Abstract
Background.
Cardiac dysfunction is present in >40% of sepsis patients and is associated with mortality rates of up to 70%. Recent evidence suggests that glycolytic metabolism plays a critical role in host defense and inflammation. Activation of Toll-like receptors on immune cells can enhance glycolytic metabolism. This study investigated whether modulation of glycolysis by inhibition of hexokinase will be beneficial to septic cardiomyopathy.
Methods.
Male C57B6/J mice were treated with a hexokinase inhibitor (2-deoxy-d-glucose [2-DG], 0.25–2 g/kg, n = 6–8) before cecal ligation and puncture (CLP) induced sepsis. Untreated septic mice served as control. Sham surgically operated mice treated with or without the 2-DG inhibitor served as sham controls. Cardiac function was assessed 6 hours after CLP sepsis by echocardiography. Serum was harvested for measurement of inflammatory cytokines and lactate.
Results.
Sepsis-induced cardiac dysfunction was significantly attenuated by administration of 2-DG. Ejection fraction and fractional shortening in 2-DG–treated septic mice were significantly (P < .05) greater than in untreated CLP mice. 2-DG administration also significantly improved survival outcome, reduced kidney and liver injury, attenuated sepsis-increased serum levels of tumor necrosis factor α and interleukin 1β as well as lactate, and enhanced the expression of Sirt1 and Sirt3 in the myocardium, which play an important role in mitochondrial function and metabolism. In addition, 2-DG administration suppresses sepsis-increased expression of apoptotic inducers Bak and Bax as well as JNK phosphorylation in the myocardium.
Conclusions.
Glycolytic metabolism plays an important role in mediating sepsis-induced septic cardiomyopathy. The mechanisms may involve regulation of inflammatory response and apoptotic signaling.
Keywords: sepsis, cardiomyopathy, glycolysis, 2-deoxy-D-glucose, inflammatory responses.
Severe sepsis and septic shock are major healthcare problems throughout the world each year [1]. Cardiovascular dysfunction contributes to sepsis-induced morbidity and mortality [2]. Activation of innate immune and inflammatory responses mediated by Toll-like receptors (TLRs) have been demonstrated to play a critical role in sepsis-induced multiple organ failure [3–5]. Importantly, targeting TLR-mediated inflammatory responses has been shown to attenuate sepsis-induced cardiovascular dysfunction and improve outcome of sepsis [3, 6].
Recent evidence suggests that metabolic reprogramming plays a critical role in host defense and inflammation [7]. Both TLRs and inflammatory cytokines involve regulation of metabolic reprogramming [8]. TLR ligands have been reported to induce metabolic switching from oxidative phosphorylation to aerobic glycolysis in macrophages [9], dendritic cells [8, 9] and lymphocytes [10]. The phenomenon of the metabolic switch from oxidative phosphorylation to aerobic glycolysis is called the Warburg effect [11]. On the other hand, aerobic glycolysis and the intermediates generated from metabolism can, in turn, regulate immune function [12].
Clinical studies have shown that the serum lactate levels positively correlate with outcome in septic patients [13, 14], indicating that sepsis and septic shock are associated with hyperlactatemia [15]. Lactate has been reported to activate innate immune and inflammatory responses via TLR-mediated NF-κB signaling and inflammasomes [16]. Yang et al have shown that lactate promotes HMGB1 acetylation and release from activated macrophages [17]. Lactate has also been reported to have a potent immunosuppressive effect on immune cells [18]. It is possible that sepsis-enhanced glycolytic metabolism could amplify innate immune and inflammatory responses in the early stage of disease and contribute to immunosuppressive effects in the later stages of sepsis. Therefore, preservation and maintenance of metabolic homeostasis could play an important role in the outcome of sepsis.
Targeting glycolysis with 2-deoxy-d-glucose (2-DG) is a therapeutic approach in cancer treatment [19]. Hexokinase is the initial and rate-limiting enzyme in glycolysis [20]. Hexokinase converts 2-DG to phosphorylated 2-DG (2-deoxy-d-glucose-6-phosphate [2-DG-6p]) [20]. However, 2-DG-6p cannot be metabolized by phosphoglucose isomerase, but it can inhibit hexokinase activity, thereby inhibiting glycolysis [20]. Tannahill et al reported that inhibition of glycolysis with 2-DG suppresses lipopolysaccharide (LPS)–induced hypoxia-inducible factor 1α (HIF-1α) and its target interleukin 1β (IL-1β) in mouse macrophages [21]. Yang et al demonstrated that inhibition of metabolic regulator pyruvate kinase M2 by shikonin markedly reduced the serum levels of lactate and HMGB1, and protected mice from lethal endotoxemia [17]. However, whether modulation of glycolysis by 2-DG will alter cardiac function in polymicrobial sepsis has not been reported.
In the present study, we observed that 2-DG administration significantly improved cardiac function and survival outcome in polymicrobial sepsis, which are positively correlated with decreased proinflammatory cytokine production and reduced myocardial apoptosis.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice were obtained from Jackson Laboratory. The experiments outlined in this manuscript conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (8th edition, 2011). The animal care and experimental protocols were approved by the Eastern Tennessee State University Committee on Animal Care.
CLP Polymicrobial Sepsis Model
Cecal ligation and puncture was performed to induce polymicrobial sepsis in mice as previously described [3, 6, 22–24]. In brief, the mice were anesthetized by 5.0% isoflurane. A midline incision was made on the anterior abdomen and the cecum was exposed and ligated with a 4-0 suture. Two punctures were made through the cecum with a 20-gauge needle and feces were extruded from the holes. For the induction of chronic CLP sepsis model, one puncture was made with a 26-gauge needle. The abdomen was then closed in 2 layers. Sham surgically operated mice served as sham control. Immediately following surgery, a single dose of resuscitative fluid (lactated Ringer’s solution, 50 mL/kg body weight) was administered by subcutaneous injection [3, 22]. Mice were treated with 2-DG (0.25, 0.5, 1, and 2 g/kg) 3 hours prior to CLP or sham surgical operation.
Echocardiography
Transthoracic 2-dimensional M-mode echocardiogram and pulsed wave Doppler spectral tracings (Toshiba Aplio 80 Imaging System, Tochigi, Japan) were used to measure left ventricular (LV) wall thickness, LV end-systolic diameter, and LV end-diastolic diameter. Percentage of fractional shortening (FS%) and ejection fraction (EF%) were calculated as described previously [3, 23].
