Abstract
Large-cell neuroendocrine carcinoma (LCNEC) is a high-grade neuroendocrine tumor. LCNECs arising from the genital organs are highly malignant and rare, with <20 cases of LCNEC developing from the uterine endometrium reported to date. We herein present the case of a patient with LCNEC of the endometrium. The patient was a 52-year-old woman, who exhibited lower abdominal pain and rapid uterine enlargement during outpatient treatment for uterine myoma. The endometrial biopsy suggested a diagnosis of poorly differentiated carcinoma or carcinosarcoma. Based on magnetic resonance imaging and positron emission tomography/computed tomography, endometrial stromal sarcoma was suspected. The serum lactate dehydrogenase level was abnormally high. Due to the suspicion of stage IIIC malignant tumor of the uterine corpus, surgery was performed. The pathological diagnosis was stage IIIC2 LCNEC of the endometrium. Recurrence occurred in the vaginal stump, and concurrent chemoradiotherapy (CCRT) was initiated 1 month after the surgery. The residual lesions markedly shrank, but metastasis to the upper abdominal region and cervix subsequently developed. CCRT was attempted, but the associated adverse effects were severe and was switched to palliative treatment. The patient eventually succumbed to the disease 309 days after surgery.
Keywords: large-cell neuroendocrine carcinoma, endometrium, concurrent chemoradiotherapy
Introduction
According to the World Health Organization (WHO) classification of digestive tract tumors, neuroendocrine cell-derived tumors are frequently encountered in the lung, digestive tract and pancreas and are roughly classified into neuroendocrine tumor (NET) and neuroendocrine carcinoma (NEC) based on proliferation kinetics (1). NECs arising from the uterine endometrium account for <1% of all uterine endometrial carcinomas. Hematogenous/lymphogenous metastasis occurs early during the course of this disease and the prognosis is poor (2). Large-cell NEC (LCNEC) arising from the uterine endometrium is particularly rare, with only 15 cases reported from 2000 onwards by Medline. We herein report the case of a LCNEC patient exhibiting rapid disease progression.
Case report
The patient was a 52-year-old woman, gravida 2 para 2, with a medical history of endometriosis, who had been followed up for myoma every 6 months for several years. The patient sought medical advice due to lower abdominal pain persisting for 1 month, with rapid uterine enlargement. Suspecting a malignant tumor of the uterine corpus, the patient was referred to the Department of Obstetrics and Gynecology of Wakayama Medical University Hospital (Wakayama, Japan).
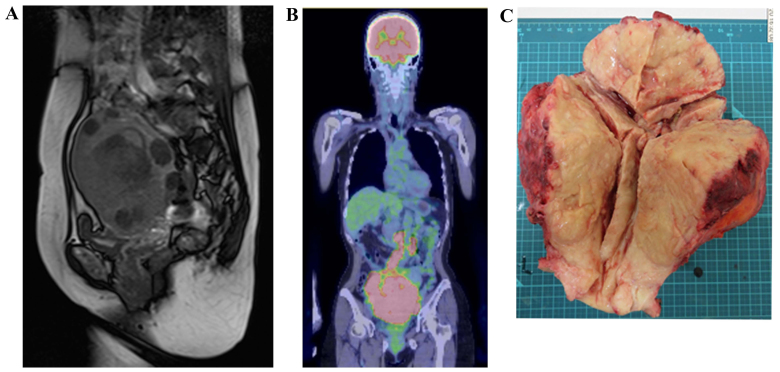
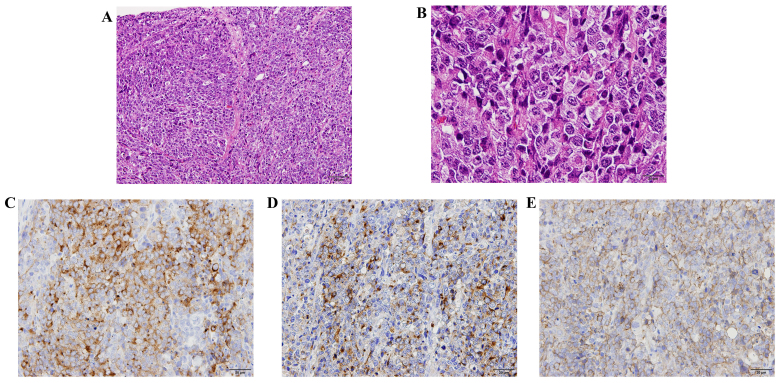
On physical examination, the uterus had enlarged to the size of a newborn's head with associated tenderness on palpation, but no postmenopausal genital bleeding was observed. Transvaginal ultrasonography and a magnetic resonance imaging (MRI) scan revealed irregular edema and marked hypertrophy of the uterine muscle layer, which was suspected to be carcinoma or sarcoma of the uterine corpus (Fig. 1A). On fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) examination, FDG uptake was increased in the uterus, bilateral ovaries and lymph nodes, from the pelvic to the para-aortic nodes (Fig. 1B). The histopathological diagnosis following endometrial biopsy