fixed with 10% formalin at 25 cm of H2O pressure, paraffin embedded, and sectioned for α-SMA/vWF staining. Hearts were fixed in 10% formalin, paraffin embedded, and sectioned for lectin and Picrosirius staining.
Adult Sprague-Dawley rats (250–300 g) purchased from Charles River (Wilmington, MA) were used for CF isolations.
Echocardiographic measurements.
The first cohort of mice were anesthetized with continuous isofluorane inhalation (1.5–3.0%), and transthoracic echocardiography was performed as described (5). A 40-MHz linear-array transducer was used (Vevo 2100; VisualSonics, Toronto, Ontario, Canada). Two-dimensional Doppler and M-mode recordings were obtained to measure left ventricular (LV) fractional shortening and LV and RV dimensions. The pulsed-wave Doppler recording at the right ventricular overflow tract was used to measure pulmonary acceleration time (PAT). Tricuspid annular plane systolic excursion (TAPSE) was measured by use of M-mode across the tricuspid valve annulus at the RV free wall. TAPSE was determined by measuring the excursion of the tricuspid annulus from its highest position to the peak descent during ventricular systole. Tissue Doppler was used to measure the early diastolic velocity of the septum (at mitral annulus) and RV lateral wall (at tricuspid annulus).
In vivo hemodynamics.
In the second cohort, RV systolic pressure, LV systolic pressure, and pulmonary artery systolic pressure (PASP) were measured under isofluorane anesthesia (1.5–2.0%) using an opened-chest technique. After the chest was opened, a high-fidelity pressure-volume 1.0-Fr catheter (PVR-1030; Millar/ADInstruments, Colorado Springs, CO) was inserted into the LV apex, and pressure measurements were recorded for 15 s. Subsequently, the same catheter was inserted into the apex of the RV, and pressure measurements were recorded for another 15 s. The catheter was then guided into the pulmonary artery just past the pulmonic valve to record the pulmonary artery pressure (PAP) for 15 s. Mean PAP was obtained by using the following equation: (PASP-2PADP)/3, where PADP is the pulmonary artery diastolic pressure. The animal was then euthanized, and tissue was collected for further analysis.
Pulmonary muscularization.
Paraffin-embedded lung tissue was sectioned and stained for α-SMA and vWF as previously described (4). Tissue sections were then scanned into an Aperio CS slide scanner at ×20 magnification. Fifty equally sized fields were marked onto the tissue image and scored for colocalized staining of α-SMA and vWF. Scoring was categorized as follows: none if no α-SMA staining colocalized with vWF, partial if <50% α-SMA staining colocalized with vWF, and complete muscularization if >50% α-SMA staining colocalized with vWF.
Picrosirius red staining and coronary vessel counts.
Paraffin-embedded heart tissue was sectioned and deparaffinized. Picrosirius-stained slides were imaged using a Nikon Eclipse-NI microscope with ×20 objective; images were taken in the red (594 nm) and green fluorescent (488 nm) channels as well as bright field. Three images each were taken from the RV, LV, and septum from each heart. Picrosirius red fluoresces in the red channel and quenches green autofluorescence. Therefore, analysis of Picrosirius red-positive stained area was performed by subtraction of the green fluorescence signal from the red fluorescence signal (Nikon Image Software Elements) similar to previously published protocols (31). ImageJ was then used to threshold the subtracted fluorescent Picrosirius red-stained image. Thresholded images were used for percentage of area quantitation, and the data were normalized to the respective room air control. For coronary microvessel analysis, antigen retrieval was performed with boiling 10 mM sodium citrate and 0.05% Tween 20, pH 6.0 for 10 min. Sections were then blocked with 5% BSA in PBS and incubated overnight with Isolectin GS-IB4, Alexa Fluor-488 Conjugate (no. I21411; Thermo Scientific, San Diego, CA), in 2.5% BSA at 4°C. Sections were then mounted with coverslips using Prolong Gold with DAPI and imaged with a fluorescent Nikon Eclipse E400 microscope. Equal-size areas were then counted manually for positive staining of lectins for microvascular vessels at ×20 magnification. Averaged vessel counts were normalized to the total area (mm2).
Collagen content measurement.
Collagen content was measured using Sircol collagen dye binding assay per the manufacturer’s instructions. Equal amounts of protein or homogenates were mixed with equal volume of Sircol dye reagent for 30 min with agitation. The collagen dye complex was centrifuged at 12,000 rpm for 10 min, and unbound dye was aspirated. The remaining pellet was washed with ice-cold acid-salt wash reagent, containing acetic acid, sodium chloride, and surfactants. Samples were then centrifuged again at 12,000 rpm for 10 min. The wash reagent was aspirated, and the remaining pellet was dissolved by adding the alkali reagent (0.5 M sodium hydroxide). After 5-min incubation, collagen content was measured using absorbance at 555 nm.
CF and myocyte isolation.
Adult rat CFs were isolated as previously described (35). Briefly, adult Sprague-Dawley rats (250–300 g) were euthanized with continuous inhalation of 5.0% isofluorane. Hearts were immediately excised, trimmed of extra-aortic tissue, and retrograde perfused for 2 min in Krebs-Henseleit (KH) buffer at 37°C and then switched to enzyme buffer 1 (KH buffer), containing 0.3 mg/ml collagenase II, 0.3 mg/ml hyaluronidase, and 50 μM CaCl2, for 18 min. After perfusion, the ventricular tissue was excised and in some cases as indicated in results (Fig. 4G) was divided into LV and RV tissue. Myocardial tissue was minced with scissors and further digested in enzyme buffer 2 (enzyme buffer 1 supplemented with 0.6 mg/ml trypsin IX, 0.6 mg/ml deoxyribonuclease, and increased [CaCl2] to 500 μM) at 37°C for 18 min in a shaking water bath. The digestion was stopped with the addition of 10 ml of DMEM supplemented with 10% FBS, penicillin, and streptomycin (complete media), filtered through a 200-μm nylon mesh, and centrifuged at 500 rpm for 5 min. The supernatant was removed and centrifuged again at 2,000 rpm for 5 min while the pellet was collected for myocytes. The second pellet was resuspended in complete media and plated into four 10-cm2 dishes. The media was changed after 2 h to remove cellular debris and unbound cells. We previously demonstrated that isolation of fibroblasts using this method provides a high percentage of cells that are vimentin positive (fibroblast) and free of vWF (endothelial) and smooth muscle myosin (smooth muscle)-positive cells (35). Cells were allowed to grow to confluency within 2 to 3 days before being passaged for in vitro experiments. Only passage 1 (P1) cells were used in this study.