In Vitro Experiments
Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial basal medium 2 supplemented with endothelial cell growth medium-2 (EGM-2) SingleQuot Kits (Lonza) [25]. After reaching 70%–80% confluence, HUVECs were treated with 2-DG at 5 µmol 12 hours before LPS stimulation. Six hours after LPS stimulation, the cells were harvested and the cellular proteins were prepared for Western blot [25].
Measurement of Lactate, Aspartate Aminotransferase, Creatine Kinase, and Creatinine
The serum levels of lactate were assessed with the Lactate Colorimetric Assay Kit (BioVision, Milpitas, California). Serum levels of aspartate aminotransferase (AST), creatine kinase, and creatinine were measured with commercially available kits according to the instructions provided by the manufacturer.
Measurement of NAD+, NADH, and NAD+/NADH Ratio
The levels of nicotinamide adenine dinucleotide (NAD+, oxidized form), NADH (reduced form), and NAD+/NADH ratio in the heart tissue were assessed with the NAD+/NADH Quantification Colorimetric Kit (BioVision ) according to the instructions provided by the manufacturer.
Tissue Accumulation of Immune Cells
Accumulation of immune cells, including neutrophils, monocytes, and macrophages in heart tissues were examined using an antibody NIMP-R14, which recognizes Ly-6G and Ly-6C of immune cells (Santa Cruz Biotechnology) [3]. Three slides from each block were evaluated, counterstained with hematoxylin, and examined with brightfield microscopy. The results are expressed as the numbers of immune cells/field (×40) [3, 24].
In Situ Apoptosis Assay
Myocardial apoptosis was examined as described previously [22] using the in situ cell death detection kit (Roche). Three slides from each block were evaluated for percentage of apoptotic cells and 4 fields on each slide were examined at the border areas using a defined rectangular field area with ×20 magnification. A total of 100 nuclei were counted. Numbers of apoptotic cardiac myocytes are presented as the percentage of total cells counted.
Western Blot
Western blot was performed as described previously [3, 22, 24]. In brief, cellular proteins were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred onto Hybond enhanced chemiluminescence (ECL) membranes (Amersham Pharmacia, Piscataway, New Jersey). The ECL membranes were incubated with the appropriate primary antibodies followed by peroxidase-conjugated secondary antibody, which was purchased from Cell Signaling Technology, Inc. The signals were quantified using the G:Box gel imaging system by Syngene (Frederick, Maryland) [3, 22, 24].
Caspase-3/7 Activity Assay
Caspase-3 /7 activity was measured using a Caspase-Glo assay kit (Promega) according to the manufacturer’s protocol as described previously.
Cytokine Assay
The levels of cytokines (tumor necrosis factor α [TNF-α], IL-1β, and interleukin 6 [IL-6]) were measured by enzyme-linked immunosorbent assay (ELISA) using OptEIA cytokine kits (BD Biosciences) as described previously [3, 24, 26].
Statistical Analysis
The data are expressed as mean ± standard error. Comparisons of data between groups were made using one-way analysis of variance (ANOVA), and Tukey procedure for multiple-range tests was performed. The log-rank test was used to compare group survival trends. Probability levels of ≤.05 were used to indicate statistical significance.
RESULTS
2-DG Administration Attenuates Sepsis-Induced Cardiac Dysfunction and Improves Survival Outcome
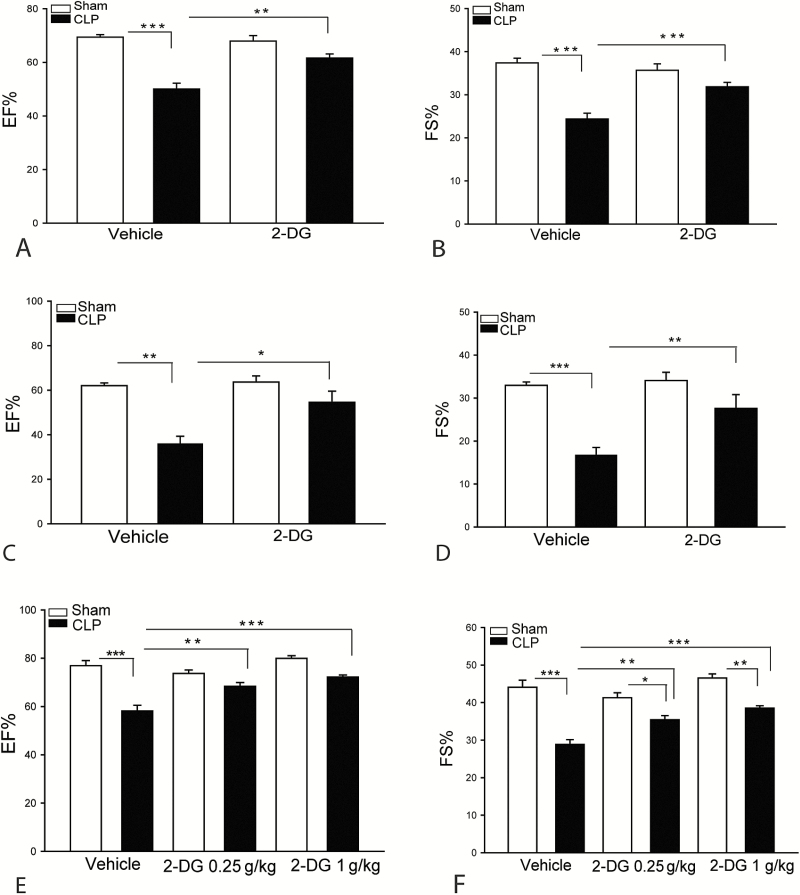
We examined whether administration of the hexokinase inhibitor, 2-DG, would alter cardiac function in polymicrobial sepsis. Figure 1A and 1B show that sepsis significantly decreased EF% by 28% and FS% by 34.8%, respectively, compared with sham control. However, 2-DG (2 kg/kg body weight) treatment significantly attenuated CLP sepsis-induced cardiac dysfunction. The EF% and FS% values in 2-DG treated septic mice were markedly increased by 23.2% and 30.7% compared with untreated CLP septic mice. Similarly, treatment of mice with 2-DG at 0.25, 0.5, and 1 g/kg also significantly attenuated CLP sepsis-induced cardiac dysfunction (Figure 1C–F).
Figure 1.
Inhibition of hexokinase attenuates sepsis-induced cardiac dysfunction. Mice were treated with a single dose of 2-deoxy-d-glucose (2-DG) (2 g/kg) 3 hours prior to cecal ligation and puncture (CLP). Cardiac function was examined by echocardiography 6 hours after CLP. A, Ejection fraction (EF%). B, Fractional shortening (FS%). C and D, 2-DG administration at 0.5 g/kg body weight also significantly attenuated CLP sepsis-induced cardiac dysfunction. E and F, 2-DG treatment at 0.25 and 1 g/kg also significantly improves cardiac function. n = 6–8/group. *P < .05, **P < .01, and ***P < .001 compared with indicated groups.
