Abstract
Background
Ketamine has recently become an agent of interest as an acute treatment for severe depression and as the anaesthetic for electroconvulsive therapy (ECT). Subanaesthetic doses result in an acute reduction in depression severity while evidence is equivocal for this antidepressant effect with anaesthetic or adjuvant doses. Recent systematic reviews call for high-quality evidence from further randomised controlled trials (RCTs).
Aims
To establish if ketamine as the anaesthetic for ECT results in fewer ECT treatments, improvements in depression severity ratings and less memory impairment than the standard anaesthetic.
Method
Double-blind, parallel-design, RCT of intravenous ketamine (up to 2 mg/kg) with an active comparator, intravenous propofol (up to 2.5 mg/kg), as the anaesthetic for ECT in patients receiving ECT for major depression on an informal basis. (Trial registration: European Clinical Trials Database (EudraCT): 2011-000396-14 and clinicalTrials.gov: NCT01306760.)
Results
No significant differences were found on any outcome measure during, at the end of or 1 month following the ECT course.
Conclusions
Ketamine as an anaesthetic does not enhance the efficacy of ECT.
Depression is among the leading causes of disability worldwide1 which in its severe forms can be life threatening. Severely depressed patients, or those who fail to respond to chemical treatment, are commonly treated with electroconvulsive therapy (ECT). ECT is highly effective and undoubtedly saves lives, but a range of factors, including side-effect profile, the necessity for hospital care and stigma, restricts its use.2 Mitigating these factors may improve the acceptability of ECT as a treatment. Ketamine is a candidate mitigation agent because of renewed interest in it as an acute treatment for severe depression3–6 and as an anaesthetic during ECT (see for example McGirr et al,6,7 Caddy et al,8 Fond et al,9 Coyle & Laws,10 and Lee et al11). McGirr et al7 pooled the results of many anaesthetic and adjunct ketamine studies and concluded that ketamine anaesthesia in ECT leads to a therapeutic improvement. Unfortunately, many variations in methodology including in electrode placement, seizure threshold, limiting the number of treatments and depression rating scales used, limit the generalisability of the conclusions that can be drawn from the work published to date. A recent Cochrane review8 concluded that higher-quality randomised controlled trials (RCTs) investigating ketamine as a treatment for depression were needed. Ketamine anaesthesia during ECT may also have additional or independent neuroprotective effects against deficits in cognitive function associated with ECT.12,13 Ketamine is a non-competitive N-methyl-d-aspartate (NMDA) receptor antagonist and activation of NMDA receptors is required for the induction of a form of synaptic plasticity called long-term potentiation (LTP),14 a potential mechanism underlying memory formation.15 Previous studies in rodents have shown that the use of ketamine anaesthesia during electroconvulsive stimulation can prevent LTP-like changes in the hippocampus.16 Consistent with the cognition-sparing hypothesis is the evidence that using ketamine as the anaesthetic in patients receiving ECT resulted in faster re-orientation time compared with methohexital,17 and also protected word recall after six treatments compared with etomidate.18
Here we present the results of the KANECT trial (‘The use of ketamine as an anaesthetic during electroconvulsive therapy (ECT) for depression: does it improve treatment outcome?’, trial registration: European Clinical Trials Database (EudraCT): 2011-000396-14 and clinicalTrials.gov: NCT01306760.). KANECT was a double-blind, parallel-design, RCT of ketamine with an active comparator, propofol (used in 88.2% of ECT treatments in Scotland19), as the anaesthetic for ECT with follow-up intervals during, immediately after and 1 month after ECT. The primary objective was to determine if using ketamine instead of propofol resulted in fewer ECT treatments and quicker improvements in ratings of depression severity. The secondary objective was to determine if using ketamine resulted in less cognitive impairment following ECT.
Method
Trial design
KANECT was a double-blind, parallel-design, randomised controlled trial of ketamine with an active comparator, prpofol, as the anaesthetic for ECT. Once consent had been obtained baseline assessments were made before patients were randomised to one of the two anaesthetics using minimisation. The minimisation variables were gender and age. No random element was employed so Taves'20 method with P = 1 was used. Treatment allocation was 1:1 ketamine:propofol. Randomisation was performed over the internet or telephone using an independent randomisation service (CHaRT, University of Aberdeen) by the principal investigator (I.C.R.) or ECT nurses and recorded in medical notes as drug A or B. An unblinding procedure was specified should the medical need arise. All post-ECT assessments were conducted by researchers masked to the anaesthetic assignment.
Recruited patients received ECT as per the routine processes at the Royal Cornhill Hospital until their consultant psychiatrist (masked to anaesthetic assignment) decided to end treatment or treatment was stopped for medical reasons. KANECT was given ethical approval by the East of Scotland Research Ethics Service (ref: 11/AL/0221) and approved by the Medicine and Healthcare products Regulatory Agency (ref: 21583/0211/001-0001).
