Abstract
Key points
The role of enteric glial cell activity in the acute regulation of epithelial barrier and secretomotor functions of the intestines under physiological conditions is not clear.
We used transgenic mice to modify glial activity and found that enteric glia significantly contribute to the neurogenic ion transport while glial activity does not appear to play a major role in the acute regulation of barrier function.
The selective activation of glial activity evoked electrogenic ion transport primarily through neural pathways and was sufficient to drive electrogenic ion transport to an extent equal to the direct activation of neurogenic ion transport.
These findings provide novel insight into the cellular mechanisms that control fluid transport homeostasis in the intestine and might provide novel therapeutic avenues for functional diarrheal diseases.
Abstract
Enteric glial cells are often implicated in the regulation of epithelial barrier and secretomotor functions of the intestines. But whether glial cell activity regulates these functions acutely under physiological conditions is not clear. We addressed this issue by using transgenic animal models to modify the activity of enteric glia, either reducing glial expression of connexin 43 in Sox10::CreERT2+/−/Cx43f/f mice or activating glial calcium responses in GFAP::hM3Dq mice, and tested the effects on colonic barrier function and electrogenic ion transport in Ussing chambers. We assessed neuronal‐dependent and ‐independent contributions by activating or inhibiting neurogenic activity with veratridine and tetrodotoxin, respectively. Our results show that the reduction of glial Cx43 expression in Sox10::CreERT2+/−/Cx43f/f mice significantly reduced neurogenic ion transport. The selective glial activation in tissues from GFAP::hM3Dq mice evoked electrogenic ion transport to an extent equal to the direct activation of neurogenic ion transport with veratridine and glial driven responses consisted of both tetrodotoxin‐sensitive and ‐insensitive components. The selective glial stimulation did not affect transmural ion conductance or cell‐impermeant dye flux but the baseline ion conductance was more variable in Sox10::CreERT2+/−/Cx43f/f tissues. Together, our findings show that glial activity contributes to the regulation of electrogenic ion transport in the intestine through effects on neurons and possibly direct effects on epithelial cells. However, glial activity does not appear to play a major role in the acute regulation of barrier function. These findings provide novel insight into the cellular mechanisms that control fluid transport homeostasis in the intestine.
Keywords: electrogenic ion transport, enteric nervous system, intestinal barrier function
Key points
The role of enteric glial cell activity in the acute regulation of epithelial barrier and secretomotor functions of the intestines under physiological conditions is not clear.
We used transgenic mice to modify glial activity and found that enteric glia significantly contribute to the neurogenic ion transport while glial activity does not appear to play a major role in the acute regulation of barrier function.
The selective activation of glial activity evoked electrogenic ion transport primarily through neural pathways and was sufficient to drive electrogenic ion transport to an extent equal to the direct activation of neurogenic ion transport.
These findings provide novel insight into the cellular mechanisms that control fluid transport homeostasis in the intestine and might provide novel therapeutic avenues for functional diarrheal diseases.
Abbreviations
- CNO
clozapine‐N‐oxide
- Cx43
connexin‐43
- Cx43‐igKO
inducible and glia‐specific Cx43 knockout
- Cx43f/f
floxed Cx43
- ERT2
mutated estrogen receptor responsive to tamoxifen
- DREADD
designer receptors exclusively activated by designer drugs
- ENS
enteric nervous system
- FS
fluorescein‐5‐(and‐6)‐sulfonate
- GFAP
glial fibrillary acidic protein
- GT
transmural conductance
- HA
haemagglutinin
- ISC
short‐circuit current
- ir
immunoreactivity
- PBS‐Triton
Triton X‐100 in PBS
- RT
room temperature
- TTX
tetrodotoxin
- WT
wild type
Introduction
The intestinal epithelium is a selectively permeable barrier that prevents the entry of luminal pathogens while permitting the movement of ions, water and nutrients (Groschwitz & Hogan, 2009). More than two blood volumes of fluid cross the mucosal epithelial surface each day (Furness et al. 2014) and this plays important roles in digestion, nutrient absorption, stool movement and host defense mechanisms (Barrett & Keely, 2000). Water movement across the intestinal epithelium is controlled by transepithelial ion transport (Barrett & Keely, 2000) which, in turn, is tightly regulated by secretomotor neurons in the submucosal plexus of the enteric nervous system (ENS) (Cooke, 1989) and extrinsic sympathetic innervation (Sjovall et al. 1986). These neurons interact with resident glial cells in the gut, called enteric glia, and increasing evidence suggests that enteric glia participate in the regulation of epithelial functions (Bush et al. 1998; Savidge et al. 2007; MacEachern et al. 2011). Yet whether enteric glia influence secretomotor or barrier functions acutely under physiological conditions is an unresolved debate (MacEachern et al. 2015; Pochard et al. 2016).
Enteric glial cells reside in all layers of the gut wall (Gulbransen & Sharkey, 2012) and respond to neurotransmitters and neuromodulators through signal transduction pathways that involve intracellular calcium (Ca2+) transients (Kimball & Mulholland, 1996; Gulbransen & Sharkey, 2009; Broadhead et al. 2012; Boesmans et al. 2013). Glial activity encoded by Ca2+ responses plays a major role in the regulation of enteric circuits that control gut motility (McClain et al. 2014, 2015). But whether the same is true for enteric circuits that control secretomotor and barrier functions is not clear. Enteric glia were indirectly implicated in the regulation of electrogenic ion transport through mechanisms that are proposed to involve interactions with neurons in the myenteric plexus (MacEachern et al. 2011). However, more recent data suggests that enteric glia play no role in the regulation of secretomotor functions under physiological conditions (MacEachern et al. 2015). These conflicting data confound a clear understanding of the role of enteric glia in the regulation of ion transport. Likewise, enteric glia are considered important stabilizers of epithelial barrier function (reviewed in Neunlist et al. 2013). Yet evidence supporting this role was largely gained from studies performed on in vitro systems that lack neuron inputs and do not show the effect of glial cell activation, or in vivo glial ablation models that are confounded by the multitude of side‐effects during glial ablation (Bush et al. 1998). Therefore, whether glial activity acutely regulates barrier and secretomotor functions of the intestine remains unknown.
Our goal in the current study was to resolve this issue by specifically studying how glial activity influences gut secretomotor and barrier functions acutely under physiological conditions. Based on the ability of glia to modulate neuron activity (McClain et al. 2014, 2015) and epithelial cell functions (Neunlist et al. 2007; Savidge et al. 2007; Bach‐Ngohou et al. 2010; Van Landeghem et al. 2011), we hypothesized that glial activity is, at least in part, required and sufficient to regulate epithelial function. We tested our hypothesis using cell‐specific approaches to selectively manipulate enteric glial activity (reviewed in Grubisic & Gulbransen, 2017) and studied the subsequent effects on secretomotor and barrier functions in full thickness preparations of mouse distal colon in Ussing chambers (reviewed in Clarke, 2009). Our results show that glial activity significantly accounts for the transepithelial ion transport stimulated by neuron depolarization but has no effect on the acute regulation of epithelial permeability. Importantly, the selective stimulation of enteric glia was sufficient to elicit neuron depolarization‐like responses of epithelial ion transport and a portion of the epithelial response to glial stimulation was not sensitive to blockade by tetrodotoxin. Together these data clearly show that glial activity plays a major role in the acute regulation of gut secretomotor functions. However, the influence of glia on gut barrier function seems to involve tonic, rather than acute, mechanisms.
Methods
Ethical approval
Animal use protocols were approved by the Michigan State University (MSU) Institutional Animal Care and Use Committee (IACUC) and were carried out in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All animals were euthanized by cervical dislocation followed by decapitation.
Animals
Mice were maintained in a temperature‐controlled environment on a 12‐hour light–dark cycle with access to water and regular chow ad libitum. Mice of both sexes, age 8–12 weeks, were used for experiments. The inducible and glia‐specific connexin‐43 knockout (Cx43‐igKO) transgenic mouse model was generated by crossing a Sox10::CreERT2 parental line in which the Sox10 promoter drives the glia‐specific expression of a fusion protein between Cre recombinase and the mutated estrogen receptor (ERT2) responsive to tamoxifen [a gift from Dr Vassilis Pachnis (Laranjeira et al. 2011)] with a floxed Cx43 (Cx43f/f) parental line containing loxP sites flanking exon 2 of Cx43 [Jackson Labs, B6.129S7‐Gja1tm1Dlg/J, RRID:IMSR_JAX:008039 (Liao et al. 2001)]. Double transgenic mice were maintained as hemizygous for Cre and homozygous for the floxed allele (Sox10::CreERT2+/−/Cx43f/f). Cre null littermates (Sox10::CreERT2–/–/Cx43f/f), hereafter referred to as Cx43f/f, served as experimental controls. Cre recombinase activity was induced by two intraperitoneal injections of tamoxifen free base (0.1 mg per 1 g of body weight; Sigma‐Aldrich, St Louis, MO, USA) per day for 2 days and experiments were performed 5 days after the last injection. GFAP::hM3Dq mice were a gift from Dr Ken McCarthy (Agulhon et al. 2013) and were bred for experiments as hemizygotes at MSU. Wild type (WT) littermates served as experimental controls. Genotyping was performed by the MSU Genomics Core Research Technology Support Facility.