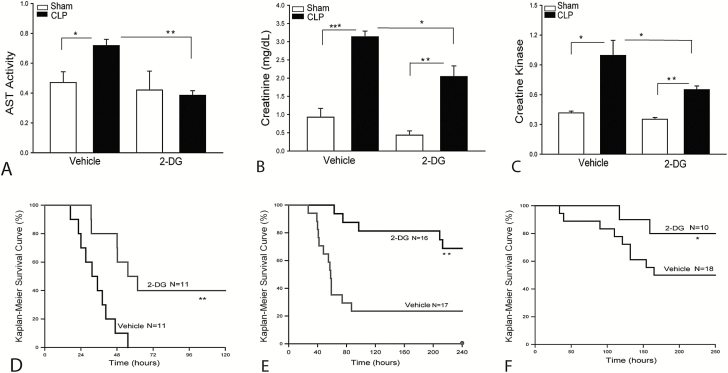
Figures 2A–C show that CLP sepsis markedly induced liver, kidney, and heart injury as evidenced by increasing serum AST, creatinine, and creatine kinase levels. In contrast, 2-DG administration at 0.25 g/kg markedly attenuated CLP sepsis-induced organ injury. In addition, administration of 2-DG markedly improved survival outcome in CLP septic mice. As shown in Figure 2D–F, the mice began to die at 17 hours after CLP. Fifty percent of septic mice died by 31 hours and 100% mortality occurred at 55 hours after CLP. In contrast, the septic mice treated with 2-DG did not show the onset of mortality until 31 hours after CLP. The median survival time (time to 50% mortality) was 55 hours and 40% of the 2-DG treated mice survived for the duration of the study (ie, 7 days after CLP). Administration of 2-DG at 0.25 g/kg also significantly improves survival outcome (Figure 2E).
Figure 2.
Administration of 2-deoxy-d-glucose (2-DG) attenuates sepsis-induced organ injury and improves survival outcome. Mice were treated with 2-DG (0.25 g/kg body weight) 3 hours before induction of cecal ligation and puncture (CLP) sepsis. Serum levels of aspartate aminotransferase (AST; A), creatinine (B), and creatine kinase (C) were measured by commercially available enzyme-linked immunosorbent assay kits. D–F, 2-DG administration improves survival outcome. Mice were treated with 2-DG (2 g/kg body weight [D] or 0.25 g/kg body weight [E]) 3 hours prior to CLP and were then re-treated with 2-DG (500 mg/kg) 24 hours after CLP. D and E, Acute CLP sepsis. F, Chronic CLP septic mice treated with 2-DG at 0.5 kg/kg body weight 3 hours prior to CLP. The animals were monitored for lethality every 2 hours for up to 250 hours following CLP. n = 10–18/group. *P < .05, **P < .01, and ***P < .001 compared with indicated groups.
Interestingly, 2-DG treatment showed a beneficial effect on the survival outcome in a chronic CLP septic model. Figure 2F shows that the chronic CLP septic mice began to die at 34 hours and 50% of septic mice died at 165 hours after CLP. Fifty percent of septic mice survived for 10 days. In 2-DG treated chronic septic mice, the time to initial mortality was 116 hours after CLP. Eighty percent of mice survived for 10 days after CLP sepsis. The data suggest that sepsis-enhanced glycolytic metabolism may contribute to sepsis-induced cardiomyopathy and mortality.
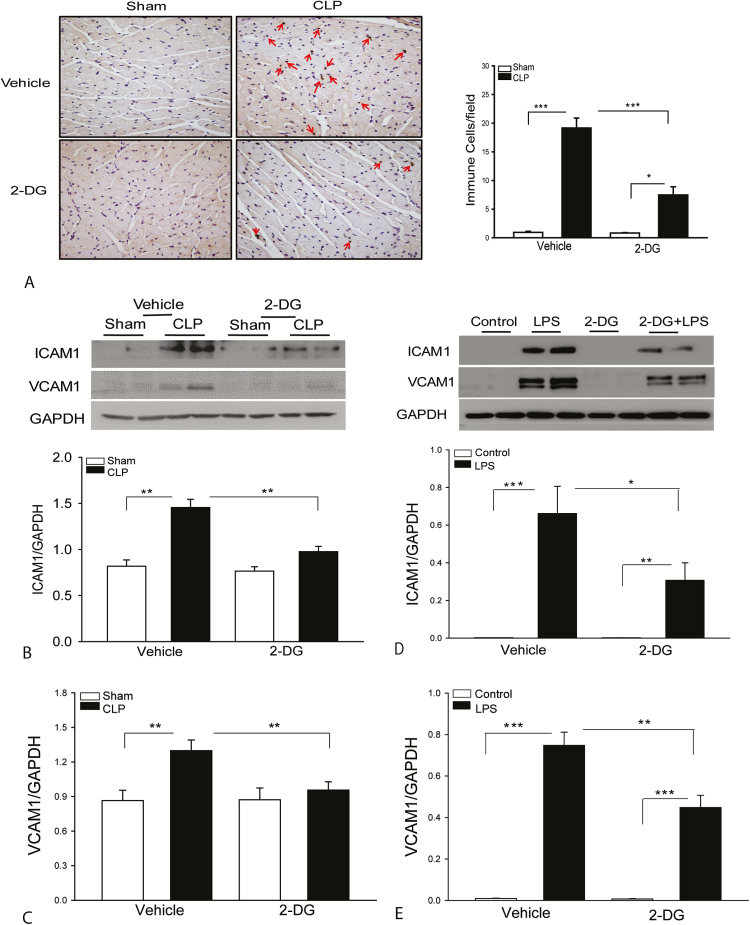
2-DG Administration Reduces the Accumulation of Immune Cells in the Myocardium and Prevents ICAM-1 and VCAM-1 Expression Following CLP Sepsis
Sepsis-induced infiltration of immune cells, in particular neutrophils, into the myocardium contributes to cardiac dysfunction [27]. Figure 3A shows that CLP sepsis significantly induced the infiltration of Ly-6G- and Ly-6C-positive immune cells, including neutrophils, monocytes, and macrophages into the myocardium compared with the sham control. In 2-DG treated septic mice, the numbers of immune cells in the myocardium were markedly reduced compared with untreated CLP septic mice. Increased expression of adhesion molecules plays a critical role in sepsis-induced infiltration of neutrophils into the myocardium [28]. We examined whether reduced accumulation of immune cells in the myocardium by 2-DG administration could be due to regulation of adhesion molecule expression in the myocardium following CLP sepsis. Figure 2B and 2C show that CLP sepsis markedly induced the expression of intercellular cell adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) compared with sham control. Thus, treatment of mice with 2-DG prevents sepsis-induced increases in the expression of adhesion molecules in the myocardium. To further confirm our observation, we performed in vitro experiments using HUVECs to examine whether 2-DG administration would alter LPS-induced expression of adhesion molecules. Figure 2D and 2E show that LPS stimulation significantly increased the expression of ICAM-1 and VCAM-1 compared with untreated control. Treatment of endothelial cells with 2-DG markedly attenuated LPS-increased expression of ICAM-1 and VCAM-1. The data suggest that CLP sepsis promotes glycolytic metabolism which, in turn, plays a role in neutrophil infiltration into the myocardium via enhanced expression of adhesion molecules on endothelial cells.
