Abstract
Isolated brain metastases prior to locoregional recurrence from hilar cholangiocarcinoma (HCCA) following curative resection are an extremely rare event. Very few reports regarding brain metastasis prior to locoregional recurrence following curative resection have been published due to the fact that to differentiate brain metastases from HCCA recurrence is challenging, particular in the early stages, since the neurological findings of brain metastasis are occult and subtle. Any patient with HCCA who has undergone radical resection and subsequently presented with a further onset of neurological symptoms should be evaluated for brain involvement. The present case study describes a patient with HCCA who underwent curative resection, and experienced isolated brain metastases prior to locoregional recurrence.
Keywords: hilar cholangiocarcinoma, brain metastases, curative resection, recurrence
Introduction
Hilar cholangiocarcinoma (HCCA), which comprises ~50% of all malignant bile duct tumors, is a type of cancer that is difficult to treat (1). Surgical resection is the only potentially curative treatment for HCCA, although it fails to reduce the recurrence rate. A previous report has revealed that the HCCA recurrence rate following primary curative resection was up to 50–75% (2). The locoregional areas that are usually regarded as the main recurrent sites have also been documented in the literature (3), and contrary to the locoregional recurrence following radical resection, very few reports regarding isolated brain metastases prior to locoregional recurrence are available. The present case study describes a patient with HCCA who underwent curative resection, and experienced isolated brain metastases prior to locoregional recurrence.
Case report
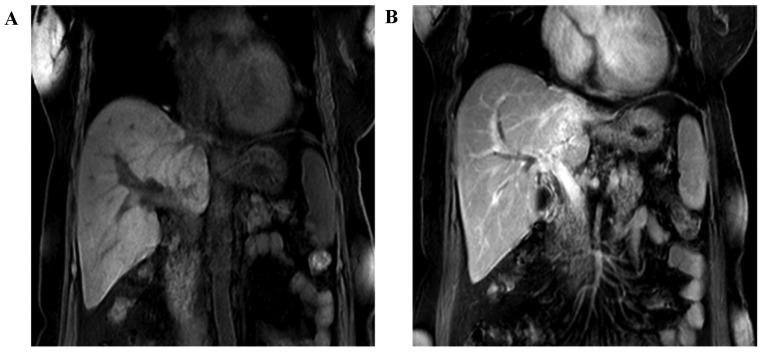
A 63-year-old female patient with a 2 weeks' history of intermittent epigastric pain was admitted to the Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China. A physical examination did not reveal any neurological symptoms, and the patient denied having any associated medical history. An magnetic resonance imaging (MRI) scan of the abdomen revealed that the left bile duct was replaced with a mass, and the intrahepatic biliary duct was dilated; furthermore, the left branch of the portal vein had been invaded (Fig. 1). Simultaneously, a laboratory examination revealed a level of the tumor marker, carcinoembryonic antigen (CEA), of 6.3 ng/ml (normal value, ≤5 ng/ml), and a cancer antigen 19-9 (CA19-9) concentration of 316 U/ml, as well as a moderately increased concentration of serum total bilirubin.
Figure 1.
Results of the MRI examination of the abdomen. (A and B) The left branch of portal vein was invaded. MRI, magnetic resonance scanning. CT, computed tomography.
A curative resection procedure of left hepatectomy with caudate lobectomy and Roux-en-Y bilioenteric anastomosis to re-establish biliary continuity were performed for this patient, with a clinical diagnosis of HCCA being made 7 days later. The patient was intraoperatively diagnosed with type IIIB HCCA, according to the Bismuth-Corlette classification system (4). Ultimately, R0 resection was achieved with negative hepatic margin and bile duct margin, confirmed by pathological examination postoperatively. None of 15 harvested lymph nodes were identified as positive. Finally, the pathological diagnosis of HCCA for this patient [according to the tumor-nodes-metastasis (TNM) system] was stage II, T2bN0M0, with well-differentiated adenocarcinoma. According to the National Comprehensive Cancer Network® (NCCN®) guidelines (5), patients with HCCA following curative resection may be free from adjuvant chemotherapy or radiotherapy. Therefore, the patient did not receive any postoperative adjuvant chemotherapy or radiation, although the present authors conformed with the appropriate follow-up schedules. The patient was released from hospital 2 weeks postoperatively.
