Abstract
The cohesin complex prevents separation of chromosomes following their duplication until the appropriate time during cell division. In vertebrates, establishment and maintenance of cohesin‐dependent linkages depend on two distinct proteins, sororin and shugoshin. New findings published in The EMBO Journal show that in Drosophila, the function of both of these cohesin regulators is carried out by a single hybrid protein, Dalmatian.
Subject Categories: Cell Cycle
The accurate segregation of chromosomes into daughter cells requires that the two newly duplicated sister chromatids are held together until they are properly attached to the spindle fibres that will pull them apart during mitosis. Cohesion established during S phase must be maintained until anaphase onset during mitosis. However, in vertebrates, the so‐called prophase pathway drives the removal of the majority of cohesin from chromosomes during and after S phase (Waizenegger et al, 2000). To ensure that sister chromatids remain cohesed during this time, a fraction of cohesin is retained on chromosomes, dependent on two factors: sororin and shugoshin (Nishiyama et al, 2010; Liu et al, 2013). Sororin counteracts the effects of the prophase pathway as cohesin is established along chromosomes in S phase, while shugoshin protects cohesin specifically in the pericentromere during mitosis. Until now, it was unclear whether cohesin maintenance factors are similarly required in organisms other than vertebrates, as no protective function had been observed for shugoshins, and sororin does not appear to be universally present in eukaryotes. The new findings from Nishiyama and colleagues (Yamada et al, 2017) demonstrate that, remarkably, the functions of sororin and shugoshin are conferred by a single hybrid protein, Dalmatian (Dmt), in Drosophila mitosis (Fig 1). The new study demonstrates the evolutionary importance of cohesin maintenance pathways and raises the intriguing possibility that variations on this theme will be identified in other organisms in the future.
Figure 1. Drosophila Dalmatian protein is a functional hybrid of the vertebrate cohesin regulators shugoshin and sororin.
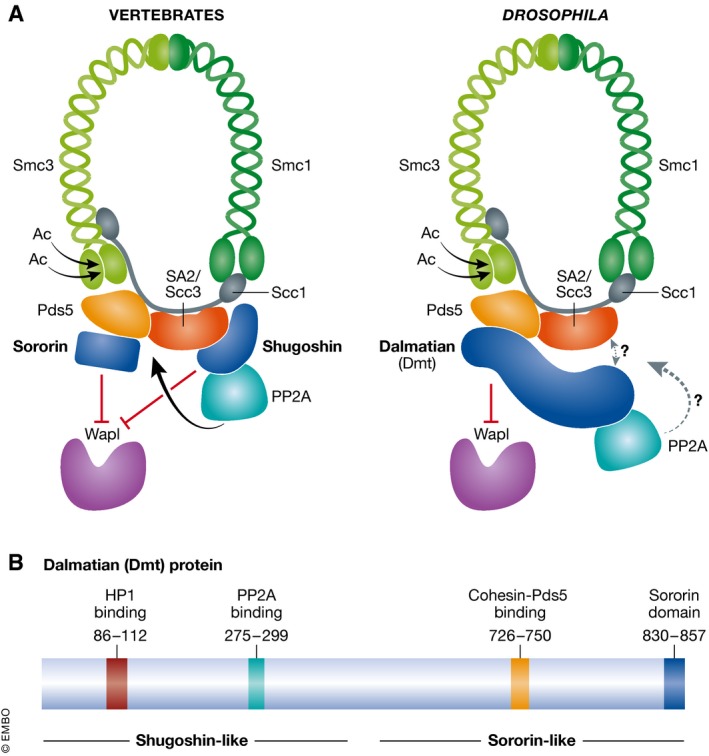
(A) The tripartite cohesin ring and its accessory proteins are conserved between vertebrates and Drosophila, but the sororin and shugoshin regulators are combined in a single protein called Dalmatian (Dmt) in flies. (B) Domain structure of Dalmatian indicating the binding domains mapped by Yamada et al (2017).