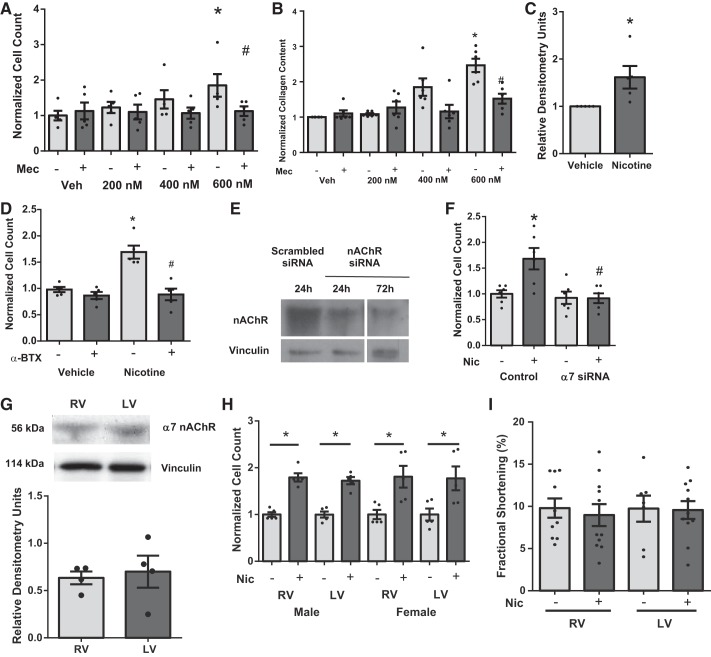
Fig. 4.
Effect of nicotine on isolated cardiac fibroblast proliferation and dependence of α7 nAChR. Nicotine increases cell counts (n = 5) (A) and collagen content (n = 5) (B) in cardiac fibroblasts through nAChR activation that was inhibited by Mec (20 μM). C: nicotine also increases procollagen expression in cardiac fibroblasts (n = 5). D: α-bungarotoxin (α-BTX; 100 nM) blocks nicotine-induced proliferation (n = 5). Knockdown of α7 nAChR with siRNA (E) blocks nicotine-induced proliferation (F) (n = 5). Quiescent cells were pretreated with either vehicle, Mec, α-BTX, or α7 nAChR siRNA, followed by vehicle or nicotine (600 nM) for 24 h. Data are normalized to vehicle treatment and presented as means ± SE. *P < 0.05 compared with Veh; #P < 0.05 compared with Mec/vehicle. G: there were no differences in expression of α7 nAChR in rat LV and RV tissues (n = 4). H: there were no changes in response to nicotine (Nic) in cardiac fibroblasts isolated from LV or RV or male or female rats. Nicotine significantly increased proliferation in all groups compared with respective untreated groups (*P < 0.05) (n = 3–4 animals/group performed in triplicate, 3-way ANOVA, Student-Newman-Keuls post hoc). I: there were no significant changes (t-test) in RV or LV cardiomyocyte contractile function with acute nicotine treatment.
Myocyte contractility.
The mechanical properties of the cardiomyocytes were assessed using an IonOptix Myocam System (IonOptix, Milton, MA) as previously described (14). Unloaded cardiomyocytes isolated from rats (as described above) were placed on a glass slide and allowed to adhere for 5 min in Tyrode buffer (at 37°C), containing (in mmol/l) 133.0 NaCl, 5.4 KCl, 5.3 MgCl2, 0.3 Na2PO4, 20.0 HEPES, 10.0 glucose, and 1.2 CaCl2 (pH7.4). Sarcomere contractility was then imaged with an inverted microscope. Cardiomyocytes were paced with 10-V, 4-ms square wave pulses at 1.0 Hz. Data are presented as a percentage of sarcomere length change.
CSE.
CSE was prepared as previously described (18). Mainstream smoke from 3R4F cigarettes was drawn into 30 ml of PBS by vacuum. Each cigarette was lit for 5 min with a total of five cigarettes being used. This was considered 100% CSE. Control solution was prepared by drawing unlit cigarettes into PBS. CSE and control solutions were used immediately. Final concentrations of CSE are expressed as percentage values (the ratio of CSE to total medium volume). The pH and color of the CSE were noted after use to maintain consistency.
In vitro experiments.
Subconfluent (60–70%) passage 1 cells were exposed to serum-free DMEM supplemented with 10 μg/ml insulin, 5.5 μg/ml transferrin, 5 ng/ml sodium selenite (ITS, Corning, Corning, NY), and penicillin and streptomycin. After 24 h, cells were pretreated with inhibitor for 30 min and then treated with either PBS (CSE vehicle)/CSE or ethanol (nicotine vehicle)/nicotine (600 nM or unless otherwise noted) for another 24 h. For proliferation assays, cells were trypsinized and counted with a hemocytometer. For cell viability assays, cells were loaded with the tetrazolium dye 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) for 2–4 h. Media was removed, and cells were washed with PBS. MTT detergent (isopropanol/HCl) was then added, and absorbance was read at 590 nm after an hour with a multiplate reader (BioTek, Winooski, VT).
Western immunoblot analysis.
Heart tissue from mice were homogenized on ice in homogenization buffer containing 20.0 mM HEPES, 250.0 mM sucrose, 100.0 mM NaCl, 0.2 mM EDTA, 0.2 mM EGTA, 200.0 μM PMSF, 0.5 mM DTT, 1.0 μM leupeptin, 1.0 μM aprotinin, and phosphatase inhibitor cocktail III. Homogenates were then centrifuged at 5,000 rpm for 10 min at 4°C. The pellet was discarded, and supernatant was then centrifuged again at 15,000 rpm for 10 min at 4°C. Supernatants were used for total protein analysis. CFs from the in vitro studies were collected in radioimmunoprecipitation assay buffer (RIPA) as previously described (29) and incubated on ice for 10 min before centrifugation for 10 min at 15,000 rpm. Protein concentration was determined using Bradford-Lowry Assay (Bio-Rad, Hercules, CA). Proteins (50 μg/lane) were resolved on 7.5% (procollagen) and 10% (Erk, PKCs, PCNA, HA, Akt, p38) separating gels by SDS-PAGE. Resolved proteins were transferred to polyvinylidene fluoride membrane (Millipore), and immunoblot analysis was performed by appropriate antibody dilution of 1:1,000 for all antibodies with the exception of procollagen and HA (1:200). Blots were then incubated with the proper horseradish peroxidase-conjugated secondary (SCBT) for 1 h at room temperature and chemiluminescence generated with Bio-Rad Clarity. Blots were imaged with a film developer or GE Healthcare ImageQuant (Piscataway, NJ). Equal loading was confirmed by probing for vinculin (1:3,000). Quantitative densitometry was performed by use of the public domain ImageJ program.
cDNA and siRNA transfections in CFs.
CFs were transiently transfected with cDNA (4 μg/well of 6-well plate) encoding dominant-negative PKC-δ (PKC-δK376R), PKC-α (PKC-αK368R), GFP and α7 siRNA (600 nM), or scrambled siRNA (600 nM) using Lipofectamine3000 according to manufacturer’s instructions. Twenty-four hours after transfection, cells were quiesced for 24 h and then used for experiments as described.
Statistical analysis.
Statistical tests were either unpaired two-tailed Student's t-test, one-way ANOVA with Dunnett’s post hoc test, or two-way ANOVA with Tukey’s post-hoc test as appropriate and unless otherwise indicated. All data are expressed as means ± SE unless otherwise indicated. A P value < 0.05 was considered statistically significant.
RESULTS
CS exposure is associated with RV dysfunction and fibrosis in absence of RV hypertrophy and pulmonary vascular remodeling.