Figure 3.
Administration of 2-deoxy-d-glucose (2-DG) attenuates sepsis-induced accumulation of immune cells and prevents intercellular cell adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) expression in the myocardium. A, Eight hours after cecal ligation and puncture (CLP)–induced sepsis, hearts were harvested and sectioned for immunostaining of immune cells with specific antibody NIMP-R1411b, which can recognize Ly-6G and Ly-6C neutrophils, monocytes, and macrophages. 2-DG prevents sepsis-induced increases in the expression of ICAM-1 (B) and VCAM-1 (C). n = 4–6 per group. 2-DG treatment attenuated lipopolysaccharide (LPS)–induced ICAM-1 (D) and VCAM-1 (E) expression in endothelial cells. Human umbilical vein endothelial cells were treated with LPS (1 µg/mL) in the presence or absence of 2-DG. The cells were harvested 12 hours after treatment. Cellular proteins were prepared for immunoblotting. n = 8 replicates in each group. *P < .05, **P < .01, and ***P < .001 compared with indicated groups.
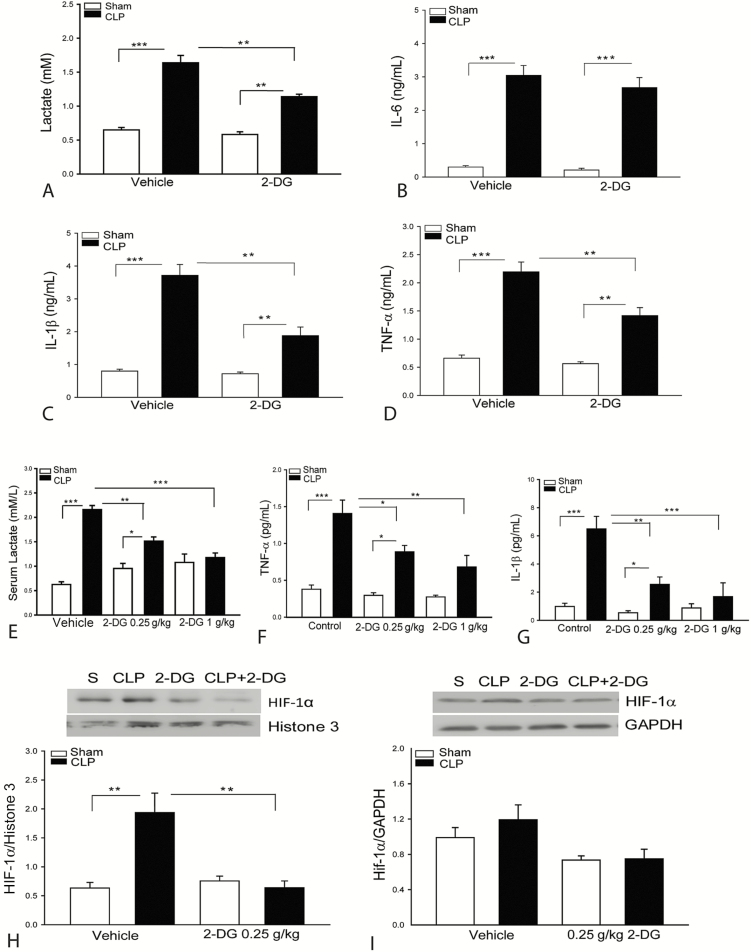
2-DG Administration Attenuates Sepsis-Induced Production of Lactate and Inflammatory Cytokines.
Lactate has been reported to induce innate immune and inflammatory responses [16]. Figure 4A–D shows that CLP sepsis significantly increases the serum levels of lactate, IL-6, IL-1β, and TNF-α compared with sham control. In contrast, 2-DG (2 g/kg) administration markedly reduced serum lactate levels by 30%, compared with untreated CLP septic mice. 2-DG administration also significantly attenuated CLP sepsis-increased levels of serum IL-1β by 49% and TNF-α by 35%. However, 2-DG administration did not alter sepsis-increased serum levels of IL-6. Administration of 2-DG at 0.25 g/kg or 1 kg/kg also markedly attenuated CLP-sepsis increased levels of lactate, TNF-α, and IL-1β in the serum (Figure 4E–G). The data suggest that glycolytic metabolism involves regulation of inflammatory cytokine production in polymicrobial sepsis. HIF-1α is involved in the regulation of IL-1β production [21]. Figure 4H shows that 2-DG administration significantly attenuates CLP sepsis-induced increases in the nuclear levels of HIF-1α. CLP-sepsis did not alter the cytosolic levels of HIF-1α (Figure 4I).
Figure 4.
Administration of 2-deoxy-d-glucose (2-DG) attenuates sepsis-induced lactate and inflammatory cytokine production. Serum was harvested 8 hours after cecal ligation and puncture (CLP) sepsis. The levels of lactate (A), tumor necrosis factor (TNF-α; B), interleukin 1β (IL-1β; C), and interleukin 6 (IL-6; D) were analyzed with enzyme-linked immunosorbent assay. Administration of 2-DG at 0.25 or 1 g/kg body weight also significantly reduced serum lactate (E), TNF-α (F), and IL-1β (G). 2-DG (0.25 g/kg) attenuated sepsis-induced increases in the nuclear hypoxia-inducible factor (HIF-1α) levels (H). CLP sepsis did not significantly alter cytosolic HIF-1α levels in the myocardium (I). n = 4–6/group. *P < .05, **P < .01, and ***P < .001 compared with indicated groups.