The core cohesin complex comprises two structural maintenance of chromosomes (SMC) subunits, Smc1 and Smc3, which, together with Scc1/Rad21, form a ring that is thought to hold the sister chromatids together by topological entrapment (Ouyang & Yu, 2017). Cohesin is loaded onto chromosomes prior to DNA replication but only becomes cohesive upon acetylation of its Smc3 subunit by the Eco1 acetyltransferase, an activity coupled to passage of the replication fork. Three accessory subunits Pds5, SA/Scc3 and Wapl associate with the Scc1 subunit of core cohesin. The accessory subunits Pds5 and Scc3 act as a platform for various cohesin regulators, including sororin and shugoshin, respectively (Hara et al, 2014; Nishiyama et al, 2010). Wapl is the key effector of the prophase pathway of cohesin removal and, in association with Pds5, releases cohesin from chromatin, most likely by opening the Smc3‐Scc1 interface (Kueng et al, 2006; Chan et al, 2012). The destabilizing activity of Wapl is counteracted by cohesin acetylation and, at least in vertebrates, the consequential recruitment of sororin. In interphase, sororin is sufficient to antagonize Wapl. Upon mitotic entry, however, sororin is phosphorylated by mitotic kinases and released from cohesin, rendering cohesin susceptible to Wapl. Protection of cohesin from Wapl in mitosis occurs only at the pericentromere and is the role of shugoshin. Shugoshin, which associates with pericentromeric heterochromatin, recruits protein phosphatase 2A to counteract the cohesin‐destabilizing phosphorylation of both sororin and cohesin by mitotic kinases. Importantly, the precocious loss of cohesion upon depletion of either sororin or shugoshin is rescued by co‐depletion of Wapl, demonstrating that the protective roles of sororin and shugoshin work by counteracting the cohesin‐destabilizing activity of Wapl (Nishiyama et al, 2010, 2013; Hara et al, 2014; Liu et al, 2013).
Whether such cohesin maintenance pathways are relevant outside vertebrates was unclear. Sororin has so far only been identified in metazoans. The Drosophila ortholog, Dmt, shows homology to sororin in its C‐terminus but also possesses an extended N‐terminus that is absent from vertebrate sororin. Additionally, the cohesin protection activity of Drosophila MEI‐S332, the founding member of the shugoshin family, is restricted to meiosis, in common with shugoshins of other non‐vertebrate organisms that also do not protect cohesin during mitosis. These observations led Yamada et al (2017) to investigate the role of Dmt in cohesion maintenance using cultured Drosophila S2 cells. Using a fluorescence in situ hybridization (FISH) assay to measure distance between sister pericentromeres, depletion of Dmt was shown to result in loss of cohesion in both interphase and mitosis. The loss of cohesion in Dmt‐depleted cells was abrogated by co‐depletion of Wapl, confirming that Drosophila Dmt, like vertebrate sororin, antagonizes Wapl‐mediated cohesin destabilization. A hint that Dmt might also have characteristics in common with shugoshins was provided by a pull‐down and mass spectrometry experiment whereby PP2A subunits were identified as Dmt binding partners. Indeed, using a version of Dmt incapable of binding the PP2A regulatory subunit, Wdb, the authors demonstrated that recruitment of PP2A by Dmt is required for proper cohesion during mitosis, but not interphase. These experiments demonstrated PP2A‐dependent antagonization of Wapl by Dmt during mitosis, similar to shugoshin. Remarkably, Yamada et al (2017) demonstrated that Dmt can substitute for shugoshin to generate robust cohesion in human cells, confirming the functional conservation between Dmt and shugoshin.
The discovery that Dalmatian is a hybrid sororin–shugoshin protein raises the question of whether it is recruited to chromosomes through features in common with these cohesin regulators. In vertebrates, sororin is reported to associate with cohesin by binding to its Pds5 accessory subunit (Nishiyama et al, 2010). Shugoshin association with chromosomes is influenced by multiple factors (Marston, 2015): first, during interphase, shugoshin associates with pericentromeric heterochromatin through association with heterochromatin protein 1 (HP1). Second, during mitosis, kinetochore‐localized Bub1 phosphorylates histone H2A on S121 and shugoshin binds directly to this mark. Third, during mitosis, shugoshin is thought to bind the SA2/Scc3‐Scc1 heterodimer of cohesin within the inner centromere. Through identification of domains of interaction and the generation of specific mutations to abolish these interactions (Fig 1), Yamada et al (2017) tested the requirements for Dmt localization to chromosomes in interphase and mitosis together with the importance of these interactions for cohesion. Interestingly, the localization of Dmt shows characteristics similar to both sororin and shugoshin. Like shugoshin, during interphase, Dmt associates with heterochromatin in a HP1‐dependent manner. In mitosis, however, binding to cohesin's Pds5 subunit is required for Dmt association with chromosomes, similarly to sororin. Furthermore, both the Dmt‐HP1 and the Dmt‐Pds5 interaction are important for mitotic cohesion. Intriguingly, localization of Dmt to heterochromatin during interphase is predominantly HP1‐dependent, yet the Dmt‐HP1 interaction appears to be dispensable for interphase cohesion. Perhaps low levels of cohesin‐bound Dmt counteract Wapl to ensure cohesion establishment during interphase, although other mechanisms cannot be ruled out. The significance of the HP1‐Dmt interaction for cohesion in mitosis is also not completely resolved. An attractive hypothesis is that HP1‐Dmt sets up the cohesion protection mechanism during interphase by recruiting PP2A to the pericentromeric heterochromatin, but further investigation is required to determine whether this is the case.
