Abstract
Key points
Following substantial bleaching of the visual pigment, the desensitization of the rod photovoltage is not as substantial as the desensitization of the rod outer segment photocurrent.
The block of cation conductances during the internal dialysis of Cs+ further desensitizes the photovoltage thereby eliminating its difference in desensitization with the rod outer segment photocurrent.
Bleached visual pigment produced an acceleration of the rod photovoltage with respect to the outer segment photocurrent, which is eliminated upon internal dialysis of Cs+.
Abstract
A majority of our visual experience occurs during the day when a substantial fraction of the visual pigment in our photoreceptor cells is bleached. Under these conditions it is widely believed that rods are saturated and do not contribute substantially to downstream signalling. However, behavioural experiments on subjects with only rod function reveals that these individuals unexpectedly retain substantial vision in daylight. We sought to understand this discrepancy by characterizing the sensitivity of rod photoresponses following exposure to bright bleaching light. Measurements of the rod outer segment photocurrent in transgenic mice, which have only rod function, revealed the well‐studied reduction in the sensitivity of rod photoresponses following pigment bleaching. However, membrane voltage measurements showed that the desensitization of the photovoltage was considerably less than that of the outer segment photocurrent following equivalent pigment bleaching. This discrepancy was largely eliminated during the blockade of cation channels due to the internal dialysis of Cs+, which increased the bleach‐induced desensitization of the photovoltage and slowed its temporal characteristics. Thus, sensitization of the photovoltage by rod inner segment conductances appears to extend the operating range of rod phototransduction following pigment bleaching.
Keywords: adaptation, photocurrent, photovoltage, retina, rod photoreceptor
Key points
Following substantial bleaching of the visual pigment, the desensitization of the rod photovoltage is not as substantial as the desensitization of the rod outer segment photocurrent.
The block of cation conductances during the internal dialysis of Cs+ further desensitizes the photovoltage thereby eliminating its difference in desensitization with the rod outer segment photocurrent.
Bleached visual pigment produced an acceleration of the rod photovoltage with respect to the outer segment photocurrent, which is eliminated upon internal dialysis of Cs+.
Abbreviations
- ERG
electroretinogram
- IS
inner segment
- MSP
microspectrophotometric
- Rh*
activated rhodopsin
Introduction
The sensitivity of our sensory systems is set fundamentally by limits imposed by sensory receptors. In dim light our vision is mediated by rod photoreceptors, whose robust single‐photon response allows exquisite sensitivity at low light levels. However, electrophysiological recordings from single rods suggest that as background light increases transduction channels close, saturating the rod photocurrent, in turn preventing the contribution of rods to vision. Surprisingly, behavioural studies on humans (Blakemore & Rushton, 1965) or mice (Naarendorp et al. 2010) lacking cone function reveal that rod vision indeed extends to bright background lights, in excess of 10 000 Rh* per rod s−1. It is difficult to reconcile how rod phototransduction that is strongly desensitized in background light can support vision under these conditions, unless single‐cell recordings fail to capture long‐time scale adaptive changes.
For decades our understanding of rod vision has centred on the phototransduction mechanism in rod outer segments responsible for converting absorbed light into changes in membrane current (Arshavsky et al. 2002; Arshavsky & Burns, 2012). More specifically, a strong focus has been placed on how the sensitivity of the rod photocurrent is controlled through modulation of the phototransduction. Following exposure to bright bleaching light, the rod photocurrent experiences a persistent desensitization, called bleaching adaptation (Cornwall et al. 1989). This form of adaptation is functionally equivalent to background adaptation (Jones et al. 1996), because bleached visual pigment constitutively activates phototransduction, although with reduced efficiency compared to light‐activated rhodopsin, Rh* (Cornwall & Fain, 1994; Matthews et al. 1996; Melia et al. 1997). The increased phototransduction activity desensitizes the photoreceptor cells beyond that expected from the depleted visual pigment and consequent loss of quantum catch. Notably, a component of this desensitization is due to compression of the light‐suppressible photocurrent as it approaches zero (Nikonov et al. 2000).
In principle, the outer segment photocurrent creates changes in membrane potential, or photovoltage, that is ultimately responsible for controlling glutamate release onto second‐order retinal neurons. Thus, inner segment conductances further shape the photovoltage (Owen & Torre, 1983; Beech & Barnes, 1989; Barnes, 1994; Barrow & Wu, 2009), but how these conductances do so during bleaching adaptation is not well established. Here we report bleach‐induced alterations in the sensitivity of rods in transgenic mice lacking cone photoresponses. Following substantial pigment bleaching, we found that the rod photocurrent measured with suction electrodes experienced a persistent reduction in sensitivity that is explained by the combined loss of quantum catch, and bleached pigment‐induced desensitization of the phototransduction cascade (see also Nymark et al. 2012). However, the rod photovoltage, whether measured from whole retina by electroretinogram (ERG), or from single rods measured with patch electrodes, did not display the same extent of desensitization as seen in photocurrent recordings. This increased sensitivity of the photovoltage was eliminated when rod inner segment cation channels were blocked by the internal dialysis of rods with a Cs+ internal solution. Thus, rod inner segment cation conductances appear to limit the sensitivity reduction of the rod output that occurs following pigment bleaching, effectively increasing the dynamic range of the rod output.
Methods
Mouse lines and preparation
While wild‐type mice (WT; C57BL6J, Jackson Laboratory, Bar Harbor, ME, USA) were used in some experiments, most experiments were performed on mice lacking cone transducin and thus lacking cone photoresponses [Gnat2cpfl3/cpfl3 (or Gnat2−/−)] bred for more than five generations in a C57BL6J background (Chang et al. 2006). The retinas of Gnat2−/− mice do not show degeneration up to 36 weeks of age, and display robust rod‐driven responses in bipolar cells (Arman & Sampath, 2012), indicating normal rod phototransduction and synaptic transmission.
