Abstract
Reprogramming adult, fully differentiated cells to pluripotency in vivo via Oct3/4, Sox2, Klf4 and c‐Myc (OSKM) overexpression has proved feasible in various independent studies and could be used to induce tissue regeneration owing to the proliferative capacity and differentiation potential of the reprogrammed cells. However, a number of these reports have described the generation of teratomas caused by sustained reprogramming, which precludes the therapeutic translation of this technology. A recent study by the Izpisúa‐Belmonte laboratory described a cyclic regime for short‐term OSKM expression in vivo that prevents complete reprogramming to the pluripotent state as well as tumorigenesis. We comment here on this and other studies that provide evidence that in vivo OSKM induction can enhance tissue regeneration, while avoiding the feared formation of teratomas. These results could inspire more research to explore the potential of in vivo reprogramming in regenerative medicine.
Subject Categories: Regenerative Medicine, Stem Cells
In vivo cell reprogramming for tissue regeneration
Reprogramming specialized cells within an injured or degenerated tissue to a pluripotent‐like state has been proposed as a novel strategy to promote regeneration while bypassing limitations of traditional cell therapies, namely those associated with ex vivo cell manipulation, transplantation and engraftment. The rationale behind this approach is to take advantage of the proliferative capacity of the reprogrammed cells, and of their ability to re‐differentiate into mature phenotypes, to repopulate the damaged tissue (de Lazaro & Kostarelos, 2014). The use of a well‐defined combination of transcription factors that are able to induce pluripotency in a variety of tissues—the Oct3/4, Sox2, Klf4 and c‐Myc (OSKM) cocktail (Takahashi & Yamanaka, 2006)—adds additional benefits of simplicity and versatility. Ultimately, in vivo reprogramming could provide new therapeutic avenues to treat a variety of conditions such as myocardial infarction, Parkinson's disease or stroke, to name a few, in which the loss of specific cell types cannot be addressed by currently established therapies.
In vitro, reprogramming is induced under defined conditions that promote and maintain pluripotency. However, an initial concern that casted doubts on the clinical translation of in vivo reprogramming is the fact that the pluripotent conversion within the organism has to overcome local pro‐differentiation signals that govern adult, fully differentiated tissues. While several independent studies have now demonstrated that the induction of pluripotency in vivo is indeed feasible via OSKM overexpression (Abad et al, 2013; Yilmazer et al, 2013; Ohnishi et al, 2014), the appearance of teratomas described by Abad et al (2013) and Ohnishi et al (2014) raised concerns about tumorigenesis and shifted the attention towards the safety of the approach. Both studies used a transgenic “reprogrammable” mouse—carrying an OSKM polycystronic cassette under the control of a doxycycline‐inducible promoter—to induce pluripotency within adult tissues. When such animals were fed with doxycycline for extended periods of time, ubiquitous and sustained expression of OSKM caused uncontrolled proliferation and disorganized differentiation followed by extensive tumorigenesis and death (Fig 1b) (Abad et al, 2013; Ohnishi et al, 2014). Both reports established a rather negative outlook for exploiting the therapeutic potential of in vivo reprogramming in regenerative medicine.
Figure 1. The fine line between tumorigenesis and regeneration upon in vivo reprogramming.
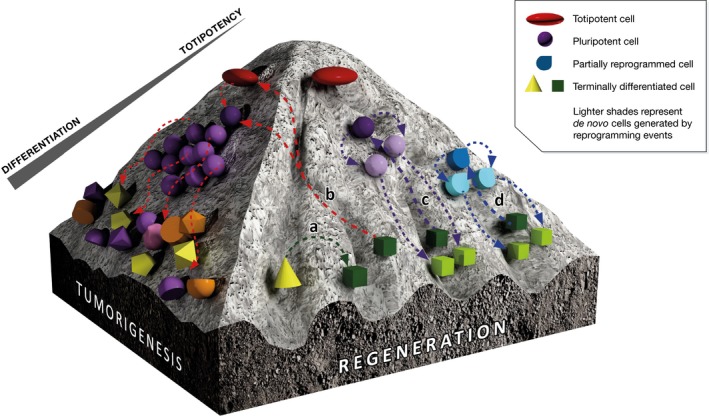
Various cell fate changes can be forced in vivo, albeit their outcomes differ. (a) Transdifferentiation drives direct conversion between specific cell types, but lacks the induction of cell division that maximizes the repopulation of an injured site; (b) sustained reprogramming to pluripotency leads to excessive and uncontrolled proliferation and random re‐differentiation into multiple lineages that results in the generation of teratomas; (c) in vivo reprogramming to a pluripotent or pluripotent‐like state may provide teratoma‐free tissue regeneration provided that the expression of pluripotency features and proliferation are strictly transient; (d) partial reprogramming is accompanied by transient proliferation that may replenish, among others, the pool of progenitor‐like cells crucial to maintain tissue homeostasis upon injury. This illustration has been adapted from the original idea of Waddington's epigenetic landscape.
