Abstract
Glioblastoma is a highly aggressive brain tumor constituted by glioma stem cell and differentiated cell populations with distinct susceptibility to cytotoxic T lymphocytes crucial for tumor immune surveillance. In this issue of The EMBO Journal, Bassoy et al (2017) show that the surface expression of certain glycans that favor recognition and killing by T cells depends on mitochondrial contacts with the endoplasmic reticulum, extending the function of this interface even to immune recognition.
Subject Categories: Cancer, Immunology, Metabolism
The key organelles in energy production, mitochondria, have also been bestowed with other crucial cellular functions, including regulation of apoptosis, metabolism, and calcium homeostasis. Mitochondria are dynamic organelles, which undergo constant fusion and fission. In mammals, mitofusins (1 and 2) and Opa1 are involved in outer and inner mitochondrial membrane fusion, whereas dynamin‐related protein 1 (Drp1) has been extensively studied for its role in mitochondrial fission, along with its receptors on the mitochondrial outer membrane mitochondrial fission factor (Mff), fission 1 (Fis1), and mitochondrial division (MiD) 49 and 51 (Pernas & Scorrano, 2016). The discovery of the molecular mediators of mitochondrial shape changes allowed the identification of the role of mitochondrial morphology in various complex cellular processes, ranging from apoptosis to metabolism to cancer susceptibility and even to differentiation (Pernas & Scorrano, 2016). Mitochondrial shape is crucial in determining identity, maintenance, and renewal of stem cells (Xie et al, 2015). While these aspects might be attributed to the tight relationship between shape and energetic output of the organelle, a surprising link between shape and the complex cardiomyocyte differentiation process extends the function of mitochondrial shape to the control of key signaling and transcriptional cascades (Kasahara et al, 2013). This control over signaling is primarily achieved by regulation of Ca2+ transients, a process that depends crucially on the juxtaposition of mitochondria with the endoplasmic reticulum (ER). The proximity between ER and mitochondria plays a crucial role in lipid and calcium signaling, apoptosis, mitochondrial fission site determination, autophagosome formation, and cellular stress response (Giacomello & Pellegrini, 2016). A study by Martinvalet and colleagues in this issue of The EMBO Journal extends the function of this interface to the unexpected control of tumor cell recognition and killing by cytotoxic T lymphocytes (Bassoy et al, 2017).
By investigating the susceptibility of the two main cellular components of glioblastoma, a highly aggressive brain tumor composed by a stem and a differentiated population to cytotoxic T lymphocytes (CTLs), Bassoy et al (2017) found that glioma stem cells (GSCs) were more readily attacked and killed in vitro and in vivo than their differentiated glioma cells (GDCs) by CTLs and that this correlated with a reduced sialylation of the surface N‐glycosylated proteins in GSCs. In an elegant time‐lapse experiment, the authors demonstrated that GSCs formed more immune synapses in a shorter duration with cytotoxic T cells when compared to GDCs.
The ability of adaptive immune cells to recognize and attack tumor cells is defined also by the precise composition of the surface N‐glycosylated proteins (known as glycocalyx) (Xiao et al, 2016). Indeed, while glycan profiling did not reveal quantitative differences between GDCs and GSCs, deep sequencing unveiled selective sialylation pathway defects in the latter, potentially explaining their reduced surface staining for sialic acid. When Bassoy et al (2017) delved deeper into the differences between GDCs and GSCs, they found that mitochondria were more elongated in the former. Recent study reported differential regulation of mitochondrial morphology and hence of “stemness” state of glioblastomas. Indeed, activated Drp1 was characteristic of GSCs, resulting in increased mitochondrial fission, and correlated with poor prognosis of glioblastoma (Xie et al, 2015). Immunotherapy, which has been found as an attractive way to treat glioblastoma, has faced challenges mainly due to the complexity of the cell surface of glioma cells (Bovenberg et al, 2013). By manipulating mitochondrial dynamics in GSCs, Bassoy et al (2017) demonstrated a correlation between mitochondrial morphology and expression of sialylated glycans on the cell surface and with immune synapse formation. However, the best proxy for sialylated glycans expression was not mitochondrial length or fusion, but extent of tethering between mitochondria and ER; indeed, Bassoy et al (2017) demonstrated reduced tethering in GSCs that correlated with reduced expression of the ER–mitochondria tether Mfn2 (Naon et al, 2016). Reintroduction of Mfn2 and even more compellingly of an artificial ER–mitochondria tether increased cell surface glycans in GSCs and reduced their killing by CTLs.