Mice were maintained on a 12 h day–night cycle and were always dark‐adapted overnight prior to experimentation. Animals were killed by cervical dislocation in accordance with protocols approved by the Institutional Animal Care and Use Committees of the University of California Los Angeles, Boston University School of Medicine and previously at the University of Southern California, in accordance with the Guide for the Care and Use of LaboratoryAnimals and the Animal Welfare Act. All experimental manipulations were performed in darkness under infrared illumination visualized with infrared image converters (BE Meyers, Redmond, WA, USA). Following death of the animal, eyes were enucleated, the lens and cornea were removed, and eyecups were stored in darkness at 32°C in Ames’ media buffered with sodium bicarbonate (Sigma, St Louis, MO, USA; Cat. No. A1420), and equilibrated with 5% CO2/95% O2. Before recordings the retinas were isolated from the retinal pigment epithelium to prevent regeneration of the visual pigment, rhodopsin. In all recordings the sensitivity of retinal cells was estimated from the flash strength that yielded a half‐maximal response (I 1/2).
Microspectrophotometry
The extent of photopigment bleaching in rods was assessed using a microspectrophotometer (Cornwall et al. 1984; Jones et al. 1993) as described previously for the mouse retina (Nymark et al. 2012). To control the amount of bleaching for the physiology experiments (see below), the fraction of bleached visual pigment was determined by two independent methods. First, the expected fractional bleach was calculated based on the photosensitivity of the visual pigment, the light intensity and the bleach duration in the respective experiments. Second, direct microspectrophotometric (MSP) measurements of pigment density were made from patches of rod outer segments that projected from edges of retinal pieces (Nymark et al. 2012; Berry et al. 2016). The concentration of bleached pigment remained constant for up to 90 min following the bleaching light exposure as expected in the absence of the retinal pigment epithelium, and the lack of pigment regeneration (Berry et al. 2016). The amounts of pigment bleaching measured using MSP were consistent with calculations of pigment bleaching used for physiological recordings.
Single cell suction electrode recordings
The sensitivity of rod outer segment current was assessed using suction electrodes as described previously (Okawa et al. 2010; Frederiksen et al. 2012; Nymark et al. 2012). Briefly, the dark‐adapted retina was chopped up into pieces, transferred to the recording chamber, and superfused with Ames’ media at a rate of ∼ 4–6 ml min−1, equilibrated with 5% CO2/95% O2 and maintained at 35–37°C. The recording chamber was placed on the stage of a modified inverted microscope (Invertoskop D; Carl Zeiss, Inc., Thornwood, NY, USA) in a light‐tight Faraday cage. Intact photoreceptors attached to retinal pieces were visualized by a CCD camera (LCL‐902HS; Watec, Middletown, NY, USA). Cells were drawn, outer segment first, into a glass micropipette filled with Ames’ media and connected to a patch‐clamp amplifier (EPC‐7; List Associates, Great Neck, NY, USA). Pipettes were fabricated from borosilicate capillary glass tubing and fire‐polished to an inner diameter of ∼1 μm, which gave a tight fit to the inner segment of the mouse rod. Light‐evoked responses to 20 ms flashes of light were measured as changes in extracellular photocurrent. In any given experiment the sensitivity in the dark‐adapted state, and after a bleach of 20, 50 or 70%, was recorded. The stimulus light and bleaching light was produced on a standard light bench described earlier (Frederiksen et al. 2012).
Whole retina ERG
Mice lacking cone phototransduction (see above) were used to isolate rod‐driven activity following pigment bleaching using transretinal‐ERGs, since cones in wild‐type (WT) mice dominate retinal sensitivity in bright light. Briefly, retinas were mounted photoreceptor side‐up over a machined 1 mm hole in a Plexiglass recording chamber, were gently flattened using forceps and were held flat using anchors. The tissue was superfused in darkness at a rate of 4–6 ml min−1 with 35–37°C Ames’ media buffered with sodium bicarbonate and equilibrated with 5% CO2/95% O2 (pH ∼ 7.4). The trans‐retinal potential change to flashes of light was measured using Ag/AgCl half‐cells connected to a differential amplifier (Model DP‐311; Warner Instruments, Hamden, CT, USA). Recordings were sampled at 1 kHz and low‐pass filtered at 30 Hz. Flashes of light, as well as bleaching light exposure, were delivered from a standard light bench (Miyagishima et al. 2009). ERG a‐waves were further isolated pharmacologically by superfusing the retina with Ames’ media containing 2 mm aspartate to eliminate the downstream contributions from the retinal circuitry and 10 mm BaCl in the solution facing the inner retina to mitigate Müller cell activity (Nymark et al. 2006).
Single cell patch clamp recordings
The photovoltage of single rods was measured with patch‐clamp electrodes from rod inner segments in 200 μm thick retinal slices, as described previously (Majumder et al. 2013, 2015). Briefly, a small piece of dark‐adapted retina was embedded in agar and cut with a vibrating microtome, transferred to a recording chamber, and superfused with Ames’ media at a rate of 4–6 ml min−1, equilibrated with 5% CO2/95% O2 and maintained at 35–37°C. The normal pipette internal solution for whole‐cell patch clamp recordings consisted of (in mm): 125 potassium aspartate, 10 KCl, 10 HEPES, 5 N‐methyl glucamine‐HEDTA, 0.5 CaCl2, 1 ATP‐Mg and 0.2 GTP‐Mg; pH was adjusted to 7.2 with N‐methyl glucamine hydroxide. For recordings of the effect of inner segment cation conductances on photovoltage the internal solution consisted of (in mm): 110 CsCH3SO3, 20 tetraethyl ammonium (TEA)‐Cl, 10 HEPES, 2 QX314 [N‐(2,6‐dimethylphenylcarbamoyl‐methyl) triethylammonium bromide] bromo, 5 N‐methyl glucamine‐HEDTA, 0.5 CaCl2, 1 ATP‐Mg and 0.2 GTP‐Mg; pH was adjusted to 7.2 with N‐methyl glucamine‐OH. The average series resistance for current clamp recordings was ∼ 15–20 MΩ and the responses were corrected for a calculated liquid junction potential of ∼10 mV (see Okawa et al. 2008). Light‐evoked responses were recorded following the delivery of 10 ms flashes from a blue LED (λmax ∼ 470 nm, full width half maximum ∼ 30 nm) whose strength varied from producing a just‐measurable response, and increased by factors of 2. Responses were low‐pass filtered at 300 Hz by an eight‐pole Bessel filter and digitized at 1 kHz. Pigment bleaching was also performed with the blue LED and slices were superfused with Ames’ media for 1 h in darkness following bleaching to allow sensitivity to reach steady‐state; recordings of the bleach‐adapted responses were then made (see Nymark et al. 2012).