Transient OSKM expression is key to prevent tumorigenesis and “rejuvenates” aged tissues
The same reports also suggested a direct relationship between the appearance of teratomas and the duration of OSKM expression. The doxycycline‐inducible promoter is in fact a convenient tool to control the on and off of OSKM expression with the administration and withdrawal of the drug; it allowed Abad et al (2013) to compare the effects of a high dose of doxycycline administered for a week to a five times lower dose over a longer interval. The shorter administration scheme resulted in lower tumorigenesis and mortality in spite of the higher dose (Abad et al, 2013). Ohnishi et al (2014) used four different induction schemes (doxycycline administration for 4, 5, 6 or 7 days) and also reported a direct relationship between the length of doxycycline feeding and the incidence of dysplastic lesions. Most of the animals fed with doxycycline for < 5 days did not show any histological aberration. Most importantly, short OSKM induction enabled re‐integration of at least some of the in vivo reprogrammed cells in the tissue. For example, after a transient de‐differentiated and proliferative phase, pancreatic cells re‐acquired their mature phenotype and physiological function and were able to produce insulin again (Ohnishi et al, 2014).
Now, a study by Izpisúa‐Belmonte and colleagues (Ocampo et al, 2016) has confirmed our earlier observations from the transient expression of OSKM in adult mouse liver (Yilmazer et al, 2013) and muscle (de Lazaro et al, 2017) that in vivo reprogramming can indeed completely escape tumorigenesis by limiting the duration of the expression of reprogramming factors. Moreover, Ocampo et al (2016) suggested that transient reprogramming erased various signs of ageing and enhanced the regenerative capacity of aged tissues. They proposed a partial reprogramming strategy in which epigenetic remodelling and active proliferation take place without reaching the pluripotent state (Fig 1d). Primary fibroblasts from progeroid reprogrammable mice—in which the onset of ageing starts at an aberrantly early age—were used to confirm that short‐term OSKM expression for 2–4 days was sufficient to trigger epigenetic changes that erased various hallmarks of the aged phenotype, but not enough to induce endogenous pluripotency markers, such as NANOG and SSEA1, and loss of cell identity. These cells were therefore “molecularly rejuvenated”, but not de‐differentiated to pluripotency.
However, maintaining the rejuvenated phenotype in progeroid cells required re‐induction of reprogramming factors, which prompted Ocampo et al (2016) to explore a short‐term but cyclic induction scheme to translate these findings to the in vivo set‐up. Progeroid reprogrammable mice were administered with doxycycline for 2 days, followed by a 5‐day withdrawal of the drug. The authors showed that six cycles of OSKM induction were sufficient to decrease various hallmarks of ageing, including histological alterations in a number of tissues and deficiencies in cardiac function, while dysplasia or teratomas were not observed for up to 35 cycles. Overall lifespan, which is severely compromised by the disease, was significantly increased in the OSKM group.
The study was also extended to physiological ageing, using 12‐month‐old, non‐progeroid mice. OSKM induction enhanced the otherwise poor regenerative capacity of both pancreas and skeletal muscle and made these tissues more resilient to a later insult. Cyclic induction of OSKM triggered proliferation of beta cells in the pancreas and satellite cells in the skeletal muscle, which are critical for the maintenance of tissue homeostasis, but whose numbers typically decrease with age. The expansion of these cell populations was thought to be the main cause to improved performance after streptozocin‐mediated ablation of beta cells (an accepted model of metabolic disease) and cardiotoxin‐induced muscle injury, a common experimental procedure to study muscle damage and regeneration. However, it would be interesting to interrogate this in vivo partial reprogramming approach in a sarcopenia model that better exemplifies the effects of physiological ageing in skeletal muscle.
Transient in vivo reprogramming to repair injured tissues
The Ocampo et al (2016) study is the first to describe the potential of OSKM to improve the resilience of aged tissues to injury, but others have also proposed OSKM overexpression to induce or enhance cell replenishment and tissue repair after injury. Gao et al (2016) used retroviral vectors to specifically overexpress OSKM in glial cells, aiming to generate de novo cells to repair traumatic brain injury. However, although a number of reprogrammed cells re‐differentiated to the neural lineage, others expressed mesoderm and endoderm markers. More importantly, clusters of pluripotent‐like cells expressing NANOG and SSEA4 actively proliferated over time and eventually generated teratomas. These results confirm the limited clinical relevance of sustained in vivo reprogramming, in this case likely linked to the use of retroviral vectors.
With the specific goal of avoiding sustained reprogramming, our laboratory has used episomal plasmid DNA to induce OSKM in vivo (Yilmazer et al, 2013). In a recent report, we showed that the generation of cells within skeletal muscle tissue that proliferate and express pluripotency markers—including NANOG—only transiently, not only eludes tumorigenesis but also enhances regeneration in a clinically relevant skeletal muscle injury model that involved laceration of the medial gastrocnemius (Fig 1c) (de Lazaro et al, 2017).
