Abstract
Progressive pulmonary fibrosis is a devastating consequence of many acute and chronic insults to the lung. Lung injury leads to alveolar epithelial cell (AEC) death, destruction of the basement membrane, and activation of transforming growth factor-β (TGF-β). There is subsequent resolution of the injury and a coordinated and concurrent initiation of fibrosis. Both of these processes may involve activation of similar intracellular signaling pathways regulated in part by dynamic changes to the extracellular matrix. Matrix signaling can augment the profibrotic fibroblast response to TGF-β. However, similar matrix/integrin signaling pathways may also be involved in the inhibition of ongoing TGF-β-induced AEC apoptosis. Focal adhesion kinase (FAK) is an integrin-associated signaling molecule expressed by many cell types. We used mice with AEC-specific FAK deletion to isolate the epithelial aspect of integrin signaling in the bleomycin model of lung injury and fibrosis. Mice with AEC-specific deletion of FAK did not exhibit spontaneous lung injury but did have significantly greater terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling-positive cells (18.6 vs. 7.1) per ×200 field, greater bronchoalveolar lavage protein (3.2 vs. 1.8 mg/ml), and significantly greater death (77 vs. 19%) after bleomycin injury compared with littermate control mice. Within primary AECs, activated FAK directly associates with caspase-8 and inhibits activation of the caspase cascade resulting in less apoptosis in response to TGF-β. Our studies support a model in which dynamic changes to the extracellular matrix after injury promote fibroblast activation and inhibition of epithelial cell apoptosis in response to TGF-β through FAK activation potentially complicating attempts to nonspecifically target this pathway for antifibrotic therapy.
Keywords: apoptosis, matrix, epithelial cell, fibrosis
persistent or repetitive injuries to the lung can initiate a progressive series of tissue and cellular responses that ultimately lead to pulmonary fibrosis (5, 39, 40, 46). During the injury phase, there is prominent epithelial cell apoptosis with denuding and destruction of the basement membrane. This loss of epithelial barrier integrity is coupled with increased capillary permeability leading to an influx of plasma proteins into the alveolar space. Plasma-derived provisional matrix proteins are rapidly deposited into the damaged alveoli transiently limiting the initial injury (44). The provisional matrix also participates in recruiting fibroblasts into the damaged areas and activating them to produce fibrillar collagens to replace the temporary provisional matrix. In limited injury, there is resolution of epithelial cell death, increased epithelial cell proliferation, restoration of epithelial integrity, and limited scar formation. In contrast, sustained epithelial cell death and exuberant matrix deposition by fibroblasts can lead to progressive fibrosis (7, 37).
The sequential changes to the extracellular matrix after injury are accompanied by increased tissue rigidity and changes in matrix receptor signaling, which can influence cell behavior during the repair and fibrotic processes (18). Integrins are among the most prominent cell surface matrix receptors. While integrins lack inherent signaling capacity, engagement to-specific extracellular matrix ligands can induce conformational changes to the integrin, which can initiate intracellular signaling through activation of signaling proteins associated with the integrin cytoplasmic tail (15, 20). Focal adhesion kinase (FAK) is a prominent integrin-associated tyrosine kinase signaling protein (32, 38). Matrix ligation leads to integrin clustering and FAK autophosphorylaton at tyrosine 397. This initial phosphorylation enables phosphorylation at other residues and by other kinases further promoting FAK activation. Activated FAK can then bind to and phosphorylate other downstream signaling molecules ultimately affecting cellular behavior.
Within fibroblasts, the dynamic changes to the matrix composition and rigidity lead to increased FAK activation promoting a number of fibroblast profibrotic activities such as increased survival, increased migration, and increased fibrotic matrix production (18). FAK activation can augment the fibroblast response to transforming growth factor-β (TGF-β), which is the most well-defined profibrotic factor (30). In some reports, TGF-β signaling can promote FAK activation and FAK signaling can augment activation of latent TGF-β leading to mutual activation of these profibrotic pathways (19, 29). Thus inhibition of FAK activity has been proposed as a therapeutic target for fibrosis (11, 23, 27). However, the cross talk between TGF-β and FAK signaling is not limited to fibroblasts and FAK activation may be involved in both the resolution of the acute injury as well as the fibrogenic process. In addition to its ability to activate fibroblasts, TGF-β is also known to induce epithelial cell apoptosis (17). We recently found that integrin signaling through the provisional matrix protein vitronectin is critical for inhibiting alveolar epithelial cell apoptosis in response to TGF-β (44). Thus matrix/integrin/FAK activation may be critical for coordinating the attenuation of lung injury with the concurrent activation of fibrosis especially in the context of robust TGF-β activation. This is critically important because we found that lung epithelial cell-specific apoptosis itself is sufficient to initiate and augment robust fibrosis. Thus nonspecific dysregulation of the coordinated epithelial and fibroblast FAK signaling pathways might be problematic for attempts to limit the fibrotic response. Recognition of both aspects of smkFAK signaling may thus be critical for optimally targeting this pathway while minimizing potential side effects.
In this report, we investigate the role of lung epithelial cell FAK specifically and isolated from the role of FAK activation in fibroblasts. We find that mice with lung epithelial cell-specific deletion of FAK are more susceptible to lung injury and have much greater death after bleomycin injury. Using a lower, nonlethal, dose of bleomycin, we find that mice with early lung epithelial cell-specific deletion of FAK are also more prone to fibrosis due to increased epithelial cell apoptosis. Finally, we define a novel mechanism for FAK protection of alveolar epithelial cell (AEC) apoptosis through direct inhibition of caspase-8 activation.
MATERIALS AND METHODS
Materials.
Antibodies to FAK, phospho-tyrosine 397 FAK, and caspase-8 were purchased from Cell Signaling Technology. The caspase-8 inhibitor Z-IETD-FMK was from R&D Systems. The FAK inhibitor PF573228 was from Tocris Biosciences. Recombinant keratinocyte growth factor (KGF) was from PeproTech. Small airway growth media (SAGM) was from Lonzo. Horseradish peroxidase-conjugated secondary antibodies were from Santa Cruz Biotechnology. Protein-A- and protein-G-conjugated agarose beads and the In Situ Cell Death Detection Kit and TMR red are from Roche. Caspase-Glo 3/7 and Caspase-Glo 8 were from Promega. Phosphatase inhibitor cocktail and human plasma fibronectin were purchased from Millipore. All other reagents were from Sigma.