2-DG Administration Attenuates Sepsis-Induced Myocardial Apoptosis
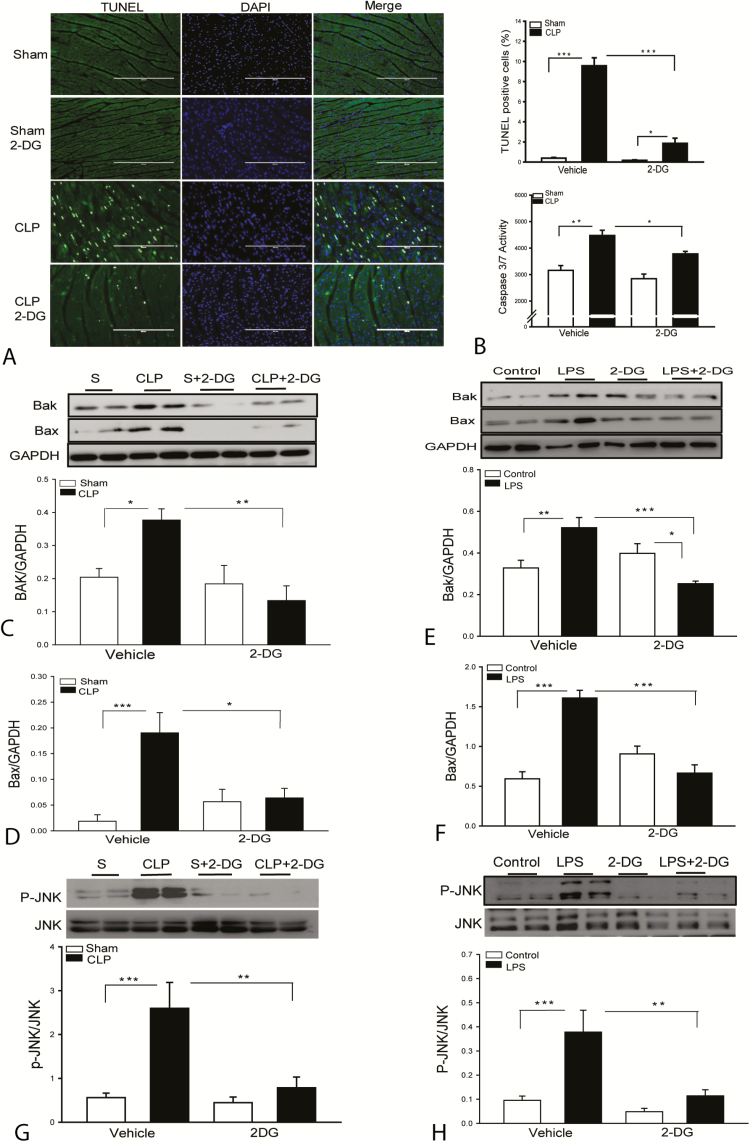
Sepsis induces cardiac myocyte apoptosis [29], which may play a role in sepsis-induced cardiac dysfunction [30]. Figure 5A shows that CLP sepsis significantly induced myocardial apoptosis as evidenced by showing increased TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling)–positive staining apoptotic cells in septic myocardial sections and myocardial caspase 3/7 activity compared with sham control. In contrast, 2-DG administration markedly attenuates sepsis-induced myocardial apoptosis and caspase 3/7 activity (Figure 5B). The data indicate that enhanced glycolytic metabolism by sepsis plays a role in sepsis-induced myocardial apoptosis.
Figure 5.
Administration of 2-deoxy-d-glucose (2-DG) attenuates sepsis-induced myocardial apoptosis. A, Hearts were harvested 8 hours after cecal ligation and puncture (CLP) sepsis and sectioned for TUNEL assay. B, Myocardial caspase-3/7 activity was analyzed by enzyme-linked immunosorbent assay. 2-DG treatment prevents sepsis-induced Bak (C) and Bax (D) expression in the myocardium. 2-DG treatment prevents lipopolysaccharide (LPS)–induced Bak (E) and Bax (F) expression in endothelial cells. n = 8 replicates/group. 2-DG treatment prevents sepsis-induced JNK phosphorylation in the myocardium (G; n = 4–6/group) and LPS-increased JNK phosphorylation in the endothelial cells (H). Human umbilical vein endothelial cells were treated with lipopolysaccharide (1 µg/mL) in the presence or absence of 2-DG. The cells were harvested 12 hours after treatment. Cellular proteins were prepared for immunoblotting. n = 8 replicates/group. *P < .05, **P < .01, and ***P < .001 compared with indicated groups.
2-DG Administration Prevents Sepsis-Induced Increases in the Expression of Bak and Bax in the Myocardium
BCL-2-associated protein x (Bax) and Bcl-2 antagonist/killer 1 (Bak) act as antagonists against antiapoptotic Bcl-2 [30]. Figure 5C and 5D show that CLP sepsis significantly increased the levels of Bax and Bak compared with sham control. In contrast, 2-DG administration prevents sepsis-induced increases in the expression of Bax and Bak in the myocardium. We also examined the effect of 2-DG on the expression of Bax and Bak in LPS-stimulated endothelial cells. As shown in Figure 5E and 5F, LPS stimulation significantly increased the levels of Bax and Bak compared with control cells. However, 2-DG administration prevents LPS induced increases in the expression of Bax and Bak. The data indicate that enhanced glycolytic metabolism by sepsis involves stimulation of the expression of apoptotic proteins that promote chromosome c release from the mitochondria, leading to apoptosis.
2-DG Administration Prevents JNK Phosphorylation in the Septic Myocardium and LPS-Stimulated Endothelial Cells
The mitogen activated protein kinase c-Jun N-terminal kinase (JNK) is a member of the MAPK family and plays a critical role in the induction of apoptosis [31]. Figure 5G shows that CLP sepsis markedly induced JNK phosphorylation, compared with sham control. However, 2-DG administration prevents sepsis-induced myocardial JNK phosphorylation. 2-DG administration also prevented LPS-induced JNK phosphorylation in endothelial cells (Figure 5H). The data suggest that sepsis-enhanced glycolytic metabolism involves activation of JNK, which may play a role in CLP sepsis-induced myocardial apoptosis.
