Abstract
Key points
A cerebellar dentate nuclei (DN) contribution to volitional oculomotor control has recently been hypothesized but not fully understood.
Cerebrotendinous xanthomatosis (CTX) is a rare neurometabolic disease typically characterized by DN damage.
In this study, we compared the ocular movement characteristics of two sets of CTX patients, with and without brain MRI evidence of DN involvement, with a set of healthy subjects.
Our results suggest that DN participate in voluntary behaviour, such as the execution of antisaccades, and moreover are involved in controlling the precision of the ocular movement.
The saccadic abnormalities related to DN involvement were independent of global and regional brain atrophy.
Our study confirms the relevant role of DN in voluntary aspects of oculomotion and delineates specific saccadic abnormalities that could be used to detect the involvement of DN in other cerebellar disorders.
Abstract
It is well known that the medial cerebellum controls saccadic speed and accuracy. In contrast, the role of the lateral cerebellum (cerebellar hemispheres and dentate nuclei, DN) is less well understood. Cerebrotendinous xanthomatosis (CTX) is a lipid storage disorder due to mutations in CYP27A1, typically characterized by DN damage. CTX thus provides a unique opportunity to study DN in human oculomotor control. We analysed horizontal and vertical visually guided saccades and horizontal antisaccades of 19 CTX patients. Results were related to the presence/absence of DN involvement and compared with those of healthy subjects. To evaluate the contribution of other areas, abnormal saccadic parameters were compared with global and regional brain volumes. CTX patients executed normally accurate saccades with normal main sequence relationships, indicating that the brainstem and medial cerebellar structures were functionally spared. Patients with CTX executed more frequent multistep saccades and directional errors during the antisaccade task than controls. CTX patients with DN damage showed less precise saccades with longer latencies, and more frequent directional errors, usually not followed by corrections, than either controls or patients without DN involvement. These saccadic abnormalities related to DN involvement but were independent of global and regional brain atrophy. We hypothesize that two different cerebellar networks contribute to the metrics of a movement: the medial cerebellar structures determine accuracy, whereas the lateral cerebellar structures control precision. The lateral cerebellum (hemispheres and DN) also participates in modulating goal directed gaze behaviour, by prioritizing volitional over reflexive movements.
Keywords: saccades, antisaccades, cerebellum, MRI, oculomotor control, volumetry
Key points
A cerebellar dentate nuclei (DN) contribution to volitional oculomotor control has recently been hypothesized but not fully understood.
Cerebrotendinous xanthomatosis (CTX) is a rare neurometabolic disease typically characterized by DN damage.
In this study, we compared the ocular movement characteristics of two sets of CTX patients, with and without brain MRI evidence of DN involvement, with a set of healthy subjects.
Our results suggest that DN participate in voluntary behaviour, such as the execution of antisaccades, and moreover are involved in controlling the precision of the ocular movement.
The saccadic abnormalities related to DN involvement were independent of global and regional brain atrophy.
Our study confirms the relevant role of DN in voluntary aspects of oculomotion and delineates specific saccadic abnormalities that could be used to detect the involvement of DN in other cerebellar disorders.
Abbreviations
- ACC
anterior cingulate cortex
- CTX
cerebrotendinous xanthomatosis
- CTXnD
CTX patients with no dentate involvement
- CTXwD
CTX patients with dentate involvement
- DLPFC
dorso‐lateral prefrontal cortex
- DN
dentate nuclei
- FEF
frontal eye field
- PEF
parietal eye field
- pre‐SMA
pre‐supplementary motor area
- SC
superior colliculus
- SEF
supplementary eye field
Introduction
Classical evidence identifies the medial cerebellum (cerebellar vermis and fastigial nuclei) as the anatomical component of the visuomotor cerebellum underlying saccadic eye movements (Noda, 1991; Voogd et al. 2012). Involvement of the lateral cerebellum (cerebellar hemispheres and DN) in ocular movements has also been supported by neurophysiological, functional and lesion studies in animals and humans (Ron & Robinson, 1973; Ohki et al. 2008; Voogd et al. 2012; Ashmore & Sommer, 2013). However, the extent and nature of their contribution to gaze control is not fully understood (Leigh & Zee, 2015). The anatomical connections of the lateral cerebellum suggest a specialized oculomotor role, likely to be different from that of the medial cerebellum (Dum & Strick, 2003). The DN, in particular, are connected through the ventrolateral thalamic nuclei to the frontal and parietal eye fields (FEF and PEF), indicating a possible involvement in complex gaze behaviour. In addition, direct projections of DN to the superior colliculus (SC) and oculomotor nuclei might possibly modulate saccadic dynamics (May, 2006). Nonetheless, studies of the influence of the DN on gaze control have led to inconclusive and sometimes contradictory results. In particular, the scarcity of studies of patients with discrete lesions of the DN has limited the understanding of the impact of this structure on eye movement control in humans. Cerebrotendinous xanthomatosis (CTX) is an autosomal recessive lipid storage disorder due to mutations in CYP27A1, encoding the mitochondrial enzyme 27‐sterol‐hydroxylase, clinically characterized by infantile‐onset diarrhoea, juvenile cataracts, tendon xanthomas, psychiatric/cognitive disturbances, pyramidal, cerebellar and extrapyramidal signs, and peripheral neuropathy (Federico & Dotti, 2003; Mignarri et al. 2014). MRI evidence of DN abnormality is reported in about 75% of patients, but no clinical differences have been reported between patients with and without DN involvement. CTX, thus, provides a unique opportunity to study the role of DN in human oculomotor control. Moreover, as the disease is treatable, the identification of neurophysiological markers could be relevant for treatment monitoring.
We analysed horizontal and vertical visually guided saccades and horizontal antisaccades of 19 CTX patients. Saccade characteristics were measured and antisaccade error and correction rates were computed. Results were related to the presence or absence of DN involvement and compared with those of a matching group of 19 healthy subjects. Moreover, since multistep saccades have been proposed as a marker of basal ganglia and/or cerebellar impairment (Optican et al. 2008; Blekher et al. 2009) we analysed the rate and characteristics of the steps composing such movements. We measured the global and regional brain atrophy volumes in both groups through a quantitative MRI assessment and correlated these volumes with the abnormal saccadic parameters, because neurodegeneration in CTX extends to other brain areas involved in oculomotor control, especially the frontal cortex. We found that DN lesions were associated with abnormal saccadic latency and precision (but not accuracy) and increased uncorrected directional errors, independently from global or regional brain atrophy.
Our results suggest that DN (and thus lateral cerebellum) participate in volitional oculomotor control through a functionally interconnected network that includes frontal cortex and basal ganglia. This network is involved in planning a proper action while simultaneously suppressing competing and reactive behaviours.
Methods
Participants
Thirty‐eight subjects volunteered for the study. Nineteen patients with genetically confirmed CTX (nine males, ten females) were recruited in our national reference centre for CTX; their mean age at examination was 41 years (range 18–63). All patients were under treatment with chenodeoxycholic acid (CDCA) supplementation at the time of the enrolment for the study. None of them were undergoing neuropsychiatric therapies. Each patient underwent complete neurological and neuro‐ophthalmological examinations. Neurological disability was assessed through modified Rankin scale (MRS) (van Swieten et al. 1988) and expanded disability status scale (EDSS) (Kurtzke, 1983). Brain MRI was obtained for all patients using an MRI 1.5T machine (Philips Gyroscan, Philips Medical System, Best, The Netherlands) and always included T1‐weighted, T2‐weighted, and fluid attenuated inversion recovery (FLAIR) images, evaluated by two experienced neuroradiologists. Based on the presence/absence of DN T2‐hyperintensity and/or FLAIR‐hyperintensity at MRI, subjects were further divided into two subgroups. The severity of the DN involvement was qualitatively assessed through a score from 0 (no involvement) to 4 (maximum involvement) by the size and confluence of MRI lesions. The control group consisted of 19 healthy age‐ and sex‐matched subjects. All subjects gave their written informed consent; the study complied with the Declaration of Helsinki and was approved by the Regional Ethics Committee.