Estimation of rod desensitization
A critical part of this work is the comparison of rod desensitization measured from the outer segment photocurrent or from the rod photovoltage (either whole retinal ERG or single cell patch clamp recordings in current clamp mode). We estimated rod desensitization following bleaching using a framework developed by Jones et al. (1996) where the sensitivity following pigment bleaching (S F) relative to the sensitivity in the dark‐adapted state (S F D) is plotted versus the fraction bleach (F) and fitted with a Weber–Fechner function scaled by a factor, k. This relation takes the form:
| (1) |
In the limit where k approaches zero, S F/S F D declines in proportion to the loss of quantum catch (i.e. a 50% bleach results in a halving in sensitivity, etc.). Thus, as k increases the extent of desensitization increases above the desensitization due to loss of quantum catch. Light intensities in each instance are reported as a 500 nm photon flux (φ μm−2), near the peak wavelength of sensitivity for rhodopsin. Although the sensitivity measured in each experimental condition reflects the same underlying processes, the differences in recording techniques (i.e in tissue preparation, angle of incidence of the light, etc.) make a direct comparison tenuous. However, the relative shift in light intensities used in different preparations between the dark‐adapted and bleach‐adapted conditions provide a level of internal consistency. Thus, the k‐value can be used as a measurement for relative desensitization, which can be compared across these preparations.
Results
Pigment bleaching desensitizes the rod photocurrent
Classical psychophysical experiments have shown that a human rod monochromat, an individual who lacks cone function, is able to function efficiently during the day with surprisingly little visual impairment (Blakemore & Rushton, 1965). Such a result appears to be at odds with measurements of light sensitivity from rods of amphibian and mammalian animal models following bright, bleaching light exposure (Cornwall & Fain, 1994; Jones et al. 1996; Woodruff et al. 2003; Fan et al. 2005; Naarendorp et al. 2010; Nymark et al. 2012). In particular, in salamander rods it has been shown that pigment bleaching causes a reduction in the sensitivity of the rod outer segment photocurrent that exceeds the prediction based on the loss of visual pigment (Cornwall et al. 1989). The extra desensitization is commonly referred to as ‘bleaching adaptation’ and is the result of persistent bleached visual pigment, or free opsin, activating phototransduction with a low efficiency (Cornwall & Fain, 1994, Melia et al. 1997). The residual phototransduction activation is functionally equivalent to exposure to background light (Jones et al. 1996), and causes a reduction in the rod's dark current that compresses light‐evoked responses. We sought to elucidate the mechanisms that allow rod photoresponses to be relayed robustly to higher visual centres despite the desensitization produced by bleaching adaptation in the rod photocurrent (see Majumder et al. 2013).
Previous work has shown that mouse rods also exhibit bleaching adaptation (Nymark et al. 2012). We estimated the magnitude of bleaching adaptation in WT mouse rods by making suction electrode recordings of the photocurrent at steady‐state following pigment bleaching while rods were bathed in bicarbonate‐buffered Ames’ media, the common extracellular solution in all experiments presented. Figure 1 A plots representative photocurrent families collected from dark‐adapted rods before, and after 1 h when steady‐state was reached following 20, 50 or 70% bleaching of the visual pigment (Rh). The relationships between the response amplitude and flash strength in each condition are plotted in Fig. 1 B, and reveal both a desensitization and compression of the photocurrent comparable to that reported previously (Nymark et al. 2012). We quantified the extent of desensitization of the photocurrent under these conditions by plotting the sensitivity (S F), normalized to the sensitivity in darkness (S F D), as a function of the fraction of bleached pigment (Fig. 1 C; see Methods). As stated above, the k‐value of the Weber–Fechner function (eqn (1)) can be used as a measure of relative desensitization following pigment bleaching. The best fit for the Weber–Fechner function was achieved with a value of k = 24 (eqn (1)), in broad agreement with the previous study (Nymark et al. 2012).
Figure 1. Measurements of photocurrent in darkness and following pigment bleaching from single rods using suction electrodes.
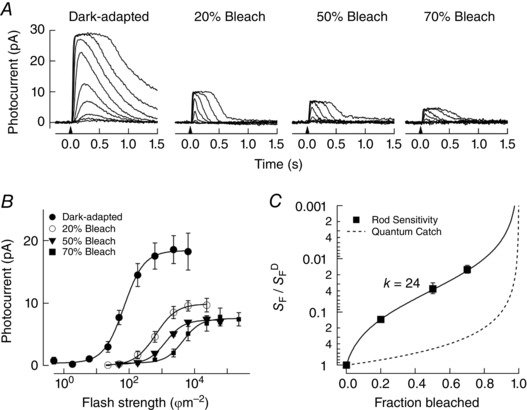
A, flash families of photocurrent in darkness (n = 7) and 1 h after bleaching 20% (n = 3), 50% (n = 3) and 70% (n = 4) of the visual pigment. Flashes at the time indicated by the downward arrow delivered 2.0–6200 photons μm−2 in darkness, 22–23 000 photons μm−2 after the 20% bleach, 22–61 000 photons μm−2 after the 50% bleach and 49–230 000 after the 70% bleach. B, the sensitivity of rod photocurrent was determined from the flash strength that yielded a half‐maximal response. C, sensitivity following pigment bleaching (S F), relative to the sensitivity in darkness (S F D), is plotted versus the fraction of the visual pigment bleached for single rods (see also Methods). The dashed line reflects the expected loss of sensitivity due to the loss of quantum catch alone. The fit line is a Weber–Fechner relationship scaled by the fraction of bleached pigment, where k = 24 (Jones et al. 1996).
