Abstract
This randomized clinical trial was designed to determine whether glutamine administration was effective in reducing the incidence and severity of mucositis and dermatitis induced by radiotherapy (RT) or chemoradiotherapy (CHRT) in patients with head and neck cancer (HNC). Fifty patients were randomized to receive orally either L-Glutamine or placebo (25 patients in each arm). In the glutamine-treated group, 10 g of oral glutamine was administered three times daily. The primary endpoint was to compare the appearance of clinical mucositis between groups at the 6th week, according to the Common Terminology Criteria for Adverse Events. Secondary endpoints were: Functional mucositis, mucositis onset, cervicofacial dermatitis, pain, weight loss and assessment of quality of life (according to the M.D. Anderson Symptom Inventory-Head and Neck). In total, 76 and 87.5% developed clinical mucositis in the glutamine and placebo group, respectively. The incidence and severity grade of mucositis at the 6th week did not exhibit statistically significantly differences between the two groups, although it had a higher value in the placebo group. Significant reduction of dermatitis incidence (P=0.038) and severity (P=0.032) was found in the glutamine group. There were no differences in other outcomes such as pain, weight loss and mucositis onset, in treatment parameters including concomitant chemotherapy, radiation dose and previous surgery, or in quality of life. The present study revealed that glutamine provided slight clinical effects compared with placebo in terms of reducing oral mucositis induced by RT or CHRT in patients with HNC at the 6th week; however, the results were not statistically significant. Although the findings suggested a significant benefit in reducing the incidence and severity of dermatitis, further confirmatory studies are required.
Keywords: glutamine, head and neck cancer, radiation therapy, chemoradiotherapy, mucositis, dermatitis
Introduction
Radiation therapy (RT) and concomitant chemoradiotherapy (CHRT) in patients with head and neck cancer (HNC) are commonly associated with complications such as mucositis and dermatitis in the oral cavity and on the cervicofacial area. Clinically, these adverse effects are able to cause severe pain and odinophagia, increase the risk of infections, skin wounds and inflammatory ulcers, limit deglutition, cause malnutrition and negatively impact the diet and overall quality of life of patients (1). The incidence rate of mucositis varies between 85 and 100%, depending on the cancer treatment regimen, with altered fractionated RT, CHRT or conventional RT (2,3), whereas the incidence of acute dermatitis varies between 7 and 25% (4,5), reaching up to 49% in patients receiving RT in combination with cetuximab (6).
Numerous efforts have been made to identify effective agents for preventing and treating mucositis and dermatitis induced by RT or CHRT. Although some standard guidelines for management are available (7,8), reliable and effective treatments are lacking (9). For mucositis, intensive oral care protocols, antimicrobial agents, anti-inflammatory agents, cytoprotective agents, recombinant keratinocyte growth factor-1, nutritional supplements, biostimulant agents or natural and homeopathic agents have been described (10–13). On the other hand, several topical or systemic formulations have been tried for dermatitis, all of them with ineffective results (5,6). The ultimate goal of these treatments is to allow patients to receive complete radiation doses while improving their quality of life, as well as avoiding unplanned discontinuation of treatments with a subsequent negative impact on the final outcome.
Glutamine is a free primary amino acid precursor for protein synthesis, involved in cell replication for rapid cell turnover, primarily in the gastrointestinal mucosa and immune system. In circumstances of intense catabolism and stress, this organic molecule becomes indispensable as its production demand exceeds the capacity of endogenous synthesis (14). In addition, patients with HNC often have a glutamine deficit accentuated by the side effects of RT or CHRT (15).
Since the first study by Skubitz and Anderson (16) in 1996 on the role of glutamine for the prevention and treatment of oral mucositis in cancer treatments, a number of studies have demonstrated that supplementation of this amino acid may reduce the incidence and severity of mucositis during treatment by reversing cellular damage and improving cellular recovery (17). Certain studies with animal models indicated that glutamine supplementation was safe and reduced cytotoxicity-induced mucositis (18,19). However, others have found no conclusive outcomes, and pointed out that more studies with higher consistency and methodological validity were needed to find more solid evidence (7). The aim of this prospective study was to determine whether oral glutamine administration was effective in reducing the incidence and severity of oral mucositis and cervicofacial dermatitis induced by RT or CHRT in patients with HNC.