Participants
Patients were eligible to participate if they were: receiving ECT on an informal basis (i.e. not detained), considered fit by an anaesthetist (American Society of Anaesthesiologists physical status classification system score of 1 or 2), had no comorbid psychiatric diagnoses recorded by the treating psychiatrist and were between the ages of 18 and 75. Patients were excluded if they had pre-existing neurological disease or cognitive impairment, pre-existing severe hypertension, severe respiratory tract disease, major cardiovascular disease, a pacemaker, significant cerebrovascular disorder or malformation, intracranial mass lesions, seizure disorder, intracranial electrode and clips, severe intra-ocular pathology, significant endocrine or metabolic disease, severe haematological disease, severe fracture, obesity, pregnancy. Patients were receiving ECT for major depression on an informal basis at the Royal Cornhill Hospital, Aberdeen, Scotland between November 2011 and December 2013. The final assessment of the final patient was completed in February 2014. The ethnicity of all patients was White British. All participants provided informed consent.
Interventions
Ketamine (Ketalar, manufactured by Pfizer), up to 2 mg/kg, intravenous (bolus) or propofol (Diprivan 1% manufactured by AstraZeneca), up to 2.5 mg/kg, intravenous (bolus) as the anaesthetic used for ECT. No restrictions were placed on the psychiatric medications and or treatments prescribed either before or during the course of the trial. The same applied for any rescue medications. The standard ECT protocol set by the Royal Cornhill Hospital and accredited by the Scottish Electroconvulsive Accreditation Network was applied to all participants. Bilateral ECT (bifrontotemporal position) was given twice per week. ECT was administered using a brief pulse constant current apparatus (Thymatron DGx). Any prescribed benzodiazepines were withheld 24 h before treatment so as to not interfere with ECT. On arrival at the treatment room patients had baseline heart rate, non-invasive blood pressure and oxygen saturation (SpO2) measured and the left arm was isolated with a pressure cuff. An intravenous cannula was inserted in the non-isolated arm. The patients in the ketamine group were administered a hypnotic dose of ketamine of up to 2 mg/kg followed by limb isolation and subsequent administration of the muscle relaxant suxamethonium (0.5–1 mg/kg). Those in the propofol group were administered a hypnotic dose of propofol up to 2.5 mg/kg followed by limb isolation and administration of suxamethonium (0.5–1 mg/kg). Heart rate, three-lead electrocardiogram (ECG), SpO2, fractional inspired oxygen (FiO2), and end-tidal carbon dioxide (EtCO2) were monitored continuously during the procedure. All participants received positive pressure ventilation with 100% oxygen during the procedure until spontaneous respiration resumed. Non-invasive blood pressure was measured before the administration of the anaesthetic, immediately post-seizure and repeated if necessary. In the recovery room patients received oxygen-enriched air via a facemask, while non-invasive blood pressure and SpO2 were monitored.
Seizure threshold was defined as the minimum dose required to produce a tonic–clonic seizure of 15 s or longer (measured by electroencephalogram (EEG)). A titration method (starting at 50 millicoulombs (mc), 100 mc, 175 mc, then increased by 50 mc) was used to determine this seizure threshold and once established the treating dose was given at twice the threshold. No seizure or one less than 15 s increased dosage by 50 mc.
Outcomes
The primary outcome was patients' illness severity as assessed using the 17-item Hamilton Rating Scale for Depression (HRSD)21 and the Montgomery–Åsberg Depression Rating Scale (MADRS).22 Both are commonly used ratings scales. The MADRS is recommended by the Scottish ECT Accreditation Network and thus is used across Scotland. The secondary outcome, patients' cognitive function, was also assessed before ECT using the Cambridge Automated Neuropsychological Test Battery Spatial Recognition Memory task (CANTAB SRM).23 Our research group have found that the CANTAB SRM is sensitive to memory deficits following ECT.24,25
Patients were assessed before the first ECT treatment (within 2–48 h), after the fourth treatment (within 24–48 h), at the end of treatment (within 24–48 h) and 1 month after the end of treatment by researchers masked to the anaesthetic employed. The pre-ECT, post-fourth (ECT4) and post-final ECT assessments were generally conducted in a quiet room in the hospital. The 1-month post-ECT assessments were completed in a quiet room in the hospital or the patient's home. Each assessment took approximately 35 min. Patients, their treating medical teams and all researchers making assessments were masked to the anaesthetic allocation. Researchers conducting ratings were trained in the administration of all measures.
Sample size
The sample size of 40 patients was based on a similarly sized sample26 where a large measurable reduction in HRSD scores was found after four ECT treatments following ketamine v. propofol anaesthesia. KANECT ended when the sample size was achieved.
