Abstract
Key points
Chloroquine (CQ) stimulates itch nerves and causes intense scratching in mice by activating the G‐protein coupled receptor (GPCR) MrgprA3; it is not known how stimulation of MrgprA3 (or other GPCRs) leads to activation of the itch nerve terminals in the skin, but previous studies have found that transient receptor potential A1 (TRPA1) gene deletion blocks CQ‐induced scratching.
In the present study we used a novel dorsal skin–nerve preparation to evaluate mechanisms underlying CQ‐ and histamine‐induced action potential discharge in itch nerve terminals.
We found that CQ activation of the nerves requires the beta3 isoform of phospholipase C, but TRPA1 or other TRP channel are not required.
Evidence is provided for a role for calcium‐activated chloride channels such as TMEM16a in GPCR‐activation of itch nerve terminals.
The mechanism by which TRP channels participate in pruritogen‐induced scratching may involve sites of action other than the primary afferent terminals.
Abstract
Chloroquine (CQ) and histamine are pruritogens commonly used to study itch in the mouse. A novel skin–nerve preparation was used to evaluate chloroquine (CQ)‐ and histamine‐induced activation of afferent nerves in the dorsal thoracic skin of the mouse. All CQ sensitive nerves were C‐fibres, and were also sensitive to histamine. The response to CQ, but not histamine, was largely absent in mrgpr‐cluster Δ−/− mice, supporting the hypothesis that CQ evokes itch largely via stimulation of MrgprA3 receptors. The CQ‐induced action potential discharge was largely absent in phospholipase Cβ3 knockout animals. The CQ and histamine responses were not influenced by removal of TRPA1, TRPV1, TRPC3 or TRPC6, nor by the TRP channel blocker Ruthenium Red. The bouts of scratching in response to CQ were not different between wild‐type and TRPA1‐deficient mice. A selective inhibitor of the calcium‐activated chloride channel TMEM16A, N‐((4‐methoxy)‐2‐naphthyl)‐5‐nitroanthranilic acid (MONNA), inhibited CQ‐induced action potential discharge at itch nerve terminals and bouts of scratching by about 50%. Although TRPA1 and TRPV1 channels may be involved in the scratching responses to intradermal pruritogens, this is unlikely to be due to an effect at the nerve terminals, where chloride channels may play a more important role.
Keywords: C‐fibers, chemosensitivity, sensory nerves, TRP channels
Key points
Chloroquine (CQ) stimulates itch nerves and causes intense scratching in mice by activating the G‐protein coupled receptor (GPCR) MrgprA3; it is not known how stimulation of MrgprA3 (or other GPCRs) leads to activation of the itch nerve terminals in the skin, but previous studies have found that transient receptor potential A1 (TRPA1) gene deletion blocks CQ‐induced scratching.
In the present study we used a novel dorsal skin–nerve preparation to evaluate mechanisms underlying CQ‐ and histamine‐induced action potential discharge in itch nerve terminals.
We found that CQ activation of the nerves requires the beta3 isoform of phospholipase C, but TRPA1 or other TRP channel are not required.
Evidence is provided for a role for calcium‐activated chloride channels such as TMEM16a in GPCR‐activation of itch nerve terminals.
The mechanism by which TRP channels participate in pruritogen‐induced scratching may involve sites of action other than the primary afferent terminals.
Abbreviations
- AITC
allyl isothiocyanate
- CQ
chloroquine
- GPCR
G‐protein coupled receptor
- KO
knockout
- Mrgpr
Mas‐related G‐protein coupled receptor
- PLC
phospholipase C
- TMEM16a
transmembrane member 16A
- TRP
transient receptor potential
Introduction
Recent studies have identified a major itch‐causing nerve in mouse skin as one defined by the expression of MrgprA3, a GPCR that when selectively activated by an agonist, e.g. chloroquine (CQ), causes intense scratching behaviour (Liu et al. 2009). The MrgprA3‐expressing neurons comprise only about 4–6% of DRG neurons. Liu et al. revealed that these neurons are a subset of TRPV1‐ and histamine H1 receptor‐expressing neurons. Selectively deleting the MrgprA3‐expressing neurons using Cre‐mediated diphtheria toxin receptor methods largely inhibited scratching in response to MrgprA3 agonists, and also inhibited scratching in response to other pruritogens including histamine, 5‐HT and those released by mast cell activation, as well as the scratching associated with contact dermatitis (Han et al. 2013). This indicates that the small subset of MrgprA3‐expressing, CQ‐sensing DRG nerves represent a major nerve subtype responsible for acute itch in the mouse.
Much of the work on the neurobiology underlying acute itch in mice has relied on behavioural assays in which bouts of scratching are quantified following injection of a pruritic substance intradermally into the nape of the neck or cheek pouch (McNeil & Dong, 2012; Akiyama & Carstens, 2013; Bautista et al. 2014; LaMotte et al. 2014; Ringkamp & Meyer, 2014). Depending on the pruritogen, acute bouts of scratching behaviour have been inhibited by TRPA1 and TRPV1 inhibitors, and reduced or even abolished in mice that have TRPA1 or TRPV1 genetically deleted. Such studies have led to the conclusion that TRPA1, and in some cases TRPV1, is essential in the itch pathways initiated by GPCR agonists like CQ and histamine (Imamachi et al. 2009; Wilson et al. 2011; Wilson & Bautista, 2014).
The general inference from these results is that TRPA1 or TRPV1 contribute to itch by acting as the cation channel that is opened downstream from GPCR signal transduction, leading to action potential discharge in the itch nerve terminals. This supports the strategy of developing peripherally acting TRPA1/TRPV1 blockers for the treatment of pathological itch. However, this hypothesis has not yet been rigorously addressed experimentally. It is possible that the TRP channels involved in the itch–scratch pathway play a role at the level of the spinal cord or in the brain in addition to, or rather than at, the primary itch nerve terminals. With respect to the brain it is worth noting that trpa1 −/− mice, or mice receiving TRPA1 antagonists, have strong behavioural abnormalities such as decreased immobility in forced swim tests, and in general behave similarly to mice on large doses of anxiolytics and antidepressants (de Moura et al. 2014). Mice lacking TRPV1 express similar behavioural abnormalities (Aguiar et al. 2014). It is possible that these alterations in behaviour contribute to the decrease in scratching associated with acute administration of a pruritogen. Indeed, antidepressants have been reported to have antipruritic activity in humans (Yosipovitch & Bernhard, 2013).
In this investigation, experiments were designed in which mouse skin was isolated along with the attending spinal nerves and DRG. Extracellular recordings from cell bodies of single DRG nerve fibres that terminate in the skin were carried out to characterize the CQ‐ and histamine‐sensitive nerve population in the dorsal thoracic skin, and to quantify the action potential discharge in response to these pruritogens. We conclude that CQ and histamine stimulate action potential discharge in the same subpopulation of cutaneous C‐fibre terminals, and that these nerves comprise about 25% of the C‐fibre population in the dorsal skin. Activation of these nerve terminals by CQ or histamine occurs independently of the presence TRPA1, TRPV1, TRPC3, TRPC6, or other Ruthenium Red‐sensitive cation channels.
Methods
Animals
All experiments carried out were approved by The Johns Hopkins Animal Care and Use Committee, and conform to The Journal’s principles and regulations, as described in Grundy (2015). Male C57BL/6J, trpa1 −/−, trpv1 −/−, trpa1/trpv1 −/−, and trpc3/trpc6−/− mice weighing 22–27 g were used. The trpa1 −/− and trpv1 −/− strains were obtained from Jackson's Laboratory. The trpa1/trpv1 −/− and mrgpr‐cluster Δ−/− animals were obtained by mating these homozygous mice in Xingzhong Dong's Laboratory. The trpc3/trpc6 −/− mice were obtained from the laboratory of Dr D. Kass (Johns Hopkins University) from a strain originally developed in the laboratory of Dr L. Birnbaumer (National Institutes of Environmental Health). The plcb3 −/− mice were developed in the laboratory of Dr M. Simon and provided by Dr K.‐W. Yau (Johns Hopkins University).
Sensitization
The allergen sensitization with ovalbumin was performed as described before (Potenzieri et al. 2012). Ovalbumin (75 μg, Sigma) with aluminum hydroxide gel (2.6 mg, Sigma) in sterile PBS (total volume 200 μl) was injected intraperitoneally three times (days 1, 3 and 5) and the mice were used for experiments at least 7 days after the last injection.