was malignancy and the differential diagnosis included small-cell (SC) and poorly differentiated carcinoma, but a definitive diagnosis was difficult to make. The serum level of lactate dehydrogenase was 1,213 IU/ml (normal range, 106–220 IU/l), of neuron-specific enolase (NSE) 84.6 ng/ml (normal range, <10 ng/ml), of carbohydrate antigen (CA)-125 158.8 U/ml (normal range, 0–35 U/ml), of carcinoembryonic antigen 33.3 ng/ml (normal range, 0–5 ng/ml) and of CA19-9 72.6 U/ml (normal range, 0–37 U/ml). The levels of pro-gastrin-releasing peptide were normal. Suspecting uterine sarcoma, carcinosarcoma or poorly differentiated carcinoma, surgery was considered to be the treatment of choice. The uterus was found to be enlarged and the serosal surface was collapsed. In addition, the lymph nodes from the pelvic to the para-aortic region were enlarged to 3 cm, in a beaded pattern. Abdominal total hysterectomy, bilateral salpingo-oophorectomy, pelvic lymph node dissection and para-aortic lymph node biopsy were performed. The excised uterus weighed 1,500 g and the tumor extensively infiltrated the cervix, parametrium and bilateral ovaries (Fig. 1C). On histopathological examination, the lesions were accompanied by extensive necrosis on hematoxylin and eosin staining, and tumor cells with a high nuclear/cytoplasmic (N/C) ratio proliferated forming mostly solid lesions (Fig. 2A). The nucleoplasm was pale, mitoses were abundant, and the N/C ratio was lower compared with that of the SC type, suggesting the LC type tumor (Fig. 2B). On immunostaining, the tumor cells were positive for the neuroendocrine markers synaptophysin, chromogranin A and CD56 (Fig. 2C-E). Based on these findings, the tumor was diagnosed as LCNEC, International Federation of Gynecology and Obstetrics stage IIIC2, pT3bN1M0.
Figure 1.
Preoperative and postoperative findings. (A) Sagittal T2-weighted images through the pelvis demonstrate abnormal diffusely infiltrative high T2 signal throughout the myometrium, with loss of the normal architecture. The rounded mass lesions with decreased T2 signal within the myometrium represent leiomyomas. (B) Positron emission tomography/computed tomography imaging demonstrated a large, solid mass in the uterine cavity. The maximum standardized uptake value of the primary lesion was 18.99, and fluorodeoxyglucose accumulation is also observed in both appendages and multiple lymph node metastases. (C) Macroscopic appearance of the surgical specimen. The uterus and cervix exhibit marked thickening and a yellow-tan color. The tumor infiltrates the full thickness of the myometrium as well as the cervix, parametrium and bilateral adnexa.
Figure 2.
Histological findings of large-cell neuroendocrine carcinoma (LCNEC). (A) The tumor cells proliferated forming loosely solid lesions accompanied by extensive necrosis [hematoxylin and eosin staining (H&E); magnification, ×100]. (B) The tumor cells were arranged in sheets without a defined border. Individual cells were large with a high nuclear/cytoplasmic ratio, severe atypia and prominent nucleoli. The cytoplasm was acidophillic and relatively ample. Numerous mitotic figures and apoptotic bodies were observed, as well as multiple, interspersed areas of necrosis. (H&E; magnification, ×400). (C-E) Immunohistochemical staining. The tumor cells were positive for (C) synaptophysin, (D) chromogranin A and (E) CD56 (original magnification, ×200).
