Abstract
Soft tissue sarcoma is a rare neoplasm of mesenchymal origin, accounting for only ~1% of all adult cancers and consisting of 75 histological subtypes. In the present report, the unique case of a 14 year-old female with metastatic malignant peripheral nerve sheath tumor (formerly, malignant melanotic schwannoma) of the parotid gland, who experienced a durable response and sustained tumor control with Rexin-G®, a tumor-targeted retroviral expression vector encoding an anti-cyclin G1 construct, is described. Post-parotidectomy, and prior to the administration of Rexin-G®, the patient received various chemotherapy regimens, including doxorubicin, ifosfamide, temozolomide, sorafenib, and an immunological therapy with interleukin-2, which only resulted in the further progression of lung metastases. The patient subsequently participated in a Phase 1/2 gene therapy study, during which she received intravenous Rexin-G® as monotherapy for two years with minimal drug-associated adverse events. Currently, the patient has no evidence of active disease 9 years after commencing the Rexin-G® treatment, and with no additional anti-cancer therapy. In conclusion, Rexin-G® may be a viable therapeutic option for malignant peripheral nerve sheath tumors, and should be further investigated in prospective histology-specific clinical trials for this type, and possibly other types, of chemotherapy-resistant sarcoma.
Keywords: targeted gene therapy vector, cell cycle control, metastatic sarcoma
Introduction
Malignant peripheral nerve sheath tumor (MPNST; formerly, melanotic schwannoma) is a rare neoplasm of Schwann cell origin capable of melanogenesis (1,2). Clinicopathologically, it is considered a distinct entity from conventional schwannoma due to genetic and clinical differences (3). Immunophenotypic indicators for schwannomas with melanotic differentiation include the presence of epitheloid cells with variably sized nuclei, and a marked accumulation of melanin (2), but differential diagnosis typically requires further analysis through ultrastructural and immunohistochemical testing. In terms of clinical management, it is of paramount importance to distinguish primary melanin-containing lesions from malignant melanoma in order to plan an appropriate therapeutic approach.
Case report
A 14 year-old female initially presented with a non-painful swelling of the right posterior mandible with right facial weakness. A magnetic resonance imaging (MRI) scan of the face and neck revealed a 5.3 cm mass of the right parotid gland, and a chest computed tomography (CT) scan revealed several small, non-specific opacities in the upper right lobe. A histopathological examination performed by core needle biopsy revealed a malignant melanotic spindle cell neoplasm, with atypical spindle cell proliferation arranged in a fascicular pattern. The tumor was histologically and immunophenotypically consistent with MPNST. The patient underwent a right radical parotidectomy and a right modified radical neck dissection with reconstructive surgery. Note was made of the extension of the neoplasm into the soft tissues around the parotid gland with perineural invasion. Immunohistochemical analysis confirmed the diagnosis, with the involvement of three out of eight tumorous lymph nodes staining positive for laminin, homatropine methylbromide 45 (HMB-45), and tyrosinase, and negative for melanoma-associated antigen recognized by T cells (MART-1) and S-100 protein. In March 2007, the patient started chemotherapy with temozolomide (75 mg/m2 given orally, daily for 45 days), and in April 2007, sorafenib (40 mg by mouth, twice daily), due to the increased sizes of nine lung lesions. In spite of these treatments, however, a repeat positron emission tomography (PET)/CT scan in June 2007 revealed diffuse progression in the lungs, thighs, and left flank. The patient was admitted for treatment with high-dose interleukin-2, but only received 7 of 14 doses due to unacceptable toxicity and disease progression. After three cycles of ifosfamide (1,800 mg/m2/day for 5 days) as continuous infusion with mesna, and doxorubicin (37.5 mg/m2/day intravenously for 2 days), a repeat PET/CT scan revealed further tumor progression; therefore, the chemotherapy was discontinued.