Eye movement recording
Eye movements were recorded with an ASL 504 eye‐tracker device (Applied Science Laboratories, Bedford, MA, USA). Data acquisition and visual stimulation were controlled by a PC (3 GHz Pentium) running custom software dedicated to real‐time data acquisition. Eye position was sampled by the eye‐tracker at a frequency of 238.1 Hz, digitized with a resolution of 16 bits, corresponding to a sensitivity of recorded eye position of 0.16 deg, and stored for off‐line analysis. Each sample was stored with an accurate time stamp. The visual stimulus was a red dot (luminance 63 cd m−2) with diameter subtending a visual angle of 0.4 deg, presented on a black background (luminance 2.5 cd m−2). The screen was a 31 × 51 cm LCD screen, with a resolution of 1024 × 768 pixels, 72 cm from the subject's eyes.
Subjects were seated in a darkened room while movements were minimized by a chinrest with a bite bar. Each recording session was preceded by an interactive calibration procedure based on nine static points and three static points of validation.
All subjects performed visually guided horizontal and vertical saccades and horizontal antisaccades.
Visually guided saccade tasks
Targets were presented at either of two horizontal eccentricities (10 deg and 18 deg, right and left) or one vertical eccentricity (8 deg, up and down). At the start of a trial, a central fixation point appeared for 1500–2500 ms. After the disappearance of the central fixation point there was a blank period (gap) of 200 ms. Then, a peripheral target appeared at one of the horizontal or vertical eccentricities for 1500 ms. Subjects were instructed to make a saccade as quickly and accurately as possible towards the target. Each saccade task consisted of a sequence of at least 40 trials for each target amplitude.
Antisaccade tasks
A target was presented at one of two possible horizontal eccentricities (10 deg and 18 deg). After the disappearance of the central fixation point and a gap of 200 ms, a peripheral target appeared randomly to the right or left for 2500 ms. Subjects were instructed not to look at the target, but to gaze in the opposite direction, making a saccade of the same amplitude. Each antisaccade task consisted of a sequence of at least 40 trials for each target amplitude.
Data analysis
All analyses were performed with custom Matlab (The Mathworks, Natick, MA, USA) scripts. Data were first resampled to a uniform step size of 4.2 ms (238.1 Hz), because the tracker occasionally dropped a sample. Signals were then denoised with the Matlab Wavelet Toolbox. A Savitzky‐Golay filter (polynomial order 2, frame size 9 samples) was used to obtain low‐pass filtered data, and their first and second order derivatives (velocity and acceleration). Data were then resampled at 1.0 ms (1 kHz sample rate) using cubic interpolation, to simplify presentation and analysis. Saccades were detected using speed and acceleration criteria. The beginning and end of the saccade were designated as the point at which the speed dropped below 10% of the peak speed. Movements were excluded if they were not saccade‐like (0.2 < amplitude < 40 deg; 5 < duration < 500 ms; 15 < peak speed < 800 deg s−1). Each trial was automatically marked as containing a single‐step or a multistep saccade. Trials with a multistep saccade were checked manually to confirm the identification of multistep saccades. Any movement that included more than one step, e.g. a corrective saccade in the same direction as the primary saccade, or a staircase of saccades to the target, was considered to be a multistep saccade.
Brain MRI volumes
We further evaluated the global and regional brain parenchymal volumes of CTXwD and CTXnD patients, measured on T1 weighted three‐dimensional gradient echo images (TR/TE = 35 ms/10256 × 256 matrix, one signal average, 250 mm field of view, 50 contiguous 3 mm slices). We used the cross‐sectional version of the SIENA software, SIENAX (http://www.fmrib.ox.ac.uk/fsl/), normalized for the subject's head size (Guerrera et al. 2010). Particularly, we obtained global measures of normalized brain volume, normalized white matter volume, normalized grey matter volume, as well as selective measurement of normalized cortical volumes. For volume measurement of specific cortical regions, through predefined standard space masks (see structural atlas in http://www.fmrib.ox.ac.uk/fsl/), we selected from the cortical grey matter the normalized volumes of the frontal, temporal, parietal, insula, and occipital lobes of both hemispheres. Furthermore, with an identical approach, we estimated the normalized volume of the cerebellum. In this case, when necessary, the cerebellum mask of the subject was manually edited by expert users (V.S., M.B., N.D.S.). Since DN and surrounding white matter are affected by various neuropathological changes, not directly related to neuronal loss, including myelin rarefaction, lipid crystal deposits, cleft fibrosis, reactive astrocytosis, scattered to widespread deposition of haemosiderin pigment, and focal calcifications (Barkhof et al. 2000), leading to various modification of their size (Guerrera et al. 2010), we did not measure the DN volumes, but we qualitatively assessed their impairment, as explained above. MRI data were compared with those obtained from a group of 23 age‐matched healthy controls.
Statistical analysis
All statistical tests were performed with the Matlab's statistics toolbox. Results were considered significant if the probability of chance occurrence, P, was less than 0.05. Measures for bar charts were compared between pairs with Student's t test if both data sets were normally distributed (by the Kolmogorov‐Smirnov test), otherwise with the Kruskal‐Wallis test. Two‐tailed tests were used.
N‐way ANOVAs were performed to look for effects and interactions among subject groups and tasks. Multiple pair‐wise comparisons (Tukey's honest significant difference criterion) were then computed to look for statistical significance. Data were grouped according to diagnosis (control or CTX with or without dentate involvement), task (visually guided or antisaccade), kind of saccade (single‐step or multistep), and target location (fixation point or eccentric target). During the antisaccade task, errors (prosaccades made to the target) and any following corrective antisaccades were grouped separately from correct antisaccades.
For the saccadic main sequences peak velocity vs. amplitude and duration vs. amplitude, curve fits were compared with a bootstrap test of the maximum difference between the two curves (Rodgers, 1999). Two curves were fitted to the original data, and the maximum difference between them was calculated. The data were then combined (because under the null hypothesis the two data samples are from the same distribution). The combined data were resampled (with replacement) to create 2000 new data sets of the same size as the original data sets. These were also fitted, producing 2000 pairs of curves. The maximum difference was calculated for each of these pairs. Fits were considered statistically different if the original maximum difference was outside the 95% confidence interval of the distribution of the resampled maximum differences.
For each measurement the data were pooled across subjects. To avoid misleading effects of outlying data and the unequal number of saccades made by each subject, the median of the results from each subject, rather than the data themselves, were pooled. If a subject made fewer than four valid saccades, that subject was eliminated from that pool. When measuring antisaccade parameters, subjects were added to the pool even if they made only one corrective antisaccade, because they were so rare.
Average performance in saccade tasks was characterized by the mean and standard deviation of the population. In particular, with respect to the amplitude, the mean value indicates the accuracy of the saccades (closeness of the average amplitude to the actual target) while the reciprocal of the standard deviation indicates the precision of the saccades (compactness of grouping, or consistency, among amplitudes).
To assess the effect of brain atrophy and other clinical and epidemiological variables a statistical analysis was performed with two‐way ANOVAs and multiple pair‐wise comparisons.
