Abstract
Key points
During lactation, prolactin promotes milk synthesis and oxytocin stimulates milk ejection.
In virgin rats, prolactin inhibits the activity of oxytocin‐secreting neurones.
We found that prolactin inhibition of oxytocin neurone activity is lost in lactation, and that some oxytocin neurones were excited by prolactin in lactating rats.
The change in prolactin regulation of oxytocin neurone activity was not associated with a change in activation of intracellular signalling pathways known to couple to prolactin receptors.
The change in prolactin regulation of oxytocin neurone activity in lactation might allow coordinated activation of both populations of neurones when required for successful lactation.
Abstract
Secretion of prolactin for milk synthesis and oxytocin for milk secretion is required for successful lactation. In virgin rats, prolactin inhibits oxytocin neurones but this effect would be counterproductive during lactation when secretion of both hormones is required for synthesis and delivery of milk to the newborn. Hence, we determined the effects of intracerebroventricular (i.c.v.) prolactin on oxytocin neurones in urethane‐anaesthetised virgin, pregnant and lactating rats. Prolactin (2 μg) consistently inhibited oxytocin neurones in virgin and pregnant rats (by 1.9 ± 0.4 and 1.8 ± 0.5 spikes s−1, respectively), but not in lactating rats; indeed, prolactin excited six of 27 oxytocin neurones by >1 spike s−1 in lactating rats but excited none in virgin or pregnant rats (χ2 2 = 7.2, P = 0.03). Vasopressin neurones were unaffected by prolactin (2 μg) in virgin rats but were inhibited by 1.1 ± 0.2 spikes s−1 in lactating rats. Immunohistochemistry showed that i.c.v. prolactin increased oxytocin expression in virgin and lactating rats and increased signal transducer and activator of transcription 5 phosphorylation to a similar extent in oxytocin neurones of virgin and lactating rats. Western blotting showed that i.c.v. prolactin did not affect phosphorylation of extracellular regulated kinase 1 or 2, or of Akt in the supraoptic or paraventricular nuclei of virgin or lactating rats. Hence, prolactin inhibition of oxytocin neurones is lost in lactation, which might allow concurrent elevation of prolactin secretion from the pituitary gland and activation of oxytocin neurones for synthesis and delivery of milk to the newborn.
Keywords: erk, lactation, oxytocin, pregnancy, stat5, vasopressin
Key points
During lactation, prolactin promotes milk synthesis and oxytocin stimulates milk ejection.
In virgin rats, prolactin inhibits the activity of oxytocin‐secreting neurones.
We found that prolactin inhibition of oxytocin neurone activity is lost in lactation, and that some oxytocin neurones were excited by prolactin in lactating rats.
The change in prolactin regulation of oxytocin neurone activity was not associated with a change in activation of intracellular signalling pathways known to couple to prolactin receptors.
The change in prolactin regulation of oxytocin neurone activity in lactation might allow coordinated activation of both populations of neurones when required for successful lactation.
Abbreviations
- CCK8S
(Tyr[SO3H]27)cholecystokinin fragment 26–33 amide
- DAB
3,3′‐diaminobenzidine
- pAkt
phosphorylated Akt
- pERK
phosphorylated extracellular regulated kinase
- PFA
paraformaldehyde
- pSTAT5
phosphorylated signal transducer and activator of transcription 5
- TH
tyrosine hydroxylase‐expressing
- TRPV1
transient receptor potential vanilloid 1
Introduction
The anterior pituitary hormone, prolactin, has over 300 ascribed functions, including regulation of reproductive function, the immune system, osmotic balance and angiogenesis (Freeman et al. 2000). In virgin rats, prolactin secretion is pulsatile and the posterior pituitary gland hormone, oxytocin, has been proposed to contribute to this pulsatile patterning as part of a negative feedback loop (Bertram et al. 2010). Oxytocin stimulates prolactin secretion in virgin rats in vivo (Egli et al. 2006), as well as from cultured anterior pituitary lactotrophs in vitro (Egli et al. 2004). In turn, prolactin enters the brain through a carrier‐mediated transport system (Walsh et al. 1987; Brown et al. 2016) and acutely inhibits the activity of oxytocin neurones (Kokay et al. 2006) by induction of a sustained hyperpolarisation (Sirzen‐Zelenskaya et al. 2011) to reduce the oxytocin‐induced secretion of prolactin.
However, during lactation the relationship between prolactin and oxytocin must change to allow these hormones to be secreted together as part of an integrated response that is required for survival of the young (Crowley, 2015). Prolactin promotes milk synthesis in the mammary gland (Grattan & Kokay, 2008) and oxytocin triggers milk delivery to the young (Brown, 2016). The co‐ordinated secretion of prolactin and oxytocin is stimulated by suckling of the young at the nipple (Higuchi et al. 1983) but there is also interdependent regulation of prolactin and oxytocin secretion; oxytocin no longer affects prolactin levels in lactating rats (Higuchi et al. 1983) but, more strikingly, prolactin seems to maintain oxytocin concentrations (Parker et al. 1991). Prolactin‐induced maintenance of oxytocin concentration is not a pharmacological phenomenon because removal of pups from lactating rats dramatically decreases plasma oxytocin concentration but this decrease is prevented by administration of prolactin (Ghosh & Sladek, 1995b). Pup removal also decreases hypothalamic oxytocin mRNA content and this too is prevented by prolactin administration (Ghosh & Sladek, 1995b). Hence, during lactation, suckling‐induced activation of oxytocin synthesis and secretion is reinforced by prolactin actions.
Because prolactin regulation of oxytocin secretion appears to depend on reproductive status, we hypothesised that the prolactin‐induced inhibition of oxytocin neurone activity evident in virgin rats would be lost in lactation. To test this hypothesis, we made extracellular single‐unit recordings from oxytocin neurones in anaesthetised rats. We found that while oxytocin neurones were consistently inhibited by intracerebroventricular (i.c.v.) administration of prolactin in virgin and pregnant rats, prolactin did not inhibit all oxytocin neurones in lactating rats. Indeed, some oxytocin neurones were clearly excited by prolactin in lactating rats. The change in prolactin‐induced regulation of oxytocin neurone activity was not associated with a change in prolactin‐induced intracellular signalling in these neurones.
Methods
Animals
Animals were purchased from the University of Otago Animal Facility. All animals were group‐housed, except lactating dams which were individually housed from mid‐pregnancy onwards, under controlled conditions (12 h light/12 h dark cycle with lights on at 07.00 h; 22 ± 1°C) and had free access to food and water. All experimental procedures were approved by the University of Otago Animal Ethics Committee and were carried out in accordance with the New Zealand Animal Welfare Act (1999) and associated guidelines. For pregnant and lactating rats, the oestrous cycle was monitored daily using vaginal cytology to examine the appearance of the epithelial cells. On pro‐oestrus, females were placed overnight in a cage with a male and on the following morning the presence of sperm indicated that mating had occurred (day 0 of gestation). Lactating rats gave birth on days 21–22 of gestation (day 1 of lactation) and pup numbers were adjusted to 10 per dam on day 2 post‐partum.
In vivo electrophysiology
On the day of electrophysiology, the rats were anaesthetised by intraperitoneal injection of 1.25 g kg−1 urethane (ethyl carbamate; Sigma, St Louis, MO, USA). For lactating rats, pups were removed prior to induction of anaesthesia, at least 4 h before experiments. Upon cessation of the flexor withdrawal reflex, a catheter was inserted into the right femoral vein for injection of (Tyr[SO3H]27)cholecystokinin fragment 26–33 amide (CCK8S; Sigma). A 22 gauge guide cannula (Plastics One, Roanoke, VA, USA) was placed in the right lateral cerebral ventricle (1.3 mm lateral and 0 mm caudal to bregma, and 3 mm ventral to the surface of the skull) and bonded to dental cement fixed to the surface of the skull by 1 mm screws for i.c.v. infusion of ovine prolactin. The pituitary stalk and the right supraoptic nucleus were exposed by transpharyngeal surgery (Brown et al. 2014). Extracellular single‐unit recordings were made via a glass recording microelectrode (15–40 MΩ) filled with 0.9% saline, using the Neurolog system and conventional electrophysiological recording techniques. A side‐by‐side SNEX‐200 stimulating electrode (Science Products GmbH, Hofheim am Taunus, Germany) was placed on the pituitary stalk to elicit antidromic action potentials in supraoptic nucleus neurones.
Neuronal activity was recorded via a CED 1401 analog–digital interface (Cambridge Electronic Design, Cambridge, UK) using Spike 2 software (Cambridge Electronic Design) and analysed offline. Neurones that fired less than one spontaneous action potential every 10 s were categorised as silent and were not recorded. Phasic activity was characterised using the ‘bursts’ script in Spike 2, with a burst being defined as activity lasting a minimum of 5 s with a minimum of 20 spikes within the burst and at least a 5 s interval between bursts, during which there was less than one spike every 5 s; phasic neurones were those for which these parameters partitioned more than 95% of spikes into bursts (Scott et al. 2009). Non‐phasic neurones were characterised as oxytocin neurones on the basis of a transient excitation of greater than 0.5 spikes s−1 averaged over 5 min following i.v. CCK8S injection (20 μg kg−1, 0.5 ml kg−1 in 0.9% saline) (Brown et al. 1996), or as vasopressin neurones by transient inhibition, or no effect, following CCK8S injection (Scott et al. 2009). During each recording, rats were given an i.c.v. injection of either 1 or 2 μg prolactin, or both 1 and 2 μg prolactin (at 1 μg μl−1 in 0.9% saline, separated by at least 10 min). The i.c.v. injections were made over 30–60 s through a 28 gauge cannula that protruded 2 mm beyond the tip of the guide cannula. At the end of the experiments the rats were killed by anaesthetic overdose.