Changes to methods after trial commencement
Several issues arose prior to completing the analysis that resulted in a change to the original analysis plan published in the trial's protocol. First, for four patients who had four or fewer ECT treatments the second assessment (post-ECT4) did not exist as this was their post-final ECT assessment, meaning that either these patients could not be included in an analysis using all four assessments or the analyses could not examine all four assessments for all patients. The trial protocol stated that the primary outcome would be assessed by examining the change in depression ratings over the course of treatment and after treatment. To this end, two sets of analyses were employed. The first set employed analysis of covariance (ANCOVA) to examine change over the acute stage of treatment using all available data (n = 35) for the pre-ECT and post-ECT4 assessments (including data from the four patients who had four or fewer ECT treatments). The second set examined change after treatment, comparing pre-ECT with post-final ECT and the 1-month follow-up, and dropping the post-ECT4 assessment. Here, data from the four patients who had four or fewer ECT treatments were included at the post-final ECT assessment.
A second issue for the second analysis set was that loss to follow-up meant that data were not available from all patients at all points (see Missing data). This meant that employing ANCOVAs to analyse change across time, as specified in the trial protocol, was not appropriate because ANCOVAs delete data list-wise, meaning any participant with missing data would be excluded. This would result in an underpowered and unrepresentative analysis. Instead, mixed models analyses were employed. These analyses have a number of advantages over ANCOVA in that they use all available data and handle missing data appropriately. Mixed models also account for correlation between repeated measurements on the same participant, and allow for the addition of the covariates used in the stratification randomisation procedure. Thus, mixed models can be thought of as examining the same thing as ANCOVA but using all available data. Our group have previously employed these analyses with similar data.24,25
Statistical analyses
All analyses were computed in SPSS 22.0 and conducted by a researcher masked to the group assignment (G.F.). An independent samples t-test was used to analyse whether there was a difference in the number of ECT treatments administered between groups.
Analyses were conducted for both depressive rating measures (HRSD and MADRS) and changes in cognitive function (CANTAB SRM performance). All analyses were run with anaesthetic and time as fixed factors and anaesthetic × time as an interaction term. Age and gender were included as covariates.
In an acute effects analysis, analysis of covariance (ANCOVA) compared outcomes pre-ECT with post-ECT4. Partial eta-squared (η2p) provided an estimate of effect size with 95% confidence intervals listed with the ketamine group reported first; and in interactions with time, the first reported confidence interval is the earliest time point. In a treatment effects analysis, linear mixed models compared outcomes assessed pre-ECT with those at post-final ECT and 1-month assessments. In each mixed model a compound symmetry covariance matrix27 was compared with a first-order autoregressive and an unstructured covariance matrix. Model fit was assessed using Akaike's Information Criterion (AIC) and the better fitting model (smallest AIC) reported. In each case this was the model with the compound symmetry covariance matrix. Estimation proceeded using restricted maximum likelihood to a maximum of 100 iterations. Residuals were examined following model fit for non-normality using Q–Q plots. All models were normal.
Missing data
Data were analysed if patients completed any follow-up assessment following pre-ECT assessment (n = 35). Data remained in the trial for analysis where appropriate despite subsequent loss to follow-up. As shown in Fig. 1, additional data were missing post-ECT4 for one patient who refused to complete the CANTAB SRM. Post-final ECT, one patient was discharged home before an assessment could be completed and a visit could not be scheduled within 2 days. A second patient was discharged home but the post-final ECT assessment was completed by telephone. CANTAB SRM data are thus missing for this patient. As four patients had four ECT treatments or fewer their data could be counted as post-ECT4 or post-final ECT, but not included as both in the same analysis. This necessitated two sets of analyses as elaborated upon above.
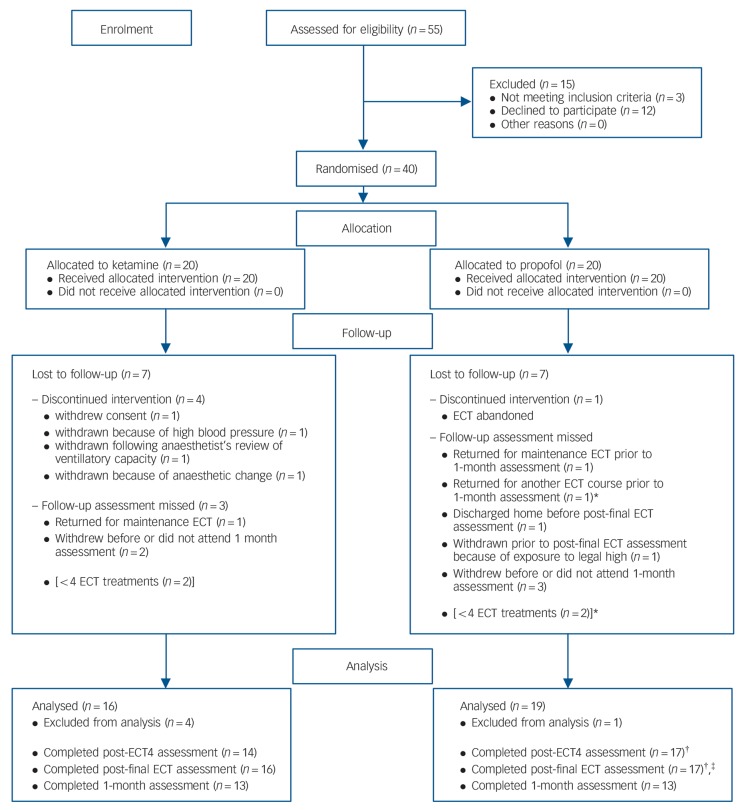
Fig. 1.