The rod photovoltage exhibits less bleach‐induced desensitization than the photocurrent
The rod output is determined by the light‐activated change in photovoltage, which is ultimately responsible for synaptic transmission to second‐order rod ON bipolar cells, and the flow of current to neighbouring photoreceptors through gap junctions. We measured the sensitivity of the average rod photovoltage using whole retina ERGs in mice lacking cone photoresponses (see Methods) to compare against these bleach‐induced changes in photocurrent sensitivity. In retinas isolated from the retinal pigment epithelium we bleached 30–70% of the visual pigment, Rh, and then estimated sensitivity changes in a pharmacologically isolated a‐wave once the active intermediates of bleaching had decayed. Figure 2 A plots representative a‐wave families collected from a dark‐adapted retinal whole mount, and in whole mounts at steady‐state following bleaching of the visual pigment as indicated. Bleach‐adapted photovoltages measured by ERG exhibited the characteristic changes expected from our measurements of extracellular photocurrent. These manifestations include response compression (Fig. 2 A) and a reduction in response amplitude per incident photon (i.e. sensitivity; Fig. 2 B), which scaled with the fraction of bleached pigment (Leibovic et al. 1987).
Figure 2. Electroretinogram recordings of average rod photovoltage in darkness and following pigment bleaching.
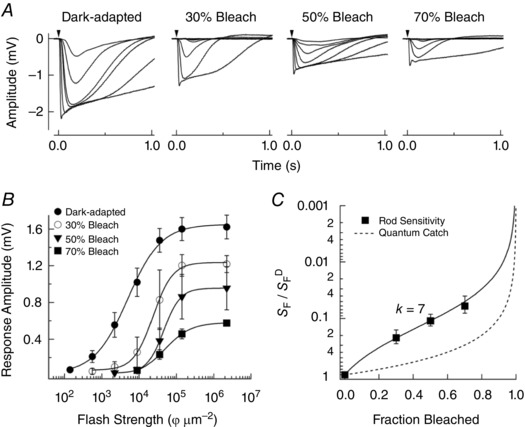
A, pharmacologically isolated ERG a‐waves measured from whole‐mount retinas of Gnat2−/− mice in darkness (n = 6) and 1 h after 30% (n = 4), 50% (n = 7) and 70% (n = 7) bleaching of the visual pigment (see Methods). Responses in darkness and after bleaching were from the same retina. Flashes at the time indicated by the downward arrow delivered 140–35 000 photons μm−2 in darkness, 550–35 000 photons μm−2 after the 30% bleach, and 2200–140 000 photons μm−2 after the 50% and 70% bleaches. B, response versus flash strength relationships for response families shown in A for the same retina in darkness and after bleaching. The sensitivity of rod photoresponses was determined from the flash strength that yielded a half‐maximal response. C, the sensitivity following pigment bleaching (S F), relative to the sensitivity in darkness (S F D), plotted versus the fraction of the visual pigment bleached and fitted as in Fig. 1. The fit line is a Weber–Fechner relationship with k = 7.
Similar to the photocurrent recordings (Fig. 1), we quantified the extent of desensitization of the a‐wave under these conditions by plotting the sensitivity (S F), normalized to the sensitivity in darkness (S F D), as a function of the fraction of bleached pigment (Fig. 2 C). The best fit for the Weber–Fechner function (eqn (1)) was achieved with a value of k = 7. Note that this scaling factor is considerably lower (P = 0.02, Student's t‐test) than the equivalent measure from the photocurrent measurements (k = 24; Fig. 1 C), indicating that the rod photovoltage appears ∼3‐fold more sensitive than the rod photocurrent following equivalent levels of pigment bleaching.
Voltage‐sensitive conductances increase rod sensitivity following pigment bleaching
We sought to understand the basis for the discrepancy between the extent of bleach‐induced sensitivity decreases in the rod photocurrent (k = 24; Fig. 1 C) versus the average rod photovoltage (k = 7; Fig. 2 C). This discrepancy reveals that the rod photovoltage is desensitized to a lesser extent than the photocurrent following pigment bleaching. It should be noted that the rod photocurrent was measured from isolated single rods, whereas the average rod photovoltage was measured from the complex ERG field potential change across the whole retina. To understand better the cellular mechanisms shaping desensitization following pigment bleaching, we measured the photovoltage from single rods in retinal slices using patch electrodes in current clamp mode. The use of Gnat2−/− mice in these recordings ensured the measured inner segment (IS) photovoltage was always from single rods. Figure 3 A plots representative photovoltage families collected from dark‐adapted rods, or after 1 h when steady‐state was reached following a 30, 70 or 90% bleach of the visual pigment. Sensitivity was again determined as the flash strength that yielded a half‐maximal response (Fig. 3 B), and the best fit for the Weber–Fechner function (eqn (1)) was achieved with a value of k = 8 (Fig. 3 C), in agreement with photovoltage recordings by ERG (Fig. 2 C, P = 0.8). Similar to ERG recordings, a reduction of the maximal response was observed consistent with a gradual hyperpolarization of the resting membrane potential when the fraction of rhodopsin bleach increased (Fig. 3 A, Table 1).
Figure 3. Measurements of bleaching adaptation from inner segments of single rods using the patch clamp technique in current clamp mode.