Results
Clinical findings (Table 1)
Table 1.
Demographic, disability scale and brain MRI volume values of cerebrotendinous xanthomatosis (CTX) patients
| Demographical data | Disability | Brain MRI volumes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt no. | Sex | Age exam. | Age diag. | MRS | EDSS | NBV | NGMV | NWMV | NCV | NFV | NTV | NPV | NIV | NOV | NCbV | DN lesions |
| 1 | F | 29 | 29 | 1 | 2 | 1530 | 681 | 849 | 555 | 211 | 128 | 123 | 20 | 73 | 170 | 1 |
| 2 | M | 56 | 31 | 3 | 4 | 1308 | 589 | 719 | 494 | 162 | 131 | 109 | 22 | 70 | 125 | 2 |
| 3 | M | 27 | 21 | 1 | 3 | 1707 | 846 | 860 | 686 | 251 | 168 | 156 | 27 | 84 | 201 | 2 |
| 4 | F | 35 | 34 | 3 | 5 | 1510 | 639 | 871 | 495 | 189 | 119 | 106 | 19 | 62 | 121 | 3 |
| 5 | M | 37 | 36 | 4 | 7 | 1492 | 649 | 843 | 498 | 193 | 106 | 118 | 22 | 59 | 123 | 3 |
| 6 | M | 48 | 45 | 4 | 7 | 1508 | 674 | 834 | 525 | 199 | 106 | 126 | 21 | 72 | 144 | 4 |
| 7 | M | 47 | 37 | 3 | 4 | 1390 | 669 | 720 | 566 | 198 | 147 | 121 | 28 | 71 | 155 | 1 |
| 8 | M | 45 | 31 | 2 | 3 | 1488 | 742 | 746 | 626 | 226 | 152 | 128 | 25 | 96 | 127 | 1 |
| 9 | F | 49 | 27 | 1 | 3 | 1466 | 662 | 804 | 531 | 172 | 151 | 112 | 25 | 72 | 165 | 1 |
| 10 | M | 44 | 43 | 2 | 4 | 1498 | 671 | 827 | 523 | 189 | 145 | 96 | 22 | 71 | 158 | 2 |
| 11 | F | 57 | 54 | 2 | 3.5 | 1452 | 634 | 818 | 489 | 181 | 117 | 103 | 24 | 64 | 152 | 1 |
| 12 | F | 63 | 36 | 3 | 3.5 | 1555 | 628 | 926 | 528 | 190 | 127 | 122 | 25 | 64 | 143 | 1 |
| 13 | F | 62 | 43 | 2 | 3 | 1441 | 614 | 827 | 521 | 180 | 131 | 115 | 23 | 72 | 180 | 1 |
| 14 | F | 62 | 48 | 1 | 1.5 | 1484 | 667 | 818 | 525 | 190 | 143 | 116 | 27 | 73 | 194 | 0 |
| 15 | F | 24 | 18 | 1 | 2 | 1729 | 863 | 865 | 699 | 253 | 171 | 160 | 26 | 88 | 153 | 0 |
| 16 | F | 33 | 32 | 2 | 3 | 1520 | 699 | 821 | 533 | 202 | 126 | 105 | 33 | 74 | 150 | 0 |
| 17 | M | 30 | 25 | 1 | 1.5 | 1635 | 797 | 838 | 698 | 235 | 167 | 156 | 33 | 98 | 167 | 0 |
| 18 | M | 20 | 13 | 1 | 1 | 1718 | 870 | 848 | 776 | 280 | 178 | 180 | 36 | 104 | 197 | 0 |
| 19 | F | 31 | 22 | 1 | 2 | 1684 | 874 | 810 | 781 | 269 | 193 | 185 | 27 | 97 | 158 | 0 |
Pt, patient; Age exam., age of the patient when examined; Age diag., age of the patient when first diagnosed; MRS, modified Rankin scale; EDSS, expanded disability status scale; NBV, normalized brain parenchimal volumes; NWMV, normalized white matter volume; NGMV, normalized grey matter volume; NCV, normalized cortical volumes; NFV, normalized frontal volume; NTV, normalized temporal volume; NPV, normalized parietal volume; NIV, normalized insula volume; NOV, normalized occipital volume; NCbV, normalized volume of the cerebellum; DN lesions, dentate nuclei lesions qualitatively assessed from 0 (no involvement) to 4 (maximum involvement). CTXwD: patients 1–13. CTXnD: patients 14–19.
Fifteen patients had a positive history for cataracts, surgically treated, but all participants were able to see the visual stimuli on the screen without correction. Beside tendon xanthomas, present in all but three patients, the other most common clinical findings were mild cognitive and mood disturbances, spasticity and cerebellar symptoms. All patients were fully able to understand the task instructions and to participate in the study. Thirteen patients (CTXwD) showed MRI abnormalities of DN (mean age 46 ± 11.6 years, range 27–63), while the remaining six patients (CTXnD) did not show any MRI DN abnormality (mean age 31 ± 11.5 years, range 18–63) (Fig. 1). The severity of DN involvement is reported in Table 1. Between the two groups, no specific clinical differences were found, except that in CTXnD the mean age was lower, and cerebellar and pyramidal signs were less prevalent (Table 1).
Figure 1. Brain MRI.
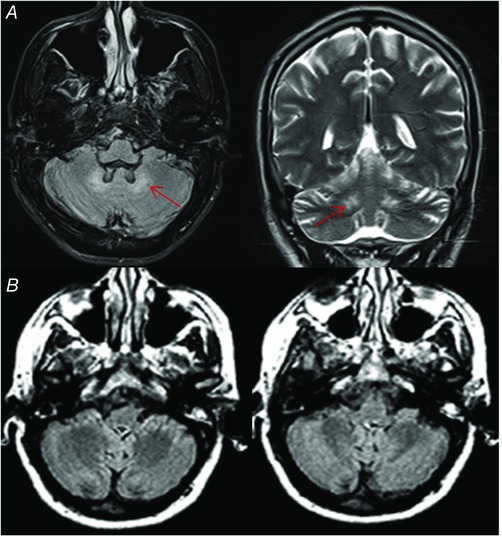
A, brain MR of patient 3 showing presence of bilateral DN hyperintensity (arrows) on axial FLAIR image (left) and coronal T2‐weighted image (right). B, brain MR of patient 14 showing absence of DN signal alteration on axial FLAIR images. [Color figure can be viewed at wileyonlinelibrary.com]
Visually guided saccades
Figure 2 shows the four types of movements used to test the saccadic system, and examples of recorded saccades from CTX patients with DN involvement (Fig. 2 A–D), CTX patients without DN involvement (Fig. 2 E–H) and controls (Fig. 2 I–L). The first column shows visually guided single‐step saccades, the second column shows visually guided multistep saccades, the third column shows correct antisaccades, and the fourth column shows prosaccade errors with corrective antisaccades.
Figure 2. Examples of saccades.