Mice.
Floxed FAK mice are previously described and were purchased from the Mutant Mouse Regional Resource Center (33). To delete FAK in lung epithelial cells, floxed FAK mice were crossed with mice expressing the surfactant protein-C reverse tetracycline transactivator (SPC-rtTA) and CMV-tetO-Cre (tetO-Cre) recombinase as previously described (21, 22) resulting in mice SPC-rtTA/tetO-Cre/FAKfl/fl mice (SC FAK) or littermate genotype controls lacking at least one of the three transgenes. Unless otherwise indicated, SC FAK and littermate control mice were started on doxycycline-containing chow (200 mg/kg) on postnatal day 28 and continued through postnatal day 42. In some experiments, mice were maintained doxycycline naïve until after injury as indicated.
Mice were injured with bleomycin as previously reported (44). Briefly, 6- to 8-wk-old mice were given 40 μl of saline or bleomycin (1 or 2 U/kg as indicated) dissolved saline via oropharyngeal aspiration (28). At the indicated time points after injection, mice were euthanized and bronchoalveolar lavage (BAL) and lung samples were collected for analysis. For BAL collection, mouse lungs were lavaged with 1 ml of PBS per mouse. Samples were centrifuged, and the supernatants were used for total protein quantification. Protein was quantified by BCA assay (Pierce). The cell pellets were resuspended for total cell counting with a hemocytometer. All mice maintained in a specific pathogen-free environment until time of death, and all animal experiments were approved by the University Animal Care and Use Committee at University of Michigan.
Lung histology.
Mice were euthanized and lungs were filled with formaldehyde to 25 cmH2O pressure. The trachea was tied, and lungs were fixed in formaldehyde overnight. The lung were then embedded in paraffin, sectioned, and stained with hematoxylin-eosin or Masson’s trichrome by the McClinchey Histology Laboratory (Stockbridge, MI). Representative images were captured with an Olympus DP-70 camera and analyzed using DP-70 software.
Hydroxyproline assay.
Whole lung hydroxyproline was measured as previously described (4). Briefly, mice were euthanized and lungs were removed. Lungs were homogenized and baked in 12 N HCl overnight at 120°C. Aliquots of the samples were neutralized with citrate buffer and incubated in chloramine T solution at room temperature. Erlich’s solution was added, and the samples were incubated at 65°C. The absorbance at 540 nm was measured, and the hydroxyproline concentration was quantified against a standard curve.
Isolation and culture of AECs and fibroblasts.
Primary murine AECs were isolated as previously described (6, 48). Briefly, mice were euthanized and the lungs were perfused and lavaged. Lungs were then filled with dispase followed by low melting-temperature agarose. The lungs were then removed and incubated in dispase for 45 min at room temperature. The lungs were teased apart, and the digested lungs were sequentially filtered through 70-, 40-, and 20-μm sterile filters. The crude lung cell mixture was then negatively selected against CD32/16 and CD45 using a magnetic separator. The remaining cells were further negatively selected by adherence to plastic petri dishes. The remaining AECs were cultured on fibronectin (10 µg/ml)-coated tissue culture plates in a 37°C, 5% CO2 incubator of SAGM (Lonza) and KGF (10 ng/ml). FAK-null AECs were derived by treating floxed FAK AECs with Cre in vitro as previously described (44).
Primary murine lung fibroblasts were isolated and cultured as previously described (24). Briefly, lungs were removed from scarified mice and minced. Minced lung pieces were placed in tissue culture plates and covered with DMEM supplemented with 10% FBS enabling lung fibroblast outgrowth. Lung fibroblast were recovered by trypsinization and filtration through a 20-µm sterile filter to remove remaining lung chunks.
For AEC cell death assays, 24 h after seeding, the media were replaced with fresh serum-free media with or without with the chemical inhibitors PF-573228 (10 µM), Z-IETD-FMK (10 µM), or DMSO vehicle control. After 2 h of pretreatment with inhibitors, TGF-β (4 ng/ml) or vehicle control was added to appropriate wells. After an additional 24 h, cells were lysed in RIPA for further analysis.
Immunoblot and immunoprecipitation.
Immunoblot was performed on tissue or cells lysed in RIPA as previously described (21). Scanned immunoblots are representative of at least three independent experiments. Immunoprecipitation was performed as previously described. Briefly, cells were lysed in RIPA supplemented with phosphatase inhibitor cocktail and centrifuged to remove cell debris. The supernatants were precleared with protein A-agarose and protein G-agarose beads for 1 h, and then the beads were removed by centrifugation. The precleared lysates were then incubated with 5 µg of antibody to FAK, caspase-8, or rabbit IgG control for 4 h at 4°C. The samples were then immunoprecipitated with a mixture of protein A- and protein G-agarose beads 4°C overnight. The beads were then centrifuged and washed, and the immunocomplexes were denatured and released from the beads by incubating in sample buffer at 70°C for 10 min. The supernatants were then analyzed by immunoblot for FAK and caspase-8.
Cell death assays.
Active caspase levels were measured in cell and lung tissue lysed in RIPA using the Caspase-Glo assay, according to manufacturer’s protocol and as previously reported (2, 43, 44). Briefly, cultured cells were washed with PBS and then immediately lysed in RIPA. For whole lung analysis, lungs were immediately frozen in liquid nitrogen and stored in −80°C. Lung tissue was then pulverized while frozen using a Cryogrinder Kit (OPS Diagnostics) and then immediately lysed in ice-cold RIPA. Samples were analyzed using a Veritas Microplate Luminometer, and values are expressed as fold differences in relative luminescence units. Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining was performed on 10-µm-thick frozen lung sections embedded in OCT or cells cultured in a chamber slide under the specified condition. Tissue or cells were stained with the In Situ Cell Death Detection Kit and TMR red per manufacturer’s protocols. Stained samples were visualized on an Olympus BX-51 fluorescence microscope, and images were captured with an Olympus DP-70 camera and analyzed using DP-70 software.
Statistical analysis.
Data are expressed as box-and-whisker plots using GraphPad Prism where the middle line represents the median, the box represents the 25 and 75th percentile, and the whiskers indicate the minimal and maximal values. For evaluation of group differences, the two-tailed Student’s t-test was used assuming equal variance. Differences in survival was determined by Mantel-Cox log rank test. P < 0.05 was accepted as significant.