Ex vivo DRG nerve–skin preparation
The mouse was injected with heparin (5 IU g−1 in saline, i.p.) and killed 15 min later by CO2 inhalation and exsanguination. The hair from the right back and side was removed using an electric shaver. Right dorsal side skin (Fig. 1 A and B) was dissected out along with the blood vessels, namely the right subscapular artery (a branch from the right axillary artery that is distributed to right mouse back skin) and its branches. The right DRG nerves and ganglia (T7, T8, T9 or T10) were separated carefully from the connective tissue. The skin was then cut to a size of approximately 3 cm × 3 cm such that the branches of the subscapular artery were positioned in the centre of the preparation. The subcutaneous tissue except for the nerves and blood vessels was dissected out to facilitate the diffusion of chemicals. If present, the vessels other than the subscapular artery were ligated to prevent a rapid exit of chemicals infused into subscapular artery. The skin was gently stretched and pinned dermis side up in a Sylgard‐lined Perspex tissue chamber. The DRG ganglion (most often T9 or T8, occasionally T7 or T10) with the nerve was pulled through a small hole to an adjacent Sylgard‐lined recording chamber and the hole was sealed with Vaseline. The tissue and recording chamber were separately superfused with Krebs solution (composed of (mm): NaCl 118, KCl 5.4, NaH2PO4 1.0, MgSO4 1.2, CaCl2 1.9, NaHCO3 25.0, and dextrose 11.1, and gassed with 95% O2–5% CO2, pH 7.4) containing indomethacin (3 μm), at a flow rate of 4 ml min−1 (temperature 32–34oC) and 3 ml min−1 (36–37°C), respectively. It is possible that mechanically probing the isolated skin can lead to prostanoid production. Indomethacin was included to reduce the potential complicating factor of endogenous cyclooxygenase products sensitizing C‐fibres over time (Emery et al. 2016). For the intra‐arterial delivery of chemicals, the 10 mm long steel tip of a 33 gauge needle attached to PE‐10 tubing (OD 0.61 mm, ID 0.28 mm) was inserted into the right subscapular artery, the blood in the skin preparation was flushed with buffer, and the needle was secured with surgical silk.
Figure 1. Extracellular recording of single fibre nerve fibre activity in isolated DRG–skin preparation.
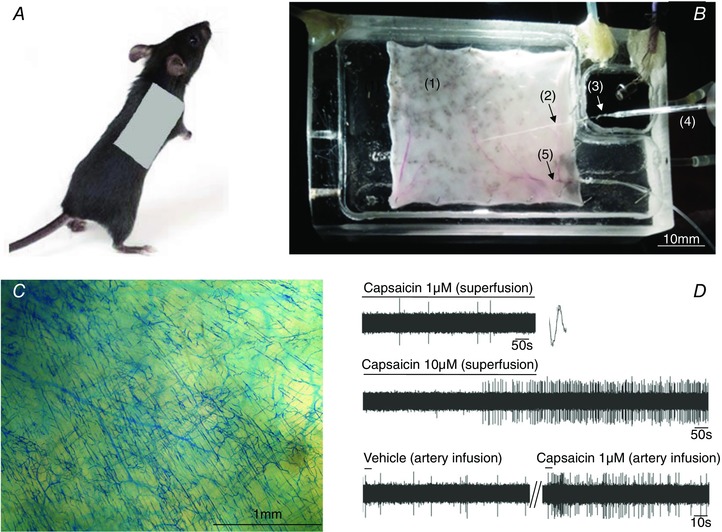
A, the location of skin used for preparation is shown on the right back side of the mouse. B, the DRG nerve–skin preparation. The skin (1) is pinned dermis (corium) side up in the tissue chamber, the T9 thoracic nerve (2) passes through a hole into the recording chamber and the T9 DRG (3) at the distal end of is pinned. Extracellular recording electrode (4) has the tip positioned in the DRG. The tissue and the recording chamber are separately superfused with warmed Krebs solution. Mechanical stimuli (von Frey hairs) are delivered to the dermis (corium) side of the skin. The drugs are delivered via cannulated right subscapular artery (5) connected to PE tubing. C, filling of the microvasculature following the infusion of Monastral Blue into the subscapular artery shows extensive distribution of the dye in the skin achieved by this route of drug delivery. D, an example showing the difference of DRG nerve fibre activation evoked by capsaicin delivered in superfusing fluid (upper two traces) and infused into subscapular artery (lower trace). The time to onset of action potential discharge was notably shorter and the magnitude of the response was higher when capsaicin was infused into subscapular artery. Note the large difference in the time scale between upper two traces and lower trace. [Color figure can be viewed at wileyonlinelibrary.com]
Extracellular recording of DRG fibres in the skin
Extracellular recordings were performed by using an aluminosilicate glass (OD 1.0 mm, ID 0.64 mm) electrode (pulled with a Flaming‐Brown micropipette puller, Sutter Instrument Company, Novade, CA, USA). The electrode was filled with 3 m sodium chloride and placed into an electrode holder connected directly to a headstage (A‐M Systems, Everett, WA, USA). A return electrode of silver–silver chloride wire and an earthed silver–silver chloride pellet were placed in the perfusion fluid of the recording chamber. The electrode resistance was approximately 2 MΩ. The recording signal was amplified (Microelectrode AC amplifier 1800, A‐M Systems) and filtered (low cut off, 0.3 kHz; high cut off, 1 kHz) and the resultant activity was displayed on an oscilloscope (TDS 3054B, Tektronix, Beaverton, OR, USA) and Apple computer. The data were stored and analysed by computer using the software TheNerveOfIt (PHOCIS, Baltimore, MD, USA). The action potential discharge in response to a stimulus was quantified for a 5 min period, by which time the response had generally ceased.
The skin was searched with a small concentric stimulating electrode. When the electrical stimulus evoked an action potential, the response to mechanical and chemical stimuli was quantified. Essentially every fibre stimulated with the non‐selective electrical stimulus was also mechanically sensitive. A mechanosensitive receptive field of a DRG fibre was identified when a punctate mechanical stimulus (von Frey hair 4.0 g) applied to the dermis surface of skin evoked a burst of action potentials. The fibre was only studied if the mechanically induced action potential discharge waveform could be clearly discriminated from background noise and other nerve activity. In rare instances two fibres with distinct action potential waveforms were recorded simultaneously. Conduction velocity was determined from electrical stimulation of the nerve trunk and/or mechanosensitive receptive field by dividing the approximate length of the nerve fibre by conduction time. The chemicals were applied via infusion into the subscapular artery (as described above) at a rate of 40 μl s−1 in a total volume of 200 μl. For every nerve studied, the vehicle was applied to ensure there was no artifactual response due to any change in mechanical force resulting from the injection. In >30 experiments we confirmed the accessibility of intraterially injected chemicals to mechanosensitive receptive filed by infusing Monastral Blue solution at the end of the experiment; in each case Monastral Blue was found saturating the receptive field (Fig. 1 C).
Antagonists were superfused over the tissue for at least 20 min prior to the application of CQ (or relevant stimulus), which was delivered in a solution that also contained the antagonist. To further ensure the antagonist was reaching the nerve terminals, it was also added intra‐arterially every 10 min as described above until the stimulus was applied.
With experience this preparation required ∼2 h to set up. The preparation was suitable for robust extracellular recordings of action potential in single C‐fibres for at least the ensuing 6 h.
Patch clamp recording
Neurons were dissociated from DRG (T7‐T10) isolated from MrgprA3‐tdTomato mice, cultured in medium and studied within 24 h. The amphotericin B‐perforated whole‐cell patch clamp technique was used to measure the membrane current, membrane potential and action potential discharge on tdTomato‐positive DRG neurons (∼5% of total) isolated from MrgprA3‐tdTomato mice. The latter were generated by mating the mice expressing MrgprA3‐Cre with mice expressing Rosa26‐loxP‐STOP‐loxP‐tdTomato. Pilot experiments showed that all tdTomato‐positive cells exhibit a robust AITC‐induced inward current at −60 mV (n = 18). In this study, only neurons with stable resting membrane potential were used. For voltage clamp experiments, the cells were held at −60 mV. The pipette solution contained (mm): KCl 40, potassium gluconate 105 and Hepes 10, pH 7.2. Amphotericin B (30 μg (100 μl)−1) was added to the pipette solution before experiments. The bath solution contained (mm): NaCl 135, KCl 5.4, MgCl2 1.0, CaCl2 1.5, Hepes 10 and glucose 10, pH 7.35. Chloroquine (1 mm) was dissolved in bath solution and applied to bath for 1–2 min. HC 030031 stock (30 mm) was prepared in DMSO, and applied to bath at 30 μm 5–10 min before application of chloroquine. All recordings were performed at room temperature. The recorded membrane potential was corrected offline for the junction potential (−13 mV).
Calcium assay
A conventional Fura‐2 based calcium assay was used to evaluate the efficacy of the trpa1 deletion in DRG neurons isolated from trpa1 −/− mice.
Behaviour study
The extent of itch in response to a pruitogen was inferred from the number of bouts of scratching as previously described (Liu et al. 2009). Mice were put in an aluminum foil enwrapped Plexiglass chamber. In front of the chamber was a video camera focused on a mirror beneath the chamber. The mice were unaware of the camera. Vehicle, or CQ were subcutaneously injected into the nape of the neck after acclimatization, and scratching behaviour was quantified for 30 min. We evaluated wild‐type with trpa1 −/− animals in a paired experimental design. The two chambers were positioned far enough part so the mice could not detect each other, in an unused laboratory in the absence of distractions. The data were subsequently quantified off‐line.