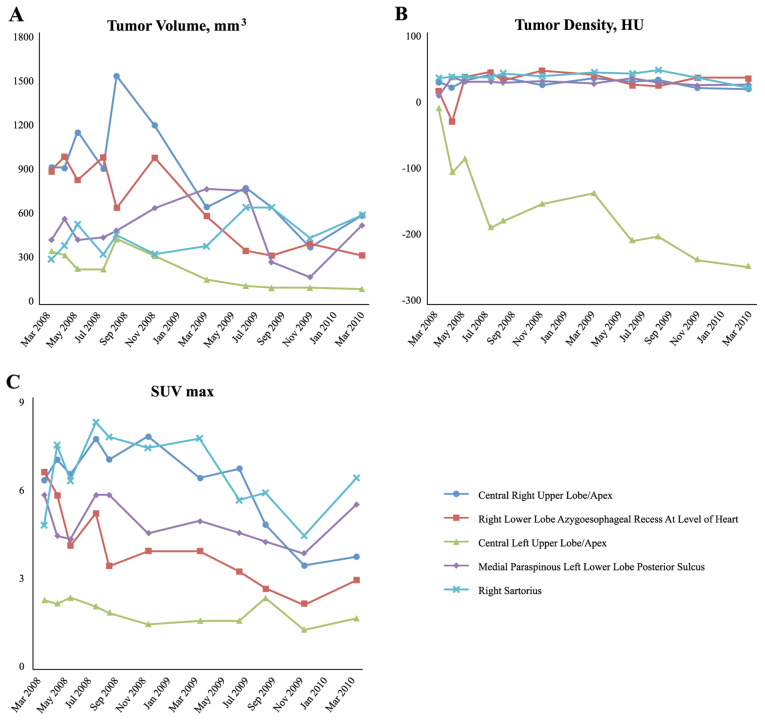
In March 2008, the patient participated in a Phase 1/2 clinical trial using intravenous Rexin-G® (developed at the USC Keck School of Medicine, Los Angeles, CA, USA) for advanced chemoresistant sarcoma (clinical trial protocol no. NCT00505713; see Table I). The patient received dose level 3 of Rexin-G® [3×10e11 colony-forming units (cfu)] three times a week as an outpatient. Objective tumor responses were evaluated by a number of parameters, including Response Evaluation Criteria In Solid Tumors (RECIST) v1 (4), tumor volume, mm3 (length × width2 × 0.52), tumor density in Hounsfeld units (HU) and the maximum standardized uptake value (SUVmax) by fluorodeoxyglucose (18-FDG) PET-CT (4,5). Based on the RECIST v1 and other radiological parameters, the patient experienced sustained disease control (Fig. 1). The patient's clinical course was complicated by an episode of nephrotic syndrome, which was attributed to the bi-weekly subcutaneous granulocyte-macrophage colony-stimulating factor (GM-CSF) injections, and therefore these were discontinued. The patient received a total of 205 Rexin-G® vector infusions over a 2-year period with minimal toxicity and no serious adverse events. Following the last infusion in June 2010, the patient underwent a PET/CT scan, comparing the results with those images taken prior to treatment. The radiology report stated that there was a marked overall improvement in the patient's pulmonary metastases, with all but one nodule being either markedly improved in size, or resolved. It was also noted that the right pleural effusion and previous significant ascites had been resolved. Currently, nine years after commencing the Rexin-G® treatment, the patient is alive and well, with no evidence of active neoplastic disease.
Table I.
USA-based clinical trials using tumor-targeted Rexin-G® for chemotherapy-resistant solid malignancies.
| Clinical trial protocol number/dose levela | Clinical site(s)/Phase | Clinical indication | No. of patients | Outcome |
|---|---|---|---|---|
| NCT00121745; dose level, minus 3-minus 1 | Rochester, MN, USA: Phase 1 | Pancreas CA | 12 | 0% 1-year OS |
| NCT00504998b; dose level, 1–3 | Santa Monica, CA, USA/Manhattan, NY, USA/(Duke) Durham, NC, USA: Phase 1/2 | Pancreas CA | 20 | 26.7% 1-year OS 13.3% 2-year OS 1 alive in sustained remission, 9-year OS |
| NCT00505713b; dose level, 1–4 | Santa Monica, CA, USA: Phase 1/2 | Bone and soft tissue sarcoma | 36 | 38.5% 1-year OS; 31% 2-year OS 1 alive with no active disease, 9-year OS |
| NCT00505271; dose level, 1–4 | Santa Monica, CA, USA/Manhattan, NY, USA: Phase 1/2 | Breast CA | 20 | 60% 1-year OS |
| NCT00572130; dose level, 1–2 | Santa Monica, CA, USA: Phase 2 | Osteosarcoma | 22 | 27.3% 1-year OS 22.7% 2-year OS 1 alive in sustained remission, 8 years |
Dose levels were calculated as follows: Dose level 1=1×10e11 cfu; dose level 2=2×10e11 cfu; dose level 3=3×10e11 cfu; and dose level 4=4×10e11 cfu.