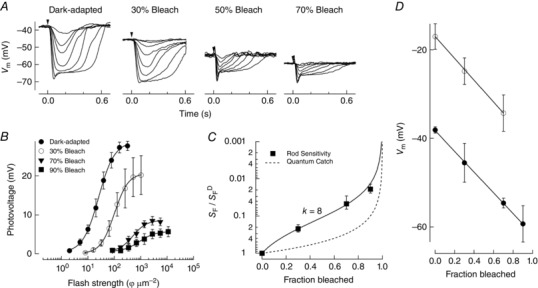
A, photovoltage responses measured from the inner segments of single rods in retinal slices of Gnat2−/− mice in darkness (n = 8) and 1 h after 30% (n = 6), 70% (n = 6) and 90% (n = 3) bleaching of the visual pigment (see Methods). Flashes at the time indicated by the downward arrow delivered 2.0–510 photons μm−2 in darkness, 8.0–1000 photons μm−2 after the 30% bleach, and 85–11 000 photons μm−2 after the 70% and 90% bleaches. B, the sensitivity of rod photoresponses was determined from the flash strength that yielded a half‐maximal response. C, sensitivity following pigment bleaching (S F), relative to the sensitivity in darkness (S F D), is plotted versus the fraction of the visual pigment bleached for Gnat2−/− retinas. The fit line is a Weber–Fechner relationship with k = 8 (see also Jones et al. 1996). D, the resting membrane potential in the dark‐adapted state, and at steady‐state in darkness after 30, 70 and 90% (solid) bleaching is plotted against the fraction bleach and fitted with a line. Note the linear relationship indicating the hyperpolarization of the resting membrane potential as a function of the extent of pigment bleached. The resting membrane potential in the dark‐adapted state and at steady‐state following 70% pigment bleaching while using a Cs+ electrode internal solution is shown as open symbols.
Table 1.
Response parameters for patch clamp recordings from rod photoreceptors
| Bleach level | I half (ϕ μm−2) | S F (1/I half) | S F/S F D | Vmax (mV) | V rest (mV) | n |
|---|---|---|---|---|---|---|
| DA | 30 ± 3.4 | 0.04 ± 0.01 | 1 | 24 ± 2.3 | −38 ± 0.7 | 8 cells 5 mice |
| DA Cs | 48 ± 2.7 | 0.02 ± 0.001 | 1 | 30 ± 5 | −17 ± 2.9 | 8 cells 3 mice |
| 30% | 130 ± 18 | 0.009 ± 0.001 | 0.2 | 21 ± 3.8 | −46 ± 4.4 | 6 cells 2 mice |
| 30% Cs | 260 ± 12 | 0.04 ± 0.001 | 0.1 | 23 ± 2 | −25 ± 2.9 | 9 cells 2 mice |
| 70% | 600 ± 55 | 0.002 ± 0.0001 | 0.05 | 9 ± 1.4 | −55 ± 1.1 | 6 cells 3 mice |
| 70% Cs | 1400 ± 160 | 0.001 ± 0.0001 | 0.02 | 7.0 ± 2.0 | −35 ± 4.1 | 5 cells 2 mice |
| 90% | 1500 ± 200 | 0.001 ± 0.0001 | 0.02 | 6.5 ± 2.5 | −60 ± 4.1 | 3 cells 1 mouse |
DA, dark‐adapted.
In response to the brightest flashes a fast negative component, or ‘nose’, can be seen in the photovoltage recordings, which arises mainly from the IS hyperpolarization‐activated cation current, I h (Fain et al. 1977; Vinberg & Koskelainen, 2010; Della Santina et al. 2012). Additionally, other cation conductances not part of the rod photocurrent (Baylor & Nunn, 1986) play a key role in shaping the rod photovoltage, including I Kx (Barnes & Hille, 1989; Beech & Barnes, 1989; Barnes, 1994). To determine whether these conductances play any role in increasing the photovoltage sensitivity, we recorded the rod photovoltage with a patch electrode internal solution where Cs+ was substituted for K+ (see Methods). As shown in Fig. 4 A, the nose component was totally absent both in the dark‐adapted state and after a 70% bleach. Sensitivity under these conditions was again estimated from the half‐maximal flash strength and normalized to the sensitivity in darkness under normal conditions (S F D) (Table 1). The Weber–Fechner equation (Fig. 4 B, dashed line) was best fit with k = 20, not significantly different from the value derived in the photocurrent measurements (k = 24, dotted line; Fig. 1 C, P = 0.5). Thus, a large part of the reduced sensitivity in the rod photocurrent compared to the rod photovoltage following pigment bleaching appears to be due to IS voltage‐dependent cation conductances.
Figure 4. Effect of Cs+ on the photovoltage following substantial bleaching of visual pigment.
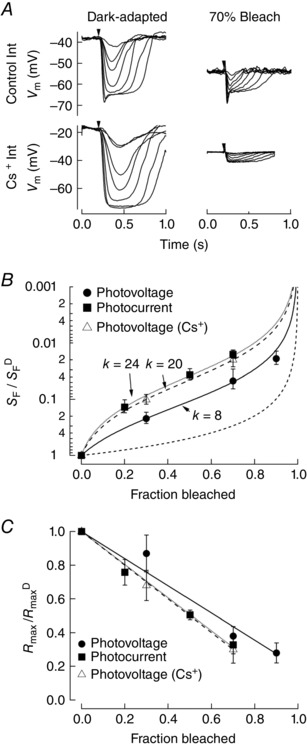
A, photovoltage was measured from the inner segments of rods in retinal slices of Gnat2−/− mice in darkness and 1 h after 70% bleaching of the visual pigment (n = 6). Inner segment K+ conductances were blocked by substituting Cs+ for K+ in the pipette solution. Flashes at the time indicated by the downward arrow delivered 2.0–510 photons μm−2 in darkness, 8.0–1000 photons μm−2 after the 30% bleach, and 85–11 000 photons μm−2 after the 70% bleach. B, the sensitivity of rod photoresponses was determined from the flash strength that yielded a half‐maximal response. Sensitivity following pigment bleaching (S F), relative to the sensitivity in darkness (S F D), is plotted versus the fraction of the visual pigment bleached. The best fit Weber–Fechner relationship for photovoltage responses with Cs+ internal solution (dashed line) is with k = 20. The Weber–Fechner relationship for photocurrent (dotted line, k = 24) and photovoltage (solid line, k = 8) are shown for comparison. C, the reduction in the maximal amplitude of the response (R max), normalized to the maximum amplitude in the dark‐adapted state (R max D), is plotted against the fraction bleached. Data were fit with a line constrained to the value of R max D at 0% bleach. Fits indicate that R max declines less steeply as a function of fraction bleach for the photovoltage compared to the photocurrent, or to the photovoltage measured with a Cs+ internal solution.
