Abstract
Key points
The nucleus reuniens (Re), a nucleus of the midline thalamus, is part of a cognitive network including the hippocampus and the medial prefrontal cortex. To date, very few studies have examined the electrophysiological properties of Re neurons at a cellular level.
The majority of Re neurons exhibit spontaneous action potential firing at rest. This is independent of classical amino‐acid mediated synaptic transmission. When driven by various forms of depolarizing current stimulus, Re neurons display considerable diversity in their firing patterns. As a result of the presence of a low threshold Ca2+ channel, spike output functions are strongly modulated by the prestimulus membrane potential.
Finally, we describe a novel form of activity‐dependant intrinsic plasticity that eliminates the high‐frequency burst firing present in many Re neurons.
These results provide a comprehensive summary of the intrinsic electrophysiological properties of Re neurons allowing us to better consider the role of the Re in cognitive processes.
Abstract
The nucleus reuniens (Re) is the largest of the midline thalamic nuclei. We have performed a detailed neurophysiological characterization of neurons in the rostral Re of brain slices prepared from adult male mice. At resting potential (−63.7 ± 0.6 mV), ∼90% of Re neurons fired action potentials, typically continuously at ∼8 Hz. Although Re neurons experience a significant spontaneous barrage of fast, amino‐acid‐mediate synaptic transmission, this was not predominantly responsible for spontaneous spiking because firing persisted in the presence of glutamate and GABA receptor antagonists. With resting potential preset to −80 mV, −20 pA current injections revealed a mean input resistance of 615 MΩ and a mean time constant of 38 ms. Following cessation of this stimulus, a significant rebound potential was seen that was sometimes sufficiently large to trigger a short burst of very high frequency (100–300 Hz) firing. In most cells, short (2 ms), strong (2 nA) current injections elicited a single spike followed by a large afterdepolarizing potential which, when suprathreshold, generated high‐frequency spiking. Similarly, in the majority of cells preset at −80 mV, 500 ms depolarizing current injections to cells led to a brief initial burst of very high‐frequency firing, although this was lost when cells were preset at −72 mV. Biophysical and pharmacological experiments indicate a prominent role for T‐type Ca2+ channels in the high‐frequency bursting of Re neurons. Finally, we describe a novel form of activity‐dependent intrinsic plasticity that persistently eliminates the burst firing potential of Re neurons.
Keywords: action potential, brain slices, calcium channel, excitability, plasticity, thalamus
Key points
The nucleus reuniens (Re), a nucleus of the midline thalamus, is part of a cognitive network including the hippocampus and the medial prefrontal cortex. To date, very few studies have examined the electrophysiological properties of Re neurons at a cellular level.
The majority of Re neurons exhibit spontaneous action potential firing at rest. This is independent of classical amino‐acid mediated synaptic transmission. When driven by various forms of depolarizing current stimulus, Re neurons display considerable diversity in their firing patterns. As a result of the presence of a low threshold Ca2+ channel, spike output functions are strongly modulated by the prestimulus membrane potential.
Finally, we describe a novel form of activity‐dependant intrinsic plasticity that eliminates the high‐frequency burst firing present in many Re neurons.
These results provide a comprehensive summary of the intrinsic electrophysiological properties of Re neurons allowing us to better consider the role of the Re in cognitive processes.
Abbreviations
- ADP
after‐depolarization potential
- αEPSC
EPSC like waveform
- AHP
afterhyperpolarization
- AP
action potential
- HCN
hyperpolarization‐activated cyclic nucleotide gated
- HPC
hippocampus
- mPFC
medial prefrontal cortex
- Re
nucleus reuniens
- TC
thalamocortical
Introduction
The nucleus reuniens (Re) is the largest of the midline thalamic nuclei. Located within the interthalamic adhesion, Re is a component of the higher‐order thalamus, a group of thalamic structures that receive little sensory input. Instead, such areas form extensive cortico‐thalamo‐cortical pathways (Vertes et al. 2015). The roles of Re have been the subject of growing interest (Cassel et al. 2013; Aggleton, 2014). In rodents, experimentally‐induced lesions to Re result in a range of cognitive outcomes (Cholvin et al. 2013; Hallock et al. 2013; Xu & Südhof, 2013), whereas, in man, this brain area is selectively compromised in Korsakoff's syndrome (amnesic‐confabulatory syndrome), a form of dementia resulting from thiamine (vitamin B1) deficiency not only often arising from alcoholism or malnutrition, but also associated with eating disorders, chemotherapy or persistent vomiting, such as that in hyperemesis gravidarum (Mair et al. 1979).
The contributions of Re to cognitive function have a sound grounding in its neural connectivity (Cassel et al. 2013; Aggleton, 2014). In particular, Re output is almost exclusively directed to the hippocampus (HPC) and limbic cortex. This includes strong monosynaptic projections to structures with key roles in cognitive function, notably area CA1 and the subiculum of (particularly ventral) HPC and much of the medial prefrontal cortex (mPFC) (Vertes, 2006; Vertes et al. 2007, 2015; Prasad & Chudasama, 2013; Varela et al. 2014). This wiring allows the Re to ‘close a synaptic loop’ between the ventral hippocampus (HPC)/subiculum and the mPFC. Indeed, afferents from the mPFC have been demonstrated to synapse on Re neurones that in turn project to CA1 (Vertes et al. 2007).
Functionally speaking, Re innervation of the hippocampus appears to be substantial. For example, it is reported that electrical stimulation of the Re in vivo produces substantial, monosynaptic latency, excitatory responses in neurons of hippocampal area CA1 (Dolleman‐Van der Weel et al. 1997). Excitation of both pyramidal cells and interneurones is reported (Dolleman‐Van der Weel et al. 1997; Dolleman‐Van der Weel & Witter, 2000). Interestingly, Re stimulation is reported to be as effective in discharging hippocampal CA1 neurons as equivalent stimulation of hippocampal area CA3, the presynaptic source of the intensively studied Schaffer collateral commissural pathway. Furthermore, Re‐CA1 synapses exhibit greater frequency facilitation than CA3–CA1 connections, and at least as much long‐term potentiation (Bertram & Zhang, 1999). The synapses responsible for this robust synaptic drive are predominantly located within the stratum lacunosum moleculare, where the distal dendrites of CA1 cells are found, as well as the temporo‐ammonic axons conveying direct excitatory input from the entorhinal cortex. Indeed, studies using extracellular electrical stimulation of hippocampal slices in an attempt to drive the temporoamonic input to CA1 cells (Giocomo & Hasselmo, 2006; Ceolin et al. 2011; Booth et al. 2016b) are probably also recruiting Re axons. Interestingly, in vivo awake tetrode recording has recently revealed that Re has ‘head direction’ cells (Jankowski et al. 2014), a neural activity more commonly associated with neurons in the entorhinal cortex and hippocampus/subiculum (Taube et al. 1990; Sargolini et al. 2006). This finding prompted further investigation of spatially responsive cells in the Re, which revealed evidence of both place cells and border cells, providing further evidence for the importance of the Re in spatial navigation (Jankowski et al. 2015).
In vivo recordings have also demonstrated the presence of Re neurons that exhibit trajectory‐dependant firing during a T‐maze based continuous alteration task, similar to neural activity observed in the CA1 region of the hippocampus and the mPFC. Interestingly, lesioning or optogenetic silencing of the Re leads to a substantial reduction in trajectory‐dependant firing in CA1. This suggests that the Re plays an important role in the transfer of information from the mPFC to CA1 that pertains to future path choices in goal‐directed behaviours (Ito et al. 2015). Another recent in vivo study (Hallock et al. 2016) has also indicated that structures in the midline thalamus are crucial for the synchronous oscillatory behaviour arising between the hippocampus and pre‐frontal cortex during working memory tasks (Jones & Wilson, 2005).
Despite the significant body of data that address the connectivity of the Re, its roles in behaviour and its pathology in disease, neurophysiological understanding of this cognitively important thalamic structure is far from comprehensive. Practically all published Re neurophysiology to date has been performed with in vivo extracellular recording methods either in anaesthetized (Dolleman‐Van der Weel et al. 1997; Bertram & Zhang, 1999) or, more recently, awake behaving rats (Jankowski et al. 2014; Ito et al. 2015; Hallock et al. 2016). Unquestionably, there have been few detailed cell‐level electrophysiological studies of Re neurons. Working in the mid‐line thalamus, which probably included Re, Graef et al. (2009) examined changes to bursting and T‐type Ca2+ channels produced in a pilocarpine‐induced epilepsy model. The same group subsequently made a similar study, in this case specifically working within Re, examining the consequences of ethanol exposure and withdrawal (Graef et al. 2011). The only other intracellular data from Re neurons of which we are aware comprises a single panel in a supplementary figure in Xu & Südhof (2013), which presents miniature IPSCs recorded from six mice at 26°C.
Given the growing interest in the neural functionality of Re, we have embarked upon a series of in vitro brain slices studies designed to better characterize the cellular neurophysiology of Re. We outline the intrinsic excitability properties of neurons in the rostral Re, using studies performed entirely using coronal brain slices prepared from adult male mice.
Methods
Ethical approval
All work in the present study was approved by the University of Exeter Animal Welfare Ethical Review Board. Animals were killed in accordance with schedule 1 of the UK Animals (Scientific Procedures) Act 1986 and the subsequent amendments regulations of 2012, as implemented in response to directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes.
Animals and tissue preparation
All tissues for the present study were obtained from male C57‐Bl/6 mice bred in the University of Exeter Biological Services Unit using founders purchased from Charles River (Margate, UK). All animals were granted ad libitum access to both food and water and were housed under a 12:12 h light/dark cycle. All animals were aged 14–18 weeks and were killed by cervical dislocation. The brain was rapidly removed and placed an in ice‐cold slicing medium consisting of (in mm): 189 sucrose, 10 d‐glucose, 26 NaHCO3, 3 KCl, 5 Mg2SO4(7H2O), 0.1 CaCl2 and 1.25 NaH2PO4. Serial 300 μm thick coronal sections were then prepared using a VT1200 vibratome (Leica Microsystems, Wetzlar, Germany). Following preparation, slices were allowed to recover for at least 1 h at room temperature in our standard recording aCSF. This was composed of (in mm):124 NaCl, 3 KCl, 24 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgSO4(7H2O) and 10 d‐glucose, gassed with carbogen (i.e. 95% O2/5% CO2)
Only one slice containing a suitable section of rostral Re could be obtained per mouse. This was centred at approximately Bregma −0.46 to −0.58. The required slice was identified with the aid of the Paxinos and Franklin mouse brain atlas (Paxinos & Franklin, 2001). In coronal slices at this level, the location of Re can be readily pinpointed as a bi‐lobed structure lying atop the third ventricle. We never use slices that had been treated previously with any drug for subsequent electrophysiological recordings. This has limited the amount of pharmacological data that we have been able to gather from Re because one pharmacological intervention at most can be studied per animal.
Electrophysiological recordings
All recordings were made using the patch clamp technique. The slice containing the rostral Re was transferred into a submerged recording chamber, which was perfused with gassed aCSF and maintained at ∼33°C. The recording chamber was mounted on the stage of an upright microscope (BX51; Olympus, Tokyo, Japan) and cells were visualized using infrared differential interference contrast optics and a CMOS USB 2.0 camera (Thor Labs, Newton, NJ, USA). Drug applications were made by addition to the perfusing aCSF.
Microelectrodes of 3–5 MΩ resistance were fabricated from borosilicate glass capillaries using a P‐97 Flaming Browning micropipette puller (Sutter Health, Sacramento, CA, USA). Pipettes were filled with a K‐gluconate based internal solution composed of (in mm): 130 K‐gluconate, 20 KCl, 10 Hepes free acid, 0.2 EGTA, 0.3 GTP‐Na salt and ATP‐Mg salt, pH adjusted to 7.3 with KOH. The 15 mV junction potential error that arises from the pairing this pipette solution with our aCSF was corrected for during the analysis.