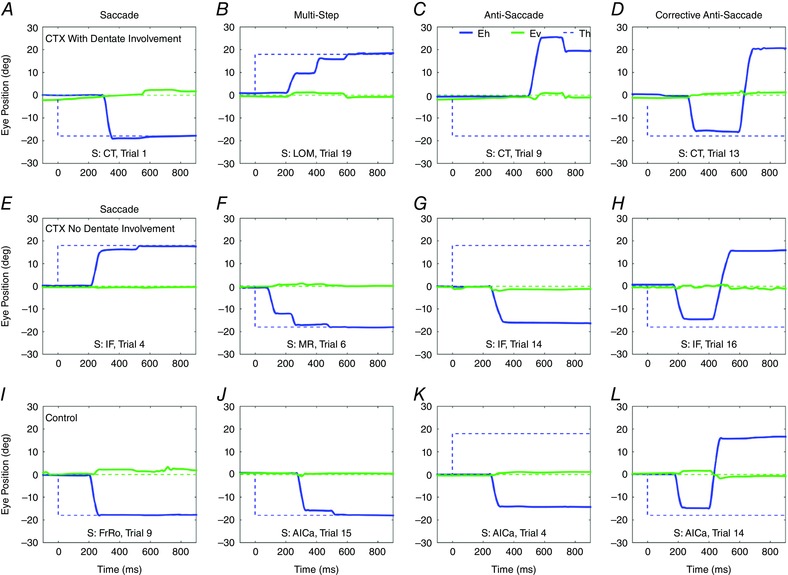
Eye movement recording from CTX patients with DN involvement (first row), CTX patients without DN involvement (second row) and controls (third row). Panels A, E and I show visually guided single‐step saccades; panels B, F and J show visually guided multistep saccades; antisaccades are shown in panels C, G and K; prosaccade errors with corrective antisaccades are shown in panels D, H and L. Blue continuous lines: horizontal gaze; green continuous line: vertical gaze; blue dotted line: horizontal target position; green dotted line: vertical target position. S is subject. [Color figure can be viewed at wileyonlinelibrary.com]
Main sequence relationships
Both horizontal and vertical saccades followed the main sequence relationship (peak velocity vs. amplitude) in all groups with no significant differences (P > 0.05). The horizontal main sequence relationships for each group are shown in Fig. 3, and their parametric fits are given in Table 2.
Figure 3. Amplitude dependence of saccadic peak velocity and duration.
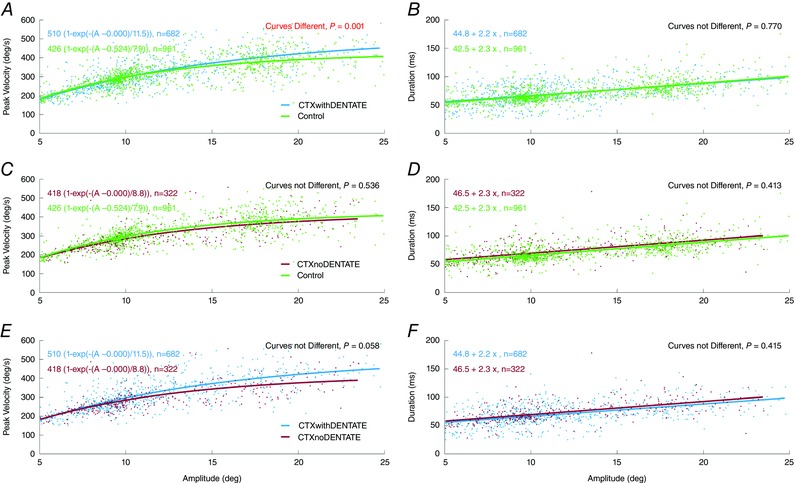
The peak velocity vs. amplitude curves and duration vs. amplitude lines for each group were compared (see Table 3). The saccade dynamics were not different among groups. [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
Main sequence fits for visually guided saccades
|
|
|
Statistical significance of comparisons | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | o | B | C | S | N | CTXnD | Control | |||
| CTXwD | 510 | 0.0 | 11.5 | 44.8 | 2.2 | 682 | P(V p) = 0.058 P(D) = 0.42 | P(V p) = 0.001 P(D) = 0.77 | ||
| CTXnD | 418 | 0.0 | 8.8 | 46.5 | 2.3 | 322 | — | P(V p) = 0.54 P(D) = 0.41 | ||
| Control | 426 | 0.5 | 7.9 | 42.5 | 2.3 | 961 | — | — | ||
Peak velocity (V p) vs. amplitude (A) was fittes with parameters for maximum velocity (M), amplitude offset (o), and angle constant (B). Duration (D) was fitted with a constant (C) and a slope (S). N is number of saccades analysed. The only significant comparison was V p vs. A for CTXwD vs. Control. If large saccades in CTXwD were faster than normal, one would expect their duration to be shorter. However, the difference in D vs. A was not significant. There were no other significant differences between the saccade characteristics in any of the groups (CTXwD, CTXnD, or Control). This suggests that saccade dynamics were essentially the same in all groups.
Latency
When all saccades were pooled, the average latency for horizontal visually guided saccades was longer in CTXwD compared to controls (Table 3, Fig. 4 A). The main effect of the diagnosis (F (2,405) = 5.23, P < 0.05) was significant. The multiple comparisons of CTXwD vs. controls (P < 0.05) and CTXwD vs. CTXnD (P < 0.05) were significant, but CTXnD vs. controls (P > 0.05) was not.
Table 3.
Parameters of horizontal and vertical visually guided saccades
| Saccade parameters | ||||
|---|---|---|---|---|
| Type of saccade | Group | Latency (ms) | Gain | Std Dev. |
| Horizontal saccades | ||||
| Single‐step + multistep | CTXwD | 235 ± 36 (12) | 0.92 ± 0.12 (13) | 0.22 ± 0.09 (13) |
| CTXnD | 212 ± 20 (6) | 0.88 ± 0.06 (6) | 0.15 ± 0.05 (6) | |
| Ctrl | 204 ± 23 (19) | 0.95 ± 0.03 (19) | 0.12 ± 0.06 (19) | |
| Single‐step saccades | ||||
| CTXwD | 243 ± 60 (11) | 1.01 ± 0.08 (12) | 0.22 ± 0.12 (12) | |
| CTXnD | 208 ± 31 (4) | 0.97 ± 0.01 (4) | 0.10 ± 0.06 (4) | |
| Ctrl | 209 ± 30 (19) | 0.97 ± 0.04 (19) | 0.09 ± 0.07 (19) | |
| Multistep saccades (first step) | ||||
| CTXwD | 225 ± 36 (11) | 0.82 ± 0.09 (12) | 0.14 ± 0.05 (12) | |
| CTXnD | 212 ± 19 (6) | 0.85 ± 0.06 (6) | 0.15 ± 0.06 (6) | |
| Ctrl | 208 ± 30 (14) | 0.91 ± 0.05 (16) | 0.12 ± 0.07 (16) | |
| Vertical saccades | ||||
| Single‐step + multistep | CTXwD | 256 ± 74 (9) | 0.88 ± 0.17 (9) | 0.26 ± 0.08 (9) |
| CTXnD | 213 ± 18 (5) | 0.98 ± 0.21 (5) | 0.28 ± 0.11 (5) | |
| Ctrl | 216 ± 29 (18) | 0.99 ± 0.10 (18) | 0.17 ± 0.08 (18) | |
| Single‐step saccades | ||||
| CTXwD | 265 ± 78 (8) | 0.98 ± 0.09 (8) | 0.26 ± 0.08 (8) | |
| CTXnD | 213 ± 19 (5) | 1.08 ± 0.16 (5) | 0.28 ± 0.15 (5) | |
| Ctrl | 220 ± 31 (17) | 0.99 ± 0.10 (17) | 0.17 ± 0.09 (17) | |
| Multistep saccades (first step) | ||||
| CTXwD | 207 ± 35 (4) | 0.60 ± 0.04 (9) | 0.13 ± 0.07 (4) | |
| CTXnD | 225 ± 35 (2) | 0.72 ± 0.13 (3) | 0.21 ± 0.12 (3) | |
| Ctrl | 211 ± 23 (10) | 0.91 ± 0.12 (11) | 0.14 ± 0.08 (18) | |
Ctrl, control subjects, CTXwD, patients with dentate involvements, CTXnD, patients without dentate involvements. Values are means ± standard deviations (number of subjects).