Furthermore, we observed a reduction in the response compression of the photovoltage compared to the photocurrent. Part of this effect is seen as a depolarization of the resting membrane potential in darkness and after bleaching (Fig. 3 D, open circles). In addition, Fig. 4 C plots the change in the maximal amplitude (R max) normalized to the maximum amplitude under dark‐adapted conditions (R max D) as a function of the fraction of visual pigment bleached and fit with a line. The photovoltage (solid) exhibited a smaller reduction in maximum amplitude compared to the photocurrent (dotted) as a function of the fraction of rhodopsin bleached. This effect was totally abolished when using a Cs+‐based patch internal solution (dashed line), indicating a non‐linear relationship between dark current and membrane voltage that is at least in part governed by inner segment cation conductances.
We finally compared the characteristics of the photocurrent and photovoltage to determine the role of cation conductances in the temporal processing of rod‐driven signals (see also Barnes & Hille, 1989; Beech & Barnes, 1989, 1994; Barrow & Wu, 2009). In Fig. 5, we plot flash families for the photocurrent, photovoltage, and the photovoltage measured with a Cs+ internal solution both for the dark‐adapted state and following a 70% bleach of the visual pigment. A speeding of the photovoltage compared to the photocurrent can be seen by inspecting these families, which appears to be reversed when cation conductances are blocked with the Cs+ internal solution (Table 1). Dark‐adapted dim flash responses under these conditions reveal that the slowed Cs+ responses were well matched to the kinetics of the photocurrent, reinforcing the notion that inner segment conductances improve the temporal sensitivity of the rod response for downstream processing (Beech & Barnes, 1989; Barrow & Wu, 2009). This effect on the temporal characteristics appears greatest for rods in the dark‐adapted state and gradually declines as the fraction of bleached rhodopsin increases, as seen for the normalized responses following 70% bleaching.
Figure 5. Effect of rod inner segment conductances on the temporal characteristics of the photovoltage.
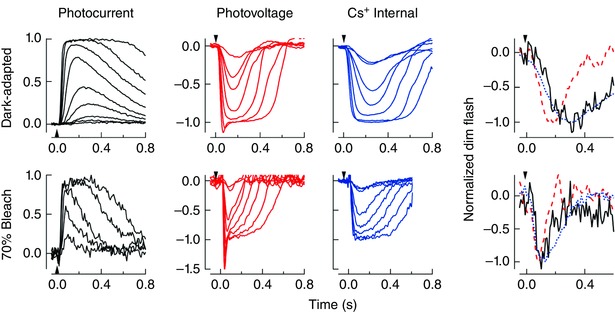
Normalized flash families are shown for the photocurrent (solid black), photovoltage (dashed red), and the photovoltage measured with a Cs+ internal solution (dotted blue) in the dark‐adapted state (top row) and following 70% bleaching of the visual pigment (bottom row). Normalized dim‐flash responses for photocurrent (solid black) and photovoltage (dashed red) under normal conditions and with Cs+ (dotted blue) are superimposed for the dark‐adapted state (top right) and following 70% bleaching (bottom right). Note that the speeding of the photovoltage with respect to the photocurrent is abolished with the Cs+ internal solution, and that this temporal filtering is less prominent at 70% bleach compared to the dark‐adapted state. The time‐to‐peak of the dim flash responses for the photocurrent, photovoltage and the photovoltage measured with a Cs+ internal solution were 300 ± 30 ms (n = 7), 300 ± 26 ms (n = 8) and 180 ± 9 ms (n = 8) for the dark‐adapted state, and 110 ± 10 ms (n = 4), 120 ± 5 ms (n = 6) and 80 ± 6 ms (n = 5) following the 70% bleach, respectively. The difference between the photovoltage and the photovoltage measured with a Cs+ internal solution was significantly different in each case (P < 0.001, Student's t test). [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
Most of our visual experience occurs during the daytime when a substantial fraction of the visual pigment in our photoreceptor cells is bleached. The reduction in the sensitivity of rods (and consequently rod vision) under these conditions will ultimately depend on several factors. First, rods will experience a loss in sensitivity by virtue of the reduced visual pigment content, or the loss of quantum catch [see also Burkhardt (1994) for cones]. Second, bleached visual pigment will further desensitize rod phototransduction due to its residual catalytic activity, a process called ‘bleaching adaptation’ (Cornwall & Fain, 1994; Jones et al. 1996; Matthews et al. 1996; Nymark et al. 2012). Finally, in bright, steady light the activation of rhodopsin will even further desensitize components of phototransduction through traditional mechanisms of background adaptation. Reduced sensitivity produced by the sum of all three factors will define the upper limits of light intensity where rods continue to contribute to visual processing. While it is appreciated that cones dominate our daytime vision, little is known about how rod sensitivity is regulated near its upper limit, and how this might influence visual processing. Here we have characterized, in the retinas of mice lacking cone photoresponses, the effects of bleaching adaptation on the sensitivity of rod photoreceptors. Two main observations arise from this work: (1) rods retain light sensitivity under conditions where a vast majority of the visual pigment has been bleached, and (2) the photovoltage, which ultimately controls synaptic transmission, is not subject to the same extent of response compression and desensitization as observed in recordings of the outer segment photocurrent.