Figure 1. Mitochondria–endoplasmic reticulum contacts define surface sialylated N‐glycoproteins and susceptibility of glioblastoma to cytotoxic T lymphocytes.
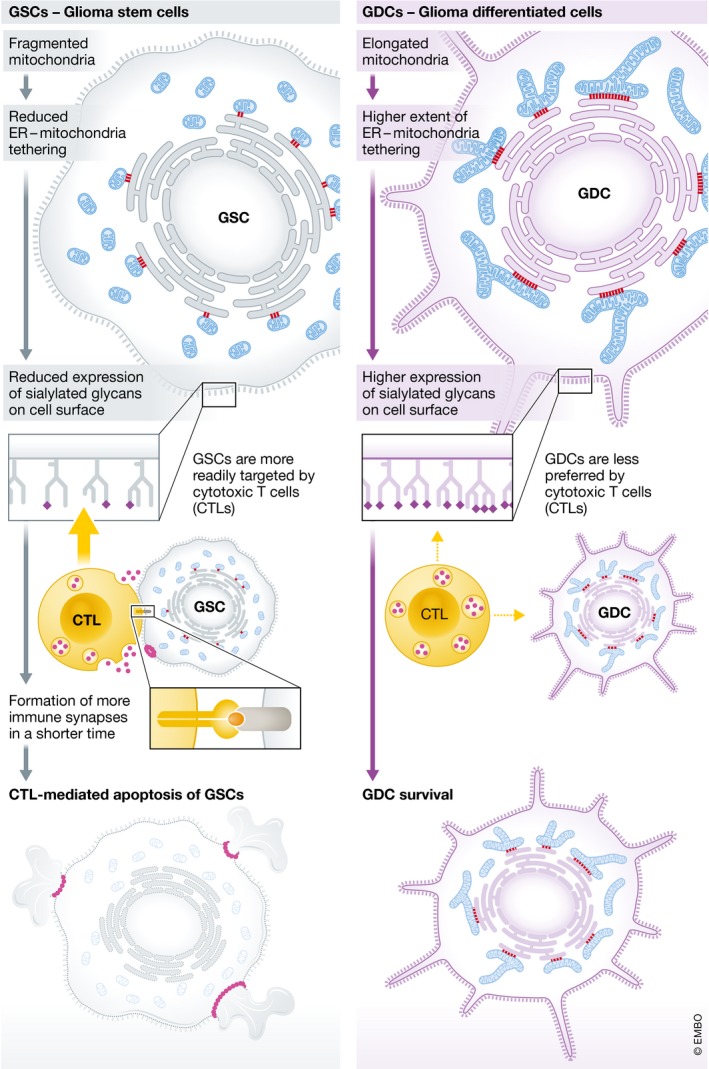
The cartoon depicts how looser ER–mitochondria contacts in glioma stem cells (GSCs, left) make them more susceptible to engagement and killing by CTLs as compared to glioma‐derived cells (GDCs, right) where contacts are tighter.
Tethering between ER and mitochondria was originally reported to participate in two defined pathways of Ca2+ exchange between ER and mitochondria and phospholipid biosynthesis. Later, the biological role of this interface was extended to several processes, including regulation of mitochondrial morphology, apoptosis, autophagy, and metabolism (Giacomello & Pellegrini, 2016). The study of Bassoy et al (2017) is the first report extending the function of ER–mitochondria tethering to a complex pathway such as production and sialylation of N‐glycosylated proteins. An open question is how tethering between ER and mitochondria can affect the sialylation of N‐glycosylated proteins. A possible clue comes from the analysis of the subcellular distribution of the process: N‐glycosylation of proteins starts in the ER, where the initial N‐glycan structure is transferred from dolichol pyrophosphate, a lipid anchor, and linked to a specific asparagine residue of the nascent recipient protein (Bieberich, 2014). ER and then the Golgi apparatus are the sites of trimming and processing of the N‐linked glycan structure that ultimately shapes the complex N‐glycoproteins. In the ER, N‐glycans are also critical for proper protein folding and quality control by chaperones in the unfolded protein response and ER‐assisted degradation (ERAD). Calnexin and calreticulin, two ER chaperones critical in ERAD, bind to monoglycosylated N‐glycoproteins: If the status of the N‐glycosylation is altered, the N‐glycan assisted calnexin/calreticulin protein folding cycle and thus the function of ERAD is altered, potentially leading to an alteration in the fate of N‐glycosylated proteins and a reduction in the exposure of specific sialylated proteins on the cell surface (Bieberich, 2014). Interestingly, calnexin shuttles from the bulk of the ER to the mitochondria‐associated membranes, the ER patches tethered to mitochondria in a process controlled by palmitoylation and by the ER–mitochondria tethering regulator PACS‐2 (Lynes et al, 2012). Thus, altered sialylation might be a consequence of disrupted intra‐ER distribution of calnexin and of malfunctioning ERAD in GSCs, albeit this possibility awaits to be experimentally tested.