Patients and methods
Patients
Patients in this trial were recruited from the Puerta del Mar University Hospital (Cadiz, Spain) between July 2010 and June 2012. The following inclusion criteria were applied: Patients with primary cancer in any head and neck location with a proven malignant biopsy, undergoing RT with or without concomitant CHRT, and 0 and I performance status grade according to ECOG (Eastern Cooperative Oncology Group Performance Status). The exclusion criteria were: Patients with a previous history of receiving RT, uncontrolled systemic or disseminated disease, presence of synchronous double malignant tumor, hypersensitivity or allergy to any of the components included in the study, uncontrolled diabetes, severe kidney or liver failure, skin diseases or autoimmune diseases.
Study design
The current study was a phase II randomized double-blind controlled study. The eligible participants were randomized into a control group or an experimental group to receive daily administration of oral glutamine or placebo for assessment of its efficacy in the management of mucositis and dermatitis following RT or concomitant CHRT. A randomization in 5 blocks of 10 patients with 1-to-1 assignment to groups was computer-generated by a statistician who was not working with the patients. These allocations were placed in sealed masked envelopes with a specific number group or an experimental group to receive a daily administration of oral glutamine or placebo. The study protocol was approved by the Ethics Committee of the Puerta del Mar University Hospital, Cadiz, Spain and by the Spanish Agency for Drugs and Health Products (number of trial registry 2009-018103-40). All patients gave written informed consent to participate.
According to the institutional protocol, the total radiation dose was fixed to 70 Gy in 35 fractions of 2 Gy, or 66 Gy in 30–33 fractions of 2 Gy in postoperative RT. Depending on the case, patients received cisplatin (100 mg/m2) or cetuximab (400 mg/m2) based on renal function and the presence of systemic complications. During the study, each patient received three daily bags to be dissolved in a glass of water (orally, distributed in the three meals), containing either 10 g of maltodextrin as the placebo (control group) or 10 g of L-Glutamine as the treatment (experimental group). Both supplements were prepared by Nutricion Medica S.L. Laboratories (Madrid, Spain) in powder form packaged in single dosage pouches indistinguishable from each other, thus ensuring double-blind masking. The patients were evaluated by the same observer at the 3rd and 6th weeks during the treatment protocol, and at the 1st and 6th months post-treatment. All patients had completed dental and oral examination prior to treatment, and underwent oral care. For symptomatic mucositis, oral paracetamol tablets 500 mg or tramadol 100 mg were administered according to the severity of pain. The need for painkillers, adverse events associated with the study drugs and patient non-adherence to treatment were recorded.
Endpoints and measures
The primary endpoint was the appearance of clinical oral mucositis at the 6th week after treatment. Secondary endpoints included evaluation of functional mucositis, onset of mucositis, cervicofacial dermatitis, pain and weight loss. Oral mucositis and cervicofacial dermatitis were assessed according to the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0). Clinical mucositis was graded from grade 0 (no mucositis) to grade 4 (symptomatic associated with life threatening consequences, tissue necrosis, significant spontaneous bleeding). Functional mucositis was evaluated from grade 0 (no mucositis) to grade 4 (symptoms associated with life-threatening consequences). Dermatitis on the cervicofacial area was assessed from grade 1 (faint erythema or dry desquamation) to grade 4 (skin necrosis or ulceration of full thickness of dermis; spontaneous bleeding from involved site). Data regarding pain were collected using a visual analog scale (VAS) from 0 (‘no pain’) to 10 (‘insupportable pain’). Evaluation of treatment tolerance was based on occurrence of adverse events during the trial. Patients fulfilled the quality of life questionnaire of the M.D. Anderson Symptom Inventory-Head and Neck (MDASI-HN), comprising 3 subscales: 13 items that rated the severity of general symptoms associated with cancer, 9 HNC-specific items that rated the severity of symptoms particularly associated with HNC and 6 items that assessed how severely symptoms interfered with daily activities. The core and HNC-specific symptoms were rated on a 0–10 scale to indicate the presence and severity of the symptom, with 0 indicating ‘not present’ and 10 indicating ‘as bad as you can imagine.’