RESULTS
Generation of mice with lung epithelial-specific deletion of FAK.
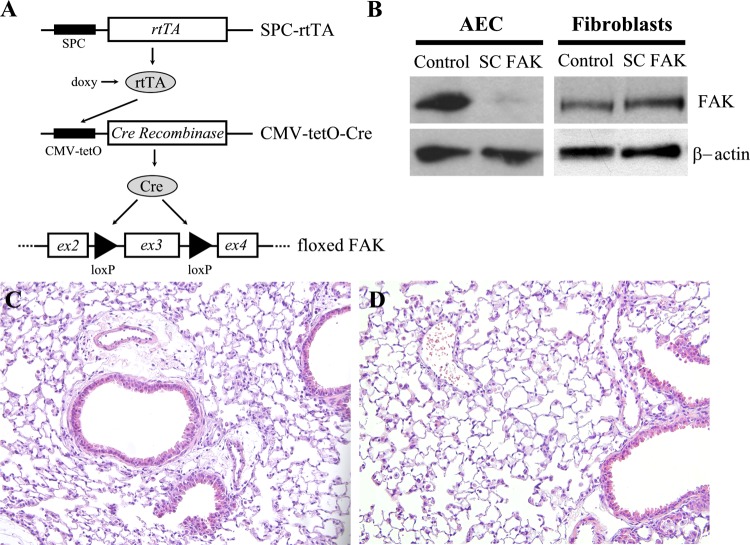
We have previously shown that the matrix signaling regulates AEC apoptosis and that in vitro inhibition or deletion of FAK within cultured AECs renders them more susceptible to TGF-β-induced apoptosis (45). Since the in vivo AEC matrix microenvironment is more complex and undergoes dynamic changes in response to injury, we therefore wanted to determine the importance of epithelial cell FAK signaling in vivo. Mice with lung epithelial cell-specific deletion of FAK were generated by crossing mice carrying the surfactant protein-C reverse tetracycline transactivator (SPC-rtTA) transgene and the CMV-tetO-Cre (tetO-Cre) transgene with floxed FAK mice. The resulting SPC-rtTA/tetO-Cre/FAKfl/fl mice (SC FAK) and littermate control mice lacking at least one of the transgenes were maintained on doxycycline containing chow from 4 to 6 wk old (Fig. 1A). Deletion of FAK was verified by immunoblot in freshly isolated AECs from SC FAK mice, which showed a ~80% loss of FAK expression compared with AECs from littermate control mice. In contrast, lung fibroblasts isolated from SC FAK mice had normal levels of FAK expression (Fig. 1B), consistent with AEC-specific deletion of FAK. Uninjured SC FAK mice were viable, were fertile, had similar weights, and had histologically normal appearing lungs compared with littermate control mice (Fig. 1, C and D).
Fig. 1.
Generation of SPC-rtTA/tetO-Cre/FAKfl/fl mice (SC FAK) mice. A: alveolar epithelial cell (AEC)-specific deletion of focal adhesion kinase (FAK) is achieved by in triple transgenic SC FAK mice carrying the surfactant protein-C promoter-reverse tetracycline transactivator (SPC-rtTA) and CMV promoter-tetO-cre recombinase (CMV-tetO-Cre) transgenes crossed into floxed FAK background. Doxycycline chow was started postnatally at 1 mo and continued for 2 wk. B: immunoblot confirming that AECs from SC FAK mice have much less FAK expression compared with littermate control mouse lacking 1 of the 3 transgenes. Fibroblasts from SC FAK mice have similar levels of FAK compared with control mice. C and D: uninjured hematoxylin-eosin-stained lung sections (×200) of littermate control (C) and SC FAK mice (D).
SC FAK mice have increased injury and death after bleomycin.
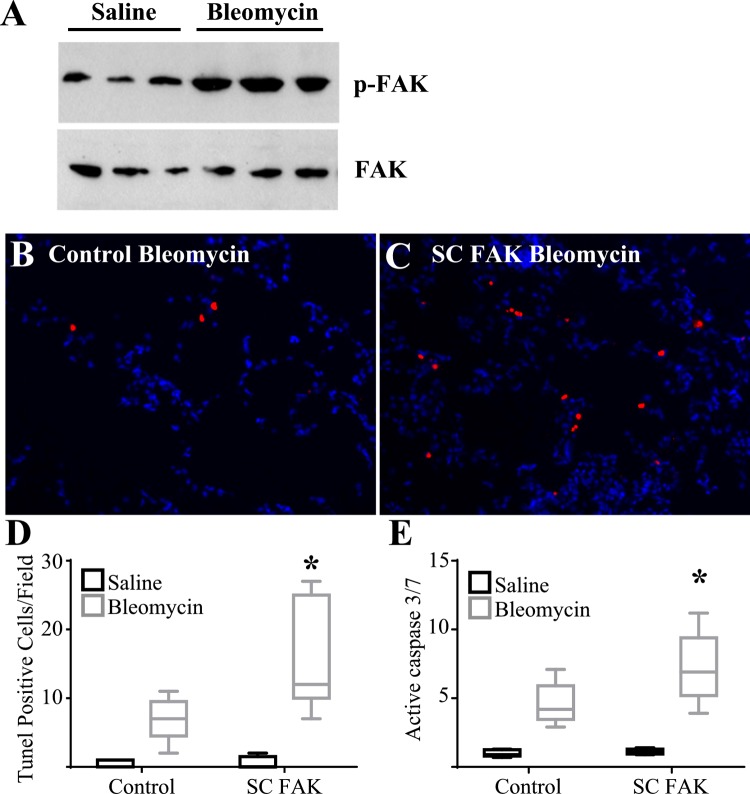
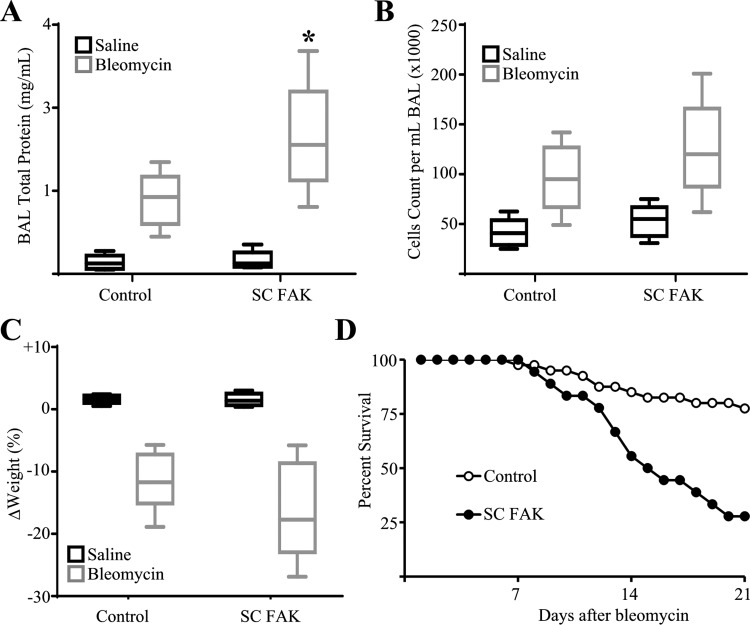
Wild-type mice were given a single intrapulmonary dose of bleomycin (2 U/kg) or saline by oropharyngeal aspiration. Five days later, whole lung lysates from mice treated with bleomycin were found to have increased levels of FAK phosphorylation compared with saline treated mice (Fig. 2A) indicating activation of FAK signaling at this early time point after injury. Next, SC FAK and littermate control mice were injured with bleomycin and analyzed for the extent of apoptosis and lung injury after 5 days. As expected, SC FAK mice exhibited more TUNEL-positive cells and significantly greater levels of active caspase-3/7 compared with littermate control mice treated with bleomycin (Fig. 2). We have previously shown that AECs represent the majority of apoptosis at this time point after bleomycin (45). Importantly, SC FAK mice treated with saline do not demonstrate elevated levels of apoptosis suggesting that that loss of FAK within AECs itself is not sufficient to induce apoptosis but rather sensitizes AECs to apoptosis triggered by the bleomycin injury. SC FAK mice exhibited signs of increased lung injury with greater protein in the BAL fluid although there was no difference in the numbers of inflammatory cells in the BAL (Fig. 3, A and B). SC FAK mice also exhibited a trend toward greater weight loss (Fig. 3C). To determine if greater AEC death might lead to persistent or progressive injury, SC FAK and littermate control mice were injured with bleomycin as before and followed for 21 days. SC FAK mice exhibited much greater mortality compared with littermate control mice with ~75% of SC FAK mice dying by day 21 compared with <20% in the littermate control group treated with bleomycin (Fig. 3D).