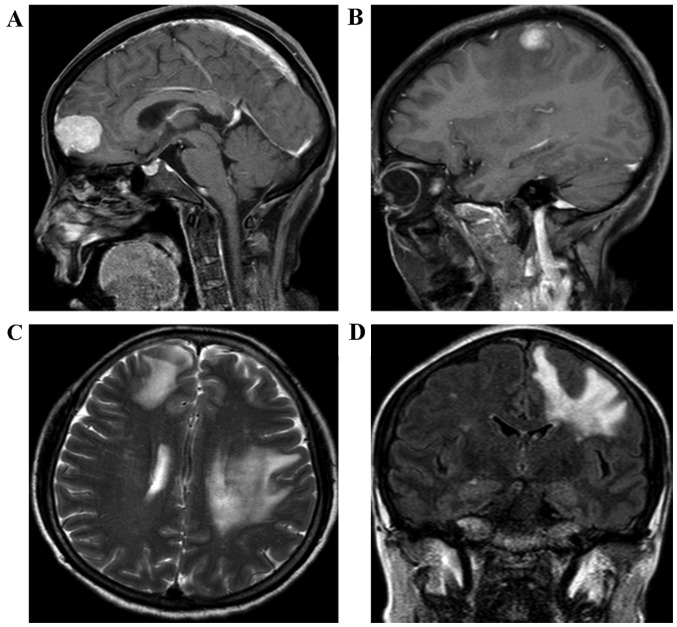
During the following-up, the level of the biomarker, CEA, was determined to be 3.2 ng/ml and the CA19-9 concentration was 32.6 U/ml 3 months after the initial radical surgery, which suggested that the patient was disease-free and that no tumor recurrence activity had been identified. The patient remained alive for 14 months without any tumors, and neither abdominal computed tomography (CT) nor a laboratory examination revealed any sign of tumor recurrence. Subsequently, the patient felt weakness of the right upper extremity and presented with mildly epigastric distention, as well as nausea, 15 months after the initial surgery. Therefore, the patient was readmitted to the Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China. A physical examination was unremarkable. Laboratory tests revealed that the level of aminotransferase was moderately increased, the level of CEA was 9.2 ng/ml, and the concentration of CA 19-9 was 207 U/ml. Furthermore, the cerebral MRI displayed enhancing lesions of the right subcortical frontal lobe and the left subcortical parietal cortex lobe (Fig. 2). It was clearly indicated that these small tumor entities were surrounded with flaky edema (Fig. 2B-D), which is a feature of metastatic encephaloma. Our multidisciplinary team discussed the presentation of the cerebral MRI images, and the radiologist and neurosurgeon considered that they were consistent with metastatic tumors, rather than a primary tumor of brain. At the same time, neither tumor recurrence nor locoregional lymph nodes metastasis was detected in the abdomen using contrasted CT (Fig. 3). A neurosurgeon was consulted for possible biopsy prior to chemotherapy or radiotherapy, but the patient refused to accept any further medical therapy. She died suddenly from a brain hernia due to progression of brain metastasis 2 months after the isolated brain metastasis.
Figure 2.
The MRI of the brain revealed an enhancing lesion of the right subcortical frontal lobe (A) and of the left subcortical parietal cortex lobe (B), respectively, 15 months after curative resection. (C and D) Lesions in the transverse and coronary position are displayed, respectively. MRI, magnetic resonance imaging.
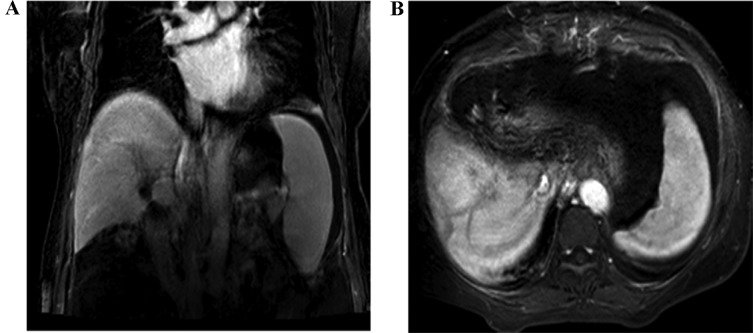
Figure 3.
Results of the abdominal contrast-CT examination of the abdomen. (A and B) The abdominal contrast-CT revealed that no tumor recurrence or locoregional lymph nodes metastasis was detected 15 months after the curative resection. CT, computed tomography.
Discussion
Brain metastases, with an incidence of ~5%, usually occurs in patients with primary carcinoma, including breast cancer, lung cancer, and melanoma (6,7). Regarding the brain metastases from HCCA, only a few cases with brain metastases can be located in the previous literature, which is predominantly associated with intrahepatic cholangiocarcinoma (ICC) rather than HCCA (6,8,9).
Surgical resection is the only potential curative option for HCCA. However, the recurrence rate following primary curative resection is currently at the level of 50–75% (2). At the same time, the main recurrent sites are predominantly located in locoregional areas, such as the hepatic resection margin, bilioenteric anastomosis, and porta hepatis (3). Non-hematogenous spread to distant organs is a rare phenomenon, particularly in the brain. In a recent study from Thailand, brain metastases occurred in 0.15% (8 out of 5,164) of patients with cholangiocarcinoma, but only 2 were patients with HCCA (10). Additionally, cholangiocarcinoma is known to be a poorly vascularized carcinoma that originates from the biliary tree, which may account for the rare occurrence of brain metastases in HCCA.