For one set of electrophysiology experiments (see Fig. 5), virgin and lactating rats were injected with the dopamine D2‐receptor agonist, bromocriptine (500 μg, s.c.), immediately after the induction of anaesthesia, at least 4 h before recording started, to eliminate endogenous circulating prolactin by inhibition of prolactin secretion from the anterior pituitary gland.
Figure 5. Prolactin effects on the activity of oxytocin neurones in virgin and lactating rats after bromocriptine treatment.
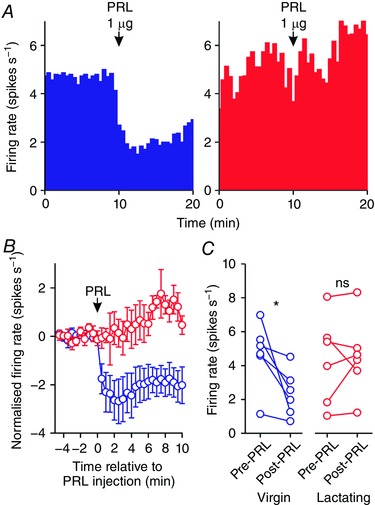
A, example ratemeter recordings of oxytocin neurone firing rate (in 30 s bins) from urethane‐anaesthetised bromocriptine‐treated virgin (left) and lactating (right) female Sprague–Dawley rats, showing that i.c.v. administration of 1 μg prolactin (PRL) reduces firing rate in the neurone from the virgin rat but not in the neurone from the lactating rat. B, mean (±SEM) oxytocin neurone firing rate (in 30 s bins) before and after i.c.v. administration of 1 μg prolactin in virgin rats (blue symbols) and lactating rats (red symbols). C, mean oxytocin neurone firing rate averaged over 5 min before and after i.c.v. administration of 1 μg prolactin in virgin rats (left panel; n = 6) and lactating rats (right panel; n = 6); * P < 0.05 (t 5 = 2.89) and ns P > 0.05 (t 5 = −0.37) vs. pre‐prolactin, paired t test. [Color figure can be viewed at wileyonlinelibrary.com]
Responses to prolactin were categorised as excitatory when they exceeded a 1 spike s−1 increase in firing rate, averaged over 5 min before and after prolactin administration; this cut‐off was selected because it is double the value that we routinely used to categorise oxytocin neurone responses to CCK8S as excitatory (Brown et al. 1996, 1997; Scott & Brown, 2011).
Intracerebroventricular cannulation for immunohistochemistry and Western blotting experiments
Rats for immunohistochemistry and Western blotting were implanted with a 22 gauge guide cannula in the right lateral cerebral ventricle (1.3 mm lateral and 0 mm caudal to bregma, and 3 mm ventral to the surface of the skull) under halothane or isoflurane anaesthesia, as previously described (Augustine & Grattan, 2008). The cannula was bonded to dental cement fixed to the surface of the skull by 1 mm screws. Surgery was performed on pro‐oestrous rats or rats on day 1–4 post‐partum. After surgery, rats were housed individually and food intake and bodyweight were monitored daily.
Dual‐label immunohistochemistry for oxytocin and phosphorylated signal transducer and activator of transcription 5 (pSTAT5)
Rats for immunohistochemistry were given three injections of bromocriptine (500 μg, s.c.) at 09.00 h and 17.00 h on the day before the experiment, and at 09.00 h on the day of experimentation and injected with vehicle or 500 ng prolactin i.c.v. in 0.9% saline, a treatment regime that we have previously shown to eliminate endogenous pSTAT5 expression in vehicle‐treated rats and to induce sub‐maximal pSTAT5 expression in prolactin‐treated rats (Sapsford et al. 2012).
At least 4 h after the final bromocriptine injection on the morning of dioestrus or day 7 post‐partum, rats were injected i.c.v. with 500 ng prolactin (2 μl of 250 ng ml−1 dissolved in saline) or 2 μl vehicle, deeply anaesthetised with pentobarbital (300 mg kg−1, i.p.) and perfused with 50 ml saline followed by 200 ml 4% paraformaldehyde (PFA) in 0.1 m PB 60 min later. Brains were immediately removed, post‐fixed overnight in 4% PFA, transferred to 30% sucrose solution in 0.1 m PB and stored at 4°C until they were infiltrated with sucrose. Brains were then frozen on dry ice and stored at −80°C until sectioning. For lactating rats, pups were removed immediately prior to induction of anaesthesia.
Coronal brain sections of 30 μm were cut through the supraoptic nucleus and paraventricular nucleus on a sliding microtome and the tissue stored in cryoprotectant. Within netwells, sections were washed in Tris‐buffered saline (TBS) and then subjected to an antigen retrieval step in Tris‐HCl (pH 10) at 90°C for 5 min. After cooling for 5 min, sections were blocked in incubation solution (TBS‐Triton, 2% normal goat serum, 0.25% BSA) for 1 h. After further washes, sections were incubated in 3% hydrogen peroxide in 40% methanol and TBS for 10 min. Sections were incubated in polyclonal rabbit anti‐pSTAT5 antibody (diluted 1:1300; Cell Signaling Technologies, Danvers, MA, USA) followed by goat anti‐rabbit IgG (1:333; Vector Laboratories, Burlingame, CA, USA) and reacted with nickel‐enhanced 3,3′‐diaminobenzidine (DAB) to produce blue/black staining in the nucleus representative of endogenous pSTAT5. Sections were then subjected to another hydrogen peroxide step followed by incubation in mouse monoclonal anti‐oxytocin antibody (1:25 000; EMD Millipore, Billerica, MA, USA) and finally by goat anti‐mouse horseradish peroxidase and reacted with DAB to produce brown cytoplasmic staining in oxytocin neurones. Sections were mounted onto gelatin‐coated slides and coverslipped with DPX mountant after going through an alcohol and xylene series. Sections were photographed under a light microscope and the numbers of pSTAT5‐positive, oxytocin‐positive and dual‐labelled cells were counted from 4–12 sections through the supraoptic and paraventricular nuclei to generate a mean number of positive cells per section in each nucleus for each rat. Negative controls were run with omission of primary antibodies and showed no non‐specific staining for pSTAT5 or oxytocin.
Western blotting for phosphorylated extracellular regulated kinase (pERK) and phosphorylated Akt (pAkt)
On day 6 post‐partum, or metoestrus for the virgin group, rats were injected with bromocriptine (500 μg in 250 μl) at 09.00 and 17.00 h. The following day (dioestrus), rats were given another dose of bromocriptine (500 μg in 250 μl) at 09.00 h, and 3–4 h later, rats were injected with i.c.v. prolactin (2 μg in 2 μl 0.9% saline) to match the higher dose used for electrophysiology, or vehicle (2 μl). Injections were carried out in conscious animals that had previously been habituated to this procedure. Rats were decapitated 7 min after i.c.v. injection and their brains were rapidly removed, frozen and stored at −80°C until further processing. For lactating rats, pups were removed immediately prior to decapitation.
Coronal brains sections (300 μm) were sliced on a cryostat and the supraoptic and paraventricular nuclei were microdissected using a micropunch technique and processed for Western blot analysis as previously described (Ladyman & Grattan, 2004). Protein concentration was determined by a modification of the Lowry method using a protein assay kit (Bio‐Rad Laboratories Inc., Hercules, CA, USA).
Samples, containing 10 μg of protein, were boiled for 4 min after the addition of loading buffer containing 2‐β mercaptoethanol, and were then separated on a 7.5% SDS‐PAGE gel and electrotransferred to nitrocellulose membrane. Membranes were incubated in LI‐COR blocking buffer (LI‐COR Biosciences, Lincoln, NE, USA) diluted 1:1 in TBS for 1 h at room temperature, then incubated overnight at 4°C with primary antibody in TBS/0.1% Tween 20/0.1% BSA. Membranes were then incubated with anti‐mouse and anti‐rabbit IgG‐IRDye‐conjugated secondary antibodies (1:10 000) in TBS (926‐32210 and 926‐32221, Millennium Science, Mulgrave, VIC, USA) for 1 h at room temperature. Fluorescence was visualised using the LI‐COR Odyssey Infrared Fluorescence Imaging system and images were quantified using the LI‐COR Odyssey software. For each sample, phosphoprotein values were normalised to the corresponding non‐phosphoprotein value. The vehicle‐treated virgin group was normalised to 1 and all data are expressed relative to this group. β‐Tubulin values were compared to ensure equivalent protein loading between samples and there was no difference in β‐tubulin expression across groups in the supraoptic nucleus (two‐way ANOVA: main effect of reproductive status: F 1,26 = 0.003, P = 0.96; main effect of prolactin: F 1,26 = 0.05, P = 0.82; interaction: F 1,26 = 0.72, P = 0.41) or paraventricular nucleus (two‐way ANOVA: main effect of reproductive status: F 1,24 = 0.64, P = 0.43; main effect of prolactin: F 1,24 = 0.36, P = 0.55; interaction: F 1,24 = 0.01, P = 0.91).