Fig. 2.
Mice with lung epithelial cell-specific deletion of FAK have greater cellular apoptosis after bleomycin. A: 5 days after intrapulmonary saline or bleomycin injury, whole lung lysate was analyzed for phospho-FAK (p-FAK) and total FAK by immunoblot. B–D: 5 days after intrapulmonary bleomycin injury, cellular apoptosis was assessed by lung sections from littermate control mice (B) and SC FAK mice (C) that were stained with terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) (×200) and TUNEL-positive cells per field were quantified (D). P < 0.05; n = 5. E: 5 days after bleomycin or saline, whole lung active caspase-3/7 was measured in SC FAK and littermate control mice; n = 5–9. *P < 0.01, compared with bleomycin control mice.
Fig. 3.
Mice with lung epithelial cell-specific deletion of FAK have greater lung injury and death after bleomycin. A and B: 5 days after bleomycin or saline, SC FAK or littermate control mice were analyzed for total bronchoalveolar lavage (BAL) protein (A) and cell count (B). SC FAK mice have greater BAL protein after bleomycin and a nonstatistically significant trend toward greater cell count compared with littermate control mice treated with bleomycin. C: SC FAK mice have a trend toward greater weight loss after bleomycin compared with littermate control mice. *P < 0.05, compared with littermate control mice treated with bleomycin; n = 5–9. D: SC FAK mice (n = 18) have less survival compared with control mice (n = 40) after bleomycin (P < 0.01).
Mice with lung epithelial deletion of FAK have more fibrosis after bleomycin.
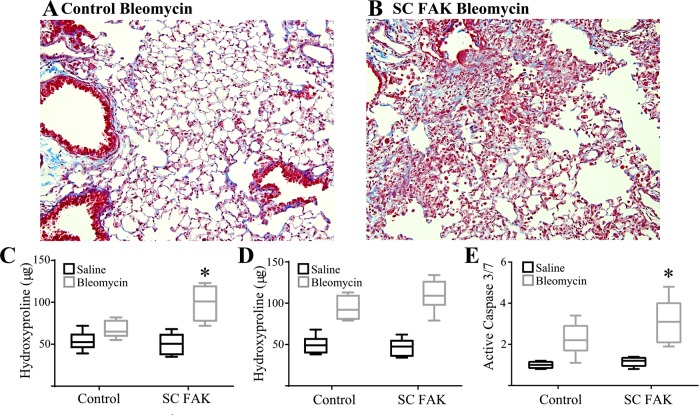
We have previously shown that specific induction of AEC death is sufficient to initiate lung fibrosis (7). This suggests that SC FAK might demonstrate a greater propensity for fibrosis after bleomycin due to greater AEC apoptosis. Since our usual dose of bleomycin resulted in an exuberance of death in mice, we used a lower dose of bleomycin. SC FAK and littermate control mice were treated with 1 U/kg of bleomycin by oropharyngeal aspiration and followed for 21 days. As opposed to the higher dose, none of the mice died during this period. Mice were analyzed for fibrosis by visualizing trichrome-stained lung sections and quantified by the hydroxyproline assay. Littermate control mice demonstrated a modest and nonsignificant trend toward greater fibrosis with this bleomycin dose. SC FAK mice, however, had an ~80% increase in hydroxyproline that was significantly greater than the hydroxyproline content of littermate control lungs after bleomycin (Fig. 4, A–C). To determine the extent to which AEC FAK activation in the early vs. late phase of injury after bleomycin contributes to the fibrotic process, doxycycline naïve SC FAK and littermate control mice were injured with saline or bleomycin (2 U/kg) and initiated and maintained on doxycycline 10 days later to delete FAK within AECs. By 3 wk, there were similar (~20%) deaths in the littermate control mice and SC FAK mice treated with bleomycin and no deaths in mice treated with saline. As expected, both littermate control mice and SC FAK mice treated with bleomycin had induction of fibrosis (Fig. 4D). SC FAK mice had significantly greater whole lung caspase-3/7 activation (Fig. 4E) and a nonstatistically significant trend (P = 0.12) toward greater fibrosis (Fig. 4D) compared with littermate control mice treated with bleomycin.