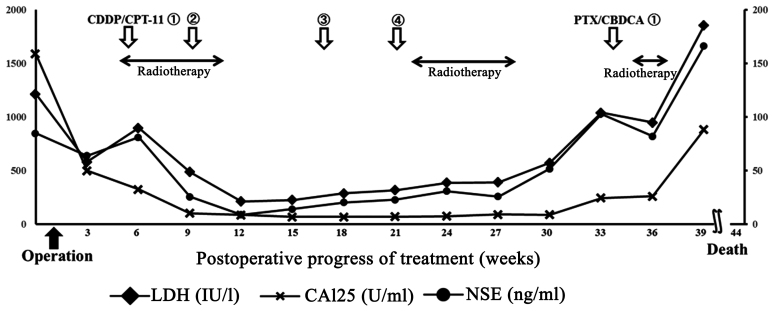
Concurrent chemoradiotherapy (CCRT) was initiated 4 weeks after surgery. Chemotherapy was performed according to the treatment applied for SC carcinoma of the lung: Irinotecan 60 mg/m2 on days 1, 8 and 15 and cisplatin 60 mg/m2 on day 1 every 4 weeks. External radiation (intensity-modulated radiation therapy) was applied to the entire pelvis over the para-aortic lymph nodes. Tumor lesions sized 2 cm were observed in the vaginal stump 1 month after surgery and expansions of peritoneal dissemination and lymph node metastases were identified on CT. These lesions markedly shrank or disappeared after treatment initiation, and tumor markers became negative (Fig. 3). However, the tumor marker levels gradually re-increased, whereas new lymph node metastases were observed above the splenic hilum located outside the irradiated region 136 days after surgery. Additional radiation of the cervix and chest resulted in markedly decreased size of the lesions, but cervicothoracic spinal metastases and paracardiac lymph node metastases developed. As renal dysfunction (increased serum creatinine level) developed after 4 cycles of chemotherapy, one cycle of the paclitaxel (175 mg/m2) and carboplatin (area under the curve 5) chemotherapy was administered. However, continuation of active treatment was difficult due to the development of severe neutropenia and thrombocytopenia: Therefore, palliative radiation therapy of the cervicothoracic spine was performed. Due to the lymph node metastasis in the upper abdominal region, endoscopic biliary stenting was performed to relieve jaundice. The patient succumbed to the disease 309 days after surgery.
Figure 3.
Postoperative course and changes in tumor marker levels. CDDP, cisplatin; CPT-11, irinotecan; PTX, paclitaxel; CBDCA, carboplatin; LDH, lactate dehydrogenase; CA, carbohydrate antigen; NSE, neuron-specific enolase.
The patient provided informed consent to publication of the details of this case and associated images.
Discussion
LCNEC originates from the lungs in the majority of the cases. In the gynecological field, the primary region is generally the uterine cervix and ovary (3–5). NETs of the endometrium include low-grade carcinoid tumors and high-grade SCNEC and LCNEC (2). Most cases of NEC of the endometrium are SCNECs, with ~90 cases reported to date (6). LCNEC arising from the endometrium is rare, and was initially reported by Erhan et al (7). Following a search through Medline, only 15 cases were found to be reported from 2,000 onwards (3–12) (Table I), and in almost all the cases the cancer was advanced. The mean age of the patients was 60 years and the main complaint was abnormal genital bleeding, similar to other endometrial carcinomas. However, some cases were only diagnosed due to pain caused by metastatic lesions. In the majority of the cases the tumor deeply infiltrated the uterine muscle layer, and ~50% of the cases were discovered at an advanced stage (6). No diagnostic criteria of LCNEC of the endometrium have been proposed to date. According to the WHO classification of pulmonary tumors, LCNEC is defined as large-cell carcinoma with NET morphology expressing the neuroendocrine markers synaptophysin, chromogranin A and/or CD56 (13). LCNEC is not included in the classification of the General Rules for Clinical and Pathological Management of Uterine Corpus Cancer in Japan (14). Clinicopathologically, the morphology of LCNEC is similar to that of SC carcinoma and LCNEC developing in the lungs, and in approximately half of the cases it coexists with endometrioid adenocarcinoma observed as part of carcinosarcoma (4). LCNEC complicated by lesions with a different histological diagnosis was also observed. The histological characteristics of LCNEC of the endometrium include cells with abundant cytoplasm, microgranular chromatin, clear nucleolus, polymorphism with various sizes, multinucleated cells, large tumor cells, ≥10 mitoses/high-power field, map-like hemorrhage and necrosis, island-like or columnar tumor cell aggregates and vascular invasion (7,8). On immunostaining, the tumor cells stained positive for synaptophysin, chromogranin A, CD56, p53 and NSE, confirming the diagnosis of LCNEC (15–18).