Figure 4. Latency, gain and precision of visually guided saccades.
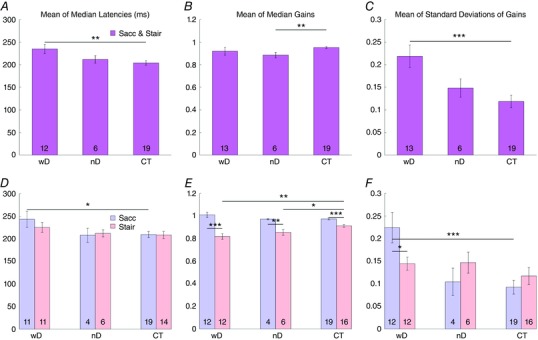
Bar graphs of the mean values of latency, gain and precision of horizontal visually guided saccades of CTX patients with DN involvement (wD), CTX patients without DN involvement (nD) and controls (CT). A–C, mean values of single‐step and multistep saccades pooled together. D–F, blue bars indicate the mean values of the single‐step saccades (Sacc); pink bars represent the mean values of the multistep saccades (Stair). Asterisks indicate the significant differences among groups (P < 0.05). [Color figure can be viewed at wileyonlinelibrary.com]
When visually guided single‐step and multistep saccades were analysed separately, only the latency of single‐step saccades was significantly longer in CTXwD than controls (Table 3 and Fig. 4 D). The main effects of single‐step or multistep (two‐way ANOVA, F (1,210) = 10.20, P < 0.01) and diagnosis (F (2,210) = 8.04, P < 0.01) were significant. There also were significant interactions between the number of steps in the saccade and the diagnosis (F (2,210) = 5.06, P < 0.05). Multiple comparisons showed that latency of single‐step saccades was longer for CTXwD than for controls (P < 0.01), but not for multistep saccades (P > 0.05).
Gain
When all saccades were pooled, the average gain (size of saccade divided by the distance from initial eye position to the target) for horizontal, visually guided saccades showed slightly hypometric saccades in both CTX patient groups with respect to controls, but the difference was significant only between CTXnD and controls (Table 3 and Fig. 4 B). The main effect of the diagnosis was not significant (F (2,540) = 1.98, P > 0.05), and no multiple comparison among CTXwD, CTXnD, and controls was significant.
When single‐step saccades and multistep saccades were analysed separately, the main effects of saccade number (F (1,299) = 186.38, P < 0.01) and diagnosis (F (2,299) = 3.66, P < 0.05) were both significant (Table 3 and Fig. 4 E). The interaction term (F (22,299) = 6.79, P < 0.01) was also significant. The multiple comparisons among single‐step or multiple step saccades and controls were not significant (P > 0.05). The gains were different for single‐step vs. multiple step saccades for CTXwD, CTXnD, and controls (P < 0.05).
Precision (reciprocal of standard deviation)
When horizontal saccades were pooled (Table 3 and Fig. 4 C), there was no effect of diagnosis (F (2,540) = 1.98, P > 0.05). However, the t test for CTXwD vs. controls was significant (P < 0.001). When single‐step and multiple step saccades were considered separately (Table 3 and Fig. 4 F), the main effects of number of steps (F (1,299) = 186.38, P < 0.01) and diagnosis (F (2,299) = 3.66, P < 0.05) were both significant, as was the interaction term (F (2,299) = 6.79, P < 0.01). The precision of single‐step vs. multistep saccades was worse in CTXwD (t test, P < 0.05). The precision of single‐step saccades in CTXwD was also worse than in controls (t test, P < 0.01, Fig. 4 F).
Frequency and number of steps of multistep saccades
The percentage of occurrence of multistep horizontal saccades was significantly higher in both CTX groups than controls (CTXwD = 45% of trials, CTXnD = 65%, Controls = 37%; multiple comparisons: CTXwD vs. controls, P < 0.001; CTXnD vs. controls, P < 0.001, CTXwD vs. CTXnD, P > 0.05, F (6,5144) = 3.11, P < 0.001). Moreover, the number of steps in multistep saccades was increased in both CTX groups compared to controls; indeed, among the total number of multistep saccades, the percentage of multistep saccades with more than two steps was 11.5% in controls; 28.5% in CTXnD; 26% in CTXwD (CTXwD vs. controls P < 0.01, CTXnD vs. controls P < 0.01). Both CTX groups also presented an increased percentage of vertical multistep saccades (CTXwD = 45%, CTXnD = 61%, Controls = 37%, CTXwD vs. controls P < 0.05, CTXwD vs. CTXnD P > 0.05, CTXnD vs. controls P > 0.05).
Antisaccades
Latency
In CTXwD, the average latency of correctly executed antisaccades was much longer than in CTXnD and controls (Table 4 and Fig. 5 A). In CTXwD and controls the latency of error saccades was much shorter than that of correct antisaccades (t test, P < 0.001).
Table 4.
Parameters of horizontal antisaccades
| Horizontal antisaccade parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| Latency (ms) | Gain | Standard deviation (precision−1) | Percentage (%) | |||||
| Group | Erroneous prosaccade | Correct antisaccade | Erroneous prosaccade | Correct antisaccade | Erroneous prosaccade | Correct antisaccade | Errors | Corrections |
| CTXwD | 209 ± 14 (10) | 558 ± 174 (6) | 0.90 ± 0.15 (10) | 1.01 ± 0.44 (9) | 0.21 ± 0.06 (10) | 0.21 ± 0.22 (9) | 74 ± 7 (10) | 19 ± 6 (10) |
| CTXnD | 208 ± 70 (6) | 266 ± 68 (6) | 0.84 ± 0.13 (6) | 0.94 ± 0.15 (6) | 0.17 ± 0.08 (6) | 0.30 ± 0.10 (6) | 46 ± 10 (6) | 42 ± 9 (6) |
| Ctrl | 188 ± 31 (17) | 264 ± 38 (19) | 0.79 ± 0.11 (17) | 0.96 ± 0.23 (19) | 0.16 ± 0.09 (17) | 0.29 ± 0.08 (19) | 18 ± 3 (19) | 43 ± 4 (17) |
Ctrl, control subjects, CTXwD, patients with dentate involvements, CTXnD, patients without dentate involvements. Values are means ± standard errors of the mean (number of subjects) for pooled single‐step and multistep saccades.
Figure 5. Latency, percentage of directional errors and corrections in the antisaccades task.
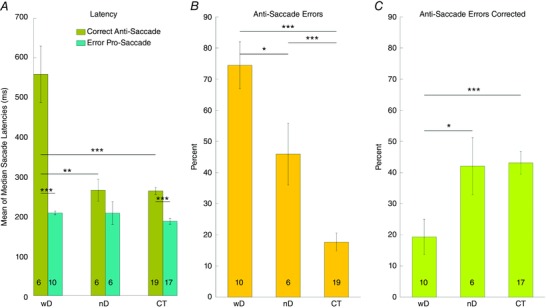
A, bar graphs of the mean latencies of correct antisaccades (olive) and erroneous prosaccades directed towards the target (teal) in CTXwD, CTXnD and CT. B, percentage of antisaccade errors (i.e. erroneous prosaccades). C, percentage of corrections after erroneous prosaccades (i.e. secondary corrective antisaccades). Asterisks indicate the significant differences among groups (P < 0.05). [Color figure can be viewed at wileyonlinelibrary.com]
Error rate
Table 4 and Fig. 5 B and C show the percentages of antisaccade errors and corrections. CTX patients showed a much higher error rate compared to controls (t test, P < 0.001). Subjects with DN involvement made significantly more errors (P < 0.05) and corrected less often (P < 0.05) than CTXnD and controls. Although CTXnD made significantly more errors than controls, they corrected just as often.