Drugs and chemicals
Chloroquine, histamine and Ruthenium Red were dissolved in distilled water (stock solution 10 mm). Capsaicin was dissolved in ethanol (stock solution 10 mm). AITC, HC‐030031, GSK2332255B, N‐((4‐methoxy)‐2‐naphthyl)‐5‐nitroanthranilic acid (MONNA) and gallein were dissolved in DMSO (stock solutions 1 m, 100 mm, 10 mm, 100 mm and 30 mm, respectively). All stock solutions were stored at −20oC and the drugs were diluted in Krebs solution to their final concentrations on the day of use. Ovalbumin for extracellular studies was directly dissolved in Krebs solution to 1 mg ml−1. All drugs were purchased from Sigma‐Aldrich except for GSK255B obtained from GSK and gallein was purchased from Tocris Bioscience.
Data analysis
The data are presented as the means ± SEM. The peak frequency of the response to stimuli is defined as the largest number of action potentials in any 1 s bin. The total number of action potentials was determined as the number of spikes occurring during 2 min after the first spike, with the exception of OVA where the spikes were counted for 10 min. By the end of these time periods the action potential discharge had returned to baseline.
The scratching behaviour was analysed after the video was downloaded onto a computer and quantified as the number of scratching bouts during the 30 min period post injection of CQ or vehicle. Student's paired and non‐paired tests were used as appropriate. The Fisher exact test was used to compare the percentage of drug‐responsive fibres between the transgenic and wild‐type mice. P values of <0.05 were considered significant.
The effect of CQ on evoking action potentials was quantified as peak frequency (Hz) and total number of spikes elicited. We carried out 228 experiments and learned that 26% of the nerve fibres responded to CQ.
The number of positive fibres evaluated was based on the coefficient of variation of the response in wild‐type animals. The coefficient of variation was 7.2% for action potential discharge frequency, so to obtain meaningful statistical analysis we carried out a sufficient number of experiments until >7 positive itch fibres were evaluated. For pharmacological analyses the data were evaluated in a paired fashion (e.g. the response of the same nerve before and after treatment). The hypothesis here is that if the ion channel blocked is the key channel for the generator potential, the drug should essentially abolish the response to CQ. Because there is no desensitization to the CQ response we were able to prove a lack of effect on CQ‐induced responses after ∼5 experiments.
Results
Intrarterial injections in a skin–nerve preparation
An electrical stimulus was used to search for single afferent nerve receptive fields in the isolated skin. We did not find many examples of ‘silent nociceptors’ in this region of the skin. For example, in the last 100 consecutive experiments, all but 9 fibres could be activated by a mechanical force applied to the mechanical receptive field (generally <2.5 mm2) with a von Frey fibre (4.0 g). Moreover, the mechanical probing was cursory to reduce damage to the preparation, so it is possible that we failed to probe precisely the receptive field of the nominally mechanically insensitive fibres. Chemical stimuli were superfused directly on to the field of the afferent nerve under study. When compared to C‐fibres in our airway–nerve preparations, where capsaicin produces immediate robust action potential discharge in a concentration range of 10 nm–1 μm (Kollarik, 2004), we noted that activation of C‐fibres in the skin with capsaicin required large agonist concentrations (10 μm), and the response inevitably had a marked delay (>400 s). We surmised that there is likely to be a substantive barrier in the underside of this region of the skin against rapid diffusion of superfused chemicals to the receptive field of the nerve. This barrier could be overcome with persistent application of a chemical, but because the rate at which a chemical stimulus reaches the terminal can influence the rate of the generator potential, and thus action potential initiation, we sought a method to bypass this barrier. By dissecting the right subscapular artery and its branches we were able to administer chemicals intra‐arterially (i.a.). Monastral Blue dye was routinely used to evaluate the vascular supply to the receptive field. When the dye was injected i.a. we noted immediate saturation of the receptive field (Fig. 1 C). When capsaicin was administered in this fashion it immediately (within 5 s, n = 76) activated the nerves, even at a concentration 10‐fold lower than needed when the drug was superfused (e.g. Fig. 1 D). Administration of vehicle i.a. was always included as a control and failed to evoke action potential discharge. We therefore evaluated the response of nerves to histamine, CQ, BAM8‐22, capsaicin and AITC delivered intra‐arterially to the terminals. The nature of the diffusion barrier was left unstudied but one might suspect that the layer of cutaneous maximus muscle that allows the skin to twitch may be involved (Pan et al. 2012). For a histological description of the potential diffusion barriers in the dorsal skin of a rodent, see Wells et al. (2010).
CQ vs. capsaicin sensitivity
All fibres that responded to CQ conducted action potentials in the C‐fibre range. A total of 228 C‐fibres were studied from 138 mice. We have previously reported that about 4% of neurons in the DRG per se are CQ sensitive (Liu et al. 2009). This population is, as expected, enriched when evaluating C‐fibre nerves specifically in the the skin. The CQ‐sensitive C‐fibres comprised 26% of the C‐fibre population. The average conduction velocity of CQ‐sensitive fibres was 0.75 ± 0.01 m s−1. This was slightly but statistically significantly (P < 0.05) slower than the conduction velocity of CQ‐insensitive C‐fibres, 0.83 ± 0.01 m s−1 (Fig. 2). The response to CQ was generally a burst of action potentials that at its peak averaged 12 ± 1 Hz. There was no desensitization between consecutive treatments of CQ on a given fibre when administered 30 min apart (P > 0.1 between response of first and second application).
Figure 2. The conduction velocity distribution of CQ‐sensitive skin DRG C‐fibres.
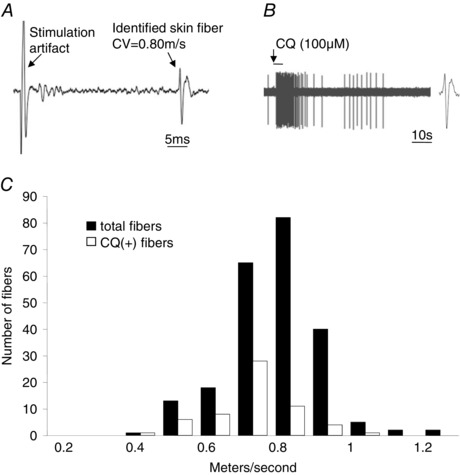
A, representative trace showing the electrical stimulation of the mechanosensitive receptive field. Conduction velocity was determined by dividing the approximate length of the nerve fibre by conduction time. B, representative trace showing the action potential discharge evoked in response to CQ delivered via artery infusion in the same fibre. Note identical action potential waveform in A and B. C, the conduction velocity distribution of CQ‐positive skin DRG C‐fibres (n = 59) and the total skin DRG C‐fibre population (n = 228).
Capsaicin sensitivity is often used as a marker of pain‐evoking C‐fibres. Among the CQ‐sensitive C‐fibres, the vast majority (73.5%) also responded to capsaicin. The capsaicin response, as quantified by the peak discharge frequency (peak number of spikes in any 1 s bin), was similar to that observed with CQ averaging, 15 ± 2 Hz. Capsaicin also stimulated CQ‐insensitive C‐fibres. We found that the capsaicin response was stronger in the CQ‐sensitive population than in the CQ‐insensitive population of C‐fibres, based on peak frequency of action potential discharge. The maximum capsaicin‐induced discharge in CQ‐insensitive C‐fibres averaged 6 ± 1 Hz (n = 41), which was significantly less than the 15 ± 2 Hz evoked in CQ‐sensitive C‐fibres (P < 0.05).
All CQ‐sensitive C‐ fibres (7 of 7) responded to the TRPA1 agonist AITC, but this required large concentrations (≥ 300 μm), and the peak frequency of the response was significantly weaker than that evoked by CQ or capsaicin, averaging only 4 ± 0.5 Hz (Fig. 3).
Figure 3. Quantification of the response to capsaicin, AITC, CQ, HA (histamine) and OVA in the skin DRG CQ‐positive C‐fibres in wild‐type mouse.
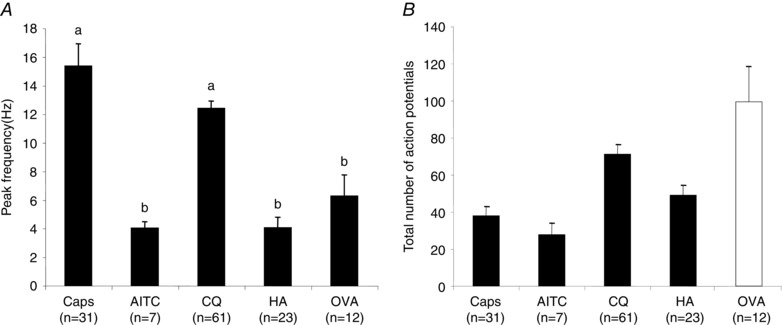
A, action potential peak frequency. B, the total number of action potentials. Capsaicin (1 μm), AITC (300 μm), CQ (100 μm) and HA (100 μm) were delivered by artery infusion and the response was recorded for 2 min. Among the CQ‐positive C‐fibres (n = 61), 31 of 40 fibres tested responded to capsaicin, all tested fibres responded to AITC (n = 7) and 23 of 25 responded to HA. Ovalbumin (OVA) was evaluated in sensitized mice. Ovalbumin (1 mg ml−1) was delivered by both arterial infusion and superfusion and the response was recorded for 30 min. Among the CQ‐positive C‐fibres 12 of 13 responded to OVA. The bars represent means ± SEM of number (n) of individual fibres studied (1 experiment per animal for OVA and capsaicin; 1–2 experiments per animal for other stimuli). An ANOVA followed by a Tukey Kramer test revealed a statistically greater peak frequency response for Caps and CQ (denoted with ‘a’) compared to the other stimuli (denoted with ‘b’).