Primary antibodies (all from Cell Signaling Technology) used were mouse anti‐ERK(1/2) (1:1000, no. 9107), rabbit anti‐phospho(Thr202/Tyr204)‐ERK(1/2) (1:1000, no. 4370), mouse anti‐Akt (1:1000, no. 2920), rabbit anti‐phospho(ser473)‐Akt (1:1000, no. 4060) and rabbit anti‐β tubulin (1:1000, no. 2146).
Data analysis and statistics
All values are reported as mean ± SEM. All statistical analyses were completed using Sigma Plot version 13 for Windows (Systat Software Inc., Chicago, IL, USA). For all statistical tests, P ≤ 0.05 was considered significant. Paired t tests were used for before‐and‐after comparisons of firing rate. One‐way (for one factor) or two‐way (for two factors) ANOVAs were used to compare multiple groups; where the F‐ratio was significant, ANOVA was followed by all‐pairwise Student–Newman–Keuls post hoc tests. Pearson Product Moment correlations were used for correlations and a chi‐square test was used for comparison of proportions.
Results
Basal firing rate of oxytocin neurones in virgin, pregnant and lactating rats
In vivo electrophysiological recordings were made from 51 oxytocin neurones in 47 urethane‐anaesthetised rats. There was no difference (F 2,48 = 0.02, P = 0.98, one‐way ANOVA) between the basal firing rate of oxytocin neurones in virgin rats (5.2 ± 0.4 spikes s−1, n = 12), pregnant rats (5.1 ± 0.6 spikes s−1, n = 12) and lactating rats (5.5 ± 0.5 spikes s−1, n = 27).
Prolactin inhibits oxytocin neurones in virgin rats
We tested the effects of i.c.v. administration of prolactin on the firing rate of 12 oxytocin neurones in 12 virgin rats; six neurones were tested with 1 and 2 μg prolactin and three neurones each were tested with either 1 or 2 μg prolactin. Consistent with our previous observations of central prolactin inhibition of oxytocin neurone activity (Kokay et al. 2006), i.c.v. administration of 1 or 2 μg prolactin inhibited oxytocin neurones in virgin rats by 0.5 ± 0.2 spikes s−1 (t 8 = 2.35, P < 0. 05, paired t test) and 1.9 ± 0.4 spikes s−1 (t 8 = 5.26, P < 0. 001), respectively (Fig. 1).
Figure 1. Prolactin inhibits oxytocin neurone activity.
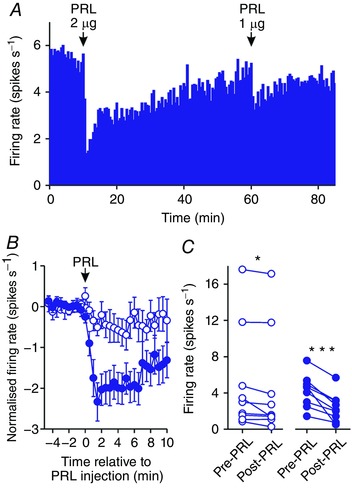
A, example ratemeter recording of oxytocin neurone firing rate (in 30 s bins) from a urethane‐anaesthetised virgin female Sprague–Dawley rat, showing clear dose‐dependent inhibition of firing rate following i.c.v. administration of 1 and 2 μg prolactin (PRL). B, mean (±SEM) oxytocin neurone firing rate (in 30 s bins) before and after i.c.v. administration of 1 μg (open symbols) and 2 μg (closed symbols) prolactin. Note that the maximal inhibition was evident over the first 5 min after prolactin administration. C, mean oxytocin neurone firing rate averaged over 5 min before and after i.c.v. administration of 1 μg (left panel; n = 9) and 2 μg (right panel; n = 9) prolactin; * P < 0.05 (t 8 = 2.35) and *** P < 0.001 (t 8 = 5.26) vs. pre‐prolactin, paired t test. [Color figure can be viewed at wileyonlinelibrary.com]
Prolactin effects on the activity of oxytocin neurones in lactating rats
To test our hypothesis that the inhibitory effects of prolactin on oxytocin neurones are lost during lactation, we tested the effects of i.c.v. administration of prolactin on the firing rate of 27 oxytocin neurones in 27 rats on days 7–17 post‐partum; six neurones were tested with 1 and 2 μg prolactin, nine neurones were tested with only 1 μg prolactin and 12 neurones were tested with only 2 μg prolactin. Overall, there was no significant effect of 1 μg (t 14 = −0.60, P = 0.56, paired t test) or 2 μg (t 17 = −1.44, P = 0.17, paired t test) i.c.v. prolactin on the mean firing rate of oxytocin neurones in lactating rats (Fig. 2). However, in contrast to the consistent inhibitory effects of i.c.v. prolactin on oxytocin neurones in virgin rats, the individual responses to i.c.v. prolactin were highly variable in lactating rats, with six of 27 neurones showing a clear excitation in response to i.c.v. prolactin (>1 spike s−1 increase in firing rate, averaged over 5 min before and after prolactin administration; e.g. Fig. 2 C). There was no correlation between the day post‐partum on which recordings were made and the change in firing rate in response to i.c.v. prolactin (Pearson Product Moment correlation coefficient, r 33 = 0.08, P = 0.65). Furthermore, there was no correlation between the pre‐prolactin firing rate and the change in firing rate in response to i.c.v. prolactin (Pearson Product Moment correlation coefficient, r 33 = −0.05, P = 0.79).
Figure 2. Prolactin effects on the activity of oxytocin neurones in lactating rats.
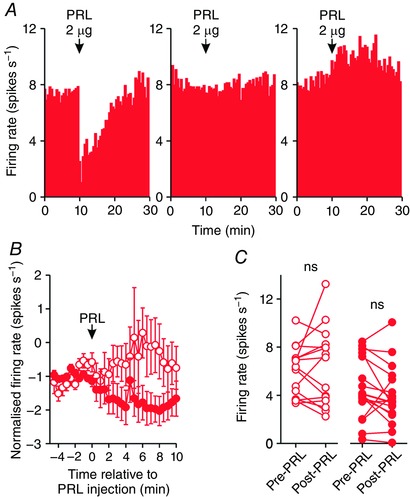
A, example ratemeter recordings of oxytocin neurone firing rate (in 30 s bins) from urethane‐anaesthetised lactating female Sprague–Dawley rats, showing inhibition of (left), no effect on (middle) and excitation of firing rate following i.c.v. administration of 2 μg prolactin (PRL). B, mean (±SEM) oxytocin neurone firing rate (in 30 s bins) before and after i.c.v. administration of 1 μg (open symbols) and 2 μg (closed symbols) prolactin in lactating rats. C, mean oxytocin neurone firing rate averaged over 5 min before and after i.c.v. administration of 1 μg (left panel; n = 15) and 2 μg (right panel; n = 18) prolactin in lactating rats; ns P > 0.05 (t 14 = −0.60 and t 17 = −1.44, for 1 and 2 μg, respectively) vs. pre‐prolactin, paired t test. [Color figure can be viewed at wileyonlinelibrary.com]
Prolactin inhibits oxytocin neurones in pregnant rats
To determine whether the loss of inhibitory prolactin effects in lactation developed over the course of gestation, we tested the effects of i.c.v. administration of 2 μg prolactin on the firing rate of 12 oxytocin neurones in eight rats on days 3–20 of gestation. Similarly to its effects in virgin rats, 2 μg i.c.v. prolactin consistently inhibited the firing rate of oxytocin neurones in pregnant rats, decreasing mean firing rate by 1.8 ± 0.5 spikes s−1 (t 11 = 3.68, P = 0.003; Fig. 3).
Figure 3. Prolactin inhibits oxytocin neurone activity in pregnant rats.
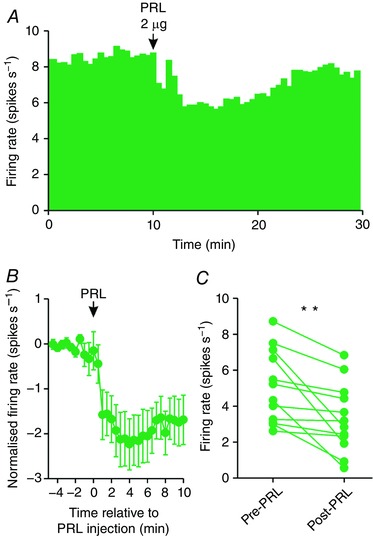
A, example ratemeter recording of oxytocin neurone firing rate (in 30 s bins) from a urethane‐anaesthetised pregnant female Sprague–Dawley rat, showing clear inhibition of firing rate following i.c.v. administration of 2 μg prolactin (PRL). B, Mean (±SEM) oxytocin neurone firing rate (in 30 s bins) before and after i.c.v. administration of 2 μg prolactin. C, mean oxytocin neurone firing rate averaged over 5 min before and after i.c.v. administration of 2 μg prolactin (n = 12); ** P < 0.01 (t 11 = 3.68) vs. pre‐prolactin, paired t test. [Color figure can be viewed at wileyonlinelibrary.com]
Prolactin effects on oxytocin neurones in virgin, pregnant and lactating rats
To determine whether the responses of oxytocin neurones to i.c.v. prolactin were different between virgin, pregnant and lactating rats, we compared the change in firing rate from 5 min before to 5 min after 2 μg i.c.v. prolactin between the three groups. Two‐way ANOVA revealed a main effect of reproductive status (F 2,59 = 4.32, P = 0.02) but no main effect of prolactin dose (F 1,59 = 1.65, P = 0.20) and no interaction between reproductive status and prolactin dose (F 2,59 = 0.53, P = 0.59); the response of oxytocin neurones to prolactin was less in lactating rats than in virgin and pregnant rats (P = 0.03 and P = 0.04, respectively, Student–Newman–Keuls post hoc tests), with no difference between the responses in virgin and pregnant rats (P = 0.38, Student–Newman–Keuls post hoc test; Fig. 4).