Cells of the rostral Re were visually identified based on their location relative to the dorsal tip of the third ventricle. All recordings were made with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA) and digitized with a Digidata 1440A interface (Molecular Devices). All data were stored on a personal computer (Hewlett‐Packard, Palo Alto, CA, USA) using pClamp, version 10.4 (Molecular Devices). This package was also used to design and deliver the various experimental protocols employed to characterize the cells.
Statistical analysis
Data were analysed using a range of custom written MATLAB scripts (MathWorks Inc., Natick, MA, USA) and pClamp 10.4 software. Statistical assessments of differences between populations were made using unpaired two‐tailed Student's t tests and both one‐ and two‐way ANOVA as appropriate. Images were prepared using Origin, version 9.1 (OriginLab Corporation, Northampton, MA, USA).
Results
Cellular properties at resting potential
Data collected from intact Re neurons using loose patch/cell‐attached recording indicated that the majority of cells exhibited a significant degree of spontaneous action potential (AP) firing. Figure 1 Aa illustrates a segment of such activity from a typical recording of this nature. The timecourse of the instantaneous frequency of AP firing for this cell recorded over 60 s is presented in Fig. 1 Ab.
Figure 1. Firing properties at rest.
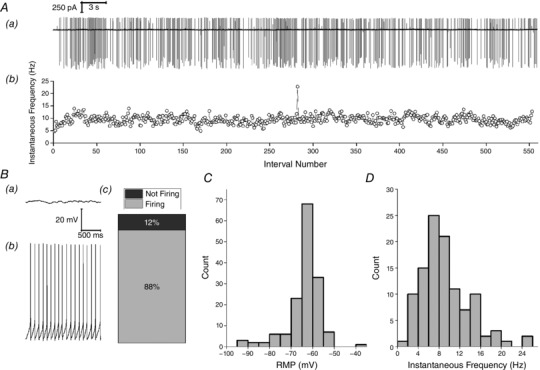
Aa, sample trace of spontaneous AP firing recorded in cell‐attached mode over a 60 s period. Ab, instantaneous frequency vs. spike interval number for this cell‐attached recording. B, sample traces from whole cell current clamp recordings illustrating (Ba) a neuron that showed no spontaneous activity and (Bb) a neuron that fired regularly at rest. Bc, cumulative column representation of the percentage of silent and spontaneously firing neurons in the Re population. C, mean resting membrane potential recorded during a 60 s epoch soon after gaining whole cell access D, the mean instantaneous AP frequency at resting membrane potential during this 60 s epoch for firing cells.
Commensurate with observations made in loose‐patch or cell‐attached mode (Fig. 1 A), robust spontaneous AP firing was also observed in the majority of Re neurons when we entered the whole cell recording mode and immediately collected a 60 s period of current clamp data with zero injected current (i.e. recording at resting potential). In 135 of 154 (∼88%) whole cell recordings of this nature, the neuron fired one or more spontaneous APs during the 60 s epoch immediately following entry into the whole cell configuration (Fig. 1 B). For all cells (i.e. both spontaneously firing and silent), the average membrane potential observed over the entire 1 min period is presented in Fig. 1 C. The 19 cells exhibiting no spontaneous spiking were characterized by a membrane potential (−70.6 ± 3.2 mV) somewhat more negative than the overall population mean (−63.7 ± 0.6 mV). The vast majority (n = 107) of the 135 cells that exhibited AP firing maintained their spiking activity throughout the entire 60 s period, with no single interspike interval longer than 5 s (and in most cells no longer than 300 ms). These cells had a mean resting potential of −61.4 ± 0.4 mV (n = 107). For these recordings, the distribution of mean AP frequency is shown in Fig. 1 D. This was compiled by calculating the average of the reciprocal of all inter‐spike intervals for each cell. The average of these mean instantaneous frequencies was 9.2 ± 0.5 Hz.
In the remaining 27 cells that fired any AP during the 60 s recording epoch, a range of spiking behaviours was observed. In nine of the cells APs initially fired regularly at 3–10 Hz, essentially in a fashion mirroring the majority group illustrated in Fig. 1 B and D, although, at some stage, this activity first slowed and then ceased, apparently as a consequence of modest and progressive (2–4 mV) hyperpolarizing shift in resting potential (Fig. 2 A). In the remaining 18 cells in which any spontaneous AP generation was seen, prolonged spike‐free periods (at least 5 s) were interrupted by brief periods of spiking. These cells fell into two clear groups. The first comprised 12 cells in which the intermittent spiking only comprised low frequency (<15 Hz) activity (Fig. 2 B), essentially similar to the spiking seen in the cells that maintained their firing for the whole 60 s (Fig. 1). The second group comprised only six cells (Fig. 2 C); in these, occasional brief bouts of spontaneous firing were observed, which included very much briefer inter‐spike intervals (as short as 3.2 ms) and thus considerably higher maximum instantaneous frequencies of spiking (75–310 Hz). A noticeable difference between the 11 cells with low‐frequency intermittent firing and the six cells with occasional high‐frequency bursting was with respect to resting potential. In the latter group, this was always more negative than −75 mV (mean −82.2 ± 2.9 mV), whereas, in the 11 cells in which the intermittent AP firing comprised only of low‐frequency spiking, the resting potential was significantly more depolarized (−66.0 ± 1.9 mV, P < 0.005, unpaired t test). This latter value lies between the average membrane potential of the 17 cells that fired no AP in 60 s (−70 mV) and the largest group of cells, namely those exhibiting maintained regular firing (−61 mV). The very negative membrane potential of the six cells in high‐frequency spiking group meant that the observed bursts rode atop a substantial transient depolarizing shift that brought the cells to threshold, the nature of which is discussed below.
Figure 2. Heterogeneous firing patterns at rest.
Sample current clamp traces. A, an initially regularly firing neuron in which cessation of firing was accompanied by a hyperpolarizing shift in resting membrane potential. B, a neuron exhibiting irregular firing at a low frequency. C, a neuron that fired irregularly in short high‐frequency bursts. The inset shows the very high‐frequency spiking present in such a burst with four APs arising in under 30 ms.
As described above, ∼70% of Re neurons fired constantly at rest with a frequency of 2–20 Hz (Fig. 1 D). Inspection of the voltage traces revealed that this firing appeared to be predominantly paced by a robust afterhyperpolarization (AHP) that followed each AP. On average, this AHP peaked 13.7 ms after the AP peak and took the membrane potential to −20.1 mV below the previous spike threshold (Fig. 3 A). As the AHP decayed and the cell depolarized again, it was able to initiate another spike; consequently, the rate of the AHP decay was appeared to be a key determinant of spontaneous firing frequency.
Figure 3. AP properties.
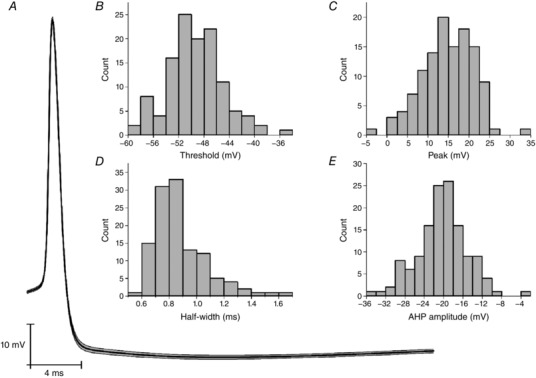
A, average trace of a spontaneous AP measured at resting membrane potential. The SEM is highlighted in grey. B–E, average spike properties of spontaneously fired APs, specifically (B) AP threshold, (C) AP peak value (i.e. zenith), (D) AP width measured at half‐height and (E) AHP amplitude.
In the 134 cells firing spontaneously at rest, we detected all of the spikes and characterized their core waveform properties. For the 120 cells firing a total of 20 or more AP, the distribution of mean AP threshold, maximum rate of rise, zenith and width at half‐height (defined as the voltage halfway between AP threshold and zenith) are presented in Fig. 3 B–E. When the distribution of AP thresholds (Fig. 3 B) is compared with the resting potential distribution (Fig. 1 C), the reason why most Re neurons exhibit spontaneous firing becomes apparent. As expected, bath application of TTX (500 nm) first slowed and then eliminated spontaneous AP firing in Re neurons (data not shown).
It was clear from close examination of current clamp recordings at rest that Re cells in vitro received a significant degree of spontaneous synaptic input (Fig. 4 A). Classical fast spontaneous synaptic activity was also apparent in recordings made in voltage clamp mode at a holding potential of −85 mV (Fig. 4 B). Indeed, spontaneous IPSCs recorded in vitro in voltage clamped Re cells at 26°C have been reported previously (Xu & Südhof, 2013).
Figure 4. Spontaneous synaptic input.

A, sample post‐synaptic potentials measured at resting membrane potential. B, sample inward going post‐synaptic currents measured at a holding potential of −85 mV. C, sample trace of miniature post‐synaptic currents measured at a holding potential of −85 mV in the presence of 500 nm TTX. Black current traces in (B) and (C) are Gaussian filtered versions of the raw grey traces.
When, during voltage clamp recordings, TTX (500 nm) was added to the perfusing aCSF to isolate miniature inward‐going synaptic events (i.e. post‐synaptic responses arising from presynaptic release of single quanta), we saw a spread of response amplitudes with a median peak of typically ∼10–15 pA. Most of these events rose very fast and decayed rapidly and exponentially with a time constant of ∼1 ms (Fig. 4 C).
We aimed to determine whether the spontaneous synaptic drive to Re cells could be a major factor in their spontaneous AP firing. We therefore made a series of recordings in the maintained presence of NBQX (5 μm), L689560 (5 μm) and gabazine (5 μm) to block AMPA/kainate, NMDA and GABAA receptors, respectively. Under these conditions, spontaneous post‐synaptic potentials were absent but the mean resting potential remained relatively depolarized (−66.7 ± 2.1 mV, n = 29) (Fig. 5 A). Furthermore, spontaneous AP electrogenisis was still apparent in the majority (20/29, 69%) of cells (Fig. 5 B). This spontaneous activity occurred with a mean frequency of (8.0 ± 1.0 Hz), which is very similar to that observed in the absence of pharmacological block of amino‐acid mediated synaptic transmission (Fig. 1 D). The various waveform properties of spontaneous APs also appeared unaffected by synaptic blockade (data not shown). This indicates that the relatively depolarized resting potentials and consequent tonic spontaneous AP firing in Re neurones in vitro arises largely from cell‐intrinsic properties, rather than synaptic drive, although the latter will not doubt shape this activity to some extent.
Figure 5. Firing properties at rest in the presence of synaptic blockers.
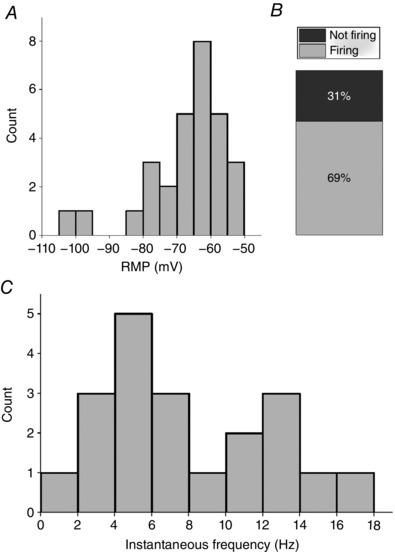
A, distribution of resting membrane potentials in Re cells recorded in the combined presence of blockers of AMPA/kainate, NMDA and GABAA receptors. B, relative proportions of spontaneously firing and silent cells under these conditions C, distribution of mean instantaneous AP frequency at resting membrane potential during a 60 s epoch recorded in whole cell configuration. All recordings were made in the presence of NBQX (5 μm), gabazine (5 μm) and L‐689560 (5 μm).
Excitability analysis from a defined pre‐set membrane potential
For a variety of underpinning biophysical reasons, the intrinsic excitability properties of all neurons depends on their resting membrane potential (see below). Consequently, to make comparisons across neurons within a population, it is helpful to set the pre‐stimulus membrane potential of each cell to a constant defined level. Furthermore, it is helpful to choose a pre‐stimulus potential at which spontaneous firing is absent because the presence of ongoing background firing complicates interpretation of any subsequent stimulus‐evoked activity. Thus, to gain a detailed insight into the intrinsic excitability of Re neurons, we initially chose to make measurements from a pre‐stimulus membrane potential of −80 mV. This pre‐stimulus potential was set by applying a suitable amount of bias current via the recording amplifier; this varied in amplitude from +46.8 to −153.7 pA, averaging −45.4 pA (n = 154).