Characterization of the CQ‐ and histamine‐sensitive nerves in skin
Histamine (100 μm) did not evoke as strong of a burst of action potentials as CQ, averaging only 4 ± 0.8 Hz at its peak (P < 0.05 compared to CQ), but the response was more persistent (Fig. 3 and representative tracing shown in Fig. 8 A). Every histamine‐sensitive fibre studied also responded to CQ and only 4 of 23 CQ‐sensitive fibres were histamine‐insensitive. In these few histamine‐insensitive fibres, CQ may have been acting independently of MrgprA3 (see below). Among 17 CQ‐insensitive C‐fibres, none responded to histamine. In other words, histamine and CQ sensitivity essentially defined the same C‐fibre subpopulation in the region of the skin we studied.
Figure 8. CQ, histamine and capsaicin‐induced stimulation of action potential discharge in C‐fibres innervating the skin of wild‐type and trpa1/trpv1 −/− double KO mice.
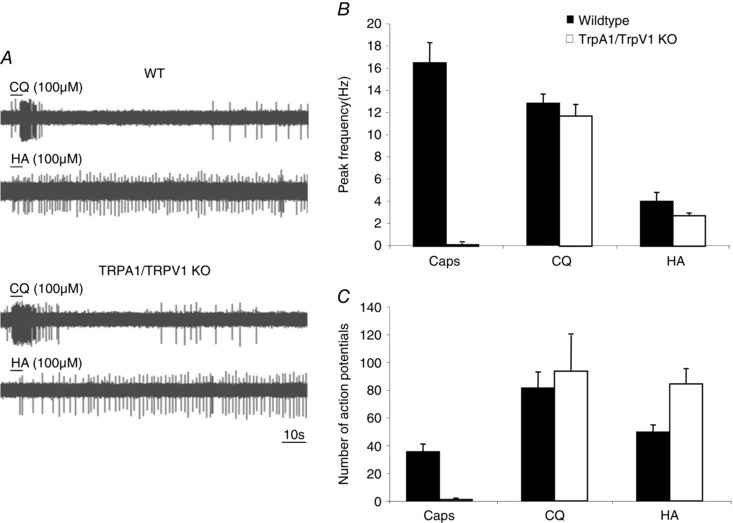
A, the pattern of response to CQ (the burst of action potentials with an onset within 10 s) was similar in the wild‐type and trpa1/trpv1 −/− mice. B and C, the peak frequency (B) and (C) the total number of action potentials evoked by capsaicin, CQ and HA in wild‐type (filled bars) and trpa1/trpv1 −/− mice. The data on wild‐type mice C‐fibres are taken from Fig. 3. In the trpa1/trpv1 −/− mice, 8 of 40 C‐fibres responded to CQ, which was not significantly different from the precentage of CQ‐sensitive fibres in wild‐type animals. Among the CQ‐positive trpa1/trpv1 −/− C‐fibres, 4 of 4 C‐fibres responded to histamine and 0 of 4 C‐fibres responded to capsaicin. The bars represent the mean ± SEM. The n values for responding nerves in wild‐type mice are as in Fig. 3; the n values for responding C‐fibres in the trpa1/trpv1 −/− skin are 4, 8 and 4 for caps, CQ, and HA, respectively (typically one nerve studied per animal).
Allergen response
We evaluated the activation of 42 skin C‐fibres by the allergen ovalbumin (OVA) in OVA‐sensitized mice (OVA has no effect on C‐fibres in non‐sensitized mice). OVA consistently evoked action potential discharge in 12 of 12 CQ‐sensitive C‐fibres, averaging 6 ± 2 Hz and 100 ± 19 action potentials over baseline (Fig. 3). In contrast, OVA modestly, if at all, stimulated action potentials in 30 CQ‐insensitive C‐fibres (1.6 ± 0.2 Hz, total spikes 23 ± 3; P < 0.01, compared to CQ‐sensitive fibres, data not shown).
Mrgpr
It has previously been noted that CQ stimulates DRG neurons via MrgprA3 (Liu et al. 2009). We evaluated 10 histamine‐responsive C‐fibres in mrgpr‐cluster Δ−/− mice. The response to histamine was not different from wild‐type mice (P > 0.1), but the response to CQ was substantially reduced (P < 0.01) (Fig. 4 A and B). Unlike studies in wild‐type animals where all histamine‐sensitive nerves responded strongly to CQ, only one of the 10 histamine‐sensitive nerves studied in mrgpr‐cluster Δ−/− mice responded strongly (243 action potentials) to CQ; two additional fibres responded with a small increase of action potential discharge over baseline (<30 action potentials, e.g. Fig. 4 A).
Figure 4. CQ and histamine (HA) stimulation in HA‐sensitive cutaneous C‐fibre in wild‐type (WT) compared to Mrgpr‐cluster Δ−/− mice.
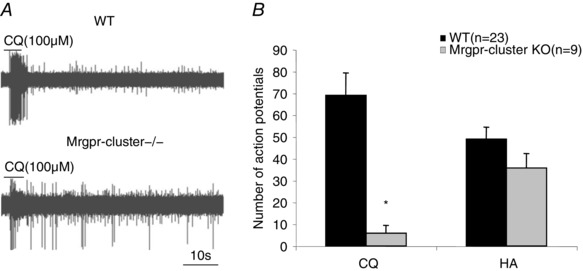
A, representative traces of the response to CQ in wild‐type and Mrgpr‐cluster Δ−/− mice. B, the mean ± SEM number of action potentials evoked by CQ and HA in HA‐sensitive C‐fibres of wild‐type (n = 23) and mrgpr‐cluster Δ−/− mice (n = 9). One of the histamine‐sensitive C‐fibres studied in mrgpr‐cluster Δ−/− mice responded with a burst of 159 action potentials after a delay post addition of CQ (and was excluded); 7 fibres did not respond and 2 responded with a low level of activation (P < 0.05, including all 10 fibres). The bars represent means ± SEM of number (n) of individual fibres studied; *significantly different from wild type (P < 0.05).
MrgprC11
DRG neurons expressing MrgprA3 largely overlap with those expressing MrgprC11 (Zylka et al. 2003). Moreover, in a calcium assay we previously noted that 3.6% of neurons in the DRG that responded to the MrgprC11 stimulant BAM8‐22, also responded to CQ (Liu et al. 2009). We evaluated the overlap in responsiveness between CQ‐ and BAM8‐22‐sensitive C‐fibres in the mouse skin. In preliminary dose–response studies we found that BAM8‐22 stimulated C‐fibres with a threshold concentration of 0.1 μm and a maximum effect at 10 μm. We evaluated 12 C‐fibres that were activated by CQ and 100% were also stimulated by BAM8‐22, with bursts of action potentials of similar intensity to CQ (Fig. 5). We evaluated 13 fibres that were CQ insensitive and found only 1 that was weakly activated by BAM8‐22. Therefore, as predicted, the CQ‐responsive and BAM8‐22‐responsive C‐fibres in the skin represent the same population of nerves.
Figure 5. Activation of CQ‐sensitive C‐fibres by the MrgprC11 agonist BAM8‐22.
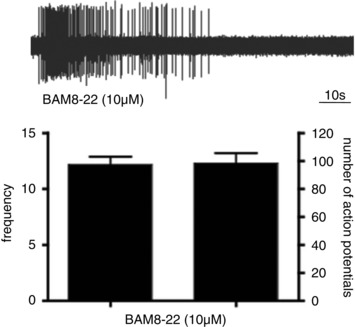
All (12 of 12 C‐fibres that responded to CQ were also strongly activated by BAM8‐22. The bar graphs are means ± SEM of n = 12 C‐fibres (frequency = peak impulse/sec). 12 of 13 C‐fibres that did not respond to CQ also failed to respond to BAM8‐22 (not shown).