Table I.
Large-cell neuroendocrine tumor of the endometrium.
| Case | Age (years) | Surgery | Stage | Histology | Treatment | IHC profile | Outcome (months) | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| 1 | 52 | ATH, BSO | IC | Pure LCNEC | RT, CDDP, VP16 | NSE, SNP | DOD3 | (7) |
| 2 | 50 | ATH, BSO, OMT, LN | IIIC | Pure LCNEC | RT, CDDP, VP16 | NSE, SNP | AWD12 | (3) |
| 3 | 80 | ATH, BSO, LN | IC | LCNEC + endometrioid | None | NSE, CGA | DOD5 | (3) |
| 4 | 77 | ATH, BSO | IIB | LCNEC + endometrioid | RT | NSE, SNP, CGA, CD56 | DOD23 | (3) |
| 5 | 79 | ATH, BSO, OM | IIIA | LCNEC + endometrioid | RT | NSE, CGA, CD56 | AWD2 | (3) |
| 6 | 88 | ATH, BSO, LN | IIIC | L/SCNEC + endometrioid | RT | NSE, CGA, CD56 | AWD1 | (3) |
| 7 | 42 | RH | IC | Pure LCNEC | CDDP, VP-16 | SNP, CGA, CD56 | AWD9 | (4) |
| 8 | 59 | RH, BSO, OM, PPALND | IIIB | LCNEC + serous | RT + CT (unknown) | NSE, SNP, CD56 | NED5 | (11) |
| 9 | 40 | ATH, BSO, PLND, OM | IB | LCNEC + sarcomatoid | None | SNP, CD56 | NED26 | (5) |
| 10 | 70 | ATH, BSO, OM | IB | Pure LCNEC | CDDP, VP-16 | SNP, CGA, CD56 | NED6 | (12) |
| 11 | 59 | ATH, BSO, OM, PPALND | IIIC2 | Pure LCNEC | CBDCA, PTX, RT, PLD, CDDP, VP-16 | NSE, SNP, CGA, CD56 | DOD12 | (10) |
| 12 | 73 | None | IVB | Pure LCNEC | None | NSE, SNP, CGA | DOD1 | (9) |
| 13 | 73 | ATH, BSO, OM, PPALND | IIIC1 | Pure LCNEC | CDDP/CPT-11 | SNP, CGA, CD56 | AWD13 | (9) |
| 14 | 71 | RH, BSO, OM, PPALND | IVB | Pure LCNEC | Planned CDDP, VP-16 | SNP, CGA | DOD1 | (8) |
| 15 | 51 | RH, BSO, OM, PPALND | IIIA | LCNEC + endometrioid | CDDP/CPT-11 | SNP, CGA, CD56 | NED18 | (6) |
| 16 | 52 | ATH, BSO, PLND | IIIC2 | Pure LCNEC | RT, CDDP/CPT-11 | SNP, CGA, CD56 | DOD10 | Present case |
ATH, abdominal total hysterectomy; BSO, bilateral salpingo-oophorectomy; OM, omentectomy; RH, radical hysterectomy; PLND, pelvic lymph node dissection; PPALND, pelvic and para-aortic lymph node dissection; RT, radiotherapy; CDDP, cisplatin; VP-16, etoposide; CBDCA, carboplatin; PTX, paclitaxel; PLD, pegylated doxorubicin; CPT-11, irinotecan; SNP, synaptophysin; CGA, chromogranin A; NSE, neuron-specific enolase; LCNEC, large-cell neuroendocrine carcinoma; SCNEC, small-cell neuroendocrine carcinoma; DOD, dead of disease; AWD, alive with disease; NED, no evidence of disease; IHC, immunohistochemical.