Electrogenic ion transport and ionic conductivity
Full thickness preparations of distal colon were mounted in Ussing chambers (aperture 0.3 cm2; EasyMount Ussing chamber system, Physiologic Instruments, San Diego, CA, USA) equipped with Multichannel Voltage/Current Clamp (Physiologic Instruments) and operated with Acquire & Analyse software version 2.3 (Physiologic Instruments) for automated data acquisition. Tissue was first equilibrated for 30 min in warmed (37°C) and oxygenated (5% CO2, 95% O2) Krebs buffer consisting of (in mm): 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3 and 11 glucose. Net movement of ions across the epithelium was recorded as short‐circuit current (I SC, μA cm−2) in voltage clamp mode. Simultaneously, transmural conductance (G T, mS cm−2) was determined by automated 5 mV pulses and recording the resulting change in the I SC (G T = I SC/5 mV). G T was used to assess the integrity of the gut wall (all preparations were intact, baseline G T < 30 mS cm−2) and to determine ionic conductance through the paracellular and transcellular pathways (Clarke, 2009). Drugs were added serosally at a final concertation of 10 μm and remained in the bath for the duration of the experiment: Clozapine‐N‐oxide (CNO, National Institute on Drug Abuse Drug Supply Program at the NIH, USA), forskolin (Tocris Bioscience, Bristol, UK), tetrodotoxin (TTX, Sigma‐Aldrich), veratridine (Tocris). To limit the possibility of off‐target effects of veratridine, we used a concentration within the range known to activate voltage‐gated sodium channels (Zhu et al. 2009) and that was comparable to prior studies of veratridine‐stimulated electrogenic ion transport in mouse colon (Hyland & Cox, 2005). Blind assessment of the effects of CNO on preparations from GFAP::hM3Dq and WT mice was performed, i.e. the person conducting experiments was not aware of the genotype of the mice. Drug responses were obtained as the difference (Δ) between the maximal response and the pre‐exposure baseline activity: ΔI SC(drug) = I SC(drug)max − I SC(baseline); ΔG T(drug) = G T(drug)max − G T(baseline). Recordings with unstable baseline were not analysed. Forskolin was added at the end of each experiment to assess tissue viability and epithelial secretory function. Tissues with forskolin responses over/below 2 standard deviations (SDs) from the overall average forskolin response (86.2 ± 41.6 μA cm−2, mean ± SD) were excluded (n = 1/24). Since the health status of the epithelium consequently predetermines I SC responses, I SC responses were normalized to the forskolin response [ΔI SC(drug)/ΔI SC(forskolin), %]. In a subset of experiments, the sodium channel blocker amiloride hydrochloride (10 μm; Tocris) was added mucosally to assess specific ion involvement.
Paracellular permeability of the gut wall
Ussing chambers were also used to assess intestinal barrier function and paracellular permeability of the distal colon preparations. After tissue equilibration a cell‐impermeant fluorescein‐5‐(and‐6)‐sulfonate (478.32 Da; Life technologies corporation, Carlsbad, CA, USA) was added mucosally (0.05 mg ml−1). Krebs buffer in the serosal chamber was sampled before the dye addition and every following 20 min (100 μl triplicates were taken and buffer was replenished). Sample fluorescence intensity was recorded in Infinite M1000 PRO microplate reader (Tecan Group Ltd, Männedorf, Switzerland) using i‐control™ microplate reader software (Tecan), version 1.6.19.2. Excitation/emission wavelengths were 495/520 nm. Dye flux reached a steady state within 1 h of the dye addition and gut wall permeability was assessed from the slope of fluorescence values of the last 4 time‐points.
Immunohistochemistry (IHC)
Colons were removed, opened along the mesentery border and pinned flat in a Sylgard (Dow‐Corning, Midland, MI, USA)‐coated dish for whole‐mount preparations or removed and flushed with phosphate‐buffered saline (PBS) for tissue sections. Tissues were preserved with Zamboni's fixative (overnight at 4°C) and subsequently washed with PBS. Whole‐mount preparations of the colonic myenteric plexus were prepared as described previously (Gulbransen et al. 2012). Briefly, the mucosa, submucosa, and circular muscle were removed with forceps to expose the myenteric plexus. Unopened fixed colons were first cryoprotected with 30% (w/v) sucrose in phosphate buffer (overnight incubation at 4°C) and then used to make 13 μm thick transverse ‘ring’‐shaped frozen sections. Both kinds of tissue preparations were processed for IHC with the antibodies shown in Table 1. Tissue preparations underwent three 10 min washes with 0.1% (v/v) Triton X‐100 in PBS (PBS‐Triton) followed by a 45 min block with blocking solution (containing 4% normal goat serum, 0.4% Triton X‐100 and 1% bovine serum albumin). Preparations were incubated in primary antibodies overnight at room temperature (RT, 22–25°C) and secondary antibodies for 2 h at RT (in blocking solution) before mounting. Dual‐labelling with primary antibodies raised in the same host was performed sequentially. Tissues were triple‐washed in PBS‐Triton after completing labelling with the first primary and secondary antibodies and then incubated overnight at RT with unlabelled goat anti‐rabbit IgG (H + L) Fab fragments (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) in PBS‐Triton (40 μg ml−1) to block all unbound antigen sites. Tissue was then triple‐washed in PBS, blocked with blocking solution and the second run of IHC was performed. In a subset of experiments, the second primary antibody was omitted to control for potential cross reactivity between the second secondary and the first primary antibodies (Fig. S1). Images were acquired through the ×60 oil immersion objective (Plan‐Apochromat, 1.42 numerical aperture) of an inverted Fluoview FV1000 confocal microscope (Olympus, Center Valley, PA, USA). Alexa Fluor 488 or 568 secondary antibodies were excited with 488 nm or 543 nm wavelengths and detected using SDM560 dichroic mirror and BA505‐525 bandpass or BA560IF longpass filter sets.
Table 1.
Details of primary and secondary antibodies used
| Antibody | Source | Dilution | RRID * | |
|---|---|---|---|---|
| Primary | Rabbit ant‐Cx43 | Sigma, St Louis, MO, USA | 1:500 | AB_476857 |
| Chicken anti‐GFAP | Abcam, Cambridge, MA, USA | 1:1000 | AB_304558 | |
| Rabbit anti‐HA# | Cell Signalling, Danvars, MA, USA | 1:500 | AB_1549585 | |
| Rabbit anti‐S100β# | Abcam, Cambridge, MA, USA | 1:200 | AB_882426 | |
| Secondary | Goat anti‐rabbit Alexa Fluor 488 | Invitrogen, Carlsbad, CA, USA | 1:400 | AB_10562715 |
| Goat anti‐chicken Alexa Fluor 568 | Invitrogen, Carlsbad, CA, USA | 1:400 | AB_10584483 | |
| Goat anti‐rabbit Alexa Fluor 568 | Invitrogen, Carlsbad, CA, USA | 1:400 | AB_10563566 | |
*RRID, Research Resource Identifier; #controlled for the cross‐reactivity (Fig. S1).
Statistical analysis
We used GB‐Stat 6.5 software (Dynamic Microsystems Inc., Silver Spring, MD, USA) for statistical analysis. All variables were first tested for normality and homogeneity of variance using Shapiro‐Wilk and Levene's tests, respectively. Since variables were normally distributed, data are presented as means ± standard error of the mean (SEM) and statistical comparisons were made using a Student's t test (separate variance version if sample variances differ significantly, otherwise pooled variance version was used) or analysis of variance (ANOVA) followed by a Tukey/Kramer post hoc test. Paired tissue samples that were run on the same day were normalized to controls and statistically compared by a one sample t test. P < 0.05 (two‐tailed) was regarded as statistically significant for all statistical comparisons.
Results
An increase in intracellular calcium (Ca2+) concentration is the hallmark of enteric glial cell activity (reviewed in Grubisic & Gulbransen, 2017). Yet how glial activity impacts the regulation of the intestinal epithelium is not known. Here, we used advanced transgenic mouse models to selectively modulate the activity of enteric glia and studied how the modulation of glial activity affects secretomotor and barrier functions using Ussing chamber techniques.