Having set the pre‐stimulus membrane potential to −80 mV, we then applied a range of defined current stimuli to probe the sub‐ and suprathreshold intrinsic properties. First, to determine an approximation of ‘rheobase’ for cells at −80 mV, we applied an incremental series of 100 ms duration, depolarizing current injections. These were increased stepwise in amplitude in small increments at 1 Hz until AP firing was observed. As shown in Fig. 6 A, cells could be divided into those which fired just one AP at rheobase and those which produce a burst of between two and six APs. The mean AP count in latter group was 2.9 ± 0.2 AP. Data summarizing the amount of current required to produce at least one AP across 107 cells is shown on the left of Fig. 6 B. Alongside this are data from a series of similar recordings made in the synaptic blocker cocktail described above. These exhibited an almost identical mean rheobase of 25.3 ± 5.1 pA (n = 25) (Fig. 6 B). For the larger drug‐free experimental group, Fig. 6 C compares the mean rheobases for those cells firing just one AP and those producing a burst of two to six APs. The latter group had a 25% lower rheobase (P = 0.01, Mann–Whitney U test) and, consequently, were both easier to bring to AP threshold and, once there, produced a greater spike output.
Figure 6. Approximation of rheobase from a pre‐stimulus potential of −80 mV.
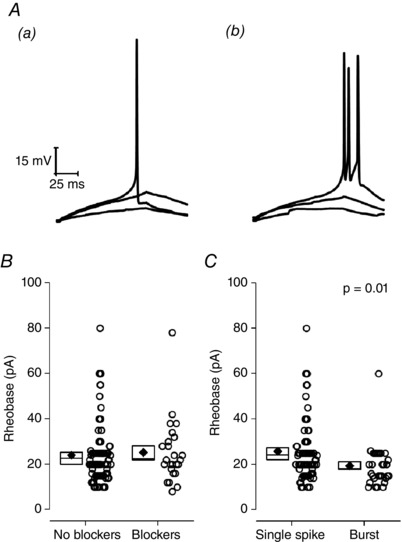
A, sample voltage traces extracted from a series of incrementally growing 100 ms current injections used to approximate the rheobase from −80 mV. Data are shown from two cells: (Aa) one with a single spike and (Ab) one with a burst. The traces shown in each are responses to the minimal current injection that elicited an AP (rheobase), rheobase – 2 pA and rheobase/2. B, comparison of rheobase distributions recorded both in the absence and presence of NBQX (5 μm), gabazine (5 μm) and L‐689560 (5 μm). C, ‘no blocker’ data from (B), broken down by cells which fired only one AP at rheobase and cells which generated a burst of two to six APs. In (B) and (C), each round symbol is a separate recording. Diamonds plot the mean, boxes indicate the standard error and the line crossing the box is the median.
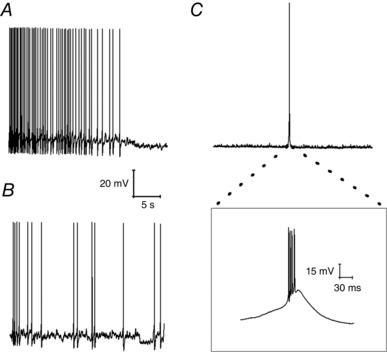
We next employed a standard incremental current injection protocol in which a series of nine consecutive 500 ms current stimuli were delivered with an inter‐stimulus interval of 10 s. The amplitude of the first current pulse was −20 pA and each subsequent pulse was +10 pA larger, such that the final pulse had an amplitude of +60 pA (Fig. 7 A). The first (i.e. −20 pA stimulus) sweep was used to determine the subthreshold intrinsic properties of Re neurons, including input resistance, membrane time constant and capacitance. Approximately 12% of Re cells exhibited a significant rebound depolarization following cessation of hyperpolarizing current injections. As reported previously (Graef et al. 2011), these rebound depolarizations were capable of driving spiking in some cells (Fig. 7 C) and, when spiking was seen, multiple AP were typically produced within a short time window. The basis of this rebound potential is described below.
Figure 7. Passive membrane properties.

A, sample voltage traces a series of current injections (−20 – +60 pA) from a set pre‐stimulus potential of −80 mV. For clarity, the responses to 30 and 60 pA stimuli have been offset. B, average voltage response to a −20 pA, 500 ms hyperpolarizing current injection from a set pre‐stimulus potential of −80 mV. The SEM is highlighted in grey. Highlighted in (C) is the high‐frequency rebound spiking behaviour observed from some cells following termination of negative current injections. D, input resistance, (E) time constant and (F) an approximation of capacitance.
Input resistance averaged 615 ± 17 MΩ (Fig. 7 D) and mean membrane time constant was 38.5 ± 1.1 ms (Fig. 7 E). The capacitance estimated by dividing the time constant by input resistance averaged 66.6 pF, indicating that, for CNS neurons, these cells were modest in size.
A slow depolarizing relaxation of the membrane potential and accompanying decrease in membrane resistance during application of a hyperpolarizing current injection is commonly known as ‘sag’. It is a prominent and important subthreshold feature of a number of CNS neurons, and arises from activation of hyperpolarization‐activated cyclic nucleotide gated (HCN) channels. The majority of Re neurons exhibited little or no sag in response to a 500 ms, 20 pA hyperpolarizing current injection, as exemplified by the average voltage traces shown in Fig. 7 B. The extent to which HCN2 channels, the predominantly expressed HCN channel in the thalamus (Santoro et al. 2000), would activate in response to a relatively short hyperpolarizing current injection is questionable given this channels long activation time constant (in the order of seconds). However, when we employed a five‐fold longer 20 pA hyperpolarizing step (2.5 s), we also observed no appreciable sag, as exemplified by the average trace in Fig. 8 A. In case HCN channels were already largely activated at −80 mV, we also examined 2.5 s 20 pA current injections at −72 mV and 500 ms current injections at −64 mV, neither of which exhibited any significant sag.
Figure 8. Absence of HCN channel‐like current.
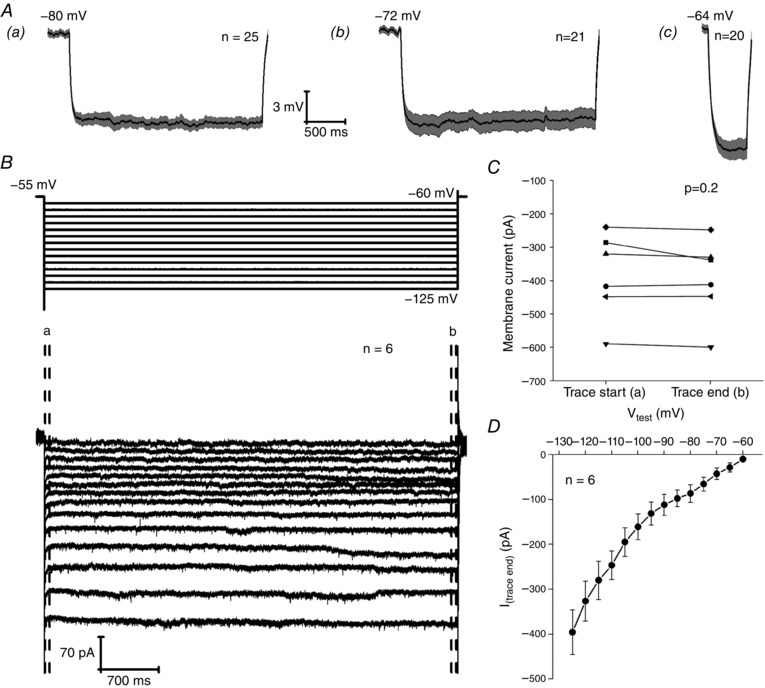
Aa and Ab, average current clamp trace showing the response to a 2.5 s, 20 pA hyperpolarizing current injection applied to cells at a prestimulus potential of (Aa) −80 mV and (Ab) −72 mV. Ac, average current clamp trace showing the response to a 500 ms, 20 pA hyperpolarizing current injection applied to cells at a prestimulus potential of −64 mV. B, voltage clamp protocol (top) and resultant average traces (bottom) of a series of 5 mV hyperpolarizing steps from a holding potential of −55 mV. C, change in membrane current between the first 60 ms (Ca) and the final 60 ms (Ca) of a voltage step to −125 mV. D, average I–V of the current response during the final 60 ms of a 5 s test pulse for a series of 5 mV hyperpolarizing voltage steps applied to six neurons held at −55 mV.
To finally confirm this apparent paucity of HCN current, and to identify any potential activation from even more depolarized prestimulus potentials, we also carried out a series of voltage clamp recordings to investigate any sign of HCN‐like conductance. Here, we applied a series of 5 s duration hyperpolarizing steps to Re cells at a holding potential of −55 mV. The test potential varied from −60 to −125 mV in 5 mV increments (Fig. 8 B). In such protocols, HCN channel activity is revealed by a slowly growing inward current that takes tens or hundred of milliseconds to come to steady‐state level. The cross‐cell average current response from six Re neurons can be seen in Fig. 8 B. In this average, there is no sign of any slow gating HCN‐like current. The presence of HCN currents can be readily revealed by looking for difference between the current level in the first few milliseconds following hyperpolarization (before the slowly gating HCN channels have opened) and that seen at the end of the voltage pulse (when the HCN channel opening probability has had time to grow). Such an analysis in presented in Fig. 8 C. This compares the inward current level recorded in the first and last 60 ms of the voltage step to −125 mV relative to the holding current. This revealed that five of the six neurons recorded exhibited no slow gating inward current whatsoever. The remaining neuron exhibited a small (∼50 pA) growth in inward current although, on average, across the six cells, there was no significant difference in current level at the start and end of the pulse (Fig. 8 C). Upon inspection of the average voltage trace (Fig. 8 B), it became clear that the amplitude grew in a non‐linear fashion across sequential voltage steps. This is also evident from Fig. 8 D, an I–V plot of the current amplitude at the end of the 5 s step vs. voltage. This form of this curve is suggestive of the presence of inwardly rectifying potassium channels because the membrane conductance increases at potentials negative to the potassium equilibrium potential.
The incrementally growing depolarizing 500 ms current stimuli (Fig. 7 A) lead to AP firing in all Re neurones. The fraction of cells firing one AP or more vs. the amplitude of the depolarizing stimulus is shown in Fig. 9 A, whereas Fig. 9 B summarizes the relationship between number of spikes elicited and current stimulus applied. Figure 9 B does not provide any information about the temporal dynamics of AP production during the 500 ms current stimulus. This feature of excitability is illustrated in Fig. 9 C, which plots of mean instantaneous frequency vs. spike interval for applied current stimuli ranging from 10 to 60 pA. We also gathered an additional dataset from a smaller group (n = 25) of Re neurons using 2.5 s long current stimuli. An example recording and pooled data from the 30 and 60 pA stimuli is shown in Fig. 9 D. These recordings show that maintained lower frequency (8–20 Hz) firing following post‐burst accommodation could continue for at least 2.5 s. In reality, we consider that this activity is ostensibly similar to the maintained firing observed at resting membrane potential in most Re neurons (Fig. 1).
Figure 9. AP production in response to depolarizing 500 ms ‘square‐wave’ current injections.