Fig. 4.
SC FAK mice are more susceptible to fibrosis after low-dose bleomycin. A and B: 3 wk after low-dose bleomycin (1 U/kg), the extent of fibrosis was visualized from trichrome stained lung sections (×200) of littermate control mice (A) and SC FAK mice (B) (×200). C: 3 wk after intrapulmonary low-dose bleomycin or saline, collagen content of littermate control mice and SC FAK mice was assessed by hydroxyproline; n = 5–9. *P < 0.05, compared with littermate control mice treated with bleomycin. D and E: 3 wk after intrapulmonary bleomycin (2 U/kg) or saline and 11 days after initiation of doxycycline chow, littermate control and SC FAK lung hydroxyproline (D) and active caspase-3/7 (E) were determined; n = 7–8. *P < 0.05.
Active FAK sequesters and prevents caspase-8 activation.
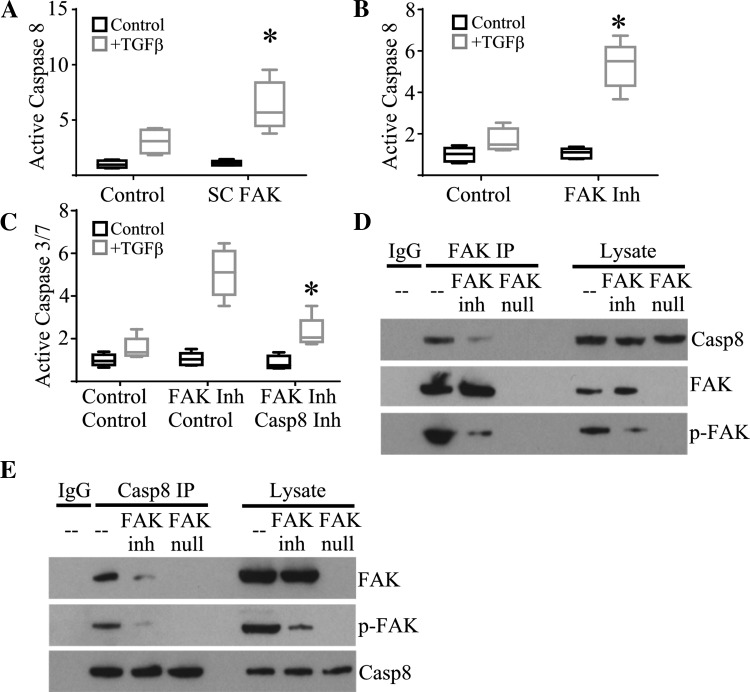
Activated FAK has previously been reported to form a direct association with caspase-8 (3). Caspase-8 activation is a key initiator of the extrinsic apoptotic cascade leading to activation of caspase-3 and subsequent apoptosis. The functional importance of caspase-8 activation on TGF-β-induced apoptosis and the presence of a FAK/caspase-8 complex has not been demonstrated in AECs. Primary AECs were cultured on tissue culture plates coated with fibronectin, which we previously reported induces FAK activation and confers resistance to TGF-β-induced apoptosis. As expected, control primary AECs had modest activation of caspase-8 after TGF-β treatment. In contrast, primary AECs from SC FAK mice had low levels of baseline caspase-8 activation but a robust increase in active caspase-8 24 h after TGF-β treatment (Fig. 5A). To further support a role for FAK activation in preventing caspase-8 activation, primary AECs from wild-type mice were cultured as above and then pretreated with a FAK inhibitor, PF-573228 (10 µM), or DMSO vehicle control before TGF-β stimulation. Similar to AECs with genetic deletion of FAK, inhibition of FAK activation resulted in augmented TGF-β-induced caspase-8 activation (Fig. 5B).
Fig. 5.
Active FAK inhibits caspase-8-mediated activation of apoptosis. A: caspase-8 activation by primary AECs from SC FAK or littermate control mice in response to transforming growth factor-β (TGF-β); n = 5. P < 0.05, compared with control AECs treated with TGF-β. B: caspase-8 activation by primary AECs from wild-type mice in response to TGF-β and treated with PF-573228 (FAK Inh) or vehicle control; n = 5. *P < 0.05, compared with AECs treated with DMSO control and TGF-β. C: caspase-3/7 activation by wild-type primary AECs in response to TGF-β and treated with PF-573228 (FAK Inh) or vehicle control and Z-IETD-FMK (Casp8 Inh) or vehicle control; n = 5. *P < 0.05, compared with AEC treated with TGF-β and FAK inhibitor without Casp8 Inh. D: immunoprecipitation (IP) for FAK from wild-type or FAK-null primary AEC lysate treated with PF573228 or vehicle control and immunoblotted for Casp8, FAK, and phospho-FAK (p-FAK). Immunoblot of 5% of lysate used for immunoprecipitation (lysate) is also shown. E: immunoprecipitation for Casp8 from wild-type or FAK-null primary AEC lysate treated with PF573228 or vehicle control and immunoblotted for Casp8, FAK, and p-FAK. Immunoblot of 5% of lysate used for immunoprecipitation (lysate) is also shown.
Caspase-3/7 activation is regarded as a final common pathway for canonical apoptosis (25). Caspase-3/7 can be activated by multiple pathways. To determine the extent to which caspase-8 activation contributes to subsequent activation of caspase-3/7, primary wild-type AECs were cultured and treated with the FAK inhibitor as above. Some wells were cotreated with a caspase-8 inhibitor, Z-IETD-FMK (10 µM), before treatment with TGF-β. As expected, inhibition of FAK led to dramatic activation of caspase-3/7; however, coinhibition of FAK and caspase-8 dramatically reduced TGF-β-induced caspase-3/7 activation (Fig. 5C).