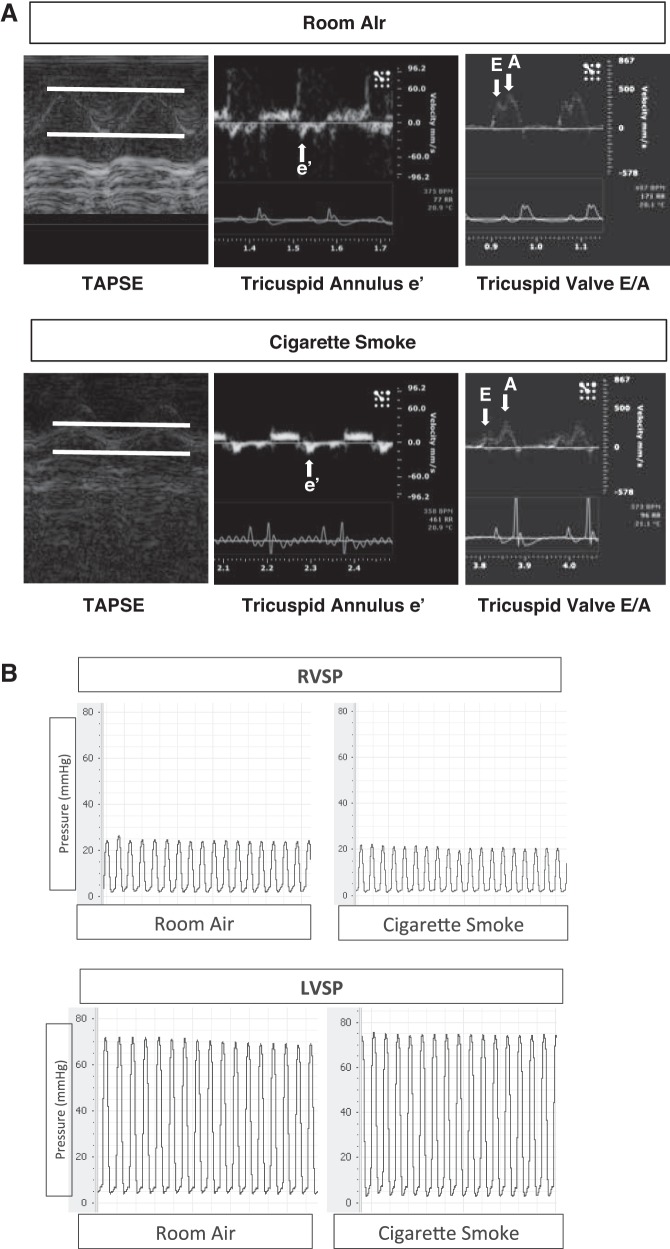
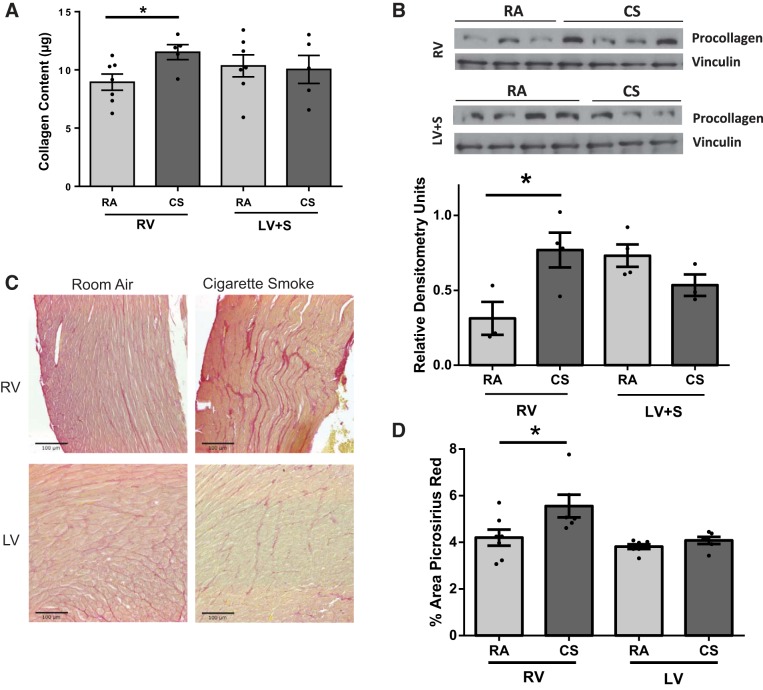
Exposure to 6 wk of CS significantly decreased RV function, as assessed by TAPSE. The tricuspid valve (TV) e′ and E-to-A ratio were also reduced, suggesting the presence of RV diastolic dysfunction (Table 1, Fig. 1A). There were no significant changes in RV or LV pressures or PAPs as assessed by invasive hemodynamics with a pressure catheter (Fig. 1B) or estimation of PAP assessed via PAT (Table 1). There were no changes in systemic blood pressure, heart rate, or other indices of LV systolic or diastolic function (Table 1, Fig. 1). There were also no changes following only 3 wk of exposure to CS (data not shown). In parallel to the changes in RV function, RV collagen content as assessed by Sircol assay was increased in the CS-exposed animals at 6 wk (Fig. 2A) but not at 3 wk (data not shown). This was confirmed by increased procollagen expression (Fig. 2B) in the RV of CS-exposed mice compared with controls. In contrast, there was no increase in collagen content or procollagen expression in the LV (Fig. 2, A and B). Collagen content of only septal tissue was also evaluated using Sircol assay, and no differences were found between CS exposure and room air (data not shown). Collagen content and fibrosis were also evaluated using Picrosirius red staining (Fig. 2C). Increased levels of collagen staining were apparent in the RV of CS-exposed mice compared with room air-exposed mice (Fig. 2C). Quantitation of Picrosirius staining demonstrated a significant increase in collagen deposition only in the RV of CS-exposed mice, similar to Sircol assay (Fig. 2D). Although collagen content was increased in the RV, there was no gross RV or LV hypertrophy (Table 2) between groups as determined by tissue weight. CS-exposed animals had overall reductions in both body and tissue weights (Table 2); however, RV and LV + S/BW ratios remained unchanged, where S is septum. There were also no changes in whole heart expression or cleavage of caspase-3 in the RV or LV of either CS or RA animals, indicating that enhanced apoptosis did not contribute to reduced RV function (data not shown). In addition, there was no notable presence of coronary microvascular rarefaction as determined by vessel counts in CS-treated animals (Table 2). There was some evidence of mild airspace disease in CS-exposed animals as shown by an increase in alveolar airspace demonstrated by mean linear intercept measured in hematoxylin and eosin-stained lung cross sections (Table 3). However, evaluation of pulmonary vascular histology did not show any smoke-induced changes in muscularization of small, medium, or large vessels compared with control (Table 2). Therefore, CS may induce direct effects on RV function and remodeling independent of significant changes in afterload, LV dysfunction, and lung vascular remodeling.
Table 1.