Statistical analysis
The sample size was calculated according to an expected clinical mucositis appearance of 100% at the 6th month. Expecting to find a reduction of 75% after prescribing glutamine (δ value of 25%), a 5% α error and an 80% β value (study power) were selected, assuming a 5% loss. With these data, the necessary number of participants to obtain statistical significance was 50 patients (25 per group). Intention to treat and per protocol analyses were performed on the population.
The results were analyzed using the SPSS version 15.0 (IBM Corp., Chicago, IL, USA). All statistical tests achieved a significance level of P<0.05 for a bilateral significance. The baseline characteristics of groups were compared using analysis of variance for continuous variables. In the case of discrete variables, the distribution of absolute frequencies and percentages were obtained. Discrete variables were compared using the χ2 statistic, and continuous variables using the Student's t-test. Comparisons between the two groups were performed using analysis of covariance. The results of VAS scores were presented as the mean, median and standard deviation.
Results
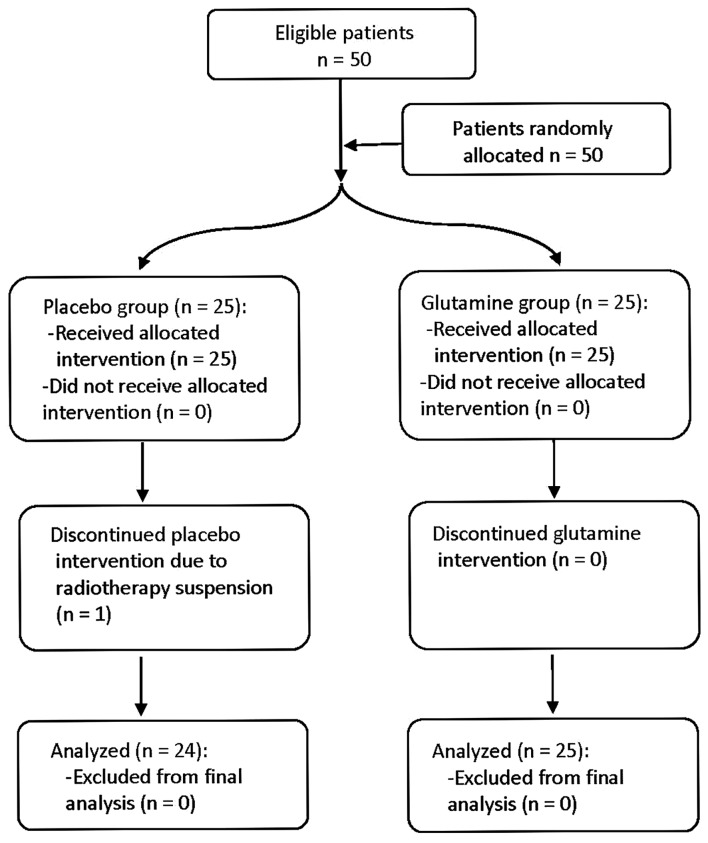
Fifty patients between 32 and 79 years of age (mean, 60.24) were included in the intention to treat analysis, 78% were male and 22% were female. One female dropped out due to RT suspension. As a result, 24 patients in the control group and 25 patients in the experimental group were included in the full per protocol analysis (Fig. 1). On conducting the statistical analysis, the intention to treat analysis and per protocol analysis produced identical results for all criterion measures; therefore, only the per protocol analysis was used to describe the results.
Figure 1.
Flow diagram of the patient treatment process.
The distribution of patients according to baseline patient characteristics, diagnostic and treatment parameters were similar between the study groups (Table I). In total, 45% of patients received RT and 55% concomitant CHRT. The dose of radiation was 70 Gy in 91.8% of patients and 66 Gy in 8.2%. Cisplatin and cetuximab was indicated in 13 and 14 patients, respectively; 63.3% of patients had previously underwent surgery.