Acute deletion of Cx43 selectively from enteric glial cells in Sox10::CreERT2+/−/Cx43f/f mice
The propagation of Ca2+ responses between enteric glia depends on adenosine triphosphate (ATP) release through hemichannels composed of connexin‐43 (Cx43) (Fig. 1 A) (Zhang et al. 2003; McClain et al. 2014). In prior work, we found that the ablation of glial Cx43 in vivo alters the fluid content of fecal matter (McClain et al. 2014). This observation led us to hypothesize that glial signalling mediated by Cx43 channels regulates secretomotor function. To test our hypothesis, we generated a mouse model to acutely ablate glial Cx43 by crossing the glial‐specific, inducible Cre driver line Sox10::CreERT2 (Laranjeira et al. 2011) with a floxed Cx43 line (Liao et al. 2001) to produce Sox10::CreERT2+/−/Cx43f/f mice [hereafter referred to as Cx43‐igKO (inducible glial knockout); Fig. 1 B]. Acute exposure to tamoxifen did not affect Cx43 immunoreactivity (Cx43‐ir) in Cre null littermate controls (Sox10::CreERT2–/–/Cx43f/f, hereafter referred as Cx43f/f) but resulted in the selective loss of Cx43‐ir from enteric glial cells in the myenteric plexus of Cx43‐igKO mice (Fig. 1 C). A severe reduction in glial Cx43‐ir was evident as early as 1 day following the completion of the tamoxifen induction (Fig. S2) and persisted until animals were collected for experiments (Fig. 1 C). In addition, we assessed glial Cx43‐ir in the submucosal plexus and the intestinal mucosa because of the importance of these compartments in controlling epithelial function (reviewed in Furness, 2006) and the abundance of glia in these areas (Liu et al. 2013) (Fig. S3). Tamoxifen‐treated Cx43‐igKO animals exhibited a loss of Cx43‐ir in submucosal glial cells (Fig. S3A) that was comparable to that observed in the myenteric plexus (Fig. 1 C). The loss of glial Cx43‐ir was less apparent in mucosal glia due to the abundance of Cx43‐ir in the surrounding epithelial cells (Leaphart et al. 2007) and subepithelial fibroblasts (Furuya et al. 2005) (Fig. S3B). Taken together, the temporal and spatial efficacy of this model indicated that this is a suitable animal model to investigate how the brief, i.e. within several days, loss of glial Cx43 expression impacts neuronally regulated intestinal epithelium functions in Ussing chamber experiments (Clarke, 2009).
Figure 1. Acute deletion of glial connexin 43 (Cx43) upon tamoxifen administration in the Cx43 inducible glia‐specific knock out (Cx43‐igKO) mouse model.
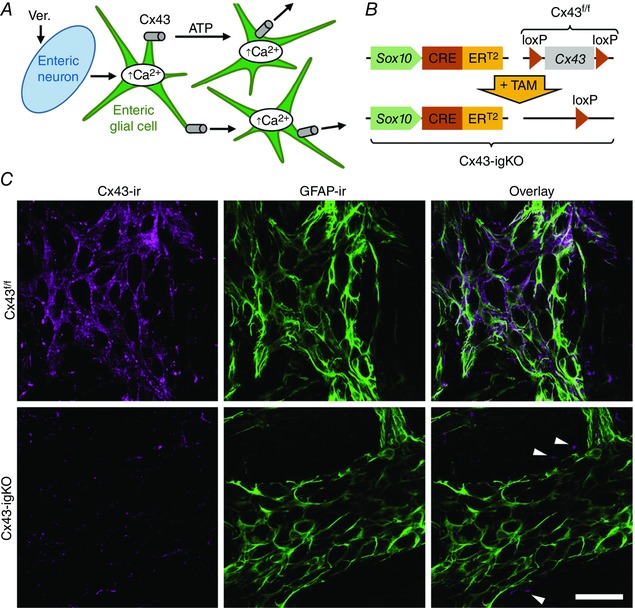
A, model of experimental paradigm. Enteric neurons were depolarized by Veratridine (Ver.), a neurotoxin that opens voltage‐dependent sodium channels and prevents their inactivation. Neuronal activity is detected by enteric glial cells and glial activity is encoded by an increase in intracellular calcium (Ca2+) concentration. The Ca2+ wave spreads through the glial network by ATP release via Cx43 hemichannels. B, Cx43‐igKO mouse model is a cross of a Sox10::CreERT2 parental line, in which the Sox10 promoter drives the glia‐specific expression of a fusion protein between Cre recombinase and the mutated estrogen receptor (ERT2) responsive to tamoxifen (TAM), with ‘floxed’ mouse line containing loxP sites flanking exon 2 of Cx43 (Cx43f/f). TAM administration to double‐positive offspring specifically activates Cre recombination in Sox10 positive glia and irreversibly excises the floxed Cx43 gene. C, confocal images of dual‐label immunohistochemistry showing immunoreactivity (ir) for Cx43 (Cx43‐ir, magenta, left) and glial fibrillary acidic protein (GFAP‐ir, green, center) in whole‐mount preparations of myenteric plexus and the adherent longitudinal muscle from the colons of tamoxifen‐treated Cx43f/f transgenic mice (top row) and their Cx43‐igKO (Sox10::CreERT2+/−/Cx43f/f) littermates (bottom row). Cx43‐ir images (left) are presented using the same fluorescence linear dynamic range. Overlays of Cx43‐ and GFAP‐ir are shown in panels at right. Two‐day tamoxifen administration reduces glia‐specific Cx43‐ir of Cx43‐igKO mice (bottom, left). Note that Cx43‐ir was not affected outside myenteric plexus (arrowheads, bottom right). Scale bar, 50 μm.
The disruption of glial Cx43 expression blunts the effect of the neuron‐regulated electrogenic ion transport
We first investigated the role of glial signalling in the regulation of active ion transport using short‐circuit current (I SC) measurements (Fig. 2). Veratridine (10 μm) was used to induce neuron depolarization while forskolin (10 μm) was applied at the end of the recording to directly drive ion secretion from epithelial cells (Fig. 2 A). Average veratridine responses were reduced in Cx43‐igKO mice compared to Cx43f/f littermate controls (37.28 ± 5.99 μA cm−2 vs. 49.16 ± 6.82 μA cm−2, mean ± SEM) but the difference was not statistically significant (P = 0.22, Student's t test, n = 6 animals per group). To reduce the possibility that variability between individual preparations was due to experimentally induced tissue damage, we normalized the neuronal component of the I SC changes induced by veratridine (ΔVer.) to the maximal epithelial response induced by forskolin (ΔForsk.) in each individual preparation (Fig. 2 B, bars and whiskers). However, we found that the majority of variability still lies in consistent differences between day‐to‐day experiments (Fig. 2 B, circles with dashed lines). Therefore, we compared pairs of preparations from Cx43‐igKO and Cx43f/f littermates that were tested simultaneously on the same day (Fig. 2 B, circles with dashed lines) and found that veratridine responses of Cx43‐igKO preparations were significantly reduced to 75 ± 5% (mean ± SEM) of the Cx43f/f littermate control responses (Fig. 2 C). This finding indicates that glial signalling significantly contributes to the neurogenic trans‐epithelial ion movement.
Figure 2. Neuron‐evoked transepithelial ion movements are reduced in Cx43‐igKO mice.
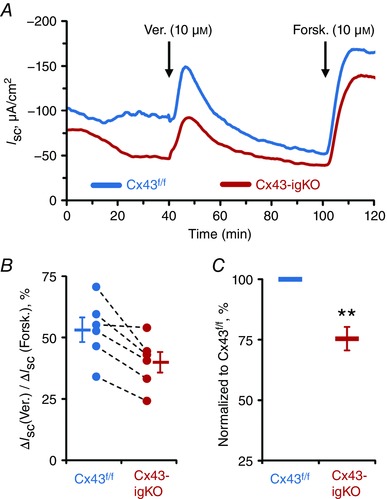
A, representative short‐circuit current (I SC) recordings from the distal colons of Cx43‐igKO (red) and Cx43f/f littermate (blue) mice responding to nerve depolarization with veratridine (Ver., 10 μm) and application of the secretagogue forskolin (Forsk., 10 μm). Drugs were added serosally at times indicated by arrows. B, veratridine responses normalized to individual reaction to forskolin. The average responses (bars and whiskers, means ± SEM) of Cx43‐igKO mice trended toward a decrease in comparison to Cx43f/f animals [P = 0.070, Student's t test (pooled variances)]. Individual veratridine responses from the paired littermate preparations are shown as circles with dashed lines. C, veratridine response of Cx43‐igKO mice normalized to the paired Cx43f/f littermate response. ** P = 0.004, one sample t test (compared from 100). Sample size, n = 6 animals per group.