Contribution of bleaching adaptation to rod signalling in bright light
The control of sensitivity by bleached visual pigment, and the effects of retinoid byproducts of phototransduction, have been studied extensively in isolated amphibian rod photoreceptors (Cornwall & Fain, 1994; Matthews et al. 1996; Ala‐Laurila et al. 2006; Kolesnikov et al. 2007; Miyagishima et al. 2009), but relatively less is known in mammals about how bleaching adaptation influences the sensitivity of rods, or signalling downstream within the retinal network. As the light levels increase the concentration of the visual pigment will gradually decrease, and will reach new steady levels as the rate of pigment degradation by light matches the rate of pigment regeneration. Under these conditions the sensitivity of rod photoresponses will reduce as a combination of the extent of bleaching adaptation (cf. Fig. 1), and adaptation owing to the catalytic activity of light‐activated Rh. Accordingly, in darkness after steady‐state has been reached following bleaching of 70–90% of the visual pigment, robust rod‐driven photo‐responses were observed from measurements of both photocurrent and photovoltage (Figs 2 and 4). This suggests that bleaching adaptation alone is insufficient to hold the outer segment current in saturation, even for the brightest bleaching exposures. Under these circumstances the role of light adaptation in further desensitization remains unknown.
The light levels where rods can no longer provide visual sensitivity would presumably be where the rate of pigment regeneration can no longer match pigment depletion. A determination of these upper limits will require quantification of the size of the available pool of 11‐cis retinal and time course of pigment regeneration in vivo. Anecdotal and experimental evidence suggests these light levels may be quite bright, perhaps exceeding 100 000 Rh* per rod−1 s−1 (Yin et al. 2006).
Inner segment conductances account for the sensitization of the photovoltage
The desensitization of the rod photocurrent is due to the combination of the desensitization of the phototransduction cascade and response compression due to closure of cGMP‐gated channels. However, what drives signalling of rods to the inner retina is synaptic release of the neurotransmitter, glutamate, from rod spherules. This, in turn, is driven by membrane potential, which derives from changes in the outer segment photocurrent and is filtered by voltage‐gated conductances (I h, I Kx) arising principally in the inner segment and ellipsoid of the cell (Baylor & Nunn, 1986; Beech & Barnes, 1989; Barrow & Wu, 2009). Here we tested the hypothesis that voltage‐gated conductances modify the membrane voltage response to increase the dynamic range of rod signalling. Inner segment cation currents may act to counteract hyperpolarization of the rod membrane potential during light‐evoked responses, perhaps to relieve the effects of bleaching adaptation in the photovoltage. Indeed, we found that during the intracellular dialysis of a Cs+ internal solution, to block cation channels, the extent of response compression decreased (Fig. 4 C). Also, during Cs+ dialysis the best fit value for the Weber–Fechner curve fit was similar (k = 20) to the value obtained from suction electrode recordings of the outer segment photocurrent (k = 24) (Fig. 4 B). The similarity of these values indicates that these rod voltage‐sensitive conductances are largely responsible for the sensitization of the photovoltage with respect to the photocurrent. The increased sensitivity of the photovoltage thus appears to allow the rod synapse to increase its dynamic range under conditions where the photocurrent remains compressed.
Additional information
Conflict of interest
None of the authors have a financial interest in these studies.
Contributions
JP designed and performed patch clamp experiments, analysed data and wrote the manuscript. RF designed and performed suction electrode experiments, analysed data and wrote the manuscript. GEP and KJM performed ERG experiments. APS and MCC conceived the study, helped design experiments and wrote the manuscript.
Funding
This work was supported by NIH Grant EY01157 (MCC), EY17606 (APS), the McKnight Endowment Fund for Neurosciences (APS), an NSF Graduate Research Fellowship (KJM) and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, UCLA.
Acknowledgements
We thank Bo Chang from the Jackson Laboratory for providing Gnat2cpfl3/cpfl3 mice, and Gordon Fain, Michael Woodruff, and Steve Barnes for comments on an earlier draft of the manuscript.
Linked articles This article is highlighted by a Perspective by Do. To read this Perspective, visit https://doi.org/10.1113/JP274146.
Contributor Information
Alapakkam P. Sampath, Email: asampath@jsei.ucla.edu.