Echocardiographic and in vivo hemodynamic parameters of room air- and cigarette smoke-exposed mice at 6 wk
| Parameter | Room Air | Cigarette Smoke |
|---|---|---|
| PAT, ms | 24.50 ± 2.60 | 25.90 ± 1.90 |
| LV ejection fraction, % | 57.90 ± 7.10 | 68.70 ± 3.50 |
| Mitral valve E/A | 1.50 ± 0.10 | 1.40 ± 0.10 |
| Mitral annulus e′, mm/s | −23.80 ± 1.90 | −19.70 ± 3.00 |
| TAPSE, mm | 1.10 ± 0.10 | 0.80 ± 0.10* |
| Tricuspid valve E/A | 0.68 ± 0.05 | 0.55 ± 0.03* |
| Tricuspid annulus e′, mm/s | −25.10 ± 0.90 | −19.40 ± 2.10* |
| Heart rate, beats/min | 370.20 ± 20.50 | 396.80 ± 24.70 |
| RVSP, mmHg | 26.30 ± 1.10 | 27.70 ± 1.70 |
| LVSP, mmHg | 79.60 ± 7.10 | 74.50 ± 8.30 |
| MPAP, mmHg | 12.00 ± 0.90 | 13.20 ± 1.30 |
Values are means ± SE; n = 6–12. PAT, pulmonary acceleration time; LV, left ventricular; TAPSE, tricuspid annular plane systolic excursion; RVSP, RV systolic pressure; LVSP, LV systolic pressure; MPAP, mean pulmonary artery pressure.
P < 0.05.
Fig. 1.
Assessment of right ventricular (RV) and left ventricular (LV) function in room air (RA)- and cigarette smoke (CS)-exposed AKR mice. A: representative images using echocardiography demonstrating tricuspid annular plane systolic excursion (TAPSE), tricuspid annulus e′, and tricuspid valve E-to-A ratio, n = 6. B: representative hemodynamic tracings obtained with a Millar pressure catheter placed into the RV and the LV. RVSP, RV systolic pressure; LVSP, LV systolic pressure.
Fig. 2.
Analysis of cardiac fibrosis in RV and LV of RA- and CS-exposed mice. RV and LV + septum (S) tissue of mice exposed to CS exhibited increased collagen content (Sircol assay, per 100 μg of protein) (A) and procollagen expression (B). Sircol assay n = 5–7, procollagen expression n = 3 to 4. *P < 0.05 vs. RA, Student’s t-test. Similar increases in fibrosis were apparent with Picrosirius red-stained paraffin-embedded sections. C: representative images of RA- and CS-exposed RV and LV. D: significantly increased Picrosirius staining in CS-exposed RV but not LV, as determined by quantitation of images. *P < 0.05 vs. RA, Student’s t-test, n = 6 to 7. Scale on images = 100 μM.
Table 2.
Indexes of cardiac hypertrophy and coronary vascular density in room air- and cigarette smoke-exposed animals
| Room Air | Cigarette Smoke | |
|---|---|---|
| Ventricular hypertrophy | ||
| Body wt, g | 31.46 ± 0.91 | 26.99 ± 0.76* |
| RV, mg | 40.18 ± 2.31 | 31.50 ± 2.41* |
| LVS, mg | 102.62 ± 3.41 | 90.32 ± 21.2* |
| RV/LVS | 0.39 ± 0.02 | 0.35 ± 0.03 |
| RV/body wt | 1.28 ± 0.04 | 1.19 ± 0.10 |
| LVS/body wt | 3.30 ± 0.11 | 3.40 ± 0.06 |
| Coronary microvessel density, vessels/mm2 | ||
| RV | 1583.20 ± 78.60 | 1676.20 ± 112.80 |
| LV | 2010.80 ± 82.90 | 1969.90 ± 120.60 |
Values are means ± SE; n = 5–9 for heart body wt, and n = 6–8 for histological analysis. S, septum.
P < 0.05.
Table 3.
Indexes of alveolar and pulmonary vascular muscularization in room air- and cigarette smoke-exposed animals
| %Vessels | Room Air | Cigarette Smoke |
|---|---|---|
| Pulmonary muscularization | ||
| None | 41.6 ± 14.9 | 45.0 ± 6.7 |
| Partial | 36.4 ± 9.4 | 38.1 ± 3.5 |
| Complete | 22.1 ± 6.1 | 17.0 ± 4.6 |
| Alveolar space | ||
| MLI | 28.9 ± 0.82 | 32.9 ± 1.18* |
Values are means ± SE; n = 4–6 for mean linear intercept (MLI), and n = 3 to 4 for histological analysis.
P < 0.05.
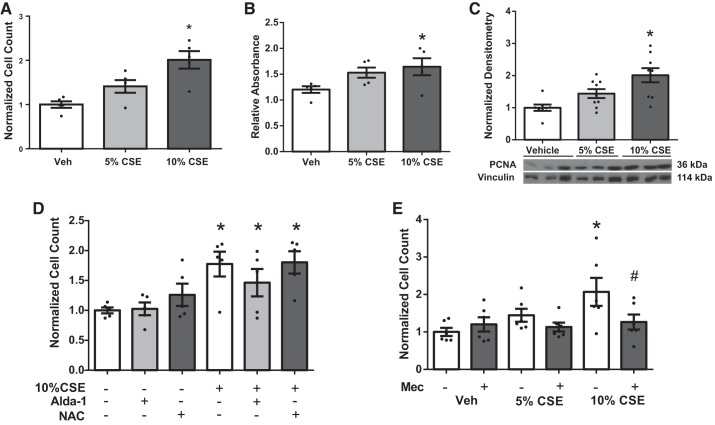
CSE induced CF proliferation.
The effects of CSE on fibroblast proliferation were assessed in adult rat CFs isolated from whole hearts (both RV and LV). Interestingly, increasing concentration of CSE resulted in CF proliferation as assessed by cell counts (Fig. 3A) and cell viability as assessed by MTT assay (Fig. 3B) in isolated fibroblasts. There was also an increase in the proliferative marker PCNA (Fig. 3C). There were no changes in the ratio of cleaved to total caspase-3, indicating that effects were not due to reduced apoptosis (data not shown).
Fig. 3.
Effects of CS extract (CSE) on fibroblast proliferation and role of nicotinic acetylcholine receptors (nAChRs). Cardiac fibroblasts isolated from rats were quiesced for 24 h, exposed to CSE for 24 h. CSE dose dependently increases cell count (n = 5) (A) and cell viability (B), as assessed by 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assay (n = 5). C: CSE increases expression of proliferation marker proliferating cell nuclear antigen (PCNA) (n = 9). Data were normalized to vinculin for loading control. D: CSE-induced cell proliferation in cardiac fibroblasts was not attenuated by activation of aldehyde dehydrogenase (Alda-1, 25 μM) or reactive oxygen species (ROS) scavenging (N-acetyl cysteine, NAC, 25 mM) (n = 5). E: mecamylamine (Mec, 20 μM) inhibited CSE-induced fibroblast proliferation (n = 5). Data are normalized to vehicle (Veh) and presented as means ± SE. *P < 0.05 compared with Veh; #P < 0.05 compared with CSE alone.
CSE contains the reactive aldehyde acrolein and ROS, which may underlie some of the cardiac effects of CS exposure. Pretreatment of CF with Alda-1, an aldehyde dehydrogenase activator, to reduce reactive aldehydes and ROS scavenging with N-acetyl cysteine (NAC) did not inhibit CSE-induced CF proliferation (Fig. 3D). Lower doses (100 nM and 10 μM) of both Alda-1 and NAC also had no effect (data not shown) on CSE-induced cell proliferation. In contrast, pretreatment with 20 μM mecamylamine (Mec), a general nAChR antagonist, attenuated the effect of CSE on CF proliferation (Fig. 3E).