Table I.
Baseline patient characteristics and diagnostic parameters.
| Placebo group (n=25) | % | Glutamine group (n=25) | % | P-value | |
|---|---|---|---|---|---|
| Sex | 0.496 | ||||
| Male | 20 | 80 | 18 | 72 | |
| Female | 5 | 20 | 7 | 28 | |
| Median age, years | 61.5 (32–81) | 59. (39–78) | 0.432 | ||
| Tobacco use | 0.358 | ||||
| No smoker | 6 | 24 | 7 | 28 | |
| <20 cigarettes | 5 | 20 | 1 | 4 | |
| 20–40 cigarettes | 6 | 24 | 7 | 28 | |
| >40 cigarettes | 2 | 8 | 5 | 20 | |
| Ex-smoker | 5 | 20 | 5 | 20 | |
| Alcohol | 0.368 | ||||
| No | 11 | 44 | 10 | 40 | |
| Yes | 12 | 48 | 11 | 44 | |
| Ex-alcoholic | 2 | 8 | 4 | 16 | |
| Median weight, kg | 76.7 (52.6–102.8) | 70.8 (37.6–96) | |||
| Median pain | 0.49 (0–8) | 0.61 (0–7) | |||
| Primary tumor site | 0.621 | ||||
| Oral cavity | 9 | 36 | 8 | 32 | |
| Oropharynx | 5 | 20 | 6 | 24 | |
| Nasopharynx | 1 | 4 | 1 | 4 | |
| Hypopharynx | 2 | 8 | 1 | 4 | |
| Larynx | 8 | 32 | 9 | 36 | |
| Tumor histopathology | 0.594 | ||||
| Squamous cell carcinoma | 21 | 84 | 22 | 88 | |
| Adenocarcinoma | 2 | 8 | 1 | 4 | |
| Mucoepidermoid | 1 | 4 | 2 | 8 | |
| Adenoid cystic carcinoma | 1 | 4 | 0 | 0 | |
| AJCC stage | 0.71 | ||||
| I | 0 | 0 | 0 | 0 | |
| II | 5 | 20 | 4 | 16 | |
| III | 7 | 28 | 5 | 20 | |
| IV | 13 | 52 | 16 | 64 | |
| ECOG-PS | 0.98 | ||||
| 0 | 24 | 96 | 25 | 100 | |
| 1 | 1 | 4 | 0 | 0 | |
AJCC, American Joint Committee on Cancer; ECOG-PS, Eastern Cooperative Oncology Group Performance Status.
The incidence of clinical mucositis was 87.5% in the placebo group and 76% in the glutamine group (81.6% of global incidence) (Table II). The incidence and severity grade of clinical and functional mucositis at the 6th week did not exhibit statistically significantly differences between the two groups. The comparison of clinical and functional mucositis had a higher value in placebo group, although without statistical difference. A direct significant statistical correlation was found between the values of the clinical and functional mucositis (P=0.01), with a coefficient of 0.71 and 0.597 at the 3rd and 6th week, respectively.
Table II.
Incidence and severity of mucositis and dermatitis.