Although, in the present case study, the diagnosis of isolated brain metastases was a clinical diagnosis made without biopsy due to the refusal of the patient, it was observed that the CEA concentration of 9.2 ng/ml (normal concentration, ≤5 ng/ml) and the CA19-9 concentration of 207 U/ml (normal, ≤34 U/ml) were markedly abnormal. Comparing these values with the CEA level of 3.2 ng/ml and the CA19-9 level of 32.6 U/ml recorded 3 months after radical surgery, this suggested that HCCA recurrence had occurred to a large extent. Furthermore, the additional primary tumor of the alimentary tract was excluded on the basis of past history and abdominal image workup. Importantly, multiple lesions, but not only one tumor entity, were detected by the brain MRI scan. In addition, these small tumor entities were surrounded with clearly flaky edema (Fig. 2B-D), which is a feature of metastatic encephaloma.
To differentiate brain metastasis from the recurrence of HCCA is challenging, particularly in the early stages, since neurological findings of brain metastasis were occult and subtle. Indeed, in this case, no remarkable sign was observed until 15 months postoperatively, when the patient developed weakness of the upper extremity. Although MRI scans may help in detection of the cerebral lesion, it is not a routine workup for HCCA during postoperative follow-up. Furthermore, hepatopancreatobiliary practitioners tend to focus on examinations of the abdominal CT and laboratory tests to detect regular recurrence sites of HCCA postoperatively, which may miss other potential solitary recurrence sites. Furthermore, to the best of our knowledge, the median survival time of brain metastasis from ICC is dismal, even after having performed whole-brain radiotherapy or chemotherapy (6,9,10). However, a similar outcome of isolated brain metastasis in HCCA following curative resection is not available. It must be admitted that our case presented a poor prognosis. Hence, early diagnosis and an associated aggressive treatment might be necessary in order to prevent irreversible neurological deficits, and to stabilize the neurological status to a certain extent.
In conclusion, isolated brain metastases prior to locoregional recurrence in HCCA following curative surgery is a fatal complication that occurs extremely rarely. Clinicians are supposed to keep their minds open and focus on the neurological symptoms, such as motor weakness, or alteration of consciousness. It is indispensable that any patient with HCCA who has undergone radical resection surgery, and subsequently presented with a further onset of neurological symptoms, should be evaluated for brain involvement (11).
Acknowledgements
The present study was supported by the ‘Young Stuff’ foundation of Sun Yat-Sen University (grant no. 15ykpy20) the Key Laboratory of Malignant Tumor Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology (grant no. [2013] 163), and the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes (grant no. KLB 09001).
References
- 1.Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut. 2012;61:1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 2.Jang JY, Kim SW, Park DJ, Ahn YJ, Yoon YS, Choi MG, Suh KS, Lee KU, Park YH. Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann Surg. 2005;241:77–84. doi: 10.1097/01.sla.0000150166.94732.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, Wagman R, Blumgart LH, Fong Y. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: Implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–1700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 4.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170–178. [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. (NCCN), corp-author Clinical Practice Guidelines in Oncology. Hepatobiliary Cancer; [Jun 27;2016 ]. (Version 2. 2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.William BM, Grem JL. Brain metastasis and leptomeningeal carcinomatosis in a patient with cholangiocarcinoma. Gastrointest Cancer Res. 2011;4:144–145. [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Z, Yang G, Yuan T, Pang X, Wang Y, Qu L, Dong L. Leptomeningeal metastasis from hepatocellular carcinoma with other unusual metastases: A case report. BMC Cancer. 2014;14:399. doi: 10.1186/1471-2407-14-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huffman JL, Yeatman TJ, Smith JB. Leptomeningial Carcinomatosis: A sequela of Cholangiocarcinoma. Am Surg. 1997;63:310–313. [PubMed] [Google Scholar]
- 9.Jacobs RE, McNeill K, Volpicelli FM, Warltier K, Iturrate E, Okamura C, Adler N, Smith J, Sigmund A, Mednick A, et al. Treatment of leptomeningeal carcinomatosis in a patient with metastatic cholangiocarcinoma. ACG Case Rep J. 2014;2:39–41. doi: 10.14309/crj.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chindaprasirt J, Sookprasert A, Sawanyawisuth K, Limpawattana P, Tiamkao S. Brain metastases from cholangiocarcinoma: A first case series in Thailand. Asian Pac J Cancer Prev. 2012;13:1995–1997. doi: 10.7314/APJCP.2012.13.5.1995. [DOI] [PubMed] [Google Scholar]
- 11.Mirrakhimov AE, Nwankwo N, Zdunek T, Bucher N. Cholangiocarcinoma and brain lesions: An extremely rare finding. BMJ Case Rep: bcr2013009235. 2013 doi: 10.1136/bcr-2013-009235. [DOI] [PMC free article] [PubMed] [Google Scholar]