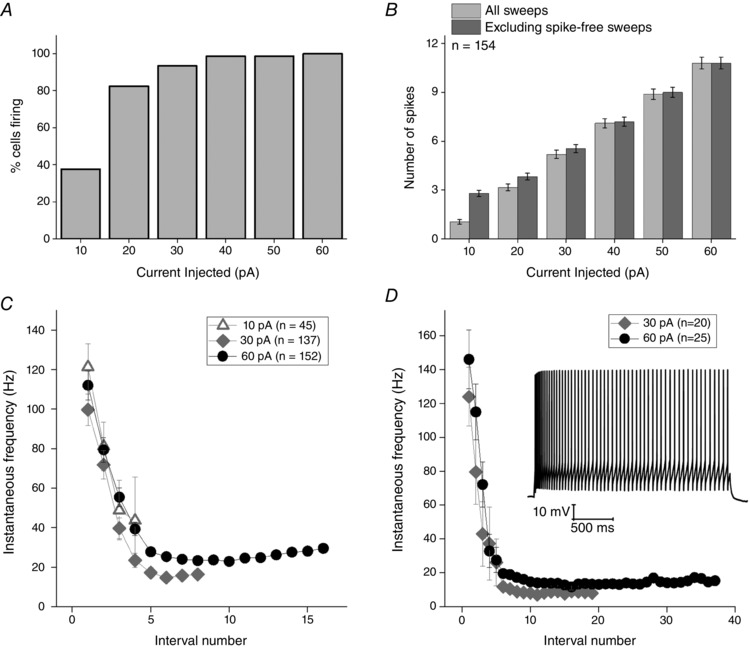
A, fraction of cells generating at least one AP and (B) the mean number of AP produced in response to a series of 6 incremental 500 ms depolarizing current injections (10–60 pA) from a set pre‐stimulus potential of −80 mV. C, instantaneous AP frequency vs. interval number for a 10, 30 and 60 pA, 500 ms current stimuli. D, instantaneous AP frequency vs. interval number and inset a sample voltage response, for a 30 and 60 pA, 2.5 s current stimuli. All error bars represent the SEM. All cells were set at −80 mV prior to current application.
It is clear from Fig. 9 C and D that, on average, when Re neurons receive current injections sufficient to induce firing, they tend to generate an initial burst of AP around 115–145 Hz followed by accommodation to maintained spiking rates ∼10–30 Hz. Although providing a useful and standard summary, the data averaged in the way shown in Fig. 9 C do not reflect the full diversity of firing behaviours that we observed in our population of Re recordings. In particular, the full spectrum of behaviours featured both highly bursty and regular spiking cellular responses to the same stimulus (Fig. 10 A). This diversity of firing patterns is illustrated in an alternative manner by the cell by cell data presented in Fig. 10 B. Here, the relative timing of APs (relative to the first = black symbols) is shown for a 60 pA depolarizing stimulus. This plot confirms that many cells fire a prominent and very high‐frequency initial AP burst, whereas other cells lack this initial strong burst and fire more regularly throughout the applied stimulus (Fig. 10 Ab).
Figure 10. Diversity of firing following a depolarizing current injection.
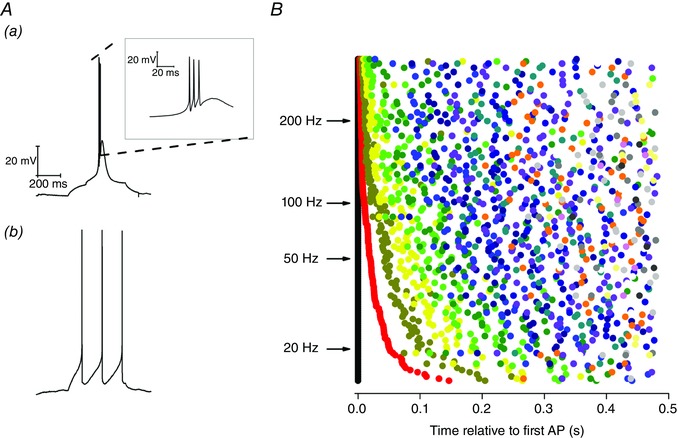
A, sample voltage traces in response to a 10 pA, 500 ms depolarizing current injection from a set pre‐stimulus membrane potential of −80 mV. Different neurons display diverse firing outcomes for this stimulus, including (Aa) high frequency bursting and (Ab) regular firing. B, heterogeneity of firing behaviour in response to a 60 pA depolarizing current step. Each row represents a different neuron and the time at which the first AP is generated for each neuron is represented by a black point at 0 ms. The timing of all subsequent APs generated is plotted relative to the first spike. The second spike is represented by a red point, the third by dark yellow, the fourth by yellow and the fifth by green, etc. Representing the data in this form illustrates the diverse array of different firing patterns present in the neuronal population.
In hippocampal pyramidal cells (Jensen et al. 1996; Yue & Yaari, 2004; Brown & Randall, 2009) and some other ‘bursty’ neurones, the presence of bursting correlates with the presence of a fast spike after‐depolarizing potential (ADP). This afterpotential is best observed when very short (1–2 ms), strong current (1–2 nA) stimuli are used to elicit a single (primary) AP. In the period after the very brief current stimulus is removed the primary AP is followed by an ADP, which can, when sufficiently large, result in production of one or more secondary APs. An ADP of this nature was present in many (67%) Re neurones. Examples of a subthreshold and a suprathreshold ADP are shown in Fig. 11 (note the intense short burst of firing produced by the latter).
Figure 11. After‐depolarizing potential following a single spike.
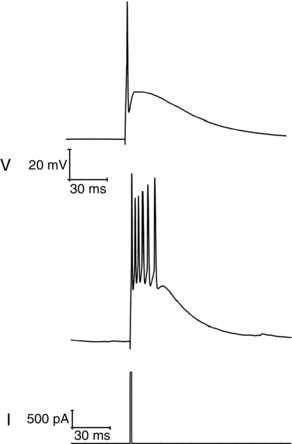
Two sample traces (top) of the voltage response following generation of a single spike elicited by a short (2 ms), strong (2 nA) square current injection (bottom).
High‐frequency firing driven by EPSP‐like stimuli
Although ‘square‐wave’ current injections in current clamp recordings are a very useful and standard means to characterize the excitability of neurons, they are not particularly representative of how cells are activated in vivo. To examine how physiological synaptic inputs might drive firing in Re neurons in vivo, we used an approach in which current stimuli with EPSC‐like waveforms (αEPSCs) were applied to Re neurons during current clamp recordings. We have previously employed such an approach to study the basis of high‐frequency bursting in hippocampal CA3 pyramidal cells (Brown & Randall, 2009). To facilitate interpretation, and to parallel the datasets described above for conventional ‘rectangular’ current stimuli (Figs 6, 7 and 9, 10, 11), these stimuli were applied from a defined pre‐stimulus membrane potential of −80 mV, at which spontaneous firing is absent. We examined stimuli in which the decay rate of the injected current was varied across a four‐fold range (Fig. 12) and also examined different stimulus peak amplitudes between 50 and 250 pA (Fig. 13).
Figure 12. αEPSC current injections can elicit high frequency burst firing.
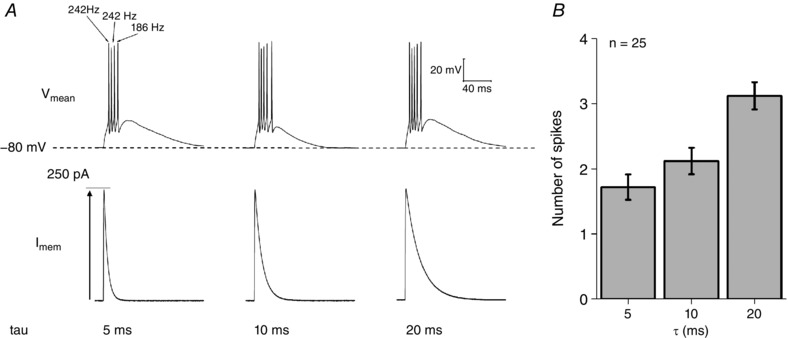
A, sample voltage responses to 250 pA αEPSC current injections with time constants of 5, 10 and 20 pA, respectively. Note the resultant high frequency bursting observed in many cells. B, number of AP elicited vs. the time constant of the αEPSC injected. As the time constant of the current injection increases, so does the number of evoked APs.
Figure 13. AP production in response to αEPSC current injections of varying sizes.
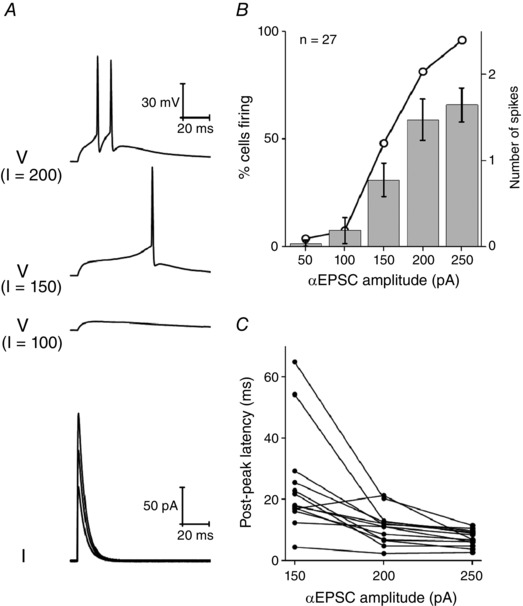
A, sample voltage responses (top) to incrementally large αEPSC injections (taudecay = 5 ms) ranging from 50−200 pA (bottom). B, mean number of spikes (column) and the percentage of neurons firing any spikes (line and symbol) in response to an αEPSC current injection with taudecay = 5 ms. C, post‐peak latency (i.e. the time after the peak of αEPSC the first AP fires) vs. αEPSC amplitude (taudecay = 5 ms).
When sufficiently large (e.g 250 pA), αEPSC stimuli produced AP generation in 100% of Re neurons maintained at a pre‐stimulus potential of −80 mV. By contrast, 50 pA stimuli almost always failed to produce APs (Fig. 13 B) and, instead, produced a subthreshold EPSP‐like waveform. With sufficiently large stimuli, the αEPSC typically produced a high‐frequency (100–250 Hz) burst of two or more AP, as exemplified by Fig. 12 A. Furthermore, as the decay of the αEPSC was slowed, the mean spike output increased, approximately doubling as the decay time constant was lengthened from 5 to 20 ms.
A feature of the spiking response to αEPSC stimuli was that the AP firing often occurred considerably after the peak of the injected current. Indeed, with just suprathreshold αEPSCs (i.e. 150 pA peak amplitude) and a 5 ms decay time constant, the first AP occurred after the αEPSC waveform had decayed practically to baseline (Fig. 13 A and C). Even with the strongest αEPSC stimuli examined (250 pA), the first AP occurred some 5–12 ms after the peak of the current injection. In either case, it was generally apparent that the αEPSC activated another subthreshold depolarizing current, which in turn drove the cells towards their AP threshold.
Changes to excitability at more depolarized membrane potentials
The excitability data described above were collected from neurons placed at a set prestimulus membrane potential of −80 mV prior to delivery of a current stimulus. This level lies in the negative tail of the range of resting potentials seen in the Re population in vitro (Fig. 1 C). In a subset of cells, we additionally collected datasets from a somewhat more depolarized pre‐stimulus potential of −72 mV, although still one at which spontaneous spiking at rest was absent (Fig. 14 A). These recordings demonstrated that quite substantial changes in aspects of the excitability profile of Re neurons arose when their resting potentials were changed by just a few mV. Perhaps somewhat surprisingly, depolarizing the pre‐stimulus membrane potential by 8 mV had no effect on the total AP output observed during the 500 ms depolarizing current pulses of various amplitudes. Thus, very similar spike numbers were observed for each level of stimulus (Fig. 14 B) and the relationship between percentage of cells firing at least one AP and injected current was almost identical (Fig. 14 C). However, on visual examination of the voltage traces, it was clear that the original high‐frequency AP burst seen in the majority of cells at −80 mV (Figs 9 and 10) was almost always absent in cells resting at −72 mV, as exemplified in Fig. 14 A. This is also reflected in the pooled data plotted in Fig. 14 D, which present the mean instantaneous frequency for the first interspike interval elicited by each level of current injection. The initial spiking rate is around five to eight times faster in the cells resting at −80 mV. In a similar vein, although 50–60% of cells at −80 mV produced a first spike pair with instantaneous frequency of 100–330 Hz, this was only ∼5% for cells at −72 mV and, in this small population, spiking did not exceed 155 Hz. The reason for the very similar total AP counts during the entirety of the 500 ms stimulus (Fig. 14 B) was that the average rate of steady state spiking in the latter part of the current stimulus was ∼10% higher in the cells starting from −72 mV.