| Placebo group (n=24) | % | Glutamine group (n=25) | % | P-value | |
|---|---|---|---|---|---|
| Incidence of clinical mucositis | 21 | 87.5 | 19 | 76 | 0.324 |
| Grade of clinical mucositis | 0.341 | ||||
| Grade 0 | 3 | 12.5 | 6 | 24 | |
| Grade 1 | 10 | 41.7 | 8 | 32 | |
| Grade 2 | 9 | 36.4 | 10 | 40 | |
| Grade 3 | 2 | 8.4 | 1 | 4 | |
| Grade 4 | 0 | 0 | 0 | 0 | |
| Incidence of functional mucositis | 18 | 75 | 19 | 76 | 0.511 |
| Grade of functional mucositis | 0.198 | ||||
| Grade 0 | 6 | 25 | 6 | 24 | |
| Grade 1 | 8 | 33.3 | 9 | 36 | |
| Grade 2 | 10 | 41.7 | 7 | 28 | |
| Grade 3 | 0 | 0 | 3 | 12 | |
| Grade 4 | 0 | 0 | 0 | 0 | |
| Incidence of dermatitis | 24 | 100 | 21 | 84 | 0.038a |
| Grade of dermatitis | 0.032a | ||||
| Grade 0 | 0 | 0 | 4 | 16 | |
| Grade 1 | 11 | 45.8 | 15 | 60 | |
| Grade 2 | 12 | 50 | 5 | 20 | |
| Grade 3 | 1 | 4.2 | 1 | 4 | |
| Grade 4 | 0 | 0 | 0 | 0 |
P<0.05.
A statistically significant reduction of the incidence (P=0.038) and severity (P=0.032) of dermatitis was found in the glutamine group at the 6th week. There were no differences between groups in the mucositis appearance according to any of treatment parameters (concomitant chemotherapy, radiation dose and previous surgery). No statistically significant differences between the two groups were identified for pain, weight loss and mucositis onset (Table III). The analysis of the quality of life questionnaire (MDASI-HN) revealed no significant differences between groups in any items at the 6th week. No patient discontinued the study medication due to adverse effects.
Table III.
Effect of glutamine on several outcomes.
| Variable | Units | Placebo group (n=24) | Glutamine group (n=25) | P-value |
|---|---|---|---|---|
| Pain | VAS (0–10) | 1.96 | 2.32 | 0.574 |
| Weight | Kg | −2.55 | −3.3 | 0.526 |
| Onset of mucositis | Days | 29.91 | 28.38 | 0.726 |
| MDASI-HN questionnaire | ||||
| General items | 2.23 | 1.7 | 0.374 | |
| Specific items | 2.6 | 2.6 | 0.48 | |
| Impact on daily activities | 3.05 | 2.58 | 0.54 | |
| Global | 2.34 | 1.85 | 0.222 | |
MDASI-H, M. D. Anderson Symptom Inventory-Head and Neck; VAS, visual analog scale.
Discussion
The effectiveness of glutamine for the prevention and treatment of oral mucositis induced by RT has been examined recently in some meta-analyses. One meta-analysis (20) identified 5 clinical randomized controlled trials that included 234 patients with HNC. The conclusions of this meta-analysis revealed that glutamine significantly reduced the risk and severity of oral mucositis induced by RT or CHRT compared with either placebo or no treatment (risk ratio 0.17; 95% CI 0.06–0.47) (21–25). Another systematic review of well-designed studies in various solid tumors and patients with hematological cancer revealed inadequate or conflicting evidence (26), as five studies did not find glutamine to be effective (27–30), while four studies did (19,31–33), suggesting a requirement for further studies on the use of oral glutamine to guide clinicians on which interventions are truly effective.
The findings of the present study did not demonstrate the primary study endpoint in a statistically significant manner. However, the incidence and severity of clinical and functional mucositis tended to be clinically lower in the glutamine group in comparison with the experimental group, although it did not reach statistical significance. The frequency of clinical mucositis was of 87.5% for the placebo group vs. 76% for the glutamine group and, of a total of 9 patients who did not show mucositis, 6 were found in the glutamine group. None of patients presented with a severity grade of 4 at the 6th week, revealing a homogeneous distribution of clinical mucositis between the two groups.
Although the present study was unable to demonstrate the effectiveness of the primary endpoint, oral glutamine significantly reduced the incidence and severity of dermatitis produced in the radiation fields, as a secondary endpoint. A previous study reported that β-hydroxy-β-methylbutyrate/arginine/glutamine supplementation was potentially effective in the prevention of radiation dermatitis in patients with HNC, as this supplementation was a protective nutrient with anti-inflammatory effects favoring the healing of inflammatory skin wounds (34).