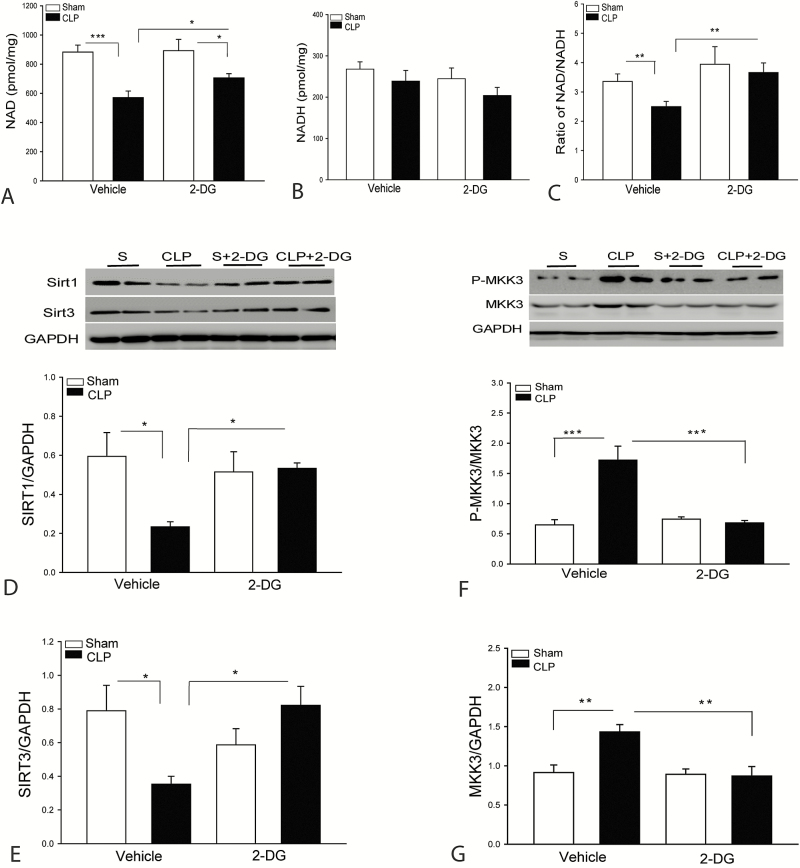
2-DG Administration Increases the Expression of Sirt1/Sirt3 in the Myocardium Following CLP Sepsis
It is well known that sepsis-induced mitochondrial dysfunction contributes to apoptosis and cardiomyopathy [32]. Sirt1/Sirt3 play a critical role in the regulation of mitochondrial function and inflammatory responses [33]. Deficiency of Sirt1 resulted in enhanced LPS-induced inflammatory response and kidney injury [34]. Sirt1 function is NAD+ dependent [32]. We observed that CLP sepsis significantly decreased the levels of NAD+ (Figure 6A) and the ratio of NAD+/NADH (Figure 6C). CLP sepsis also markedly decreased the levels of myocardial Sirt1 (Figure 6D) and Sirt3 (Figure 6E), compared with sham control. Interestingly, 2-DG administration significantly attenuated sepsis-decreased levels of NAD+ and the ratio of NAD+/NADH and prevented sepsis-induced decreases in the levels of Sirt1 (Figure 6D) and Sirt3 (Figure 6E) in the myocardium. The data suggest that increased expression of Sirt1/Sirt3 by 2-DG administration could be an important mechanism for the attenuation of sepsis-induced apoptosis and inflammatory response in polymicrobial sepsis.
Figure 6.
Administration of 2-deoxy-d-glucose (2-DG) attenuated sepsis-induced decreases in the levels of nicotinamide adenine dinucleotide (NAD+, oxidized form), Sirt1, and Sirt3 in the myocardium. Hearts were harvested 8 hours after cecal ligation and puncture (CLP) for preparation of cellular proteins. NAD+ (A) and nicotinamide adenine dinucleotide (NADH, reduced form) (B) levels were examined by commercially available kits. C, Ratio of NAD+/NADH. D and E, 2-DG treatment increased the levels of Sirt1 (D) and Sirt3 (E) in the myocardium. F and G, 2-DG treatment prevents MKK3 phosphorylation and expression in the myocardium. n = 4–6/group. *P < .05, **P < .01, and ***P < .001 compared with indicated groups.
2-DG Administration Significantly Attenuates Sepsis-Increased MKK3 Expression in the Myocardium
MAP kinase kinase 3 (MKK3) has been reported to regulate inflammation in sepsis [35, 36]. Figure 6F and 6G show that CLP sepsis significantly increased the levels of phosphorylated and total MKK3 compared with sham control. However, 2-DG administration prevented sepsis-increased levels of phosphorylated and total MKK3 in the myocardium. The data suggest that enhanced glycolytic metabolism by sepsis involves increased expression of MKK3, which contributes to apoptosis and inflammatory response in sepsis.
DISCUSSION
The present study showed that polymicrobial sepsis enhances glycolytic metabolism, which plays a role in sepsis-induced cardiomyopathy and mortality. Specifically, we observed that CLP sepsis significantly enhanced glycolysis as evidenced by increased serum levels of lactate. Modulation of glycolytic metabolism by administration of 2-DG, an inhibitor for hexokinase-2 which is the initial kinase for glycolysis, markedly improves cardiac function and survival outcomes in CLP septic mice. Our observation indicates that modulation of glycolytic metabolism in the early phase of polymicrobial sepsis could be an appropriate approach for sepsis/septic shock.
Clinical studies have shown that the serum levels of lactate are associated with outcomes of septic patients [13, 14]. In the early phase of sepsis, reduced serum lactate levels are positively correlated with better survival outcome [37]. Recently, Wang et al reported that 2-DG administration significantly improves survival outcome in LPS-induced sepsis [38]. We observed that modulation of glycolysis by administration of 2-DG markedly improved cardiac function and survival outcome. Administration of 2-DG also attenuated sepsis-induced liver, kidney, and heart injury, indicating that 2-DG could have beneficial effect on sepsis-induced organ injury. 2-DG administration also significantly reduced the levels of serum lactate and inflammatory cytokines, suggesting that enhanced glycolytic metabolism by sepsis involves stimulation of innate immune and inflammatory responses. Activation of innate immune and inflammatory responses mediated by TLRs plays a critical role in polymicrobial sepsis [3–5]. Targeting the TLR-mediated NF-κB activation pathway has been reported to improve cardiac function and survival outcome [3, 6]. Interestingly, recent studies have shown that glycolytic metabolism may play an important role in mediating innate immune and inflammatory responses [7–10]. The TLR4 ligand LPS [8] and the TLR3 ligand Poly I:C [39] have been reported to induce a metabolic switch from oxidative phosphorylation to glycolysis in macrophages. On the other hand, activated macrophages exhibited increased glycolysis accompanied by decreased oxygen consumption [40]. In addition, enhanced glycolysis has also been observed in activated lymphocytes [10]. Collectively, the data indicate that glycolytic metabolism involves activation of innate immune and inflammatory responses in polymicrobial sepsis.