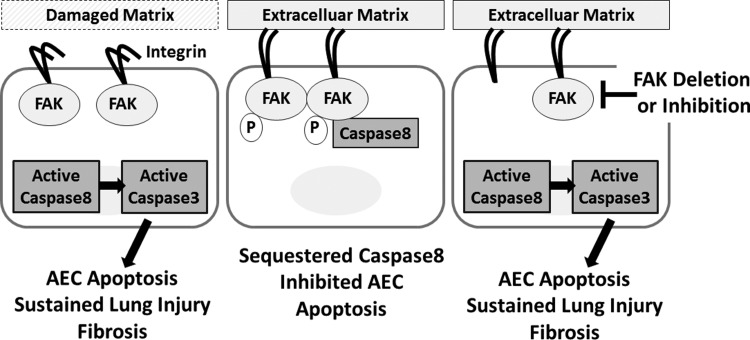
Finally, wild-type and FAK-null AECs were cultured as above and immunoprecipated with FAK or caspase-8. We found that matrix-activated FAK coimmunopreciptitates with caspase-8 confirming a direct interaction between active FAK and caspase-8. In contrast, inhibition of FAK activity and autophosphorylation with PF-573228 significantly inhibited FAK coimmunoprecipitation with caspase-8 (Fig. 5, D and E). Immunoblot for p-FAK also indicates that at least some of the FAK in association with caspase-8 is in the phosphorlyated form. As expected, deletion of FAK completely abrogated FAK coprecipitation with caspase-8. Collectively, these results suggest that matrix-mediated FAK activation may function to sequester or otherwise inhibit activation of caspase-8, which in turn inhibits activation of caspase-3/7 and subsequent apoptosis (Fig. 6).
Fig. 6.
Proposed mechanism. Injury promotes damage to the underlying alveolar matrix leading to weak integrin-mediated FAK activation and AEC susceptibility to apoptosis. Strong integrin-matrix engagement leads to increased FAK phosphorylation and direct inhibition of caspase-8-mediated activation of caspase-3 and subsequent apoptosis. FAK inhibition or AEC-specific FAK deletion leads to AEC susceptibility to apoptosis promoting sustained lung injury and fibrosis.
DISCUSSION
Our results support a model in which injury initiates a coordinated repair process involving activation of similar intracellular signaling pathways within different cell types (5, 30, 39, 40). After injury, there is early epithelial cell death and destruction of the underlying tissue architecture. The subsequent repair process is coordinated and staged. There are sequential changes to the extracellular matrix with loss of the underlying basement membrane, rapid deposition of a plasma-derived provisional matrix followed by cellular generation of fibrillar collagen. There are also dynamic changes to the cellular composition coordinated by programmed cell death, cellular proliferation, differentiation, and recruitment. TGF-β is a primary driver of both the initial injury repair process and subsequent fibrosis, in part through stimulation of ongoing epithelial cell death and fibrogenic activation of fibroblasts leading to myofibroblast differentiation (10, 13, 14, 34). We and others have recently demonstrated a prominent role for matrix/integrin signaling in coordinating changes in the extracellular matrix with cell behavior during these processes (18, 22, 36, 44, 47). As matrix remodeling progresses, there is increased matrix stiffness leading to increased activation of matrix-related intracellular signaling pathways. These common pathways are activated on multiple cell types aiding in a coordinated shift to the next stage of the response to injury with increased activation of TGF-β, suppressed epithelial cell apoptosis in areas with sufficient matrix remodeling and ongoing epithelial cell apoptosis in persistently injured areas and augmented activation of fibrogenic effector cells. Integrin engagement to the extracellular matrix activates signaling through a number of integrin associated pathways such as FAK, Src, and Rho (15, 20). Activation of these matrix related signaling pathways are known to augment fibroblast activation. This has fostered interest in targeting these pathways to inhibit progressive fibrosis. However, generalized inhibition of FAK will likely influence cell behavior beyond fibroblast activation with unexpected or problematic outcomes within a coordinated multicellular process. A similar dilemma has been identified with attempts to target other prominent fibroblast survival and activation pathways. For example, the abl kinase inhibitor imatinib was shown to inhibit fibroblast activation; however, subsequent studies demonstrated a prominent role for abl kinase activity in AEC survival. Imatinib treatment attenuated fibrosis in animal models, but this multicellular complexity likely contributed to the negative clinical trial of imatinib in idiopathic pulmonary fibrosis (8, 9, 41). Similarly, generalized inhibition of FAK has been shown to attenuate fibrosis in animal models, but concurrent activation of profibrotic pathways may complicate attempts at targeting this pathway for idiopathic pulmonary fibrosis therapy.
Epithelial cell death is a prominent feature of acute lung injury, and there is also strong evidence for continued epithelial cell apoptosis in progressive fibrosis (2, 31, 42). Histologic samples from patients with fibrotic lung diseases, including idiopathic pulmonary fibrosis, demonstrate marked AEC apoptosis (35). While AEC apoptosis may be a necessary step for clearing away the damaged tissue and facilitating deposition of the provisional and fibrotic tissue, AEC apoptosis can also stimulate further changes to the lung repair process (7, 37). Numerous studies have demonstrated an active role for AEC apoptosis in stimulating ongoing fibrosis. Inhibition of apoptosis through genetic manipulation or chemical inhibition has been shown to attenuate fibrosis while AEC-specific induction of apoptosis is sufficient to initiate robust fibrosis (35). We have previously shown that FAK activation can regulate matrix-mediated AEC resistance to apoptosis (45).
FAK is a nonreceptor tyrosine kinase that is associated with the cytoplasmic tail of integrins (15, 20). Engagement to the matrix leads to integrin clustering and FAK autophosphorylation at tyrosine 397. This phosphorylation induces conformational changes allowing FAK phosphorylation by other kinases such as Src resulting in greater FAK activation. FAK interaction with caspases has previously been shown, and this interaction may reciprocally influence the downstream signaling events of each partner (1, 12, 16, 33, 38, 49). Some studies have suggested that caspase proteolytic activation leads to FAK degradation promoting cell detachment and greater mobility. Conversely, matrix engagement is known to inhibit programmed cell death in part due to FAK activation. In our SC FAK mice, lung epithelial cells did not spontaneously undergo programmed cell death or anoikis, rather deletion of FAK led to greater sensitivity to induction of apoptosis. Our studies suggest that active FAK forms a direct association with caspase-8 and prevents activation of the apoptotic cascade through this caspase.
Although TGF-β is known to induce epithelial cell apoptosis, the exact mechanism remains unclear. Apoptosis primarily occurs through canonical extrinsic or intrinsic pathways ultimately leading to activation of caspase-3 (25, 26). The extrinsic pathway is initiated by a death receptors, such as Fas, and is mediated through caspase-8. TGF-β-mediated activation of caspase-8 suggests at least some involvement of the extrinsic pathway, but the TGF-β receptor has not been described as a death receptor and likely activates this pathway indirectly. Caspase-8 has previously been shown to associate directly with integrins, and TGF-β signaling can converge with integrin signaling at many foci. Thus an integrin/FAK/caspase-8 complex may represent an important regulatory point for epithelial cell death depending on the matrix conditions.