Activation of α7 nAChR mediated CF proliferation.
Because effects of CSE were mediated via activation of nAChRs, we examined whether nicotine was sufficient to promote CF proliferation and increase collagen through nAChR. Similar to CSE, nicotine increased CF proliferation (Fig. 4A). Nicotine also increased collagen content in a dose-dependent manner (Fig. 4B). Nicotine (600 nM) also increased procollagen expression (Fig. 4C). The effective doses of nicotine to induce proliferation were similar to those previously measured in the blood of smokers (25–444 nM) (23). Nicotine-induced proliferation was blocked by pretreatment with Mec (20 μM) (Fig. 4, A and B). Similarly, the effect of nicotine was blocked by α-BTX (100 nM), a specific α7 nAChR antagonist (Fig. 4D). Knockdown of α7 nAChR receptors with siRNA (Fig. 4E) also blocked nicotine-induced proliferation (Fig. 4F). To investigate one potential cause of RV-selective CS-induced fibrosis, α7 nAChR expression was next examined in RV and LV tissue. There were no changes in the expression of α7 nAChR (Fig. 4G). As fibroblasts used in all the previous experiments were from mixed RV and LV populations, we isolated fibroblasts specifically from the LV and RV of male and female rats. We found no difference in nicotine-induced proliferation in fibroblasts isolated from LV or RV of male or female rats (Fig. 4H) (3-way ANOVA, effect of nicotine, P < 0.01; effect of chamber, P = 0.85; and effect of sex, P = 0.89; there were no significant interactions). The consistent response to nicotine in fibroblasts isolated from whole hearts or specific chambers indicates that there are additional physiological factors or possibly other constituents in CS that may positively (RV) or negatively (LV) modulate fibroblast proliferation in vivo following CS exposure. In addition, acute nicotine treatment (1 h) did not cause any direct effects on LV or RV cardiomyocyte contractile function (Fig. 4I), further supporting the concept that nicotine-induced fibroblast proliferation is responsible for impacting cardiac function.
Nicotine-induced CF proliferation is PKC-α and -δ dependent.
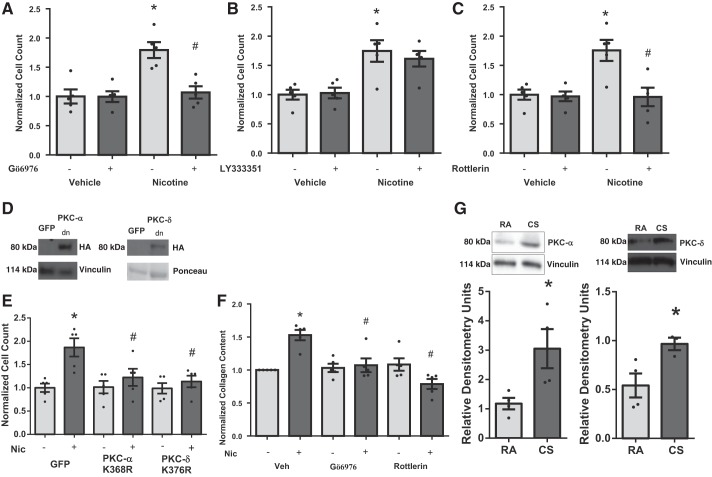
CFs were pretreated with either Gö6976 (100 nM), LY333531 (50 nM), or rottlerin (3 μM) to inhibit classical PKCs, PKC-β, or PKC-δ, respectively (Fig. 5, A–C). Inhibition of PKC-α/β with Gö6976 (Fig. 5A) attenuated nicotine-induced CF proliferation. This effect was absent using the PKC-β-specific inhibitor LY333531 alone (Fig. 5B), indicating that effects of Gö6976 were attributable to inhibition of PKC-α. PKC-δ inhibition (Fig. 5C) with rottlerin also attenuated nicotine-mediated CF proliferation. Transfection of CF with dominant-negative plasmids of PKC-α and -δ (Fig. 5, D and E) also blunted nicotine-induced CF proliferation, confirming results with small molecule inhibitors. Gö6976 and rottlerin also blocked the nicotine-induced increase in collagen content (Fig. 5F). In addition, immunoblots of RV lysates from smoke-exposed mice confirmed a role for PKC signaling in vivo with increased expression of PKC-α and -δ compared with room air controls (Fig. 5, G, top and bottom). We did not detect any change in PKC expression in the LV (data not shown).
Fig. 5.
Role of PKC signaling in nicotine-induced cardiac fibroblast proliferation. Small-molecule inhibitors of PKC-α (100 nM) (A) or -δ (3 μM) (C), but not PKC-β (50 nM) (B), block nicotine-induced cardiac fibroblast proliferation (n = 5). Expression of dominant-negative (Κ368Ρ) PKC-α (D) or dominant-negative (K376R) PKC-δ (E) also blocks nicotine-induced cardiac fibroblast proliferation (n = 5). GFP, green fluorescent protein; HA, hemagglutinin. Chemical inhibition of PKC-α and -δ blocks nicotine-induced increased collagen content (F). Cells were quiesced in serum-free medium for 24 h before nicotine stimulation. *P < 0.05 vs. Veh/Veh, and #P < 0.05 vs. Veh/Nic using 2-way ANOVA, Student-Newman-Keuls post hoc test. CS-exposed mice have increased expression of PKC-α and -δ in RV tissue. G, top: representative blot is shown. Bottom: quantitation of data in G (n = 4). *P < 0.05 vs. RA.
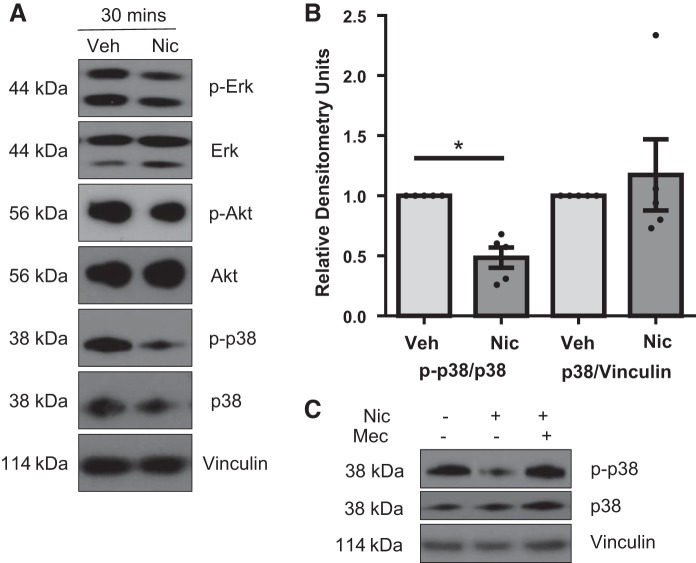
Nicotine reduces p38 MAPK activation but does not promote other common proliferation signals.