Another fascinating issue is the potential role of Bub1 kinase and histone H2A‐S121‐P in localizing Dmt in Drosophila. Initial experiments suggested that Bub1 is not required for Dmt localization onto chromosomes: though depletion of Bub1 caused loss of cohesion, these effects are likely indirect. This indicates that some features of Dmt are distinct from shugoshin and vice versa. Several functions have been reported for shugoshins, distinct from their cohesin protection activity, and it is possible that these roles have been split between Dmt and MEI‐S332, the canonical shugoshin ortholog, which does not function in cohesion protection. However, the current study did not find any evidence that MEI‐S332 participates in one such reported activity of shugoshins in yeast and vertebrates, sister kinetochore biorientation.
Cohesin is the best‐understood member of a family of chromosome organizer complexes that is conserved from bacteria to humans (Uhlmann, 2016). At the heart of these complexes is a highly conserved dimer of SMC proteins, whose ATPase heads are bridged by additional, more variant subunits. The minimal functional unit of cohesin is the tripartite ring, while in vivo, a plethora of regulatory factors alter its association with chromatin and ability to hold chromosomes together. As exemplified by this fascinating discovery of a hybrid cohesin protector in Drosophila, variations on a theme are likely to emerge, revealing commonalities and surprises in distinct organisms, cell types and functional contexts. Future work in a wide variety of organisms promises to yield further fascinating insights and surprises into the workings of these ancient chromosome organizers.
Acknowledgements
Wellcome support my laboratory through a Senior Research Fellowship [107827] and core funding for the Wellcome Centre for Cell Biology [203149]. I am grateful to Stefan Galander for helpful comments and other members of the laboratory for discussions.
See also: T Yamada et al (June 2017)
References
- Chan K‐L, Roig MB, Hu B, Beckouët F, Metson J, Nasmyth K (2012) Cohesin's DNA exit gate is distinct from its entrance gate and is regulated by acetylation. Cell 150: 961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Zheng G, Qu Q, Liu H, Ouyang Z, Chen Z, Tomchick DR, Yu H (2014) Structure of cohesin subcomplex pinpoints direct shugoshin‐Wapl antagonism in centromeric cohesion. Nat Struct Mol Biol 21: 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, Peters JM (2006) Wapl controls the dynamic association of cohesin with chromatin. Cell 127: 955–967 [DOI] [PubMed] [Google Scholar]
- Liu H, Rankin S, Yu H (2013) Phosphorylation‐enabled binding of SGO1‐PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat Cell Biol 15: 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston AL (2015) Shugoshins: tension‐sensitive pericentromeric adaptors safeguarding chromosome segregation. Mol Cell Biol 35: 634–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T, Ladurner R, Schmitz J, Kreidl E, Schleiffer A, Bhaskara V, Bando M, Shirahige K, Hyman AA, Mechtler K, Peters JM (2010) Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell 143: 737–749 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Sykora MM, Huis In ‘t Veld PJ, Mechtler K, Peters JM (2013) Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating sororin. Proc Natl Acad Sci USA 110: 13404–13409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Yu H (2017) Releasing the cohesin ring: a rigid scaffold model for opening the DNA exit gate by Pds5 and Wapl. BioEssays 39: 1600207 [DOI] [PubMed] [Google Scholar]
- Uhlmann F (2016) SMC complexes: from DNA to chromosomes. Nat Rev Mol Cell Biol 17: 399–412 [DOI] [PubMed] [Google Scholar]
- Waizenegger IC, Hauf S, Meinke A, Peters JM (2000) Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103: 399–410 [DOI] [PubMed] [Google Scholar]
- Yamada T, Tahara E, Kanke M, Kuwata K, Nishiyama T (2017) Drosophila Dalmatian combines sororin and shugoshin roles in establishment and protection of cohesion. EMBO J 36: 1513–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]