It is well known that increased expression of adhesion molecules on endothelial cells plays a critical role in mediating the infiltration of immune cells, including neutrophils, monocytes, and macrophages into the myocardium, thereby contributing to cardiac dysfunction in sepsis [28]. Importantly, we observed that 2-DG administration markedly attenuated sepsis-induced immune cell infiltration into the myocardium by preventing sepsis-induced adhesion molecule expression in the myocardium. 2-DG treatment also prevented LPS-induced increases in the expression of adhesion molecule on endothelial cells in vitro. The data indicate that glycolytic metabolism involves sepsis-induced endothelial adhesion molecule expression. At the present, we do not understand the mechanisms by which glycolysis regulates adhesion molecule expression. However, recent studies have shown that, in addition to proinflammatory cytokines, lactate has been reported to activate the TLR4-mediated NF-κB pathway [16]. Yang et al have shown that lactate stimulates HMGB1 acetylation and release [17] and that attenuation of glycolysis by suppressing PKM2 activity significantly improves outcome of sepsis [17]. Therefore, it is possible that lactate and inflammatory cytokines as well as intermediates generated from enhanced glycolysis may involve regulation of adhesion molecule expression via activation of the NF-κB pathway [41].
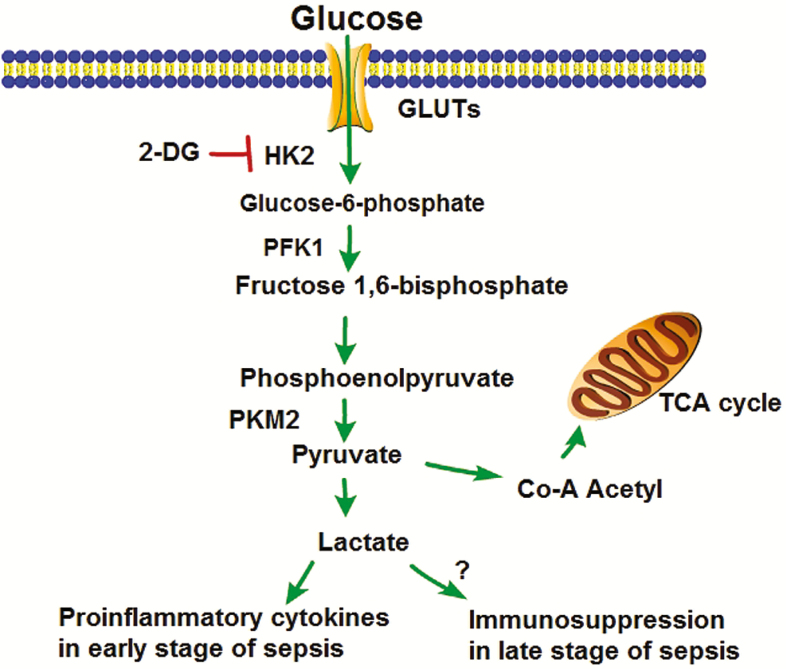
Figure 7.
Two-deoxy-d-glucose (2-DG) suppresses HK2 activity, thus reducing lactate production. Abbreviations: GLUT, glucose transport protein; HK2, hexokinase 2; PFK1, phosphofrutokinase 1; PKM2, pyruvate kinase isozymes M1/2; TCA, tricarboxylic acid.
Myocardial apoptosis contributes to cardiac dysfunction in sepsis [29, 30]. We observed that sepsis significantly increased expression of Bax and Bak in the myocardium, which promotes cytochrome c release from mitochondria [42] resulting in activation of apoptotic signaling. We observed that 2-DG administration prevents sepsis-induced increases in the expression of myocardial Bax and Bak in the myocardium. Similarly, 2-DG treatment also prevents LPS-induced Bax and Bak expression in endothelial cells. In addition, 2-DG administration prevents sepsis-induced JNK phosphorylation, indicating that enhanced glycolytic metabolism by sepsis activates apoptotic signaling. At present, we do not understand how enhanced glycolysis will activate apoptotic signaling. Interestingly, we observed that 2-DG administration prevents sepsis-induced decreases in the levels of myocardial Sirt1 and Sirt3. It is well known that Sirt1 and Sirt3 play a critical role in preservation of mitochondrial function and improve cellular metabolism [33, 43]. Sepsis-induced mitochondrial dysfunction [44] contributes to activate apoptotic signaling. Although we did not analyze mitochondrial function in the present study, increased expression of Sirt1 and Sirt3 by 2-DG may involve preservation of mitochondrial function during sepsis.
In addition, Sirt1 has also been reported to protect against myocardial ischemic injury [45] and have anti-inflammatory effects [46] through inhibition of activity of NF-κB RelB/p65 [47]. Gao et al reported that deficiency of Sirt1 results in enhanced proinflammatory response and aggravation of acute kidney injury by endotoxin [34]. We observed that administration of 2-DG to septic mice significantly increased expression of myocardial Sirt1, which is consistent with the data showing that 2-DG treatment markedly attenuated sepsis-increased serum levels of inflammatory cytokines. Currently, we do not understand how 2-DG administration enhanced expression of Sirt1 and Sirt3 in the myocardium. Recently, Wang et al reported that treatment of endothelial cells with 2-DG significantly improved cell viability and induced autophagy via AMPK-mediated mechanism [48]. AMPK has been demonstrated to interact with Sirt1 for the balance of energy metabolism and anti-inflammatory response [49]. Although we did not examine the effect of 2-DG administration on AMPK activity in septic mice, we observed that 2-DG treatment markedly suppressed MKK3 phosphorylation in the myocardium of septic mice. MKK3 has been shown to regulate Sirt1 activity in sepsis [35]. Mannam et al reported that sepsis or LPS stimulation significantly increased MKK3 activity and decreased Sirt1 expression [35]. Deficiency of MKK3 resulted in increases in the expression of Sirt1 in the lung and in endothelial cells [35], which are positively correlated with survival outcome [32]. Collectively, suppressed MKK3 phosphorylation and increased Sirt1 expression by 2-DG could be an important mechanism for attenuating inflammatory response and decreasing apoptosis by 2-DG in polymicrobial sepsis.
In summary, the present study demonstrated that glycolysis is involved in sepsis-induced cardiomyopathy and mortality. Modulation of sepsis-enhanced glycolysis with 2-DG significantly attenuated sepsis-induced cardiac dysfunction and improved survival outcome. The mechanisms involve attenuation of sepsis-induced proinflammatory responses and myocardial apoptosis through decreasing MKK3 phosphorylation and increasing Sirt1 and Sirt3 expression.