Specific imaging findings of uterine LCNEC have not been reported to date. Makihara et al reported that the MRI findings of LCNEC are similar to those of other poorly differentiated endometrial cancers and sarcoma (9). In the present case, the preoperative diagnosis of LCNEC based on MRI and PET/CT was difficult.
LCNEC of the endometrium is treated similar to other endometrial carcinomas, i.e., surgical resection, radiation and chemotherapy, as no standard therapy has been established to date due to the small number of reported cases. The regimen is selected based on LCNEC of other organs. Cases of LCNEC of the lungs and digestive tract that responded to irinotecan and cisplatin therapy (19) and pulmonary SC carcinoma patients with a significant response to cisplatin and etoposide therapy (20) have been reported. In addition, cases treated with a combination of chemotherapy and an octreotide similar to somatostatin were reported (10). Inhibition of tumor growth by somatostatin analogues has been shown in animal models and human tumor cell lines. Its mechanism is reported to be inhibition of secretion of insulin-like growth factor-1, other tumor growth factors and vascularization, and specific direct tumor inhibition through the somatostatin receptor, which is strongly expressed in NETs (10,21). Partial response was observed in a SCNEC of the endometrium treated with octreotide (22), but other cases exhibited tumor enlargement and adverse effects, such as repeated hypo/hyperglycemia (10). Thus, despite these investigations, LCNECs of the endometrium rapidly progress and the prognosis is very poor. In the present case, the tumor recurred 4 weeks after surgery. CCRT was initiated, but the disease relapsed and metastasized; thus, continuation of active treatment was no longer feasible and the patient succumbed to the disease 309 days after surgery.
In conclusion, LCNEC of the endometrium is a very rare, highly malignant tumor, which is difficult to diagnose as its imaging and pathological findings are non-specific. The prognosis of LCNEC is poor due to its rapid progression and lack of established therapy. Designing an effective therapy protocol through the accumulation and investigation of reported cases is urgently required.
References
- 1.Bosman FT, Carneiro F, Hruban RH, Theise ND. Nomenclature and classification of neuroendocrine neoplasma of the digestive system; WHO Classification of Tumours of the Digestive System; 2010.Lyon: IARC; pp. 13–14. [Google Scholar]
- 2.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classification of Tumours of Female Reproductive Organs. 4th. Lyon: IARC; 2014. p. 122. [Google Scholar]
- 3.Mulvany NJ, Allen DG. Combined large cell neuroendocrine and endometrioid carcinoma of the endometrium. Int J Gynecol Pathol. 2008;27:49–57. doi: 10.1097/pgp.0b013e31806219c5. [DOI] [PubMed] [Google Scholar]
- 4.Albores-Saavedra J, Martinez-Benitez B, Luevano E. Small cell carcinomas and large cell neuroendocrine carcinomas of the endometrium and cervix: Polypoid tumors and those arising in polyps may have a favorable prognosis. Int J Gynecol Pathol. 2008;27:333–339. doi: 10.1097/PGP.0b013e31815de006. [DOI] [PubMed] [Google Scholar]
- 5.Terada T. Large cell neuroendocrine carcinoma with sarcomatous changes of the endometrium: A case report with immunohistochemical studies and molecular genetic study of KIT and PDGFRA. Pathol Res Pract. 2010;206:420–425. doi: 10.1016/j.prp.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto H, Nasu K, Kai K, Nishida M, Narahara H, Nishida H. Combined large-cell neuroendocrine carcinoma and endometrioid adenocarcinoma of the endometrium: A case report and survey of related literature. J Obstet Gynaecol Res. 2016;42:206–210. doi: 10.1111/jog.12881. [DOI] [PubMed] [Google Scholar]
- 7.Erhan Y, Dikmen Y, Yucebilgin MS, Zekioglu O, Mgoyi L, Terek MC. Large cell neuroendocrine carcinoma of the uterine corpus metastatic to brain and lung: Case report and review of the literature. Eur J Gynaecol Oncol. 2004;25:109–112. [PubMed] [Google Scholar]