Phospholipase β3
The Mrgprs are GPCRs that are often linked to Gq. Gq‐coupled receptors stimulate phospholipase C (PLC). However, studies at the cell soma have indicated that CQ activates the neurons independently of PLC (Wilson et al. 2011). An RNAseq analysis of MrgprA3 expressing neurons revealed that the dominant PLC isoform expressed is the PLCβ3 isoform (Usoskin et al. 2015). We therefore evaluated the effect of CQ in PLCβ3 knockout (KO) animals. We evaluated 26 C‐fibres in 8 plcβ3 −/− mice. There was no action potential discharge in 23 of these C‐fibres. In the 3 that responded the total number of action potentials elicited were only 5, 10, and 5 (Fig. 6 A and B). As a positive control, capsaicin stimulated C‐fibres from plcβ3 −/− mice (15 ± 5.1 Hz); the number of action potentials evoked by capsaicin was not statistically different from wild‐type mice (Fig. 6). Gallein, an inhibitor of G‐protein βγ signalling had no effect on CQ‐induced action potential discharge (Fig. 10).
Figure 6. CQ stimulation in cutaneous C‐fibre in wild‐type compared to PLCβ3 KO mice.
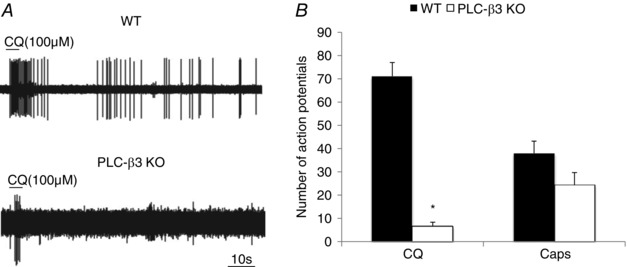
A, representative traces of the response to CQ in wild‐type and PLCβ3 KO mice. B, the number of action potentials evoked by CQ in wild‐type and PLCβ3 KO mice. The wild‐type data are the same as presented in Fig. 3. 26 C‐fibres in 8 PLCβ3 KO mice were evaluated. Among these fibres 23 had no response and 3 had a minimal activation. The histogram reflects the mean ± SEM of 61 and the 3 CQ responding fibres in WT and PLCβ3 KO mice, respectively. As a positive control, the number of action potentials evoked by capsaicin was not statistically different from wild‐type mice.
Figure 10. The response to CQ is not inhibited following 20 min treatment with gallein, Ruthenium Red, HC030031 and XE991.
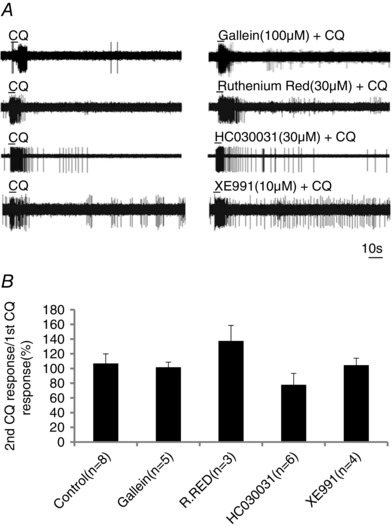
A, representative traces of the response to CQ in the absence and presence of gallein (100 μm), Ruthenium Red (30 μm), HC030031 (30 μm) and XE991 in WT mice. B, the response to repeated administration of CQ in the absence and presence of the various inhibitors. The data are presented as means ± SEM of the ratio of the second CQ response to the first CQ response (the response quantified as number of action potentials evoked). No drug significantly inhibited the CQ response (P > 0.1).
Lack of TRP channel involvement in CQ‐induced action potential discharge
Previous studies have shown that CQ‐induced scratching is strongly dependent on TRPA1 (Wilson et al. 2011). We evaluated 15 cutaneous C‐fibres from trpa1 −/− mice. As with C‐fibres in wild‐type mice, about 27% (4/15) responded to CQ and histamine. The peak response and total number of action potentials were not different from that observed in wild type. OVA stimulated action potential discharge in 3 of 3 CQ‐sensitive C‐fibres in the skin of trpa1 −/− mice in a fashion similar to wild type, averaging 5 ± 1 Hz. AITC (300 μm) failed to activate C‐fibres in the trpa1 −/− mice, whereas, as mentioned above, this concentration of AITC activated C‐fibres studied in wild‐type mice. These data are summarized in Fig. 7 A. Consistent with these observations, we found no difference in the bouts of scratching in response to intradermal CQ between 18 pairs of wild‐type vs. trpa1 −/− mice (Fig. 7 B).
Figure 7. CQ, OVA and AITC‐induced stimulation of action potential discharge in C‐fibres innervating the skin of wild‐type and trpa1 −/− mice.
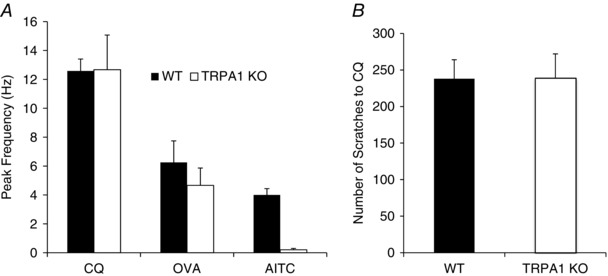
A, the bar graphs represent the means ± SEM peak action potential discharge frequency observed in C‐fibres innervating the skin of wild‐type (filled bars) and trpa1 −/− mice (open bars). CQ (100 μm) activated 4 of 15 C‐fibres in trpa1 −/− mice, which was not different from the percentage of CQ‐sensitive C‐fibres in wild‐type mice (P > 0.1). The OVA (1 mg ml−1) and AITC (300 μm) data were derived from C‐fibres that were previously determined to be CQ sensitive. The respective n values for wild‐type and trpa1 −/− animals were: CQ, 33 and 4; OVA, 12 and 3; AITC, 7 and 3. B, scratching bouts in wild‐type (WT) compared to trpa1 −/− mice in response to CQ injection. Each day one wild‐type and one trpa1 −/− animal was evaluated. The bars represent the means ± SEM number of bouts evoked over a 30 min period in n = 18 pairs of mice.
To further substantiate the completeness of the trpa1 deletion we quantified the effect of the TRPA1 agonist cinnamaldehyde (250 μm) and capsaicin (150 nm) in elevating calcium in neurons isolated from the DRG. Capsaicin increased calcium in 27% of the neurons (51/190) whereas cinnamaldehyde failed to increase calcium in any neurons from trpa1 −/− mice (data not shown).
A more thorough investigation was carried out in 20 trpa1/trpv1 −/− double knockout mice. The proportion of C‐fibres that responded to CQ (8 of 40) was again not different from wild type (P > 0.5). Neither the peak frequency of discharge nor total number of action potentials evoked was different from that found in wild‐type animals (Fig. 8 A–C). Likewise, the response to histamine was not different in CQ‐sensitive C‐fibres from wild‐type and trpa1/trpv1 −/− animals (Fig. 8 A–C)). Capsaicin was added at the end of all experiments and none of the fibres evaluated in the trpa1/trpv1 −/− animals responded with action potential discharge (Fig. 8 B and C).
We next evaluated the response to CQ in C‐fibres isolated from trpc3/trpc6 −/− mice. Among the 20 C‐fibres studied 30% responded to CQ and the peak discharge frequency and total number of action potentials evoked were not different from wild type (Fig. 9 A).
Figure 9. The response to CQ is not altered in trpC3/6 −/− double KO or by TRPC3/6 antagonist in trpa1/trpv1 −/− double KO mice.
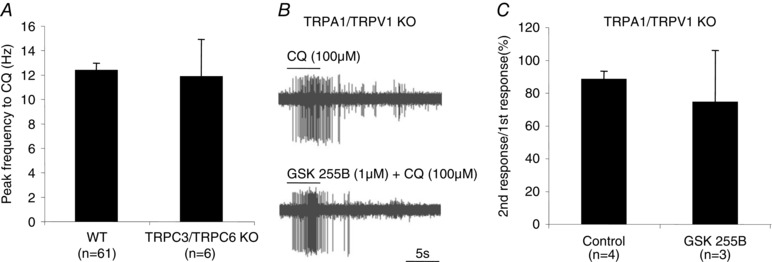
A, bar graphs showing the mean ± SEM of the peak frequency (Hz) evoked by CQ in wild‐type and TRPC3/C6 KO mice. B, representative traces of response to CQ in the absence and presence of the TRPC3/6 antagonist GSK255B in trpa1/trpv1 −/− mice. C, the response to repeated administration of CQ in the absence and presence of the TRPC3/6 antagonist GSK255B in the trpa1/trpv1 −/− mice. The bar graphs depict the means ± SEM of the number of action potentials evoked presented as the ratio of the second CQ response to the first CQ response.
We addressed the hypothesis of redundancy among the TRP channels such that any one channel is sufficient to evoke action potential discharge. The response of the potent TRPC3/TRPC6 antagonist GSK2332255B (Seo et al. 2014) was evaluated on the CQ response in C‐fibres isolated from trpa1/trpv1 −/− animals. The response in the presence of GSK2332255B averaged 84 ±13% of the initial response studied in the absence of GSK2332255B (P > 0.5) (Fig. 9 B and C).