Figure 14. Firing behaviour in response to square current injections are dependent on membrane holding potential.
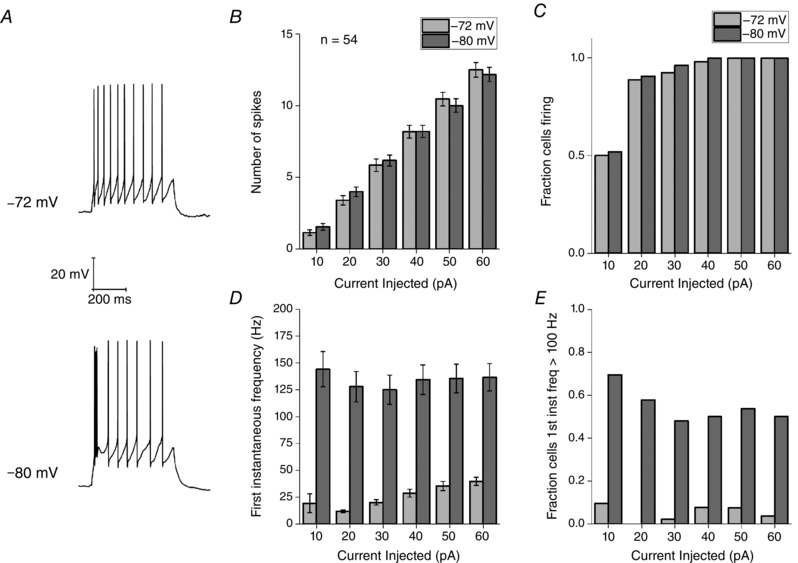
A, sample voltage responses to a 60 pA square current injection applied from pre‐stimulus membrane potentials of −72 and −80 mV. B, the average number of spikes fired in response to six depolarizing current injections (10–60 pA) from a Vm of −72 or −80 mV. Error bars represent the SEM. C, fraction of cells firing one or more AP for each current injection. Number of spikes fired or the fraction of cells firing in response to current injections were unaffected by V m. D, average instantaneous frequency between the first two spikes fired in response to a depolarizing stimulus of the indicated amplitude. E, fraction of cells firing two APs at a frequency greater than 100 Hz. Both the average first instantaneous frequency and fraction of cells firing with a first instantaneous frequency > 100 Hz were significant decreased by depolarizing the membrane by 8 mV.
Electrophysiological and pharmacological evidence for a T‐type Ca2+ conductance
The presence of both hyperpolarization‐induced rebound firing in the absence of significant sag (Figs 7 and 8) and the subthreshold depolarizing potentials that trigger subsequent high‐frequency firing when Re neurons are depolarized from more negative potentials (Figs 9 and 10, 11, 12) are suggestive of the presence of T‐type (CaV3 family) Ca2+ channels. Classically, these channels have a low threshold for activation (more negative than AP threshold) and are readily inactivated when the resting potential becomes slightly depolarized (Perez‐Reyes, 2003). Commensurate with this, the Allen Brain Atlas (http://mouse.brain‐map.org) (Lein et al. 2007) indicates robust expression of the CaV3.1 isoform of low threshold Ca2+ channel in Re. Furthermore, two previous studies (Graef et al. 2009, 2011) have demonstrated expression of T‐type Ca2+ channel mRNA and classical low threshold currents in the midline thalamus.
The size and geometrical complexity of CNS neurons in brain slices or in vivo, combined with their typically very large current densities can greatly hinder high fidelity voltage clamp analysis of voltage‐gated ionic conductances. However, with sufficient care and suitable protocols, it is possible to gather some useful information particularly with regards to modest sized, slower‐gating conductances, which suffer less from space clamp associated issues. Voltage clamp recordings were made pairing our standard pipette solution and our standard aCSF supplemented with 500 nm TTX (to eliminate voltage‐gated sodium currents). Initially, we applied an incremental series of voltage steps from a holding potential of −85 mV, this produced mixed currents such as those presented in Fig. 15 A. Notably, with modest depolarizations (e.g. to −63 mV), only inward currents were observed, whereas, once the test potential became depolarized beyond −45 mV, large outward‐going, partially inactivating currents began to dominate the response. These outward currents, which are carried by K+ ions, rapidly increased in size to many nanoamps if depolarization was increased further. The voltage‐dependence and waveform kinetics of the inward current elicited with modest depolarizations was highly suggestive of a classical low threshold, T‐type current. Using a series of small voltage steps separated by 2 mV increments, we further profiled the inward current within the narrow voltage range where it was the predominate current (Fig. 15 B). The current activated at the negative voltages expected for a T‐type current and largely inactivated over ∼60 ms of depolarization. We also gathered steady state inactivation data for these channels by varying the holding potential prior to an invariant step to −65 mV (Fig. 15 D) and profiled the rate of recovery from inactivation with a standard variable interval two step protocol (Fig. 15 E).
Figure 15. Gating properties of low‐threshold inward currents.

A, voltage clamp protocol (top) and resultant current responses for a series of 8 mV incremental depolarizing voltage steps applied from a holding potential of −85 mV. B, sample voltage trace and the subsequent current response to a series of 2 mV incremental depolarizing voltage steps (from −75 to −47 mV) from a pre‐stimulus holding potential of −85 mV. C, average I–V of the observed peak inward current in response to this series of 2 mV voltage steps. D, inactivation curve showing the average voltage at which the T‐type calcium current inactivates in Re neurons. E, line and symbol plot showing the rate of recovery from inactivation of T‐type Ca2+ current determined with a standard variable interval two pulse protocol.
The pivotal neurophysiological roles of low threshold Ca2+ channels have been widely studied in other thalamic neurons for many years (Pape et al. 2004), although the availability of reasonably potent and selective T‐type channel blockers is a more recent advance (Xiang et al. 2011). Application of one such molecule ML‐218 (3 μm) caused a substantial decrease in the amplitude of the ADP elicited following a single short strong current injection (Fig. 16 A). The drug also reduced the instantaneous frequency of the first pair of spikes produced by a 500 ms depolarizing current step, without changing the total spike output from the stimulus. Finally, the drug eliminated the rebound firing produced when Re neurons resting at −70 mV (a membrane potential where 59% of neurons exhibit rebound firing following a hyperpolarizing step) were hyperpolarized for 500 ms with a −50 pA current stimulus. The data in Figs 15 and 16 together indicate that T‐type channels play a key role in the ability of Re neurones to produce very high‐frequency firing after transient sojourns at negative membrane potentials.
Figure 16. Pharmacological inhibition of T‐type calcium channels alters firing properties.
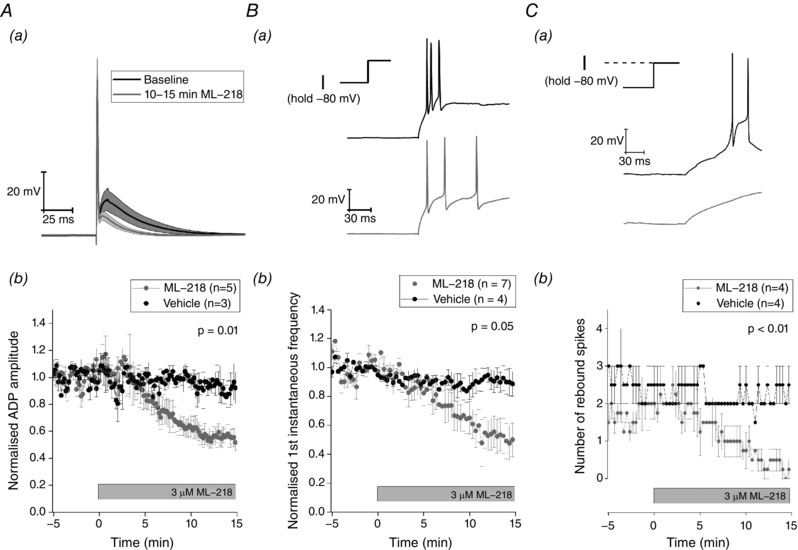
A, application of the T‐type calcium blocker ML‐218 (3 μm) reduces the amplitude of the ADP that arises following a single spike. Aa, average trace of the waveform of a single spike elicited by a short, strong current injection (2 ms, 2 nA) recorded during a 5 min baseline (black), and during the last 5 min of a 15 min exposure to 3 μm of T‐type calcium channel blocker ML‐218. Shaded area represents SEM. Ab, time course of the effect of ML‐218 on the average ADP amplitude normalized to the pre‐drug baseline. Ba, sample trace of the voltage response to a 100 pA depolarizing current injection just prior to application of ML‐218 (black) and following 15 min of exposure to ML‐218 (grey). Bb, effect of ML‐218 on the first instantaneous frequency normalized to the predrug baseline. Ca, sample traces showing the repolarization driven rebound spiking following a −50 pA hyperpolarizing current injection just prior to application of ML‐218 (black) and following 15 min of exposure to ML‐218 (grey). Cb, effect of ML‐218 on the average number of rebound spikes observed following repolarization. All P values represent paired comparisons of the selected measurement between the pre‐drug baseline and the final 5 min of the drug application.
Reduction in ‘burstyness’ through activity‐dependent intrinsic plasticity
The data in Figs 1 to 16 outline core aspects of the neurophysiology of rostral Re neurons, in particular their intrinsic excitability properties. Although often tonically firing at around 8 Hz at rest, after short periods at sufficiently negative potentials (i.e. −80 mV), the cells are capable of producing very high‐frequency burst firing, sometimes reaching instantaneous frequencies in excess of 300 Hz. The presence of an ML‐218‐sensitive, low threshold Ca2+ conductance appears to be central to the production of this high‐frequency burst firing. Intrinsic neuronal plasticity describes how the excitability properties of neurons can be modified by ‘experience’ delivered experimentally as a conditioning stimulus of some form. For example, we have previously described how the ‘burstyness’ of hippocampal pyramidal neurons can be persistently reduced either by brief cell‐intrinsic activity patterns or via the activation of metabotropic glutamate receptors at synapses (Brown & Randall, 2009; Brown et al. 2011).
We were interested in whether we could identify a similar means to transform the excitability of Re neurons. In vivo, the nucleus reuniens exhibits a strong theta oscillation and the firing of many neurons is synchronized to this (Jankowski et al. 2014, 2015; Ito et al. 2015). We therefore decided to employ a theta‐paced burst firing protocol as a candidate conditioning stimulus for induction of intrinsic plasticity in Re neurons. Notably, a similar protocol has proved effective in persistently altering excitability of hippocampal neurons (Brown & Randall, 2009). In whole cell current clamp recordings, we maintained the prestimulus membrane potential at −80 mV and collected a data sweep every 10 s. In all sweeps, we first applied a 2 nA, 2 ms stimulus to evoke a single spike and subsequent ADP (as in Figs 11 and 16 A); notably, we did not use cells in which the ADP triggered AP firing because this complicates quantification of this afterpotential and consequently experimental interpretation. In every second sweep, the short‐strong ADP eliciting stimulus was followed 1.5 s later by a 500 ms, 50 pA hyperpolarizing current pulse, which could be used to measure passive properties (Fig. 7 B). We ran this protocol for at least 5 min to collect baseline data prior to applying the conditioning stimulus. The conditioning stimulus consisted of using short (2 ms) strong (2 nA) current injections to drive the cells to spike 5 times at 150 Hz (Fig. 17 A); this was repeated continuously every 200 ms for 15 s (thus, a total of 75 bursts of five spikes in 15 s). During application of the conditioning stimulus, we allowed the membrane potential to follow its desired trajectory rather than endeavouring to keep it at the fixed prestimulus level of −80 mV. Following application of the 15 s conditioning stimulus, we returned to the stimulus protocol employed in the pre‐conditioning period and followed the cell for a further 15 min. In this post‐conditioning period, as in the preconditioning period, the interstimulus membrane potential was again maintained at −80 mV.