Figure 4. Prolactin effects on oxytocin neurone activity in virgin, pregnant and lactating rats.
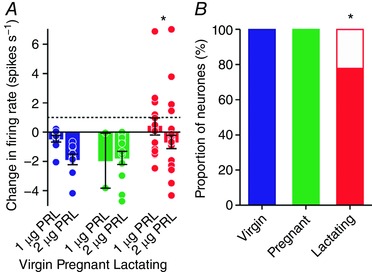
A, mean (±SEM) change in oxytocin neurone firing rate before and after i.c.v. administration of 1 or 2 μg prolactin. Circles represent individual data points from single injections of prolactin and the dashed line indicates the 1 spike s−1 increase set as the threshold for an excitatory response. There was a main effect of reproductive status (F 2,59 = 4.32, P = 0.02, two‐way ANOVA) but no main effect of prolactin dose (F 1,59 = 1.65, P = 0.20) and no interaction between reproductive status and prolactin dose (F 2,59 = 0.53, P = 0.59); * P < 0.05 vs. virgin and pregnant groups, Student–Newman–Keuls post hoc tests. B, stacked bar chart showing the proportion of oxytocin neurones excited (white) or not excited (dark) by i.c.v. administration of prolactin to virgin, pregnant and lactating rats; * P < 0.05, chi‐square test. [Color figure can be viewed at wileyonlinelibrary.com]
Categorising the responses of individual neurones to prolactin as excitatory (>1 spike s−1 increase in firing rate, averaged over 5 min before and after prolactin administration) or not excitatory (<1 spike s−1 increase) revealed that more oxytocin neurones were excited by prolactin in lactating rats (6 of 27) than in virgin rats (0 of 12) and pregnant rats (0 of 12; χ2 2 = 7.2, P = 0.03, chi‐square test, Fig. 4).
Prolactin inhibits oxytocin neurones in virgin rats but not in lactating rats after bromocriptine treatment
Because the prolactin‐induced inhibition of oxytocin neurones in pregnant rats was indistinguishable from that evident in virgin rats, pregnant rats were not included in further experiments to reduce the number of animals used. To determine whether the changes evident in the responses of oxytocin neurones to i.c.v. prolactin were dependent upon endogenous prolactin levels in virgin and lactating rats, we tested the effects of i.c.v. administration of 1 μg prolactin on the firing rate of six oxytocin neurones in five bromocriptine‐treated virgin rats and six oxytocin neurones from four bromocriptine‐treated rats on days 7–12 post‐partum. Consistent with its effects in non‐bromocriptine‐treated rats, 1 μg i.c.v. prolactin consistently inhibited the firing rate of oxytocin neurones in virgin rats (by 2.3 ± 0.8 spikes s−1; t 5 = 2.89, P = 0.03, paired t test) but not in lactating rats (+0.2 ± 0.6 spikes s−1 change; t 5 = −0.37, P = 0.73, paired t test; Fig. 5). One of six oxytocin neurones in lactating rats increased its firing rate by more than 1 spike s−1 after prolactin, whereas all neurones from virgin rats decreased their firing rate after prolactin.
Prolactin inhibits vasopressin neurones in lactating rats but not in virgin rats
To determine whether the inhibitory effects of prolactin were specific to oxytocin neurones, we tested the effects of 1 and 2 μg prolactin i.c.v. on the firing rates of vasopressin neurones in 21 virgin and lactating rats. In virgin rats, vasopressin neurone firing rate was not affected by i.c.v. prolactin administration (−0.4 ± 0.3 spikes s−1 change between the 5 min before and after 1 μg prolactin i.c.v.; n = 14; t 13 = 1.15, P = 0.27, paired t test; −0.4 ± 0.3 spikes s−1 change after 2 μg prolactin i.c.v.; n = 11; t 10 = 0.26, P = 0.80, paired t test). In lactating rats, vasopressin neurone firing rate was not affected by 1 μg prolactin i.c.v. (−1.0 ± 0.6 spikes s−1 change; n = 8; t 7 = 1.84, P = 0.11, paired t test) but was inhibited by 1.1 ± 0.2 spikes s−1 after 2 μg prolactin i.c.v. (n = 6; t 5 = 4.4, P = 0.006, paired t test; Fig. 6).
Figure 6. Prolactin effects on vasopressin neurone activity in virgin and lactating rats.
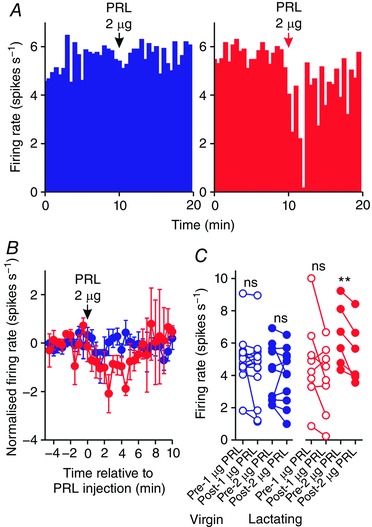
A, example ratemeter recordings of vasopressin neurone firing rate (in 30 s bins) from urethane‐anaesthetised virgin (left) and lactating (right) female Sprague–Dawley rats, showing that i.c.v. administration of 2 μg prolactin (PRL) reduces firing rate in the neurone from the lactating rat but not in the neurone from the virgin rat. B, mean (±SEM) vasopressin neurone firing rate (in 30 s bins) before and after i.c.v. administration of 2 μg prolactin in virgin rats (blue symbols) and lactating rats (red symbols); data from 1 μg prolactin injections have been omitted for clarity. C, mean vasopressin neurone firing rate averaged over 5 min before and after i.c.v. administration of 1 and 2 μg prolactin in virgin rats (left panel) and lactating rats (right panel); ns P > 0.05 (t 13 = 1.15 after 1 μg in virgin rats, t 10 = 0.26 after 2 μg in virgin rats and t 7 = 1.84 after 1 μg in lactating rats), ** P < 0.01 (t 5 = 4.40) vs. pre‐prolactin, paired t test. [Color figure can be viewed at wileyonlinelibrary.com]
Eight vasopressin neurones exhibited spontaneous phasic activity (four in virgin rats and four in lactating rats). In virgin rats, i.c.v. prolactin (1 or 2 μg) did not affect the mean firing rate (t 3 = 0.07, P = 0.95, paired t test), intra‐burst firing rate (t 3 = 1.07, P = 0.36, paired t test), burst duration (t 3 = −2.33, P = 0.19, paired t test) or inter‐burst interval (t 3 = −1.69, P = 0.41, paired t test) of phasic neurones. Similarly, in lactating rats, i.c.v. prolactin (1 or 2 μg) did not affect the mean firing rate (t 3 = 0.96, P = 0.12, paired t test), intra‐burst firing rate (t 3 = −1.00, P = 0.39, paired t test), burst duration (t 3 = 0.74, P = 0.51, paired t test) or inter‐burst interval (t 3 = −0.79, P = 0.48, paired t test) of phasic neurones.
Prolactin increases pSTAT5 expression in oxytocin neurones in virgin and lactating rats
To determine whether plasticity in the effects of prolactin on oxytocin neurones was associated with a change in canonical prolactin receptor intracellular signal transduction, we measured pSTAT5 expression in oxytocin neurones that were given three injections of bromocriptine over 28–4 h prior to the experiment. As expected, i.c.v. prolactin increased the expression of pSTAT5 in the supraoptic and paraventricular nuclei (Table 1). Remarkably, i.c.v. prolactin also caused a modest increase in the number of oxytocin‐positive neurones in the supraoptic nucleus, but not the paraventricular nucleus (Table 1). There was no pSTAT5 expression in supraoptic or paraventricular nuclei of vehicle‐treated virgin and lactating rats, but similar high pSTAT5 expression levels after i.c.v. prolactin in both virgin and lactating rats (Table 1). In the supraoptic nucleus, there were no pSTAT5‐expressing neurones in vehicle‐treated rats and most neurones with pSTAT5 after i.c.v. prolactin were oxytocin neurones, with only ∼20% being non‐oxytocin neurones (Fig. 7). In the paraventricular nucleus, there were no pSTAT5‐expressing neurones in vehicle‐treated rats and many neurones with pSTAT5 after i.c.v. prolactin were oxytocin neurones but almost half were non‐oxytocin neurones (Fig. 8).
Table 1.