Consistent with the finding with trpa1 −/− mice, in wild‐type mice we found that the CQ‐induced response was not significantly inhibited by the TRPA1 antagonist HC030031 (30 μm) (Fig. 10). HC030031 did, however, inhibit the response to AITC (300 μm). In the presence HC0031, AITC evoked 0.8 ± 0.8 Hz stimulation (n = 5) compared to 4 ± 0.4 Hz (n = 7) in untreated tissue (P < 0.05). We evaluated the effect of Ruthenium Red (30 μm), a substance that non‐selectively blocks many TRP channels and other cation channels. The response to CQ was not inhibited following 20 min treatment with Ruthenium Red (Fig. 10 A and B).
Patch clamp studies on cell bodies
Previous studies have indicated that the electrophysiological effects of CQ on the cell soma require TRPA1. Based on the data presented above, this would suggest a distinction between the mechanisms occurring at the cell soma vis‐à‐vis terminals. To directly address this hypothesis we evaluated the response of the MrgprA3‐expressing neurons to CQ using MrgprA3‐tdTomato mice. At a holding potential of −60 mV, 100 μm CQ elicited an inward current (0.46 ± 0.19 pA pF−1, n = 6). At 1 mm CQ this current was increased to 3.0 ± 1.6 pA pF−1 (n = 13). In current clamp mode, 1 mm CQ caused a membrane depolarization by 8.8 ± 0.7 mV (n = 9). In 7 of 9 neurons this was sufficient to evoke action potential discharge ranging from 3 to 61 action potentials (averaging 24 ± 8). We next evaluated the effect of the TRPA1 antagonist HC‐030031 on the responses at the cell bodies. We found that the CQ‐evoked current was reduced by 52.5 ± 10.5% (n = 6) upon the second stimulation with 1 mm CQ (10 min interval between two CQ applications). We therefore evaluated the effects of HC 030031 in a non‐paired fashion. In neurons pretreated with HC 030031, the CQ‐induced membrane depolarization was the same as control, averaging 9.6 ± 1.2 mV (n = 9). In 6 of 9 HC‐treated neurons, CQ evoked action potential discharges that were not different from control (ranging from 2 to 57 and averaging 22 ± 7). These data are illustrated in Fig. 11. Although TRPA1 does not appear to be required for CQ‐induced membrane depolarization, the MrgprA3‐expressing neurons were, however, responsive to AITC. In fact, when studied in voltage clamp mode, the inward current evoked by AITC (100 μm) averaged 22.3 ± 2.8 pA pF−1, which was ∼7‐fold larger than the 3.0 ± 1.6 pA pF−1 evoked by 1 mm CQ (P < 0.01; data not shown).
Figure 11. Whole‐cell patch clamp recording of mrgprA3‐tdTomato‐expressing neurons.
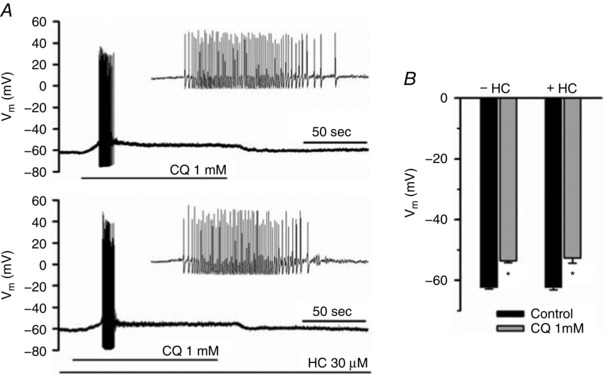
A, studies in current clamp mode showing the membrane depolarization and action potential discharge evoked by CQ (1 mm) in a control neuron (top) and a neuron pretreated for 5 min with HC 030031 (30 μm) (bottom). The number of action potentials was not influenced by the TRPA1 antagonist (mean data are presented in the text). B, the depolarizing effect of CQ (1 mm) on the membrane potential in control neurons and neurons treated with 30 μm HC 030031. * P < 0.01 relative to control, bars represent the mean ± SEM of 9 experiments.
M‐current
Blocking of neuronal M‐type potassium channels (KCNQ/Kv7) provides for a mechanism by which GPCR/PLC can evoke a membrane depolarization and an increase in excitability of neurons (Hernandez et al. 2008; Linley et al. 2008). We found that the effect of CQ was not mimicked by an effective Kv7 blocker XE991, nor was the peak frequency or total number of action potentials evoked by CQ altered in the presence Kv7 blockade (Fig. 10).
TMEM16A
In cell bodies of neurons isolated from the DRG and vagal sensory ganglia, bradykinin evokes a depolarizing current by activating calcium‐activated chloride channels (Oh & Weinreich, 2004; Lee et al. 2005; Liu et al. 2010). At the level of C‐fibre terminals in the isolated trachea, the bradykinin‐induced action potential discharge is inhibited by non‐selective chloride channel blockers. TMEM16a is a calcium‐activated chloride channel that is expressed in MrgA3‐expressing DRG neurons. Using an siRNA strategy to knockdown TMEM16a expression in small‐diameter DRG neurons decreases bradykinin‐induced depolarizing currents in the rat Liu et al. 2010. Recently, MONNA has been described as a potent and selective inhibitor of TMEM16A (S. J. Oh et al. 2013). We found that MONNA (10 μm) inhibited CQ‐induced action potential discharge from itch nerves in the skin by approximately 50% (Fig. 12 A). Consistent with the electrophysiological studies was the finding that MONNA inhibited the number of scratching bouts by about 50% (Fig. 12 B).
Figure 12. The effect of MONNA on CQ‐induced action potential discharge and bouts of scratching.
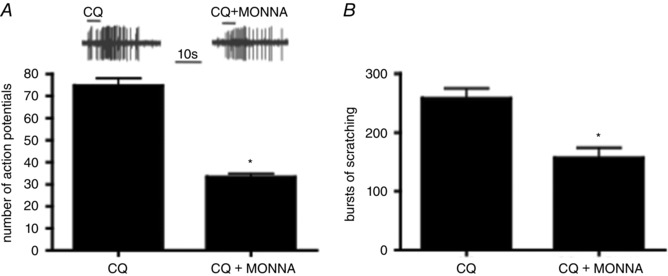
A, the mean ± SEM of CQ in the absence and presence of MONNA (10 μm, 15 min), in 10 CQ‐sensitive C‐fibres (* P < 0.05). B, the means ± SEM of the number of bouts of scratching over 30 min in the presence of CQ (10 mm; n = 7) or CQ + MONNA (1 mm; n = 5) (* P < 0.01).
Discussion
The results show that the CQ‐induced activation of itch fibres terminating in mouse skin depends on Mrgpr GPCRs and the stimulation of PLCβ3 isozymes. The data also show that the ion channels downstream from GPCR actvation with CQ or histamine that are required for membrane depolarization and action potential discharge at the peripheral terminals of itch nerves are unlikely to include TRP channels, but may involve, at least partially, calcium‐activated chloride channels.
We found little evidence for a CQ‐sensitive, histamine‐insensitive nerve population in the skin. Likewise, we found little evidence for a histamine‐sensitive, CQ‐insensitive nerve population. These findings are consistent with the hypothesis that either CQ or histamine can be used as a marker of the MrgprA3‐expressing itch fibres in this region of the skin. Our observations that CQ‐sensitive fibres are histamine‐and capsaicin‐sensitive C‐fibres is in agreement with nerve recording from the mouse skin in vivo (Han et al. 2013). The conclusion that CQ‐sensitive nerves in the skin are the same population as histamine sensitive fibres is also consistent with the observation that selective deletion of MrgprA3‐expressing neurons reduces the scratching in response not only to CQ, but also to histamine (Han et al. 2013). This conclusion is at odds with findings in the cheek where histamine‐ and CQ‐sensitive nerves appear to represent distinct populations (Roberson et al. 2013). This may represent a difference between itch neurons in the trigeminal ganglia vs. DRG. We used concentrations of histamine and CQ (100 μm) that, based on other studies, are expected to be saturating for histamine receptors and MrgprA3. It remains possible that there may be a nerve subtype in the mouse skin that responds only to larger concentrations of histamine or CQ.
Our previous studies showed that among the Mrgpr receptors, CQ selectively stimulated MrgprA3 (Liu et al. 2009). The results with the C‐fibres in the mrgpr cluster Δ−/− animals are consistent with this conclusion. It has previously been reported that MrgprA3 and MrgprC11 are expressed in the same overlapping population of DRG neurons (Zylka et al. 2003). In 12 experiments where the C‐fibre responded strongly to CQ, the nerve also responded strongly to the MrgprC11 agonist BAM8‐22, whereas CQ‐insensitive neurons were also insensitive to BAM8‐22. This provides additional support for the assumption that C‐fibres activated by CQ are those that express MrgprA3. BAM8‐22 is a pruritogen in human volunteers presumably due to its effect on MrgrX1, the human functional orthologue for MrgprC11 (Dong et al. 1990), although BAM8‐22 may also have Mrgpr‐independent sensitizing on C‐fibre afferent nerves (Hager et al. 2008). It is worth noting that in one histamine‐sensitive fibre, CQ caused a robust discharge of action potentials in the absence of MrgprA3. An additional two other fibres also responded to CQ, with a subtle but noticeable increase in action potential discharge over baseline. Caution is therefore required when using CQ as a specific MrgprA3 agonist. This is also the case for behavioural assays; CQ causes significant bouts of scratching in mrgpr‐cluster Δ−/− animals (Liu et al. 2009) and in mice in which MrgA3‐expressing neurons are deleted (Han et al. 2013), although significantly less than in wild‐type animals.