In the current study, there were no differences in other outcomes including pain, weight loss and mucositis onset, and in treatment parameters such as concomitant chemotherapy, radiation dose and previous surgery. This study was not able to demonstrate an improvement in the quality of life of patients as no statistical differences were also found between the two groups in the assessment of cancer-associated symptoms with the MDASI-HN quality of life questionnaire.
Very few clinical trials on oral glutamine administration for the prevention of oral mucositis in patients with HNC have been reported (21–24). In general terms, the majority of studies have described favorable results concerning oral glutamine administration, and consequently the findings of the present study are not fully consistent with the indexed literature. However, the comparison with these previous studies is challenging, as the choice of the primary endpoint, the δ value and the magnitude of the clinically relevant difference, were not revealed in the previous studies. In the present study, mucositis and dermatitis were considered as qualitative variables, which gave more verisimilitude to clinical reality unlike other studies in which they were classified as quantitative, thus losing much data analysis.
The results of the present study must be interpreted with caution due to the short follow-up period and the reduced study sample size. The sample size depended upon the 25% δ value, estimated based on the assumption that the placebo group should have a 100% incidence of clinical mucositis at the 6th week, which did not occur. Consequently, a lower δ value may have favored a larger sample size. This study was a single center study performed in the authors' setting. Although the distribution of patients was similar between groups and treatment parameters such as chemotherapy, radiation dose or previous surgery showed no difference between groups, a subgroup of analysis could have been performed if the number of subjects were not too low. Consequently, multicenter and large-scale studies are warranted. The strengths of this study were based on its randomized double-blind placebo-controlled study design. Throughout the trial, the main researcher monitored the patient test adherence, which resulted in a 100% adherence, and also performed a count of bags used and surplus.
Therefore, it is only possible to conclude that, in this double-blind, randomized study, oral glutamine provided slight clinical effects compared with placebo in reducing oral mucositis induced by RT or CHRT in patients with HNC at the 6th week, although the results were not statically significant. While the findings of the study suggested a significant benefit in reducing the incidence and severity of dermatitis, further confirmatory studies with a new primary endpoint and a larger sample size are required.
Acknowledgements
The present study formed part of a thesis and L-glutamine and oral nutrition supplements were gifts of Nutrition Medica S.L. Laboratories (Madrid, Spain).
References
- 1.Moslemi D, Nokhandani AM, Otaghsaraei MT, Moghadamnia Y, Kazemi S, Moghadamnia AA. Management of chemo/radiation-induced oral mucositis in patients with head and neck cancer: A review of the current literature. Radiother Oncol. 2016;120:13–20. doi: 10.1016/j.radonc.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, Komaroff E, Nalysnyk L, Zilberberg MD. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/S0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 3.Harada K, Ferdous T, Horinaga D, Uchida K, Mano T, Mishima K, Park S, Hanazawa H, Takahashi S, Okita A, et al. Efficacy of elemental diet on prevention for chemoradiotherapy-induced oral mucositis in patients with oral squamous cell carcinoma. Support Care Cancer. 2016;24:953–959. doi: 10.1007/s00520-015-2866-7. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 5.Elliott EA, Wright JR, Swann RS, Nguyen-Tân F, Takita C, Bucci MK, Garden AS, Kim H, Hug EB, Ryu J, et al. Phase III trial of an emulsion containing trolamine for the prevention of radiation dermatitis in patients with advanced squamous cell carcinoma of the head and neck: Results of radiation therapy oncology group trial 99–13. J Clin Oncol. 2006;24:2092–2097. doi: 10.1200/JCO.2005.04.9148. [DOI] [PubMed] [Google Scholar]