CONSORT flow diagram showing patient eligibility and inclusion in the trial.
*One patient is the same patient; †Patient refused Cambridge Automated Neuropsychological Test Battery Spatial Recognition Memory task (CANTAB SRM) (n = 1); ‡Completed over telephone so no CANTAB (n = 1). ECT, electroconvulsive therapy.
Results
Recruitment
In total, 55 patients were screened eligible to participate in KANECT. Of these, 52 were approached to participate and 40 consented, of whom 22 were female. As patients were randomly assigned to receive ketamine or propofol stratified by gender and age (⩽65 and >65), baseline demographic characteristics of each group were not statistically significant. Both groups contained equal numbers of males (n = 9) and females (n = 11). Mean age was 51.76 (s.d. = 9.97) and 49.88 (s.d. = 12.53) in the ketamine and propofol groups respectively. Baseline depression ratings and CANTAB performance are displayed in Table 1.
Table 1.
Means scores on each dependent measure by group (standard deviation).
| Mean (s.d.) |
||||
|---|---|---|---|---|
| Pre-ECT | Post-ECT4 | Post-final ECT | 1 month post-ECT | |
| Hamilton Rating Scale for Depression | ||||
| Ketamine | 27.19 (6.47) | 17.25 (6.88) | 13.50 (9.32) | 14.08 (8.08) |
| Propofol | 24.79 (8.50) | 13.58 (5.71) | 8.41 (4.70) | 12.08 (9.86) |
| Montgomery–Åsberg Depression Rating Scale | ||||
| Ketamine | 36.38 (8.29) | 23.81 (11.20) | 18.69 (16.48) | 17.85 (13.15) |
| Propofol | 35.68 (8.39) | 18.74 (9.44) | 8.18 (6.27) | 17.15 (13.75) |
| Cambridge Automated Neuropsychological Test Battery Spatial Recognition Memory task |
||||
| Ketamine | 0.71 (0.11) | 0.60 (0.12) | 0.60 (0.16) | 0.70 (0.11) |
| Propofol | 0.72 (0.15) | 0.63 (0.10) | 0.64 (0.11) | 0.65 (0.12) |
ECT, electroconvulsive therapy.
Over the course of the study 14 patients were lost to follow-up or withdrew. One patient withdrew before ECT was completed, four were withdrawn either because of ECT being abandoned or medical decisions to change the anaesthetic. One patient was withdrawn from the trial following the end of ECT but before any post-final ECT assessment because of their exposure to ‘legal high’ substances. A further two patients withdrew from the trial prior to the 1-month assessment, and three more did not respond to attempts to arrange the 1-month assessment. One patient returned for a further course of ECT, and two others were prescribed maintenance ECT before the 1-month assessment. Fig. 1 shows these withdrawals. The five patients (four from the ketamine group) who withdrew or were withdrawn after commencement of ECT but before a follow-up assessment were significantly older, t(38) = −2.11, P<0.05 (x̄ = 60.32) than those who continued (x̄ = 49.46) but there was no significant difference in their HRSD (x̄ = 21.6 v. 25.91), MADRS (x̄ = 31.0 v. 36.0) or CANTAB SRM (x̄ = 0.68 v. 0.72) scores. Data from these patients were not examined further. The number of ECT treatments received by patients in each group (ketamine: x̄ = 7.88, s.d.= 3.18; propofol: x̄ = 7.26, s.d. = 2.23) was compared using an independent samples t-test. No significant difference was found, t(33)<1.0, P>0.05, d= 0.23.
Acute effects analysis (between pre-ECT and post-ECT4)
For the HRSD, the ANCOVA found no main effect of anaesthetic (F(1,31) = 2.93, mean square error (MSE) = 25.10, P>0.05, η2p = 0.09, ketamine: 95% CI 19.60–24.83, propofol: 95% CI 16.80–21.58), a main effect of time (F(1,31) = 12.77, MSE = 46.97, P<0.01, η2p = 0.29, pre-ECT: 95% CI 23.29–28.60, post-ECT4: 95% CI 13.31–17.60) where pre-ECT scores were higher than post-ECT4 scores, and no interaction between anaesthetic and time (F(1,31) <1.0, η2p = 0.03, pre-ECT, ketamine: 95% CI 22.63–30.63, propofol: 95% CI 21.60–28.91; post-ECT4, ketamine: 95% CI 14.57–21.04, propofol: 95% CI 10.16–16.07). No interaction was found between time and gender (F(1,31)<1.0) but an interaction between time and age group was found (F(1,31) = 4.53, MSE = 46.97, P<0.05). Examination of mean scores showed that HRSD scores decreased over time for younger adults (x̄ = 26.50–14.91; n = 32) but not for older adults (x̄ = 19.33–19.0; n = 3). Removing the covariates, as advocated by Gilmore28 and IBM,29 resulted in a larger main effect of time (F(1,33) = 38.40, MSE = 50.58, P<0.01).