Oxytocin and pSTAT5 expression in the supraoptic and paraventricular nuclei of rats given three s.c. injections of bromocriptine 28–4 h before i.c.v. vehicle/prolactin
| Supraoptic nucleus oxytocin | Supraoptic nucleus pSTAT5 | Paraventricular nucleus oxytocin | Paraventricular nucleus pSTAT5 | |
|---|---|---|---|---|
| Dioestrus + vehicle | 55 ± 2 (n = 5) | 0 ± 0 (n = 5) | 100 ± 4 (n = 5) | 0 ± 0 (n = 5) |
| Lactating + vehicle | 56 ± 2 (n = 5) | 0 ± 0 (n = 5) | 96 ± 7 (n = 4) | 0 ± 0 (n = 4) |
| Dioestrus + prolactin | 58 ± 2 (n = 7) | 67 ± 4 (n = 7) | 107 ± 8 (n = 7) | 154 ± 12 (n = 7) |
| Lactating + prolactin | 67 ± 4 (n = 6) | 65 ± 6 (n = 6) | 123 ± 9 (n = 6) | 143 ± 25 (n = 6) |
| Main effect of status | F 1,22 = 2.92 | F 1,22 = 0.06 | F 1,21 = 0.48 | F 1,21 = 0.11 |
| P = 0.10 | P = 0.81 | P = 0.50 | P = 0.75 | |
| Main effect of prolactin | F 1,22 = 4.81 | F 1,22 = 255.04 | F 1,21 = 3.76 | F 1,21 = 71.32 |
| P = 0.04 | P < 0.001 | P = 0.07 | P < 0.001 | |
| Interaction | F 1,22 = 1.50 | F 1,22 = 0.06 | F 1,21 = 1.3 | F 1,21 = 0.11 |
| P = 0.24 | P = 0.81 | P = 0.27 | P = 0.75 |
Data shown are the mean number (±SEM) of oxytocin‐positive neurones and pSTAT5‐positive neurones per section in the supraoptic and paraventricular nuclei after 500 ng i.c.v. prolactin after three 500 μg s.c. bromocriptine injections 28–4 h beforehand. Significant P‐values are in bold.
Figure 7. Prolactin activates pSTAT5 in the supraoptic nucleus of virgin and lactating rats.
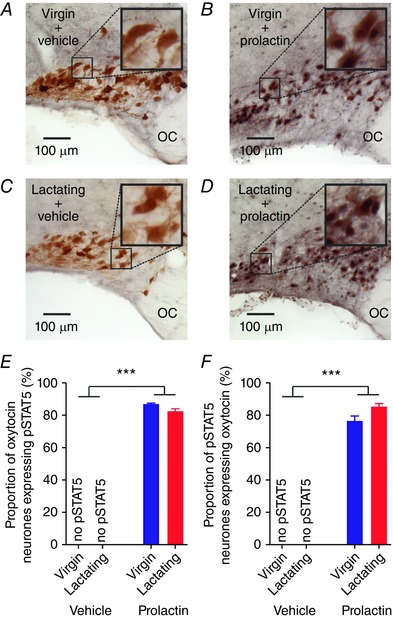
A–D, representative images of pSTAT5 (nuclear staining) and oxytocin immunostaining (cytoplasmic staining) in the supraoptic nucleus of virgin and lactating rats after three injections of bromocriptine over 28–4 h before the experiment. E, mean (±SEM) proportion of oxytocin neurones expressing pSTAT5 in the supraoptic nucleus of virgin and lactating rats given either i.c.v. vehicle (both n = 5) or 500 ng prolactin (n = 7 and 6, respectively) after three injections of bromocriptine over 28–4 h before the experiment. Two‐way ANOVA: main effect of reproductive status: F 1,22 = 3.12, P = 0.09; main effect of prolactin: F 1,22 = 4474.41, P < 0.001; interaction: F 1,22 = 3.12, P = 0.09. F, mean (±SEM) proportion of pSTAT5‐positive neurones expressing oxytocin in the supraoptic nucleus of virgin and lactating rats given either i.c.v. vehicle (both n = 5) or 500 ng prolactin (n = 7 and 6, respectively) after three injections of bromocriptine over 28–4 h before the experiment. Two‐way ANOVA: main effect of reproductive status: F 1,22 = 2.78, P = 0.11; main effect of prolactin: F 1,22 = 912.12, P < 0.001; interaction: F 1,22 = 2.78, P = 0.11. OC, optic chiasm. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 8. Prolactin activates pSTAT5 in the paraventricular nucleus of virgin and lactating rats.
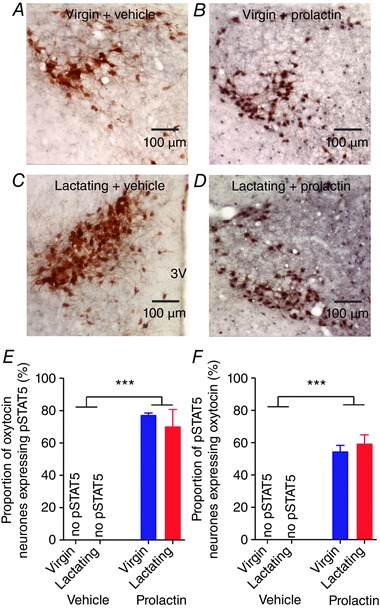
A–D, representative images of pSTAT5 (nuclear staining) and oxytocin immunostaining (cytoplasmic staining) in the paraventricular nucleus of virgin and lactating rats after three injections of bromocriptine over 28–4 h before the experiment. E, mean (±SEM) proportion of oxytocin neurones expressing pSTAT5 in the paraventricular nucleus of virgin and lactating rats given either i.c.v. vehicle (n = 5 and 4, respectively) or 500 ng prolactin (n = 7 and 6, respectively) after three injections of bromocriptine over 28–4 h before the experiment. Two‐way ANOVA: main effect of reproductive status: F 1,21 = 0.27, P = 0.61; main effect of prolactin: F 1,21 = 118.78, P < 0.001; interaction: F 1,21 = 0.27, P = 0.61. F, mean (±SEM) proportion of pSTAT5‐positive neurones expressing oxytocin in the paraventricular nucleus of virgin and lactating rats given either i.c.v. vehicle (n = 5 and 4, respectively) or 500 ng prolactin (n = 7 and 6, respectively) after three injections of bromocriptine over 28–4 h before the experiment. Two‐way ANOVA: main effect of reproductive status: F 1,21 = 2.28, P = 0.60; main effect of prolactin: F 1,21 = 155.90, P < 0.001; interaction: F 1,21 = 2.28, P = 0.60. 3V: 3rd ventricle. [Color figure can be viewed at wileyonlinelibrary.com]
Prolactin does not affect pERK1, pERK2 or pAkt expression in the supraoptic and paraventricular nuclei of virgin and lactating rats
To determine whether plasticity in the effects of 2 μg i.c.v. prolactin on oxytocin neurones was associated with a change in other intracellular signal transduction pathways known to be coupled to the prolactin receptor, we measured ERK1, pERK1, ERK2, pERK2, Akt and pAkt expression in the supraoptic and paraventricular nuclei using Western blotting (Fig. 9 A). There were no changes in the expression of any of the intracellular signalling molecules in the supraoptic nucleus (Table 2) or paraventricular nucleus (Table 3) and there was no change in the ratio of the phosphorylated proteins to non‐phosphorylated proteins in the supraoptic or paraventricular nuclei (Fig. 9 B–D).
Figure 9. Prolactin does not affect phosphorylation of ERK and Akt in virgin and lactating rats.