Prior studies have shown that generalized inhibition of FAK with chemical inhibitors can block fibroblast activation and attenuate fibrosis (11, 27). In a complex multicellular process, disruption of part of the process may be sufficient to generally attenuate fibrosis even if it also results in activation of profibrotic pathways. Our results suggest that signaling induced by the remodeling matrix may lead to activation of FAK and other matrix-associated signaling proteins in different cell types as part of a coordinated process in which there is suppression of epithelial cell apoptosis with attenuation of continued lung injury with concurrent activation of fibroblast deposition of collagen. Deletion of FAK before injury led to augmented fibrosis after low-dose bleomycin consistent with the notion that AEC apoptosis itself can activate profibrotic pathways. TGF-β activation persists through the later stages of fibrosis and is an important initiator of AEC apoptosis indicating the potential need for suppressed AEC apoptosis during the later stages of fibrosis. We found that deletion of FAK 10 days after bleomycin injury led to increased caspase activation 21 days after injury consistent with the notion that even in the later stages of fibrosis, there is still FAK-mediated suppression of AEC apoptosis. We did not observe a statistically significant increase in fibrosis with deletion of FAK after injury suggesting a greater effect of AEC apoptosis and AEC FAK signaling during the early stages of injury on subsequent fibrosis. Targeting FAK deletion or inhibition at different time points after injury are currently being pursued and may help clarify the mechanism. In a tightly controlled setting, generalized inhibition of FAK and matrix signaling achieves the desired antifibrotic effect despite activation of potentially counterproductive pathways. Generalized inhibition of FAK in established fibrosis may be sufficient for treatment of fibrosis, but further studies should focus on the influence of matrix signaling on multiple cell types during injury and fibrosis to more precisely target profibrotic pathways. Our studies also suggest that antifibrotic therapy would be significantly enhanced by identification of fibroblast and epithelial cell-specific pathways and methods, such as nanoparticle technology, to deliver therapeutic agents to a specific cell type.
In summary, we have demonstrated that AEC activation of FAK is necessary to limit lung injury and AEC apoptosis in a model of lung fibrosis. Active FAK associates with caspase-8 and prevents activation of the caspase cascade. Because AEC apoptosis is known to be sufficient to drive fibrosis, generalized inhibition of FAK may lead to antifibrotic and profibrotic influences on the fibrotic process.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-108904 (to K. K. Kim).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K.W., M.A., and K.K.K. conceived and designed research; A.K.W., M.A., S.J., and K.K.K. performed experiments; A.K.W., M.A., S.J., and K.K.K. analyzed data; A.K.W., M.A., S.J., and K.K.K. interpreted results of experiments; A.K.W. and K.K.K. prepared figures; A.K.W. and K.K.K. drafted manuscript; A.K.W., M.A., S.J., and K.K.K. edited and revised manuscript; A.K.W., M.A., S.J., and K.K.K. approved final version of manuscript.
REFERENCES
- 1.Alisi A, Arciello M, Petrini S, Conti B, Missale G, Balsano C. Focal adhesion kinase (FAK) mediates the induction of pro-oncogenic and fibrogenic phenotypes in hepatitis C virus (HCV)-infected cells. PLoS One 7: e44147, 2012. doi: 10.1371/journal.pone.0044147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley SL, Sisson TH, Wheaton AK, Kim KK, Wilke CA, Ajayi IO, Subbotina N, Wang S, Duckett CS, Moore BB, Horowitz JC. Targeting inhibitor of apoptosis proteins protects from bleomycin-induced lung fibrosis. Am J Respir Cell Mol Biol 54: 482–492, 2016. doi: 10.1165/rcmb.2015-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbero S, Mielgo A, Torres V, Teitz T, Shields DJ, Mikolon D, Bogyo M, Barilà D, Lahti JM, Schlaepfer D, Stupack DG. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer Res 69: 3755–3763, 2009. doi: 10.1158/0008-5472.CAN-08-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauman KA, Wettlaufer SH, Okunishi K, Vannella KM, Stoolman JS, Huang SK, Courey AJ, White ES, Hogaboam CM, Simon RH, Toews GB, Sisson TH, Moore BB, Peters-Golden M. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest 120: 1950–1960, 2010. doi: 10.1172/JCI38369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackwell TS, Tager AM, Borok Z, Moore BB, Schwartz DA, Anstrom KJ, Bar-Joseph Z, Bitterman P, Blackburn MR, Bradford W, Brown KK, Chapman HA, Collard HR, Cosgrove GP, Deterding R, Doyle R, Flaherty KR, Garcia CK, Hagood JS, Henke CA, Herzog E, Hogaboam CM, Horowitz JC, King TE Jr, Loyd JE, Lawson WE, Marsh CB, Noble PW, Noth I, Sheppard D, Olsson J, Ortiz LA, O’Riordan TG, Oury TD, Raghu G, Roman J, Sime PJ, Sisson TH, Tschumperlin D, Violette SM, Weaver TE, Wells RG, White ES, Kaminski N, Martinez FJ, Wynn TA, Thannickal VJ, Eu JP. Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am J Respir Crit Care Med 189: 214–222, 2014. doi: 10.1164/rccm.201306-1141WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol 14: 309–315, 1996. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 7.Courey AJ, Horowitz JC, Kim KK, Koh TJ, Novak ML, Subbotina N, Warnock M, Xue B, Cunningham AK, Lin Y, Goldklang MP, Simon RH, Lawrence DA, Sisson TH. The vitronectin-binding function of PAI-1 exacerbates lung fibrosis in mice. Blood 118: 2313–2321, 2011. doi: 10.1182/blood-2010-12-324574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR; Imatinib-IPF Study Investigators . Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am J Respir Crit Care Med 181: 604–610, 2010. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 9.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest 114: 1308–1316, 2004. doi: 10.1172/JCI200419603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, Boomershine CS, Ortiz C, Sherrill TP, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. TGFβ signaling in lung epithelium regulates bleomycin-induced alveolar injury and fibroblast recruitment. Am J Physiol Lung Cell Mol Physiol 300: L887–L897, 2011. doi: 10.1152/ajplung.00397.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan GP, Wang W, Zhao H, Cai L, Zhang PD, Yang ZH, Zhang J, and Wang X. Pharmacological inhibition of focal adhesion kinase attenuates cardiac fibrosis in mice cardiac fibroblast and post-myocardial-infarction models. Cell Physiol Biochem 37: 515–526, 2015. doi: 10.1159/000430373. [DOI] [PubMed] [Google Scholar]