In summary, the study by Bassoy et al (2017) reveals a novel function for ER–mitochondria contacts in defining susceptibility of glioma cells to immune effector cells. Their findings open the exciting possibility that treatment of glioblastoma with molecules that directly inhibit ER–mitochondria linkers, such as the newly developed Mfn2 inhibitors (Franco et al, 2016), or with agents that inhibit ERAD, might improve immunotherapy against this very aggressive cancer.
Acknowledgements
L.S. is a Senior Scientist of the Dulbecco‐Telethon Institute. This study was supported by AIRC Italy, ERC FP7‐282280, and FP7 CIG PCIG13‐GA‐2013‐618697; Italian Ministry of Research FIRB RBAP11Z3YA_005; and Italian Ministry of Health GR 05109.
See also: EY Bassoy et al (June 2017)
References
- Bassoy EY, Kasahara A, Chiusolo V, Jacquemin G, Boydell E, Zamorano S, Riccadonna C, Pellegatta S, Hulo N, Dutoit V, Derouazi M, Dietrich PY, Walker PR, Martinvalet D (2017) ER–mitochondria contacts control surface glycan expression and sensitivity to killer lymphocytes in glioma stem‐like cells. EMBO J 36: 1493–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich E (2014) Synthesis, processing, and function of N‐glycans in N‐glycoproteins In Glycobiology of the nervous system, Yu RK, Schengrund C‐L. (eds), pp 47–70. New York, NY: Springer New York; [Google Scholar]
- Bovenberg MSS, Degeling MH, Tannous BA (2013) Cell‐based immunotherapy against gliomas: from bench to bedside. Mol Ther 21: 1297–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A, Kitsis RN, Fleischer JA, Gavathiotis E, Kornfeld OS, Gong G, Biris N, Benz A, Qvit N, Donnelly SK, Chen Y, Mennerick S, Hodgson L, Mochly‐Rosen D, Dorn GW II (2016) Correcting mitochondrial fusion by manipulating mitofusin conformations. Nature 540: 74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Pellegrini L (2016) The coming of age of the mitochondria‐ER contact: a matter of thickness. Cell Death Differ 23: 1417–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara A, Cipolat S, Chen Y, Dorn GW, Scorrano L (2013) Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science 342: 734–737 [DOI] [PubMed] [Google Scholar]
- Lynes EM, Bui M, Yap MC, Benson MD, Schneider B, Ellgaard L, Berthiaume LG, Simmen T (2012) Palmitoylated TMX and calnexin target to the mitochondria‐associated membrane. EMBO J 31: 457–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naon D, Zaninello M, Giacomello M, Varanita T, Grespi F, Lakshminaranayan S, Serafini A, Semenzato M, Herkenne S, Hernandez‐Alvarez MI, Zorzano A, De Stefani D, Dorn GW II, Scorrano L (2016) Critical reappraisal confirms that Mitofusin 2 is an endoplasmic reticulum‐mitochondria tether. Proc Natl Acad Sci USA 113: 11249–11254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernas L, Scorrano L (2016) Mito‐morphosis: mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu Rev Physiol 78: 505–531 [DOI] [PubMed] [Google Scholar]
- Xiao H, Woods EC, Vukojicic P, Bertozzi CR (2016) Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci USA 113: 10304–10309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Wu Q, Horbinski CM, Flavahan WA, Yang K, Zhou W, Dombrowski SM, Huang Z, Fang X, Shi Y, Ferguson AN, Kashatus DF, Bao S, Rich JN (2015) Mitochondrial control by DRP1 in brain tumor initiating cells. Nat Neurosci 18: 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]