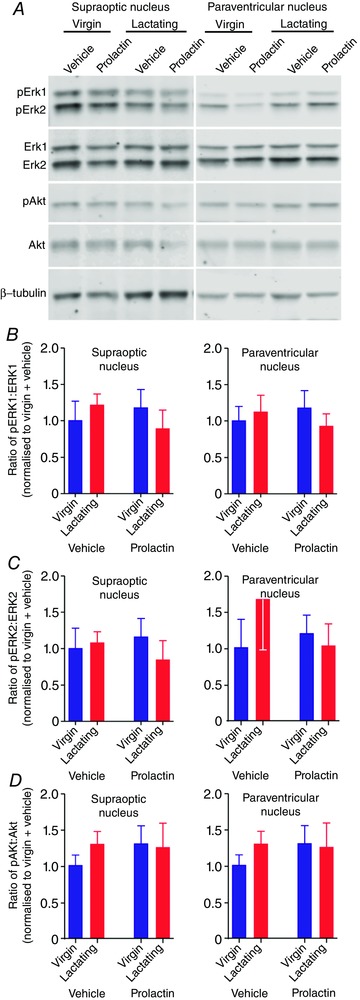
A and B, images showing representative immunoblots of ERK1, pERK1, ERK2, pERK2, Akt, pAkt and β‐tubulin in supraoptic nucleus (left) and paraventricular nucleus (right) tissue samples from virgin and lactating rats injected with 2 μg prolactin or vehicle. Bands were analysed using Image Studio Software (LI‐COR). Phosphoprotein levels were normalised to non‐phosphoprotein levels and expressed as a proportion of the levels in the vehicle‐treated virgin rats (n = 6–9 per group). B, mean ratio of pERK1:ERK1 (±SEM) in the supraoptic nucleus (left) and paraventricular nucleus (right) of virgin and lactating rats. For the pERK1:ERK1 ratio in the supraoptic nucleus, two‐way ANOVA showed no main effect of reproductive status (F 1,26 = 0.02, P = 0.89) or prolactin (F 1,26 = 1.00, P = 0.76) and no interaction (F 1,26 = 1.07, P = 0.31). Similarly, for the pERK1:ERK1 ratio in the paraventricular nucleus, two‐way ANOVA showed no main effect of reproductive status (F 1,24 = 0.53, P = 0.47) or prolactin (F 1,24 = 0.06, P = 0.80) and no interaction (F 1,24 = 0.68, P = 0.42). C, mean ratio of pERK2:ERK2 (±SEM) in the supraoptic nucleus (left) and paraventricular nucleus (right) of virgin and lactating rats. For the pERK2:ERK2 ratio in the supraoptic nucleus, two‐way ANOVA showed no main effect of reproductive status (F 1,26 = 0.22, P = 0.64) or prolactin (F 1,26 = 0.02, P = 0.88) and no interaction (F 1,26 = 0.62, P = 0.44). Similarly, for the pERK2:ERK2 ratio in the paraventricular nucleus, two‐way ANOVA showed no main effect of reproductive status (F 1,24 = 0.35, P = 0.56) or prolactin (F 1,24 = 0.28, P = 0.60) and no interaction (F 1,24 = 1.00, P = 0.33). D, mean ratio of pAkt:Akt (±SEM) in the supraoptic nucleus (left) and paraventricular nucleus (right) of virgin and lactating rats. For the pAkt:Akt ratio in the supraoptic nucleus, two‐way ANOVA showed no main effect of reproductive status (F 1,22 = 0.08, P = 0.78) or prolactin (F 1,22 = 0.07, P = 0.78) and no interaction (F 1,22 = 0.36, P = 0.56). Again similarly, for the pAkt:Akt ratio in the paraventricular nucleus, two‐way ANOVA showed no main effect of reproductive status (F 1,24 = 0.24, P = 0.63) or prolactin (F 1,24 = 0.27, P = 0.60) and no interaction (F 1,24 = 0.49, P = 0.49). [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
ERK1, pERK1, ERK2, pERK2, Akt, pAkt and β‐tubulin expression in the supraoptic nucleus
| ERK1 | pERK1 | ERK2 | pERK2 | Akt | pAkt | β‐Tubulin | |
|---|---|---|---|---|---|---|---|
| Dioestrus + vehicle | 33 582 ± 3886 | 67 504 ± 12 294 | 82 527 ± 11 135 | 1 36 186 ± 26 810 | 13 183 ± 1502 | 24 783 ± 2395 | 97 857 ± 14 998 |
| (n = 7) | (n = 7) | (n = 7) | (n = 7) | (n = 6) | (n = 6) | (n = 7) | |
| Lactating + vehicle | 28 492 ± 3886 | 82 094 ± 12 294 | 76 328 ± 11 135 | 1 58 925 ± 26 810 | 12 018 ± 1502 | 24 433 ± 2395 | 84 761 ± 14 998 |
| (n = 7) | (n = 7) | (n = 7) | (n = 7) | (n = 6) | (n = 6) | (n = 7) | |
| Dioestrus + prolactin | 29 140 ± 3635 | 73 092 ± 11 500 | 73 859 ± 10 416 | 1 40 454 ± 25 078 | 11 715 ± 1301 | 22 288 ± 2074 | 82 157 ± 14 029 |
| (n = 8) | (n = 8) | (n = 8) | (n = 8) | (n = 8) | (n = 8) | (n = 8) | |
| Lactating + prolactin | 29 477 ± 3635 | 60 502 ± 11 500 | 71 451 ± 10 416 | 1 08 821 ± 25 078 | 10 607 ± 1502 | 18 603 ± 2395 | 93 664 ± 14 029 |
| (n = 8) | (n = 8) | (n = 8) | (n = 8) | (n = 6) | (n = 6) | (n = 8) | |
| Main effect of status | F 1,26 = 0.39 | F 1,26 = 0.007 | F 1,26 = 0.16 | F 1,26 = 0.03 | F 1,22 = 0.61 | F 1,22 = 0.76 | F 1,26 < 0.01 |
| P = 0.53 | P = 0.93 | P = 0.69 | P = 0.87 | P = 0.44 | P = 0.39 | P = 0.96 | |
| Main effect of prolactin | F 1,26 = 0.21 | F 1,26 = 0.45 | F 1,26 = 0.40 | F 1,26 = 0.78 | F 1,22 = 0.98 | F 1,22 = 3.22 | F 1,26 = 0.05 |
| P = 0.65 | P = 0.51 | P = 0.54 | P = 0.38 | P = 0.33 | P = 0.09 | P = 0.82 | |
| Interaction | F 1,26 = 0.52 | F 1,26 = 1.30 | F 1,26 = 0.03 | F 1,26 = 1.10 | F 1,22 < 0.01 | F 1,22 = 0.52 | F 1,26 = 0.72 |
| P = 0.48 | P = 0.26 | P = 0.86 | P = 0.30 | P = 0.98 | P = 0.48 | P = 0.40 |
Data shown are densitometry measurements (arbitrary units ± SEM) from Western blots for ERK1, pERK1, ERK2, pERK2, Akt, pAkt and β‐tubulin expression in the supraoptic nucleus after 2 μg i.c.v. prolactin.
Table 3.
ERK1, pERK1, ERK2, pERK2, Akt, pAkt and β‐tubulin expression in the paraventricular nucleus
| ERK1 | pERK1 | ERK2 | pERK2 | Akt | pAkt | β‐Tubulin | |
|---|---|---|---|---|---|---|---|
| Dioestrus + vehicle | 13 940 ± 1971 | 55 228 ± 17 841 | 46 986 ± 5604 | 1 81 329 ± 48 184 | 21 143 ± 2917 | 61 857 ± 8419 | 1 83 186 ± 37 256 |
| (n = 7) | (n = 7) | (n = 7) | (n = 7) | (n = 7) | (n = 7) | (n = 7) | |
| Lactating + vehicle | 14 108 ± 2129 | 84 183 ± 19 270 | 47 567 ± 6053 | 2 50 833 ± 52 045 | 18 667 ± 3151 | 71 683 ± 9094 | 1 57 300 ± 40 241 |
| (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | |
| Dioestrus + prolactin | 16 312 ± 1738 | 84 000 ± 15 734 | 47 044 ± 4943 | 2 25 489 ± 42 495 | 21 267 ± 2573 | 78 322 ± 7425 | 1 64 789 ± 32 857 |
| (n = 9) | (n = 9) | (n = 9) | (n = 9) | (n = 9) | (n = 9) | (n = 9) | |
| Lactating + prolactin | 14 863 ± 2129 | 63 917 ± 19 271 | 49 017 ± 6053 | 1 93 083 ± 52 045 | 26 333 ± 3151 | 80 467 ± 9094 | 1 30 217 ± 40 241 |
| (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | |
| Main effect of status | F 1,24 = 0.10 | F 1,24 = 0.06 | F 1,24 = 0.05 | F 1,24 = 0.14 | F 1,24 = 0.19 | F 1,24 = 0.49 | F 1,24 = 0.64 |
| P = 0.75 | P = 0.81 | P = 0.82 | P = 0.71 | P = 0.66 | P = 0.49 | P = 0.43 | |
| Main effect of prolactin | F 1,24 = 0.61 | F 1,24 = 0.06 | F 1,24 = 0.02 | F 1,24 = 0.02 | F 1,24 = 1.74 | F 1,24 = 2.19 | F 1,24 = 0.36 |
| P = 0.44 | P = 0.82 | P = 0.90 | P = 0.89 | P = 0.20 | P = 0.15 | P = 0.55 | |
| Interaction | F 1,24 = 0.16 | F 1,24 = 1.84 | F 1,24 = 0.01 | F 1,24 = 1.09 | F 1,24 = 1.63 | F 1,24 = 0.20 | F 1,24 = 0.01 |
| P = 0.69 | P = 0.19 | P = 0.90 | P = 0.31 | P = 0.21 | P = 0.66 | P = 0.91 |
Data shown are densitometry measurements (arbitrary units ± SEM) from Western blots for ERK1, pERK1, ERK2, pERK2, Akt, pAkt and β‐tubulin expression in the paraventricular nucleus after 2 μg i.c.v. prolactin.
Discussion
Here, we found that prolactin‐induced inhibition of oxytocin neurones was maintained in pregnancy but was lost in lactation. The maintenance of prolactin inhibition of oxytocin neurones in pregnancy probably allows elevated prolactin and placental lactogen to complement other mechanisms known to dampen oxytocin neurone excitability during pregnancy, such as neurosteroids and opioids (Brown & Russell, 2004; Brunton et al. 2014), to restrain oxytocin secretion and thereby prevent premature uterine contraction. Remarkably, prolactin inhibition of oxytocin neurones was lost in lactation when secretion of both hormones in response to suckling is required to support lactation.
While the overall change in response was a loss of prolactin inhibition in lactation, this concealed a large heterogeneity in the responses from lactating rats; prolactin still inhibited some oxytocin neurones in lactating rats, while other neurones were little affected and some were clearly excited. The secretion of oxytocin in response to action potential invasion of the terminals in the posterior pituitary gland is highly non‐linear due to frequency facilitation of oxytocin release at the terminals, whereby increasing action potential frequency causes a progressively greater secretion of oxytocin secretion per action potential (Bicknell, 1988). Hence, the excited neurones would probably impact oxytocin secretion to a greater degree than the inhibited neurones. Consistent with this speculation, central administration of prolactin robustly increases oxytocin secretion in male rats (Vega et al. 2010) but prolactin excites only a small proportion of supraoptic nucleus neurones in hypothalamic slices from male rats (Townsend et al. 2005).