- 8.Nguyen ML, Han L, Minors AM, Bentley-Hibbert S, Pradhan TS, Pua TL, Tedjarati SS. Rare large cell neuroendocrine tumor of the endometrium: A case report and review of the literature. Int J Surg Case Rep. 2013;4:651–655. doi: 10.1016/j.ijscr.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makihara N, Maeda T, Nishimura M, Deguchi M, Sonoyama A, Nakabayashi K, Kawakami F, Itoh T, Yamada H. Large cell neuroendocrine carcinoma originating from the uterine endometrium: A report on magnetic resonance features of 2 cases with very rare and aggressive tumor. Rare Tumors. 2012;4:e37. doi: 10.4081/rt.2012.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahabi S, Pellicciotta I, Hou J, Graceffa S, Huang GS, Samuelson RN, Goldberg GL. Clinical utility of chromogranin A and octreotide in large cell neuro endocrine carcinoma of the uterine corpus. Rare Tumors. 2011;3:e41. doi: 10.4081/rt.2011.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posligua L, Malpica A, Liu J, Brown J, Deavers MT. Combined large cell neuroendocrine carcinoma and papillary serous carcinoma of the endometrium with pagetoid spread. Arch Pathol Lab Med. 2008;132:1821–1824. doi: 10.5858/132.11.1821. [DOI] [PubMed] [Google Scholar]
- 12.Deodhar KK, Kerkar RA, Suryawanshi P, Menon H, Menon S. Large cell neuroendocrine carcinoma of the endometrium: An extremely uncommon diagnosis, but worth the efforts. J Cancer Res Ther. 2011;7:211–213. doi: 10.4103/0973-1482.82942. [DOI] [PubMed] [Google Scholar]
- 13.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of Lung, Pleura, Thymus and Heart. 4th. Lyon: IARC; 2015. Large cell neuroendocrine carcinoma; pp. 69–72. [DOI] [PubMed] [Google Scholar]
- 14.Tsukamoto N. The general rules for clinical and pathological management of uterine corpus cancer. Nihon Rinsho. 2004;10:285–289. (In Japanese) [PubMed] [Google Scholar]
- 15.Gilks CB, Young RH, Gersell DJ, Clement PB. Large cell neuroendocrine [corrected] carcinoma of the uterine cervix: A clinicopathologic study of 12 cases. Am J Surg Pathol. 1997;21:905–914. doi: 10.1097/00000478-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 16.McCluggage WG, Kennedy K, Busam KJ. An immunohistochemical study of cervical neuroendocrine carcinomas: Neoplasms that are commonly TTF1 positive and which may express CK20 and P63. Am J Surg Pathol. 2010;34:525–532. doi: 10.1097/PAS.0b013e3181d1d457. [DOI] [PubMed] [Google Scholar]
- 17.Krivak TC, McBroom JW, Sundborg MJ, Crothers B, Parker MF. Large cell neuroendocrine cervical carcinoma: A report of two cases and review of the literature. Gynecol Oncol. 2001;82:187–191. doi: 10.1006/gyno.2001.6254. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Shimamoto T, Amada S, Asada Y, Hayashi T. Large cell neuroendocrine carcinoma of the uterine cervix: A clinicopathological study of six cases. Int J Gynecol Pathol. 2003;22:226–230. doi: 10.1097/01.PGP.0000071046.12278.D1. [DOI] [PubMed] [Google Scholar]
- 19.Tanimoto H, Hamasaki A, Akimoto Y, Honda H, Takao Y, Okamoto K, Teramoto M, Teramoto H, Kaneko M, Oshita T. A case of large cell neuroendocrine carcinoma (LCNEC) of the uterine cervix successfully treated by postoperative CPT-11+CDDP chemotherapy after non-curative surgery. Gan To Kagaku Ryoho. 2012;39:1439–1441. (In Japanese) [PubMed] [Google Scholar]
- 20.Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 21.Scully RE, Aguirre P, DeLellis RA. Argyrophilia, serotonin, and peptide hormones in the female genital tract and its tumors. Int J Gynecol Pathol. 1984;3:51–70. doi: 10.1097/00004347-198403010-00005. [DOI] [PubMed] [Google Scholar]
- 22.Verschraegen CF, Matei C, Loyer E, Malpica A, Tornos C, Kudelka AP, Kavanagh JJ. Octreotide induced remission of a refractory small cell carcinoma of the endometrium. Int J Gynecol Cancer. 1999;9:80–85. doi: 10.1046/j.1525-1438.1999.09886.x. [DOI] [PubMed] [Google Scholar]