We next evaluated nicotine-induced changes in downstream pathways related to acute nAChR signaling, including Erk, Akt, and p38-MAPK. Although no changes were noted in phosphorylation of Erk or Akt (Fig. 6A), nicotine decreased p38-MAPK phosphorylation after 30-min nicotine exposure (Fig. 6, A and B). Decreased p38 phosphorylation in response to nicotine was attenuated by pretreatment with the general nAChR antagonist Mec (Fig. 6C).
Fig. 6.
Effects of nicotine on phosphorylation of Akt, Erk, and p38-MAPK. A: nicotine treatment for 30 min does not change Akt or Erk phosphorylation but does acutely decrease phosphorylated p38-MAPK. Representative blots are shown. B: quantitation of the ratio of p-p38 to total p38 and total p38 to the loading control vinculin from A. Data are normalized to Veh. C: reduced p38-MAPK phosphorylation is blocked by the nAChR antagonist Mec. A representative blot is shown, n = 5. *P < 0.05 vs. vehicle, unpaired t-test.
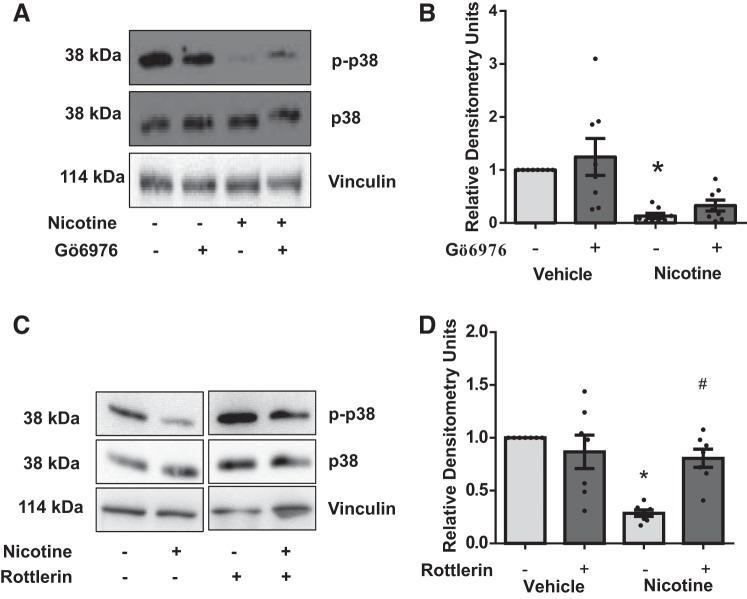
Nicotine-reduced p38-MAPK activation is mediated by PKC-δ but not PKC-α.
Pretreatment of rat CFs with the PKC-α/β inhibitor Gö6976 did not block nicotine-induced reduction in p38-MAPK activity (Fig. 7, A and B). In contrast, treatment of cells with rottlerin significantly attenuated nicotine-induced reduction in p38-MAPK activity (Fig. 7, C and D).
Fig. 7.
Determination of upstream signals regulating nicotine-dependent reduced p38-MAPK phosphorylation. A: chemical inhibition of PKC-α does not reduce p38-MAPK phosphorylation. A representative blot is shown. B: quantitation of results in A, n = 8. C: nicotine-mediated reduction of p38-MAPK phosphorylation is blocked by rottlerin. D: quantitation of results in C, n = 8. *P < 0.05 vs. control/vehicle group. #P < 0.05 from both vehicle and nicotine control groups; 2-way ANOVA, Tukey’s test. Any samples in cropped images were run on the same gel with groups irrelevant to the study removed.
DISCUSSION
The principal findings of this study were the following: 1) 6-wk CS exposure induced RV dysfunction and fibrosis in mice independent of overt pulmonary vascular remodeling, 2) CS-induced profibrotic changes were not found in LV tissue, and 3) nicotine directly induced CF proliferation and collagen content through α7 nAChR-dependent PKC-α and -δ signaling.
Our results indicate that CS directly changes RV function and collagen content. Importantly, depressed RV contractile function and enhanced fibrosis observed in this study appeared to be independent of remodeling of the pulmonary circulation and increased afterload (as determined by PAT, histological examination, and unchanged PAP and RV pressure). In addition, we believe that nicotine is likely the causative factor of increased RV fibrosis because nicotine application to CFs increased cell proliferation and collagen content. The effects of nicotine on proliferation are cell and tissue dependent with enhanced proliferation in certain cancers and increased apoptosis in endothelial cells (6, 24, 25). In contrast to endothelial cells, nicotine is known to directly increase proliferation in numerous cell types, potentially through activation of nAChR (8, 10, 13). Multiple fibroblast types express α7 nAChR (1, 13). To our knowledge, this is the first study demonstrating direct effects of nicotine on cardiac ventricular fibroblasts mediated via nAChR. General or specific blockade of α7 nAChR with Mec and α-BTX, respectively, or specific knockdown of α7 nAChR was capable of blocking the CSE/nicotine-induced CF proliferation and profibrotic phenotype.
Importantly, in the CS-exposed mice, enhanced RV fibrosis and depressed function were not associated with an increase in gross RV hypertrophy, as assessed by RV-to-body weight and RV-to-LV + S ratios. This further indicates that the enhanced RV fibrosis and fibroblast proliferation stimulated by CS were independent of pulmonary vascular remodeling because increased pulmonary pressures and elevated RV afterload would likely be associated with RV hypertrophy and failure, as seen in models of COPD and pulmonary hypertension (30). CS-induced effects were likely mediated by direct nicotine stimulation of fibroblast α7 nAChR. This was supported by direct stimulation of CF proliferation and collagen expression by nicotine in vitro. In addition, to our knowledge, this is the first report of increased RV fibrosis and dysfunction in the absence of simultaneous RV hypertrophy. In this study, we used AKR mice that are prone to CS-induced pulmonary injury. There are no data on use of AKR mice to study pulmonary vascular remodeling and RV function that we are aware of to compare with our model. Others have reported that after 5 mo of CS exposure, significant vascular remodeling and RV hypertrophy were present in certain strains of mice (e.g., A/J). We believe that our model may provide a unique insight into development of RV dysfunction in the absence of pulmonary hypertension in settings of CS exposure, as noted in patients with COPD and normal PAPs (26). Further studies are needed to explore the temporal aspect of progression of airspace disease, vascular remodeling, and RV dysfunction to precisely characterize these relationships in our model of CS exposure.
Another important finding of these studies was that depressed cardiac function and enhanced fibrotic signals in response to CS were unique to the RV. It is presently unclear why depressed function and fibrotic responses were limited to the RV in CS-exposed mice. Numerous differences have been described between RV and LV cardiomyocytes as well as whole heart responses to different agonists and stimuli (9, 22). However, no studies have documented differences in fibroblast-specific responses in the two ventricles. Nevertheless, our data clearly indicate that CS induces RV fibrosis and RV dysfunction. Importantly, in our isolated cell experiments, fibroblasts isolated from either the RV or LV had similar response to nicotine. In numerous disease states, the RV is considerably more prone to fibrosis and changes in afterload than the LV (9, 19, 21). Potential explanations for differences in LV and RV fibrosis may be that fibroblast proliferative responses are dependent on chamber-specific hemodynamic stress in vivo, possibly related to airspace disease, localized neurohumoral or immune differences, and any potential up- or downregulated systems that interact with nAChR signaling. It is also possible that species-specific differences in CF signaling in response to nicotine exist between the mice hearts and isolated rat CFs used in this study. Finally, cross talk between CFs and cardiomyocytes plays an important role in modulating cardiomyocyte structure and function (15, 28). Although nicotine does not have any direct effect on cardiomyocyte function, it is possible that the nicotine-exposed CFs may modulate cardiomyocyte structure and function, contributing to the RV dysfunction observed in CS-exposed animals. Future studies will be needed to differentiate these possibilities and the important differences observed in LV and RV fibrotic responses in response to CS.