A randomization test on the log-rank statistic using 20,000 random samples revealed a dose-response association between overall survival and the Rexin-G dosage (P=0.002 for sarcoma and 0.04 for pancreas cancer). Analysis was done using NCSS software (Number Cruncher Statistical Systems, Kaysville, UT, USA). Statistical analysis was performed by a biostatistician who was not otherwise involved in the study. CA, carcinoma; cfu colony forming units; OS, overall survival.
Figure 1.
Various tumor parameters of the patient monitored over time. (A) Individual tumor volume measurements over time. Tumor volumes of individual target lesions (measured by CT imaging), in mm3, were calculated using O'Reilly's formula (length × width2 × 0.52). (B) Individual tumor density measurements over time. Tumor densities of individual target lesions, in HU, were calculated by radiographic image analysis. (C) Individual FDG avidity measurements over time. Avidities of individual target lesions for 18-FDG (SUVmax) were measured in individual target lesions using a PET/CT scan. CT, computed tomography; PET, positron emission tomography; HU, Hounsfeld units; FDG, fluorodeoxyglucose; SUVmax, maximum standardized uptake value.
Discussion
Peripheral nerve sheath tumor (PNST) is a rare subset of soft tissue sarcomas displaying an immunophenotype consistent with that of conventional schwannomas, along with cytoplasmic melanin deposition characteristic of melanomas (3,6,7). These tumors occur most frequently at nerve roots, although other locations along the peripheral nervous system have been described (8). Tumors originating from the bone, soft tissues, heart, mouth, esophagus, bronchus, retroperitoneum, uterine cervix, orbit, parotid gland, as well as the spinal cord, acoustic nerve, cerebellum, and sympathetic chain, have been reported (1,9). Statistics have revealed a 1.1:1 male: female ratio (9). The ages of those afflicted vary between 10 and 84 years (7,9), although the peak incidence occurs with patients in their fourth decade (7). There have been fewer than 200 cases of PNST reported since it was first described in 1932 (10,11), with approximately 40 reported malignancies as of 2014 (12). To date, there have been no published cases of MPNST originating from the parotid gland. Therefore, this case is unique and worthy of report, particularly with respect to the patient's impressive response to an innovative tumor-targeted gene therapy vector, designated Rexin-G®.
To make a differential diagnosis between MPNST and spindle cell melanoma is very difficult, particularly in small biopsy specimens, due to the diverse range of tumor derivations from a Schwann cell lineage (13) and similar histological features, including cell pleomorphisms with prominent nucleoli (1). In the present case study, immunohistochemical staining and ultrastructural examinations proved to be useful in guiding the diagnostic process. All reported cases of MPNST, including the present patient's case, have revealed positive gene expression of HMB-45 and tyrosinase, indicative of melanocytic differentiation, but negative expression of MART-1 and S-100 protein, thereby eliminating a diagnosis of melanoma (14). Further studies for the expression of laminin, which was displayed intensely in two-thirds of the reported cases (15), concluded that the tumor represented a Schwannian differentiation, based on the biphasic pattern (i.e. individual cell and nested) in the external lamina.
The prognosis for PNST is unpredictable at best, with 10% of all cases developing metastasis (8,10,14). Although generally considered benign, the tumor is prone to malignancy and recurrence, occurring in ~20% of patients (1,8,16). Surgery has been the primary treatment option (9), followed by radiation therapy or adjuvant chemotherapy. For the patient described in the present case report, a right parotidectomy was performed, and systemic chemotherapy was administered post-surgery to treat the metastatic lung lesions, albeit without success.