M. Carter Cornwall, Email: cornwall@bu.edu.
References
- Ala‐Laurila P, Kolesnikov AV, Crouch RK, Tsina E, Shukolyukov SA, Govardovskii VI, Koutalos Y, Wiggert B, Estevez ME & Cornwall MC (2006). Visual cycle: dependence of retinol production and removal on photoproduct decay and cell morphology. J Gen Physiol 128, 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arman AC & Sampath AP (2012). Dark‐adapted response threshold of OFF ganglion cells is not set by OFF bipolar cells in the mouse retina. J Neurophysiol 107, 2649–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky VY & Burns ME (2012). Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem 287, 1620–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky VY, Lamb TD & Pugh EN (2002). G proteins and phototransduction. Annu Rev Physiol 64, 153–187. [DOI] [PubMed] [Google Scholar]
- Barnes S (1994). After transduction: response shaping and control of transmission by ion channels of the photoreceptor inner segments. Neuroscience 58, 447–459. [DOI] [PubMed] [Google Scholar]
- Barnes S & Hille B (1989). Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol 94, 719–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow AJ & Wu SM (2009). Complementary conductance changes by I Kx and I h contribute to membrane impedance stability during the rod light response. Channels (Austin) 3, 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA & Nunn BJ (1986). Electrical properties of the light‐sensitive conductance of rods of the salamander Ambystoma tigrinum . J Physiol 371, 115–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ & Barnes S (1989). Characterization of a voltage‐gated K+ channel that accelerates the rod response to dim light. Neuron 3, 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J, Frederiksen R, Yao Y, Nymark S, Chen J & Cornwall C (2016). Effect of rhodopsin phosphorylation on dark adaptation in mouse rods. J Neurosci 36, 6973–6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore CB & Rushton WA (1965). Dark adaptation and increment threshold in a rod monochromat. J Physiol 181, 612–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt DA (1994). Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J Neurosci 14, 1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Dacey MS, Hawes NL, Hitchcock PF, Milam AH, Atmaca‐Sonmez P, Nusinowitz S & Heckenlively JR (2006). Cone photoreceptor function loss‐3, a novel mouse model of achromatopsia due to a mutation in Gnat2. Invest Ophthalmol Vis Sci 47, 5017–5021. [DOI] [PubMed] [Google Scholar]
- Cornwall MC & Fain GL (1994). Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol 480, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall MC, MacNichol EF & Fein A (1984). Absorptance and spectral sensitivity measurements of rod photoreceptors of the tiger salamander, Ambystoma tigrinum . Vision Res 24, 1651–1659. [DOI] [PubMed] [Google Scholar]
- Cornwall MC, Ripps H, Chappell RL & Jones GJ (1989). Membrane current responses of skate photoreceptors. J Gen Physiol 94, 633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Santina L, Piano I, Cangiano L, Caputo A, Ludwig A, Cervetto L & Gargini C (2012). Processing of retinal signals in normal and HCN deficient mice. PLoS One 7, e29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Granda AM & Maxwell JM (1977). Voltage signal of photoreceptors at visual threshold. Nature 265, 181–183. [DOI] [PubMed] [Google Scholar]
- Fan J, Woodruff ML, Cilluffo MC, Crouch RK & Fain GL (2005). Opsin activation of transduction in the rods of dark‐reared Rpe65 knockout mice. J Physiol 568, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen R, Boyer NP, Nickle B, Chakrabarti KS, Koutalos Y, Crouch RK, Oprian D & Cornwall MC (2012). Low aqueous solubility of 11‐cis‐retinal limits the rate of pigment formation and dark adaptation in salamander rods. J Gen Physiol 139, 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Cornwall MC & Fain GL (1996). Equivalence of background and bleaching desensitization in isolated rod photoreceptors of the larval tiger salamander. J Gen Physiol 108, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Fein A, MacNichol EF & Cornwall MC (1993). Visual pigment bleaching in isolated salamander retinal cones. Microspectrophotometry and light adaptation. J Gen Physiol 102, 483–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov AV, Ala‐Laurila P, Shukolyukov SA, Crouch RK, Wiggert B, Estevez ME, Govardovskii VI & Cornwall MC (2007). Visual cycle and its metabolic support in gecko photoreceptors. Vision Res 47, 363–374. [DOI] [PubMed] [Google Scholar]
- Leibovic KN, Dowling JE & Kim YY (1987). Background and bleaching equivalence in steady‐state adaptation of vertebrate rods. J Neurosci 7, 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder A, Pahlberg J, Boyd KK, Kerov V, Kolandaivelu S, Ramamurthy V, Sampath AP & Artemyev NO (2013). Transducin translocation contributes to rod survival and enhances synaptic transmission from rods to rod bipolar cells. Proc Natl Acad Sci USA 110, 12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder A, Pahlberg J, Muradov H, Boyd KK, Sampath AP & Artemyev NO (2015). Exchange of cone for rod phosphodiesterase 6 catalytic subunits in rod photoreceptors mimics in part features of light adaptation. J Neurosci 35, 9225–9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR, Fain GL & Cornwall MC (1996). Role of cytoplasmic calcium concentration in the bleaching adaptation of salamander cone photoreceptors. J Physiol 490, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia TJ, Cowan CW, Angleson JK & Wensel TG (1997). A comparison of the efficiency of G protein activation by ligand‐free and light‐activated forms of rhodopsin. Biophys J 73, 3182–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima KJ, Cornwall MC & Sampath AP (2009). Metabolic constraints on the recovery of sensitivity after visual pigment bleaching in retinal rods. J Gen Physiol 134, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naarendorp F, Esdaille TM, Banden SM, Andrews‐Labenski J, Gross OP & Pugh EN (2010). Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. J Neurosci 30, 12495–12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov S, Lamb TD & Pugh EN (2000). The role of steady phosphodiesterase activity in the kinetics and sensitivity of the light‐adapted salamander rod photoresponse. J Gen Physiol 116, 795–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymark S, Frederiksen R, Woodruff ML, Cornwall MC & Fain GL (2012). Bleaching of mouse rods: microspectrophotometry and suction‐electrode recording. J Physiol 590, 2353–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymark S, Haldin C, Tenhu H & Koskelainen A (2006). A new method for measuring free drug concentration: retinal tissue as a biosensor. Invest Ophthalmol Vis Sci 47, 2583–2588. [DOI] [PubMed] [Google Scholar]
- Okawa H, Miyagishima KJ, Arman AC, Hurley JB, Field GD & Sampath AP (2010). Optimal processing of photoreceptor signals is required to maximize behavioural sensitivity. J Physiol 588, 1947–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa H, Sampath AP, Laughlin SB & Fain GL (2008). ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol 18, 1917–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen WG & Torre V (1983). High‐pass filtering of small signals by retinal rods. Ionic studies. Biophys J 41, 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinberg F & Koskelainen A (2010). Calcium sets the physiological value of the dominant time constant of saturated mouse rod photoresponse recovery. PLoS One 5, e13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Estevez ME, Cornwall MC & Kefalov VJ (2009). Intra‐retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci 12, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL & Lem J (2003). Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet 35, 158–164. [DOI] [PubMed] [Google Scholar]
- Yin L, Smith RG, Sterling P & Brainard DH (2006). Chromatic properties of horizontal and ganglion cell responses follow a dual gradient in cone opsin expression. J Neurosci 26, 12351–12361. [DOI] [PMC free article] [PubMed] [Google Scholar]