Nicotine-dependent CF proliferation and collagen expression were dependent on α7 nAChR signaling through PKCs. α7 nAChRs are ligand-gated ion channels, which regulate Ca2+ influx in response to acetylcholine or nicotine (1, 8, 13). This study supports the idea that nicotine in CS activates α7 nAChR-mediated Ca2+ influx, leading to specific PKC isoform activation and subsequent proliferation and fibrosis. The nAChR-mediated Ca2+ influx has been associated with activation of Ca2+-dependent kinases to mediate downstream proliferative effects (1). We found that nicotine-induced CF proliferation required the Ca2+-dependent PKC-α but did not require the similarly regulated PKC-βII. In addition, the novel PKC isoform PKC-δ was also required for nicotine-induced CF proliferation (Fig. 5). Upregulation of PKCs has been implicated in the pathogenesis of LV heart failure and fibrosis (17, 20), and our data indicate that similar PKC signals may be present during CS- and nicotine-induced RV dysfunction.
Nicotine-induced increases in CF proliferation and collagen expression were associated with decreased p38-MAPK phosphorylation, which was PKC-δ dependent. The literature regarding p38-MAPK and fibrosis is conflicting; however, multiple studies support the notion that decreasing p38 activity in CFs is profibrotic. In a hypoxia-induced model of pulmonary hypertension in rats, we showed that pulmonary hypertension-induced RV fibrosis is associated with increased RV expression of PKC-βII and PKC-δ (5). RV fibrosis in this model was also associated with decreased p38-MAPK phosphorylation. In addition, angiotensin II (ANG II), which contributes to hypoxia-dependent pulmonary hypertension, directly increases CF proliferation and collagen expression. The ANG II-induced fibrotic phenotype in CFs also required PKC-dependent downregulation of p38-MAPK (5). Furthermore, cardiomyocyte p38-MAPK activation was previously shown to be a negative regulator of cardiac hypertrophy. Braz and colleagues (3) demonstrated hypertrophy and decreased function in DN-p38-MAPK, DN-MKK3, and DN-MKK6 transgenic mice as well as enhanced hypertrophic responses to aortic banding. These authors also found that DN-p38-MAPK and DN-MKK3 mouse hearts exhibited elevated interstitial fibrosis, supporting our hypothesis that nicotine-mediated decreases in p38-MAPK activity may be a key signal to promote RV fibrosis. Inhibition of p38-MAPK also enhanced nuclear factor of activated T-cell (NFAT) signaling, which is a known mediator of hypertrophy. Nicotine was recently found to increase NFAT activity in neonatal rat cardiomyocytes (16). In contrast, a role for profibrotic activities of p38-MAPK has been proposed based on numerous studies identifying p38-MAPK signaling as a downstream effector of the profibrotic cytokine TGF-β (32). However, some well-characterized p38-MAPK inhibitors are also known to inhibit the TGF-β receptor kinase, thus limiting interpretation of these studies (33). Future studies are needed to elucidate the mechanism and role of reduced p38-MAPK activity in nicotine-induced fibroblast proliferation.
Finally, it is noteworthy that nicotine itself is the constituent of CS capable of inducing proliferation and fibrotic responses in CFs. This could have serious implications for chronic nicotine replacement therapy for smoking cessation or recreational use. Future studies will need to determine whether nicotine alone can induce the RV fibrosis changes found in CS-exposed mice to help identify whether continuous nicotine replacement is a safe method of smoking cessation or for recreational use. Further inquiry into the direct effects of nicotine on RV function and fibrosis in relevant animal models and patients will be required.
GRANTS
This material is based on work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development: Biomedical Laboratory Research and Development Service (MERIT Review Award to G. Choudhary, 5IO1BX000711), National Heart, Lung, and Blood Institute Grant (1R01HL128661 awarded to G. Choudhary), and support from the Department of Medicine, Alpert Medical School of Brown University. R. Clements is supported by an AHA Grant-in Aid (GRNT20460376). H. Chichger was supported by American Heart Association Grant (13POST16860031). A. Allawzi was supported by NIH 5R25GM083270 and 5T32GM077995. K. O’Connell and P. Sakhatskyy were supported by National Heart, Lung, and Blood Institute Grant T32 HL094300-05. E. Jeong and S. Dudley were supported by National Institutes of Health Grant R01 HL104025. Q. Lu was supported by American Thoracic Society/Pulmonary Hypertension research award PH-05-015, R01 HL130230, and P20 GM 103652. P. Zhang was supported by P20 GM 103652. S. Rounds was supported by P20 GM 103652 and VA MERIT Review HL64936. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the National Institutes of Health.
DISCLOSURES
G. Choudhary is the principal investigator on an investigator-initiated study funded by Novartis. No other conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.V., R.T.C., N.K., A.A., K.A.O., E.-M.J., P.S., and G.C. performed experiments; A.V., R.T.C., K.A.O., E.-M.J., P.S., and G.C. analyzed data; A.V., R.T.C., H.C., N.K., A.A., K.A.O., S.D., P.S., Q.L., P.Z., S.R., and G.C. interpreted results of experiments; A.V. and R.T.C. prepared figures; A.V., R.T.C., and G.C. drafted manuscript; A.V., R.T.C., H.C., A.A., K.A.O., E.-M.J., S.D., P.S., Q.L., P.Z., S.R., and G.C. edited and revised manuscript; A.V., R.T.C., H.C., N.K., A.A., K.A.O., E.-M.J., S.D., P.S., Q.L., P.Z., S.R., and G.C. approved final version of manuscript.