Indeed, the existence of a transient proliferative phase upon reprogramming via OSKM induction, either as partial reprogramming (Fig 1d) or as a transient pluripotent‐like state (Fig 1c), may be more efficient in repopulating the injured site compared to transdifferentiation strategies (Fig 1a) that use cell‐specific transcription factors to mediate direct conversion between mature cell types, but lack the induction of cell division (Fig 1a). In addition, two studies have recently suggested that senescence signals, triggered by tissue damage and ageing, could favour and increase the efficiency of in vivo reprogramming (Chiche et al, 2016; Mosteiro et al, 2016). This interaction may reinforce the potential of the OSKM cocktail to assist regeneration of injured and aged tissues.
Future directions and concluding remarks
Collectively, all these studies confirm that in vivo overexpression of OSKM can induce cell reprogramming without increasing the risk of teratoma formation. However, transient expression of such factors is key to avoid sustained pluripotency, uncontrolled proliferation and tumorigenesis, and should therefore be a prerequisite for clinical applications, either via partial in vivo reprogramming or through the generation of transient pluripotent‐like intermediate cells. The potential of the OSKM cocktail to enhance regeneration is encouraging in both strategies. To further explore therapeutic approaches, therefore, it is imperative that the field moves beyond any presumed association of in vivo reprogramming with teratoma formation and instead focuses on resolving the remaining challenges towards clinical translation.
Among those, the in vivo delivery of OSKM—or other reprogramming factors—will be a major issue and will greatly depend on the specific condition to be treated. In the context of a systemic disease, such as progeria, it may be unrealistic to raise expectations for a vector able to transduce all cells in a patient. Instead, it will be crucial to investigate whether reprogramming specific tissues, such as vital organs as the brain or liver, might be sufficient to halt or delay the life‐limiting progress of the disease. Even to reprogram a particular organ, tissue or cell type, engineering delivery vectors that provide targeted, efficient, transient and repeated expression of OSKM factors will be a crucial requirement to move from biologically interesting, yet clinically irrelevant, transgenic mouse models to clinical studies. In addition, as with any other therapeutic intervention, it will be important to account for potential species‐specific differences when aiming to identify the balance between efficient reprogramming and potential undesired effects. The history of clinical gene therapy offers many examples of strategies that worked very efficiently in animal models, but had detrimental outcomes in human patients (Wilson, 2009). Eventually, this will only be overcome with early‐stage, well‐designed and cautious human clinical studies.
In conclusion, we believe that the proof of concept of in vivo reprogramming for tissue regeneration and the strategies to elude tumorigenesis discussed here represent two initial prerequisites that should encourage further research towards clinical use. They will hopefully build enough confidence to continue on the long road to clinical translation of transient in vivo cell reprogramming for tissue regeneration and rejuvenation.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to acknowledge Ms Noemí López Martínez for her help in the three‐dimensional design of Fig 1.
Contributor Information
Giulio Cossu, Email: giulio.cossu@manchester.ac.uk.
Kostas Kostarelos, Email: kostas.kostarelos@manchester.ac.uk.
References
- Abad M, Mosteiro L, Pantoja C, Canamero M, Rayon T, Ors I, Grana O, Megias D, Dominguez O, Martinez D et al (2013) Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 502: 340–345 [DOI] [PubMed] [Google Scholar]
- Chiche A, Le Roux I, von Joest M, Sakai H, Aguin SB, Cazin C, Salam R, Fiette L, Alegria O, Flamant P et al (2016) Injury‐induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell 20: 407–414 [DOI] [PubMed] [Google Scholar]
- Gao X, Wang X, Xiong W, Chen J (2016) In vivo reprogramming reactive glia into iPSCs to produce new neurons in the cortex following traumatic brain injury. Sci Rep 6: 22490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lazaro I, Kostarelos K (2014) In vivo cell reprogramming to pluripotency: exploring a novel tool for cell replenishment and tissue regeneration. Biochem Soc Trans 42: 711–716 [Google Scholar]
- de Lazaro I, Yilmazer A, Nam Y, Qubisi S, Razak F, Cossu G, Kostarelos K (2017) Non‐viral induction of transient cell reprogramming in skeletal muscle to enhance tissue regeneration. bioRxiv doi: 10.1101/101188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteiro L, Pantoja C, Alcazar N, Marion RM, Chondronasiou D, Rovira M, Fernandez‐Marcos PJ, Munoz‐Martin M, Blanco‐Aparicio C, Pastor J et al (2016) Tissue damage and senescence provide critical signals for cellular reprogramming in vivo . Science 354: aaf4445 [DOI] [PubMed] [Google Scholar]
- Ocampo A, Reddy P, Martinez‐Redondo P, Platero‐Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E et al (2016) In vivo amelioration of age‐associated hallmarks by partial reprogramming. Cell 167: 1719–1733.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi K, Semi K, Yamamoto T, Shimizu M, Tanaka A, Mitsunaga K, Okita K, Osafune K, Arioka Y, Maeda T et al (2014) Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation. Cell 156: 663–677 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Wilson JM (2009) Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol Genet Metab 96: 151–157 [DOI] [PubMed] [Google Scholar]
- Yilmazer A, de Lazaro I, Bussy C, Kostarelos K (2013) In vivo cell reprogramming towards pluripotency by virus‐free overexpression of defined factors. PLoS ONE 8: e54754 [DOI] [PMC free article] [PubMed] [Google Scholar]