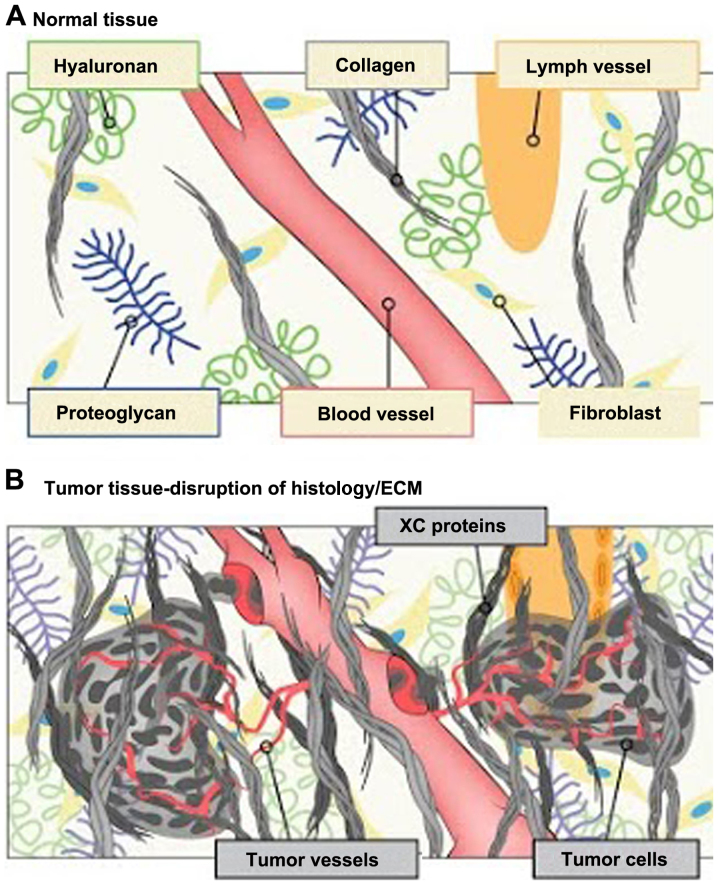
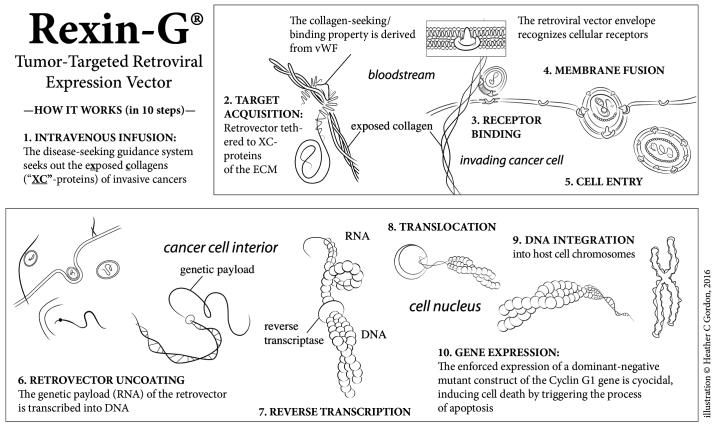
Rexin-G® is the first tumor-targeted gene therapy vector that has been tested in the clinic (4). Injected intravenously, the targeted retroviral particles operate within the vascular system via a high-affinity collagen-binding motif derived from von Willebrand coagulation factor (17). Atypical amounts of exposed collagenous (XC) proteins are located in areas of tumor invasion, neoangiogenesis and stroma formation, possibly resulting from exposure to protease activity within the tumor microenvironment (TME; Fig. 2). Rexin-G® accumulates in these metastatic deposits by seeking out the abnormal XC proteins, thereby increasing effective vector concentration in the TME in close association with the cancer cells. The function of the genetic payload (a dominant negative cyclin G1 construct is encoded in Rexin-G®) is to halt the G1 phase of the cell cycle, thus inducing cell death via apoptosis-mediated pathways (Fig. 3) (17–20).
Figure 2.
Illustration of the tumor microenvironment compared with normal tissues. (A) Normal tissue. (B) The XC-targeting motif enables the vector to seek out and accumulate in the tumor microenvironment by binding to abnormally exposed proteins found abundantly in tumors, e.g., as a result of tumor invasion, ECM remodeling and neoangiogenesis, but not in normal tissues. Xc, exposed collagenous; ECM, extracellular matrix.
Figure 3.