Circulating prolactin is elevated in lactation in response to suckling (Grattan & Kokay, 2008; Grattan et al. 2008) and prolactin itself enhances oxytocin release from oxytocin neurone terminals in the posterior pituitary gland of lactating rats (Parker et al. 1991). Hence, suckling‐induced prolactin release might increase oxytocin levels in the circulation via a combination of increased activity in some oxytocin neurones (after prolactin transport into the brain) and amplification of secretion by circulating prolactin effects at the oxytocin neurone terminals.
Mechanisms of prolactin actions on oxytocin neurones
The simplest explanation for a loss of prolactin inhibition in lactation, when circulating prolactin is elevated (Grattan & Kokay, 2008; Grattan et al. 2008), is desensitisation of prolactin actions on oxytocin neurones; it is unlikely that this desensitisation is due to downregulation of prolactin receptors in the supraoptic nucleus because mRNA expression for the long form of the prolactin receptor is similar in the supraoptic nuclei of virgin, pregnant and lactating rats (Kokay et al. 2006). Hence, any desensitisation would be more likely to be due to changes in intracellular signal transduction downstream of the prolactin receptor.
We have previously shown that prolactin administration phosphorylates STAT5 in oxytocin neurones of virgin rats (Sapsford et al. 2012). Here, we found that the induction of pSTAT5 by prolactin was similar in virgin and lactating rats, suggesting that the canonical prolactin receptor signal transduction pathway can be fully activated by prolactin administration in lactating rats. Therefore, there appears to be no desensitisation of STAT5 signal transduction in oxytocin neurones during lactation. The lack of change in induction of pSTAT5 in lactating rats is not surprising because prolactin induction of pSTAT5 modulates gene expression (Lerant et al. 2001) and prolactin administration is known to increase oxytocin mRNA expression (Ghosh & Sladek, 1995a,b; Donner & Neumann, 2009). Here, we found that the number of oxytocin‐positive neurones was increased after prolactin, suggesting that the increased oxytocin mRNA expression is acutely translated into increased protein expression to bring more oxytocin neurones above the threshold for detection. The effects of prolactin on firing rate seen here were maximal within several minutes, long before changes in gene expression would be expected to impact activity. Hence, the activation of pSTAT5 is probably involved in modulation of gene expression within oxytocin neurones, rather than a direct modulation of electrical activity.
Prolactin receptors can activate multiple intracellular signalling pathways, particularly through ERK and phosphatidylinositol 3 kinase–Akt signalling (Bole‐Feysot et al. 1998). Prolactin rapidly increases pERK expression in the supraoptic and paraventricular nuclei of virgin female rats (Blume et al. 2009; Sjoeholm et al. 2011) and inhibition of the Akt signalling pathway reduces oxytocin secretion from hypothalamo‐neurohypophysial explants in response to other stimuli (Song et al. 2014). In contrast to previous work using immunohistochemistry for pERK (Blume et al. 2009; Sjoeholm et al. 2011), prolactin did not affect pERK1 or 2 expression in the supraoptic or paraventricular nuclei of virgin or lactating rats. Similarly, pAkt expression was unaffected by prolactin in the supraoptic and paraventricular nuclei of virgin or lactating rats, suggesting that the ERK and Akt signalling pathways do not mediate the prolactin‐induced inhibition of firing rate in virgin rats, or underpin the change in the firing rate response to prolactin evident in lactation.
Prolactin induces a robust hyperpolarisation in oxytocin neurones from virgin female rats in vitro by increasing the potassium leak conductance (Sirzen‐Zelenskaya et al. 2011), which probably underpins the inhibition evident in vivo. A reduced prolactin‐induced inhibition of oxytocin neurones in lactation might be underpinned by a reduced activation of the potassium leak conductance. However, this cannot explain the prolactin‐induced excitation seen in some oxytocin neurones. This excitation might result from the emergence of a depolarising action of prolactin on oxytocin neurones in lactation that has yet to be identified. Prolactin sensitises transient receptor potential vanilloid 1 (TRPV1) channels to capsaicin activation in sensory neurones (Patil et al. 2013). Supraoptic nucleus neurones express TRPV1 channels that can be stimulated by neuropeptides (Chakfe & Bourque, 2000; Naeini et al. 2006) and TRPV1 appears to be among the most highly upregulated genes in the supraoptic nucleus in lactation (Augustine et al. 2016). Hence, TRPV1 channels provide a potential mechanism by which prolactin could increase oxytocin neurone activity.
An alternative, or additional, potential explanation is that the prolactin‐induced excitation of oxytocin neurones is indirect. We administered prolactin i.c.v. and so it will have access to other populations of neurones in the brain, including those that project to oxytocin neurones. In virgin and lactating rats, hypothalamic arcuate nucleus tyrosine hydroxylase‐expressing (TH) neurons express prolactin receptors (Kokay & Grattan, 2005) and are excited by prolactin (Romano et al. 2013). However, these neurones down‐regulate TH expression in lactation (Romano et al. 2013) and have recently been shown to directly inhibit paraventricular nucleus neurons via GABA release (Zhang & van den Pol, 2016). Remarkably, during lactation, GABA inhibition of oxytocin neurones is converted into excitation (Lee et al. 2015). Hence, prolactin could potentially excite oxytocin neurones in lactating rats via GABA release from arcuate nucleus TH neurones.
The wide heterogeneity of responses to prolactin in oxytocin neurones, particularly evident in lactating rats, might reflect differing strengths of the opposing excitatory and inhibitory influences in each neurone. Further work will be required to determine the mechanisms by which prolactin excites oxytocin neurones in lactating rats.
Prolactin effects on vasopressin neurones
While the focus of this work was on prolactin regulation of oxytocin neurones, we confirmed previous observations of a lack of response of vasopressin neurones to prolactin in virgin rats (Kokay et al. 2006). However, we were surprised to find that the higher dose of prolactin consistently inhibited vasopressin neurones in lactating rats because almost no vasopressin neurones express mRNA for the long form of the prolactin receptor, even in lactation (Kokay et al. 2006), suggesting that the prolactin inhibition of vasopressin neurones might be mediated by afferent inputs.
When plasma osmolality is increased, vasopressin promotes water reabsorption via activation of kidney V2 receptors to increase insertion of aquaporin‐2 into the luminal membrane of collecting duct principal cells (Marples et al. 1999). While pregnancy and lactation are most often associated with plasticity in oxytocin neurones, a reduced osmotic set‐point for vasopressin secretion is established in lactation, leading to a paradoxical increase in vasopressin levels in lactating rats in the face of reduced plasma osmolality (Suzuki et al. 2000). The emergence of prolactin inhibition of vasopressin neurones in lactation might be part of a compensatory mechanism by which elevated circulating prolactin helps to prevent over‐activation of the vasopressin system when the osmotic set‐point is reduced.
Concluding remarks
During lactation, prolactin and oxytocin are secreted together in pulses in response to suckling (Higuchi et al. 1983). Here, we have confirmed that prolactin consistently inhibits oxytocin neurones in virgin rats and have shown that this inhibition is maintained during pregnancy but is lost in lactation, presumably facilitating the secretion of prolactin and oxytocin to support delivery of sufficient milk that is essential for survival of the young. The mechanisms that underpin this change in prolactin regulation of oxytocin neurone activity are yet to be identified but appear unlikely to involve the most common intracellular signalling pathways known to be activated by prolactin receptors.
Additional information
Competing interests
The authors have no competing interests.
Author contributions
All authors contributed to the design of the experiments. RAA, SRL, GTB, YA, TJS, VS, ICK and CHB completed the experiments and analyses. CHB, RAA, SRL, ICK and DRG wrote the manuscript and all authors critically reviewed the manuscript for intellectual content.
Funding
This work was supported by the Marsden Fund of the Royal Society of New Zealand (UOO0905) and the Health Research Council of New Zealand (14/568). YA was supported by a Saudi Arabian Ministry of Higher Education scholarship.
Translational perspective.
The World Health Organisation recommends breastfeeding as the optimal source of nutrition for babies. However, lactation failure and difficulties are commonplace. While non‐pharmacological interventions are used first, many cases of lactation failure are treated with drugs (Forinash et al. 2012), including the use of dopamine receptor antagonists to increase prolactin secretion. Our findings suggest that dopamine receptor antagonists might overcome lactation failure by also promoting milk secretion via prolactin activation of oxytocin neurons.
Acknowledgements
None.