Figure 17. Re neurons exhibit intrinsic plasticity of the ADP.
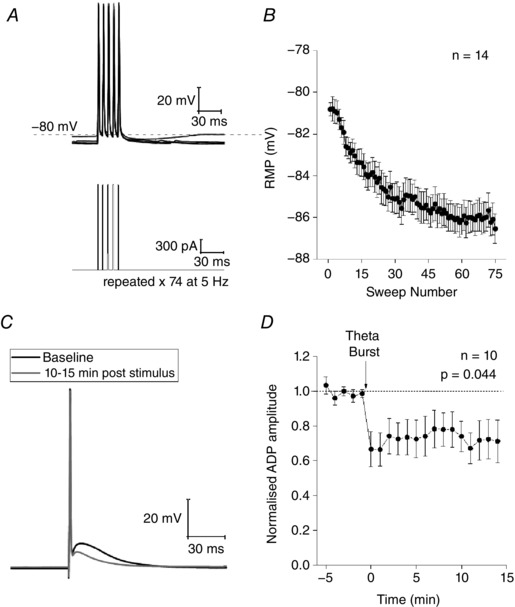
A, sample voltage (top) and current (bottom) traces of a theta burst induction protocol used to induce intrinsic plasticity. Briefly, each burst was driven induced with five short, strong (2 ms, 2 nA) current injections at a frequency of 150 Hz. Seventy‐five bursts were induced at 5 Hz to induce plasticity. B, modest hyperpolarization in the resting membrane potential that developed as the induction protocol progressed. C, voltage trace from an example experiment showing the average response to a single spike during the baseline period (black) and 10–15 min after the conditioning protocol (grey). D, timecourse of the baseline‐normalized ADP amplitude from 10 experiments.
As shown in Fig. 17 A and B, application of the 75 spike, theta‐patterned conditioning protocol caused a progressive, acute change in the membrane potential of Re neurons. Thus, the cells on average hyperpolarized by over 6 mV, a change that followed an exponential trajectory that was largely complete after around nine theta burst cycles (i.e. 45 AP). Notably, this change did not stop the current stimuli from producing AP, although the post‐burst AHP was lost, probably as a result of the decreased driving force for K+ ion fluxes that mediate the AHP. Notably, this change was transient and cells returned to their pre‐conditioning membrane potential within < 1 min. In line with this, the bias current used to keep the cells at −80 mV in the pre‐ and post‐conditioning periods was not different.
More interestingly, following the conditioning stimulus, a persistent change in the intrinsic excitability of Re neurons was observed. This was manifest as a decrease in the amplitude of the ADP observed following a single AP (Fig. 17 C and D). Indeed, the ADP was largely absent in most cells and the amplitude measured was just that afforded by the need for the membrane to discharge (Brown and Randall, 2009). The conditioning protocol acutely caused a 10–15% decrease in input resistance, although this disappeared within 40 s and no lasting changes to input resistance were observed. Thus, in the last 5 min of the experiment, the input resistance was 100 ± 3% of its baseline value (P > 0.98).
Discussion
Thalamic neurons that project to the cortex, thalamocortical (TC) neurons, have been the subject of substantial neurophysiological investigation over the last 40 years. Amongst the most widely studied TC neurons are those conveying sensory information within either the visual or somatosensory systems; for example, those whose cell bodies reside in the lateral geniculate and ventrobasal nuclei. Such studies have produced a general view of the intrinsic electrical properties of TC and how these shape the contribution of these neurons to network activities associated with sensory processing (Jahnsen & Llinás, 1984; McCormick & Huguenard, 1992; Turner et al. 1997; Williams et al. 1997; Hughes et al. 1999; Pape et al. 2004; Llinas & Steriade, 2006; Huguenard & McCormick, 2007).
In the present study, we have characterized cell‐level neurophysiological properties of Re neurons. Unlike classical TC neurons of sensory pathways, these cells of the midline thalamus receive little if any direct sensory information, although they still both send and receive extensive cortical connections (Vertes et al. 2015). We specifically focussed the present study on neurons located in the rostral portion of Re because this is the predominant source of the afferent projections to the hippocampal and prefrontal circuits that have recently generated considerable interest (Varela et al. 2014). Furthermore, from an anatomical perspective, the rostral Re is also easy to identify unequivocally in murine brain slices. Although beyond the scope of this initial study, in the future, it would be of interest to compare the properties of the rostral and caudal aspects of Re. Also, given the diversity of intrinsic properties observed, it would be of interest to examine whether there is a relationship between defined post synaptic target of these cells, established with retrograde labelling methods, as well as their neurophysiological properties at the cellular level.
Brain slice preparations encompassing the rostral nucleus reuniens are somewhat limiting for those aiming to examine the effects of pharmacological agents, especially in mice. This is because the region is both relatively small and resides on the midline, meaning that each mouse provides only one slice (unlike, for example, the hippocampus or cerebellum where perhaps 10 unilateral slices can be obtained per animal). We stringently avoid recording from cells in slices that have been previously treated with a drug during a prior recording. Consequently, this typically limits pharmacological analysis to studying the effects of one acute drug exposed cell per animal. Our only exception to this is studying cells in slices chronically treated with drugs to establish a particular background condition; for example, the use of synaptic blockers throughout all recordings.
In whole cell recordings, the majority of Re neurons were found to be relatively depolarized at rest. A major consequence of this was the presence of robust spontaneous firing of fast rising and decaying AP in the majority of cells. This firing often, although not always, occurred in a tonic fashion with a mean frequency of ∼8–9 Hz. These observations of spontaneous tonic firing in the majority of neurons are in agreement with Re recordings that we have performed from slices obtained from both younger (6 weeks) and older mice (∼1 year and 2 years; D.A Walsh, J.T Brown and A.D Randall, unpublished data).
The depolarized resting potential and resultant spiking does not appear to arise as a consequence of entering the whole cell mode because spontaneous spiking was also clearly evident in loose patch or cell attached recordings in which the cytoplasmic contents are undisturbed. Furthermore, in our laboratory, exactly the same recording solutions and conditions result in much more negative resting potentials in other neurons; for example, both CA1 and CA3 pyramidal cells (Brown & Randall, 2009; Booth et al. 2016b), as well as various cortical neurons (Tamagnini et al. 2014; Dawson et al. 2015; Booth et al. 2016a). Interestingly, the depolarized membrane potential also contrasts starkly with the hyperpolarized membrane potential observed in both first and higher order thalamic nuclei (Jahnsen & Llinás, 1984; Varela & Sherman, 2007; Kolaj et al. 2012). Indeed, to the best of our knowledge, in no other thalamic relay nuclei have the majority of neurons been reported to fire spontaneously when recorded during the light stage of a 12:12 h light/dark cycle. Although not supported by our loose patch/cell‐attached observations, in which the cytoplasmic contents are undisturbed, we were interested in whether the depolarized resting potential seen may have reflected the relatively depolarized Cl− equilibrium potential our intracellular solution produces combined with a significant level of tonic GABAergic drive. We found, however, that blocking GABAA receptors with GABAzine produced little effect on either resting potential or firing behaviours. Furthermore, blocking GABAA receptors did not appear to result in substantial increases in overall network activity in the Re, whereas, in the hippocampus and many areas of the cortex, pharmacological elimination of ongoing GABAergic inhibition generally produces huge hypersynchronous epileptiform burst events (Brown et al. 2003).
Although the properties of synaptic inputs to Re neurons are not a major feature of the present study, which instead focusses on intrinsic properties, it is notable that Re cells receive a significant ongoing spontaneous synaptic barrage. Indeed, even when spontaneous firing of neurons throughout the slice was eliminated with TTX, Re neurones received a substantial barrage of spontaneous miniature synaptic input. The quantal sizes of many of these events measured in voltage clamp, combined with the relatively high input resistance of Re neurons, would indicate that they are individually capable of transiently driving the membrane potential to be depolarized by a few millivolts. A thorough study of the properties and plasticity of key defined synaptic input pathways to Re neurons is required to better understand how this component of the higher order thalamus contributes to circuit function. Optogenetic approaches would appear to be highly suited to this objective. Nevertheless, our work reveals that blocking all spontaneous ionotropic, amino acid‐mediated synaptic function had no discernible effect on the rates of spontaneous spiking or the distribution of resting potentials within the reduced complexity of a brain slice preparation.
The predominant spontaneous firing frequencies of Re neurons corresponds well with the mean firing rates of Re neurons described in vivo (Ito et al. 2015), as well as the robust theta rhythm that can be recorded in vivo with electrodes implanted into Re. Indeed, autocorrelations of in vivo extracellular recordings of the firing of single Re neurons also reveal a strong phase locking to theta rhythms (Jankowski et al. 2014). Importantly, in brain slices, normal CNS circuit connectivity and resultant network activity is lost; consequently, the observation of spontaneous firing at rates in the theta band suggest that Re could be an intrinsic theta generator and may in turn relay this activity to the limbic structures to which it is connected. The fact that we found many Re neurons fired tonically at ∼8 Hz in blockers of glutamatergic and GABAergic synaptic transmission lends further support this concept of a theta range oscillator intrinsic to Re neurons. Full testing of the idea that Re can act as a theta generator will require in vivo experiments; for example, using optogenetic or pharmacogenetic manipulation of Re neurons at the same time as recording network behaviours in Re and defined projection targets. One initial study of this nature indicates that theta activity in hippocampal area CA1 is preserved when Re is lesioned with ibotenic acid or firing rates are optogenetically reduced (Ito et al. 2015). This may be because the primary hippocampal targets of Re projections appears to be interneurons, rather than pyramidal cells (M. Craig and C. McBain, personal communication).
Although able to intrinsically fire spikes tonically in the theta frequency range in the absence of synaptic drive, the spiking behaviour of Re neurons in vivo will unquestionably be modulated and modified by various forms of excitatory and inhibitory synaptic drive into these cells. These synaptic influences will arises from multiple sources (McKenna & Vertes, 2004), most notably various components of the limbic system. Synaptic inhibition probably pivotally involves input from the reticular nucleus of the thalamus and the zona incerta (Cassel et al. 2013). As outlined above, to achieve an understanding of the contribution of Re to CNS circuits, the functional properties of these afferent inputs to Re are worthy of much more experimental consideration in the future.
Compared to the cortical and hippocampal principal cells, we have recorded in vitro using similar solutions at similar temperatures, Re neurons have a much higher input resistance. Hence, the average value in the present study was over 600 MΩ, whereas, for example, we find adult CA1 pyramidal cells are typically ∼130 MΩ, pyramidal cells in layer 2 of perirhinal cortex average 100 MΩ (Tamagnini et al. 2014) and layer 2/3 stellate cells in the dorsal end of entorhinal cortex average 40 MΩ (Booth et al. 2016). To some extent, their high input resistance probably reflects the smaller size of Re neurons (capacitance ∼50% lower), although it also indicates a relative paucity of active background K+ channels. Rodent TC neurons in other nuclei recorded with broadly similar methods (patch clamp in slices, ∼32–35 C, Kgluconate pipette solution) typically have an input resistance of ∼200 MΩ and resting potentials of −60 to −70 mV (Brunton & Charpak, 1998; Augustinaite & Heggelund, 2007; Samios & Inoue, 2014). It is notable, however, that we have been unable to find any methodologically equivalent (i.e. patch clamp at circa 33°C with Kgluconate intracellular solution) studies of other thalamic nuclei at the same adult age as our mice (∼4 months). Indeed, many TC neuron studies employ much younger rodents often ≤ 3–4 weeks of age. Cells in such animals might be expected to have higher input resistances than those of true adults because this feature of membrane physiology generally decreases with age.