Gain and precision
Gain and precision of correctly executed antisaccades, and error prosaccades, were not different among the three groups (Table 4).
Disability scores and dentate involvement score
No significant correlation was found among saccade latency, gain or precision with MRS, EDSS, and qualitative dentate involvement scores (P > 0.05). Lack of correlation might be due to the subjective nature of the evaluation of these scales, the limited number of the items assessed and the limited range of scores.
Brain MRI volumes
Brain MRI (Table 1) showed significantly reduced normalized brain, grey matter and cortical volumes in CTXwD compared to CTXnD and controls (P < 0.001). No difference was found between CTXnD and controls. No differences were found when comparing white matter volumes among CTXwD, CTXnD, and controls. We infer from these results and lack of clinical symptoms of extra‐cortical grey matter impairment that the significant brain and grey matter volume reduction that we have observed is due to cortical atrophy. Although the cortex was globally reduced, individual areas (frontal, parietal, occipital, insula and cerebellar volumes) tended to be smaller, but this reduction did not reach significance. To exclude the possibility that the saccadic abnormalities in CTXwD were associated with (and thus more likely due to) atrophy in specific brain areas other than the DN we looked at the correlations between the main significantly abnormal saccadic parameters (single step saccade latency, gain and precision) and volumes of different areas in CTXwD. We did not find any significant correlation (P > 0.05).
Discussion
In this study, for the first time, we have quantified and compared saccadic parameters and brain MRI volumes of a cohort of CTX patients. Our study indicates that CTX patients executed normally accurate saccades with normal main sequence relationships; they made more frequent multistep saccades and directional errors during the antisaccade task than controls. Moreover, CTX with DN damage showed increased latency of both reflexive and voluntary saccades, normal accuracy but impaired precision of eye movements, and increased directional errors not followed by corrections, independently of disability scores. Although patients with DN involvement showed greater global brain and cortical atrophy than the other groups, no significant correlation was found among measures of brain volumes and these specific eye movement features, which could then help in understanding the contribution of DN to oculomotion.
All CTX patients showed normally accurate (gain close to 1) and fast saccades, thus indicating that in CTX, the medial cerebellum, superior colliculus and brainstem, involved in the generation of reflexive saccades, are macroscopically and functionally spared.
Also, all CTX patients made more multistep saccades and significantly more directional errors than normal in the antisaccade task. Abnormally frequent or fragmented multistep saccades may occur in neurodegenerative diseases (Optican et al. 2008; Blekher et al. 2009), but also in healthy subjects. Multistep saccades with shorter latencies than single‐step saccades have been observed in young subjects, possibly reflecting an immature development of cortical frontal areas that inhibit subcortical saccade‐generating structures (van Donkelaar et al. 2007). Thus, multistep saccades in CTX could indicate a facilitation of more automatic eye movements due to reduced control by an impaired frontal cortex. Analogously, antisaccade directional errors in CTX may represent automatic reflexive eye movements rather than erroneously planned saccades (Bowling et al. 2012); indeed, they had, in all subjects, shorter latencies than correct antisaccades, with values similar to those of multistep saccades. Therefore, both increased frequency of multistep saccades and antisaccadic directional errors point towards impaired inhibition by frontal areas in CTX. An increased rate of premature reflexive saccades follows FEF inactivation (Dias & Segraves, 1999), and inhibitory control of reflexive saccades is impaired after lesions of FEF and dorsolateral prefrontal cortex (DLPFC) (Pierrot‐Deseilligny et al. 2003; Ploner et al. 2005). Neural activity controlling the correct antisaccade planning instead of a reflexive prosaccade execution is also seen in supplementary eye field (SEF), pre‐supplementary motor area (pre‐SMA) and anterior cingulate cortex (ACC) of medial frontal cortex. These areas have a well‐defined role in monitoring the context‐dependent control of the movement, inhibition of reflexive behaviour and conflict resolution respectively (Amador et al. 2004; Stuphorn, 2015; Rushworth et al. 2007). Diffuse cortical atrophy, including the frontal areas, is observed during the evolution of neurodegeneration in CTX. In our study, however, we found significantly reduced normalized brain, grey matter and cortical volumes only in CTXwD. Thus, in CTXwD the increased global cortical atrophy might have contributed to the higher frequency of directional errors in the antisaccade task. Moreover, although each single cortical area was slightly thinner in CTXwD than CTXnD and controls, the differences did not reach the level of significance. Thus, while we could not identify a single cortical area in which atrophy was more prominent in our patients, we cannot rule out a functional impairment that might have affected some regions more than others as for example the frontal lobes. Finally, the mild global cognitive and mood disturbances shown by our patients might also have accounted for the increased antisaccade error rate.
In our study, a role of DN in saccade motor planning within a network involving the frontal cerebral cortex is suggested by the saccadic behaviour observed in CTXwD. Anatomically, the DN can be divided into two parts, supposed to participate in dissociable functional loops (Salmi et al. 2010): a dorso‐rostral ‘motor’ portion projecting to the pre‐motor and motor cortex, and a ventro‐caudal ‘cognitive’ portion, connected with frontal and parietal associative areas, including oculomotor regions. In non‐human primates, the ventral DN projects through the thalamus to prefrontal areas including the dorsolateral prefrontal cortex (DLPFC), the frontal and supplementary eye fields (FEF and SEF), the medial and lateral intraparietal areas (MIP and LIP), and to the SC and the oculomotor nucleus (Middleton & Strick, 2001; Dum & Strick, 2003; May, 2006; Glickstein et al. 2011). Phylogenetically, the ventral DN has evolved in parallel with the cerebellar hemispheres and the frontal associative cortex (Balsters et al. 2010). Ventral DN activity has been related to higher cognitive functions that are typically associated with the fronto‐parietal cortex (Dum & Strick, 2003; Prevosto et al. 2010; Salmi et al. 2010; Kuper et al. 2011).
Several studies suggest that the lateral cerebellum is actually involved in motor planning and timing of saccades, and visuospatial abilities (Voogd et al. 2012). In monkeys, visually triggered saccade‐related neural activity in the cerebellar hemispheres has been reported (Mano et al. 1996; Miles et al. 2006). Lesions of left cerebellar hemisphere in monkeys has led to increased saccade latency and trial‐to‐trial amplitude variability, but not to dysmetria, contrary to what is usually found in lesions of the oculomotor vermis (Ohki et al. 2008). DN activity has also been correlated with saccade initiation and direction, with different time courses for self‐timed saccades and visually guided saccades (Ashmore & Sommer, 2013). Recently, inactivation of DN nuclei in monkeys has been associated with increased erroneous prosaccades in the antisaccade trial, while successful antisaccades during inactivation tended to have a shorter latency, indicating a possible regulation of antisaccade generation by DN through the pathways to the frontal cortex (Kunimatsu et al. 2016).