The MADRS ANCOVA found no main effect of anaesthetic (F(1,31) = 1.42, MSE = 50.73, P>0.05, η2p = 0.04, ketamine: 95% CI 26.44–33.87, propofol: 95% CI 23.76–30.54), a main effect of time (F(1,31) = 10.40, MSE = 76.12, P<0.01, η2p = 0.25, pre-ECT: 95% CI 33.12–38.87, post-ECT4: 95% CI 17.71–24.93) with pre-ECT scores higher than post-ECT4 scores, and no interaction between anaesthetic and time (F(1,31) = 2.05, P>0.05, η2p = 0.06, pre-ECT, ketamine: 95% CI 31.60–40.26, propofol: 95% CI 32.10–40.02; post-ECT4, ketamine: 95% CI 18.95–29.82, propofol: 95% CI 13.29–23.23). There were no interactions between time and gender (F(1,31)<1.0) or time and age (F(1,31) = 2.08, Ps>0.05). Removing the covariates again resulted in a larger within-participants effect of time (F(1,33) = 48.78, MSE = 77.53).
The ANCOVA examining mean CANTAB SRM proportion correct from all patients for whom there was data pre-ECT and post-ECT4 (n = 34) found no main effects of anaesthetic (F(1,30)<1.0, MSE = 0.01, η2p = 0.03, ketamine: 95% CI 0.61–0.70, propofol: 95% CI 0.64–0.72) or time (F(1,30)<1.0, MSE<0.02, η2p = 0.02, pre-ECT: 95% CI 0.68–0.77, post-ECT4: 95% CI 0.57–0.65), nor was there an interaction between anaesthetic and time (F <1.0, η2(1,30) p<0.01, pre-ECT, ketamine: 95% CI 0.65–0.78, propofol: 95% CI 0.67–0.80; post-ECT4, ketamine: 95% CI 0.54–0.66, propofol: 95% CI 0.57–0.68). There were no interactions between time and gender or time and age (both Fs(1,30)<1.0). Removing the covariates resulted in a significant within-participants effect of time (F(1,32) = 14.54, MSE<0.02, P<0.01): post-ECT4 scores were lower.
Treatment effects analysis (between pre-ECT, post-final ECT and 1-month post-ECT)
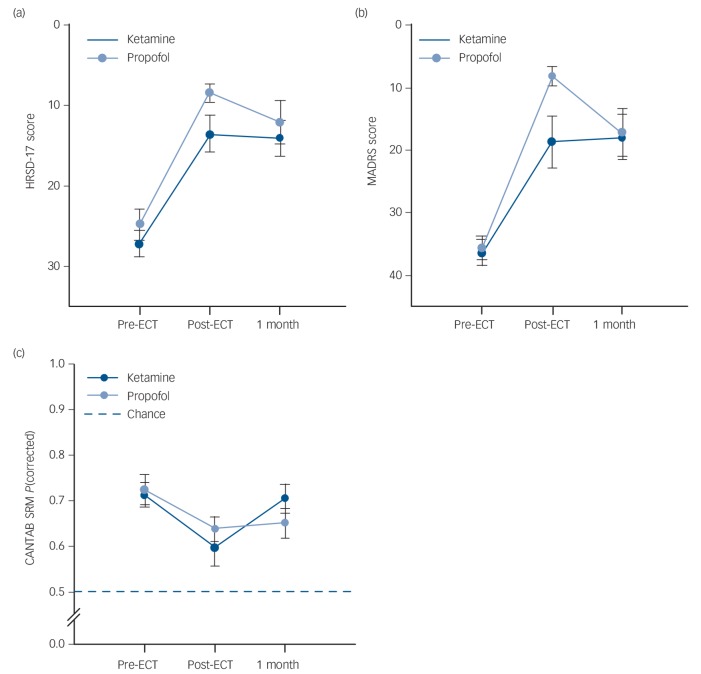
Linear mixed models examined group differences in depressive symptoms or cognitive function over time (pre-ECT, post-final ECT and at the 1-month follow-up). These data are displayed in Fig. 2. For HRSD ratings, there was no significant effect of anaesthetic (F(1) = 2.54, P>0.05) but a significant effect of time was found (F(2) = 46.92, P<0.01). Across patients HRSD scores were reduced following ECT. The interaction between factors was not significant (F(2)<1.0, P>0.05). For MADRS ratings, there was also no significant effect of anaesthetic (F(1) = 1.45, P>0.05) and a significant effect of time (F(2) = 55.03, P<0.01). Across patients MADRS scores were reduced following ECT. The interaction between factors was not significant (F(2) = 2.92, P = 0.06) but Fig. 2(b) shows this almost significant interaction is because of the steeper reduction in MADRS scores post-final ECT in the propofol group. On the CANTAB SRM, there was no significant effect of anaesthetic (F(1)<1.0, P>0.05) but a significant effect of time was found (F(2) = 5.84, P<0.01). Pairwise comparisons revealed that post-final ECT CANTAB SRM proportion correct was lower than the pre-ECT baseline. The interaction between factors was not significant (F(2)<1.0, P>0.05).