An illustration of the mechanism of action of Rexin-G®. The Rexin-G® nanoparticle displays an XC-targeting motif, derived from the coagulation factor, vWF, on its surface amphotropic gp70 envelope protein. When injected intravenously, Rexin-G® seeks out and accumulates in cancerous lesions by binding to exposed collagenous (XC) proteins. This chimeric retrovector has the innate property of binding to a tumor's natural viral/cell receptor, fusing, entering, uncoating, and integrating randomly into the chromosomes of only actively dividing cells (i.e., cancer cells). This selective property spares all terminally differentiated and/or non-replicative cells of normal organs, including hepatocytes, neuronal cells and myocardial cells. Rexin-G® carries a cytocidal dominant negative cyclin G1 construct, which causes cell death via apoptosis. vWF, von Willebrand factor; XC, exposed collagenous.
Based on a critical evaluation of its safety and potential efficacy, as well as the unmet medical need, Rexin-G® was granted Orphan Drug status for soft tissue sarcoma and osteosarcoma by the US Food and Drug Administration (FDA) in 2008 (21); and in 2010, Phase 1 and Phase 2 clinical trials using Rexin-G® for chemotherapy-resistant soft tissue sarcoma and osteosarcoma, respectively, were successfully completed (5,21). The results of these studies demonstrated the overall safety and, in clinical trials for sarcoma and pancreatic cancer, the dose-dependent efficacy in controlling tumor growth and improving survival rates with the use of Rexin-G®, particularly at the higher dose levels (Table I). Accordingly, long-term survival follow-up (up to 15 years post-treatment) is required by the US FDA for investigational gene therapy products; to date, there have been no reports of delayed or late adverse events associated with Rexin-G® treatment.
In summary, in the present report, the unique case of a 14 year-old patient with widely metastatic MPNST of the parotid gland, who experienced a durable response and sustained tumor control (and minimal toxicity) with an innovative therapy treatment of Rexin-G®, an XC-/tumor-targeted retrovector bearing a cytocidal gene construct, is described. On the basis of these results, the continued development of Rexin-G® for this rare type of mesenchymal cancer, and potentially other chemoresistant sarcomas, is highly recommended.
Acknowledgements
The authors are grateful to Heather C. Gordon (Art Consultant, Sarcoma Oncology Center, Santa Monica, CA, USA) for graphic illustrations and editorial assistance in the writing of this manuscript (see www.heathergordondrawings.com).
Glossary
Abbreviations
- CT
computed tomography
- MRI
magnetic resolution imaging
- MPNST
malignant peripheral nerve sheath tumor
- PNST
peripheral nerve sheath tumor
- PET
positron emission tomography
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- XC
exposed collagenous
- TME
tumor microenvironment
References
- 1.Vallat-Decouvelaere AV, Wassef M, Lot G, Catala M, Moussalam M, Caruel N, Mikol J. Spinal melanotic schwannoma: A tumour with poor prognosis. Histopathology. 1999;35:558–566. doi: 10.1046/j.1365-2559.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: Diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295–319. doi: 10.1007/s00401-012-0954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 4.Chawla SP, Chawla NS, Quon D, Chua-Alcala V, Blackwelder WC, Hall FL, Gordon EM. An advanced phase 1/2 study using an XC-targeted gene therapy vector for chemotherapy resistant sarcoma. Sarcoma Res Int. 2016;3:1024. [Google Scholar]
- 5.Gordon EM, Cornelio GH, Lorenzo CC, III, Levy JP, Reed RA, Liu L, Hall FL. First clinical experience using a ‘pathotropic’ injectable retroviral vector (Rexin-G) as intervention for stage IV pancreatic cancer. Int J Oncol. 2004;24:177–185. [PubMed] [Google Scholar]