Loss of glial Cx43 causes dysregulation of transmural ionic conductivity
Given that enteric glia are known regulators of the epithelial barrier (reviewed in Neunlist et al. 2013) and the loss of glia increases gut wall permeability (Savidge et al. 2007), we hypothesized that glial activity dynamically regulates barrier function and that the acute reduction of glial signalling through Cx43 (hemi)channels would affect transmural permeability of intestine. To test this hypothesis, we assessed the ionic conductivity of distal colon preparations by transmural conductance (G T) recordings. G T measures ion conductivity through both trans‐ and paracellular pathways (Clarke, 2009). G T was not significantly affected by veratridine in preparations from either Cx43f/f or Cx43‐igKO mice [ΔG T = 0.27 ± 0.83 mS cm−2 in Cx43f/f vs. −1.26 ± 1.49 mS cm−2 in Cx43‐igKO (mean ± SD), P = 0.553 and 0.056, respectively, one sample t test (compared from 0)]. However, the baseline G T values of Cx43‐igKO preparations exhibited significantly higher variability in comparison to their Cx43f/f littermate controls (Fig. 3 B) and this effect was not due to day‐to‐day experimental variation (Fig. 3 C). Paracellular permeability of the intestinal preparations assessed by the flux of cell‐impermeant fluorescein‐5‐(and‐6)‐sulfonate (FS, 478.32 Da) was also not significant different between the groups (Fig. 4). Taken together, our data indicate that although neuronal depolarization does not have a major effect on transmural ion conductance, glial pathways involving Cx43 do participate in maintaining the physiological range of transmural ion conductivity.
Figure 3. Colonic preparations of Cx43‐igKO mice exhibit more variable ionic conductivity.
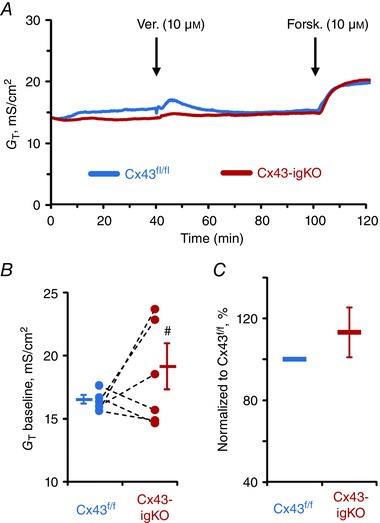
A, representative transmural conductance (G T) recordings from the distal colons of Cx43‐igKO (red) and Cx43f/f littermate (blue) mice responding to nerve depolarization with veratridine (Ver., 10 μm) and application of the secretagogue forskolin (Forsk., 10 μm). Veratridine did not cause a significant response in either group (see text for details). B, average baseline G T values (bars and whiskers, means ± SEM) of preparations from Cx43‐igKO mice exhibited higher variability in comparison to their Cx43f/f littermates. Individual baseline G T values from the paired littermate preparations are shown as circles with dashed lines. # P = 0.002, Levene's test of homogeneity of variances; P = 0.294 Student's t test of equality of means (separate variances). C, individual baseline G T values of Cx43‐igKO mice normalized to the paired Cx43f/f littermate values showed no significant difference [P = 0.29, one sample t test (compared from 100)]. Sample size, n = 6 animals per group.
Figure 4. Gut wall paracellular permeability is not affected by the glial‐specific deletion of Cx43.
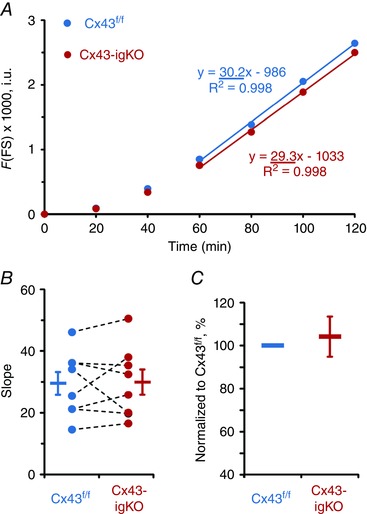
A, cell‐impermeant fluorescein‐5‐(and‐6)‐sulfonate (FS, 478.32 Da) was added mucosally and sampled in the serosal chamber. Circles depict average FS fluorescence (F) values measured in intensity units (i.u.) in experiments with tissues from Cx43‐igKO mice (red) and Cx43f/f littermates (blue). Permeability is determined by assessing the slope obtained during steady‐state dye flux (from the last four measurements). B, there was no significant difference between the average Cx43‐igKO and Cx43f/f slopes [bars and whiskers, means ± SEM; P = 0.939, Student's t test (pooled variances)]. Individual slope values from the paired Cx43‐igKO and Cx43f/f littermate preparations are shown as circles with dashed lines. C, individual Cx43‐igKO slope normalized to the paired Cx43f/f littermate slope showed no significant difference [P = 0.669, one sample t test (compared from 100)]. Sample size, n = 8 animals per group.
Transmural expression of hM3Dq ‘DREADD’ receptors by enteric glia in colon of the GFAP::hM3Dq mice
Our results show that neuron‐regulated active ion transport is significantly blunted when expression of glial Cx43 is reduced (Fig. 2), suggesting that glial activity might be sufficient to activate electrogenic ion transport. We tested this hypothesis using a chemogenetic approach (Armbruster et al. 2007) to selectively activate enteric glial cells in GFAP::hM3Dq mice (Fig. 5 A and B). In this model, the glial‐specific GFAP promoter drives the expression of the Gq‐coupled DREADD (designer receptors exclusively activated by designer drugs) hM3Dq (Agulhon et al. 2013) that is only activated by the physiologically inert, synthetic ligand, clozapine N‐oxide (CNO). Therefore, the application of CNO selectively triggers Ca2+ responses in glia. We recently showed that the hM3Dq is exclusively expressed in enteric glial cells at the level of the myenteric plexus in this model and that CNO application elicits robust Ca2+ responses in myenteric glia (McClain et al. 2015). But whether other populations of enteric glial cells (Boesmans et al. 2015; Rao et al. 2015), such as those associated with secretomotor neurons in the submucosal plexus or those associated with the epithelium, also express the transgene is not clear. We used immunohistochemistry to broadly assess hM3Dq expression by all populations of glia throughout the gut wall. Given that hM3Dq is expressed as a fusion protein with a haemagglutinin (HA) protein tag (Fig. 5 B), we took HA immunoreactivity (ir) as acceptable evidence for hM3Dq protein expression and localization. We observed HA‐ir throughout the colon wall and, more importantly, that HA‐ir is confined to GFAP‐ and S100β‐immunoreactive enteric glial cells (Fig. 5 C and D). As expected, HA‐ir colocalized more fully with S100β‐ir than GFAP‐ir because of the subcellular localization of the proteins (cytoskeletal GFAP, plasma membrane HA‐hM3Dq and cytosolic S100β) (Fig. 5 D). Of note, we observed HA‐ir in mucosal glia that are in proximity to colonic crypts (arrowheads and crossed double arrows in Fig. 5 C and D). These results show that hM3Dq receptors are expressed by all subtypes of enteric glia in the colons of GFAP::hM3Dq mice, including those in positions that could influence secretomotor and barrier functions.
Figure 5. Chemogenetic approach to stimulate enteric glial cells.
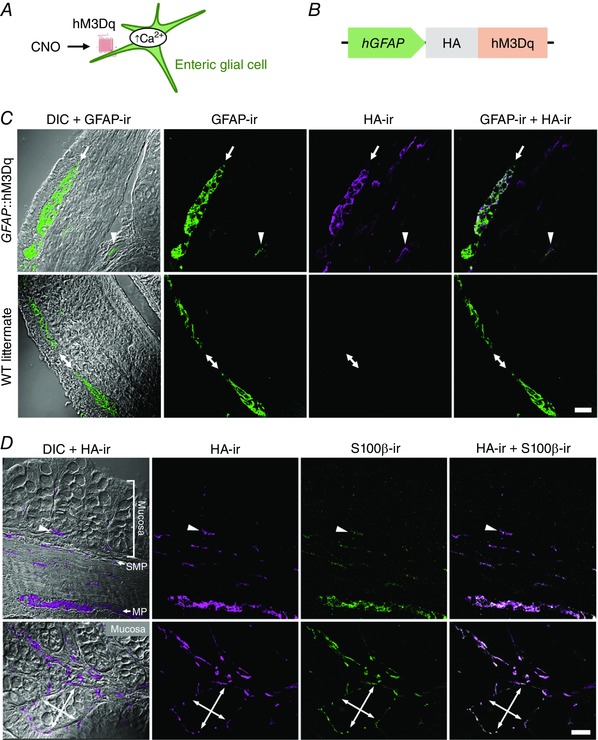
A, model of experimental paradigm: hM3Dq is a Gq‐coupled DREADD (designer receptors exclusively activated by designer drugs) that is only activated by the physiologically inert, synthetic ligand, clozapine N‐oxide (CNO). B, schematic diagram of transgene: HA‐tagged hM3Dq protein is driven by the 2.2 kB human glial fibrillary acidic protein (hGFAP) promoter. This permits selectively triggering Ca2+ responses in glia by applying CNO (A). C and D, confocal images of dual‐label immunohistochemistry for the HA‐tagged hM3Dq protein (HA‐ir, magenta in C and D) and enteric glia (GFAP‐ir and S100β‐ir, green in C and D, respectively) in transversal ‘ring’ sections of colons from GFAP::hM3Dq transgenic mice (C and D) or their WT littermates (C). Overlays of HA‐ir and glia‐specific markers are shown in panels at right. Differential interference contrast (DIC) was used for better orientation within the gut wall. C, in GFAP::hM3Dq sections (upper row) HA‐ir colocalized with GFAP‐ir within the myenteric plexus (MP, arrow) and mucosa (arrowhead) and HA‐ir was absent in WT preparations (bottom row, MP indicated by double arrows). Note that GFAP and HA‐tagged hM3Dq protein have different subcellular localization (cytoskeletal vs. plasma membrane) so they are not expected to completely colocalize. D, transmural (top) and mucosal (bottom) sections of colons from GFAP::hM3Dq mice. Note the HA‐ and S100β‐ir positive cells in proximity to the colonic crypts, either at their base (arrowheads, top) or surrounding them (crossed double arrows, bottom). SMP, submucosal plexus. Scale bars, 25 μm.