- 12.Fanucchi S, Veale RB. Delayed caspase-8 activation and enhanced integrin β1-activated FAK underpins anoikis in oesophageal carcinoma cells harbouring mt p53-R175H. Cell Biol Int 35: 819–826, 2011. doi: 10.1042/CBI20100894. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc 9: 111–116, 2012. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 14.Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans 35: 661–664, 2007. doi: 10.1042/BST0350661. [DOI] [PubMed] [Google Scholar]
- 15.Giancotti FG, Ruoslahti E. Integrin signaling. Science 285: 1028–1032, 1999. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg RS, Bernstein AM, Benezra M, Gelman IH, Taliana L, Masur SK. FAK-dependent regulation of myofibroblast differentiation. FASEB J 20: 1006–1008, 2006. doi: 10.1096/fj.05-4838fje. [DOI] [PubMed] [Google Scholar]
- 17.Hagimoto N, Kuwano K, Inoshima I, Yoshimi M, Nakamura N, Fujita M, Maeyama T, Hara N. TGF-beta 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. J Immunol 168: 6470–6478, 2002. doi: 10.4049/jimmunol.168.12.6470. [DOI] [PubMed] [Google Scholar]
- 18.Hinz B. Matrix mechanics and regulation of the fibroblast phenotype. Periodontol 2000 63: 14–28, 2013. doi: 10.1111/prd.12030. [DOI] [PubMed] [Google Scholar]
- 19.Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal 19: 761–771, 2007. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 21.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 103: 13180–13185, 2006. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, Chapman HA. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest 119: 213–224, 2009. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinoshita K, Aono Y, Azuma M, Kishi J, Takezaki A, Kishi M, Makino H, Okazaki H, Uehara H, Izumi K, Sone S, Nishioka Y. Antifibrotic effects of focal adhesion kinase inhibitor in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol 49: 536–543, 2013. doi: 10.1165/rcmb.2012-0277OC. [DOI] [PubMed] [Google Scholar]
- 24.Kleaveland KR, Velikoff M, Yang J, Agarwal M, Rippe RA, Moore BB, Kim KK. Fibrocytes are not an essential source of type I collagen during lung fibrosis. J Immunol 193: 5229–5239, 2014. doi: 10.4049/jimmunol.1400753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuwano K. Epithelial cell apoptosis and lung remodeling. Cell Mol Immunol 4: 419–429, 2007. [PubMed] [Google Scholar]
- 26.Kuwano K. Involvement of epithelial cell apoptosis in interstitial lung diseases. Intern Med 47: 345–353, 2008. doi: 10.2169/internalmedicine.47.0713. [DOI] [PubMed] [Google Scholar]
- 27.Lagares D, and Kapoor M. Targeting focal adhesion kinase in fibrotic diseases. BioDrugs 27: 15–23, 2013. doi: 10.1007/s40259-012-0003-4. [DOI] [PubMed] [Google Scholar]
- 28.Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp Lung Res 32: 181–199, 2006. doi: 10.1080/01902140600817465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leask A. Focal adhesion kinase: a key mediator of transforming growth factor beta signaling in fibroblasts. Adv Wound Care (New Rochelle) 2: 247–249, 2013. doi: 10.1089/wound.2012.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 18: 816–827, 2004. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 31.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, Homer RJ, Elias JA. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med 200: 377–389, 2004. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6: 56–68, 2005. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 33.Owen KA, Abshire MY, Tilghman RW, Casanova JE, Bouton AH. FAK regulates intestinal epithelial cell survival and proliferation during mucosal wound healing. PLoS One 6: e23123, 2011. doi: 10.1371/journal.pone.0023123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-beta is a critical mediator of acute lung injury. J Clin Invest 107: 1537–1544, 2001. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 3: 364–372, 2006. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 36.Sheppard D. Modulation of acute lung injury by integrins. Proc Am Thorac Soc 9: 126–129, 2012. doi: 10.1513/pats.201112-052AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 181: 254–263, 2010. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 14: 598–610, 2014. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thannickal VJ, Toews GB, White ES, Lynch JP III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417, 2004. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 40.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: ultimate and proximate causes. J Clin Invest 124: 4673–4677, 2014. doi: 10.1172/JCI74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vittal R, Zhang H, Han MK, Moore BB, Horowitz JC, Thannickal VJ. Effects of the protein kinase inhibitor, imatinib mesylate, on epithelial/mesenchymal phenotypes: implications for treatment of fibrotic diseases. J Pharmacol Exp Ther 321: 35–44, 2007. doi: 10.1124/jpet.106.113407. [DOI] [PubMed] [Google Scholar]
- 42.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 43.Warsinske HC, Wheaton AK, Kim KK, Linderman JJ, Moore BB, Kirschner DE. Computational modeling predicts simultaneous targeting of fibroblasts and epithelial cells is necessary for treatment of pulmonary fibrosis. Front Pharmacol 7: 183, 2016. doi: 10.3389/fphar.2016.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheaton AK, Velikoff M, Agarwal M, Loo TT, Horowitz JC, Sisson TH, Kim KK. The vitronectin RGD motif regulates TGF-β-induced alveolar epithelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol 310: L1206–L1217, 2016. doi: 10.1152/ajplung.00424.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol 201: 343–354, 2003. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol 87: 601–615, 2008. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Velikoff M, Agarwal M, Disayabutr S, Wolters PJ, Kim KK. Overexpression of inhibitor of DNA-binding 2 attenuates pulmonary fibrosis through regulation of c-Abl and Twist. Am J Pathol 185: 1001–1011, 2015. doi: 10.1016/j.ajpath.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zouq NK, Keeble JA, Lindsay J, Valentijn AJ, Zhang L, Mills D, Turner CE, Streuli CH, Gilmore AP. FAK engages multiple pathways to maintain survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J Cell Sci 122: 357–367, 2009. doi: 10.1242/jcs.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]