- 6.Er U, Kazanci A, Eyriparmak T, Yigitkanli K, Senveli E. Melanotic schwannoma. J Clin Neurosci. 2007;14:676–678. doi: 10.1016/j.jocn.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Kurtkaya-Yapicier O, Scheithauer B, Woodruff JM. The pathobiologic spectrum of Schwannomas. Histol Histopathol. 2003;18:925–934. doi: 10.14670/HH-18.925. [DOI] [PubMed] [Google Scholar]
- 8.Welling LC, Guirado VM, Tessari M, Felix AR, Zanellato C, Figueiredo EG, Taricco MA, Teixeira MJ. Spinal melanotic schwannomas. Arq Neuropsiquiatr. 2012;70:156–157. doi: 10.1590/S0004-282X2012000200018. [DOI] [PubMed] [Google Scholar]
- 9.Faria MH, Dória-Netto RH, Osugue GJ, Lde S Queiroz, Chaddad-Neto FE. Melanotic schwannoma of the cervical spine progressing with pulmonary metastasis: Case report. Neurol Med Chir (Tokyo) 2013;53:712–716. doi: 10.2176/nmc.cr2012-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan SY, Cheng YC, Kao TH. Intramedullary melanotic schwannoma: Case report and review of the literature. Surg Neurol Int. 2014;5(Suppl 4):S181–S184. doi: 10.4103/2152-7806.136742. [DOI] [Google Scholar]
- 11.Khoo M, Pressney I, Hargunani R, Tirabosco R. Melanotic schwannoma: An 11-year case series. Skeletal Radiol. 2016;45:29–34. doi: 10.1007/s00256-015-2256-8. [DOI] [PubMed] [Google Scholar]
- 12.Torres-Mora J, Dry S, Li X, Binder S, Amin M, Folpe AL. Malignant melanotic schwannian tumor: A clinicopathologic, immunohistochemical, and gene expression profiling study of 40 cases, with a proposal for the reclassification of ‘melanotic schwannoma’. Am J Surg Pathol. 2014;38:94–105. doi: 10.1097/PAS.0b013e3182a0a150. [DOI] [PubMed] [Google Scholar]
- 13.Röhrich M, Koelsche C, Schrimpf D, Capper D, Sahm F, Kratz A, Reuss J, Hovestadt V, Jones DT, Bewerunge-Hudler M, et al. Methylation-based classification of benign and malignant peripheral nerve sheath tumors. Acta Neuropathol. 2016;131:877–887. doi: 10.1007/s00401-016-1540-6. [DOI] [PubMed] [Google Scholar]
- 14.Küsters-Vandevelde HV, van Engen-van Grunsven IA, Küsters B, van Dijk MR, Groenen PJ, Wesseling P, Blokx WA. Improved discrimination of melanotic schwannoma from melanocytic lesions by combined morphological and GNAQ mutational analysis. Acta Neuropathol. 2010;120:755–764. doi: 10.1007/s00401-010-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang HY, Park N, Erlandson RA, Antonescu CR. Immunohistochemical and ultrastructural comparative study of external lamina structure in 31 cases of cellular, classical, and melanotic schwannomas. Appl Immunohistochem Mol Morphol. 2004;12:50–58. doi: 10.1097/00129039-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Killeen RM, Davy CL, Bauserman SC. Melanocytic schwannoma. Cancer. 1988;62:174–183. doi: 10.1002/1097-0142(19880701)62:1<174::AID-CNCR2820620127>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Hall FL, Liu L, Zhu NL, Stapfer M, Anderson WF, Beart RW, Gordon EM. Molecular engineering of matrix-targeted retroviral vectors incorporating a surveillance function inherent in von Willebrand factor. Hum Gene Ther. 2000;11:983–993. doi: 10.1089/10430340050015293. [DOI] [PubMed] [Google Scholar]
- 18.Chawla SP, Chua VS, Fernandez L, Quon D, Blackwelder WC, Gordon EM, Hall FL. Advanced phase I/II studies of targeted gene delivery in vivo: Intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic cancer. Mol Ther. 2010;18:435–441. doi: 10.1038/mt.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon EM, Levy JP, Reed RA, Petchpud WN, Liu L, Wendler CB, Hall FL. Targeting metastatic cancer from the inside: A new generation of targeted gene delivery vectors enables personalized cancer vaccination in situ. Int J Oncol. 2008;33:665–675. [PubMed] [Google Scholar]
- 20.Gordon EM, Lopez FF, Cornelio GH, Lorenzo CC, III, Levy JP, Reed RA, Liu L, Bruckner HW, Hall FL. Pathotropic nanoparticles for cancer gene therapy Rexin-G IV: Three-year clinical experience. Int J Oncol. 2006;29:1053–1064. [PubMed] [Google Scholar]
- 21.Gordon EM, Hall FL. Rexin-G, a targeted genetic medicine for cancer. Expert Opin Biol Ther. 2010;10:819–832. doi: 10.1517/14712598.2010.481666. [DOI] [PubMed] [Google Scholar]