However, DN show striking expansion and changes in neuronal organization in humans with respect to other primates, thus conclusions from animal studies might not easily translate to humans. Functional brain MRI studies on healthy subjects showed activation of the cerebellar hemispheres and/or DN during various oculomotor and visuospatial tasks (Dieterich et al. 2000; Schraa‐Tam et al. 2009; Kuper et al. 2011; Gerardin et al. 2012). Lateral cerebellar lesions have been inconsistently associated with impaired saccadic adaptation (Straube et al. 2001; Panouilleres et al. 2012, 2013), dysmetria (Straube et al. 2001; Filippopulos et al. 2013; Panouilleres et al. 2013), increased saccadic latency, impaired saccade sequencing (Filippopulos et al. 2013). Finally, very few human studies have focused on eye movement in lesions involving predominantly the DN. In SCA20, a spinocerebellar ataxia with DN calcifications, saccades were qualitatively reported as hypermetric (Knight et al. 2004). In two patients with olivo‐dentato‐rubral system degeneration, saccades showed increased latency and reduced velocity and amplitude (Wiest et al. 1995). Thus, while an involvement of DN in oculomotion seems well supported, its role remains unclear.
In our study we found that patients with DN damage showed higher latency of both reflexive and voluntary saccades, impaired precision and greater directional errors not followed by corrections in antisaccades than either CTXnD or controls. A longer latency could reflect longer processing of the visual stimulus and/or longer motor planning after the stimulus is identified. In CTX, the underlying metabolic defect leads to intraocular and visual pathway abnormalities that could slow the processing of the visual stimulus (Cruysberg et al. 1995; Dotti et al. 2001). However, only CTXwD had longer latencies, which are better explained by longer motor planning required to execute saccades.
An increase in saccade latency of reflexive and voluntary saccades was also observed in PEF and FEF dysfunctions, respectively (Dias & Segraves, 1999; Noudoost et al. 2014). Moreover, CTXwD patients corrected their errors less frequently than controls or CTXnD subjects. Uncorrected antisaccade errors could be caused by loss of antisaccade planning (Bowling et al. 2012), as also observed in FEF lesions (Boxer et al. 2012).
DN damage was also associated with less precise saccades in CTX patients. Frontal areas with which the DN are connected seem to have a role in modulating saccade amplitude. Discharge of FEF neurons correlates with saccade amplitude and direction; however, it does not dynamically encode the motor error of visually guided movements, but is supposed to store the location of the movement end‐point, as computed by the parietal lobes, and use it for non‐reflexive movements, which are indeed clearly hypometric after FEF lesions (Yang & Kapoula, 2011; Noudoost et al. 2014). Lesions of FEF increase the end‐point scatter of visually guided saccades, causing less precise saccades (Dias & Segraves, 1999; Noudoost et al. 2014).
However, the lack of correlation with regional cortical atrophy indicates that the neuronal loss in the frontal lobes cannot completely account for the observed saccadic abnormalities. In contrast, these abnormalities were correlated with DN degeneration, which suggests that DN and frontal cortex are strongly interconnected functionally.
Patients with CTXwD tended to have more cerebellar symptoms than those with CTXnD. However, there were no differences in the cerebellar volumes between the two groups. Neither did we find any correlations between cerebellar volume and saccade abnormalities. We hypothesize on the basis of our results that two networks contribute to select the target of a movement and define the amplitude of a saccade. One network, involving the medial cerebellum, would localize the target of a visually guided movement determining the accuracy of the saccade (which is the average of the movement end‐points). A second network, including the lateral cerebellum, would refine the selected location, increasing the precision of the movement (i.e. how scattered the end‐points are, by reducing the standard deviation of the end‐points).
In conclusion, our study shows that CTX patients have well‐spared saccadic dynamics, which indicates preserved medial cerebellum and brainstem activity. Patients with CTX also show a release of premature reflexive saccades instead of correct antisaccades, which could indicate a reduction in monitoring from cortical frontal areas of subcortical structures during the preparatory period.
DN could participate in oculomotor control through a circuit that is essential for executing precise, context‐appropriate, movements. DN damage may contribute to extended motor planning, leading to the execution of accurate, but imprecise, saccades and more frequent, and uncorrected, errors in antisaccades.
Additional information
Competing interests
The authors have no conflicts of interest to report.
Author contributions
F.R., E.P. and A.R: conception and design of the work; acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content. A.M.: acquisition, interpretation of clinical data for the work; revising the work critically for important intellectual content. L.M.O,: analysis and interpretation of data for the work; drafting the work and revising it critically for important intellectual content. V.S.: analysis and interpretation of data for the work; drafting the work. N.D.S.: acquisition and analysis of data for the wok; revising the work critically for important intellectual content. M.B.: analysis and interpretation of data for the work; revising the work critically for important intellectual content. L.M.: MRI acquisition, and interpretation of data for the work; revising the work critically for important intellectual content. M.T.D.: acquisition of data for the work; revising the work critically for important intellectual content. A.F.: interpretation of data for the work; revising the work critically for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was in part funded by the Intramural Research Program of NEI and EC FP7‐PEOPLE‐IRSES‐CERVISO 269263.
Acknowledgements
We thank the patients and their families for their participation in the study. We also thank Dr Susan Vitale (Division of Epidemiology and Clinical Applications, NEI, NIH) for statistical consulting.
F. Rosini and E. Pretegiani contributed equally to this paper.
References
- Amador N, Schlag‐Rey M & Schlag J (2004). Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. J Neurophysiol 91, 1672–1689. [DOI] [PubMed] [Google Scholar]
- Ashmore RC & Sommer MA (2013). Delay activity of saccade‐related neurons in the caudal dentate nucleus of the macaque cerebellum. J Neurophysiol 109, 2129–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters JH, Cussans E, Diedrichsen J, Phillips KA, Preuss TM, Rilling JK & Ramnani N (2010). Evolution of the cerebellar cortex: the selective expansion of prefrontal‐projecting cerebellar lobules. Neuroimage 49, 2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhof F, Verrips A, Wesseling P, van Der Knaap MS, van Engelen BG, Gabreels FJ, Keyser A, Wevers RA & Valk J (2000). Cerebrotendinous xanthomatosis: the spectrum of imaging findings and the correlation with neuropathologic findings. Radiology 217, 869–876. [DOI] [PubMed] [Google Scholar]
- Blekher T, Weaver M, Rupp J, Nichols WC, Hui SL, Gray J, Yee RD, Wojcieszek J & Foroud T (2009). Multiple step pattern as a biomarker in Parkinson disease. Parkinsonism Relat Disord 15, 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling AC, Hindman EA & Donnelly JF (2012). Prosaccade errors in the antisaccade task: differences between corrected and uncorrected errors and links to neuropsychological tests. Exp Brain Res 216, 169–179. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Garbutt S, Seeley WW, Jafari A, Heuer HW, Mirsky J, Hellmuth J, Trojanowski JQ, Huang E, DeArmond S, Neuhaus J & Miller BL (2012). Saccade abnormalities in autopsy‐confirmed frontotemporal lobar degeneration and Alzheimer disease. Arch Neurol 69, 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruysberg JR, Wevers RA, van Engelen BG, Pinckers A, van Spreeken A & Tolboom JJ (1995). Ocular and systemic manifestations of cerebrotendinous xanthomatosis. Am J Ophthalmol 120, 597–604. [DOI] [PubMed] [Google Scholar]