A paucity of active background K+ channels is also a probable contributor to the depolarized resting potential of Re neurons compared to various cortical and hippocampal pyramidal cells studied under identical conditions. The combination of depolarized resting potentials and high input resistances found in Re neurons mean subthreshold cells need little depolarizing current to initiate firing. For example, even when set at −80 mV, which is around the 95% percentile of resting potentials, only 20 pA of current is required to bring ∼80% of the cells to firing threshold (Figs 6 and 9). The large input resistance of Re cells also means they have a comparatively long membrane time constant averaging almost 40 ms. This means that voltage changes in either direction are relatively slow. This may be particularly pertinent for shaping the decay of synaptic potentials and also spike after‐potentials. For example, the timecourse over which the AHP declines appears to pace firing at rest and anything that reduced input resistance without causing substantial hyperpolarization could speed firing. This could potentially occur, for example, through a GABAergic shunting conductance if the chloride equilibrium potential was not too negative. Additional studies utilizing gramicidin‐perforated patch or dynamic clamp could be very informative in this regard.
The Ih current and the sag that it produces in voltage recordings is a cardinal feature of other TC neurons. This conductance plays a role in the determination of resting potential (Meuth et al. 2006; Amarillo et al. 2014) and its gating has long been considered crucial for facilitating transitions between hyperpolarized potentials where burst firing predominates and more depolarized modes of tonic firing. It is also considered crucial to the emergence of rhythmic activities in the thalamus (McCormick & Pape, 1990; Pape, 1996). Notably, Re neurons appear to lack entirely hyperpolarization‐activated sag and Ih‐like currents (Figs 7 B and 8), although these exact same recording conditions are capable of supporting robust Ih‐mediated currents in other cells types (Booth et al. 2016a,b). The absence of Ih‐like currents and sag is also in line with the relative lack of HCN channel expression in Re as reported in the Allen brain atlas (www.mouse.brain‐map.org).
By contrast to other TC cells (Meuth et al. 2006; Amarillo et al. 2014), the lack of Ih and associated sag in Re neurons means that other mechanisms must account for their depolarized resting membrane potential. The lack of Ih probably has consequences with respect to how Re neurons contribute to their cognitive networks, which suggests substantial differences from the behaviours of other more widely studied TC neurons.
Some degree of diversity was seen in the patterns of spontaneous firing in the Re. Unquestionably, most cells fired in a tonic fashion at 2–16 Hz, although more intermittent or bursty patterns of firing were seen in a minority of cells. Notably, the only cells that spontaneously fired high‐frequency spike bursts were those with very negative mean resting potentials. In these cells, the occasional spontaneous bursts of spikes that could exceed instantaneous frequencies of 200 Hz were seen atop an initiating depolarizing transient, which probably reflects a burst of feedforward activation of low threshold Ca2+ channels.
To investigate the intrinsic properties underlying the neurophysiological profile of Re neurons, we extended our studies to investigate how various current stimuli triggered AP production. To reduce the variability that arises from cell to cell differences in resting potential, we initially set the prestimulus resting potential to −80 mV because, at this negative level, there is no ongoing spontaneous firing, which also simplifies the interpretation of data. From this hyperpolarized prestimulus membrane potential, sufficiently large depolarizing current injections produced firing. For over 80% of cells, only 20 pA of applied current was required to produce spiking. In many cells, the first two to four spikes arrived in the form of an initial high‐frequency spike burst, such that the mean instantaneous frequency of the first spike pair was ∼120 Hz (Figs 9 and 10, 11, 12, 13, 14), although observations in excess of 200 Hz were not uncommon. This was the case with either the weakest or strongest suprathreshold stimuli (Figs 9 and 10). Indeed, with the weaker current stimuli, often only an initial high‐frequency burst of spiking was observed (Figs 7 A and 9 C), whereas, with stronger stimuli (e.g. 30 or 60 pA), the initial burst was typically followed by more tonic spiking at 20–30 Hz (Figs 9 and 10). This behaviour is typical of TC neurons. Figure 10 highlights the variety of responses that we observed. The initial frequency of firing of a Re neuron correlates significantly with the size of its observed ADP (r = 0.62, P < 0.001) suggesting that the variable expression of T‐type calcium channels in these neurons could be responsible for the observed diversity. Neurons in the Re have been classified into four groups based on the differential expression of Ca2+ binding proteins calbindin and calretinin (Bokor et al. 2002). As such, it also plausible that differential calcium buffering capabilities between groups could contribute to the observed diversity. Given that the Re appears to be first thalamic nucleus in which such variety of responses from a fixed potential has been reported, future studies should focus on the underlying cellular mechanisms responsible.
High‐frequency burst spiking was also notable when cells at −80 mV were activated with short (2 ms) strong (1–2 nA) current stimuli that rapidly elicited a single primary AP (Fig. 11) or αEPSC current stimuli (Figs 12 and 13). In these cases, the spike burst occurred after the current stimulus was over, indicating that pro‐spiking neurophysiological processes initiated during the current stimulus continued. These processes were apparent in the ADP that followed the single spike driven by short strong current injections (Fig. 11) or the deviations from a typical EPSP waveform in the αEPSC‐driven burst firing (Figs 12 and 13).
The high‐frequency burst firing in response to 500 ms depolarizing stimuli was eliminated when the Re cells where placed at −72 mV prior to application of the current stimulus. At this potential, the low threshold inward current with classical T‐type channel biophysics seen in voltage clamped Re neurons is essentially completely inactivated, whereas at −80 mV some 15% of the channels appear to be available (Fig. 15 D). In line with this, high‐frequency bursting driven by depolarizing current stimuli, both short strong (Fig. 16 A) and longer and weaker (Fig. 16 B) as well as from an ‘anodal break’ following hyperpolarization are also attenuated by the selective T‐type blocker ML‐218.
Thus, Re neurons exhibit radically different firing outputs to depolarizing stimuli depending on their membrane potential prior to the arrival of the stimulus. A shift of only 8 mV completely reconfigures the resultant spiking response. This appears to be predominantly a result of the presence of low threshold, T‐type Ca2+ channels. The currents arising from these voltage‐gated channels have long be shown to play a prominent role in the neurophysiological profile of various thalamic neurons, in particular those of the reticular nucleus and the lateral geniculate. Furthermore, two of the very few previous studies employing cellular recordings in the midline thalamic nuclei have focussed on T‐type channels and their modulation in rodent models of epilepsy and alcoholism (Graef et al. 2009, 2011). T‐type Ca2+ channels are effectively activated by EPSP‐like waveforms (Warre et al. 2002), and T‐type currents have been shown to contribute to the depolarizing envelope of EPSPs in cerebellar Purkinje cells (Ly et al. 2016).
The ability of Re neurons to fire short bursts of AP at very high frequencies either in response to depolarizing current stimuli or through ‘rebound’ following a negative current stimulus is important when considering consequences for the synapses these cells form in the hippocampus, prefrontal cortex and elsewhere. As a result of the effects of short‐term synaptic plasticity, the glutamatergic drive generated from a Re afferent pathway exhibiting occasional bursts of spikes at 100–300 Hz will certainly be very different from that produced by tonic 8 Hz firing. Notably, nothing is known about the short‐term plasticity of Re projections and related features of synaptic function such as their probability of release and post‐synaptic response kinetics.
The synaptic terminations of Re projections into area CA1 of the hippocampus are concentrated in the stratum lacunosum moleculare, which is also where most temporoammonic inputs from the entorhinal cortex terminate. In this distal dendritic region far from the CA1 pyramidal cell layer, electrical stimulation of axons with small numbers of high‐frequency bursts produces robust long‐term potentiation of excitatory synapses on hippocampal neurons (Remondes & Schuman, 2003; Booth et al. 2014, 2016b). Although this long‐term synaptic plasticity is frequently attributed to temporoammonic afferents, unquestionably a component of such responses arises from stimulation of Re neurons that have long been known to produce a robust synaptic response in area CA1 (Dolleman‐Van der Weel et al. 1997; Dolleman‐Van der Weel & Witter, 2000) and also to exhibit robust LTP (Bertram & Zhang, 1999). On this basis, high‐frequency burst firing of presynaptic Re neurons would appear to be ideal for driving synaptic potentiation of their inputs to the hippocampus.
Importantly, our data suggest that the means by which Re neurons will communicate with their downstream synaptic partners depends strongly on their resting potential. Cells of around −65 mV may be tonically firing in the theta frequency range. Assuming these spikes generated in the perisomatic region successfully complete their journey to downstream synapses, post‐synaptic responses will be produced every so often, largely dependent on the probability of release. Not much in the way of EPSP summation or short‐term synaptic plasticity would be expected. Re neurons resting more depolarized than −72 mV but not spontaneously firing will not produce high‐frequency spike bursts when activated, for example, by excitatory synaptic drive, and thus may only produce single spikes or a period of low‐frequency regular spiking. By contrast, Re cells sat at −80 mV or below will probably generate a robust multispike burst on activation that could drive a substantial summated, frequency facilitated EPSP in post‐synaptic cells, a response of the sort that might induce synaptic plasticity if repeated a few times. Notably, Re cells do not need to spend long at negative potentials for T‐type channels to deinactivate and the ability to generate high‐frequency spike bursts to emerge. For example, 300 ms at −85 mV deinactivates ∼80% of T‐type channels (Fig. 15 E).
For the reasons described above, both gaining an understanding of the actual membrane potentials of Re neurons in vivo and determining the key factors that control membrane potential are crucial to understanding how Re contributes to the function of the CNS circuits in which it sits. For example, metabotropic receptor‐mediated IPSPs (produced by activation of GABAB, dopamine D2 or noradrenaline α2 receptors, coupled to GIRK K+ channels) last at least 500 ms and can often last many seconds (North & Surprenant, 1985), which is certainly sufficient time to deinactivate T‐type channels and enable burst firing. Notably, Re receives inputs from the ventral tegmental area and the locus coeruleus (Cassel et al. 2013) and there are correlations between the expression of α2 receptors in Re and behaviour (Wilmot et al. 1988)
In vitro electrophysiological recordings are generally performed with the expectation, or at least hope, that the recorded neurophysiological outcomes relate to those of the corresponding cells in vivo. With confidence in this expectation, it is possible to use the data obtained in vitro to consider how the neurophysiological properties of the investigated cell shape its functional contributions the various neural circuits of which it forms part. Such interpretations can be performed at various levels, from those based on the general ideas highlighted in the present study to those utilizing the data to aid in the building of computational models. By performing the first substantial cellular level study of Re neurones, the present study aimed to obtain insights of this nature; accordingly, we have discussed some of the ways the properties that we have observed in vitro may shape the behaviour of Re neurons in CNS circuits. Furthermore, based on our data, we are also currently starting to build mathematical models of Re neurons.
We feel, however, it is prudent to introduce a note of caution; it is certainly possible that the cellular properties we have documented are not facsimiles of what would be seen with cellular recordings performed in vivo. Indeed, as we have discussed previously (Brown & Randall 2009), the very process of preparing brain slices could induce significant and long‐lasting changes to intrinsic and/or synaptic neurophysiological features of a neuron. However, the task of obtaining over 100 in vivo patch clamp (or intracellular) recordings from a deep and relatively small midline structure in the murine brain would be daunting and, if data gathered without the confounds of anaesthesia were required (for example from head‐fixed recordings), the challenge might best be described as Herculean.
Consequently, in the absence of equivalent in vivo data, we consider this dataset to be valuable for those who aim to better understand the contribution of Re to various CNS processes, particularly so when considered in conjunction with what we have learnt from recent in vivo investigations of the activity of these neurons in behaving rodents (Jankowski et al. 2014, 2015; Ito et al. 2015) as well as studies that outline changes to Re neurons in disease models (Graef et al. 2009, 2011).
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
DAW, JTB and ADR were responsible for the conception and design of experiments; collection, assembly, analysis and interpretation of data; and the drafting of the article and critically revising it for critical intellectual content. All authors approved the final version of the manuscript submitted for publication. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This research was supported by a PhD studentship to DW co‐supported by Eli Lilly and Company and the University of Exeter.
Acknowledgements
The authors wish to thank Hannah Smithers who provided some of the brain slices used in these studies because she was better at getting out of bed than DAW.