- 6.Giro C, Berger B, Bölke E, Ciernik IF, Duprez F, Locati L, Maillard S, Ozsahin M, Pfeffer R, Robertson AG, et al. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: Results of a survey in EORTC institutes. Radiother Oncol. 2009;90:166–171. doi: 10.1016/j.radonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Mead GM. Management of oral mucositis associated with cancer chemotherapy. Lancet. 2002;359:815–816. doi: 10.1016/S0140-6736(02)07960-6. [DOI] [PubMed] [Google Scholar]
- 8.Vélez I, Tamara LA, Mintz S. Management of oral mucositis induced by chemotherapy and radiotherapy: An update. Quintessence Int. 2004;35:129–136. [PubMed] [Google Scholar]
- 9.Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120:1453–1461. doi: 10.1002/cncr.28592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stokman MA, Spijkervet FK, Boezen HM, Schouten JP, Roodenburg JL, de Vries EG. Preventive intervention possibilities in radiotherapy- and chemotherapy-induced oral mucositis: Results of meta-analyses. J Dent Res. 2006;85:690–700. doi: 10.1177/154405910608500802. [DOI] [PubMed] [Google Scholar]
- 11.Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE. Mucositis Study Section of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology: Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820–831. doi: 10.1002/cncr.22484. [DOI] [PubMed] [Google Scholar]
- 12.Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, Salzwimmer M, Lizambri R, Emmerson L, Chen MG, Berger D. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: A randomized, placebo-controlled trial. J Clin Oncol. 2011;29:2815–2820. doi: 10.1200/JCO.2010.32.4103. [DOI] [PubMed] [Google Scholar]
- 13.Xu JL, Xia R, Sun ZH, Sun L, Min X, Liu C, Zhang H, Zhu YM. Effects of honey use on the management of radio/chemotherapy-induced mucositis: A meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg. 2016;45:1618–1625. doi: 10.1016/j.ijom.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Wischmeyer PE. Clinical applications of L-glutamine: Past, present, and future. Nutr Clin Pract. 2003;18:377–385. doi: 10.1177/0115426503018005377. [DOI] [PubMed] [Google Scholar]
- 15.Neu J, DeMarco V, Li N. Glutamine: Clinical applications and mechanisms of action. Curr Opin Clin Nutr Metab Care. 2002;5:69–75. doi: 10.1097/00075197-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Skubitz KM, Anderson PM. Oral glutamine to prevent chemotherapy induced stomatitis: A pilot study. J Lab Clin Med. 1996;127:223–228. doi: 10.1016/S0022-2143(96)90082-7. [DOI] [PubMed] [Google Scholar]
- 17.Silverman S., Jr Diagnosis and management of oral mucositis. J Support Oncol. 2007;5(2 Suppl 1):S13–S21. [PubMed] [Google Scholar]
- 18.Savarese DM, Savy G, Vahdat L, Wischmeyer PE, Corey B. Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treat Rev. 2003;29:501–513. doi: 10.1016/S0305-7372(03)00133-6. [DOI] [PubMed] [Google Scholar]
- 19.Choi K, Lee SS, Oh SJ, Lim SY, Lim SY, Jeon WK, Oh TY, Kim JW. The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin Nutr. 2007;26:57–62. doi: 10.1016/j.clnu.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Leung HW, Chan AL. Glutamine in alleviation of radiation-induced severe oral mucositis: A meta-analysis. Nutr Cancer. 2016;68:734–742. doi: 10.1080/01635581.2016.1159700. [DOI] [PubMed] [Google Scholar]
- 21.Huang EY, Leung SW, Wang CJ, Chen HC, Sun LM, Fang FM, Yeh SA, Hsu HC, Hsiung CY. Oral glutamine to alleviate radiation-induced oral mucositis: A pilot randomized trial. Int J Radiat Oncol Biol Phys. 2000;46:535–539. doi: 10.1016/S0360-3016(99)00402-2. [DOI] [PubMed] [Google Scholar]