- Dias EC & Segraves MA (1999). Muscimol‐induced inactivation of monkey frontal eye field: effects on visually and memory‐guided saccades. J Neurophysiol 81, 2191–2214. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Bucher SF, Seelos KC & Brandt T (2000). Cerebellar activation during optokinetic stimulation and saccades. Neurology 54, 148–155. [DOI] [PubMed] [Google Scholar]
- Dotti MT, Rufa A & Federico A (2001). Cerebrotendinous xanthomatosis: heterogeneity of clinical phenotype with evidence of previously undescribed ophthalmological findings. J Inherit Metab Dis 24, 696–706. [DOI] [PubMed] [Google Scholar]
- Dum RP & Strick PL (2003). An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol 89, 634–639. [DOI] [PubMed] [Google Scholar]
- Federico A & Dotti MT (2003). Cerebrotendinous xanthomatosis: clinical manifestations, diagnostic criteria, pathogenesis, and therapy. J Child Neurol 18, 633–638. [DOI] [PubMed] [Google Scholar]
- Filippopulos F, Eggert T & Straube A (2013). Effects of cerebellar infarcts on cortical processing of saccades. J Neurol 260, 805–814. [DOI] [PubMed] [Google Scholar]
- Gerardin P, Miquee A, Urquizar C & Pelisson D (2012). Functional activation of the cerebral cortex related to sensorimotor adaptation of reactive and voluntary saccades. Neuroimage 61, 1100–1112. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Sultan F & Voogd J (2011). Functional localization in the cerebellum. Yale J Biol Med 47, 59–80. [DOI] [PubMed] [Google Scholar]
- Guerrera S, Stromillo ML, Mignarri A, Battaglini M, Marino S, Di Perri C, Federico A, Dotti MT & De Stefano N (2010). Clinical relevance of brain volume changes in patients with cerebrotendinous xanthomatosis. J Neurol Neurosurg Psychiatry 81, 1189–1193. [DOI] [PubMed] [Google Scholar]
- Knight MA, Gardner RJ, Bahlo M, Matsuura T, Dixon JA, Forrest SM & Storey E (2004). Dominantly inherited ataxia and dysphonia with dentate calcification: spinocerebellar ataxia type 20. Brain 127, 1172–1181. [DOI] [PubMed] [Google Scholar]
- Kunimatsu J, Suzuki TW & Tanaka M (2016). Implications of lateral cerebellum in proactive control of saccades. J Neurosci 36, 7066–7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper M, Dimitrova A, Thurling M, Maderwald S, Roths J, Elles HG, Gizewski ER, Ladd ME, Diedrichsen J & Timmann D (2011). Evidence for a motor and a non‐motor domain in the human dentate nucleus – an fMRI study. Neuroimage 54, 2612–2622. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF ( 1983). Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33, 1444–1452. [DOI] [PubMed] [Google Scholar]
- Leigh RJ & Zee DS (2015). The Neurology of Eye Movements. Oxford University Press, Oxford. [Google Scholar]
- Mano N, Ito Y & Shibutani H (1996). Context dependent discharge characteristics of saccade‐related Purkinje cells in the cerebellar hemispheres of the monkey. Prog Brain Res 112, 423–430. [DOI] [PubMed] [Google Scholar]
- May PJ ( 2006). The mammalian superior colliculus: laminar structure and connections. Prog Brain Res 151, 321–378. [DOI] [PubMed] [Google Scholar]
- Middleton FA & Strick PL (2001). Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 21, 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignarri A, Gallus GN, Dotti MT & Federico A (2014). A suspicion index for early diagnosis and treatment of cerebrotendinous xanthomatosis. J Inherit Metab Dis 37, 421–429. [DOI] [PubMed] [Google Scholar]
- Miles OB, Cerminara NL & Marple‐Horvat DE (2006). Purkinje cells in the lateral cerebellum of the cat encode visual events and target motion during visually guided reaching. J Physiol 571, 619–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H (1991). Cerebellar control of saccadic eye movements: its neural mechanisms and pathways. Jpn J Physiol 41, 351–368. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Clark KL & Moore T (2014). A distinct contribution of the frontal eye field to the visual representation of saccadic targets. J Neurosci 34, 3687–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki M, Kitazawa H, Hiramatsu T, Kaga K, Kitamura T, Yamada J & Nagao S (2008). Role of primate cerebellar hemisphere in voluntary eye movement control revealed by lesion effects. J Neurophysiol 101, 934–947. [DOI] [PubMed] [Google Scholar]
- Optican LM, Rucker JC, Keller EL & Leigh RJ (2008). Mechanism of interrupted saccades in patients with late‐onset Tay‐Sachs disease. Prog Brain Res 171, 567–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panouilleres M, Alahyane N, Urquizar C, Salemme R, Nighoghossian N, Gaymard B, Tilikete C & Pelisson D (2013). Effects of structural and functional cerebellar lesions on sensorimotor adaptation of saccades. Exp Brain Res 231, 1–11. [DOI] [PubMed] [Google Scholar]
- Panouilleres M, Neggers SF, Gutteling TP, Salemme R, van der Stigchel S, van der Geest JN, Frens MA & Pelisson D (2012). Transcranial magnetic stimulation and motor plasticity in human lateral cerebellum: dual effect on saccadic adaptation. Hum Brain Mapp 33, 1512–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot‐Deseilligny C, Muri RM, Ploner CJ, Gaymard B & Rivaud‐Pechoux S (2003). Cortical control of ocular saccades in humans: a model for motricity. Prog Brain Res 142, 3–17. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard BM, Rivaud‐Pechoux S & Pierrot‐Deseilligny C (2005). The prefrontal substrate of reflexive saccade inhibition in humans. Biol Psychiatry 57, 1159–1165. [DOI] [PubMed] [Google Scholar]
- Prevosto V, Graf W & Ugolini G (2010). Cerebellar inputs to intraparietal cortex areas LIP and MIP: functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb Cortex 20, 214–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JL (1999). The bootstrap, the jackknife, and the randomization test: A sampling taxonomy. Multivariate Behav Res 34, 441–456. [DOI] [PubMed] [Google Scholar]
- Ron S & Robinson DA (1973). Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol 36, 1004–1022. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME & Bannerman DM (2007). Functional organization of the medial frontal cortex. Curr Opin Neurobiol 17, 220–227. [DOI] [PubMed] [Google Scholar]
- Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O & Carlson S (2010). Cognitive and motor loops of the human cerebro‐cerebellar system. J Cogn Neurosci 22, 2663–2676. [DOI] [PubMed] [Google Scholar]
- Schraa‐Tam CK, van Broekhoven P, van der Geest JN, Frens MA, Smits M & van der Lugt A (2009). Cortical and cerebellar activation induced by reflexive and voluntary saccades. Exp Brain Res 192, 175–187. [DOI] [PubMed] [Google Scholar]
- Straube A, Deubel H, Ditterich J & Eggert T (2001). Cerebellar lesions impair rapid saccade amplitude adaptation. Neurology 57, 2105–2108. [DOI] [PubMed] [Google Scholar]
- Stuphorn V (2015). The role of supplementary eye field in goal-directed behavior. J Physiol Paris 109, 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar P, Saavedra S & Woollacott M (2007). Multiple saccades are more automatic than single saccades. J Neurophysiol 97, 3148–3151. [DOI] [PubMed] [Google Scholar]
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ & van Gijn J (1988). Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19, 604–607. [DOI] [PubMed] [Google Scholar]
- Voogd J, Schraa‐Tam CK, van der Geest JN & De Zeeuw CI (2012). Visuomotor cerebellum in human and nonhuman primates. Cerebellum 11, 392–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest G, Mueller C, Wessely P, Steinhoff N, Trattnig S & Deecke L (1995). Oculomotor abnormalities in Dyssynergia cerebellaris myoclonica. Acta Otolaryngol Suppl 520, 392–394. [PubMed] [Google Scholar]
- Yang Q & Kapoula Z (2011). Distinct control of initiation and metrics of memory‐guided saccades and vergence by the FEF: a TMS study. PloS One 6, e20322. [DOI] [PMC free article] [PubMed] [Google Scholar]