Since the strong activation by CQ appears to depend on Mrgpr activation, it is likely that GPCR–Gq signalling is involved (Han et al. 2002). A consequence of GPCR–Gq activation is the stimulation of PLC. There are six families of PLC that comprise thirteen isozymes. An RNAseq analysis of MrgprA3‐expressing mouse DRG neurons revealed that the isoform expressed is almost exclusively limited to PLCβ3 (Usoskin et al. 2015). CQ caused no (or very minimal) action potential discharge in C‐fibres terminating in the skin of PLCβ3‐deficient mice; a finding consistent with a GPCR–Gq–PLC‐dependent nerve terminal activation.
The ionic mechanisms downstream of PLCβ3 activation that underlie action potential discharge are not known. A surprising finding of the present study was the lack of influence of TRPA1 or TRPV1 in the activation of the C‐fibre terminals by CQ or histamine. Other studies have found that stimulation of a depolarizing current in DRG cell bodies, or elevations in intracellular calcium by CQ in nerve cell bodies dissociated from DRG is strictly dependent on TRPA1. Moreover, the scratching response to intradermal injections of CQ were virtually absent in some studies with trpa1 −/− mice (Wilson et al. 2011). The present results show that TRPA1 is not required for the action potential response to CQ at the C‐fibre nerve terminals. Our patch clamp analysis also revealed no electrophysiological evidence for an important role of TRPA1 in the CQ‐induced membrane depolarization and action potential discharge in the cell soma.
It is possible that TRPA1 may be selectively involved at lower levels of GPCR stimulations, and its role was surmounted by the 100 μm concentration of CQ used in the present study. This is unlikely, however, as our previous concentration–response analysis has noted that 100 μm is not a supramaximal concentration for MrgprA3 activation (Liu et al. 2009). We also saw a lack of TRPA1 involvement in the action potential discharge evoked by endogenous ‘physiological concentrations’ of pruritogens released as a consequence of allergen (OVA)‐activated mast cells. The mechanism of antigen activation of the CQ‐sensitive nerves was not studied, but in this context it is worth noting that upon immunological activation mast cells can release mediators that stimulate MrgprC11 (Lee et al. 2008).
Our results, considered along with the findings of others that TRPA1 is involved in CQ‐induced scratching, would indicate that the location of the TRPA1 channels involved in the scratch reflex in healthy mice may be somewhere other than the nerve terminals, most likely in the central nervous system (Akiyama & Carstens, 2014). Mice in which trpa1 is genetically deleted, or that are treated with TRPA1 antagonists have strongly altered behaviour responses to stressors, similar to mice treated with large doses of benzodiazepines or antidepressant drugs (de Moura et al. 2014). Consistent with our findings with respect to itch nerve terminal activation, we failed to find a difference in the CQ‐induced bouts of scratching between wild‐type and trpa1 −/− mice. Therefore, the effect of TRPA1 on scratching may depend on subtleties of experimental design, a conclusion more in keeping with a central site of action rather than a dependency on TRPA1 for action potential generation at the itch nerve terminals.
Agonists of GPCRs, notably Gq‐coupled receptors, often lead to TRPV1 activation (Veldhuis et al. 2015). The CQ‐sensitive terminals are more responsive to TRPV1 stimulation than TRPA1, therefore we thought it likely that TRPV1 may be involved in the CQ‐induced action potential discharge. This hypothesis was not supported by the data showing that the percentage of neurons responding and the magnitude of the action potential discharge induced by either CQ or histamine was not different between wild‐type and trpa1/trpv1 −/− double knockout animals, nor inhibited by Ruthenium Red.
Than et al. noted that CQ stimulates calcium increases in a subset of DRG neurons independently of TRPA1 or TRPV1. In these neurons, the calcium rise was inhibited by TRPC3 blockade (Than et al. 2013). The MrgprA3‐expressing neurons in mouse DRG express TRPC3 and TRPC6 mRNA (Usoskin et al. 2015). Our observation that the action potential discharge in the terminals was the same in wild‐type mice and trpc3/trpc6 −/− mice indicates that these channels are not critical for the action potential responses. The lack of an inhibitory effect of removing TRPA1, TRPV1, TRPC3 or TRPC6 is not likely to be due to strong redundancy among these channels, because the potent TRPC3/TRPC6 inhibitor GSK2332255B (Seo et al. 2014) had little effect on CQ‐induced responses, even when evaluated in the skin from trpa1/trpv1 −/− animals.
Gq–GPCR activation of PLC can lead to neuronal membrane depolarization by blocking the so‐called M‐current carried by KCNQ/Kv7 channels (Hernandez et al. 2008; Linley et al. 2008). An effective M‐current blocker did not mimic the effect of CQ or histamine, nor did it alter the action potential discharge evoked by CQ. Moreover, the MrgprA3‐expressing subpopulation of neurons in the DRG express relatively little KCNQ mRNA (Usoskin et al. 2015).
It is known that adult primary sensory nerves concentrate intracellular Cl− such that the equilibrium potential for Cl− (E Cl)is more positive than the resting membrane potential (Rohrbough & Spitzer, 1996). Previous studies at the soma of sensory neurons, and nerve terminals of vagal C‐fibres revealed that a component of bradykinin B2 receptor‐mediated nerve stimulation is inhibited by chloride channel blockers (Oh & Weinreich, 2004; Lee et al. 2005), and in the case of small‐diameter DRG neurons by specifically inhibiting TMEM16a expression (Liu et al. 2010). The MrgprA3‐expressing nerves express the calcium‐activated chloride channels including TMEM16A (Aon‐1) (Usoskin et al. 2015), and this channel has been likened to GPCR–PLC‐dependent stimulation (Stolz et al. 2015). Recently MONNA has been characterized as a selective TMEM16A blocker (S. J. Oh et al. 2013). The finding that this antagonist inhibited CQ‐induced C‐fibre stimulation and bouts of scratching by 50% supports the hypothesis that chloride efflux through TMEM16A channels contributes the CQ‐induced generator potential in itch nerves; as has been previously hypothesized for bradykinin‐induced activation of DRG neurons in the rat (Liu et al. 2010). This conclusion is based on a single antagonist, and TMEM16A blockers including MONNA may have relevant non‐selective effects (Boedtkjer et al. 2015). Therefore, this hypothesis needs to be cautiously considered until independent verification is obtained.
The data presented here argue against a strategy targeting TRP channels at the level of the itch nerve terminals to control GPCR‐dependent itch. It should be kept in mind, however, that all studies were carried out in healthy animals and the pruritogens were limited to an evaluation of histamine and CQ. Inhibition of TRPA1 inhibits bile acid‐induced scratching in mice, and stimulation of the TGR5 receptor with bile acid can lead to TRPA1 activation in cell systems (Lieu et al. 2014). It is possible that TRPA1 may play more of a role in itch associated with inflamed skin. TRPA1 is expressed in multiple cell types, and inflammation associated with dermatitis is associated with elevation of TRPA1 expression. Blocking TRPA1 inhibits scratching associated with dry skin (Wilson et al. 2013), atopic (M. H. Oh et al. 2013) and contact dermatitis (Liu et al. 2013). This, however, was associated with inhibition of the inflammatory response and production of pruritogens, and may not necessarily have been due to direct regulation of action potential generation at the nerve terminals (Liu et al. 2013; Wilson et al. 2013).
Additional information
Competing interests
The authors declare no competing interests.
Author contributions
F.R. works in laboratories at the JHU Asthma Centre. He was involved with the conception of the study, experimental design, data acquisition, and interpretation, as well as editing the paper for content. H.S. works in laboratories at the JHU Asthma Centre. She was involved experimental design aspects, all patch clamp recordings, data interpretation, and manuscript preparation. R.H. works in laboratories at the JHU Asthma Centre. He was involved with experimental design, scratching and electrophysiological data acquisition and manuscript editing. J.M. works in laboratories at the JHU Department of Neuroscience. He was involved with experimental design, scratching data acquisition and manuscript preparation. X.D. works in laboratories at the JHU Department of Neuroscience. He was involved with experimental design, conceptual aspects, development of genetically modified mice, and manuscript preparation. B.U. works in laboratories at the JHU Asthma Centre. He conceived of the project, was involved with experimental design, data interpretation, and drafted the manuscript.
Funding
This work was supported by National Institutes of Health [R01NS054791] and by BioMed Martin, Martin, Slovakia [ITMS 26220220187].
Acknowledgements
We acknowledge the excellent technical assistant of Ms Sonya Meeker.
Linked articles This article is highlighted by a Perspective by Gamper. To read this Perspective, visit https://doi.org/10.1113/JP274199.