References
- Augustine RA, Bouwer GT, Seymour AJ, Grattan DR & Brown CH (2016). Reproductive regulation of gene expression in the hypothalamic supraoptic and paraventricular nuclei. J Neuroendocrinol 28, 10.1111/jne.12350. [DOI] [PubMed] [Google Scholar]
- Augustine RA & Grattan DR (2008). Induction of central leptin resistance in hyperphagic pseudopregnant rats by chronic prolactin infusion. Endocrinology 149, 1049–1055. [DOI] [PubMed] [Google Scholar]
- Bertram R, Helena CV, Gonzalez‐Iglesias AE, Tabak J & Freeman ME (2010). A tale of two rhythms: the emerging roles of oxytocin in rhythmic prolactin release. J Neuroendocrinol 22, 778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell RJ (1988). Optimizing release from peptide hormone secretory nerve terminals. J Exp Biol 139, 51–65. [DOI] [PubMed] [Google Scholar]
- Blume A, Torner L, Liu Y, Subburaju S, Aguilera G & Neumann ID (2009). Prolactin activates mitogen‐activated protein kinase signaling and corticotropin releasing hormone transcription in rat hypothalamic neurons. Endocrinology 150, 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole‐Feysot C, Goffin V, Edery M, Binart N & Kelly PA (1998). Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19, 225–268. [DOI] [PubMed] [Google Scholar]
- Brown CH (2016). Magnocellular neurons and posterior pituitary function. Compr Physiol 6, 1701–1741. [DOI] [PubMed] [Google Scholar]
- Brown CH, Han SY, Moaddab M, Scott V & Schwenke DO (2014). Peptidergic control of oxytocin and vasopressin neurons and its role in reproductive and hypertension‐associated plasticity In Neurophysiology of Neuroendocrine Neurons, ed. Armstrong WE. & Tasker JG, pp. 65–86. Wiley‐Blackwell, Oxford, UK. [Google Scholar]
- Brown CH, Munro G, Johnstone LE, Robson AC, Landgraf R & Russell JA (1997). Oxytocin neurone autoexcitation during morphine withdrawal in anaesthetized rats. Neuroreport 8, 951–955. [DOI] [PubMed] [Google Scholar]
- Brown CH, Munro G, Murphy NP, Leng G & Russell JA (1996). Activation of oxytocin neurones by systemic cholecystokinin is unchanged by morphine dependence or withdrawal excitation in the rat. J Physiol 496, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH & Russell JA (2004). Cellular mechanisms underlying neuronal excitability during morphine withdrawal in physical dependence: lessons from the magnocellular oxytocin system. Stress 7, 97–107. [DOI] [PubMed] [Google Scholar]
- Brown RS, Wyatt AK, Herbison RE, Knowles PJ, Ladyman SR, Binart N, Banks WA & Grattan DR (2016). Prolactin transport into mouse brain is independent of prolactin receptor. FASEB J 30, 1002–1010. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA & Hirst JJ (2014). Allopregnanolone in the brain: protecting pregnancy and birth outcomes. Prog Neurobiol 113, 106–136. [DOI] [PubMed] [Google Scholar]
- Chakfe Y & Bourque CW (2000). Excitatory peptides and osmotic pressure modulate mechanosensitive cation channels in concert. Nat Neurosci 3, 572–579. [DOI] [PubMed] [Google Scholar]
- Crowley WR (2015). Neuroendocrine regulation of lactation and milk production. Compr Physiol 5, 255–291. [DOI] [PubMed] [Google Scholar]
- Donner N & Neumann ID (2009). Effects of chronic intracerebral prolactin on the oxytocinergic and vasopressinergic system of virgin ovariectomized rats. Neuroendocrinology 90, 315–322. [DOI] [PubMed] [Google Scholar]
- Egli M, Bertram R, Sellix MT & Freeman ME (2004). Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology 145, 3386–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Bertram R, Toporikova N, Sellix MT, Blanco W & Freeman ME (2006). Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. Am J Physiol Endocrinol Metab 290, E566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forinash AB, Yancey AM, Barnes KN & Myles TD (2012). The use of galactogogues in the breastfeeding mother. Ann Pharmacother 46, 1392–1404. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A & Nagy G (2000). Prolactin: structure, function, and regulation of secretion. Physiol Rev 80, 1523–1631. [DOI] [PubMed] [Google Scholar]
- Ghosh R & Sladek CD (1995a). Prolactin modulates oxytocin mRNA during lactation by its action on the hypothalamo‐neurohypophyseal axis. Brain Res 672, 24–28. [DOI] [PubMed] [Google Scholar]
- Ghosh R & Sladek CD (1995b). Role of prolactin and gonadal steroids in regulation of oxytocin mRNA during lactation. Am J Physiol Endocrinol Metab 269, E76–84. [DOI] [PubMed] [Google Scholar]
- Grattan DR & Kokay IC (2008). Prolactin: a pleiotropic neuroendocrine hormone. J Neuroendocrinol 20, 752–763. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Steyn FJ, Kokay IC, Anderson GM & Bunn SJ (2008). Pregnancy‐induced adaptation in the neuroendocrine control of prolactin secretion. J Neuroendocrinol 20, 497–507. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Honda K, Fukuoka T, Negoro H, Hosono Y & Nishida E (1983). Pulsatile secretion of prolactin and oxytocin during nursing in the lactating rat. Endocrinol Jpn 30, 353–359. [DOI] [PubMed] [Google Scholar]
- Kokay IC, Bull PM, Davis RL, Ludwig M & Grattan DR (2006). Expression of the long form of the prolactin receptor in magnocellular oxytocin neurons is associated with specific prolactin regulation of oxytocin neurons. Am J Physiol Regul Integr Comp Physiol 290, R1216–1225. [DOI] [PubMed] [Google Scholar]
- Kokay IC & Grattan DR (2005). Expression of mRNA for prolactin receptor (long form) in dopamine and pro‐opiomelanocortin neurones in the arcuate nucleus of non‐pregnant and lactating rats. J Neuroendocrinol 17, 827–835. [DOI] [PubMed] [Google Scholar]
- Ladyman SR & Grattan DR (2004). Region‐specific reduction in leptin‐induced phosphorylation of signal transducer and activator of transcription‐3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology 145, 3704–3711. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim YB, Kim JS, Kim WB, Kim YS, Han HC, Colwell CS, Cho YW & In Kim Y (2015). GABAergic inhibition is weakened or converted into excitation in the oxytocin and vasopressin neurons of the lactating rat. Mol Brain 8, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerant A, Kanyicska B & Freeman ME (2001). Nuclear translocation of STAT5 and increased expression of Fos related antigens (FRAs) in hypothalamic dopaminergic neurons after prolactin administration. Brain Res 904, 259–269. [DOI] [PubMed] [Google Scholar]
- Marples D, Frokiaer J & Nielsen S (1999). Long‐term regulation of aquaporins in the kidney. Am J Physiol 276, F331–339. [DOI] [PubMed] [Google Scholar]
- Naeini RS, Witty MF, Seguela P & Bourque CW (2006). An N‐terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci 9, 93–98. [DOI] [PubMed] [Google Scholar]
- Parker SL, Armstrong WE, Sladek CD, Grosvenor CE & Crowley WR (1991). Prolactin stimulates the release of oxytocin in lactating rats: evidence for a physiological role via an action at the neural lobe. Neuroendocrinology 53, 503–510. [DOI] [PubMed] [Google Scholar]
- Patil MJ, Ruparel SB, Henry MA & Akopian AN (2013). Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex‐dependent manner: contribution of prolactin receptor to inflammatory pain. Am J Physiol Endocrinol Metab 305, E1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano N, Yip SH, Hodson DJ, Guillou A, Parnaudeau S, Kirk S, Tronche F, Bonnefont X, Le Tissier P, Bunn SJ, Grattan DR, Mollard P & Martin AO (2013). Plasticity of hypothalamic dopamine neurons during lactation results in dissociation of electrical activity and release. J Neurosci 33, 4424–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapsford TJ, Kokay IC, Ostberg L, Bridges RS & Grattan DR (2012). Differential sensitivity of specific neuronal populations of the rat hypothalamus to prolactin action. J Comp Neurol 520, 1062–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott V, Bishop VR, Leng G & Brown CH (2009). Dehydration‐induced modulation of κ‐opioid inhibition of vasopressin neurone activity. J Physiol 587, 5679–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott V & Brown CH (2011). Kisspeptin activation of supraoptic nucleus neurons in vivo. Endocrinology 152, 3862–3870. [DOI] [PubMed] [Google Scholar]
- Sirzen‐Zelenskaya A, Gonzalez‐Iglesias AE, Boutet de Monvel J, Bertram R, Freeman ME, Gerber U & Egli M (2011). Prolactin induces a hyperpolarising current in rat paraventricular oxytocinergic neurones. J Neuroendocrinol 23, 883–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoeholm A, Bridges RS, Grattan DR & Anderson GM (2011). Region‐, neuron‐, and signaling pathway‐specific increases in prolactin responsiveness in reproductively experienced female rats. Endocrinology 152, 1979–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Levin BE, Stevens W & Sladek CD (2014). Supraoptic oxytocin and vasopressin neurons function as glucose and metabolic sensors. Am J Physiol Regul Integr Comp Physiol 306, R447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Koizumi N, Hirose H, Hokao R, Takemura N & Motoyoshi S (2000). Changes in plasma arginine vasopressin concentration during lactation in rats. Comp Med 50, 277–280. [PubMed] [Google Scholar]
- Townsend J, Cave BJ, Norman MR, Flynn A, Uney JB, Tortonese DJ & Wakerley JB (2005). Effects of prolactin on hypothalamic supraoptic neurones: evidence for modulation of STAT5 expression and electrical activity. Neuro Endocrinol Lett 26, 125–130. [PubMed] [Google Scholar]
- Vega C, Moreno‐Carranza B, Zamorano M, Quintanar‐Sttjp12284ano A, Mendez I, Thebault S, Martinez de la Escalera G & Clapp C (2010). Prolactin promotes oxytocin and vasopressin release by activating neuronal nitric oxide synthase in the supraoptic and paraventricular nuclei. Am J Physiol Regul Integr Comp Physiol 299, R1701–1708. [DOI] [PubMed] [Google Scholar]
- Walsh RJ, Slaby FJ & Posner BI (1987). A receptor‐mediated mechanism for the transport of prolactin from blood to cerebrospinal fluid. Endocrinology 120, 1846–1850. [DOI] [PubMed] [Google Scholar]
- Zhang X & van den Pol AN (2016). Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat Neurosci 19, 1341–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]