The selective activation of enteric glia emulates neuron‐driven I SC responses and directly stimulates electrogenic ion transport
We started by testing the role of glial activation on electrogenic ion transport using I SC recordings. CNO (10 μm) was applied first to selectively activate enteric glial cells while subsequent application of veratridine and forskolin served as controls for the responsiveness of the ENS and epithelial cells, respectively. Veratridine and forskolin elicited comparable responses in tissues from GFAP::hM3Dq and WT mice and the vehicle control had no effect on the I SC baseline in either (Fig. 6 A and B, blue traces). Since these tissues had similar functional characteristics, i.e. I SC baseline and responses to veratridine and forskolin, we pooled their data in subsequent analysis. CNO had no effect on preparations from WT mice [Fig. 6 B and C, blue traces; ΔI SC = 3.03 ± 2.49 mS cm−2 (mean ± SD), P = 0.093, one sample t test (compared from 0)]. In contrast, CNO elicited robust responses in preparations from GFAP::hM3Dq mice (Fig. 6 A–C, red traces) that were significantly different from the CNO‐treated WT and vehicle‐treated GFAP::hM3Dq preparations (Fig. 6 D). Furthermore, CNO‐induced responses were on average equal in magnitude to veratridine‐induced responses from control preparations (74.27 vs. 75.32% of the forskolin response) (Fig. 6 D). These data show that the selective activation of glial cells alone is sufficient to stimulate electrogenic ion transport to an extent equal to direct neuron depolarization.
Figure 6. Glial stimulation emulates neuron‐evoked changes in transepithelial ion movement and is not completely blocked by tetrodotoxin.
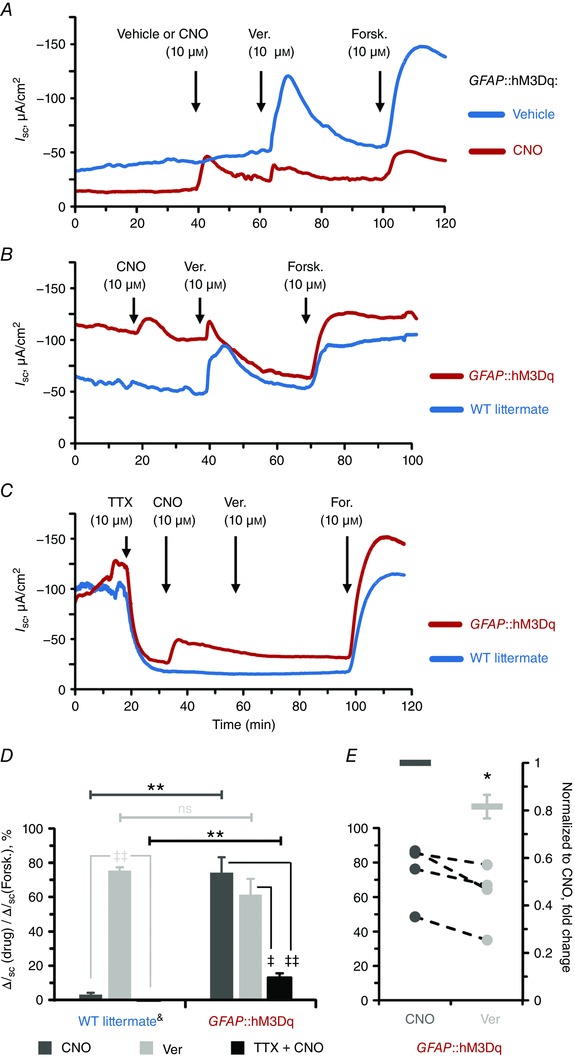
A and B, representative I SC recordings from distal colon preparations of GFAP::hM3Dq mice in response to vehicle (blue) or the hM3Dq agonist clozapine‐N‐oxide (CNO, 10 μm, red) (A) and CNO‐treated GFAP::hM3Dq mice (red) and their wild‐type (WT) littermates (blue) (B). Neuron depolarization and epithelial cell stimulation were induced by veratridine (Ver., 10 μm) and the secretagogue forskolin (Forsk., 10 μm), respectively. Drugs were added at times indicated by arrows. Note that CNO, veratridine and forskolin elicit robust I SC responses in GFAP::hM3Dq mice (A and B) while vehicle‐treated GFAP::hM3Dq mice (A) and CNO‐treated WT littermates (B) only exhibit responses to veratridine and forskolin. C, representative I SC recordings from distal colon preparations pretreated with the voltage‐gated sodium channel inhibitor tetrodotoxin (TTX, 10 μm). Other drugs were added as mentioned above. Note that CNO still elicits response in GFAP::hM3Dq mice although veratridine response is completely blocked. D, CNO (dark grey), veratridine (light grey) and TTX‐preincubated CNO (black)‐induced responses of WT littermate (left) and GFAP::hM3Dq preparations (right) normalized to the individual forskolin responses. Horizontal bars at the top mark comparisons of the same drug treatment between the genotypes (Student's t test): ** P < 0.01; ns, not significant. Angled bars mark comparisons of different drug treatments within the same genotype (analysis of variance (ANOVA) followed by Tukey/Kramer post hoc test): ‡ P < 0.05; ‡‡ P < 0.01. &CNO and Ver responses are pooled from vehicle‐treated GFAP::hM3Dq mice and CNO‐treated WT littermates (blue traces in A and B; see text for details). Sample size, n = 3–6 animals per group. E, paired responses to sequential application of CNO and veratridine from individual GFAP::hM3Dq preparations (circles, left axis, title as in D) and veratridine normalized to CNO responses (bars, right axis). * P = 0.034, one sample t test (compared from 1). Sample size, n = 4 animals per group.
As mentioned above, we observed that mucosal glia residing in close proximity to colonic crypts express the hM3Dq receptor in this mouse line (arrowheads and crossed double arrows in Fig. 5 C and D). Therefore, we hypothesized that a portion of the effect of glial activation on I SC could be due to direct effects of glia on epithelial cells rather than solely through neuronal circuits. We tested this hypothesis by assessing the efficacy of CNO in the presence of tetrodotoxin (TTX, 10 μm) to block neuronal depolarization. TTX caused a decrease in the I SC amplitude and abolished responses to veratridine (Fig. 6 C). Interestingly, TTX only partly inhibited CNO‐induced responses (Fig. 6 C) and CNO application in the presence TTX still induced significant responses (13.06 ± 2.45% of the forskolin response, mean ± SEM) in comparison to the WT preparations (Fig. 6 D). This finding strongly suggests that enteric glial cells regulate electrogenic ion transport both by influencing neuronal circuits and perhaps by more direct communication with epithelial cells. In support, veratridine responses were reduced in GFAP::hM3Dq preparations following responses driven by CNO (Fig. 6 D, right). Indeed, comparison between the two responses in the same preparations (Fig. 6 E, circles with dashed lines, left axis) revealed significant reduction of the veratridine response amplitude (Fig. 6 E, bars and whiskers, right axis). These data suggest that neuronal signalling pathways driven by glial cells are rendered refractory due to their preceding activation with CNO. In support, the amplitude of the observed effect is comparable to the previously mentioned TTX‐resistant CNO responses. Together, our data suggest that mucosal glia might serve as an intermediary between the neurons and epithelial cells of the intestines.
To test the specific type of ion involved in the observed I SC changes, we treated a subset of tissues with the sodium channel inhibitor amiloride and then subsequently TTX and CNO (data not shown). The direct glial response with or without amiloride pretreatment (12.61 ± 2.89% vs. 13.06 ± 4.25% of forskolin I SC response (mean ± SEM), n = 3) was not significantly different [P = 0.886, Student's t test (pooled variances)]. This outcome suggests that glial activation does not primarily affect epithelial cells through mechanisms that involve amiloride‐sensitive sodium ion transport.