References
- Aguiar DC, Moreira FA, Terzian AL, Fogaca MV, Lisboa SF, Wotjak CT & Guimaraes FS (2014). Modulation of defensive behavior by Transient Receptor Potential Vanilloid Type‐1 (TRPV1) Channels. Neurosci Biobehav Rev 46, 418–428. [DOI] [PubMed] [Google Scholar]
- Akiyama T & Carstens E (2013). Neural processing of itch. Neuroscience 250, 697–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T & Carstens E (2014). Spinal coding of itch and pain In Itch: Mechanisms and Treatment, eds Carstens E. & Akiyama T. CRC Press, Boca Raton, FL, USA. [PubMed] [Google Scholar]
- Bautista DM, Wilson SR & Hoon MA (2014). Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci 17, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtkjer DM, Kim S, Jensen AB, Matchkov VM & Andersson KE (2015). New selective inhibitors of calcium‐activated chloride channels … T16Ainh‐A01, CaCCinh‐A01 and MONNA … what do they inhibit? Br J Pharmacol 172, 4158–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura JC, Noroes MM, Rachetti Vde P, Soares BL, Preti D, Nassini R, Materazzi S, Marone IM, Minocci D, Geppetti P, Gavioli EC & Andre E (2014). The blockade of transient receptor potential ankirin 1 (TRPA1) signalling mediates antidepressant‐ and anxiolytic‐like actions in mice. Br J Pharmacol 171, 4289–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong A, Huang P & Caughey WS (1990). Protein secondary structures in water from second‐derivative amide I infrared spectra. Biochemistry 29, 3303–3308. [DOI] [PubMed] [Google Scholar]
- Emery EC, Luiz AP, Sikandar S, Magnusdottir R, Dong X & Wood JN (2016). In vivo characterization of distinct modality‐specific subsets of somatosensory neurons using GCaMP. Sci Adv 2, e1600990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager UA, Hein A, Lennerz JK, Zimmermann K, Neuhuber WL & Reeh PW (2008). Morphological characterization of rat Mas‐related G‐protein‐coupled receptor C and functional analysis of agonists. Neuroscience 151, 242–254. [DOI] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH & Dong X (2013). A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16, 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Dong X, Hwang JI, Zylka MJ, Anderson DJ & Simon MI (2002). Orphan G protein‐coupled receptors MrgA1 and MrgC11 are distinctively activated by RF‐amide‐related peptides through the Galpha q/11 pathway. Proc Natl Acad Sci USA 99, 14740–14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CC, Zaika O, Tolstykh GP & Shapiro MS (2008). Regulation of neural KCNQ channels: signalling pathways, structural motifs and functional implications. J Physiol 586, 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI & Han SK (2009). TRPV1‐expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA 106, 11330–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik MUB (2004). Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild‐type and TRPV1−/− mice. J Physiol 555, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Dong X & Ringkamp M (2014). Sensory neurons and circuits mediating itch. Nature Rev Neurosci 15, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Dong X, Liu Q, Patel KN, Choi OH, Vonakis B & Undem BJ (2008). Agonists of the MAS‐related gene (Mrgs) orphan receptors as novel mediators of mast cell‐sensory nerve interactions. J Immunol 180, 2251–2255. [DOI] [PubMed] [Google Scholar]
- Lee MG, Macglashan DW Jr & Undem BJ (2005). Role of chloride channels in bradykinin‐induced guinea‐pig airway vagal C‐fibre activation. J Physiol 566, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu T, Jayaweera G, Zhao P, Poole DP, Jensen D, Grace M, McIntyre P, Bron R, Wilson YM, Krappitz M, Haerteis S, Korbmacher C, Steinhoff MS, Nassini R, Materazzi S, Geppetti P, Corvera CU & Bunnett NW (2014). The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology 147, 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley JE, Rose K, Patil M, Robertson B, Akopian AN & Gamper N (2008). Inhibition of M current in sensory neurons by exogenous proteases: a signaling pathway mediating inflammatory nociception. J Neurosci 28, 11240–11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, Sui A, McKay MC, McAlexander MA, Herrick CA & Jordt SE (2013). TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J 27, 3549–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H & Gamper N (2010). The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M‐type K+ channels and activation of Ca2+‐activated Cl− channels. J Clin Invest 120, 1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ & Dong X (2009). Sensory neuron‐specific GPCR Mrgprs are itch receptors mediating chloroquine‐induced pruritus. Cell 139, 1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil B & Dong X (2012). Peripheral mechanisms of itch. Neurosci Bull 28, 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EJ & Weinreich D (2004). Bradykinin decreases K+ and increases Cl− conductances in vagal afferent neurones of the guinea‐pig. J Physiol 558, 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Oh SY, Lu J, Lou H, Myers AC, Zhu Z & Zheng T (2013). TRPA1‐dependent pruritus in IL‐13‐induced chronic atopic dermatitis. J Immunol 191, 5371–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, Hwang SJ, Jung J, Yu K, Kim J, Choi JY, Hartzell HC, Roh EJ & Lee CJ (2013). MONNA, a potent and selective blocker for transmembrane protein with unknown function 16/anoctamin‐1. Mol Pharmacol 84, 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Grunewald B, Nguyen T, Farah M, Polydefkis M, McDonald J, Schramm LP, Toyka KV, Hoke A & Griffin JW (2012). The lateral thoracic nerve and the cutaneous maximus muscle – a novel in vivo model system for nerve degeneration and regeneration studies. Exp Neurol 236, 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenzieri C, Meeker S & Undem BJ (2012). Activation of mouse bronchopulmonary C‐fibers by serotonin and allergen‐ovalbumin challenge. J Physiol 590, 5449–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringkamp M & Meyer R (2014). Pruriceptors In Itch: Mechanisms and Treatment, eds Carstens E. & Akiyama T. CRC Press, Boca Raton, FL, USA. [Google Scholar]
- Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, Binshtok AM & Woolf CJ (2013). Activity‐dependent silencing reveals functionally distinct itch‐generating sensory neurons. Nat Neurosci 16, 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J & Spitzer NC (1996). Regulation of intracellular Cl− levels by Na+‐dependent Cl− cotransport distinguishes depolarizing from hyperpolarizing GABAA receptor‐mediated responses in spinal neurons. J Neurosci 16, 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo K, Rainer PP, Shalkey Hahn V, Lee DI, Jo SH, Andersen A, Liu T, Xu X, Willette RN, Lepore JJ, Marino JP Jr, Birnbaumer L, Schnackenberg CG & Kass DA (2014). Combined TRPC3 and TRPC6 blockade by selective small‐molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc Natl Acad Sci USA 111, 1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz M, Klapperstuck M, Kendzierski T, Detro‐Dassen S, Panning A, Schmalzing G & Markwardt F (2015). Homodimeric anoctamin‐1, but not homodimeric anoctamin‐6, is activated by calcium increases mediated by the P2Y1 and P2X7 receptors. Pflugers Arch 467, 2121–2140. [DOI] [PubMed] [Google Scholar]
- Than JY, Li L, Hasan R & Zhang X (2013). Excitation and modulation of TRPA1, TRPV1, and TRPM8 channel‐expressing sensory neurons by the pruritogen chloroquine. J Biol Chem 288, 12818–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling‐Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S & Ernfors P (2015). Unbiased classification of sensory neuron types by large‐scale single‐cell RNA sequencing. Nat Neurosci 18, 145–153. [DOI] [PubMed] [Google Scholar]
- Veldhuis NA, Poole DP, Grace M, McIntyre P & Bunnett NW (2015). The G protein‐coupled receptor‐transient receptor potential channel axis: molecular insights for targeting disorders of sensation and inflammation. Pharmacol Rev 67, 36–73. [DOI] [PubMed] [Google Scholar]
- Wells MY, Voute H, Bellingard V, Fisch C, Boulifard V, George C & Picaut P (2010). Histomorphology and vascular lesions in dorsal rat skin used as injection sites for a subcutaneous toxicity study. Toxicol Pathol 38, 258–266. [DOI] [PubMed] [Google Scholar]
- Wilson SR & Bautista DM (2014). Role of transient receptor potential channels in acute and chronic itch In Itch: Mechanisms and Treatment, eds Carstens E. & Akiyama T. CRC Press, Boca Raton, FL, USA. [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck‐Fisher A, Liu Q, Patel KN, Dong X & Bautista DM (2011). TRPA1 is required for histamine‐independent, Mas‐related G protein‐coupled receptor‐mediated itch. Nat Neurosci 14, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA & Bautista DM (2013). The ion channel TRPA1 is required for chronic itch. J Neurosci 33, 9283–9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosipovitch G & Bernhard JD (2013). Clinical practice. Chronic pruritus. N Engl J Med 368, 1625–1634. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Dong X, Southwell AL & Anderson DJ (2003). Atypical expansion in mice of the sensory neuron‐specific Mrg G protein‐coupled receptor family. Proc Natl Acad Sci USA 100, 10043–10048. [DOI] [PMC free article] [PubMed] [Google Scholar]