- 22.Cerchietti LC, Navigante AH, Lutteral MA, Castro MA, Kirchuk R, Bonomi M, Cabalar ME, Roth B, Negretti G, Sheinker B, Uchima P. Double-blinded, placebo-controlled trial on intravenous L-alanyl-L-glutamine in the incidence of oral mucositis following chemoradiotherapy in patients with head and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;65:1330–1337. doi: 10.1016/j.ijrobp.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Chattopadhyay S, Saha A, Azam M, Mukherjee A, Sur PK. Role of oral glutamine in alleviation and prevention of radiation-induced oral mucositis: A prospective randomized study. South Asian J Cancer. 2014;3:8–12. doi: 10.4103/2278-330X.126501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsujimoto T, Yamamoto Y, Wasa M, Takenaka Y, Nakahara S, Takagi T, Tsugane M, Hayashi N, Maeda K, Inohara H, et al. L-glutamine decreases the severity of mucositis induced by chemoradiotherapy in patients with locally advanced head and neck cancer: A double-blind, randomized, placebo-controlled trial. Oncol Rep. 2015;33:33–39. doi: 10.3892/or.2014.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vidal-Casariego A, Calleja-Fernández A, Ballesteros-Pomar MD, Cano-Rodríguez I. Efficacy of glutamine in the prevention of oral mucositis and acute radiation induced esophagitis: A retrospective study. Nutr Cancer. 2013;65:424–429. doi: 10.1080/01635581.2013.765017. [DOI] [PubMed] [Google Scholar]
- 26.Yarom N, Ariyawardana A, Hovan A, Barasch A, Jarvis V, Jensen SB, Zadik Y, Elad S, Bowen J, Lalla RV. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO): Systematic review of natural agents for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21:3209–3221. doi: 10.1007/s00520-013-1869-5. [DOI] [PubMed] [Google Scholar]
- 27.Jebb SA, Osborne RJ, Maughan TS, Mohideen N, Mack P, Mort D, Shelley MD, Elia M. 5-fluorouracil and folinic acid induced mucositis: No effect of oral glutamine supplementation. Br J Cancer. 1994;70:732–735. doi: 10.1038/bjc.1994.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson PM, Schroeder G, Skubitz KM. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer. 1998;83:1433–1439. doi: 10.1002/(SICI)1097-0142(19981001)83:7<1433::AID-CNCR22>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Okuno SH, Woodhouse CO, Loprinzi CL, Sloan JA, LaVasseur BI, Clemens-Schutjer D, Swan D, Axvig C, Ebbert LP, Tirona MR, et al. Phase III controlled evaluation of glutamine for decreasing stomatitis in patients receiving fluorouracil (5-FU)-based chemotherapy. Am J Clin Oncol. 1999;22:258–261. doi: 10.1097/00000421-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Ward E, Smith M, Henderson M, Reid U, Lewis I, Kinsey S, Allgar V, Bowers D, Picton SV. The effect of high-dose enteral glutamine on the incidence and severity of mucositis in paediatric oncology patients. Eur J Clin Nutr. 2009;63:134–140. doi: 10.1038/sj.ejcn.1602894. [DOI] [PubMed] [Google Scholar]
- 31.Rubio IT, Cao Y, Hutchins LF, Westbrook KC, Klimberg VS. Effect of glutamine on methotrexate efficacy and toxicity. Ann Surg. 1998;227:772–778; 778–780. doi: 10.1097/00000658-199805000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cockerham MB, Weinberger BB, Lerchie SB. Oral glutamine for the prevention of oral mucositis associated with high-dose paclitaxel and melphalan for autologous bone marrow transplantation. Ann Pharmacother. 2000;34:300–303. doi: 10.1345/aph.19168. [DOI] [PubMed] [Google Scholar]
- 33.Peterson DE, Jones JB, Petit RG., II Randomized, placebo-controlled trial of Saforis for prevention and treatment of oral mucositis in breast cancer patients receiving anthracycline-based chemotherapy. Cancer. 2007;109:322–331. doi: 10.1002/cncr.22384. [DOI] [PubMed] [Google Scholar]
- 34.Imai T, Matsuura K, Asada Y, Sagai S, Katagiri K, Ishida E, Saito D, Sadayasu R, Wada H, Saijo S. Effect of HMB/Arg/Gln on the prevention of radiation dermatitis in head and neck cancer patients treated with concurrent chemoradiotherapy. Jpn J Clin Oncol. 2014;44:422–427. doi: 10.1093/jjco/hyu027. [DOI] [PubMed] [Google Scholar]