Notes
Financial support. This work was supported by the National Institutes of Health (grant numbers HL071837 to C. L., GM083016 to C. L. and D. W., GM53522 to D. W., GM119197 to D. W.), and C06RR0306551.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014; 5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernandes CJ, Jr, de Assuncao MS. Myocardial dysfunction in sepsis: a large, unsolved puzzle. Crit Care Res Pract 2012; 2012:896430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao M, Ha T, Zhang X, et al. Toll-like receptor 3 plays a central role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med 2012; 40:2390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feng Y, Zou L, Zhang M, Li Y, Chen C, Chao W. MyD88 and Trif signaling play distinct roles in cardiac dysfunction and mortality during endotoxin shock and polymicrobial sepsis. Anesthesiology 2011; 115:555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zou L, Feng Y, Chen YJ, et al. Toll-like receptor 2 plays a critical role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med 2010; 38:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams DL, Ha T, Li C, et al. Modulation of tissue Toll-like receptor 2 and 4 during the early phases of polymicrobial sepsis correlates with mortality. Crit Care Med 2003; 31:1808–18. [DOI] [PubMed] [Google Scholar]
- 7. Cheng SC, Joosten LA, Netea MG. The interplay between central metabolism and innate immune responses. Cytokine Growth Factor Rev 2014; 25:707–13. [DOI] [PubMed] [Google Scholar]
- 8. Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 2010; 115:4742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med 2016; 213:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng SC, Scicluna BP, Arts RJW, et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 2016; 17:406–13. [DOI] [PubMed] [Google Scholar]
- 11. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loftus RM, Finlay DK. Immunometabolism: cellular metabolism turns immune regulator. J Biol Chem 2016; 291:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chertoff J, Chisum M, Garcia B, Lascano J. Lactate kinetics in sepsis and septic shock: a review of the literature and rationale for further research. J Intensive Care 2015; 3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009; 37:1670–7. [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care 2014; 18:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Samuvel DJ, Sundararaj KP, Nareika A, Lopes-Virella MF, Huang Y. Lactate boosts TLR4 signaling and NF-kappaB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J Immunol 2009; 182:2476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang L, Xie M, Yang M, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun 2014; 5:4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sánchez-García FJ. Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol 2016; 7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene 2006; 25:4646. [DOI] [PubMed] [Google Scholar]
- 20. Parniak M, Kalant N. Incorporation of glucose into glycogen in primary cultures of rat hepatocytes. Can J Biochem Cell Biol 1985; 63:333–40. [DOI] [PubMed] [Google Scholar]
- 21. Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013; 496:238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ha T, Hua F, Grant D, et al. Glucan phosphate attenuates cardiac dysfunction and inhibits cardiac MIF expression and apoptosis in septic mice. Am J Physiol Heart Circ Physiol 2006; 291:H1910–8. [DOI] [PubMed] [Google Scholar]
- 23. Ha T, Lu C, Liu L, et al. TLR2 ligands attenuate cardiac dysfunction in polymicrobial sepsis via a phosphoinositide 3-kinase-dependent mechanism. Am J Physiol Heart Circ Physiol 2010; 298:H984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao M, Ha T, Zhang X, et al. The Toll-like receptor 9 ligand, CpG oligodeoxynucleotide, attenuates cardiac dysfunction in polymicrobial sepsis, involving activation of both phosphoinositide 3 kinase/Akt and extracellular-signal-related kinase signaling. J Infect Dis 2013; 207:1471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu C, Wang X, Ha T, et al. Attenuation of cardiac dysfunction and remodeling of myocardial infarction by microRNA-130a are mediated by suppression of PTEN and activation of PI3K dependent signaling. J Mol Cell Cardiol 2015; 89(pt A):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang X, Ha T, Liu L, et al. Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc Res 2013; 97:432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006; 368:157–69. [DOI] [PubMed] [Google Scholar]
- 28. Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem 1991; 266:2282–9. [PubMed] [Google Scholar]
- 29. Nevière R, Fauvel H, Chopin C, Formstecher P, Marchetti P. Caspase inhibition prevents cardiac dysfunction and heart apoptosis in a rat model of sepsis. Am J Respir Crit Care Med 2001; 163:218–25. [DOI] [PubMed] [Google Scholar]
- 30. Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J 2001; 15:879–92. [DOI] [PubMed] [Google Scholar]
- 31. Pizzino G, Bitto A, Pallio G, et al. Blockade of the JNK signalling as a rational therapeutic approach to modulate the early and late steps of the inflammatory cascade in polymicrobial sepsis. Mediators Inflamm 2015; 2015:591572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Exline MC, Crouser ED. Mitochondrial mechanisms of sepsis-induced organ failure. Front Biosci 2008; 13:5030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vachharajani VT, Liu T, Wang X, Hoth JJ, Yoza BK, McCall CE. Sirtuins link inflammation and metabolism. J Immunol Res 2016; 2016:8167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao R, Chen J, Hu Y, et al. Sirt1 deletion leads to enhanced inflammation and aggravates endotoxin-induced acute kidney injury. PLoS One 2014; 9:e98909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mannam P, Shinn AS, Srivastava A, et al. MKK3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2014; 306:L604–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Srivastava A, Shinn AS, Lee PJ, Mannam P. MKK3 mediates inflammatory response through modulation of mitochondrial function. Free Radic Biol Med 2015; 83:139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care 2013; 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang A, Huen SC, Luan HH, et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 2016; 166:1512–1525.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woolard MD, Kevil CG. Paying the toll for glucose regulation: a central role for TLR3. Diabetes 2015; 64:3345–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 2015; 25:771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lockyer JM, Colladay JS, Alperin-Lea WL, Hammond T, Buda AJ. Inhibition of nuclear factor-kappaB-mediated adhesion molecule expression in human endothelial cells. Circ Res 1998; 82:314–20. [DOI] [PubMed] [Google Scholar]
- 42. Vaseva AV, Moll UM. The mitochondrial p53 pathway. Biochim Biophys Acta 2009; 1787:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 2014; 25:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 2004; 4:729–41. [DOI] [PubMed] [Google Scholar]
- 45. Hsu CP, Zhai P, Yamamoto T, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 2010; 122:2170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Y, Wang P, Yang X, et al. SIRT1 inhibits inflammatory response partly through regulation of NLRP3 inflammasome in vascular endothelial cells. Mol Immunol 2016; 77:148–56. [DOI] [PubMed] [Google Scholar]
- 47. Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 2013; 25:1939–48. [DOI] [PubMed] [Google Scholar]
- 48. Wang Q, Liang B, Shirwany NA, Zou MH. 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoS One 2011; 6:e17234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl) 2011; 89:667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]