References
- Aggleton JP (2014). Looking beyond the hippocampus: old and new neurological targets for understanding memory disorders. Proc Biol Sci 281, doi: 10.1098/rspb.2014.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarillo Y, Zagha E, Mato G, Rudy B & Nadal MS (2014). The interplay of seven subthreshold conductances controls the resting membrane potential and the oscillatory behavior of thalamocortical neurons. J Neurophysiol 112, 393–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinaite S & Heggelund P (2007). Changes in firing pattern of lateral geniculate neurons caused by membrane potential dependent modulation of retinal input through NMDA receptors. J Physiol 582, 297–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram EH & Zhang DX (1999). Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience 92, 15–26. [DOI] [PubMed] [Google Scholar]
- Bokor H, Csáki A, Kocsis K & Kiss J (2002). Cellular architecture of the nucleus reuniens thalami and its putative aspartatergic/glutamatergic projection to the hippocampus and medial septum in the rat. Eur J Neurosci 16, 1227–1239. [DOI] [PubMed] [Google Scholar]
- Booth CA, Brown JT & Randall AD (2014). Neurophysiological modification of CA1 pyramidal neurons in a transgenic mouse expressing a truncated form of disrupted‐in‐schizophrenia 1. Eur J Neurosci 39, 1074–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CA, Ridler T, Murray TK, Ward MA, de Groot E, Goodfellow M, Phillips KG, Randall AD & Brown JT (2016a). Electrical and network neuronal properties are preferentially disrupted in dorsal, but not ventral, medial entorhinal cortex in a mouse model of tauopathy. J Neurosci 36, 312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CA, Witton J, Nowacki J, Tsaneva‐Atanasova K, Jones MW, Randall AD & Brown JT (2016b). Altered intrinsic pyramidal neuron properties and pathway‐specific synaptic dysfunction underlie aberrant hippocampal network function in a mouse model of tauopathy. J Neurosci 36, 350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JT, Booth CA & Randall AD (2011). Synaptic activation of mGluR1 generates persistent depression of a fast after‐depolarizing potential in CA3 pyramidal neurons. Eur J Neurosci 33, 879–889. [DOI] [PubMed] [Google Scholar]
- Brown JT, Gill CH, Farmer CE, Lanneau C, Randall AD, Pangalos MN, Collingridge GL & Davies CH (2003). Mechanisms contributing to the exacerbated epileptiform activity in hippocampal slices of GABAB1 receptor subunit knockout mice. Epilepsy Res 57, 121–136. [DOI] [PubMed] [Google Scholar]
- Brown JT & Randall AD (2009). Activity‐dependent depression of the spike after‐depolarization generates long‐lasting intrinsic plasticity in hippocampal CA3 pyramidal neurons. J Physiol 587, 1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J & Charpak S (1998). Mu‐opioid peptides inhibit thalamic neurons. J Neurosci 18, 1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel J‐C, Pereira de Vasconcelos A, Loureiro M, Cholvin T, Dalrymple‐Alford JC & Vertes RP (2013). The reuniens and rhomboid nuclei: neuroanatomy, electrophysiological characteristics and behavioral implications. Prog Neurobiol 111, 34–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceolin L, Kantamneni S, Barker GRI, Hanna L, Murray L, Warburton EC, Robinson ESJ, Monn JA, Fitzjohn SM, Collingridge GL, Bortolotto ZA & Lodge D (2011). Study of novel selective mGlu2 agonist in the temporo‐ammonic input to CA1 neurons reveals reduced mGlu2 receptor expression in a wistar substrain with an anxiety‐like phenotype. J Neurosci 31, 6721–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De Sa Nogueira D, Raingard H, Robelin L, Kelche C, Pereira de Vasconcelos A & Cassel J‐C (2013). The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. J Neurosci 33, 8772–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson N, Kurihara M, Thomson DM, Winchester CL, McVie A, Hedde JR, Randall AD, Shen S, Seymour PA, Hughes ZA, Dunlop J, Brown JT, Brandon NJ, Morris BJ & Pratt JA (2015). Altered functional brain network connectivity and glutamate system function in transgenic mice expressing truncated disrupted‐in‐schizophrenia 1. Transl Psychiatry 5, e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman‐Van der Weel MJ, Lopes da Silva FH & Witter MP (1997). Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J Neurosci 17, 5640–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman‐Van der Weel MJ & Witter MP (2000). Nucleus reuniens thalami innervates γ aminobutyric acid positive cells in hippocampal field CA1 of the rat. J Neurosci 17, 5640–5650. [DOI] [PubMed] [Google Scholar]
- Giocomo LM & Hasselmo ME (2006). Difference in time course of modulation of synaptic transmission by group II versus group III metabotropic glutamate receptors in region CA1 of the hippocampus. Hippocampus 16, 1004–1016. [DOI] [PubMed] [Google Scholar]
- Graef JD, Huitt TW, Nordskog BK, Hammarback JH & Godwin DW (2011). Disrupted thalamic T‐type Ca2+ channel expression and function during ethanol exposure and withdrawal. J Neurophysiol 105, 528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef JD, Nordskog BK, Wiggins WF & Godwin DW (2009). An acquired channelopathy involving thalamic T‐type Ca2+ channels after status epilepticus. J Neurosci 29, 4430–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Wang A & Griffin AL (2016). Ventral midline thalamus is critical for hippocampal‐prefrontal synchrony and spatial working memory. J Neurosci 36, 8372–8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Shaw CL & Griffin AL (2013). Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working‐memory dependent tactile‐visual conditional discrimination task. Behav Neurosci 127, 860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Tóth TI, Williams SR & Crunelli V (1999). All thalamocortical neurones possess a T‐type Ca2+ ‘window’ current that enables the expression of bistability‐mediated activities. J Physiol 517, 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR & McCormick DA (2007). Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci 30, 350–356. [DOI] [PubMed] [Google Scholar]
- Ito HT, Zhang S‐J, Witter MP, Moser EI & Moser M‐B (2015). A prefrontal‐thalamo‐hippocampal circuit for goal‐directed spatial navigation. Nature 522, 50–55. [DOI] [PubMed] [Google Scholar]
- Jahnsen H & Llinás R (1984). Electrophysiological properties of guinea‐pig thalamic neurones: an in vitro study. J Physiol 349, 205–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MM, Islam MN, Wright NF, Vann SD, Erichsen JT, Aggleton JP & O'Mara SM (2014). Nucleus reuniens of the thalamus contains head direction cells. Elife 3, doi: 10.7554/eLife.03075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MM, Passecker J, Islam MN, Vann S, Erichsen JT, Aggleton JP & O'Mara SM (2015). Evidence for spatially‐responsive neurons in the rostral thalamus. Front Behav Neurosci 9, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MS, Azouz R & Yaari Y (1996). Spike after‐depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol 492, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW & Wilson MA (2005). Theta rhythms coordinate hippocampal‐prefrontal interactions in a spatial memory task. PLoS Biol 3, e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj M, Zhang L, Ronnekleiv OK & Renaud LP (2012). Midline thalamic paraventricular nucleus neurons display diurnal variation in resting membrane potentials, conductances, and firing patterns in vitro. J Neurophysiol 107, 1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES et al (2007). Genome‐wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. [DOI] [PubMed] [Google Scholar]
- Llinas RR & Steriade M (2006). Bursting of thalamic neurons and States of Vigilance. J Neurophysiol 95, 3297–3308. [DOI] [PubMed] [Google Scholar]
- Ly R, Bouvier G, Szapiro G, Prosser HM, Randall AD, Kano M, Sakimura K, Isope P, Barbour B & Feltz A (2016). Contribution of postsynaptic T‐type calcium channels to parallel fibre‐Purkinje cell synaptic responses. J Physiol 594, 915–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair WGP, Warrington EK & Weiskrantz L (1979). Memory disorder in korsakoff’ s psychosis a neuropathological and neuropsychological investigation of two cases. Brain 102, 749–783. [DOI] [PubMed] [Google Scholar]
- McCormick DA & Huguenard JR (1992). A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol 68, 1384–1400. [DOI] [PubMed] [Google Scholar]
- McCormick DA & Pape HC (1990). Properties of a hyperpolarization‐activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol 431, 291–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT & Vertes RP (2004). Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol 480, 115–142. [DOI] [PubMed] [Google Scholar]
- Meuth SG, Kanyshkova T, Meuth P, Landgraf P, Munsch T, Ludwig A, Hofmann F, Pape H‐C & Budde T (2006). Membrane resting potential of thalamocortical relay neurons is shaped by the interaction among TASK3 and HCN2 channels. J Neurophysiol 96, 1517–1529. [DOI] [PubMed] [Google Scholar]
- North RA & Surprenant A (1985). Inhibitory synaptic potentials resulting from alpha 2‐adrenoceptor activation in guinea‐pig submucous plexus neurones. J Physiol 358, 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H‐C (1996). Queer current and pacemaker: the hyperpolarization‐activated cation current in neurons. Annu Rev Physiol 58, 299–327. [DOI] [PubMed] [Google Scholar]
- Pape H‐C, Munsch T & Budde T (2004). Novel vistas of calcium‐mediated signalling in the thalamus. Pflügers Arch Eur J Physiol 448, 131–138. [DOI] [PubMed] [Google Scholar]
- Paxinos G & Franklin KBJ (2001). The Mouse Brain in Stereotaxic Coordinates., 2nd edn. Academic Press, San Diego. [Google Scholar]
- Perez‐Reyes E (2003). Molecular physiology of low‐voltage‐activated t‐type calcium channels. Physiol Rev 83, 117–161. [DOI] [PubMed] [Google Scholar]
- Prasad JA & Chudasama Y (2013). Viral tracing identifies parallel disynaptic pathways to the hippocampus. J Neurosci 33, 8494–8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remondes M & Schuman EM (2003). Molecular mechanisms contributing to long‐lasting synaptic plasticity at the temporoammonic‐CA1 synapse. Learn Mem 10, 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samios VN & Inoue T (2014). Interleukin‐1β and interleukin‐6 affect electrophysiological properties of thalamic relay cells. Neurosci Res 87, 16–25. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR & Siegelbaum SA (2000). Molecular and functional heterogeneity of hyperpolarization‐activated pacemaker channels in the mouse CNS. J Neurosci 20, 5264–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser M‐B & Moser EI (2006). Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762. [DOI] [PubMed] [Google Scholar]
- Tamagnini F, Scullion S, Brown JT & Randall AD (2014). Low concentrations of the solvent dimethyl sulphoxide alter intrinsic excitability properties of cortical and hippocampal pyramidal cells. PLoS ONE 9, e92557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU & Ranck JB (1990). Head‐direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JP, Anderson CM, Williams SR & Crunelli V (1997). Morphology and membrane properties of neurones in the cat ventrobasal thalamus in vitro. J Physiol 505, 707–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Kumar S, Yang JY & Wilson MA (2014). Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct 219, 911–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C & Sherman SM (2007). Differences in response to muscarinic activation between first and higher order thalamic relays. J Neurophysiol 98, 3538–3547. [DOI] [PubMed] [Google Scholar]
- Vertes RP (2006). Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142, 1–20. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti‐Buck K & Leranth C (2007). Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull 71, 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB & Hoover WB (2015). Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev 54, 89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warre RC., McNaughton NC. & Randall A. (2002). Differential discrimination of fast and slow synaptic waveforms by two low‐voltage‐activated calcium channels. Neuroscience 110, 375–388. [DOI] [PubMed] [Google Scholar]
- Williams SR, Turner JP, Hughes SW & Crunelli V (1997). On the nature of anomalous rectification in thalamocortical neurones of the cat ventrobasal thalamus in vitro. J Physiol 505, 727–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot CA, Sullivan AC & Levin BE (1988). Effects of diet and obesity on brain α1‐ and α2‐noradrenergic receptors in the rat. Brain Res 453, 157–166. [DOI] [PubMed] [Google Scholar]
- Xiang Z et al (2011). The discovery and characterization of ML218: a novel, centrally active T‐type calcium channel inhibitor with robust effects in STN neurons and in a rodent model of parkinson's disease. ACS Chem Neurosci 2, 730–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W & Südhof TC (2013). A neural circuit for memory specificity and generalization. Science 339, 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C & Yaari Y (2004). KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci 24, 4614–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]