Fig. 2.
Mean scores on the dependent measures across time (pre-electroconvulsive therapy (ECT), post-final ECT and 1-month post-ECT) by anaesthetic group (treatment effects analysis).
(a) Group differences in Hamilton Rating Scale for Depression (HRSD) ratings across follow-up, (b) group differences in Montgomery–Åsberg Depression Rating Scale (MADRS) ratings across follow-up, (c) group differences in Cambridge Automated Neuropsychological Test Battery Spatial Recognition Memory task (CANTAB SRM) scores across follow-up. Error bars are the standard error of the mean.
Harms
One serious adverse event (prolonged hospital admission) was recorded in the propofol group when a participant developed an elevated mood following their ECT course. No adverse events related to exposure to ketamine occurred. It is also worth noting anecdotally that the psychotomimetic effects of ketamine were absent in our sample.
Discussion
Main findings and comparison with findings from other studies
Contrary to our hypotheses, when ketamine was used as the anaesthetic for ECT there was no significant difference in the number of ECT treatments, ratings of depression severity or cognitive function when compared with propofol. Indeed, although patients in both groups improved with treatment, examination of mean depression ratings on both measures actually show higher, albeit not significantly different, scores in the ketamine group.
These results join recent evidence7 that suggests ketamine's antidepressant effect does not emerge when it is used as the anaesthetic in ECT, despite promising prospects in other contexts on an acute timescale (for example Zarate et al,3 Berman et al,5 and Kudoh et al30). Yoosefi et al31 have recently reported a randomised, double-blind clinical trial comparing ketamine and thiopental as the anaesthetics for ECT. Patients in both groups received six ECT treatments (by design). Although patients who were administered ketamine had lower scores on the HRSD following the first ECT and before the second, there was no between-group difference in depression ratings at the end of treatment or 1 month after. Similarly, Rasmussen et al32 compared ketamine with methohexital but did not find any significant differences in depression ratings between the two anaesthetics. Consistently, when ketamine is used in addition to thiopentone anaesthetic no differences in depressive symptoms after the initial and sixth ECT,33 nor at the end of treatment,34 have been found.
The KANECT study found no significant difference between ketamine and propofol on a cognitive measure previously shown to detect post-ECT impairments. Again, other recent studies support this finding. Loo et al34 reported no difference in a large number of neuropsychological outcomes, whereas Rasmussen et al32 and Yoosefi et al31 report conflicting results with the Mini-Mental State Examination (MMSE).35 Rasmussen et al found no differences whereas Yoosefi et al found an improvement 1 month after the final ECT only in the ketamine group. These differences may reflect the insensitivity of the MMSE to specific cognitive deficits, and its susceptibility to changes based on patient orientation.
This trial was adequately powered to detect a difference between anaesthetic groups should it have occurred. The sample size (n = 40) was based on Okamoto et al26 where a large group difference in symptom reduction after the fourth treatment was found. They found a large effect based on an average 24-point drop in HRSD scores in the ketamine group (n = 8) v. a 14-point drop in the propofol group (n = 1). KANECT's power calculation was based on these results determining that given a large effect size 20 patients per group would be needed (two-tailed, power of 90%, critical t of 2.02). We achieved the same sample size as Okamoto et al26 but the drop in mean HRSD scores was equivalent between groups (ketamine 9.94, propofol 11.21). The equivalent critical t-value was also smaller: 1.71 (F(1,31) = 2.93, where t = √F). Crucially, there were methodological and statistical improvements in KANECT that may explain this difference. Okamoto et al randomised on the basis of patient preference and used repeated t-tests to compare scores after each ECT. In KANECT, patients and raters were masked to anaesthetic allocation, randomisation was minimised by gender and age (decreasing the degrees of freedom), and type-I error inflation was controlled appropriately. For these reasons, it is doubtful that a group difference would have been found should a larger sample been recruited.
Okamoto et al26 kept the number of ECTs constant between groups. This was a dependent variable in the current trial and fewer ECTs were administered in both groups although the numbers were broadly equivalent (8 in Okamoto et al v. 7.88 and 7.26 for ketamine and propofol, respectively here). This trial found no difference in the number of ECT treatments between anaesthetic groups and although this mirrored the depression ratings results, one potential source of bias for this measure was that the number of treatments was determined by the prescribing clinician rather than the study protocol. Although there is no reason to doubt that depression severity was the primary reason to continue ECT, and the prescribing clinician was masked to anaesthetic allocation, it is possible that other unknown factors had an influence.