Glial stimulation has no effect on transmural ion conductivity and gut wall permeability
Finally, we measured G T and FS flux following the activation of glia to investigate the effect of glial activity on transmural permeability in the mouse distal colon (Figs. 7–8). CNO did not cause a significant G T responses in either GFAP::hM3Dq or control preparations [ΔG T = −0.30 ± 0.78 and −0.11 ± 0.23 mS cm−2 (mean ± SD), P = 0.399 and 0.166, one sample t test (compared from 0)] and other drug combinations also had no significant effects (Fig. 7 D). Similarly, CNO application did not significantly change FS flux in the colons of either GFAP::hM3Dq or WT littermates (Fig. 8 B–C). Together, these results show that the selective activation of glia does not play a significant role in acute regulation of transmural ion conductance and paracellular permeability of the gut wall.
Figure 7. Selective activation of glial Ca2+ responses does not affect trasmural ionic conductivity.
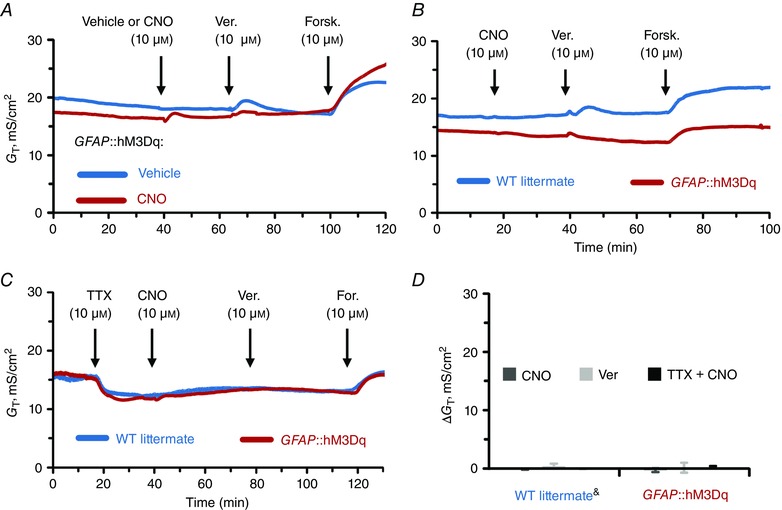
A–D, individual G T recordings from distal colon preparations (A–C) and average G T responses (D) of GFAP::hM3Dq and WT littermate preparations (genotype, drug and other labels as described in previous figure legend). &CNO and Ver responses are pooled from vehicle‐treated GFAP::hM3Dq mice and CNO‐treated WT littermates (blue traces in A and B, see text for details). Sample size, n = 3–6 animals per group.
Figure 8. Gut wall paracellular permeability is not affected by the glial‐specific activity.
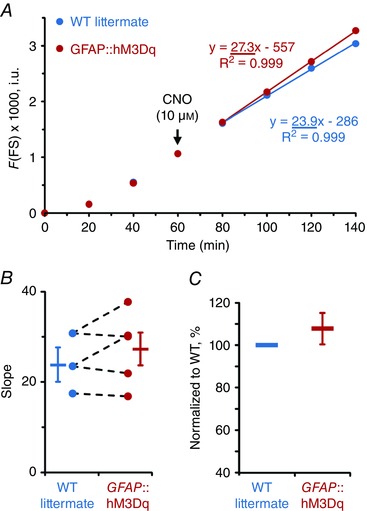
A, average FS fluorescence (F) measured in intensity units (i.u.) of GFAP::hM3Dq (red) and WT littermate (blue) preparations. CNO was applied as indicated by arrow. Permeability was determined by assessing the slope obtained during the last four measurements. B, there was no significant difference in average slopes (bars and whiskers, means ± SEM) between the genotypes (P = 0.559, Student's t test (pooled variances)). Individual slope values from the paired GFAP::hM3Dq and WT littermate preparations are shown as circles with dashed lines. C, individual GFAP::hM3Dq slope normalized to the paired WT littermate response showed no significant difference [P = 0.354, one sample t test (compared from 100)]. Sample size, n = 3 and 5 animals per group.
Discussion
Our results provide the first direct evidence for the regulation of colonic electrogenic ion transport by glial activity (Fig. 9). Glial activity had an impressive influence over the neural control of secretomotor activities in our studies. For example, the selective activation of glia was sufficient to elicit I SC responses of equal magnitude as those driven by directly activating neurons; and neurogenic I SC responses were impaired when we interfered with glial Cx43‐mediated intercellular signalling. Interestingly, glial activation appears to influence secretomotor activities by modulating neuron activity and through TTX‐insensitive pathways that may include direct signalling between mucosal glia and intestinal epithelial cells. In contrast, we did not detect a major glial influence in the acute regulation of intestinal permeability.
Figure 9. Role of enteric glial activity in electrogenic ion transport of mouse colon.
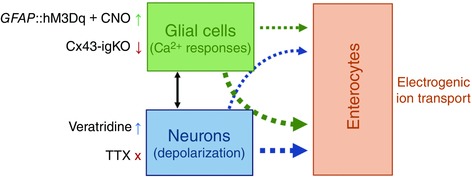
Glial Ca2+ responses are central in the close, bi‐directional, interactions between enteric neurons and glial cells. In this study we selectively activated (GFAP::hM3Dq + CNO) or inhibited (Cx43‐igKO) glial Ca2+ responses in concert with neuron depolarization (veratridine) or inhibition of neuron action potentials (TTX, tetrodotoxin). We found that selective stimulation of enteric glia and neuron depolarization have equal effects on electrogenic ion transport. Neuron depolarization‐induced electrogenic ion transport significantly relies on the activity of enteric glia (narrow blue arrow) while glia‐stimulated secretory response is mostly mediated via neurons (thick green arrow) and additional mechanisms that may include direct signalling between enteric glial cells and enterocytes (narrow green arrow). Further experiments are needed to show direct interaction with enterocytes (dotted arrows). Width of arrows depicts the relative amplitude of the responses. This schematic diagram is not drawn to scale.
Enteric glial cells are intimate companions on neurons (Gabella, 1972) and were traditionally considered passive support cells due to their electrical inexcitability (Hanani et al. 2000). Glial activity, however, is encoded by changes in intracellular Ca2+ concentration and enteric glial cells both respond to and influence enteric neurotransmission through pathways that involve Ca2+ signalling (reviewed in Grubisic & Gulbransen, 2017). Our earlier work showed that the selective manipulation of glial Ca2+ responses modifies gut motility through effects on enteric neurons because the glial‐driven effects on motility were abolished in the presence of TTX (McClain et al. 2014, 2015). In contrast, the effects of glial Ca2+ responses on electrogenic ion transport were not completely abolished by even high concentrations of TTX in our current study (Fig. 6 C). This TTX‐insensitive component could be due to residual neural activity mediated by enteric neurons expressing TTX‐resistant sodium channels such as Na(v)1.9 (Padilla et al. 2007) or through non‐neuronal pathways. Given the close proximity of glial cells to the base of colonic crypts in the mouse (Fig. 5 D) and human (Liu et al. 2013) intestine, it is easy to speculate that direct interactions between the mucosal glia and enterocytes are responsible for the TTX‐resistant component of the responses. The precise mechanisms that might mediate signalling between the glia and enterocytes are currently unclear but could include gliotransmission via Cx43 hemichannels. For example, enteric glia utilize Cx43 hemichannels to release ATP into the extracellular space (Zhang et al. 2003; Brown et al. 2016) and purines are known pro‐secretory mediators (Kottgen et al. 2003). In either case, the selective activation of glia appears to influence enterocyte chloride ion secretion because epithelial cells from the base of the colonic crypts are predominantly involved in chloride ion secretion (reviewed in Geibel, 2005). Likewise, the glial influence on electrogenic ion transport was not affected by amiloride, a broad sodium channel inhibitor (see Results for details).
Interestingly, we observed significantly smaller veratridine responses following glial‐driven responses (Fig. 6 E). Desensitization of neurons, glia or enterocytes could all contribute to this phenomenon. For example, large neuronal responses driven by glial stimulation may cause these circuits to fatigue and rundown to occur with successive stimulation. Alternatively, the stimulation of mucosal glia by CNO may render them refractory to subsequent neuronal stimulation. In support, similar effects were observed in studies of retinal glial cells (Newman & Zahs, 1997). This scenario would imply that glia act as intermediaries between neuronal innervation and enterocytes and definitely supports the concept of the digestive ‘neuronal–glial–epithelial unit’ (Neunlist et al. 2013). However, this does not imply direct cell‐to‐cell communication between glia and enterocytes as other cells residing in the gut mucosa, e.g. subepithelial fibroblast, might be also involved. In any case, these data raise many questions about intercellular interactions at the level of the intestinal mucosa that should be further investigated.