REFERENCES
- 1.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89: 73–120, 2009. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 120: 1951–1960, 2009. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 3.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest 111: 1475–1486, 2003. doi: 10.1172/JCI200317295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casserly B, Mazer JM, Vang A, Harrington EO, Klinger JR, Rounds S, Choudhary G. C-type natriuretic peptide does not attenuate the development of pulmonary hypertension caused by hypoxia and VEGF receptor blockade. Life Sci 89: 460–466, 2011. doi: 10.1016/j.lfs.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chichger H, Vang A, O’Connell KA, Zhang P, Mende U, Harrington EO, Choudhary G. PKC d and bII regulate angiotensin II-mediated fibrosis through p38: a mechanism of RV fibrosis in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L827–L836, 2015. doi: 10.1152/ajplung.00184.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damico R, Simms T, Kim BS, Tekeste Z, Amankwan H, Damarla M, Hassoun PM. p53 mediates cigarette smoke-induced apoptosis of pulmonary endothelial cells: inhibitory effects of macrophage migration inhibitor factor. Am J Respir Cell Mol Biol 44: 323–332, 2011. doi: 10.1165/rcmb.2009-0379OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das A, Dey N, Ghosh A, Das S, Chattopadhyay DJ, Chatterjee IB. Molecular and cellular mechanisms of cigarette smoke-induced myocardial injury: prevention by vitamin C. PLoS One 7: e44151, 2012. doi: 10.1371/journal.pone.0044151. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci 29: 151–158, 2008. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation 129: 1033–1044, 2014. doi: 10.1161/CIRCULATIONAHA.113.001375. [DOI] [PubMed] [Google Scholar]
- 10.Goette A, Lendeckel U, Kuchenbecker A, Bukowska A, Peters B, Klein HU, Huth C, Röcken C. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart 93: 1056–1063, 2007. doi: 10.1136/hrt.2005.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Arroyo J, Santos-Martinez LE, Aranda A, Pulido T, Beltran M, Muñoz-Castellanos L, Dominguez-Cano E, Sonnino C, Voelkel NF, Sandoval J. Differences in right ventricular remodeling secondary to pressure overload in patients with pulmonary hypertension. Am J Respir Crit Care Med 189: 603–606, 2014. doi: 10.1164/rccm.201309-1711LE. [DOI] [PubMed] [Google Scholar]
- 12.Hilde JM, Skjørten I, Grøtta OJ, Hansteen V, Melsom MN, Hisdal J, Humerfelt S, Steine K. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol 62: 1103–1111, 2013. doi: 10.1016/j.jacc.2013.04.091. [DOI] [PubMed] [Google Scholar]
- 13.Jensen K, Nizamutdinov D, Guerrier M, Afroze S, Dostal D, Glaser S. General mechanisms of nicotine-induced fibrogenesis. FASEB J 26: 4778–4787, 2012. doi: 10.1096/fj.12-206458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong EM, Chung J, Liu H, Go Y, Gladstein S, Farzaneh-Far A, Lewandowski ED, Dudley SC Jr. Role of mitochondrial oxidative stress in glucose tolerance, insulin resistance, and cardiac diastolic dysfunction. J Am Heart Assoc 5: 5, 2016. doi: 10.1161/JAHA.115.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamo T, Akazawa H, Komuro I. Cardiac nonmyocytes in the hub of cardiac hypertrophy. Circ Res 117: 89–98, 2015. doi: 10.1161/CIRCRESAHA.117.305349. [DOI] [PubMed] [Google Scholar]
- 16.Li N, Si B, Ju JF, Zhu M, You F, Wang D, Ren J, Ning YS, Zhang FQ, Dong K, Huang J, Yu WQ, Wang TJ, Qiao B. Nicotine induces cardiomyocyte hypertrophy through TRPC3-mediated Ca(2+)/NFAT signalling pathway. Can J Cardiol 32: 1260, 2016. doi: 10.1016/j.cjca.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q, Molkentin JD. Protein kinase Cα as a heart failure therapeutic target. J Mol Cell Cardiol 51: 474–478, 2011. doi: 10.1016/j.yjmcc.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Q, Sakhatskyy P, Grinnell K, Newton J, Ortiz M, Wang Y, Sanchez-Esteban J, Harrington EO, Rounds S. Cigarette smoke causes lung vascular barrier dysfunction via oxidative stress-mediated inhibition of RhoA and focal adhesion kinase. Am J Physiol Lung Cell Mol Physiol 301: L847–L857, 2011. doi: 10.1152/ajplung.00178.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part One. Am J Respir Crit Care Med 150: 833–852, 1994. doi: 10.1164/ajrccm.150.3.8087359. [DOI] [PubMed] [Google Scholar]
- 20.Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov 11: 937–957, 2012. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modesti PA, Vanni S, Bertolozzi I, Cecioni I, Lumachi C, Perna AM, Boddi M, Gensini GF. Different growth factor activation in the right and left ventricles in experimental volume overload. Hypertension 43: 101–108, 2004. doi: 10.1161/01.HYP.0000104720.76179.18. [DOI] [PubMed] [Google Scholar]
- 22.Reddy S, Bernstein D. Molecular mechanisms of right ventricular failure. Circulation 132: 1734–1742, 2015. doi: 10.1161/CIRCULATIONAHA.114.012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell MA, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. BMJ 280: 972–976, 1980. doi: 10.1136/bmj.280.6219.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakhatskyy P, Gabino Miranda GA, Newton J, Lee CG, Choudhary G, Vang A, Rounds S, Lu Q. Cigarette smoke-induced lung endothelial apoptosis and emphysema are associated with impairment of FAK and eIF2α. Microvasc Res 94: 80–89, 2014. doi: 10.1016/j.mvr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res 12: 14–23, 2014. doi: 10.1158/1541-7786.MCR-13-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeger W, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galiè N, Ghio S, Gibbs S, Martinez FJ, Semigran MJ, Simonneau G, Wells AU, Vachiéry JL. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 62, Suppl: D109–D116, 2013. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Soh JW, Weinstein IB. Roles of specific isoforms of protein kinase C in the transcriptional control of cyclin D1 and related genes. J Biol Chem 278: 34709–34716, 2003. doi: 10.1074/jbc.M302016200. [DOI] [PubMed] [Google Scholar]
- 28.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis. Circ Res 118: 1021–1040, 2016. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vang A, Mazer J, Casserly B, Choudhary G. Activation of endothelial BKCa channels causes pulmonary vasodilation. Vascul Pharmacol 53: 122–129, 2010. doi: 10.1016/j.vph.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 30.van de Veerdonk MC, Bogaard HJ, Voelkel NF. The right ventricle and pulmonary hypertension. Heart Fail Rev 21: 259–271, 2016. doi: 10.1007/s10741-016-9526-y. [DOI] [PubMed] [Google Scholar]
- 31.Vogel B, Siebert H, Hofmann U, Frantz S. Determination of collagen content within picrosirius red stained paraffin-embedded tissue sections using fluorescence microscopy. MethodsX 2: 124–134, 2015. doi: 10.1016/j.mex.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao H, Zhang YY. Understanding the role of transforming growth factor-beta signalling in the heart: overview of studies using genetic mouse models. Clin Exp Pharmacol Physiol 35: 335–341, 2008. doi: 10.1111/j.1440-1681.2007.04876.x. [DOI] [PubMed] [Google Scholar]
- 33.Yakymovych I, Engström U, Grimsby S, Heldin CH, Souchelnytskyi S. Inhibition of transforming growth factor-beta signaling by low molecular weight compounds interfering with ATP- or substrate-binding sites of the TGF beta type I receptor kinase. Biochemistry 41: 11000–11007, 2002. doi: 10.1021/bi025936u. [DOI] [PubMed] [Google Scholar]
- 34.Yamada S, Zhang XQ, Kadono T, Matsuoka N, Rollins D, Badger T, Rodesch CK, Barry WH. Direct toxic effects of aqueous extract of cigarette smoke on cardiac myocytes at clinically relevant concentrations. Toxicol Appl Pharmacol 236: 71–77, 2009. doi: 10.1016/j.taap.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P, Su J, King ME, Maldonado AE, Park C, Mende U. Regulator of G protein signaling 2 is a functionally important negative regulator of angiotensin II-induced cardiac fibroblast responses. Am J Physiol Heart Circ Physiol 301: H147–H156, 2011. doi: 10.1152/ajpheart.00026.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]