Although no evidence of an enhanced antidepressive effect of ketamine was found, it was well-tolerated for ECT. No adverse events related to exposure to ketamine occurred. It is also worth noting anecdotally that the psychotomimetic effects of ketamine were absent in our participants. However, Caddy et al8 reported that ketamine caused more confusion and emotional blunting compared with placebo, with the former conclusion also reached by McGirr et al.7
Limitations
Our results are limited by a number of factors. First, because of patient attrition we were unable to compare treatment effects at all follow-ups for our entire sample meaning the analysis lost some power. Our use of linear mixed models goes some way to mitigate this limitation as such models use all available data and appropriately account for within-participant correlations in measurements. Second, although three of the four patients whose participation was discontinued for medical reasons were in the ketamine group, these withdrawals were made for medical reasons rather than as a result of anaesthetic assignment. Third, a feature of our trial design was that the decision on management of the ECT course was taken by the patients' treating clinicians, who were masked to anaesthetic assignment. Therefore, treatment failures (new ECT course or maintenance ECT prior to 1-month follow-up, n = 3) were outside our control and we are confident are independent of anaesthetic assignment. Fourth, we placed no restrictions on concomitant medication prescribing during the course of the trial except that benzodiazepines were withdrawn prior to ECT. Although it is plausible that other medication may have affected our results, randomisation means that these should be similarly distributed between our groups.
In conclusion, the results of this double-blind, parallel-design, RCT demonstrate that ketamine as an anaesthetic for ECT does not enhance the efficacy of ECT. Although patients in both groups improved with treatment, neither of the primary outcomes of the number of ECT treatments received nor the change in depression symptom severity showed any differences between anaesthetics. Nor was a difference found in the degree of impairment on the cognitive measure we employed.
Acknowledgments
The trial protocol is available for download here: http://www.abdn.ac.uk/ims/research/neuroscience/mood-disorders.php. This manuscript reports the results of the final clinical trial led by I.C.R. at the University of Aberdeen. Despite his untimely death in 2014, this work represents the culmination of his academic interest in the use and mechanisms of action underlying electroconvulsive therapy (ECT), which started with preclinical research published back in 1993. I.C.R. was always a champion of rigorous research into the causes of and treatments for mental illness and his influence in the field will be sorely missed. Our thanks go to all the patients who participated in the trial; Alison Campbell for pre-ECT patient assessment, recruitment, anaesthetic management during the trial and assistance in data collection; Ellen Robertson and Kate Ferries for their help in patient care and data collection; Jennifer Adams for her help with data collection, and to members of the independent Trial Steering Committee: Keith Matthews, Ian Anderson, John Norrie and Darren Gibson.
Footnotes
Declaration of interest
C.AS. reports grants from Vifor Pharma, outside the submitted work. I.C.R. (deceased) declared personal fees from AstraZeneca, Sanofi Aventis and Sunovion, and non-financial support from Lundbeck, between 2009 and 2014 and all outside the submitted work.
Funding
The Chief Scientists Office (CSO) of Scotland funded this research.
References
- 1. World Health Organization Depression Fact sheet N369. WHO, 2015. (http://www.who.int/mediacentre/factsheets/fs369/en/). [Google Scholar]
- 2. Waite J, Easton A. The ECT Handbook (3rd edn). Royal College of Psychiatrists, 2013. [Google Scholar]
- 3. Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–64. [DOI] [PubMed] [Google Scholar]
- 4. Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 2013; 170: 1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–4. [DOI] [PubMed] [Google Scholar]
- 6. McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 2015; 45: 693–704. [DOI] [PubMed] [Google Scholar]
- 7. McGirr A, Berlim MT, Bond DJ, Neufeld NH, Chan PY, Yatham LN, et al. A systematic review and meta-analysis of randomized controlled trials of adjunctive ketamine in electroconvulsive therapy: efficacy and tolerability. J Psychiatr Res 2015; 62: 23–30. [DOI] [PubMed] [Google Scholar]
- 8. Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev 2015; 9: CD011611. [DOI] [PubMed] [Google Scholar]
- 9. Fond G, Loundou A, Rabu C, Macgregor A, Lançon C, Brittner M, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology 2014; 231: 3663–76. [DOI] [PubMed] [Google Scholar]