The TTX‐sensitive component of glial regulated electrogenic ion transport (Fig. 6 D) clearly arises from glial interactions with enteric neurons. Some of the mechanisms involved in enteric neuron‐glia interactions have recently been identified (reviewed in Grubisic & Gulbransen, 2017) and it is clear that glia are both capable of responding to neurotransmitters and releasing neuroactive substances. Glial release of ATP through Cx43 hemichannels is involved in the propagation of Ca2+ responses through the network of enteric glial cells (Kimball & Mulholland, 1996; McClain et al. 2014) and also serves as a neurotransmitter in the ENS (reviewed in Ren & Bertrand, 2008). This feed‐forward mechanism of glial stimulation probably accounts for the ability of glia to drive responses of equal magnitude to neuron depolarization‐evoked responses (Fig. 6 D). In support, the reduction of glial Cx43 expression in submucosal (Fig. S3A) and myenteric (Fig. 1 C) ganglia significantly reduces neurogenic ion transport (Fig. 2 C). Together, these observations indicate that enteric glial cells have a prominent interaction with nerve pathways that drive secretion.
Gut secretomotor function is also influenced by extrinsic innervation and sympathetic innervation has a prominent role in the inhibition of secretion (reviewed in Furness, 2006). These extrinsic neurons are accompanied by peripheral glial cells that express Sox10 (Britsch et al. 2001) and the deletion of Cx43 in these cells could contribute to altered secretomotor function in our model system. Although sympathetic ganglia are absent in our preparations, the axons of sympathetic neurons are present and could affect our findings. These axons are accompanied by myelinating Schwann cells that express Sox10. Very little is known about how Schwann cells influence the activity of sympathetic axons in the periphery. What is known suggests that myelinating Schwann cells do share some common features with enteric glia such as the expression of Sox10 and the ability to release neuroactive substances like ATP through hemichannels (Nualart‐Marti et al. 2013). However, there are also important differences between the specific mechanisms used by the two cell types. Importantly, Schwann cells that myelinate peripheral nerves release ATP through mechanisms that involve connexin 32 (Cx32) hemichannels (see Nualart‐Marti et al. 2013) whereas enteric glia release ATP through Cx43 hemichannels. This distinct difference in the mechanisms between the two cell types makes it unlikely that our model of Cx43 ablation significantly affected the interactions between Schwann cells and sympathetic nerves although we cannot entirely rule out this possibility. Enteric glia in the myenteric plexus are also influenced by sympathetic innervation (Gulbransen et al. 2010) so it is possible that these interactions could influence our current outcomes. However, in this study we show that glial activity enhances secretomotor function while sympathetic innervation has known inhibitory effects on fluid secretion (reviewed in Furness, 2006). It is tempting to speculate that glial activity acts as feed‐back mechanism to inhibit sympathetic nerve terminals but we do not have any current evidence to support this speculation.
We originally hypothesized that glial activity would modulate gut barrier function based on the clear influence of glial mediators on epithelial function in cell culture models (Neunlist et al. 2007; Savidge et al. 2007; Bach‐Ngohou et al. 2010; Van Landeghem et al. 2011). Therefore, we were surprised to find that neither the activation of enteric glia nor neurons caused acute changes in gut permeability (Fig. 7 D). Despite the fact that measurements of dye flux in intact gut preparations are not as sensitive as measurements obtained using cell monolayers in vitro, intact preparations represent a more physiologically relevant system and there is no good evidence to suggest that the measures used in our study would be insufficient to detect changes in gut permeability. The more likely explanation for this discrepancy is that tight junction rearrangement requires more time to have a measurable effect. In support, the previously published data supporting a role for glia in barrier modulation were mainly gained from experiments that were performed several days after the experimental manipulation and we similarly showed that Cx43‐igKO animals, tested 5 days after the tamoxifen induction (Fig. S2), do have disrupted ion conductance (Fig. 3 B). It is unlikely that the variability of these G T responses is due to inconsistent tamoxifen induction because we also measured I SC in these preparations and did not observe large variability in this measure. Therefore, the change in G T in Cx43‐igKO animals does indicate that glial Cx43 plays a role in regulation of the gut permeability. Together, these observations suggest that enteric glial cells affect gut barrier function through tonic, rather than acute, mechanisms. Glial Cx43 could play an important role in the chronic regulation of barrier function by regulating the release of modulators of epithelial function (reviewed in Neunlist et al. 2013) such as prostaglandins (Cherian et al. 2005).
Our data show that glia play an important role in the regulation of water movement across the gut epithelium through effects on electrogenic ion transport. One major implication of these findings is that changes to glial cells during infection or inflammation could be important mechanisms in the pathogenesis of many common gastrointestinal diseases. Chloride transport from epithelial cells is the major determinant of fluid secretion (Barrett & Keely, 2000) and mucosal secretion of fluid is important component of host defenses. Enteric glial cells express receptors to detect components of bacteria (Barajon et al. 2009) and inflammatory stimuli (Stoffels et al. 2014), so the acute activation of enteric glia could participate in the clearing of pathogens by stimulating fluid secretion. However, the prolonged and/or excessive exposure to these signals prompts a phenotypic switch of glial cells into ‘reactive glia’ and reactive gliosis is observed in many gastrointestinal disorders (Linan‐Rico et al. 2016). Reactive enteric glia, for example, have abnormal Ca2+ signalling and a 5‐fold increase in ATP release (Linan‐Rico et al. 2016). Taken together with our results, reactive glia might lead to increased chloride secretion from the gut epithelium and contribute to secretory diarrhea. Similar glial hyperactivity could contribute to other diarrheal diseases without known overt pathology such as diarrhea predominant irritable bowel syndrome and functional diarrhea. In these situations, glia‐targeted strategies could be important new therapeutic approaches.
In conclusion, our data show a novel role for enteric glia in the physiological regulation of the intestinal epithelium. Although glial Ca2+ responses did not acutely affect gut wall permeability, glial Cx43 participates in the long‐term regulation of the barrier function. Our findings show that glial activity works in concert with neuronal activity to regulate electrogenic ion transport. Glia also influence ion transport through TTX‐insensitive pathways that may involve direct interactions with epithelial cells. These observations raise the possibility that enteric glial activation plays a key role in functional diarrheal diseases and that the selective manipulation of glia could be beneficial to improve treatment and patient quality of life.
Additional information
Competing interests
The authors have no financial, professional or personal conflicts that are relevant to the manuscript.
Author contributions
Overall project conceptualization and design was developed by V.G. under the supervision of B.D.G. Experiments and analysis were performed by V.G. The manuscript was written by V.G. and edited by V.G. and B.D.G. Both authors agreed to the final version.
Funding
Dr Gulbransen's research is currently supported by grants from the Crohn's and Colitis Foundation of America (CCFA; Senior Research Award; Grant No. 327058) and the National Institutes of Health (NIH; RO1DK103723).
Supporting information
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Figure S1. Control for immunohistochemistry protocol using primary antibodies raised in the same host (Fig. 5D).
Figure S2. Dynamic expression of the connexin 43 (Cx43) in the Cx43 inducible and glia‐specific knock out (Cx43‐igKO) animal model.
Figure S3. Expression of glial Cx43 in the submucosal plexus and mucosa.
Acknowledgements
The authors thank Dr Susanne Mohr for access to a microplate reader and Jonathon L. McClain for help with animal husbandry and experimental blinding procedures.
Linked articles This article is highlighted by a Perspective by Boesmans & Vanden Berghe. To read this Perspective, visit https://doi.org/10.1113/JP273975.
This is an Editor's Choice article from the 1 June 2017 issue.