- 10. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol 2015; 30: 152–63. [DOI] [PubMed] [Google Scholar]
- 11. Lee EE, Della Selva MP, Liu A, Himelhoch S. Ketamine as a novel treatment for major depressive disorder and bipolar depression: A systematic review and quantitative meta-analysis. Gen Hosp Psychiatry 2015; 37: 178–84. [DOI] [PubMed] [Google Scholar]
- 12. Brakemeier E, Berman R, Prudic J, Zwillenberg K, Sackeim HA. Self-evaluation of the cognitive effects of electroconvulsive therapy. J ECT 2011; 27: 59–66. [DOI] [PubMed] [Google Scholar]
- 13. Robertson H, Pryor R. Memory and cognitive effects of ECT: informing and assessing patients. Adv Psychiatr Treat 2006; 12: 228–37. [Google Scholar]
- 14. Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol 1983; 334: 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 1986; 319: 774–6. [DOI] [PubMed] [Google Scholar]
- 16. Stewart CA, Reid IC. Ketamine prevents ECS-induced synaptic enhancement in rat hippocampus. Neurosci Lett 1994; 178: 11–4. [DOI] [PubMed] [Google Scholar]
- 17. Krystal AD, Weiner RD, Dean MD, Lindahl VH, Tramontozzi LA, III, Falcone G, et al. Comparison of seizure duration, ictal EEG, and cognitive effects of ketamine and methohexital anesthesia with ECT. J Neuropsychiatry Clin Neurosci 2003; 15: 27–34. [DOI] [PubMed] [Google Scholar]
- 18. McDaniel WW, Sahota AK, Vyas BV, Laguerta N, Hategan L, Oswald J. Ketamine appears associated with better word recall than etomidate after a course of 6 electroconvulsive therapies. J ECT 2006; 22: 103–6. [DOI] [PubMed] [Google Scholar]
- 19. Scottish ECT Accreditation Network Scottish ECT Accreditation Network Annual Report 2015: A Summary of ECT in Scotland for 2014. ISD Scotland Publications, 2015. [Google Scholar]
- 20. Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther 1974; 15: 443–53. [DOI] [PubMed] [Google Scholar]
- 21. Williams JBW, Kobak KA, Beck P, Engelhardt N, Lipsitz J, Olin J, et al. The GRID-HAMD: standardisation of the Hamilton depression rating scale. Int Clin Psychopharmacol 2008; 23: 120–9. [DOI] [PubMed] [Google Scholar]
- 22. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–9. [DOI] [PubMed] [Google Scholar]
- 23. Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 1995; 33: 1–24. [DOI] [PubMed] [Google Scholar]
- 24. Fernie G, Bennett DM, Currie J, Perrin JS, Reid IC. Detecting objective and subjective cognitive effects of electroconvulsive therapy: intensity, duration and test utility in a large clinical sample. Psychol Med 2014; 44: 2985–94. [DOI] [PubMed] [Google Scholar]
- 25. Falconer DW, Cleland J, Fielding S, Reid IC. Using the Cambridge neuropsychological test automated battery (CANTAB) to assess the cognitive impact of electroconvulsive therapy on visual and visuospatial memory. Psychol Med 2010; 40: 1017–25. [DOI] [PubMed] [Google Scholar]
- 26. Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J ECT 2010; 26: 223–7. [DOI] [PubMed] [Google Scholar]
- 27. IBM Problem Subject: Repeated Measures with Constant Covariates in GLM. SPSS Knowledgebase Resolution Number 22133. IBM, 2010. (http://www-01.ibm.com/support/docview.wss?uid=swg21477023). [Google Scholar]
- 28. Gilmore GC. Inappropriate use of covariate analysis renders meaningless results. J Int Neuropsychol Soc 2007; 13: 370. [DOI] [PubMed] [Google Scholar]
- 29. IBM Repeated Measures ANCOVA with a Constant Covariate in MIXED in SPSS. SPSS Knowledgebase Resolution Number 22273. IBM, 2010. (http://www-01.ibm.com/support/docview.wss?uid=swg21477006). [Google Scholar]
- 30. Kudoh A, Takahira Y, Katagai H, Takazawa T. Small-dose ketamine improves the postoperative state of depressed patients. Anesth Analg 2002; 95: 114–8. [DOI] [PubMed] [Google Scholar]
- 31. Yoosefi A, Sepehri AS, Kargar M, Akhondzadeh S, Sadeghi M, Rafei A, et al. Comparing effects of ketamine and thiopental administration during electroconvulsive therapy in patients with major depressive disorder: a randomized, double-blind study. J ECT 2014; 30: 15–21. [DOI] [PubMed] [Google Scholar]
- 32. Rasmussen KG, Kung S, Lapid MI, Oesterle TS, Geske JR, Nuttall GA, et al. A randomized comparison of ketamine versus methohexital anesthesia in electroconvulsive therapy. Psychiatry Res 2014; 215: 362–5. [DOI] [PubMed] [Google Scholar]
- 33. Abdallah CG, Fasula M, Kelmendi B, Sanacora G, Ostroff R. Rapid antidepressant effect of ketamine in the electroconvulsive therapy setting. J ECT 2012; 28: 157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loo CK, Katalinic N, Garfield JBB, Sainsbury K, Hadzi-Pavlovic D, Mac-Pherson R. Neuropsychological and mood effects of ketamine in electroconvulsive therapy: a randomised controlled trial. J Affective Disord 2012; 142: 233–40. [DOI] [PubMed] [Google Scholar]
- 35. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]