References
- Agulhon C, Boyt KM, Xie AX, Friocourt F, Roth BL & McCarthy KD (2013). Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein‐coupled receptor activation in vivo . J Physiol 591, 5599–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S & Roth BL (2007). Evolving the lock to fit the key to create a family of G protein‐coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104, 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach‐Ngohou K, Mahe MM, Aubert P, Abdo H, Boni S, Bourreille A, Denis MG, Lardeux B, Neunlist M & Masson D (2010). Enteric glia modulate epithelial cell proliferation and differentiation through 15‐deoxy‐12,14‐prostaglandin J2. J Physiol 588, 2533–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A & Rumio C (2009). Toll‐like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem 57, 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett KE & Keely SJ (2000). Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62, 535–572. [DOI] [PubMed] [Google Scholar]
- Boesmans W, Cirillo C, Van den Abbeel V, Van den Haute C, Depoortere I, Tack J & Vanden Berghe P (2013). Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol Motil 25, e151–160. [DOI] [PubMed] [Google Scholar]
- Boesmans W, Lasrado R, Vanden Berghe P & Pachnis V (2015). Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 63, 229–241. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C & Wegner M (2001). The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev 15, 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhead MJ, Bayguinov PO, Okamoto T, Heredia DJ & Smith TK (2012). Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J Physiol 590, 335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IA, McClain JL, Watson RE, Patel BA & Gulbransen BD (2016). Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin‐43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2, 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH & Sofroniew MV (1998). Fulminant jejuno‐ileitis following ablation of enteric glia in adult transgenic mice. Cell 93, 189–201. [DOI] [PubMed] [Google Scholar]
- Cherian PP, Siller‐Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E & Jiang JX (2005). Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell 16, 3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LL (2009). A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol 296, G1151–G1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke HJ (1989). Role of the “little brain” in the gut in water and electrolyte homeostasis. FASEB J 3, 127–138. [DOI] [PubMed] [Google Scholar]
- Furness JB (2006). The Enteric Nervous System. Blackwell Publishing, Inc. [Google Scholar]
- Furness JB, Callaghan BP, Rivera LR & Cho HJ (2014). The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol 817, 39–71. [DOI] [PubMed] [Google Scholar]
- Furuya S, Furuya K, Sokabe M, Hiroe T & Ozaki T (2005). Characteristics of cultured subepithelial fibroblasts in the rat small intestine. II. Localization and functional analysis of endothelin receptors and cell‐shape‐independent gap junction permeability. Cell Tissue Res 319, 103–119. [DOI] [PubMed] [Google Scholar]
- Gabella G (1972). Fine structure of the myenteric plexus in the guinea‐pig ileum. J Anat 111, 69–97. [PMC free article] [PubMed] [Google Scholar]
- Geibel JP (2005). Secretion and absorption by colonic crypts. Annu Rev Physiol 67, 471–490. [DOI] [PubMed] [Google Scholar]
- Groschwitz KR & Hogan SP (2009). Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124, 3–20; quiz 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubisic V & Gulbransen BD (2017). Enteric glia: the most alimentary of all glia. J Physiol 595, 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Bains JS & Sharkey KA (2010). Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J Neurosci 30, 6801–6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ & Sharkey KA (2012). Activation of neuronal P2X7 receptor‐pannexin‐1 mediates death of enteric neurons during colitis. Nat Med 18, 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD & Sharkey KA (2009). Purinergic neuron‐to‐glia signaling in the enteric nervous system. Gastroenterology 136, 1349–1358. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD & Sharkey KA (2012). Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9, 625–632. [DOI] [PubMed] [Google Scholar]
- Hanani M, Francke M, Hartig W, Grosche J, Reichenbach A & Pannicke T (2000). Patch‐clamp study of neurons and glial cells in isolated myenteric ganglia. Am J Physiol Gastrointest Liver Physiol 278, G644–G651. [DOI] [PubMed] [Google Scholar]
- Hyland NP & Cox HM (2005). The regulation of veratridine‐stimulated electrogenic ion transport in mouse colon by neuropeptide Y (NPY), Y1 and Y2 receptors. Br J Pharmacol 146, 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball BC & Mulholland MW (1996). Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C‐dependent mechanism. J Neurochem 66, 604–612. [DOI] [PubMed] [Google Scholar]
- Kottgen M, Loffler T, Jacobi C, Nitschke R, Pavenstadt H, Schreiber R, Frische S, Nielsen S & Leipziger J (2003). P2Y6 receptor mediates colonic NaCl secretion via differential activation of cAMP‐mediated transport. J Clin Invest 111, 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P & Pachnis V (2011). Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest 121, 3412–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaphart CL, Qureshi F, Cetin S, Li J, Dubowski T, Baty C, Beer‐Stolz D, Guo F, Murray SA & Hackam DJ (2007). Interferon‐gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology 132, 2395–2411. [DOI] [PubMed] [Google Scholar]
- Liao Y, Day KH, Damon DN & Duling BR (2001). Endothelial cell‐specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci USA 98, 9989–9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linan‐Rico A, Turco F, Ochoa‐Cortes F, Harzman A, Needleman BJ, Arsenescu R, Abdel‐Rasoul M, Fadda P, Grants I, Whitaker E, Cuomo R & Christofi FL (2016). Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype: implications for GI infection, IBD, POI, neurological, motility, and GI disorders. Inflamm Bowel Dis 22, 1812–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YA, Chung YC, Pan ST, Shen MY, Hou YC, Peng SJ, Pasricha PJ & Tang SC (2013). 3‐D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterol Motil 25, e324–338. [DOI] [PubMed] [Google Scholar]
- McClain JL, Fried DE & Gulbransen BD (2015). Agonist‐evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol 1, 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JL, Grubisic V, Fried D, Gomez‐Suarez RA, Leinninger GM, Sevigny J, Parpura V & Gulbransen BD (2014). Ca2+ responses in enteric glia are mediated by connexin‐43 hemichannels and modulate colonic transit in mice. Gastroenterology 146, 497–507.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEachern SJ, Patel BA, Keenan CM, Dicay M, Chapman K, McCafferty DM, Savidge TC, Beck PL, MacNaughton WK & Sharkey KA (2015). Inhibiting inducible nitric oxide synthase in enteric glia restores electrogenic ion transport in mice with colitis. Gastroenterology 149, 445–455.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEachern SJ, Patel BA, McKay DM & Sharkey KA (2011). Nitric oxide regulation of colonic epithelial ion transport: a novel role for enteric glia in the myenteric plexus. J Physiol 589, 3333–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, Naveilhan P, Ruhl A, Lardeux B, Savidge T, Paris F & Galmiche JP (2007). Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF‐beta1‐dependent pathway. Am J Physiol Gastrointest Liver Physiol 292, G231–G241. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Van Landeghem L, Mahe MM, Derkinderen P, des Varannes SB & Rolli‐Derkinderen M (2013). The digestive neuronal‐glial‐epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol 10, 90–100. [DOI] [PubMed] [Google Scholar]
- Newman EA & Zahs KR (1997). Calcium waves in retinal glial cells. Science 275, 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nualart‐Marti A, del Molino EM, Grandes X, Bahima L, Martin‐Satue M, Puchal R, Fasciani I, Gonzalez‐Nieto D, Ziganshin B, Llobet A, Barrio LC & Solsona C (2013). Role of connexin 32 hemichannels in the release of ATP from peripheral nerves. Glia 61, 1976–1989. [DOI] [PubMed] [Google Scholar]
- Padilla F, Couble ML, Coste B, Maingret F, Clerc N, Crest M, Ritter AM, Magloire H & Delmas P (2007). Expression and localization of the Nav1.9 sodium channel in enteric neurons and in trigeminal sensory endings: implication for intestinal reflex function and orofacial pain. Mol Cell Neurosci 35, 138–152. [DOI] [PubMed] [Google Scholar]
- Pochard C, Coquenlorge S, Jaulin J, Cenac N, Vergnolle N, Meurette G, Freyssinet M, Neunlist M & Rolli‐Derkinderen M (2016). Defects in 15‐HETE production and control of epithelial permeability by human enteric glial cells from patients with Crohn's disease. Gastroenterology 150, 168–180. [DOI] [PubMed] [Google Scholar]
- Rao M, Nelms BD, Dong L, Salinas‐Rios V, Rutlin M, Gershon MD & Corfas G (2015). Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia 63, 2040–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J & Bertrand PP (2008). Purinergic receptors and synaptic transmission in enteric neurons. Purinergic Signal 4, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R & Sofroniew MV (2007). Enteric glia regulate intestinal barrier function and inflammation via release of S‐nitrosoglutathione. Gastroenterology 132, 1344–1358. [DOI] [PubMed] [Google Scholar]
- Sjovall H, Abrahamsson H, Westlander G, Gillberg R, Redfors S, Jodal M & Lundgren O (1986). Intestinal fluid and electrolyte transport in man during reduced circulating blood volume. Gut 27, 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffels B, Hupa KJ, Snoek SA, van Bree S, Stein K, Schwandt T, Vilz TO, Lysson M, Veer CV, Kummer MP, Hornung V, Kalff JC, de Jonge WJ & Wehner S (2014). Postoperative ileus involves interleukin‐1 receptor signaling in enteric glia. Gastroenterology 146, 176–187.e1. [DOI] [PubMed] [Google Scholar]
- Van Landeghem L, Chevalier J, Mahe MM, Wedel T, Urvil P, Derkinderen P, Savidge T & Neunlist M (2011). Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol 300, G976–G987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Segura BJ, Lin TR, Hu Y & Mulholland MW (2003). Intercellular calcium waves in cultured enteric glia from neonatal guinea pig. Glia 42, 252–262. [DOI] [PubMed] [Google Scholar]
- Zhu HL, Wassall RD, Takai M, Morinaga H, Nomura M, Cunnane TC & Teramoto N (2009). Actions of veratridine on tetrodotoxin‐sensitive voltage‐gated Na currents, Na1.6, in murine vas deferens myocytes. Br J Pharmacol 157, 1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Figure S1. Control for immunohistochemistry protocol using primary antibodies raised in the same host (Fig. 5D).
Figure S2. Dynamic expression of the connexin 43 (Cx43) in the Cx43 inducible and glia‐specific knock out (Cx43‐igKO) animal model.
Figure S3. Expression of glial Cx43 in the submucosal plexus and mucosa.


